Structural Alterations of Human Erythrocytes Induced by Minocycline
Abstract
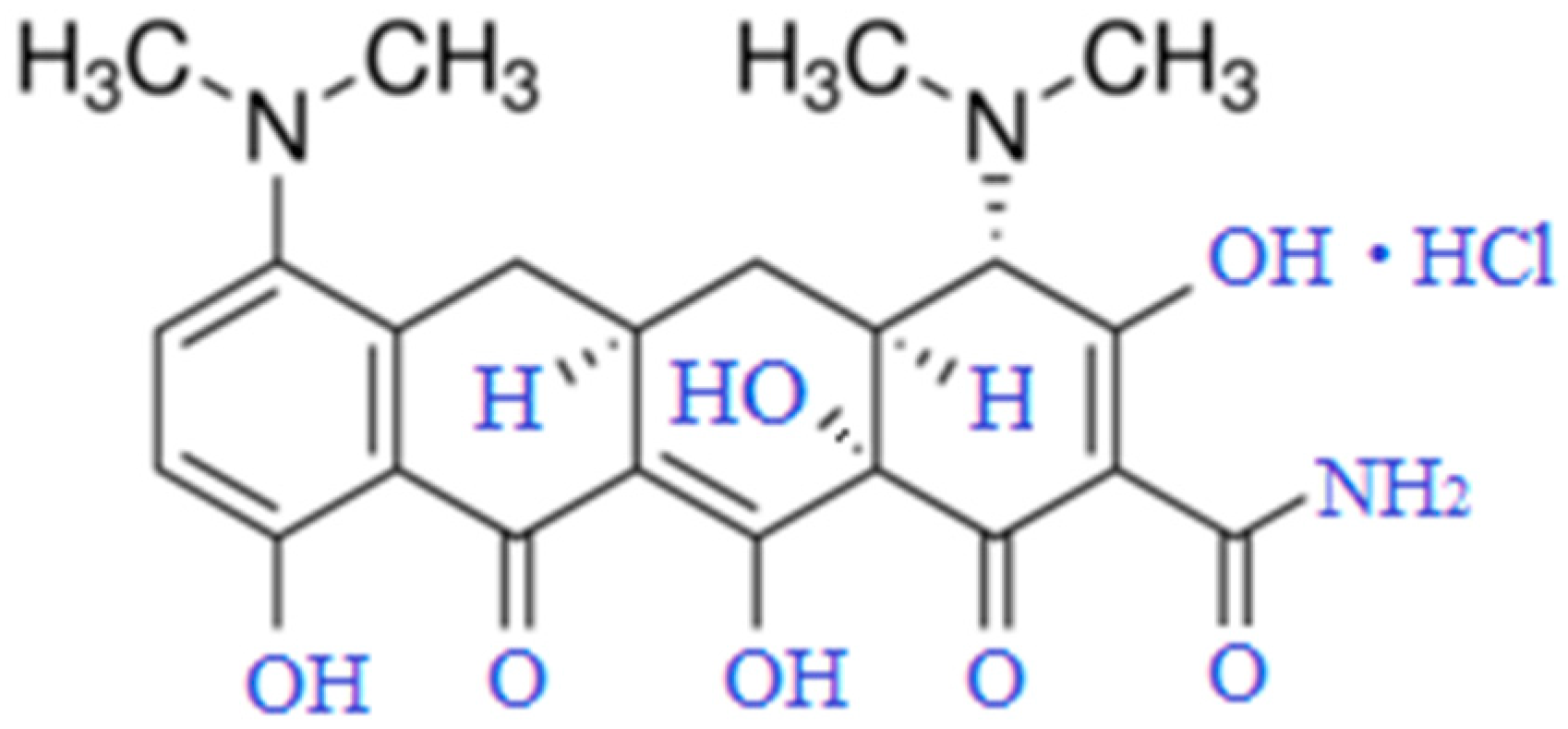
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Blood Collection
2.3. Separation of Erythrocytes
2.4. Preparing RBC Suspension
2.5. SEM
2.6. BSA Washing
2.7. Spectrophotometry
- Shimadzu UV-2401PC Recording Spectrophotometer:
- Wavelength repeatability: ±0.1 nm.
- Wavelength accuracy: ±0.3 nm.
- Wavelength scanning speed: slow.
- Photometric system: Double-beam, direct ratio system with dynode feedback.
- Photometric range: Absorbance −4~5 Abs. (0.001 Abs. increments).
- Photometric accuracy ±0.002 Abs. (0~0.5 Abs.) with NIST 930D Filter (Gaithersburg, MD, USA). ±0.004 Abs. (0.5~1 Abs.) with NIST 930D Filter. ±0.3%T (0~100%T) with NIST 930D Filter.
- Photometric repeatability: ±0.001 Abs. (0~1.0 Abs.) ±0.1%T.
- Baseline flatness: Within ±0.001 Abs. (excluding noise; 2 nm slit width and SLOW wavelength scanning speed).
2.8. Molecular Docking
2.9. Statistical Analysis
3. Results and Discussion
3.1. Scanning Electron Microscopy of Erythrocytes in the Presence of MC
3.2. Spectral Characteristics of Intraerythrocyte Hemoglobin Solutions
3.3. Molecular Docking
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TGF-β | Transforming growth factor beta |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| IKKα/β | IkappaB kinase |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| VEGF | Vascular endothelial growth factor |
| eIF2α | Eukaryotic initiation factor 2 |
| NMDA | N-methyl-D-aspartate receptors |
| TNF-α | Tumor necrosis factor-alpha |
| mTOR | Mechanistic target of rapamycin |
| IL | Interleukin |
| MCP-1 | Monocyte chemoattractant protein 1 |
| MIP-1α | Macrophage inflammatory protein |
References
- Luger, A.L.; Sauer, B.; Lorenz, N.I.; Engel, A.L.; Braun, Y.; Voss, M.; Harter, P.N.; Steinbach, J.P.; Ronellenfitsch, M.W. Doxycycline Impairs Mitochondrial Function and Protects Human Glioma Cells from Hypoxia-Induced Cell Death: Implications of Using Tet-Inducible Systems. Int. J. Mol. Sci. 2018, 19, 1504. [Google Scholar] [CrossRef]
- Patel, A.; Khande, H.; Periasamy, H.; Mokale, S. Immunomodulatory Effect of Doxycycline Ameliorates Systemic and Pulmonary Inflammation in a Murine Polymicrobial Sepsis Model. Inflammation 2020, 43, 1035–1043. [Google Scholar] [CrossRef]
- Chang, C.H.; Liu, W.T.; Hung, H.C.; Gean, C.Y.; Tsai, H.M.; Su, C.L.; Gean, P.W. Synergistic inhibition of tumor growth by combination treatment with drugs against different subpopulations of glioblastoma cells. BMC Cancer 2017, 17, 905. [Google Scholar] [CrossRef]
- Dijk, S.N.; Protasoni, M.; Elpidorou, M.; Kroon, A.M.; Taanman, J.W. Mitochondria as target to inhibit proliferation and induce apoptosis of cancer cells: The effects of doxycycline and gemcitabine. Sci. Rep. 2020, 10, 4363. [Google Scholar] [CrossRef]
- Lamb, R.; Ozsvari, B.; Lisanti, C.L.; Tanowitz, H.B.; Howell, A.; Martinez-Outschoorn, U.E.; Sotgia, F.; Lisanti, M.P. Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: Treating cancer like an infectious disease. Oncotarget 2015, 6, 4569–4584. [Google Scholar] [CrossRef]
- Garrido-Mesa, N.; Zarzuelo, A.; Gálvez, J. Minocycline: Far beyond an antibiotic. Br. J. Pharmacol. 2013, 169, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Levkovitch-Verbin, H.; Waserzoog, Y.; Vander, S.; Makarovsky, D.; Piven, I. Minocycline upregulates pro-survival genes and downregulates pro-apoptotic genes in experimental glaucoma Graefes. Arch. Clin. Exp. Ophthalmol. 2014, 252, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Levkovitch-Verbin, H.; Waserzoog, Y.; Vander, S.; Makarovsky, D.; Ilia, P. Minocycline mechanism of neuroprotection involves the Bcl-2 gene family in optic nerve transection. Int. J. Neurosci. 2014, 124, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Yong, V.W.; Wells, J.; Giuliani, F.; Casha, S.; Power, C.; Metz, L.M. The promise of minocycline in neurology. Lancet Neurol. 2004, 3, 744–751. [Google Scholar] [CrossRef]
- Yrjänheikki, J.; Tikka, T.; Keinänen, R.; Goldsteins, G.; Chan, P.H.; Koistinaho, J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc. Natl. Acad. Sci. USA 1999, 96, 13496–13500. [Google Scholar] [CrossRef]
- Zhang, R.; Yong, V.W.; Xue, M. Revisiting Minocycline in Intracerebral Hemorrhage: Mechanisms and Clinical Translation. Front. Immunol. 2022, 13, 844163. [Google Scholar] [CrossRef] [PubMed]
- Motaghinejad, M.; Farokhi, N.; Motevalian, M.; Safari, S. Molecular, histological and behavioral evidences for neuroprotective effects of minocycline against nicotine-induced neurodegeneration and cognition impairment: Possible role of CREB-BDNF signaling pathway. Behav. Brain Res. 2020, 386, 112597. [Google Scholar] [CrossRef] [PubMed]
- Adembri, C.; Selmi, V.; Vitali, L.; Tani, A.; Margheri, M.; Loriga, B.; Carlucci, M.; Nosi, D.; Formigli, L.; De Gaudio, A.R. Minocycline but not tigecycline is neuroprotective and reduces the neuroinflammatory response induced by the superimposition of sepsis upon traumatic brain injury. Crit. Care Med. 2014, 42, e570–e582. [Google Scholar] [CrossRef] [PubMed]
- Shayan, M.; Mehri, S.; Razavi, B.M.; Hosseinzadeh, H. Minocycline as a Neuroprotective Agent in Arsenic-Induced Neurotoxicity in PC12 Cells. Biol. Trace Elem. Res. 2023, 201, 2955–2962. [Google Scholar] [CrossRef]
- Bian, C.; Wan, Y.; Koduri, S.; Hua, Y.; Keep, R.F.; Xi, G. Iron-Induced Hydrocephalus: The Role of Choroid Plexus Stromal Macrophages. Transl. Stroke Res. 2023, 14, 238–249. [Google Scholar] [CrossRef]
- Suárez-Rivero, J.M.; López-Pérez, J.; Muela-Zarzuela, I.; Pastor-Maldonado, C.; Cilleros-Holgado, P.; Gómez-Fernández, D.; Álvarez-Córdoba, M.; Munuera-Cabeza, M.; Talaverón-Rey, M.; Povea-Cabello, S.; et al. Neurodegeneration, Mitochondria, and Antibiotics. Metabolites 2023, 13, 416. [Google Scholar] [CrossRef]
- Griffin, M.O.; Fricovsky, E.; Ceballos, G.; Villarreal, F. Tetracyclines: A pleitropic family of compounds with promising therapeutic properties, Review of the literature. Am. J. Physiol. Cell Physiol. 2010, 299, 539–548. [Google Scholar] [CrossRef]
- Klein, N.C.; Cunha, B.A. Tetracyclines. Med. Clin. N. Am. 1995, 79, 789–801. [Google Scholar] [CrossRef]
- Xie, L.-X.; Zhou, J.; Ledesma, K.R.; Merlau, P.R.; Tam, V.H. The impact of serum protein binding on bacterial killing of minocycline. J. Glob. Antimicrob. Resist. 2020, 21, 252–254. [Google Scholar] [CrossRef]
- Fares, M.; Abedi-Valugerdi, M.; Hassan, M.; Potácová, Z. DNA damage, lysosomal degradation and Bcl-xL deamidation in doxycycline- and minocycline-induced cell death in the K562 leukemic cell line. Biochem. Biophys. Res. Commun. 2015, 463, 268–274. [Google Scholar] [CrossRef][Green Version]
- Shen, H.X.; Liu, C.; Lin, H.J.; Xu, L.J.; Wang, G.Y.; Yan, M.X. The efficacy and safety of minocycline as adjuvant therapy in refractory mycoplasma pneumonia in Chinese children: A meta-analysis. Ital. J. Pediatr. 2022, 48, 176. [Google Scholar] [CrossRef]
- Gardner, K.; Bennett, V. A new erythrocyte membrane-associated protein with calmodulin binding activity. Identification and purification. J. Biol. Chem. 1986, 261, 1339–1348. [Google Scholar] [CrossRef]
- Dey, S.; Reddy Nandigam, A.; Kumar Kancharla, A.K.; Mohammad, S.T.; Shankar Gummaluri, S.; Doppalapudi, H.; Mahapatra, A.; Padala, S. Scanning electron microscopy study to evaluate and compare fibrin clot adhesion over the root surface treated with tetracycline, doxycycline and minocycline. Dent. Med. Probl. 2023, 60, 267–272. [Google Scholar] [CrossRef]
- Singh, S.; Khanna, D.; Kalra, S. Minocycline and Doxycycline: More Than Antibiotics. Curr. Mol. Pharmacol. 2021, 14, 1046–1065. [Google Scholar] [CrossRef]
- Hoefnagel, J.J.; van Leeuwen, R.L.; Mattie, H.; Bastiaens, M.T. Bijwerkingen van minocycline in de behandeling van acne vulgaris [Side effects of minocycline in the treatment of acne vulgaris]. Ned. Tijdschr. Geneeskd. 1997, 141, 1424–1427. [Google Scholar] [PubMed]
- Gordon, M.M.; Porter, D. Minocycline induced lupus: Case series in the West of Scotland. J. Rheumatol. 2001, 28, 1004–1006. [Google Scholar] [PubMed]
- Shapiro, L.E.; Knowles, S.R.; Shear, N.H. Comparative safety of tetracycline, minocycline, and doxycycline. Arch. Dermatol. 1997, 133, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Antunes, A.; Davril, A.; Tréchot, P.; Grandidier, M.; Truchetet, F.; Cuny, J.F. Syndrome d’hypersensibilité à la minocycline [Minocycline hypersensitivity syndrome]. Ann. Dermatol. Venereol. 1999, 126, 518–521. [Google Scholar]
- Skverchinskaya, E.; Levdarovich, N.; Ivanov, A.; Mindukshev, I.; Bukatin, A. Anticancer Drugs Paclitaxel, Carboplatin, Doxorubicin, and Cyclophosphamide Alter the Biophysical Characteristics of Red Blood Cells, In Vitro. Biology 2023, 12, 230. [Google Scholar] [CrossRef]
- Tikhomirova, I.A.; Muravyov, A.V.; Petrochenko, E.P.; Kislov, N.V.; Cheporov, S.V.; Peganova, E.V. Alteration of red blood cell microrheology by anti-tumor chemotherapy drugs. Biochem. Moscow Suppl. Ser. 2016, 10, 135–141. [Google Scholar] [CrossRef]
- Sanchez-Fito, M.T.; Oltra, E. Optimized treatment of heparinized blood fractions to make them suitable for analysis. Biopreserv. Biobank. 2015, 13, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Phosphate Buffered Saline (PBS). Available online: http://www.medicago.se/sites/default/files/pdf/productsheets/PBS_Buffer_v._01.pdf (accessed on 20 October 2024).
- Baeva, E.S.; Artyukhov, V.G. Non-antibacterial effects of antibiotics: Results of our research. Proc. VSU Ser Chem. Biol. Pharm. 2018, 4, 53–58. [Google Scholar]
- Colina, J.R.; Suwalsky, M.; Petit, K.; Contreras, D.; Manrique-Moreno, M.; Jemiola-Rzeminska, M.; Strzalka, K. In vitro evaluation of the protective effect of crocin on human erythrocytes. Biophys. Chem. 2022, 281, 106738. [Google Scholar] [CrossRef]
- Suwalsky, M.; Manrique, M.; Villena, F.; Sotomayor, C.P. Structural effects in vitro of the anti-inflammatory drug diclofenac on human erythrocytes and molecular models of cell membranes. Biophys. Chem. 2009, 141, 34–40. [Google Scholar] [CrossRef]
- Hamidi, M.; Zarei, N.; Zarrin, A.; Mohammadi-Samani, S. Preparation and validation of carrier human erythrocytes loaded by bovine serum albumin as a model antigen/protein. Drug Deliv. 2007, 14, 295–300. [Google Scholar] [CrossRef]
- Reinhart, W.H.; Piety, N.Z.; Deuel, J.W.; Makhro, A.; Schulzki, T.; Bogdanov, N.; Goede, J.S.; Bogdanova, A.; Abidi, R.; Shevkoplyas, S.S. Washing stored red blood cells in an albumin solution improves their morphologic and hemorheologic properties. Transfusion 2015, 55, 1872–1881. [Google Scholar] [CrossRef]
- Abdelgawad, M.A.; Labib, M.B.; Ali, W.A.M.; Kamel, G.; Azouz, A.A.; El-Nahass, E.S. Design, synthesis, analgesic, anti-inflammatory activity of novel pyrazolons possessing aminosulfonyl pharmacophore as inhibitors of COX-2/5-LOX enzymes: Histopathological and docking studies. Bioorg. Chem. 2018, 78, 103–114. [Google Scholar] [CrossRef]
- Holyavka, M.G.; Makin, S.M.; Kondratyev, M.S.; Abdullatypov, A.V.; Kovaleva, T.A.; Artyukhov, V.G. Supramolecular Organization of Inulinases from Aspergillus awamori, Aspergillus ficuum and Kluyveromyces marxianus: A Comparative Aspect. Biophysics 2018, 63, 866–875. [Google Scholar] [CrossRef]
- Dey, K.; van Cromvoirt, A.M.; Hegemann, I.; Goede, J.S.; Bogdanova, A. Role of Piezo1 in Terminal Density Reversal of Red Blood Cells. Cells 2024, 13, 1363. [Google Scholar] [CrossRef]
- Bogdanova, A.; Kaestner, L. Advances in Red Blood Cells Research. Cells 2024, 13, 359. [Google Scholar] [CrossRef]
- Livshits, L.; Peretz, S.; Bogdanova, A.; Zoabi, H.; Eitam, H.; Barshtein, G.; Galindo, C.; Feldman, Y.; Pajić-Lijaković, I.; Koren, A.; et al. The Impact of Ca2+ on Intracellular Distribution of Hemoglobin in Human Erythrocytes. Cells 2023, 12, 2280. [Google Scholar] [CrossRef]
- Mahadeva, M.; Niestępski, S.; Kowacz, M. Dependence of cell’s membrane potential on extracellular voltage observed in Chara globularis. Biophys. Chem. 2024, 307, 107199. [Google Scholar] [CrossRef]
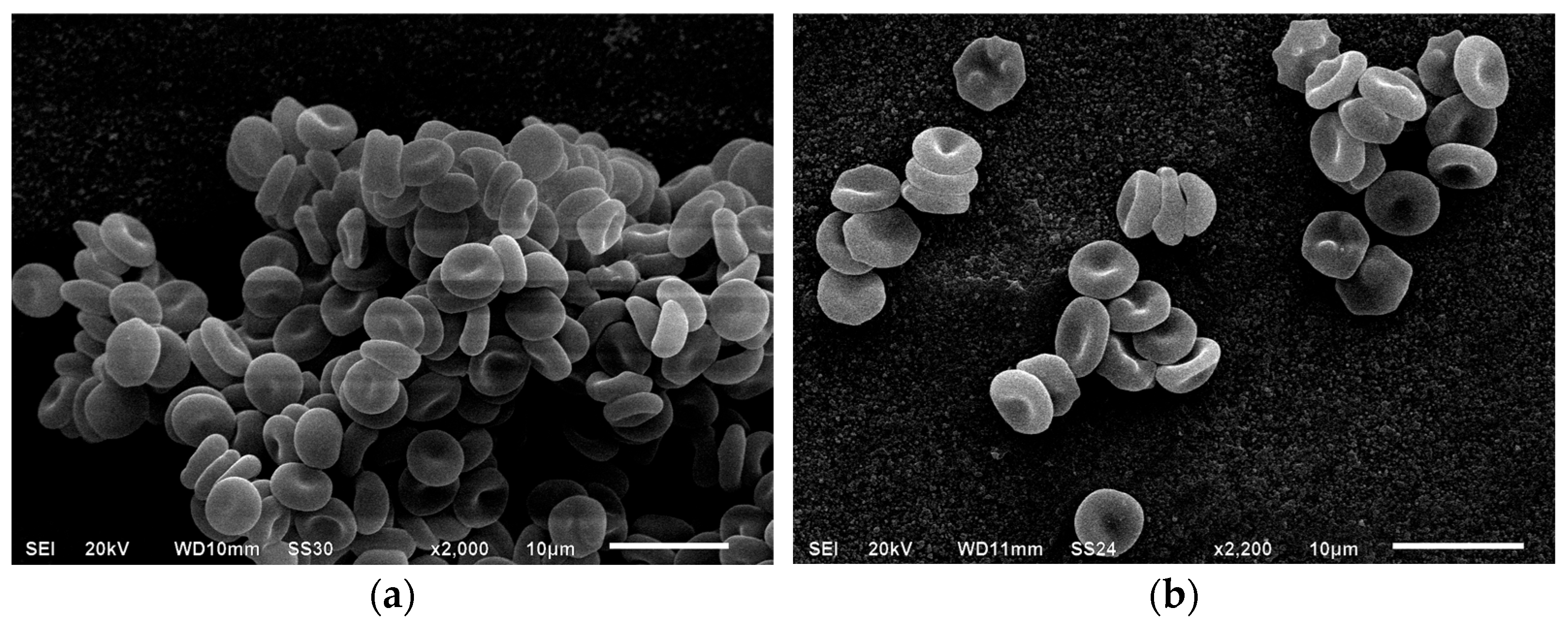
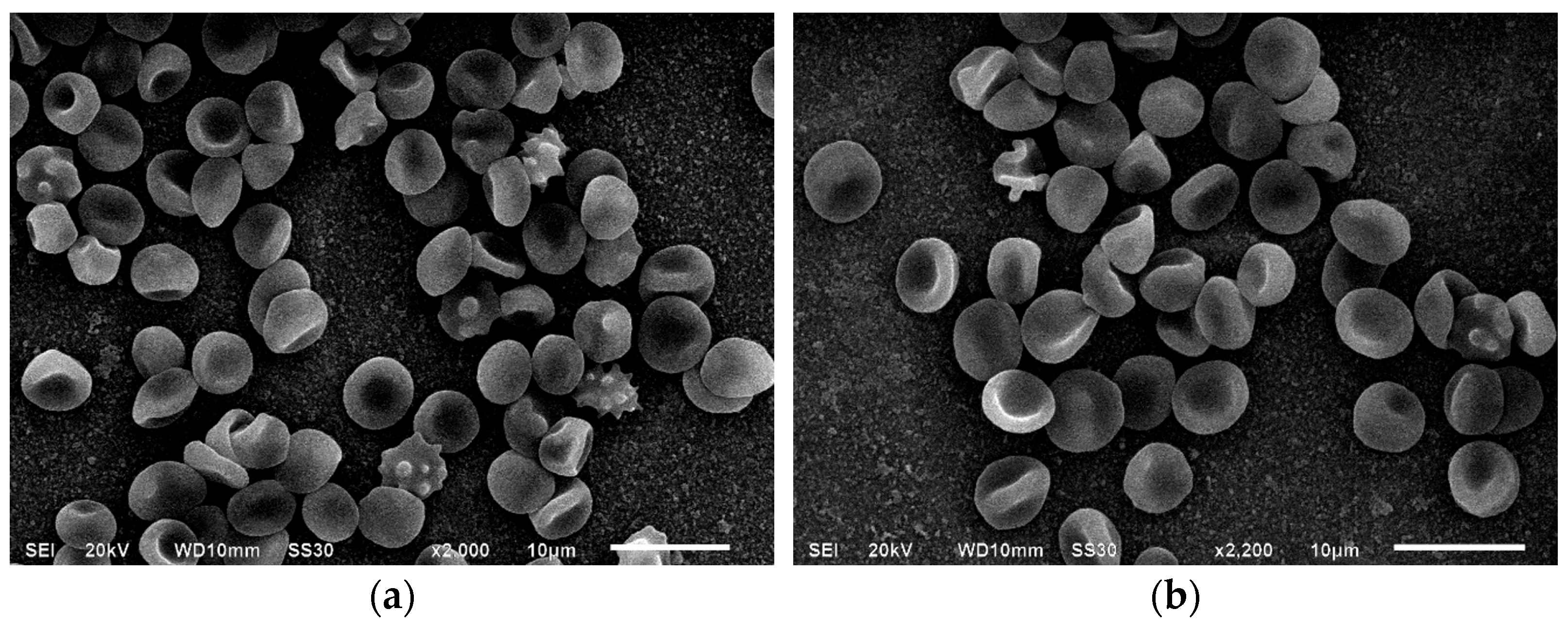
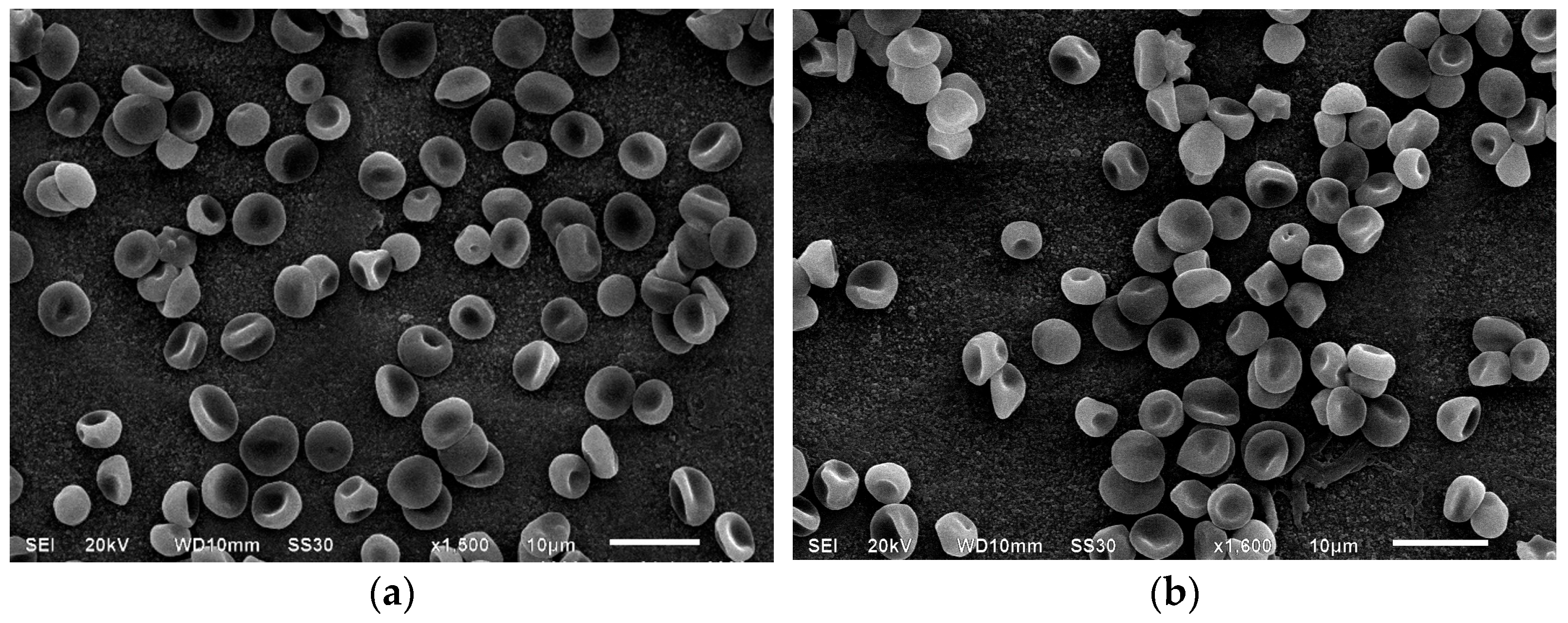
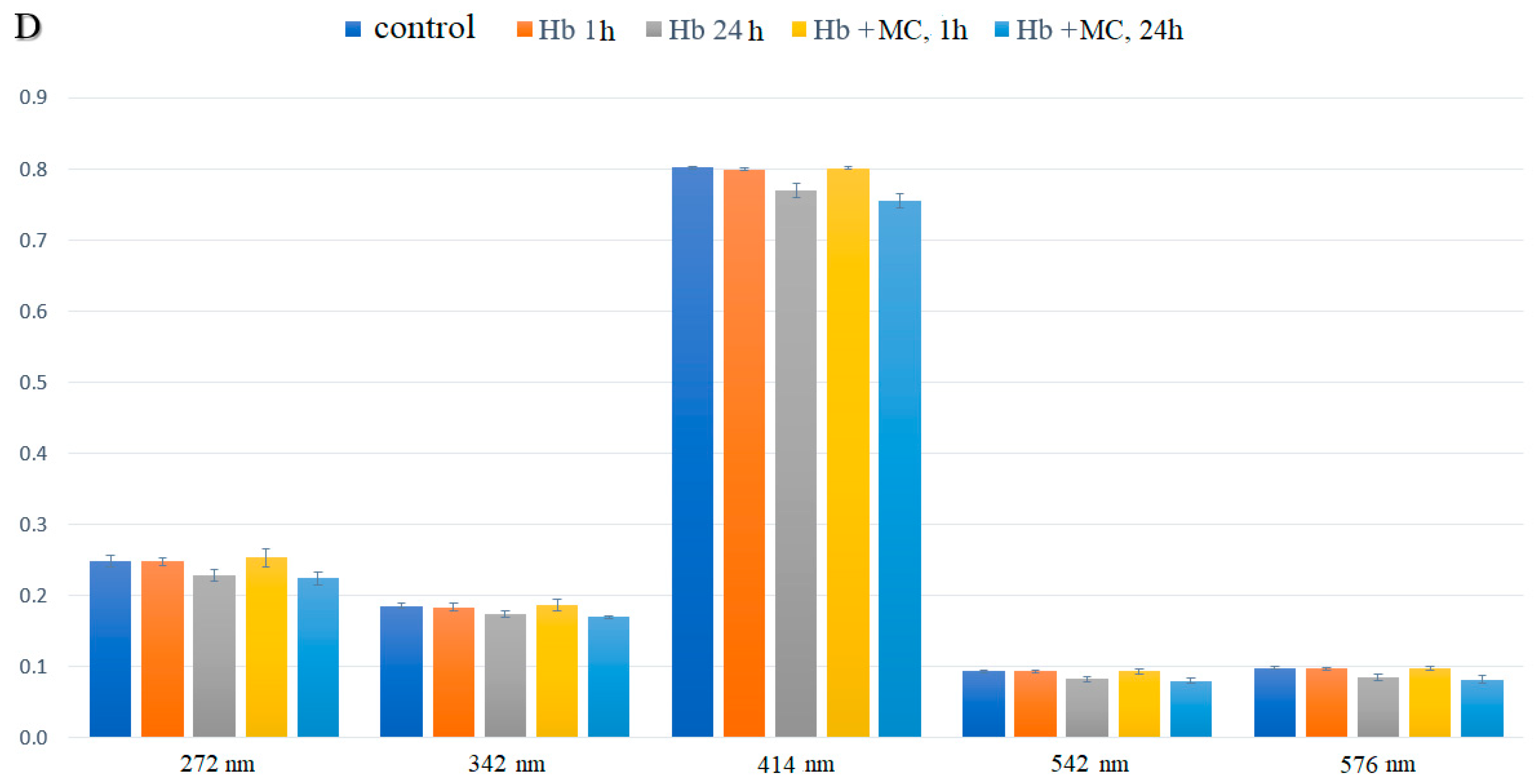
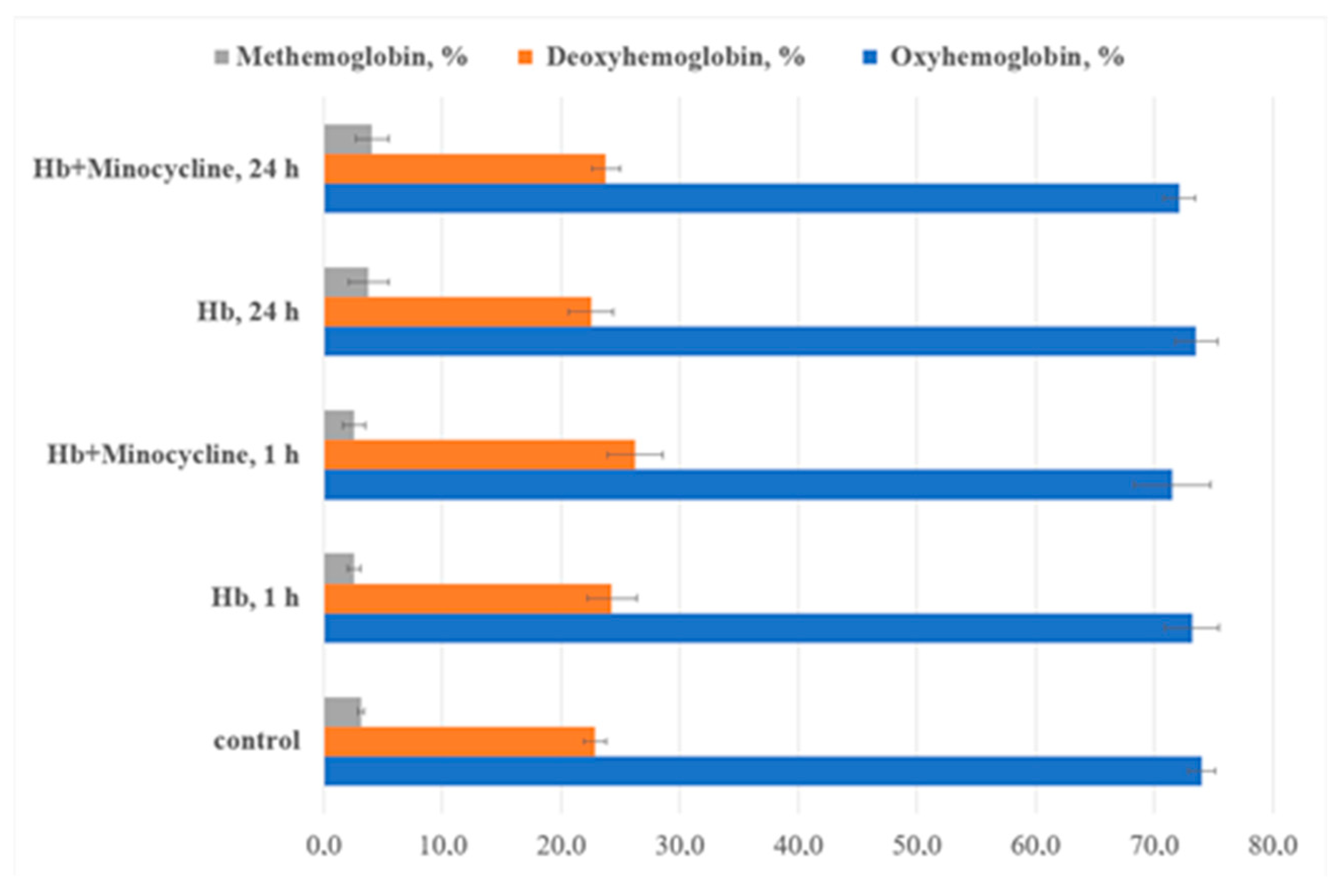
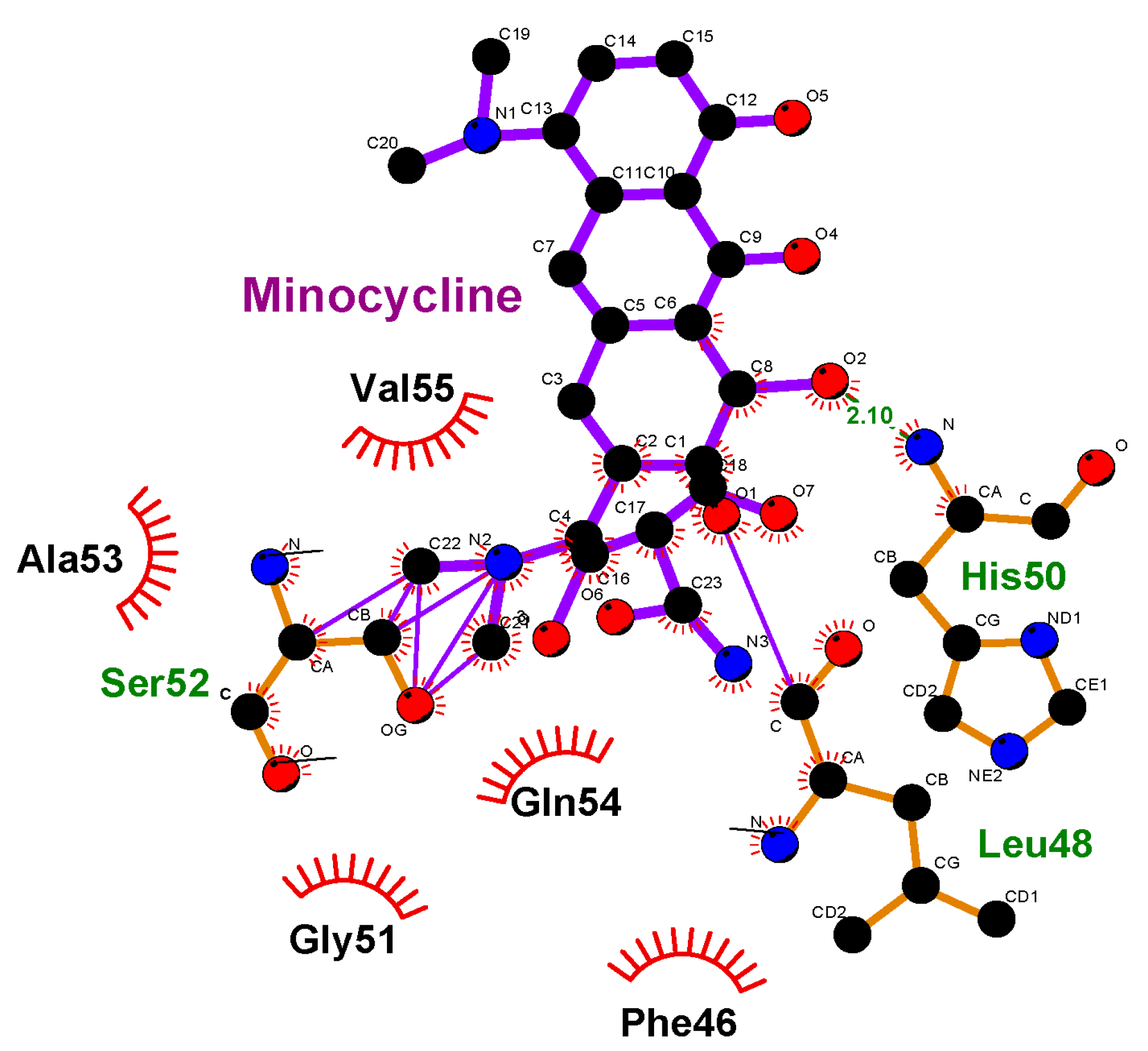
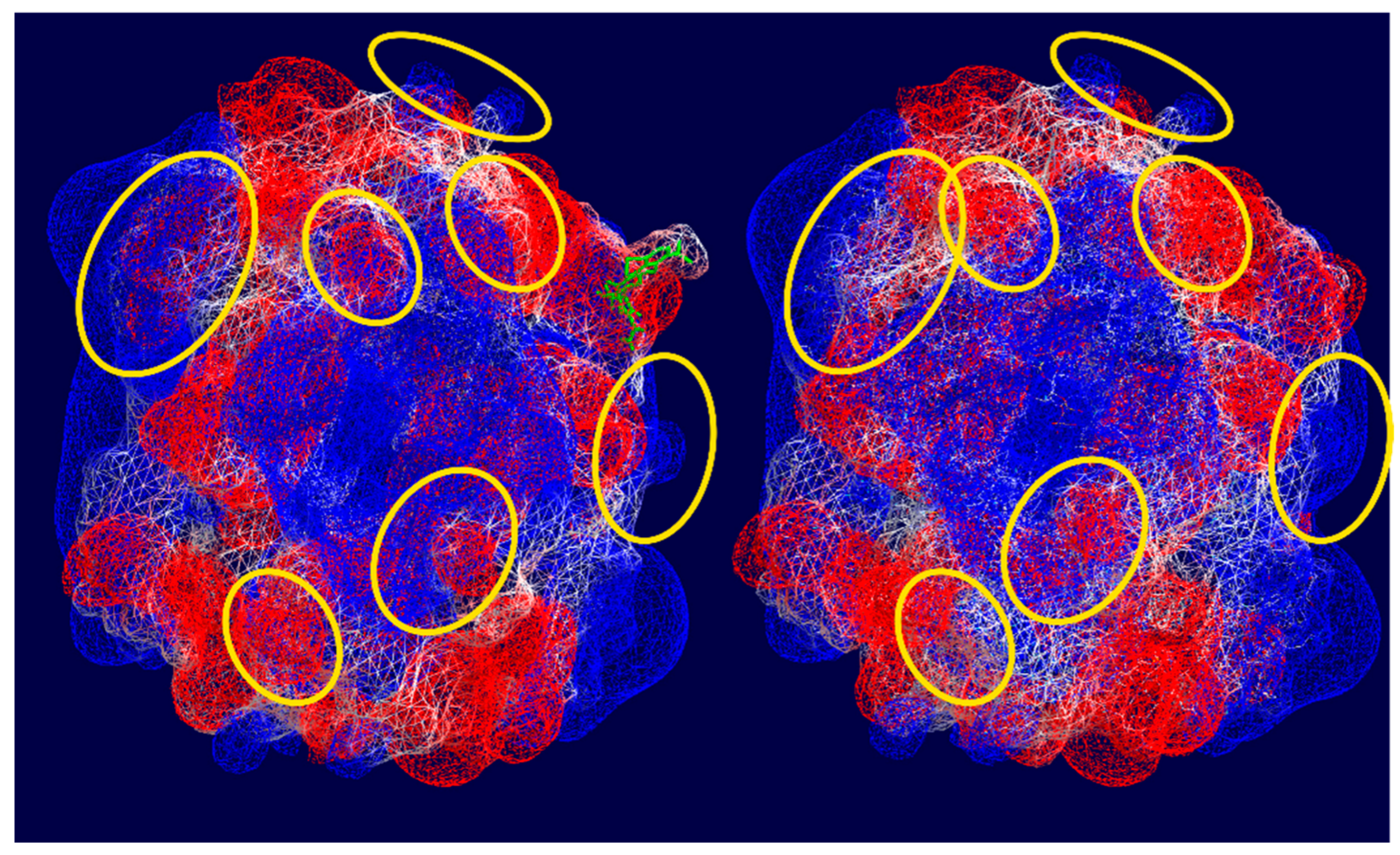
| Indices, % | Control | Incubation with MC, 1 h | Incubation with MC + BSA, 1 h | Incubation with MC, 24 h | Incubation with MC + BSA, 24 h |
|---|---|---|---|---|---|
| Discocytes | 91.1 ± 1.1 | 85.2 ± 1.5 | 88.7 ± 1.1 | 81.4 ± 0.9 | 85.9 ± 1.2 |
| Echinocytes | 3.5 ± 0.5 | 5.2 ± 0.2 | 1.5 ± 0.7 | 7.4 ± 1.4 | 4.0 ± 0.9 |
| Discocytes with a “ridge” | 3.1 ± 0.3 | 6.1 ± 0.3 | 6.0 ± 0.6 | 6.2 ± 0.4 | 5.6 ± 0.9 |
| Reversibly deformed | 97.6 ± 0.8 | 96.4 ± 0.6 | 96.2 ± 0.6 | 95.0 ± 0.6 | 95.4 ± 0.7 |
| “deflated ball” | 0.2 ± 0.02 | 1.1 ± 0.9 | 1.2 ± 0.4 | 1.6 ± 0.1 | 1.6 ± 0.4 |
| “dome-shaped” | 1.2 ± 1.07 | 1.2 ± 0.5 | 1.8 ± 0.6 | 1.3 ± 0.2 | 1.2 ± 0.4 |
| Spherocytes | 0.0 ± 0.0 | 0.06 ± 0.02 | 0.09 ± 0.0 | 0.5 ± 0.2 | 0.5 ± 0.1 |
| Stomatocytes | 0.0 ± 0.0 | 0.02 ± 0.01 | 0.01 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 |
| Degenerative forms | 0.5 ± 0.0 | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.7 ± 0.3 | 0.7 ± 0.2 |
| Spherocytes with spikes | 0.4 ± 0.1 | 0.5 ± 0.02 | 0.1 ± 0.0 | 0.7 ± 0.02 | 0.2 ± 0.1 |
| Irreversibly deformed | 2.4 ± 0.8 | 3.5 ± 0.3 | 3.7 ± 0.5 | 5.1 ± 0.1 | 4.5 ± 0.2 |
| Transformation index | 1.1 ± 0.02 | 1.2 ± 0.02 | 1.1 ± 0.01 | 1.2 ± 0.02 | 1.2 ± 0.01 |
| Index of reversible transformation | 1.1 ± 0.03 | 1.1 ± 0.02 | 1.1 ± 0.01 | 1.2 ± 0.01 | 1.1 ± 0.04 |
| Index of irreversible transformation | 0.03 ± 0.02 | 0.04 ± 0.01 | 0.04 ± 0.02 | 0.06 ± 0.03 | 0.05 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baeva, E.; Holyavka, M.; Artyukhov, V.; Kondratyev, M. Structural Alterations of Human Erythrocytes Induced by Minocycline. Cells 2025, 14, 1787. https://doi.org/10.3390/cells14221787
Baeva E, Holyavka M, Artyukhov V, Kondratyev M. Structural Alterations of Human Erythrocytes Induced by Minocycline. Cells. 2025; 14(22):1787. https://doi.org/10.3390/cells14221787
Chicago/Turabian StyleBaeva, Elena, Marina Holyavka, Valery Artyukhov, and Maxim Kondratyev. 2025. "Structural Alterations of Human Erythrocytes Induced by Minocycline" Cells 14, no. 22: 1787. https://doi.org/10.3390/cells14221787
APA StyleBaeva, E., Holyavka, M., Artyukhov, V., & Kondratyev, M. (2025). Structural Alterations of Human Erythrocytes Induced by Minocycline. Cells, 14(22), 1787. https://doi.org/10.3390/cells14221787










