Highlights
What are the main findings?
- Only 2.3% (52 total) of all human antisense genes have been examined in head and neck cancer (HNC) thus far.
- HOXA10-AS, LEF1-AS1, MSC-AS1, and ZEB2-AS1 have clinical promise for future biomarker development.
What are the implications of the main findings?
- Robust cross-validations on antisense gene aberrations will promote biomarker development and implementation in the HNC field, as genetic biomarkers are lacking.
- Antisense gene aberrations and treatment responses are underexplored and warrant future studies.
Abstract
Antisense genes (usually suffixed by -AS) represent a class of long non-coding RNAs (lncRNAs) transcribed from the opposite strand of annotated human genes or exon(s). A total of ~2236 human antisense genes exist in the human genome. Their genomic locations with respect to the corresponding sense genes, their dysregulated expression patterns in cancer specimens, and clinical associations with patient outcomes reveal their potential importance in clinical settings. As of today, there lacks a comprehensive review of HNC-associated antisense genes/transcripts to help move forward the antisense field for genetic biomarker development or future drug research. In total, 2.3% (52/2236 antisense genes) of all known human antisense genes have been investigated in head and neck cancer (HNC). Thus, we perform a comprehensive review of the genomic aberrations (mutations, copy number changes, RNA-expression dysregulation, and single nucleotide polymorphisms) associated with HNC patient prognosis, disease progression, cancer cell signaling, drug sensitivity, and radio-resistance. Four antisense genes, namely HOXA10-AS, LEF1-AS1, MSC-AS1, and ZEB2-AS1, have been clinically cross-validated and have consistently demonstrated to be associated with patient outcomes in multiple independent cohorts by different research teams, with clear evidence for the prioritization of clinical biomarker development in HNC. Single nucleotide polymorphisms (SNPs) of antisense genes with evidence for HNC risk or outcomes should be further validated in different ethnic groups, for potential global HNC applications.
1. Introduction
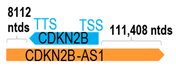
Based on the most up-to-date HUGO Gene Nomenclature Committee gene list in 2025, there is a total of 2236 human antisense genes in the entire human genome (https://www.genenames.org/; accessed on 13 May 2025). All such antisense genes, standardized with the -AS, -AS1, or -AS2 suffixes (with a few exceptions), represent long non-coding RNA genes that are transcribed from the opposite strand of annotated human genes or exon(s). For example, the CDKN2B-AS1 gene encodes a long non-coding RNA that is transcribed from the opposite (reversed) strand of the human CDKN2B gene, hereafter called the corresponding sense gene. Interestingly, among all the human antisense genes, some are found to span the entire opposite strand of the corresponding human gene, while some only encompass part of an exon or several exons, or encompass the immediate 5′- or the 3′-untranslated region (UTR) of a human gene. As the genomic locations of most antisense genes are universally situated in either the exons, 5′-, or 3′-UTRs of specific human genes (Figure 1A), antisense genes often distinguish themselves from another class of long non-coding RNA called LINCs (long intergenic non-coding RNAs), which are non-coding RNAs greater than 200 nucleotides long with no overlapping annotated coding genes.
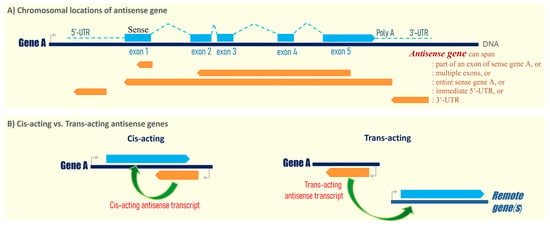
Figure 1.
(A) Schematic showing the chromosomal location of a sense gene (gene A, for example) and the antisense gene found in human. Theoretically, an antisense gene runs in the opposite direction with sequence overlapping with either the exonic region(s) (e.g., overlapping with part of an exon, mostly exon 1; or overlapping with multiple exons), the 5′-untranslated region (5′-UTR) or 3′-untranslated region (3′-UTR) of its corresponding human gene. Sense gene transcript is shown with exons depicted as blue boxes, and introns in the blue dotted line. Orange arrows represent possible locations of the antisense gene (DNA). (B) Cis-acting vs. trans-acting antisense genes in gene regulation. A cis-acting antisense gene regulates the expression of its sense gene that overlaps or is immediately adjacent to the antisense gene, whereas a trans-acting antisense gene can regulate the expression of other gene(s) at different chromosomal location(s) in a remote manner. The antisense gene transcript is shown in orange arrow, while the sense gene transcript in light blue arrow.
As of today, it remains to be fully characterized if all 2236 human antisense genes are primarily involved in the regulation of their sense gene expression in cis. As defined, a cis-acting antisense gene regulates the expression of its sense gene that overlaps or is immediately adjacent to the antisense gene (Figure 1B). Studies have shown that some antisense genes can regulate exon-specific splicing, thus controlling the expression of various RNA isoforms, and can regulate the mRNA’s stability and location of the sense gene [1]. However, a trans-acting antisense gene can regulate the expression of other gene(s) at different chromosomal location(s) in a remote manner (Figure 1B). For example, some antisense genes can function as sponges for various cellular RNAs or microRNAs, thus regulating the expressions of multiple target genes simultaneously. Thus, both cis-acting and trans-acting antisense genes could potentially exhibit diverse cellular functions, and even global effects on cell growth, differentiation, and cell death, etc. The cancer field has begun to examine how human antisense genes contribute to human carcinogenesis, and whether they can serve as therapeutic targets for human cancers.
Head and neck cancer (HNC) is the sixth most common cancer in the world. The World Health Organization (WHO) projects > 1.08 million new cases per year by 2030 [2]. HNC encompasses cancers arising from the oral cavity, pharynx, and larynx. Etiologies include smoking, alcohol consumption, air pollution, and genetics, as well as infection with oncogenic viruses in specific parts of the pharynx, such as the Human Papillomavirus (HPV) for oropharyngeal cancer, and the Epstein–Barr virus (EBV) for nasopharyngeal cancer. Recent findings show that some human antisense genes are found to contribute to HNC tumorigenesis, cancer cell aggressiveness, and signaling regulation, as well as drug sensitivity. As of today, there lacks a comprehensive review of HNC-associated antisense genes/transcripts to help move forward the field for the development of genetic biomarkers or future drug research. Thus, we hereby summarize all recent clinical and biological findings on such 52 HNC-related antisense genes/transcripts, with the objective of identifying potential antisense genes as targets for future biomarker and drug development in HNC.
2. Only 2.3% of All Human Antisense Genes Explored in HNC
There is a rising interest in the study of human antisense genes in both normal and disease contexts. In the past decade, more than 2270 studies have been published on human antisense genes in human models and human tissues, as well as human cancers, based on the PubMed database. This has far exceeded the number of published studies (535) on “intergenic, non-exon-associated” long non-coding RNAs “LINCs”. Among all published human antisense gene studies, 61 studies on 52 antisense genes reported on HNC tumors or cell models (based on PubMed data captured on 13 May 2025). The 61 studies were identified using the keywords “antisense genes or transcripts” and “head and neck cancer” (including each HNC subtype each time, including oral, laryngeal, pharyngeal, salivary gland, oropharyngeal, or nasopharyngeal cancer) using the PubMed literature search engine on https://pubmed.ncbi.nlm.nih.gov/. All extracted information was cross-checked by two or all three authors of this review.
From a total of 2236 human antisense genes annotated by HuGO (listed in Supplementary Table S1), only approximately 2.3% of them have been studied in HNC (52/2236 genes). In order to have a deeper understanding of the plausible roles of this interesting class of genes in HNC carcinogenesis, we first summarize their aberrant expression patterns in HNC per the published reports, followed by analysis of their chromosomal locations in relation to specific exons of the corresponding paired sense genes.
2.1. Chromosomal Locations of 52 HNC-Related Antisense Genes in Relation to Respective Sense Gene/Exon(s)
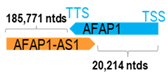
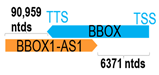
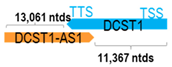
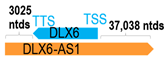
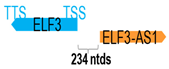
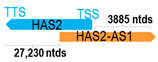
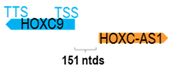
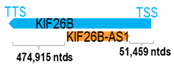
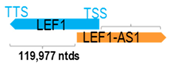
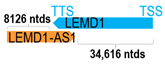
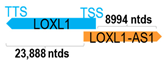
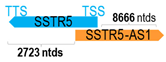
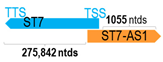
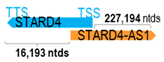
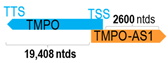
A human antisense gene, transcribed from the antisense orientation of a specific sense gene or exon(s), is believed to have a presumed role in the regulation of the gene or exon of interest. Yet, such a presumed role still needs to be experimentally proven for all antisense genes. To aid future functional validation studies, we listed the detailed chromosomal locations of the 52 HNC-reported antisense genes, the exact lengths and locations in relation to the sense target genes/exon(s), the reported number of antisense gene isoforms (based on UCSC Genome Browser information), and the length ratios of the antisense vs. sense gene in Table 1.

Table 1.
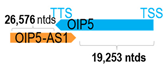
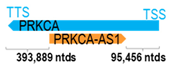
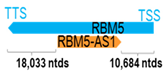
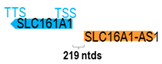
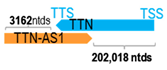
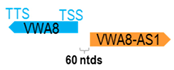
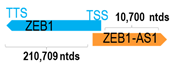
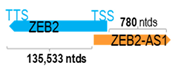
Genomic information of 52 HNC-related antisense genes in relation to corresponding sense genes (based on NCBI GenBank information with GRCh38p.14 reference genome and NCBI RefSeq annotations from https://www.ncbi.nlm.nih.gov/gene/; retrieved on 11 December 2025). NR number is listed below each antisense transcript. Distance to transcription start site (TSS) or transcription termination site (TTS) of the sense gene is listed for each antisense gene graphically. Ratio of antisense/sense lengths = number of nucleotides of antisense gene/number of nucleotides of the corresponding sense gene. n.d.: not determined.
First, the 52 HNC-studied antisense genes have lengths ranging from 989 to 227,501 nucleotides, with the longest being STARD4-AS1, and the shortest being HOXC-AS1. Second, based on the GenBank and UCSC Genome Browser data, most of these antisense genes have only one isoform, while 19 of them are known to have multiple isoforms (ranging from 2 to 32 isoforms; Table 1). Further, we found that irrespective of the length of the antisense gene, none of these 52 antisense genes cover all exons of the presumed target/corresponding sense gene. In fact, when we compared the ratio of the antisense gene/presumed target gene lengths, 39 such antisense genes were shorter than their sense target genes, among which, 16 of them bear less than 10% of the target gene length. Overall, 13 antisense genes are longer than their corresponding sense genes, with MSC-AS1 being 74.72 times longer than the corresponding MSC gene (musculin; a very small human gene with only two exons). MSC-AS1 resides in the antisense orientation, overlaps with exon 1, the 5′-UTR, and is far ahead of the MSC gene (Table 1). It is likely that the MSC-AS1 gene, whose length far exceeds that of MSC, plays a regulatory role, both locally and beyond. This significant length difference suggests that MSC-AS1 may regulate MSC gene expression, likely by short-range and long-range mechanisms such as chromatin remodeling and transcriptional interference, etc. Interestingly, a closer examination of the nearby genes or enhancers covered by MSC-AS1 reveals that it overlaps with a major part of the TRPA1 gene (transient receptor potential cation channel subfamily A member 1), with a not-yet-identified function, as well as more than 10 enhancers, including two H3K4me1 hESC enhancers (LOC127459754 and LOC127459755), the BRD4-independent group 4 enhancer (LOC126860417), the MED14-independent group 3 enhancer (LOC126860416), and multiple enhancers with unknown gene interactions, as mapped by a massive ATAC-STARR-seq based on NCBI gene information (www.ncbi.nlm.nih.gov). It is plausible that MSC-AS1 may have a much wider enhancer-related regulatory function on other genes in addition to MSC. This may warrant further investigations.
2.2. Antisense Genes on Exon 1, Early Introns, or Promoter Region of Sense Genes
Sixteen of the fifty-two antisense genes (30.8%) have sequence complementarity to the first exon of the corresponding sense genes, which is noteworthy for sense gene regulation. First, exon 1 is usually positioned immediately downstream of the promoter region, where transcription is initiated. Antisense gene transcription (RNA expression) with exon 1 sequence complementarity can significantly influence sense gene expression through promoter interference, chromatin remodeling, and transcriptional collision [1]; or, it can affect the mRNA stability of the sense gene by mediating its degradation [56]. Additionally, many human promoters are inherently bidirectional, meaning that they possess the capacity to initiate transcription in both sense and antisense directions from the same genomic locus. This bidirectional transcription often results in the production of antisense RNAs originating in close proximity to the transcription start site (TSS) of protein-coding genes, frequently leading to overlap with exon 1 or the 5′-UTR of the corresponding sense gene [57,58]. This genomic arrangement allows antisense genes to engage in cis-regulatory mechanisms that modulate the expression of their corresponding sense genes. By overlapping or residing near promoter regions, antisense transcripts can influence promoter activity by altering the accessibility or configuration of transcription factor binding sites. They can also affect the recruitment and progression of RNA polymerase II, thereby impacting transcription initiation and elongation. Furthermore, antisense transcription can induce local histone modifications, such as changes in methylation or acetylation marks, leading to chromatin remodeling that either facilitates or represses gene expression [1,56].
Six of the fifty-two antisense genes (11.5%) have sequence complementarity to intron 1 or intron 2 of the corresponding sense genes. These are as follows: FAS-AS1, FUT8-AS1, KIF26B-AS1, PRKCA-AS1, LHFPL3-AS1, and IGF2BP2-AS1. Introns are mostly known to be involved in splicing; therefore, effects on introns by these antisense genes may likely regulate splicing (thus, altering splice variant expression patterns) and sense gene expression by interference with spliceosomes and RNA polymerase recruitment, respectively [1,59]. Further, recent findings reveal that introns can also increase sense gene expression without serving as binding sites for transcriptional factors; rather, through intron-mediated enhancement mechanisms, they can alter the transcription rate, nuclear export, and transcript stability of the sense gene factors [60], thus regulating the dynamics of sense gene expressions.
Interestingly, 17 of the 52 antisense genes (32.7%) do not overlap with but only reside adjacent to their corresponding sense genes at the genomic level. These include the following: FGD5-AS1, FOXD2-AS1, HNF1A-AS1, HOXC-AS1, HOXA11-AS, KTN1-AS1, COX10-AS1, MNX1-AS1, RASAL2-AS1, RHPN1-AS1, SLC16A1-AS1, USP2-AS1, VWA8-AS1, ZFAS1, RGMB-AS1, ZNF667-AS1, and ELF3-AS1. It is plausible that the antisense genes residing at the 5′-UTR of the sense genes may affect the promoter or enhancer activity of the adjacent sense genes, while those residing at the 3′-UTR of the sense genes may affect mRNA stability, processing, and localization of the sense genes [61]. Lastly, these antisense genes could potentially exert their regulatory effects through non-sequence complementary mechanisms, such as transcriptional interference, chromatin remodeling, or long-range regulation on the adjacent sense genes and even some other genes nearby as well [60].
2.3. Additional HNC-Relevant Antisense Genes Revealed by TCGA-HNC Recurrent Focal Amplified/Deleted Regions
Recently, the Cancer Genome Atlas (TCGA) characterization of HNC patient tumors (N = 279 cases) revealed multiple notable recurrent focal amplified and deleted chromosomal regions in which 27 antisense genes are located [62]. Table 2 lists the location of TCGA-reported HNC recurrent focal amplification or deleted regions bearing 27 residing antisense genes with reported q values in the cohort [62]. Among which, four have been previously studied in HNC (AFAP1-AS1, DLX6-AS1, OIP5-AS1, and MRPL23-AS1). Consistent with TCGA data, DLX6-AS1 expression has been previously reported to be up-regulated in Laryngeal Squamous Cell Carcinoma (LSCC) and nasopharyngeal carcinoma (NPC) tissues [8,63]. However, inconsistency exists for AFAP1-AS1, OIP5-AS1, and MRPL23-AS1, which were reported to reside within the recurrent focal deleted loci by TCGA-HNC cohort; N = 279, yet, they have been previously reported to be frequently upregulated in head and neck tumor tissues, including NPC, oral squamous cell carcinoma (OSCC), tongue squamous cell carcinoma (TSCC), salivary adenoid cystic carcinoma (SACC), and LSCC [3,30,34,64,65,66,67,68]. Future investigations are warranted in larger and more ethnically diverse HNC cohorts as TCGA cohort mainly comprises Caucasian HNC patients.

Table 2.
TCGA-reported recurrent focal amplified and deleted chromosomal regions with antisense genes located within the detected peak boundaries (peaks defined by GISTIC 2.0 version; data extracted from Cancer Genome Atlas 2015 [62]; N = 279 cases).
For the remaining 23 TCGA-associated antisense genes, five of them have been studied in other cancer types. For instance, DICER-AS1 downregulation was reported to be associated with advanced staging in gastric cancer [69]; NPPA-AS1 downregulation was associated with tumor aggressiveness in cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC) [70]; and tumor-specific downregulation of ENTPD3-AS1 was reported to be associated with poorer patient overall survival (OS) and relapse-free survival (RFS) in LUAD [71], followed by the subsequent biological demonstration of ENTPD3-AS1′s suppressive role in LUAD cell growth and migration. Lastly, BVES-AS1, which is located at a recurrently deleted genomic region of HNC (TCGA-HNC) [62], has also been reported to be downregulated in colorectal cancer (CRC), with a significant association with TNM staging in CRC patients [72]. Overall, the aberrant downregulation of these antisense genes in the published TCGA-HNC cohort and additional cancer types implicate that certain antisense genes may worth future clinical and biological investigations for biomarker purposes.
3. Clinical Significance of 52 Antisense Genes in HNC
3.1. Prognostic Findings on Antisense Genes in HNC Patient Cohorts
Since the biological roles of antisense genes (or their transcripts) in humans can be complex and multifaced, cancer researchers often examine their clinical values, mainly by examining their RNA expression (i.e., the “antisense transcripts”) in patient cohorts prior to subsequent functional investigations. As summarized in Table 3, a total of 27 antisense genes/transcripts have been examined for their potential clinical significance in either TCGA-HNC cohort (the largest cohort to date) or independent cohorts. First, 24 such antisense transcripts were found to be overexpressed in HNC patient tumors compared to adjacent normal tissues or normal control tissues, and were reported to be associated with poorer overall survival (OS) or poorer disease-free survival (DFS), implicating their potential involvement in HNC tumorigenesis or progression. Notably, the prognostic significance of HOXA10-AS, LEF1-AS1, MSC-AS1, and ZEB2-AS1 has been independently reported in multiple cohorts with consistent patient outcome associations and will be discussed at length below. For the antisense genes that predict patient outcomes in TCGA-HNC cohort, we believe that future clinical validations would be important to help classify them as “validated biomarkers” for HNC. One side note, only two antisense transcripts, namely COX10-AS1 and SBF2-AS1, were found to have specific downregulation in HNC and were associated with poorer OS (Table 3).

Table 3.
Specific upregulation or downregulation of antisense RNA transcripts with significance association with head and neck cancer patient outcomes from various cohorts. Published Hazard Ratios (HRs) for overall survival were extracted from the published references. N.A.: not available.
In the section below, we will focus on four antisense transcripts (HOXA10-AS, LEF1-AS1, MSC-AS1, and ZEB2-AS1), which have been cross-validated in multiple independent HNC patient cohorts, and which can be harnessed for future clinical biomarker development with confidence.
HOXA10-AS (also known as HOXA-AS4) is a relatively novel antisense gene. It is predicted by Alliance of Genome Resources to be involved in miRNA-mediated post-transcriptional gene silencing and is likely part of the RISC complex. As shown in Table 3, independent studies showed that HOXA10-AS overexpression was significantly associated with poorer OS in oral and laryngeal SCC patients [18,75,76,80]. Its overexpression was also found to be associated with poorer survival in other malignancies, including low-grade glioma [81], lung adenocarcinoma [82], gastric cancer [83], and leukemia [84]. Using two oral cancer cell lines, SAS and SCC25, it has been demonstrated that the specific knockdown of HOXA10-AS mRNA impeded cancer cell growth, migration, and clonogenicity in vitro. Further, overexpression and specific knockdown of HOXA10-AS transcript in SAS cells resulted in increased and reduced tumor growth in vivo, respectively, confirming HOXA10-AS’s role in promoting oral cancer growth. Bioinformatics analyses showed that HOXA10-AS could be linked to TP63 mRNA processing. Though the potential activity of HOXA10-AS on its presumed sense gene has not been studied in HNC, the regulatory activity of HOXA10-AS on HOXA10 has been demonstrated in esophageal cancer and glioblastoma models [85,86]. Interestingly, in lung adenocarcinoma models, HOXA10-AS was linked to Wnt/beta-catenin signaling, and its expression was regulated by the transcriptional factor ELK1 [82]. In gastric cancer cells, HOXA10-AS serves as an oncogene, promoting gastric tumor growth by sponging up miRNA-6509-5p, thus enhancing YBX1 signaling for tumorigenic activities [83].
LEF1-AS1 (also known as LEF1NAT) is a known oncogene in glioblastoma, hepatocellular carcinoma, and oral cavity SCC (OSCC) [24,87,88]. Using 88 pairs of OSCC tissues, Zhang et al. showed that LEF1-AS1 was significantly upregulated in OSCC and such an upregulation was associated with poorer prognosis in patients [77]. This finding was consistent with observations from Fan et al. using TCGA-HNC database [77]. Subsequent functional studies demonstrated that LEF1-AS1 interacted with LATS1 to suppress Hippo signaling and promote YAP nuclear translocation and function in OSCC [77]. In glioblastoma, LEF1-AS1 has been demonstrated to act as a competing endogenous RNA (ceRNA) to sponge and downregulate miR-543, resulting in the upregulation of EN2 (engrailed homeobox 2) and increased tumor aggressiveness [89]. In hepatocellular carcinoma cells, LEF1-AS1 can modulate the miR-10a-5p level to upregulate MSI1 (Musashi1) expression and activate AKT signaling, contributing to chemoresistance [90]. These findings suggest that LEF1-AS1 could serve as a prognostic biomarker and therapeutic target in multiple cancer types.
MSC-AS1 (also known as MAT1) is overexpressed in HNC and NPC. Its overexpression was found to be associated with poorer OS in NPC patients and in TCGA-HNC patients [32]. Functional studies showed that MSC-AS1 promoted NPC cell proliferation, invasion, and epithelial-to-mesenchymal transition (EMT), while inhibiting NPC cell apoptosis. Mechanistically, MSC-AS1 acts as a molecular sponge for miR-524-5p, leading to upregulation of the NR4A2 oncogene in NPC. In other cancers (gastric and lung cancer), MSC-AS1 has been shown to have proto-oncogenic roles, including the promotion of cancer cell proliferation, migration, and drug resistance. For example, MSC-AS1 has been demonstrated to regulate the expression of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (FKFB3), thus affecting glycolysis and gastric cancer cell proliferation [91], while in lung cancer cells, it sponges up miR-33b-5ps, thus altering the glycerol-3-phosphate acyltransferase, mitochondrial (GPAM), which has been implied in glycerolipid biosynthesis [92]. In summary, MSC-AS1 may potentially have diagnostic implications for HNC and some other cancers.
ZEB2-AS1 (also known as ZEB2-AS, ZEB2AS, or ZEB2NAT) is a naturally occurring antisense transcript located at the 5′-UTR of zinc finger E-box binding homeobox 2 (ZEB2). It is known that activation of the transforming growth factor-beta pathway could drive ZEB2-AS1 transcription [93]. Diao et al. reported the tumor-specific upregulation of the ZEB2-AS1 transcript in 71 paired normal–tumor Asian HNC tissues [52]. They reported that patients with tumoral overexpression of the ZEB2-AS1 transcript had significantly poorer OS and DFS compared to those with low ZEB2-AS1 expression. Functional data showed that specific knockdown of ZEB2-AS1 overexpression by the oligonucleotide approach resulted in a reduction in HNC cell growth, cell migration, and clonogenicity, with increased cell death, confirming its tumor-promoting roles in HNC. They further demonstrated that ZEB2-AS1 could regulate the mRNA stability of its sense gene, ZEB2, which is a key transcription factor mediating EMT. In addition to HNC, ZEB2-AS1 has also been found to be upregulated in hepatocellular carcinoma [94] and bladder [95], lung [96], and gastric cancers [97], etc. With further validations in additional patient cohorts in HNC and other cancers, ZEB2-AS1 expression may serve as a prognostic biomarker for these cancers.
3.2. Clinical Significance of Single Nucleotide Polymorphism (SNP) of Antisense Genes in HNC
In addition to gene upregulation or downregulation, single-nucleotide polymorphisms (SNPs) of several HNC-studied antisense genes have been reported, including those of CDKN2B-AS1, HOTAIR, and FAS-AS1. Here, we summarize the potential clinical importance of their associated SNPs in HNC development.
CDKN2B-AS1 SNPs: At least eight CDKN2B-AS1 SNPs have been reported in various HNC subtypes thus far. In a genome-wide association (GWA) meta-analysis for oral cancer risk, Lesseur et al. identified a susceptibility locus at CDKN2B-AS1 on 9p21.3. The associated SNP rs8181047 was significantly associated with increased risk of oral cancer in Europe [odds ratio (OR) = 1.18], North America (OR = 1.25), and South America (OR = 1.39), with an overall calculated OR of 1.16 (p = 7 × 10−7) [98]. However, a subsequent study analyzing five CDKN2B-AS1 SNPs (rs564398, rs1333048, rs1537373, rs2151280, and rs8181047) in 1060 OSCC cases and 1183 controls in Taiwan found no direct association of rs8181047 with overall OSCC risk, while carriers of a minor allele of rs1333048 (AA changed to CA/CC genotypes) had a higher likelihood of developing advanced-stage OSCC (stage III/IV vs. stage I/II), especially in betel quid chewers and cigarette smokers [99]. There could be potential differences in oral cancer risk associated with rs8181047 among different ethnic groups, which would require further investigations. In EBV-associated NPC, Wang et al. showed that in Southern China, the rs2069418 of CDKN2B-AS1, in particular, the CC genotype, was associated with increased NPC risk compared to individuals bearing the heterozygote GC genotype (the cohort comprised 10,472 NPC cases and 6907 control cases). Further, such a CC genotype may promote the expression of CDKN2B-AS1 in NPC cells by increasing the enhancer activity of CRE3 [100]. Subsequent overexpression and specific gene knockdown experiments demonstrated that CDKN2B-AS1 likely acts as an oncogene in NPC.
HOTAIR SNPs: A total of six HOTAIR SNPs have been investigated in HNC. Su et al. examined the potential association of four HOTAIR SNPs, namely rs920778, rs1899663, rs4759314, and rs12427129, in a Taiwanese oral cancer cohort (907 OSCC vs. 1200 control cases). They found that rs1899663 (TT genotype) was significantly associated with an increased risk of OSCC with an adjusted odds ratio (AOR) of 2.227. Among the OSCC cases were 193 non-betel quid users. Subsequent analysis focusing on non-betel quid users’ risk, with rs1899663 (TT genotype) significantly associated with increased OSCC risk with an AOR of 2.380, and AORs of 1.437 and 1.436 for rs920778 (TC and TC + CC genotypes, respectively) [101]. In a Chinese HNC cohort (366 HNC and 732 control cases), HOTAIR’s SNP rs4759314 was found to be associated with increased risk of HNC, especially among older individuals and alcohol consumers. rs4759314 was associated with a significantly increased risk of HNC among the Chinese population [GG vs. AA: adjusted odds ratio (OR) = 1.23, 95% confidence interval (CI) = 1.01–1.50; additive model: OR = 1.21, 95% CI = 1.01–1.46]. Stratified analysis showed that this association remained significant among older individuals (adjusted OR = 1.32, 95% CI = 1.01–1.72, p = 0.040) and drinkers (adjusted OR = 1.50, 95% CI = 1.02–2.19, p = 0.040). No significant associations were observed between rs874945 or rs7958904 and HNC risk [102]. It is plausible that some HOTAIR SNPs may contribute to HNC development.
FAS-AS1 SNP: In a cohort of 684 NPC and 823 control subjects, Guo et al. reported that individuals carrying the CC genotype of the FAS-AS1 SNP, rs6586163, were associated with reduced risk of NPC (CC vs. AA genotype, OR = 0.645, p = 0.006) and better OS (AC + CC vs. AA genotype, HR = 0.667, p = 0.030) compared to those with the AA genotype. Further, it was demonstrated that overexpression of the rs6586163 variant (CC genotype) led to FAS-AS1 upregulation in NPC cell models, which in turn altered Fas isoform splicing, reduced NPC cell growth, and promoted apoptosis [11].
These reported SNPs of the above antisense genes may have the potential to aid the identification of susceptible individuals for HNC prevention purposes upon future validations in independent cohorts.
4. Functional Roles of Antisense Genes/Transcripts in HNC
A subset of the 52 antisense genes has been subjected to some levels of functional studies in HNC models and found to be involved in HNC growth, cell growth, invasiveness, and signaling alteration, as well as drug or radiotherapy responses. Antisense genes with such demonstrated biological functions are listed in Table 4 and discussed below. Most of these functional studies were conducted using overexpression or specific gene knockdown approaches in HNC cell models or animal models.

Table 4.
Functional roles of antisense genes/transcripts in HNC as demonstrated by experimental evidence.
4.1. Proliferation, Cell Cycle, and Cell Death Regulation in HNC
Using overexpression or knockdown approaches (siRNA or shRNA), 39 antisense genes/transcripts have been found to promote HNC cell growth or cell cycle progression or regulate cell death in vitro. In fact, most of these antisense genes have been demonstrated to have potential proto-oncogenic roles in HNC. Except for FAS-AS1, COX10-AS1, NR2F2-AS1, SLC16A1-AS1, and SSTR5-AS1, their overexpression in HNC cells resulted in the suppression of cell growth, indicative of their potential tumor-suppressive roles in HNC.
Imbalances between cell proliferation and cell death could contribute to tumorigenesis. In addition to promoting cell proliferation, three antisense genes/transcripts have been known to reduce apoptosis in HNC models. These include BBOX-1-AS1, TTN-AS1, and MSC-AS1. Zhao et al. reported that BBOX-1-AS1 silencing could result in the inhibition of OSCC cell proliferation accompanied by an increase in apoptosis [4]. Fu et al. reported TTN-AS1 upregulation in OSCC cells and specific knockdown of TTN-AS1 significantly inhibited cell proliferation with increased apoptosis [47]. Thus, these antisense genes/transcripts could promote HNC tumorigenesis, potentially via the inhibition of apoptosis.
4.2. Epithelial–Mesenchymal Transition (EMT), Migration, and Invasion in HNC
EMT is a key process by which epithelial cells gain invasion and metastatic potential via transitioning to a mesenchymal state. Studies have shown that various antisense genes can regulate EMT in HNC cells by modulating the expression and stability of EMT-related genes, including E-cadherin, N-cadherin, vimentin, fibronectin, and matrix-metalloproteases (MMP proteins), etc. It is important to note that EMT-promoting antisense genes are potential targets for anti-metastatic therapy in HNC.
As shown in Table 4, 45 antisense genes/transcripts have been demonstrated to promote EMT or invasiveness of HNC cells. For example, FUT8-AS1 depletion has been shown to suppress OSCC cell migration and EMT via the modulation of a complex miR-944/FUS/TCF4 (transcription factor 4) feedback loop. Specific knockdown of FUT8-AS1 resulted in increased expression of E-cadherin and reduced expression of N-cadherin, vimentin, MMP2, and MMP7, which was accompanied by a reduction in cellular migration activities [14].
In OSCC, HNF1A-AS1 has also been reported to promote cancer cell aggressiveness and EMT, as HNF1A-AS1 silencing resulted in EMT inhibition in OSCC cells [16]. Similarly, several other antisense genes, including VIM-AS1, ZEB1-AS1, and ZEB2-AS1, have also been shown to promote EMT in OSCC models [49,51,95]. In contrast, NR2F2-AS1 inhibits TGF-beta-induced EMT, migration, and invasion, as well as inhibiting angiogenesis, indicating its role in EMT suppression in OSCC [33].
In LSCC and NPC, five antisense genes, namely MSC-AS1, KTN1-AS1, SBF2-AS1, TM4SF19-AS1, and ZEB2-AS1, have been reported to regulate EMT. In particular, SBF2-AS1 has been shown to suppress EMT and migration of LSCC cells in vitro and LSCC metastasis in vivo. SBF2-AS1 serves as competing endogenous RNA (ceRNA) to sponge up miR-302b-3p (which acts to downregulate TGFBR2 by binding to the 3′-UTR of TGFBR2), thus resulting in the upregulation of both TGFBR2 mRNA and protein in LSCC cells. It was postulated that such an SBF2-AS1-mediated TGFBR2 upregulation likely suppresses EMT and migration in LSCC [40]. In contrast to SBF2-AS1, the remaining four antisense genes (MSC-AS1, KTN1-AS1, TM4SF19-AS1, and ZEB2-AS1) promote EMT, implicating their potential roles in promoting cancer progression. MSC-AS1 has been shown to be upregulated in NPC tissues and cells. Functional studies showed that MSC-AS1 could enhance NPC cell invasion and EMT, in addition to its effects on promoting NPC cell proliferation. Mechanistically, MSC-AS1 acts as a ceRNA, sponging up the tumor-suppressive miR-524-5p and resulting in miR-542-5p downregulation in NPC cells [32]. Thus, MSC-AS1 upregulation in NPC could elicit oncogenic effects via downregulating the tumor suppressor miRNA-542-5p with its associated target gene effects.
Similarly to MSC-AS1, KTN1-AS1 has also been shown to serve as a ceRNA in HNC, promoting cell proliferation, migration, invasion, and EMT by sponging and depleting miR-153-3p, thus resulting in the dysregulation of two EMT genes, SNAI1 and ZEB2, in HNC cells [23].
4.3. Signaling Pathway Regulation or Interactions
Many antisense genes are known to be key regulators of their respective sense genes. Multiple mechanisms could be involved, including the antisense transcripts’ ability to interfere with the sense gene’s transcription by forming RNA-DNA hybrids that would affect sense gene transcription initiation, promoter competition, DNA methylation, and transcriptional interference by collision of RNA polymerases/transcriptional machinery (between the antisense and sense transcription), etc. They could also affect sense mRNA splicing/isoform expressions, protein translation, and even RNA degradation due to their base complementary characteristics with the sense genes/transcripts. Though antisense RNA transcripts usually do not code for proteins, they can contain domains that can interact with DNA, RNA, and even proteins due to their RNA structure/folding flexibility. Thus, they can serve as dynamic scaffolds to bind other DNA, RNA, miRNA, or proteins, and even form functional complexes [1] and alter various other signaling potentials. As illustrated by SBF2-AS1 above, antisense can serve as a ceRNA, such as SBF2-AS1 (as discussed above), sponging up the miR-302b-3p responsible for modulating the expression or stability of many mRNAs (thus, proteins), including TGFBR2 and potentially others. Further, if antisense transcripts affect transcriptional factors, such as homeobox transcription factors (e.g., HOXA11), expressions of various target genes of those transcription factors would be affected, which could in turn impact a wide range of signaling events associated with such transcription factors. In summary, antisense transcripts can exhibit their effects on specific sense genes (i.e., in a cis-acting manner) or exert global effects (in a trans-acting manner) on various DNA (e.g., interacting with enhancers or promoters of its own or of other genes), RNA (e.g., affecting RNA stability, splicing, and sponging up miRNAs, etc.), and proteins (e.g., affecting protein stability or function, etc.), thus potentially affecting various signaling pathways in cells. Therefore, it is important to realize that a single antisense transcript could theoretically regulate multiple signaling events, given its wide range of dynamic “molecular switch” effects. A summary of the potential mechanisms of gene regulation by antisense genes/transcripts is graphically depicted in Figure 2.
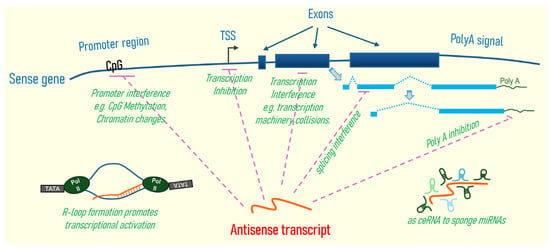
Figure 2.
Schematic depicting plausible mechanisms of gene regulation by antisense transcripts. (1) Promoter interference includes interaction of antisense transcript with DNA or DNA methyltransferases, resulting in alteration of CpG methylation status of the sense gene promoter, antisense transcript’s interaction, or recruitment of histone-modifying enzymes, thus altering chromatin structure and epigenetic control of the sense gene [56]. (2) Interference of sense gene transcription by blockade of transcription initiation at the transcription start site (TSS), exons, or even introns of the sense gene via collision of RNA polymerases/transcriptional machineries during sense and antisense gene transcription [1]. In addition, binding of an antisense RNA transcript to sense RNA transcript can also mediate sense RNA transcript degradation. (3) Splicing interference of sense gene transcripts can occur when antisense transcript blocks the access of, for example, splicing factors that are necessary for splicing of nascent sense transcripts. (4) Polyadenylation of sense mRNA transcripts can be affected if the antisense transcript overlaps with such polyA signals of the sense gene [103]. (5) R-loop formation by antisense transcript can promote sense gene transcription. The antisense transcript can form part of a hybrid DNA:RNA structure known as the R loop, which can facilitate the opening of the chromosome to help optimal binding of various transcriptional activators of the sense gene [104]. (6) Antisense transcripts can serve as scaffold ceRNAs (competing endogenous RNAs) competing for microRNAs (miRNAs) in the cell, thus altering the availability of miRNAs and affecting the degradation of a variety of cellular mRNAs. Sense gene transcripts are shown with exons depicted as light blue boxes, and introns with blue dotted line; antisense gene transcripts are depicted as orange curves; purple dotted lines indicate inhibition. TATA boxes, and Polymerase II (Pol II) are shown for R-loop.
Though our understanding of the full range effects of all 52 HNC-related antisense genes/transcripts on HNC signaling is minimal, several of them have been shown to regulate or modulate crucial HNC signaling pathways, including the EGFR/MAPK, PI3K/Akt, NF-κB, Notch, TGF-beta, and HIF-1α/VEGF pathways, etc. By regulating key signaling molecules within these pathways, the antisense genes can regulate HNC cell proliferation, invasion, angiogenesis, and even sensitivity to specific inhibitors, etc. Furthermore, we found that 13 out of the 52 antisense genes are known/putative transcription factors (e.g., homeobox proteins and zinc finger proteins) (Table 1); it is likely that they could exert extensive signaling effects in HNC cells by altering multitudes of transcriptional events.
For example, in tongue SCC, FOXD2-AS1 has been reported to be significantly upregulated in tumor tissues based on TCGA-HNC dataset. Its elevated expression was found to be significantly associated with advanced staging and lymphatic metastasis. Specific knockdown of FOXD2-AS1 in two cell lines, namely SCC-9 and CAL-27, were found to inhibit p65 expression and phosphorylation (key NF-κB players) and inhibit the MAPK pathway by inhibiting Erk1 phosphorylation [73]. Similarly, Ai et al. showed that DCST1-AS1 (which promotes HNC migration and invasion) could also regulate NF-κB signaling in HNC cells and in THP-1 differentiated macrophages by downregulating p65 protein expression [7].
Hypoxia-inducible factor 1-alpha (HIF-1α) is an important transcription factor regulating cellular adaptation in hypoxia, a condition that is essentially found in the inner hypoxic core of most human tumors. Recently, Tang et al. showed that USP2-AS1 was specifically upregulated in HNC cells during hypoxia, and it was a direct transcriptional target of HIF-1α. Further, this hypoxia-inducible USP2-AS1 could functionally promote HNC cell growth and invasion in vitro, and tumor growth in vivo. It was postulated that USP2-AS1 might affect E3 ubiquitin ligase DCAF13 expression and, thus, affect the associated signaling in HNC cells [48].
KEAP1 is an important oxidative stress sensor. It is known that KEAP/Nrf2 signaling enables cells to cope with oxidative stress. Zhao et al. showed that HOXA10-AS could modulate KEAP/Nrf2 signaling in human HNC xenograft models. They showed that tumors established from AMC-HN-8 cells with HOXA10-AS knockdown by shRNA had increased KEAP1 compared to tumors expressing control shRNA, suggesting HOXA10-AS as a potential regulator of KEAP1 in HNC [75]. Further, overexpression of MNX1-AS1 and LSAMP-AS1 in HNC cells (AMC-HN-8 and Tu177) could result in moderate activation of the PI3K/AKT pathway via Akt phosphorylation [105].
As discussed earlier, ceRNAs, via sponge effects, may play a role in signaling pathway regulation. In HNC cell models, SBF2-AS1, KTN1-AS1, and MSC-AS1 antisense transcripts could serve as ceRNAs to sponge up and thus downregulate the expression of miR-302b-3p, miR-153-3p, and miR-524-5p, respectively. Sponge effects of these antisense transcripts require further investigation regarding whether they regulate classic signaling pathways such as the EGFR/MAPK, PI3K/Akt, NF-κB, Notch, TGF-β, and HIF-1α/VEGF pathways, etc., just as other antisense transcripts can also serve as ceRNAs to modulate key signaling events in HNC tumorigenesis.
4.4. Modulation of Drug Responses and Radio-Resistance
As some antisense genes can regulate specific signaling events (or multiple signaling events via ceRNA activity), alter mRNA splicing and stability, and regulate expression levels of the sense proteins, it is plausible that they may be able to alter resistance/sensitivity to drugs, especially for targeted therapies in cancer treatment.
EGFR tyrosine kinase inhibitors (TKIs) have minimal clinical activity in HNC patients. Tan et al. reported two clinical exceptional responders to gefitinib (a first-generation EGFR TKI) with the EGFR exon 20 (Q787Q) (c.2361G>A; rs10251977) synonymous mutation [9]. Importantly, this EGFR mutation rests on the overlapping genomic sequence of the EGFR-AS1 gene, and this specific mutation on the EGFR-AS1 variant can shift the dynamics of EGFR mRNA splicing toward the EGFR isoform D (rather than isoform A). Such a shift resulted in EGFR pathway activation and EGFR dependency in HNC cells bearing this mutation, thus conferring sensitivity to gefitinib in mutant HNC cells. They postulated that in HNC cells bearing the homozygous A/A genotype for rs10251977 would be more sensitive to EGFR TKI than cells with the G/A or G/G genotypes. It is plausible yet remains to be further tested in larger HNC cohorts, whether such a mutated EGFR-AS1 status could impact EGFR TKI sensitivity in HNC by altering EGFR mRNA spliced isoform balances.
FOXD2-AS1 (overexpressed in laryngeal SCC) and LHFPL3-AS1 (overexpressed in OSCC), have been shown to confer cisplatin resistance in HNC models [13,26]. Specifically, Li et al. showed that FOXD2-AS1 displayed a tumor-specific upregulation (compared to paired adjacent normal; ~2-fold increase; N = 24 cases), and its upregulation was further found to be associated with disease relapse upon cisplatin-based chemotherapy in a small HNC cohort (N = 14 cases, 9 cases with relapse and 5 cases without relapse), which uncovered the direct involvement of FOXD2-AS1 in mediating cisplatin resistance in HNC. Mechanistic studies with two HNC cell models (Hep-2 and Tu-212) showed that FOXD2-AS1 overexpression could mediate cisplatin resistance via STAT3-induced stemness in these cells. In fact, FOXD2-AS1 has also been shown to confer cisplatin resistance in NSCLC cells as well [106]. As for LHFPL3-AS1, it has been demonstrated to be upregulated in multiple HNC cell lines (SCC9, CAL27, SCC25, and HSC3 vs. normal NOK cells), as well as in OSCC tissues (N = 51) greater than 2-fold when compared to adjacent normal cell lines. In cisplatin-resistant HNC cells (SCC9-R and CAL27-R), LHFPL3-AS1 was upregulated for 2.5–3-fold when compared to parental cells, and siRNA-specific knockdown of LHFPL3-AS1 reversed the cisplatin resistance of these two models in vitro [26]. Wang et al. showed that LHFPL3-AS1 was upregulated in EBV-positive radioresistant NPC cells, and siRNA targeting of LHFPL3-AS1 could reverse radioresistance in vitro [107]. Peng et al. showed that ZFAS1 contributed to intrinsic resistance to low-dose radiation (at 8Gy) in NPC cell models (C666-1 and SUNE-1), as specific siRNA knockdown of ZFAS1 by siRNA resulted in increased apoptosis upon radiation treatment in vitro, and such resistance could be mediated via mir-7-5p [108].
Lastly, it would be important to further explore if other HNC-related antisense genes with demonstrated clinical significance may also affect patient outcomes by driving treatment resistance.
5. Conclusions
Four antisense genes, namely HOXA10-AS, LEF1-AS1, MSC-AS1, and ZEB2-AS1, have been clinically cross-validated in multiple cohorts, with clear evidence to support clinical biomarker development in HNC. SNPs of several major antisense genes with evidence for HNC risk or outcomes should be further validated in different ethnic groups, for potential global applications in HNC, including desperate EBV-associated NPC, HPV-associated oropharyngeal cancer, and HNCs unrelated to viruses. Lastly, too little is known about their potential mechanisms on HNC pathogenesis, and more signaling investigations should be conducted to help dissect their molecular contributions to HNC carcinogenesis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells15010009/s1, Supplementary Table S1. All annotated antisense genes approved by the HUGO Gene Nomenclature Committee (https://www.genenames.org/; accessed on 13 May 2025).
Author Contributions
J.Y.: Data Curation, Formal Analysis, Investigation, Methodology, Writing—Original Draft, and Review and Editing; S.M.A.: Validation, Writing—Original Draft, and Review and Editing; Y.-K.N.: Validation and Visualization; V.W.Y.L.: Conceptualization, Data Curation, Formal Analysis, Funding Acquisition, Investigation, Methodology, Supervision, Writing—Original Draft, and Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Georgia Cancer Center Start-up Fund to VWYL. The sponsor had no role in the study’s design; collection, analysis, and interpretation of data; writing of the report; and decision to submit the article for publication.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable. All data were extracted from the original articles cited.
Conflicts of Interest
All authors do not have any conflicts of interest to disclose.
References
- Pelechano, V.; Steinmetz, L.M. Gene regulation by antisense transcription. Nat. Rev. Genet. 2013, 14, 880–893. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92, Correction in Nat. Rev. Dis. Primers 2023, 9, 4. [Google Scholar] [CrossRef]
- Lian, Y.; Xiong, F.; Yang, L.; Bo, H.; Gong, Z.; Wang, Y.; Wei, F.; Tang, Y.; Li, X.; Liao, Q.; et al. Long noncoding RNA AFAP1-AS1 acts as a competing endogenous RNA of miR-423-5p to facilitate nasopharyngeal carcinoma metastasis through regulating the Rho/Rac pathway. J. Exp. Clin. Cancer Res. 2018, 37, 253. [Google Scholar] [CrossRef]
- Zhao, C.; Shi, W.; Chen, M. Long non-coding RNA BBOX1-antisense RNA 1 enhances cell proliferation and migration and suppresses apoptosis in oral squamous cell carcinoma via the miR-3940-3p/laminin subunit gamma 2 axis. Bioengineered 2022, 13, 11138–11153. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xiao, Y.; Ma, L.; Wang, J. Regulating of cell cycle progression by the lncRNA CDKN2B-AS1/miR-324-5p/ROCK1 axis in laryngeal squamous cell cancer. Int. J. Biol. Markers 2020, 35, 47–56. [Google Scholar] [CrossRef]
- Deng, J.; Wu, M. COX10-AS1-mediated miR-361-5p regulated cell invasion and migration by targeting SPRY1 in oral squamous cell carcinoma. Am. J. Transl. Res. 2023, 15, 2191–2206. [Google Scholar] [PubMed]
- Ai, Y.; Liu, S.; Luo, H.; Wu, S.; Wei, H.; Tang, Z.; Li, X.; Zou, C. lncRNA DCST1-AS1 Facilitates Oral Squamous Cell Carcinoma by Promoting M2 Macrophage Polarization through Activating NF-kappaB Signaling. J. Immunol. Res. 2021, 2021, 5524231. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Jia, L.; Ren, H.; Jin, C.; Ren, Q.; Zhang, H.; Hu, D.; Zhang, H.; Hu, L.; Xie, T. LncRNA DLX6-AS1 increases the expression of HIF-1alpha and promotes the malignant phenotypes of nasopharyngeal carcinoma cells via targeting MiR-199a-5p. Mol. Genet. Genom. Med. 2020, 8, e1017. [Google Scholar] [CrossRef]
- Tan, D.S.W.; Chong, F.T.; Leong, H.S.; Toh, S.Y.; Lau, D.P.; Kwang, X.L.; Zhang, X.; Sundaram, G.M.; Tan, G.S.; Chang, M.M.; et al. Long noncoding RNA EGFR-AS1 mediates epidermal growth factor receptor addiction and modulates treatment response in squamous cell carcinoma. Nat. Med. 2017, 23, 1167–1175. [Google Scholar] [CrossRef]
- Chu, H.; Li, Z.; Gan, Z.; Yang, Z.; Wu, Z.; Rong, M. LncRNA ELF3-AS1 is involved in the regulation of oral squamous cell carcinoma cell proliferation by reprogramming glucose metabolism. Onco Targets Ther. 2019, 12, 6857–6863. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Z.; Zhang, M.; Bao, M.; He, B.; Zhou, X. LncRNA FAS-AS1 upregulated by its genetic variation rs6586163 promotes cell apoptosis in nasopharyngeal carcinoma through regulating mitochondria function and Fas splicing. Sci. Rep. 2023, 13, 8218. [Google Scholar] [CrossRef]
- Ge, C.; Dong, J.; Chu, Y.; Cao, S.; Zhang, J.; Wei, J. LncRNA FGD5-AS1 promotes tumor growth by regulating MCL1 via sponging miR-153-3p in oral cancer. Aging 2020, 12, 14355–14364. [Google Scholar] [CrossRef]
- Li, R.; Chen, S.; Zhan, J.; Li, X.; Liu, W.; Sheng, X.; Lu, Z.; Zhong, R.; Chen, L.; Luo, X.; et al. Long noncoding RNA FOXD2-AS1 enhances chemotherapeutic resistance of laryngeal squamous cell carcinoma via STAT3 activation. Cell Death Dis. 2020, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Huang, Y.; Wu, S.; Zhu, D.; Li, W. FUT8-AS1/miR-944/Fused in Sarcoma/Transcription Factor 4 Feedback Loop Participates in the Development of Oral Squamous Cell Carcinoma through Activation of Wnt/beta-Catenin Signaling Pathway. Am. J. Pathol. 2023, 193, 233–245. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, S.; Chen, J.; Wang, Z.; Liang, X.; Wang, X.; Jiang, J.; Lang, J.; Li, L. Long noncoding RNA HAS2-AS1 mediates hypoxia-induced invasiveness of oral squamous cell carcinoma. Mol. Carcinog. 2017, 56, 2210–2222. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, H.; Fan, S.; Lin, H.; Lian, W. STAT3-induced upregulation of long noncoding RNA HNF1A-AS1 promotes the progression of oral squamous cell carcinoma via activating Notch signaling pathway. Cancer Biol. Ther. 2019, 20, 444–453. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, L.; Zhang, L.; Wang, Y.; Li, H.; Ren, X.; Wei, F.; Yu, W.; Liu, T.; Wang, X.; et al. Long non-coding RNA HOTAIR promotes tumor cell invasion and metastasis by recruiting EZH2 and repressing E-cadherin in oral squamous cell carcinoma. Int. J. Oncol. 2015, 46, 2586–2594. [Google Scholar] [CrossRef]
- Wang, D. Promotive effects of HOXA10 antisense RNA on the stemness of oral squamous cell carcinoma stem cells through a microRNA-29a/MCL-1/phosphatidyl inositol 3-kinase/protein kinase B axis. Arch. Oral Biol. 2021, 126, 105114. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Yang, B.; Liu, F.; Fang, Q. LncRNA HOXA11-AS promotes OSCC progression by sponging miR-98-5p to upregulate YBX2 expression. Biomed. Pharmacother. 2020, 121, 109623. [Google Scholar] [CrossRef]
- Tang, Z.; Zeng, X.; Li, J.; Qiu, S.; Zhao, H.; Wang, Z.; Zheng, Y. LncRNA HOXC-AS1 promotes nasopharyngeal carcinoma (NPC) progression by sponging miR-4651 and subsequently upregulating FOXO6. J. Pharmacol. Sci. 2021, 147, 284–293. [Google Scholar] [CrossRef]
- Tong, S.; Wang, X.; Guo, X.; Lu, Z. Knockdown of lncRNA IGF2BP2-AS1 inhibits proliferation and migration of oral squamous cell carcinoma cells via the Wnt/beta-catenin pathway. J. Oral Pathol. Med. 2022, 51, 272–280. [Google Scholar] [CrossRef]
- Li, L.; Han, J.; Zhang, S.; Dong, C.; Xiao, X. KIF26B-AS1 Regulates TLR4 and Activates the TLR4 Signaling Pathway to Promote Malignant Progression of Laryngeal Cancer. J. Microbiol. Biotechnol. 2022, 32, 1344–1354. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, K.; Cao, W.; Xu, Q.; Wang, X.; Qin, X.; Wang, X.; Li, Y.; Zhang, J.; Chen, W. Long noncoding RNA KTN1-AS1 promotes head and neck squamous cell carcinoma cell epithelial-mesenchymal transition by targeting miR-153-3p. Epigenomics 2020, 12, 487–505. [Google Scholar] [CrossRef]
- Fan, J.; Wang, C.; Zhai, X.; Li, J.; Ju, J.; Zhu, Y.; Zheng, S.; Ren, N.; Huang, B.; Jiang, X.; et al. lncRNA LEF1-AS1 Acts as a Novel Biomarker and Promotes Hypopharyngeal Squamous Cell Carcinoma Progression and Metastasis by Targeting the miR-221-5p/GJA1 Axis. Dis. Markers 2022, 2022, 3881310. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Wu, J.; Li, N.; Jiang, C. Long Noncoding RNA LEMD1-AS1 Increases LEMD1 Expression and Activates PI3K-AKT Pathway to Promote Metastasis in Oral Squamous Cell Carcinoma. Biomed. Res. Int. 2022, 2022, 3543948. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Zhang, D.; Lv, H.; Lei, X. LncRNA LHFPL3-AS1 Promotes Oral Squamous Cell Carcinoma Growth and Cisplatin Resistance Through Targeting miR-362-5p/CHSY1 Pathway. Onco Targets Ther. 2021, 14, 2293–2300. [Google Scholar] [CrossRef]
- He, G.; Yao, W.; Li, L.; Wu, Y.; Feng, G.; Chen, L. LOXL1-AS1 contributes to the proliferation and migration of laryngocarcinoma cells through miR-589-5p/TRAF6 axis. Cancer Cell Int. 2020, 20, 504. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Luo, X.; Wu, W.; Li, Y.; Yu, H.; Wang, Y.; Yan, J. The long non-coding RNA MACC1-AS1 promotes nasopharyngeal carcinoma cell stemness via suppressing miR-145-mediated inhibition on SMAD2/MACC1-AS1 axis. Biomed. Pharmacother. 2020, 125, 109986. [Google Scholar] [CrossRef]
- Cui, X.; Yu, H.; Yu, T.; Xiao, D.; Wang, X. LncRNA MNX1-AS1 drives aggressive laryngeal squamous cell carcinoma progression and serves as a ceRNA to target FoxM1 by sponging microRNA-370. Aging 2021, 13, 9900–9910. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Fu, M.; Du, Z.H.; Zhao, F.; Yang, W.W.; Xu, L.H.; Li, S.L.; Ge, X.Y. Long Noncoding RNA MRPL23-AS1 Promotes Adenoid Cystic Carcinoma Lung Metastasis. Cancer Res. 2020, 80, 2273–2285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, Q.; Cheng, W.; Dong, W.; Kou, B. YTHDC1-Mediated lncRNA MSC-AS1 m6A Modification Potentiates Laryngeal Squamous Cell Carcinoma Development via Repressing ATXN7 Transcription. Mol. Biotechnol. 2025, 67, 1659–1673. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Yang, L.; Tian, L.; Guo, Y.; Li, Y. LncRNA MSC-AS1 aggravates nasopharyngeal carcinoma progression by targeting miR-524-5p/nuclear receptor subfamily 4 group A member 2 (NR4A2). Cancer Cell Int. 2020, 20, 138. [Google Scholar] [CrossRef]
- Qin, S.Y.; Li, B.; Liu, J.M.; Lv, Q.L.; Zeng, X.L. LncRNA NR2F2-AS1 inhibits the progression of oral squamous cell carcinoma by mediating the miR-32-5p/SEMA3A axis. Kaohsiung J. Med. Sci. 2024, 40, 877–889. [Google Scholar] [CrossRef]
- Tang, J.; Fu, C.; Li, Y.; Chen, S.; Jiang, X.; Xu, W.; Xie, H. Long Noncoding RNA OIP5-AS1 Promotes the Disease Progression in Nasopharyngeal Carcinoma by Targeting miR-203. Biomed. Res. Int. 2021, 2021, 9850928. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, R.; Liu, Q.; Deng, C.; Zhou, Q.; Wen, W.; Chai, L. Study on the expression of lncRNA PRKCA-AS1 in oral squamous cell carcinoma. Transl. Cancer Res. 2024, 13, 5202–5213. [Google Scholar] [CrossRef]
- Rong, M.; Zhang, M.; Dong, F.; Wu, K.; Cai, B.; Niu, J.; Yang, L.; Li, Z.; Lu, H.Y. LncRNA RASAL2-AS1 promotes METTL14-mediated m6A methylation in the proliferation and progression of head and neck squamous cell carcinoma. Cancer Cell Int. 2024, 24, 113. [Google Scholar] [CrossRef]
- Li, C.; Ye, J.; Zhang, Z.; Gong, Z.; Lin, Z.; Ding, M. Long non-coding RNA RBM5-AS1 promotes the aggressive behaviors of oral squamous cell carcinoma by regulation of miR-1285-3p/YAP1 axis. Biomed. Pharmacother. 2020, 123, 109723. [Google Scholar] [CrossRef]
- Xu, Z.; Xi, K. LncRNA RGMB-AS1 promotes laryngeal squamous cell carcinoma cells progression via sponging miR-22/NLRP3 axis. Biomed. Pharmacother. 2019, 118, 109222. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, H.; Dong, W. LncRNA RHPN1-AS1 promotes the progression of nasopharyngeal carcinoma by targeting CELF2 expression. Exp. Mol. Pathol. 2021, 122, 104671. [Google Scholar] [CrossRef]
- Li, Y.; Tang, B.; Lyu, K.; Yue, H.; Wei, F.; Xu, Y.; Chen, S.; Lin, Y.; Cai, Z.; Guo, X.; et al. Low expression of lncRNA SBF2-AS1 regulates the miR-302b-3p/TGFBR2 axis, promoting metastasis in laryngeal cancer. Mol. Carcinog. 2022, 61, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Zhang, X.; Lai, W.; Wang, J. Long non-coding RNA SLC16A1-AS1: Its multiple tumorigenesis features and regulatory role in cell cycle in oral squamous cell carcinoma. Cell Cycle 2020, 19, 1641–1653. [Google Scholar] [CrossRef]
- Qin, H.; Xu, J.; Gong, L.; Jiang, B.; Zhao, W. The long noncoding RNA ST7-AS1 promotes laryngeal squamous cell carcinoma by stabilizing CARM1. Biochem. Biophys. Res. Commun. 2019, 512, 34–40. [Google Scholar] [CrossRef]
- Li, J.; Qiao, Z.; Li, Y.; Lu, X.; Shao, T.; Lv, X. Bioinformatic analysis indicated that STARD4-AS1 might be a novel ferroptosis-related biomarker of oral squamous cell carcinoma. Heliyon 2024, 10, e33193. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Niu, Y.; Cui, Z.; Jin, L.; Peng, S.; Dong, Z. Long noncoding RNA TFAP2A-AS1 promotes oral squamous cell carcinoma cell growth and movement via competitively binding miR-1297 and regulating TFAP2A expression. Mol. Carcinog. 2022, 61, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, X.; Niu, K.; Sun, L.; Li, D. LncRNA TM4SF19-AS1 exacerbates cell proliferation, migration, invasion, and EMT in head and neck squamous cell carcinoma via enhancing LAMC1 expression. Cancer Biol. Ther. 2022, 23, 1–9. [Google Scholar] [CrossRef]
- Mokhtarinejad, M.; Pirhoushiaran, M.; Heidarzadehpilehrood, R.; Hesami, S.; Azmoudeh-Ardalan, F.; Farahani, A.S. Upregulated long non-coding RNAs TMPO-AS1, DDX11-AS1, and POLE gene expression predict poor prognosis in head and neck squamous cell carcinoma (HNSCC). Gene Rep. 2024, 36, 101942, Corrigendum in Gene Rep. 2024, 37, 102095. [Google Scholar] [CrossRef]
- Fu, S.W.; Zhang, Y.; Li, S.; Shi, Z.Y.; Zhao, J.; He, Q.L. LncRNA TTN-AS1 promotes the progression of oral squamous cell carcinoma via miR-411-3p/NFAT5 axis. Cancer Cell Int. 2020, 20, 415. [Google Scholar] [CrossRef]
- Tang, J.; Wu, Z.; Wang, X.; Hou, Y.; Bai, Y.; Tian, Y. Hypoxia-Regulated lncRNA USP2-AS1 Drives Head and Neck Squamous Cell Carcinoma Progression. Cells 2022, 11, 3407. [Google Scholar] [CrossRef]
- Bozgeyik, E.; Ege, B.; Koparal, M.; Ceylan, O. Clinical significance of Vimentin Antisense RNA 1 and its correlation with other epithelial to mesenchymal transition markers in oral cancers. Pathol. Res. Pract. 2022, 232, 153807. [Google Scholar] [CrossRef] [PubMed]
- Srisathaporn, S.; Pientong, C.; Heawchaiyaphum, C.; Nukpook, T.; Aromseree, S.; Ekalaksananan, T. The Oncogenic Role of VWA8-AS1, a Long Non-Coding RNA, in Epstein-Barr Virus-Associated Oral Squamous Cell Carcinoma: An Integrative Transcriptome and Functional Analysis. Int. J. Mol. Sci. 2024, 25, 12565. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Wang, C.; Wang, Q.; Yan, G. Long Noncoding RNA ZEB1-AS1 Downregulates miR-23a, Promotes Tumor Progression, and Predicts the Survival of Oral Squamous Cell Carcinoma Patients. Onco Targets Ther. 2021, 14, 2699–2710. [Google Scholar] [CrossRef]
- Diao, P.; Ge, H.; Song, Y.; Wu, Y.; Li, J.; Li, Z.; Yang, J.; Wang, Y.; Cheng, J. Overexpression of ZEB2-AS1 promotes epithelial-to-mesenchymal transition and metastasis by stabilizing ZEB2 mRNA in head neck squamous cell carcinoma. J. Cell Mol. Med. 2019, 23, 4269–4280. [Google Scholar] [CrossRef]
- Kolenda, T.; Guglas, K.; Kopczynska, M.; Teresiak, A.; Blizniak, R.; Mackiewicz, A.; Lamperska, K.; Mackiewicz, J. Oncogenic Role of ZFAS1 lncRNA in Head and Neck Squamous Cell Carcinomas. Cells 2019, 8, 366. [Google Scholar] [CrossRef]
- Meng, W.; Cui, W.; Zhao, L.; Chi, W.; Cao, H.; Wang, B. Aberrant methylation and downregulation of ZNF667-AS1 and ZNF667 promote the malignant progression of laryngeal squamous cell carcinoma. J. Biomed. Sci. 2019, 26, 13. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, Y.; Shi, D.; Nie, C.; Luo, Y.; Guo, L.; Zou, Y.; Xie, C. LncRNA ZNF667-AS1 Promotes ABLIM1 Expression by Adsorbing micro RNA-1290 to Suppress Nasopharyngeal Carcinoma Cell Progression. Onco. Targets Ther. 2020, 13, 4397–4409. [Google Scholar] [CrossRef] [PubMed]
- Faghihi, M.A.; Wahlestedt, C. Regulatory roles of natural antisense transcripts. Nat. Rev. Mol. Cell Biol. 2009, 10, 637–643. [Google Scholar] [CrossRef]
- Trinklein, N.D.; Aldred, S.F.; Hartman, S.J.; Schroeder, D.I.; Otillar, R.P.; Myers, R.M. An abundance of bidirectional promoters in the human genome. Genome Res. 2004, 14, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.; Kanhere, A.; Wahlestedt, C.; Mattick, J.S. Natural antisense transcripts as versatile regulators of gene expression. Nat. Rev. Genet. 2024, 25, 730–744. [Google Scholar] [CrossRef]
- Lapidot, M.; Pilpel, Y. Genome-wide natural antisense transcription: Coupling its regulation to its different regulatory mechanisms. EMBO Rep. 2006, 7, 1216–1222. [Google Scholar] [CrossRef]
- Shaul, O. How introns enhance gene expression. Int. J. Biochem. Cell Biol. 2017, 91, 145–155. [Google Scholar] [CrossRef]
- Mayr, C. What Are 3’ UTRs Doing? Cold Spring Harb. Perspect. Biol. 2019, 11, a034728. [Google Scholar]
- Cancer Genome Atlas, N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef]
- Yang, Q.; Sun, J.; Ma, Y.; Zhao, C.; Song, J. LncRNA DLX6-AS1 promotes laryngeal squamous cell carcinoma growth and invasion through regulating miR-376c. Am. J. Transl. Res. 2019, 11, 7009–7017. [Google Scholar]
- Li, B.J.; Ren, F.H.; Zhang, C.; Zhang, X.W.; Jiao, X.H. LncRNA AFAP1-AS1 Promotes Oral Squamous Cell Carcinoma Development by Ubiquitin-Mediated Proteolysis. Int. Dent. J. 2024, 74, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, G.; Anand, S.; Raksha, P.; Dhamodharan, S.; Prasanna Srinivasa Rao, H.; Subbiah, S.; Murugan, A.K.; Munirajan, A.K. LncRNA OIP5-AS1 is overexpressed in undifferentiated oral tumors and integrated analysis identifies as a downstream effector of stemness-associated transcription factors. Sci. Rep. 2018, 8, 7018. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Fu, M.; Song, Y.Q.; Li, S.L.; Ge, X.Y. Long non-coding RNA MRPL23-AS1 suppresses anoikis in salivary adenoid cystic carcinoma in vitro. Oral Dis. 2023, 29, 1588–1601. [Google Scholar] [CrossRef]
- Tang, M.; Wu, H.; Zhang, H.; Xu, X.; Jiang, B.; Chen, Q.; Wei, Y.; Qian, H.; Han, L. Actin filament-associated protein 1-antisense RNA1 promotes the development and invasion of tongue squamous cell carcinoma via the AFAP1-AS1/miR-133a-5p/ZIC2 axis. J. Gene Med. 2024, 26, e3654. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Xia, X.; Ye, B. Long non-coding RNA OIP5-AS1 serves as an oncogene in laryngeal squamous cell carcinoma by regulating miR-204-5p/ZEB1 axis. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 2177–2184. [Google Scholar] [CrossRef]
- Afrough, H.; Ghafouri-Fard, S.; Yousefi, H.; Pakzad, P.; Kholghi Oskooei, V.; Taheri, M. DICER-AS1 lncRNA: A putative culprit in the pathogenesis of gastric cancer. Exp. Mol. Pathol. 2020, 116, 104490. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Xie, B.; Wei, W. REST-repressed lncRNA NPPA-AS1 regulates cervical cancer progression by modulating miR-302e/DKK1/Wnt/beta-catenin signaling pathway. J. Cell Biochem. 2021, 122, 16–28. [Google Scholar] [CrossRef]
- Chiang, H.H.; Ong, C.T.; Chang, C.Y.; Wu, K.L.; Wu, Y.Y.; Lai, J.C.; Shen, T.Y.; Hung, J.Y.; Lee, H.C.; Tsai, Y.M.; et al. Downregulated antisense lncRNA ENTPD3-AS1 contributes to the development of lung adenocarcinoma. Am. J. Cancer Res. 2024, 14, 854–868. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Deng, Q.; Chen, Z.; Chen, Y.; Fu, Z. BVES-AS1 suppresses the colorectal cancer progression via the miR-1269a/b-SVEP1-PI3K/AKT axis. Adv. Clin. Exp. Med. 2024, 33, 1217–1236. [Google Scholar] [CrossRef]
- Zhou, G.; Huang, Z.; Meng, Y.; Jin, T.; Liang, Y.; Zhang, B. Upregulation of long non-coding RNA FOXD2-AS1 promotes progression and predicts poor prognosis in tongue squamous cell carcinoma. J. Oral Pathol. Med. 2020, 49, 1011–1018. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, W.; Lin, C.; Wang, X.; Zhang, X.; Zhang, Y.; Yang, R.; Chen, W.; Cao, W. Dysregulation of FOXD2-AS1 promotes cell proliferation and migration and predicts poor prognosis in oral squamous cell carcinoma: A study based on TCGA data. Aging 2020, 13, 2379–2396. [Google Scholar] [CrossRef]
- Zhao, K.; Chen, L.; Xie, Y.; Ren, N.; Li, J.; Zhai, X.; Zheng, S.; Liu, K.; Wang, C.; Qiu, Q.; et al. m6A/HOXA10-AS/ITGA6 axis aggravates oxidative resistance and malignant progression of laryngeal squamous cell carcinoma through regulating Notch and Keap1/Nrf2 pathways. Cancer Lett. 2024, 587, 216735. [Google Scholar] [CrossRef]
- Yan, X.; Cong, B.; Chen, Q.; Liu, L.; Luan, X.; Du, J.; Cao, M. Silencing lncRNA HOXA10-AS decreases cell proliferation of oral cancer and HOXA10-antisense RNA can serve as a novel prognostic predictor. J. Int. Med. Res. 2020, 48, 300060520934254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Bao, C.; Zhang, X.; Lin, X.; Pan, D.; Chen, Y. Knockdown of lncRNA LEF1-AS1 inhibited the progression of oral squamous cell carcinoma (OSCC) via Hippo signaling pathway. Cancer Biol. Ther. 2019, 20, 1213–1222. [Google Scholar] [CrossRef]
- Yang, L.; Lu, P.; Yang, X.; Li, K.; Chen, X.; Qu, S. Excavating novel diagnostic and prognostic long non-coding RNAs (lncRNAs) for head and neck squamous cell carcinoma: An integrated bioinformatics analysis of competing endogenous RNAs (ceRNAs) and gene co-expression networks. Bioengineered 2021, 12, 12821–12838. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, H.; Yu, B.; Chen, W.; Zhai, L.; Li, X.; Fang, Y. Long noncoding RNA ZEB2-AS1 facilitates laryngeal squamous cell carcinoma progression by miR-6840-3p/PLXNB1 axis. Onco Targets Ther. 2019, 12, 7337–7345. [Google Scholar] [CrossRef]
- Chen, Y.T.; Kan, C.H.; Liu, H.; Liu, Y.H.; Wu, C.C.; Kuo, Y.P.; Chang, I.Y.; Chang, K.P.; Yu, J.S.; Tan, B.C. Modular scaffolding by lncRNA HOXA10-AS promotes oral cancer progression. Cell Death Dis. 2022, 13, 629. [Google Scholar] [CrossRef] [PubMed]
- Isaev, K.; Jiang, L.; Wu, S.; Lee, C.A.; Watters, V.; Fort, V.; Tsai, R.; Coutinho, F.J.; Hussein, S.M.I.; Zhang, J.; et al. Pan-cancer analysis of non-coding transcripts reveals the prognostic onco-lncRNA HOXA10-AS in gliomas. Cell Rep. 2021, 37, 109873. [Google Scholar] [CrossRef]
- Sheng, K.; Lu, J.; Zhao, H. ELK1-induced upregulation of lncRNA HOXA10-AS promotes lung adenocarcinoma progression by increasing Wnt/beta-catenin signaling. Biochem. Biophys. Res. Commun. 2018, 501, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lu, C.; Li, X.; Li, F.; Zhao, Y.; Xu, M.; Jia, H.; Yuan, S. LncRNA HOXA10-AS functions as an oncogene by binding miR-6509-5p to upregulate Y-box binding protein 1 in gastric cancer. Bioengineered 2022, 13, 11373–11387. [Google Scholar] [CrossRef]
- Al-Kershi, S.; Bhayadia, R.; Ng, M.; Verboon, L.; Emmrich, S.; Gack, L.; Schwarzer, A.; Strowig, T.; Heckl, D.; Klusmann, J.H. The stem cell-specific long noncoding RNA HOXA10-AS in the pathogenesis of KMT2A-rearranged leukemia. Blood Adv. 2019, 3, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.Y.; Cui, J.; Li, D.H.; Li, Q.; Hong, X.Y. HOXA10-AS: A novel oncogenic long non-coding RNA in glioma. Oncol. Rep. 2018, 40, 2573–2583. [Google Scholar] [CrossRef]
- Kuai, J.; Wu, K.; Han, T.; Zhai, W.; Sun, R. LncRNA HOXA10-AS promotes the progression of esophageal carcinoma by regulating the expression of HOXA10. Cell Cycle 2023, 22, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wang, G.; Zhu, W.; Luo, C.; Guo, Z. LEF1-AS1 accelerates tumorigenesis in glioma by sponging miR-489-3p to enhance HIGD1A. Cell Death Dis. 2020, 11, 690. [Google Scholar] [CrossRef]
- Dong, H.; Jian, P.; Yu, M.; Wang, L. Silencing of long noncoding RNA LEF1-AS1 prevents the progression of hepatocellular carcinoma via the crosstalk with microRNA-136-5p/WNK1. J. Cell Physiol. 2020, 235, 6548–6562. [Google Scholar] [CrossRef]
- Zeng, S.; Zhou, C.; Yang, D.H.; Xu, L.S.; Yang, H.J.; Xu, M.H.; Wang, H. LEF1-AS1 is implicated in the malignant development of glioblastoma via sponging miR-543 to upregulate EN2. Brain Res. 2020, 1736, 146781. [Google Scholar] [CrossRef]
- Gao, J.; Dai, C.; Yu, X.; Yin, X.B.; Zhou, F. Long noncoding RNA LEF1-AS1 acts as a microRNA-10a-5p regulator to enhance MSI1 expression and promote chemoresistance in hepatocellular carcinoma cells through activating AKT signaling pathway. J. Cell Biochem. 2021, 122, 86–99. [Google Scholar] [CrossRef]
- Jin, X.; Qiao, L.; Fan, H.; Liao, C.; Zheng, J.; Wang, W.; Ma, X.; Yang, M.; Sun, X.; Zhao, W. Long non-coding RNA MSC-AS1 facilitates the proliferation and glycolysis of gastric cancer cells by regulating PFKFB3 expression. Int. J. Med. Sci. 2021, 18, 546–554. [Google Scholar] [CrossRef]
- Li, S.; Yang, S.; Qiu, C.; Sun, D. LncRNA MSC-AS1 facilitates lung adenocarcinoma through sponging miR-33b-5p to up-regulate GPAM. Biochem. Cell Biol. 2021, 99, 241–248. [Google Scholar] [CrossRef]
- Mahboobeh, Z.; Pegah, M.; Fatemeh, S.; Elham, K.; Hanieh, A.; Milad, R.; Mohammad, S. lncRNA ZEB2-AS1: A promising biomarker in human cancers. IUBMB Life 2020, 72, 1891–1899. [Google Scholar] [CrossRef]
- Lan, T.; Chang, L.; Wu, L.; Yuan, Y. Downregulation of ZEB2-AS1 decreased tumor growth and metastasis in hepatocellular carcinoma. Mol. Med. Rep. 2016, 14, 4606–4612. [Google Scholar] [CrossRef]
- Wu, X.; Yan, T.; Wang, Z.; Wu, X.; Cao, G.; Zhang, C. LncRNA ZEB2-AS1 promotes bladder cancer cell proliferation and inhibits apoptosis by regulating miR-27b. Biomed. Pharmacother. 2017, 96, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Hu, Y.; Hu, M.; He, J.; Li, B. Long non-coding RNA ZEB2-AS1 promotes proliferation and inhibits apoptosis in human lung cancer cells. Oncol. Lett. 2018, 15, 5220–5226. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Gao, H.; Liu, K.; Gao, B.; Ren, H.; Li, Z.; Liu, F. The lncRNA ZEB2-AS1 is upregulated in gastric cancer and affects cell proliferation and invasion via miR-143-5p/HIF-1alpha axis. Onco Targets Ther. 2019, 12, 657–667. [Google Scholar] [CrossRef]
- Lesseur, C.; Diergaarde, B.; Olshan, A.F.; Wunsch-Filho, V.; Ness, A.R.; Liu, G.; Lacko, M.; Eluf-Neto, J.; Franceschi, S.; Lagiou, P.; et al. Genome-wide association analyses identify new susceptibility loci for oral cavity and pharyngeal cancer. Nat. Genet. 2016, 48, 1544–1550. [Google Scholar] [CrossRef]
- Yeh, J.C.; Chen, Y.T.; Chou, Y.E.; Su, S.C.; Chang, L.C.; Chen, Y.L.; Lin, C.W.; Yang, S.F. Interactive effects of CDKN2B-AS1 gene polymorphism and habitual risk factors on oral cancer. J. Cell Mol. Med. 2023, 27, 3395–3403. [Google Scholar] [CrossRef]
- Wang, T.M.; Xiao, R.W.; He, Y.Q.; Zhang, W.L.; Diao, H.; Tang, M.; Mai, Z.M.; Xue, W.Q.; Yang, D.W.; Deng, C.M.; et al. High-throughput identification of regulatory elements and functional assays to uncover susceptibility genes for nasopharyngeal carcinoma. Am. J. Hum. Genet. 2023, 110, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Su, S.C.; Hsieh, M.J.; Lin, C.W.; Chuang, C.Y.; Liu, Y.F.; Yeh, C.M.; Yang, S.F. Impact of HOTAIR Gene Polymorphism and Environmental Risk on Oral Cancer. J. Dent. Res. 2018, 97, 717–724. [Google Scholar] [CrossRef]
- Wu, B.; Liu, J.; Wang, B.; Liao, X.; Cui, Z.; Ding, N. Association on polymorphisms in LncRNA HOTAIR and susceptibility to HNSCC in Chinese population. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 702–706. [Google Scholar]
- Gu, R.; Zhang, Z.; DeCerbo, J.N.; Carmichael, G.G. Gene regulation by sense-antisense overlap of polyadenylation signals. RNA 2009, 15, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Boque-Sastre, R.; Soler, M.; Oliveira-Mateos, C.; Portela, A.; Moutinho, C.; Sayols, S.; Villanueva, A.; Esteller, M.; Guil, S. Head-to-head antisense transcription and R-loop formation promotes transcriptional activation. Proc. Natl. Acad. Sci. USA 2015, 112, 5785–5790. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Cao, H.; Yang, J.; Liu, T.; Wang, B. PI3K/Akt signalling pathway-associated long noncoding RNA signature predicts the prognosis of laryngeal cancer patients. Sci. Rep. 2023, 13, 14764. [Google Scholar] [CrossRef]
- Ge, P.; Cao, L.; Yao, Y.J.; Jing, R.J.; Wang, W.; Li, H.J. lncRNA FOXD2-AS1 confers cisplatin resistance of non-small-cell lung cancer via regulation of miR185-5p-SIX1 axis. Onco Targets Ther. 2019, 12, 6105–6117. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Z.; Li, Y.; Gu, A.; Wang, Y.; Cai, Y.; Yu, Y.; Deng, X. Down-regulated long non-coding RNA LHFPL3 antisense RNA 1 inhibits the radiotherapy resistance of nasopharyngeal carcinoma via modulating microRNA-143-5p/homeobox A6 axis. Bioengineered 2022, 13, 5421–5433. [Google Scholar] [CrossRef]
- Peng, J.; Liu, F.; Zheng, H.; Wu, Q.; Liu, S. IncRNA ZFAS1 contributes to the radioresistance of nasopharyngeal carcinoma cells by sponging hsa-miR-7-5p to upregulate ENO2. Cell Cycle 2021, 20, 126–141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.