Losing the Filter: How Kynurenine Pathway Dysregulation Impairs Habituation
Highlights
- Kynurenine pathway (KP) dysregulation may impair habituation by disrupting synaptic plasticity, redox balance, and glial signaling.
- KP metabolites 3-HK and QA drive oxidative and excitotoxic stress, while excess KYNA interferes with neurotransmission, weakening habituation circuits.
- KMO upregulation promotes inflammatory KP activity undermining the neural flexibility required for habituation in aging.
- KMO deletion may restore KP balance and preserve olfactory and novelty habituation in aged mice.
Abstract
1. Introduction
2. Mechanistic Effects of KP Dysregulation on Habituation
3. Synaptic Plasticity Impairment
4. Neurotransmitter Disruption
5. Oxidative Stress and Mitochondrial Dysfunction
6. Neurogenesis Impairment
7. Circuit-Level Disruption
8. Inflammation-Driven Feedback Loops
9. AhR Activation and Glial Crosstalk
10. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colwill, R.M.; Lattal, K.M.; Whitlow, J.W.; Delamater, A.R. Habituation: It’s Not What You Think It Is. Behav. Processes 2023, 207, 104845. [Google Scholar] [CrossRef]
- Lamiré, L.-A.; Haesemeyer, M.; Engert, F.; Granato, M.; Randlett, O. Inhibition Drives Habituation of a Larval Zebrafish Visual Response. Elife 2023, 12, RP84926. [Google Scholar] [CrossRef]
- Castellucci, V.; Pinsker, H.; Kupfermann, I.; Kandel, E.R. Neuronal Mechanisms of Habituation and Dishabituation of the Gill-Withdrawal Reflex in Aplysia. Sci. New Ser. 1970, 167, 1745–1748. [Google Scholar]
- Thompson, R.F. Habituation: A History. Neurobiol. Learn. Mem. 2009, 92, 127–134. [Google Scholar] [CrossRef]
- McDiarmid, T.A.; Yu, A.J.; Rankin, C.H. Habituation Is More Than Learning to Ignore: Multiple Mechanisms Serve to Facilitate Shifts in Behavioral Strategy. BioEssays 2019, 41, 1900077. [Google Scholar] [CrossRef]
- Rankin, C.H.; Abrams, T.; Barry, R.J.; Bhatnagar, S.; Clayton, D.F.; Colombo, J.; Coppola, G.; Geyer, M.A.; Glanzman, D.L.; Marsland, S.; et al. Habituation Revisited: An Updated and Revised Description of the Behavioral Characteristics of Habituation. Neurobiol. Learn. Mem. 2009, 92, 135–138. [Google Scholar] [CrossRef]
- Blok, L.E.R.; Boon, M.; Van Reijmersdal, B.; Höffler, K.D.; Fenckova, M.; Schenck, A. Genetics, Molecular Control and Clinical Relevance of Habituation Learning. Neurosci. Biobehav. Rev. 2022, 143, 104883. [Google Scholar] [CrossRef]
- Kepler, L.D.; McDiarmid, T.A.; Rankin, C.H. Habituation in High-Throughput Genetic Model Organisms as a Tool to Investigate the Mechanisms of Neurodevelopmental Disorders. Neurobiol. Learn. Mem. 2020, 171, 107208. [Google Scholar] [CrossRef]
- McDiarmid, T.A.; Bernardos, A.C.; Rankin, C.H. Habituation Is Altered in Neuropsychiatric Disorders—A Comprehensive Review with Recommendations for Experimental Design and Analysis. Neurosci. Biobehav. Rev. 2017, 80, 286–305. [Google Scholar] [CrossRef]
- Das, S.; Sadanandappa, M.K.; Dervan, A.; Larkin, A.; Lee, J.A.; Sudhakaran, I.P.; Priya, R.; Heidari, R.; Holohan, E.E.; Pimentel, A.; et al. Plasticity of Local GABAergic Interneurons Drives Olfactory Habituation. Proc. Natl. Acad. Sci. USA 2011, 108. [Google Scholar] [CrossRef]
- Randlett, O.; Haesemeyer, M.; Forkin, G.; Shoenhard, H.; Schier, A.F.; Engert, F.; Granato, M. Distributed Plasticity Drives Visual Habituation Learning in Larval Zebrafish. Curr. Biol. 2019, 29, 1337–1345.e4. [Google Scholar] [CrossRef]
- Çevik, M.Ö. Habituation, Sensitization, and Pavlovian Conditioning. Front. Integr. Neurosci. 2014, 8. [Google Scholar] [CrossRef]
- Kaiser, H.; Parker, E.; Hamrick, M.W. Kynurenine Signaling through the Aryl Hydrocarbon Receptor: Implications for Aging and Healthspan. Exp. Gerontol. 2020, 130, 110797. [Google Scholar] [CrossRef]
- Pocivavsek, A.; Schwarcz, R.; Erhardt, S. Neuroactive Kynurenines as Pharmacological Targets: New Experimental Tools and Exciting Therapeutic Opportunities. Pharmacol. Rev. 2024, 76, 978–1008. [Google Scholar] [CrossRef]
- Morrens, M. The Role of the Kynurenine Pathway in Cognitive Function in Brain Disorders: Insights and Challenges. Brain. Behav. Immun. 2025, 127, 110–111. [Google Scholar] [CrossRef]
- Pathak, S.; Nadar, R.; Kim, S.; Liu, K.; Govindarajulu, M.; Cook, P.; Watts Alexander, C.S.; Dhanasekaran, M.; Moore, T. The Influence of Kynurenine Metabolites on Neurodegenerative Pathologies. Int. J. Mol. Sci. 2024, 25, 853. [Google Scholar] [CrossRef] [PubMed]
- Mithaiwala, M.N.; Santana-Coelho, D.; Porter, G.A.; O’Connor, J.C. Neuroinflammation and the Kynurenine Pathway in CNS Disease: Molecular Mechanisms and Therapeutic Implications. Cells 2021, 10, 1548. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Clanchy, F.I.L.; Huang, Y.-S.; Chiang, N.-Y.; Darlington, L.G.; Williams, R.O. An Integrated Cytokine and Kynurenine Network as the Basis of Neuroimmune Communication. Front. Neurosci. 2022, 16, 1002004. [Google Scholar] [CrossRef] [PubMed]
- Maddison, D.C.; Giorgini, F. The Kynurenine Pathway and Neurodegenerative Disease. Semin. Cell Dev. Biol. 2015, 40, 134–141. [Google Scholar] [CrossRef]
- Bakker, L.; Choe, K.; Eussen, S.J.P.M.; Ramakers, I.H.G.B.; Van Den Hove, D.L.A.; Kenis, G.; Rutten, B.P.F.; Verhey, F.R.J.; Köhler, S. Relation of the Kynurenine Pathway with Normal Age: A Systematic Review. Mech. Ageing Dev. 2024, 217, 111890. [Google Scholar] [CrossRef]
- Campbell, B.M.; Charych, E.; Lee, A.W.; Möller, T. Kynurenines in CNS Disease: Regulation by Inflammatory Cytokines. Front. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Sorgdrager, F.J.H.; Vermeiren, Y.; Van Faassen, M.; Van Der Ley, C.; Nollen, E.A.A.; Kema, I.P.; De Deyn, P.P. Age- and Disease-specific Changes of the Kynurenine Pathway in Parkinson’s and Alzheimer’s Disease. J. Neurochem. 2019, 151, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Vohra, M.; Lemieux, G.A.; Lin, L.; Ashrafi, K. Kynurenic Acid Accumulation Underlies Learning and Memory Impairment Associated with Aging. Genes Dev. 2018, 32, 14–19. [Google Scholar] [CrossRef]
- Garrison, A.M.; Parrott, J.M.; Tuñon, A.; Delgado, J.; Redus, L.; O’Connor, J.C. Kynurenine Pathway Metabolic Balance Influences Microglia Activity: Targeting Kynurenine Monooxygenase to Dampen Neuroinflammation. Psychoneuroendocrinology 2018, 94, 1–10. [Google Scholar] [CrossRef]
- Hughes, T.D.; Güner, O.F.; Iradukunda, E.C.; Phillips, R.S.; Bowen, J.P. The Kynurenine Pathway and Kynurenine 3-Monooxygenase Inhibitors. Molecules 2022, 27, 273. [Google Scholar] [CrossRef] [PubMed]
- Parrott, J.M.; Redus, L.; O’Connor, J.C. Kynurenine Metabolic Balance Is Disrupted in the Hippocampus Following Peripheral Lipopolysaccharide Challenge. J. Neuroinflammation 2016, 13, 124. [Google Scholar] [CrossRef]
- Salminen, A. Role of Indoleamine 2,3-Dioxygenase 1 (IDO1) and Kynurenine Pathway in the Regulation of the Aging Process. Ageing Res. Rev. 2022, 75, 101573. [Google Scholar] [CrossRef]
- Hahn, B.; Reneski, C.H.; Pocivavsek, A.; Schwarcz, R. Prenatal Kynurenine Treatment in Rats Causes Schizophrenia-like Broad Monitoring Deficits in Adulthood. Psychopharmacology 2018, 235, 651–661. [Google Scholar] [CrossRef]
- Milosavljevic, S.; Smith, A.K.; Wright, C.J.; Valafar, H.; Pocivavsek, A. Kynurenine Aminotransferase II Inhibition Promotes Sleep and Rescues Impairments Induced by Neurodevelopmental Insult. Transl. Psychiatry 2023, 13, 106. [Google Scholar] [CrossRef]
- Tufvesson-Alm, M.; Schwieler, L.; Schwarcz, R.; Goiny, M.; Erhardt, S.; Engberg, G. Importance of Kynurenine 3-Monooxygenase for Spontaneous Firing and Pharmacological Responses of Midbrain Dopamine Neurons: Relevance for Schizophrenia. Neuropharmacology 2018, 138, 130–139. [Google Scholar] [CrossRef]
- McMullen, E.; de la Flor, M.A.; Gunarante, G.; O’Connor, J.C.; Roman, G. Opynfield: An Open-Source Python Package for the Analysis of Open Field Exploration Data. J. Neuroinformatics 2025. [Google Scholar]
- de la Flor, M.A.; Kuhn-Sandoval, N.; Santana-Coelho, D.; Lozano, D.; Harrison, S.J.; Bucknor, M.C.; Mithaiwala, M.N.; OConnor, J.; Kokovay, E. KMO Deletion Preserves Non-Associative Learning and SVZ Neurogenesis in Aging Mice. SSRN 2025. [Google Scholar] [CrossRef]
- Notarangelo, F.M.; Pocivavsek, A. Elevated Kynurenine Pathway Metabolism during Neurodevelopment: Implications for Brain and Behavior. Neuropharmacology 2017, 112, 275–285. [Google Scholar] [CrossRef]
- González Esquivel, D.; Ramírez-Ortega, D.; Pineda, B.; Castro, N.; Ríos, C.; Pérez De La Cruz, V. Kynurenine Pathway Metabolites and Enzymes Involved in Redox Reactions. Neuropharmacology 2017, 112, 331–345. [Google Scholar] [CrossRef]
- Mor, A.; Tankiewicz-Kwedlo, A.; Krupa, A.; Pawlak, D. Role of Kynurenine Pathway in Oxidative Stress during Neurodegenerative Disorders. Cells 2021, 10, 1603. [Google Scholar] [CrossRef]
- Lugo-Huitrón, R.; Ugalde Muñiz, P.; Pineda, B.; Pedraza-Chaverrí, J.; Ríos, C.; Pérez-de La Cruz, V. Quinolinic Acid: An Endogenous Neurotoxin with Multiple Targets. Oxid. Med. Cell. Longev. 2013, 2013, 1–14. [Google Scholar] [CrossRef]
- Rahman, A.; Rao, M.S.; Khan, K.M. Intraventricular Infusion of Quinolinic Acid Impairs Spatial Learning and Memory in Young Rats: A Novel Mechanism of Lead-Induced Neurotoxicity. J. Neuroinflammation 2018, 15, 263. [Google Scholar] [CrossRef]
- Zwilling, D.; Huang, S.-Y.; Sathyasaikumar, K.V.; Notarangelo, F.M.; Guidetti, P.; Wu, H.-Q.; Lee, J.; Truong, J.; Andrews-Zwilling, Y.; Hsieh, E.W.; et al. Kynurenine 3-Monooxygenase Inhibition in Blood Ameliorates Neurodegeneration. Cell 2011, 145, 863–874. [Google Scholar] [CrossRef]
- Abudahab, S.; Price, E.T.; Dozmorov, M.G.; Deshpande, L.S.; McClay, J.L. The Aryl Hydrocarbon Receptor, Epigenetics and the Aging Process. J. Nutr. Health Aging 2023, 27, 291–300. [Google Scholar] [CrossRef]
- Summers, B.S.; Thomas Broome, S.; Pang, T.W.R.; Mundell, H.D.; Koh Belic, N.; Tom, N.C.; Ng, M.L.; Yap, M.; Sen, M.K.; Sedaghat, S.; et al. A Review of the Evidence for Tryptophan and the Kynurenine Pathway as a Regulator of Stem Cell Niches in Health and Disease. Int. J. Tryptophan Res. 2024, 17, 11786469241248287. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hatabayashi, K.; Arita, M.; Yajima, N.; Takenaka, C.; Suzuki, T.; Takahashi, M.; Oshima, Y.; Hara, K.; Kagawa, K.; et al. Kynurenine Signaling through the Aryl Hydrocarbon Receptor Maintains the Undifferentiated State of Human Embryonic Stem Cells. Sci. Signal 2019. [Google Scholar] [CrossRef]
- Blossom, V.; Ullal, S.D.; D’Souza, M.M.; Ranade, A.V.; Kumar, N.A.; Rai, R. Implicating Neuroinflammation in Hippocampus, Prefrontal Cortex and Amygdala with Cognitive Deficit: A Narrative Review. 3 Biotech 2025, 15, 320. [Google Scholar] [CrossRef]
- Höglund, E.; Øverli, Ø.; Winberg, S. Tryptophan Metabolic Pathways and Brain Serotonergic Activity: A Comparative Review. Front. Endocrinol. 2019, 10, 158. [Google Scholar] [CrossRef]
- Muneer, A. Kynurenine Pathway of Tryptophan Metabolism in Neuropsychiatric Disorders: Pathophysiologic and Therapeutic Considerations. Clin. Psychopharmacol. Neurosci. 2020, 18, 507–526. [Google Scholar] [CrossRef]
- Wichers, M.C.; Maes, M. The Role of Indoleamine 2,3-Dioxygenase (IDO) in the Pathophysiology of Interferon-α-Induced Depression. J. Psychiatry Neurosci. 2004, 29, 11–17. [Google Scholar] [CrossRef]
- Cooke, S.F.; Bear, M.F. Visual Recognition Memory: A View from V1. Curr. Opin. Neurobiol. 2015, 35, 57–65. [Google Scholar] [CrossRef]
- Imbeault, S.; Gubert Olivé, M.; Jungholm, O.; Erhardt, S.; Wigström, H.; Engberg, G.; Jardemark, K. Blockade of KAT II Facilitates LTP in Kynurenine 3-Monooxygenase Depleted Mice. Int. J. Tryptophan Res. 2021, 14, 11786469211041368. [Google Scholar] [CrossRef]
- Stone, T.W.; Darlington, L.G. The Kynurenine Pathway as a Therapeutic Target in Cognitive and Neurodegenerative Disorders. Br. J. Pharmacol. 2013, 169, 1211–1227. [Google Scholar] [CrossRef]
- Pocivavsek, A.; Elmer, G.I.; Schwarcz, R. Inhibition of Kynurenine Aminotransferase II Attenuates Hippocampus-dependent Memory Deficit in Adult Rats Treated Prenatally with Kynurenine. Hippocampus 2019, 29, 73–77. [Google Scholar] [CrossRef]
- Fukuwatari, T. Possibility of Amino Acid Treatment to Prevent the Psychiatric Disorders via Modulation of the Production of Tryptophan Metabolite Kynurenic Acid. Nutrients 2020, 12, 1403. [Google Scholar] [CrossRef]
- Oh, C.; Nakamura, T.; Zhang, X.; Lipton, S.A. Redox Regulation, Protein S-Nitrosylation, and Synapse Loss in Alzheimer’s and Related Dementias. Neuron 2024, 112, 3823–3850. [Google Scholar] [CrossRef]
- Wen, T.H.; Binder, D.K.; Ethell, I.M.; Razak, K.A. The Perineuronal ‘Safety’ Net? Perineuronal Net Abnormalities in Neurological Disorders. Front. Mol. Neurosci. 2018, 11, 270. [Google Scholar] [CrossRef]
- Wilson, C.; González-Billault, C. Regulation of Cytoskeletal Dynamics by Redox Signaling and Oxidative Stress: Implications for Neuronal Development and Trafficking. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef]
- Bondulich, M.K.; Fan, Y.; Song, Y.; Giorgini, F.; Bates, G.P. Ablation of Kynurenine 3-Monooxygenase Rescues Plasma Inflammatory Cytokine Levels in the R6/2 Mouse Model of Huntington’s Disease. Sci. Rep. 2021, 11, 5484. [Google Scholar] [CrossRef]
- Wang, D.; Lang, Z.; Wei, S.; Wang, W.; Zhang, H. Targeting Brain-derived Neurotrophic Factor in the Treatment of Neurodegenerative Diseases: A Review. Neuroprotection 2024, 2, 67–78. [Google Scholar] [CrossRef]
- Flores-Barrera, E.; Thomases, D.R.; Cass, D.K.; Bhandari, A.; Schwarcz, R.; Bruno, J.P.; Tseng, K.Y. Preferential Disruption of Prefrontal GABAergic Function by Nanomolar Concentrations of the α7nACh Negative Modulator Kynurenic Acid. J. Neurosci. 2017, 37, 7921–7929. [Google Scholar] [CrossRef]
- Potter, M.C.; Elmer, G.I.; Bergeron, R.; Albuquerque, E.X.; Guidetti, P.; Wu, H.-Q.; Schwarcz, R. Reduction of Endogenous Kynurenic Acid Formation Enhances Extracellular Glutamate, Hippocampal Plasticity, and Cognitive Behavior. Neuropsychopharmacology 2010, 35, 1734–1742. [Google Scholar] [CrossRef] [PubMed]
- Kutlu, M.G.; Zachry, J.E.; Melugin, P.R.; Tat, J.; Cajigas, S.; Isiktas, A.U.; Patel, D.D.; Siciliano, C.A.; Schoenbaum, G.; Sharpe, M.J.; et al. Dopamine Signaling in the Nucleus Accumbens Core Mediates Latent Inhibition. Nat. Neurosci. 2022, 25, 1071–1081. [Google Scholar] [CrossRef]
- Trisal, S.; Aranha, M.; Chodankar, A.; VijayRaghavan, K.; Ramaswami, M. A Drosophila Circuit for Habituation Override. J. Neurosci. 2022, 42, 2930–2941. [Google Scholar] [CrossRef] [PubMed]
- Martos, D.; Tuka, B.; Tanaka, M.; Vécsei, L.; Telegdy, G. Memory Enhancement with Kynurenic Acid and Its Mechanisms in Neurotransmission. Biomedicines 2022, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, D.; Iyer, M.; Narayanasamy, A.; Siva, K.; Vellingiri, B. Kynurenine Pathway in Parkinson’s Disease—An Update. eNeurologicalSci 2020, 21, 100270. [Google Scholar] [CrossRef]
- Bacqué-Cazenave, J.; Bharatiya, R.; Barrière, G.; Delbecque, J.-P.; Bouguiyoud, N.; Di Giovanni, G.; Cattaert, D.; De Deurwaerdère, P. Serotonin in Animal Cognition and Behavior. Int. J. Mol. Sci. 2020, 21, 1649. [Google Scholar] [CrossRef]
- Leussis, M.; Bolivar, V. Habituation in Rodents: A Review of Behavior, Neurobiology, and Genetics. Neurosci. Biobehav. Rev. 2006, 30, 1045–1064. [Google Scholar] [CrossRef]
- Mayford, M.; Siegelbaum, S.A.; Kandel, E.R. Synapses and Memory Storage. Cold Spring Harb. Perspect. Biol. 2012, 4, a005751. [Google Scholar] [CrossRef]
- Li, Y.; Hu, N.; Yang, D.; Oxenkrug, G.; Yang, Q. Regulating the Balance between the Kynurenine and Serotonin Pathways of Tryptophan Metabolism. FEBS J. 2017, 284, 948–966. [Google Scholar] [CrossRef]
- López-Schier, H. Neuroplasticity in the Acoustic Startle Reflex in Larval Zebrafish. Curr. Opin. Neurobiol. 2019, 54, 134–139. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Sehgal, A.; Singh, S.; Bhatia, S.; Al-Harrasi, A.; Zengin, G.; Bumbu, A.G.; Andronie-Cioara, F.L.; Nechifor, A.C.; et al. The Footprint of Kynurenine Pathway in Neurodegeneration: Janus-Faced Role in Parkinson’s Disorder and Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 6737. [Google Scholar] [CrossRef]
- Savitz, J. The Kynurenine Pathway: A Finger in Every Pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tong, J.; Zhang, P.; Zhou, Y.; Cui, Y.; Tan, S.; Wang, Z.; Yang, F.; Kochunov, P.; Chiappelli, J.; et al. Effects of Neuroactive Metabolites of the Tryptophan Pathway on Working Memory and Cortical Thickness in Schizophrenia. Transl. Psychiatry 2021, 11, 198. [Google Scholar] [CrossRef]
- Mezo-González, C.E.; Daher Abdi, A.; Reyes-Castro, L.A.; Olvera Hernández, S.; Almeida, C.; Croyal, M.; Aguesse, A.; Gavioli, E.C.; Zambrano, E.; Bolaños-Jiménez, F. Learning Deficits Induced by High-Calorie Feeding in the Rat Are Associated with Impaired Brain Kynurenine Pathway Metabolism. Int. J. Tryptophan Res. 2022, 15, 11786469221111116. [Google Scholar] [CrossRef] [PubMed]
- Yeap, J.; Crouch, B.; Riedel, G.; Platt, B. Sequential Habituation to Space, Object and Stranger Is Differentially Modulated by Glutamatergic, Cholinergic and Dopaminergic Transmission. Behav. Pharmacol. 2020, 31, 652–670. [Google Scholar] [CrossRef] [PubMed]
- Sathyasaikumar, K.V.; Stachowski, E.K.; Wonodi, I.; Roberts, R.C.; Rassoulpour, A.; McMahon, R.P.; Schwarcz, R. Impaired Kynurenine Pathway Metabolism in The Prefrontal Cortex of Individuals with Schizophrenia. Schizophr. Bull. 2011, 37, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Castellano-Gonzalez, G.; Jacobs, K.R.; Don, E.; Cole, N.J.; Adams, S.; Lim, C.K.; Lovejoy, D.B.; Guillemin, G.J. Kynurenine 3-Monooxygenase Activity in Human Primary Neurons and Effect on Cellular Bioenergetics Identifies New Neurotoxic Mechanisms. Neurotox. Res. 2019, 35, 530–541. [Google Scholar] [CrossRef]
- Șerban, M.; Toader, C.; Covache-Busuioc, R.-A. The Redox Revolution in Brain Medicine: Targeting Oxidative Stress with AI, Multi-Omics and Mitochondrial Therapies for the Precision Eradication of Neurodegeneration. Int. J. Mol. Sci. 2025, 26, 7498. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.; Redondo-Flórez, L.; Beltrán-Velasco, A.; Ramos-Campo, D.; Belinchón-deMiguel, P.; Martinez-Guardado, I.; Dalamitros, A.; Yáñez-Sepúlveda, R.; Martín-Rodríguez, A.; Tornero-Aguilera, J. Mitochondria and Brain Disease: A Comprehensive Review of Pathological Mechanisms and Therapeutic Opportunities. Biomedicines 2023, 11, 2488. [Google Scholar] [CrossRef]
- Olesen, M.A.; Torres, A.K.; Jara, C.; Murphy, M.P.; Tapia-Rojas, C. Premature Synaptic Mitochondrial Dysfunction in the Hippocampus during Aging Contributes to Memory Loss. Redox Biol. 2020, 34, 101558. [Google Scholar] [CrossRef]
- Comyn, T.; Preat, T.; Pavlowsky, A.; Plaçais, P.-Y. Mitochondrial Plasticity: An Emergent Concept in Neuronal Plasticity and Memory. Neurobiol. Dis. 2024, 203, 106740. [Google Scholar] [CrossRef]
- Massaad, C.A.; Klann, E. Reactive Oxygen Species in the Regulation of Synaptic Plasticity and Memory. Antioxid. Redox Signal. 2011, 14, 2013–2054. [Google Scholar] [CrossRef]
- Song, N.; Mei, S.; Wang, X.; Hu, G.; Lu, M. Focusing on Mitochondria in the Brain: From Biology to Therapeutics. Transl. Neurodegener. 2024, 13, 23. [Google Scholar] [CrossRef]
- Netto, M.B.; De Oliveira Junior, A.N.; Goldim, M.; Mathias, K.; Fileti, M.E.; Da Rosa, N.; Laurentino, A.O.; De Farias, B.X.; Costa, A.B.; Rezin, G.T.; et al. Oxidative Stress and Mitochondrial Dysfunction Contributes to Postoperative Cognitive Dysfunction in Elderly Rats. Brain. Behav. Immun. 2018, 73, 661–669. [Google Scholar] [CrossRef]
- Jones, S.P.; Guillemin, G.J.; Brew, B.J. The Kynurenine Pathway in Stem Cell Biology. Int. J. Tryptophan Res. 2013, 6, IJTR.S12626. [Google Scholar] [CrossRef]
- Siddiqui, T.; Bhattarai, P.; Popova, S.; Cosacak, M.I.; Sariya, S.; Zhang, Y.; Mayeux, R.; Tosto, G.; Kizil, C. KYNA/Ahr Signaling Suppresses Neural Stem Cell Plasticity and Neurogenesis in Adult Zebrafish Model of Alzheimer’s Disease. Cells 2021, 10, 2748. [Google Scholar] [CrossRef]
- Chen, W.-C.; Chang, L.-H.; Huang, S.-S.; Huang, Y.-J.; Chih, C.-L.; Kuo, H.-C.; Lee, Y.-H.; Lee, I.-H. Aryl Hydrocarbon Receptor Modulates Stroke-Induced Astrogliosis and Neurogenesis in the Adult Mouse Brain. J. Neuroinflammation 2019, 16, 187. [Google Scholar] [CrossRef]
- Roman, Á.C.; Carvajal-Gonzalez, J.M.; Merino, J.M.; Mulero-Navarro, S.; Fernández-Salguero, P.M. The Aryl Hydrocarbon Receptor in the Crossroad of Signalling Networks with Therapeutic Value. Pharmacol. Ther. 2018, 185, 50–63. [Google Scholar] [CrossRef]
- Trikha, P.; Lee, D.A. The Role of AhR in Transcriptional Regulation of Immune Cell Development and Function. Biochim. Biophys. Acta BBA-Rev. Cancer 2020, 1873, 188335. [Google Scholar] [CrossRef]
- Vogel, C.F.A. The Aryl Hydrocarbon Receptor Repressor - More than a Simple Feedback Inhibitor of AhR Signaling: Clues for Its Role in Inflammation and Cancer. Curr. Opin. Toxicol. 2017, 2, 109–119. [Google Scholar] [CrossRef]
- Pham, H.; Ono, M.; Hara, E.; Nguyen, H.; Dang, A.; Do, H.; Komori, T.; Tosa, I.; Hazehara-Kunitomo, Y.; Yoshioka, Y.; et al. Tryptophan and Kynurenine Enhances the Stemness and Osteogenic Differentiation of Bone Marrow-Derived Mesenchymal Stromal Cells In Vitro and In Vivo. Materials 2021, 14, 208. [Google Scholar] [CrossRef]
- Chess, A.C.; Simoni, M.K.; Alling, T.E.; Bucci, D.J. Elevations of Endogenous Kynurenic Acid Produce Spatial Working Memory Deficits. Schizophr. Bull. 2007, 33, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, W.-Y.; Figarella, K.; Garaschuk, O. Olfactory Impairment in Men and Mice Related to Aging and Amyloid-Induced Pathology. Pflüg. Arch. Eur. J. Physiol. 2021, 473, 805–821. [Google Scholar] [CrossRef] [PubMed]
- Apple, D.M.; Fonseca, R.S.; Kokovay, E. The Role of Adult Neurogenesis in Psychiatric and Cognitive Disorders. Brain Res. 2017, 1655, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Portalés, A.; Chamero, P.; Jurado, S. Natural and Pathological Aging Distinctively Impacts the Pheromone Detection System and Social Behavior. Mol. Neurobiol. 2023, 60, 4641–4658. [Google Scholar] [CrossRef]
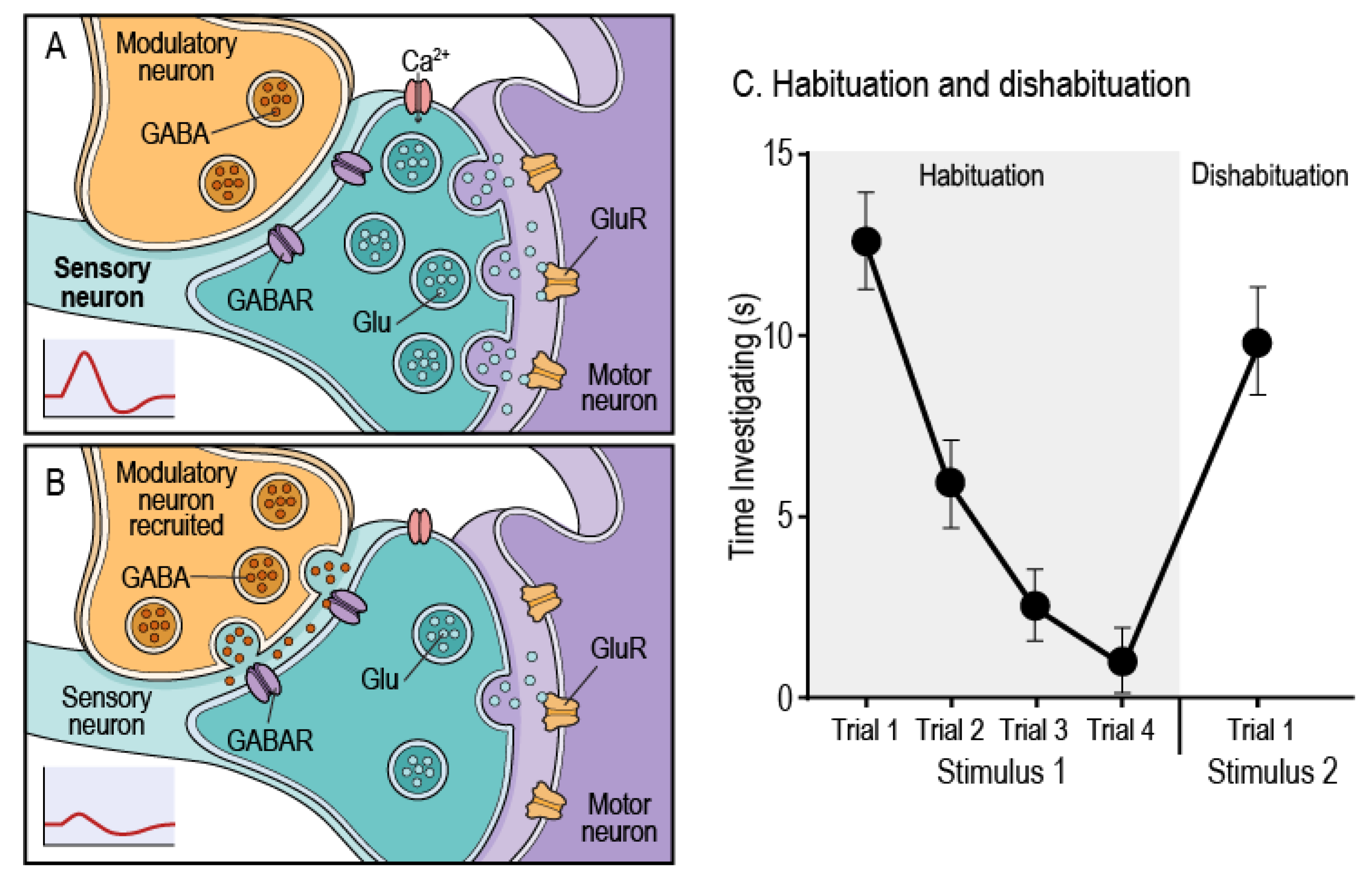

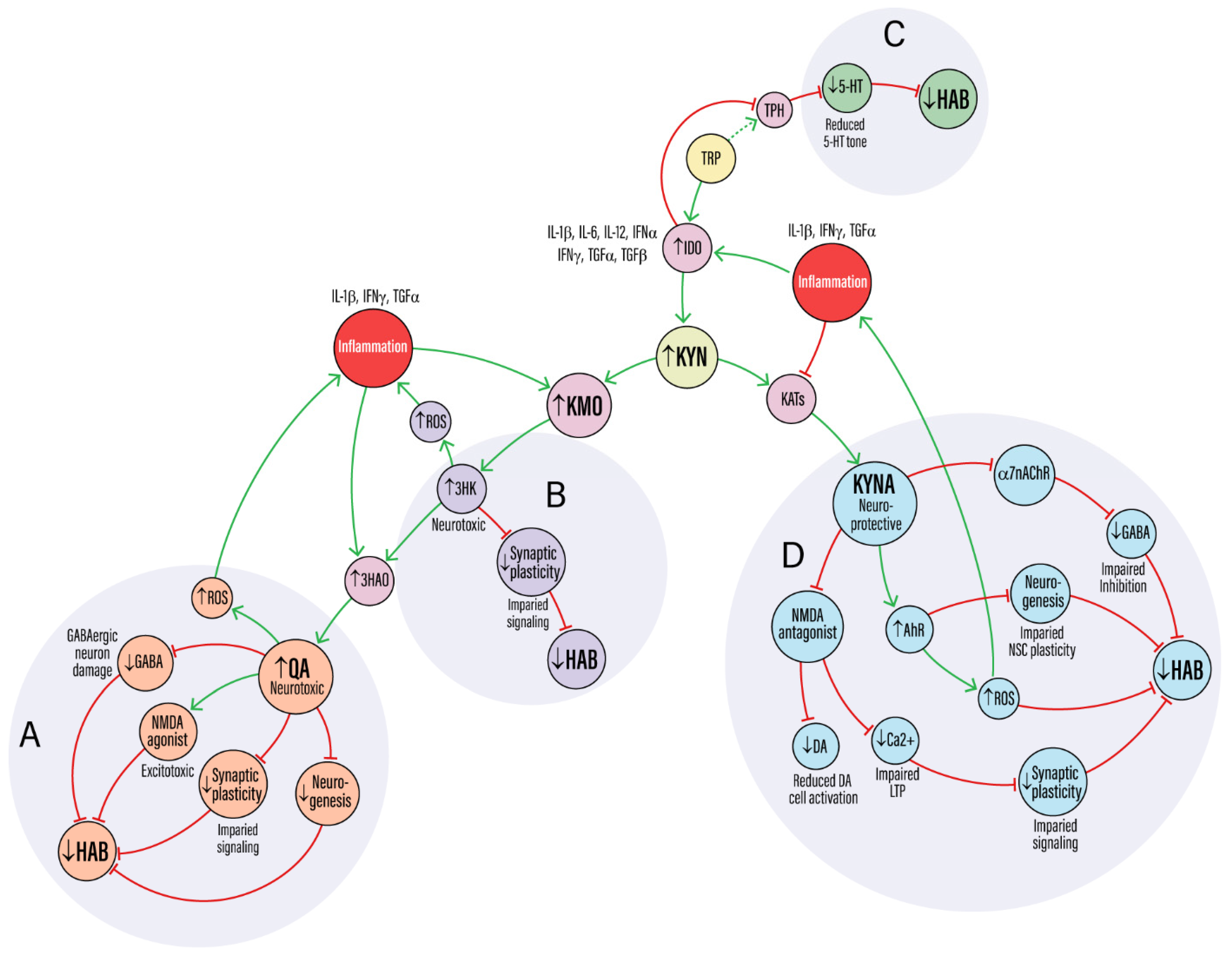
| Metabolite | Mechanisms Affected | Key References | Impact on Habituation |
|---|---|---|---|
| Kynurenic acid (KYNA) | Antagonist at NMDA and α7nACh receptors; suppresses glutamate release; reduces excitatory drive to GABAergic interneurons; activates AhR signaling | [14,17,18,33] | Dose-dependent suppression of LTP; disrupted glutamate–GABA balance; reduced DA signaling; excessive AhR activation reduces neurogenesis and circuit stability → impairs habituation |
| 3-Hydroxykynurenine (3-HK) | Undergoes auto-oxidation and redox cycling, generating ROS; contributes to oxidative stress and lipid peroxidation | [16,25,34,35] | Oxidative damage to redox-sensitive synaptic proteins and cytoskeletal elements; impaired mitochondrial function and synaptic plasticity → impairs habituation |
| Quinolinic acid (QA) | NMDA receptor agonist; drives calcium overload and excitotoxicity; inhibits glutamate uptake; induces cytotoxicity in GABAergic neurons | [36,37,38] | Excitotoxicity and loss of inhibitory tone destabilize circuits; suppresses neurogenesis; chronic E/I imbalance → strongly impairs habituation |
| Kynurenine (KYN) | Precursor metabolite; activates AhR signaling to maintain stem-cell identity and pluripotency; modulates immune tone | [17,39,40,41] | Context-dependent: supports regenerative potential under basal conditions but may suppress differentiation and neurogenesis during aging/inflammation → mixed effects on habituation |
| ↑ IDO activity (↓ 5-HT) | Inflammation-driven IDO activation diverts tryptophan away from serotonin (5-HT) synthesis | [42,43,44,45] | Decreased 5-HT availability impairs sensory gating, salience detection, and cognitive flexibility → impairs habituation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Flor, M.A.; O’Connor, J.C. Losing the Filter: How Kynurenine Pathway Dysregulation Impairs Habituation. Cells 2025, 14, 1786. https://doi.org/10.3390/cells14221786
de la Flor MA, O’Connor JC. Losing the Filter: How Kynurenine Pathway Dysregulation Impairs Habituation. Cells. 2025; 14(22):1786. https://doi.org/10.3390/cells14221786
Chicago/Turabian Stylede la Flor, Miguel A., and Jason C. O’Connor. 2025. "Losing the Filter: How Kynurenine Pathway Dysregulation Impairs Habituation" Cells 14, no. 22: 1786. https://doi.org/10.3390/cells14221786
APA Stylede la Flor, M. A., & O’Connor, J. C. (2025). Losing the Filter: How Kynurenine Pathway Dysregulation Impairs Habituation. Cells, 14(22), 1786. https://doi.org/10.3390/cells14221786








