Targeting ATF5, CEBPB, and CEBPD with Cell-Penetrating Dpep Sensitizes Tumor Cells to NK-92MI Cell Cytotoxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Peptides and Reagents
- Dpep: RQIKIWFQNRRMKWKKLVELSAENEKLHQRVEQLTRDLAGLRQFFK;
- Dpep-mut: RQIKIWFQNRRMKWKKLVEGSAENEKGHQRVEQGTRDGAGGRQFFK
2.3. Cell Viability and Experimental Designs
2.3.1. Cell Viability Assays on Tumor Cells
2.3.2. Effect of Dpep on NK-92MI Cell Viability and Cytotoxic Activity
2.3.3. Effect of Secreted Factors of NK-92MI Cytotoxic Activity
2.3.4. NK-92MI Cell Inactivation Studies
2.3.5. Tumor Cell Resistance to NK-92MI Cell Cytotoxicity
2.4. Detection of Apoptosis by Flow Cytometry
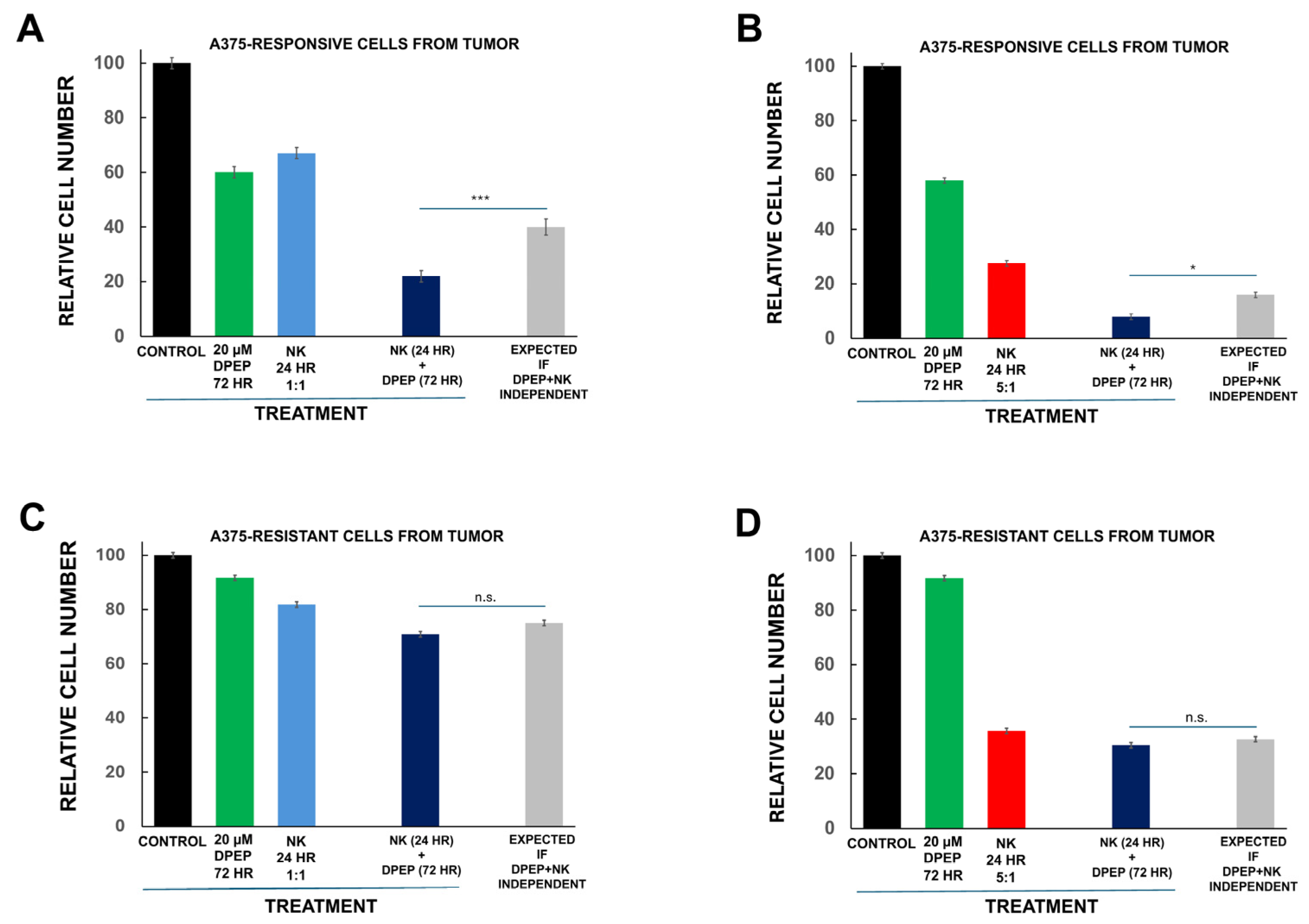
2.5. Independence Assessment
2.6. Plate-Seq Data Analysis
2.7. Statistical Analyses
3. Results
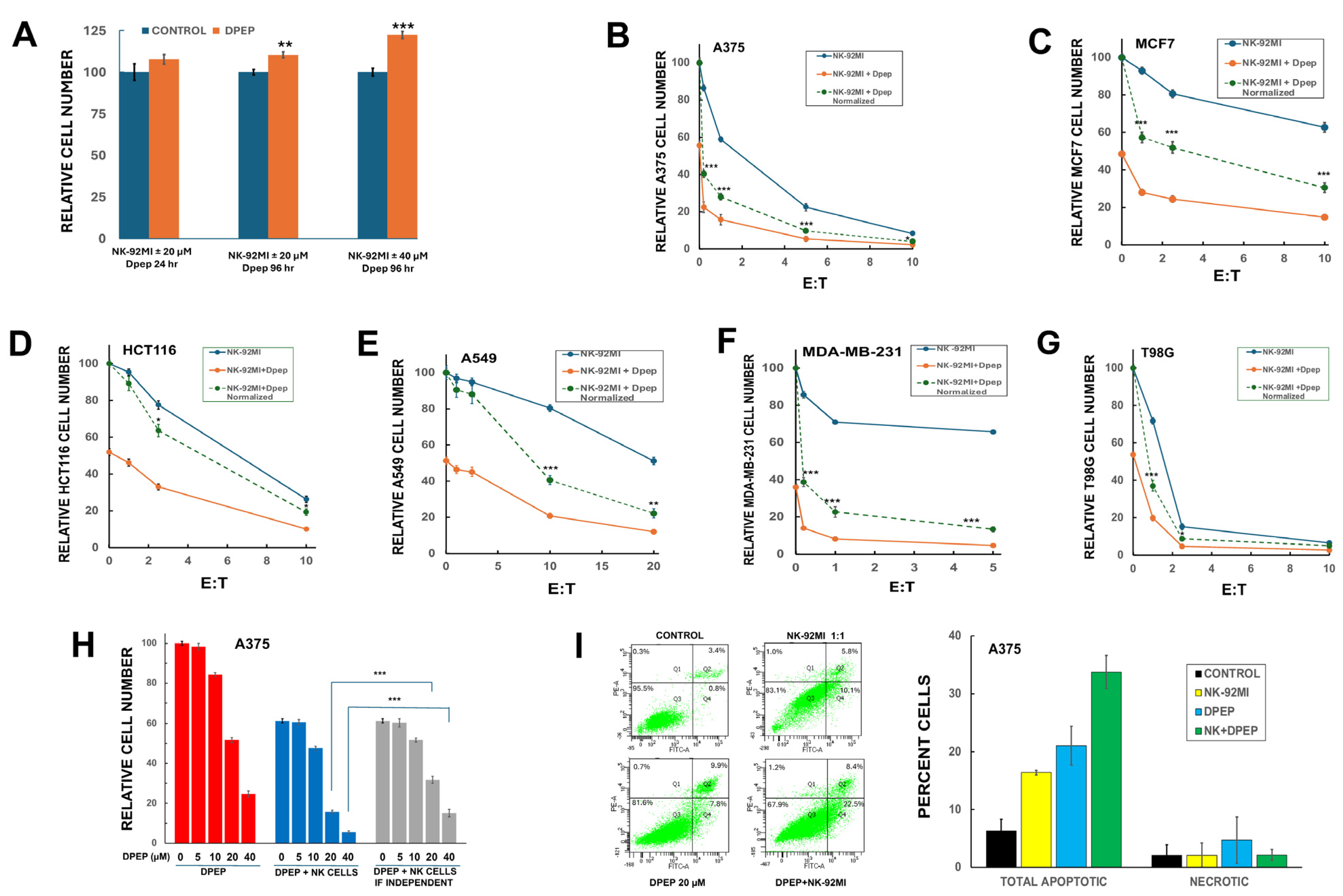
3.1. Dpep Does Not Adversely Affect NK-92MI Cell Growth/Survival
3.2. Dpep Sensitizes Multiple Tumor Cell Lines to NK-92MI Cell Killing
3.3. Dpep Sensitizes Tumor Cells to NK-92MI Cells in a Dose-Dependent Manner
3.4. Sensitization of Tumor Cells to NK-92MI Cells Requires Active Peptide
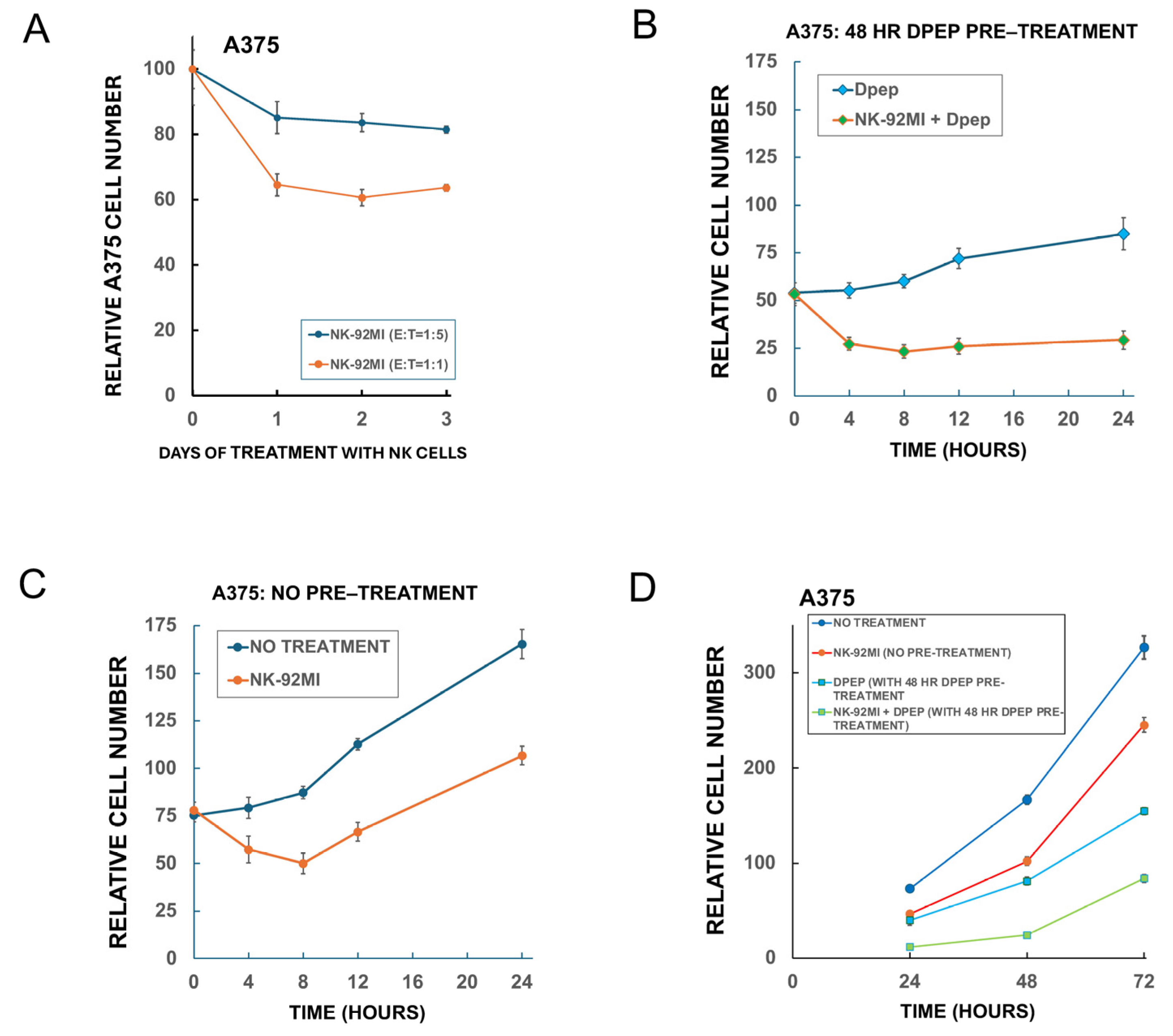
3.5. Sensitization of Tumor Cells to NK-92MI Cells Increases as a Function of Dpep Pre-Treatment Time
3.6. Medium Conditioned by Dpep-Treated Tumor Cells Does Not Affect NK-92MI Cell Cytotoxicity
3.7. Direct Treatment of NK-92MI Cells with Dpep Does Not Substantially Affect Their Activity
3.8. Tumor Cell Sensitization to NK-92MI Cells Requires Susceptibility to Dpep Killing
3.9. Dpep and NK Cell Inactivation
3.10. Dpep-Treated Tumor Cells Respond to Serial Treatments with NK-92MI Cells
4. Discussion
5. Limitations
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NK cells | Natural killer cells |
| CAR-NK | Chimeric Antigen Receptor-engineered NK cells |
| FBS | Fetal bovine serum |
| E:T | Effector-to-target |
| FITC | Fluorescein isothiocyanate |
| PI | Propidium iodide |
References
- Laskowski, T.J.; Biederstadt, A.; Rezvani, K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat. Rev. Cancer 2022, 22, 557–575. [Google Scholar] [CrossRef] [PubMed]
- Wolf, N.K.; Kissiov, D.U.; Raulet, D.H. Roles of natural killer cells in immunity to cancer, and applications to immunotherapy. Nat. Rev. Immunol. 2023, 23, 90–105. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, H.; Jounaidi, Y. Comprehensive snapshots of natural killer cells functions, signaling, molecular mechanisms and clinical utilization. Signal Transduct. Target. Ther. 2024, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Franks, S.E.; Wolfson, B.; Hodge, J.W. Natural Born Killers: NK Cells in Cancer Therapy. Cancers 2020, 12, 2131. [Google Scholar] [CrossRef]
- Klingemann, H. The NK-92 cell line-30 years later: Its impact on natural killer cell research and treatment of cancer. Cytotherapy 2023, 25, 451–457. [Google Scholar] [CrossRef]
- Zhang, C.; Oberoi, P.; Oelsner, S.; Waldmann, A.; Lindner, A.; Tonn, T.; Wels, W.S. Chimeric Antigen Receptor-Engineered NK-92 Cells: An Off-the-Shelf Cellular Therapeutic for Targeted Elimination of Cancer Cells and Induction of Protective Antitumor Immunity. Front. Immunol. 2017, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Tam, Y.K.; Maki, G.; Miyagawa, B.; Hennemann, B.; Tonn, T.; Klingemann, H.G. Characterization of genetically altered, interleukin 2-independent natural killer cell lines suitable for adoptive cellular immunotherapy. Hum. Gene Ther. 1999, 10, 1359–1373. [Google Scholar] [CrossRef]
- Tam, Y.K.; Miyagawa, B.; Ho, V.C.; Klingemann, H.G. Immunotherapy of malignant melanoma in a SCID mouse model using the highly cytotoxic natural killer cell line NK-92. J. Hematother 1999, 8, 281–290. [Google Scholar] [CrossRef]
- Fabian, K.P.; Hodge, J.W. The emerging role of off-the-shelf engineered natural killer cells in targeted cancer immunotherapy. Mol. Ther. Oncolytics 2021, 23, 266–276. [Google Scholar] [CrossRef]
- Suck, G.; Odendahl, M.; Nowakowska, P.; Seidl, C.; Wels, W.S.; Klingemann, H.G.; Tonn, T. NK-92: An ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol. Immunother. 2016, 65, 485–492. [Google Scholar] [CrossRef]
- Lamers-Kok, N.; Panella, D.; Georgoudaki, A.M.; Liu, H.; Ozkazanc, D.; Kucerova, L.; Duru, A.D.; Spanholtz, J.; Raimo, M. Natural killer cells in clinical development as non-engineered, engineered, and combination therapies. J. Hematol. Oncol. 2022, 15, 164. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Wang, M.; Yan, Y.; Jin, X.; Ning, L.; Xu, B.; Wang, Y.; Hao, Y.; Luo, Z.; Guo, C.; et al. Challenges in the Development of NK-92 Cells as an Effective Universal Off-the-Shelf Cellular Therapeutic. J. Immunol. 2024, 213, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Maki, G.; Krystal, G.; Dougherty, G.; Takei, F.; Klingemann, H.G. Induction of sensitivity to NK-mediated cytotoxicity by TNF-alpha treatment: Possible role of ICAM-3 and CD44. Leukemia 1998, 12, 1565–1572. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.H.; Kim, M.J.; Kim, S.J.; Park, S.J.; Chung, J.S.; Bae, J.H.; Kang, C.D. EGFR inhibitors enhanced the susceptibility to NK cell-mediated lysis of lung cancer cells. J. Immunother. 2011, 34, 372–381. [Google Scholar] [CrossRef]
- Sawasdee, N.; Wattanapanitch, M.; Thongsin, N.; Phanthaphol, N.; Chiawpanit, C.; Thuwajit, C.; Yenchitsomanus, P.T.; Panya, A. Doxorubicin sensitizes breast cancer cells to natural killer cells in connection with increased Fas receptors. Int. J. Mol. Med. 2022, 49, 40. [Google Scholar] [CrossRef]
- Raja, R.; Wu, C.; Bassoy, E.Y.; Rubino, T.E., Jr.; Utagawa, E.C.; Magtibay, P.M.; Butler, K.A.; Curtis, M. PP4 inhibition sensitizes ovarian cancer to NK cell-mediated cytotoxicity via STAT1 activation and inflammatory signaling. J. Immunother. Cancer 2022, 10, e005026. [Google Scholar] [CrossRef]
- Son, C.H.; Keum, J.H.; Yang, K.; Nam, J.; Kim, M.J.; Kim, S.H.; Kang, C.D.; Oh, S.O.; Kim, C.D.; Park, Y.S.; et al. Synergistic enhancement of NK cell-mediated cytotoxicity by combination of histone deacetylase inhibitor and ionizing radiation. Radiat. Oncol. 2014, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Karpel-Massler, G.; Horst, B.A.; Shu, C.; Chau, L.; Tsujiuchi, T.; Bruce, J.N.; Canoll, P.; Greene, L.A.; Angelastro, J.M.; Siegelin, M.D. A Synthetic Cell-Penetrating Dominant-Negative ATF5 Peptide Exerts Anticancer Activity against a Broad Spectrum of Treatment-Resistant Cancers. Clin. Cancer Res. 2016, 22, 4698–4711. [Google Scholar] [CrossRef]
- Zhou, Q.; Sun, X.; Pasquier, N.; Jefferson, P.; Nguyen, T.T.T.; Siegelin, M.D.; Angelastro, J.M.; Greene, L.A. Cell-Penetrating CEBPB and CEBPD Leucine Zipper Decoys as Broadly Acting Anti-Cancer Agents. Cancers 2021, 13, 2504. [Google Scholar] [CrossRef]
- Greene, L.A.; Zhou, Q.; Siegelin, M.D.; Angelastro, J.M. Targeting Transcription Factors ATF5, CEBPB and CEBPD with Cell-Penetrating Peptides to Treat Brain and Other Cancers. Cells 2023, 12, 581. [Google Scholar] [CrossRef]
- Zhou, Q.; Greene, L.A. Dpep Inhibits Cancer Cell Growth and Survival via Shared and Context-Dependent Transcriptome Perturbations. Cancers 2023, 15, 5318. [Google Scholar] [CrossRef] [PubMed]
- Demidenko, E.; Miller, T.W. Statistical determination of synergy based on Bliss definition of drugs independence. PLoS ONE 2019, 14, e0224137. [Google Scholar] [CrossRef]
- Melaiu, O.; Lucarini, V.; Cifaldi, L.; Fruci, D. Influence of the Tumor Microenvironment on NK Cell Function in Solid Tumors. Front. Immunol. 2019, 10, 3038. [Google Scholar] [CrossRef]
- Ran, G.H.; Lin, Y.Q.; Tian, L.; Zhang, T.; Yan, D.M.; Yu, J.H.; Deng, Y.C. Natural killer cell homing and trafficking in tissues and tumors: From biology to application. Signal Transduct. Target. Ther. 2022, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Zhi, L.; Wang, X.; Gao, Q.; He, W.; Shang, C.; Guo, C.; Niu, Z.; Zhu, W.; Zhang, X. Intrinsic and extrinsic factors determining natural killer cell fate: Phenotype and function. Biomed. Pharmacother. 2023, 165, 115136. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Tian, Z. NK Cell Exhaustion. Front. Immunol. 2017, 8, 760. [Google Scholar] [CrossRef]
- Jia, H.; Yang, H.; Xiong, H.; Luo, K.Q. NK cell exhaustion in the tumor microenvironment. Front. Immunol. 2023, 14, 1303605. [Google Scholar] [CrossRef]
- Cozar, B.; Greppi, M.; Carpentier, S.; Narni-Mancinelli, E.; Chiossone, L.; Vivier, E. Tumor-Infiltrating Natural Killer Cells. Cancer Discov. 2021, 11, 34–44. [Google Scholar] [CrossRef]
- Yan, Y.; Steinherz, P.; Klingemann, H.G.; Dennig, D.; Childs, B.H.; McGuirk, J.; O’Reilly, R.J. Antileukemia activity of a natural killer cell line against human leukemias. Clin. Cancer Res. 1998, 4, 2859–2868. [Google Scholar]
- Sun, X.; Angelastro, J.M.; Merino, D.; Zhou, Q.; Siegelin, M.D.; Greene, L.A. Dominant-negative ATF5 rapidly depletes survivin in tumor cells. Cell Death Dis. 2019, 10, 709. [Google Scholar] [CrossRef]
- Pan, K.; Farrukh, H.; Chittepu, V.C.S.R.; Xu, H.; Pan, C.X.; Zhu, Z. CAR race to cancer immunotherapy: From CAR T, CAR NK to CAR macrophage therapy. J. Exp. Clin. Cancer Res. 2022, 41, 119. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Jimenez-Cortegana, C.; Tay, A.H.M.; Wickstrom, S.; Galluzzi, L.; Lundqvist, A. NK cells and solid tumors: Therapeutic potential and persisting obstacles. Mol. Cancer 2022, 21, 206. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, J.M.; Rehman, S.; Virumbrales-Munoz, M.; McMinn, P.H.; Geiger, P.; Fitzgerald, C.; Heaster, T.; Skala, M.C.; Beebe, D.J. Microfluidic tumor-on-a-chip model to evaluate the role of tumor environmental stress on NK cell exhaustion. Sci. Adv. 2021, 7, eabc2331. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Siegelin, M.D.; Greene, L.A. Targeting ATF5, CEBPB, and CEBPD with Cell-Penetrating Dpep Sensitizes Tumor Cells to NK-92MI Cell Cytotoxicity. Cells 2025, 14, 667. https://doi.org/10.3390/cells14090667
Zhou Q, Siegelin MD, Greene LA. Targeting ATF5, CEBPB, and CEBPD with Cell-Penetrating Dpep Sensitizes Tumor Cells to NK-92MI Cell Cytotoxicity. Cells. 2025; 14(9):667. https://doi.org/10.3390/cells14090667
Chicago/Turabian StyleZhou, Qing, Markus D. Siegelin, and Lloyd A. Greene. 2025. "Targeting ATF5, CEBPB, and CEBPD with Cell-Penetrating Dpep Sensitizes Tumor Cells to NK-92MI Cell Cytotoxicity" Cells 14, no. 9: 667. https://doi.org/10.3390/cells14090667
APA StyleZhou, Q., Siegelin, M. D., & Greene, L. A. (2025). Targeting ATF5, CEBPB, and CEBPD with Cell-Penetrating Dpep Sensitizes Tumor Cells to NK-92MI Cell Cytotoxicity. Cells, 14(9), 667. https://doi.org/10.3390/cells14090667






