TNFR1 Suppression by XPro1595 Reduces Peripheral Neuropathies Associated with Perineural Invasion in Female Mice
Highlights
- In a mouse model of perineural invasion, blocking soluble TNFα signaling with XPro1595—but not TNFR1 knockout—reduced tumor burden, mechanical allodynia, and locomotor deficits, primarily in females.
- XPro1595 may exert its beneficial effects via promoting mitochondrial function and myelination while suppressing inflammatory, extracellular matrix, and tumor progression pathways.
- Pharmacologically targeting soluble TNFα with XPro1595 may represent a promising, sex-dependent therapeutic strategy to alleviate cancer-associated pain and nerve injury linked to PNI.
- TNFα signaling contributes to both tumor progression and nerve pathology in a sex-specific manner, highlighting the need for precision approaches in cancer pain management.
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. The Mouse PNI Model
2.3. Von Frey Paw Withdrawal Assay
2.4. Hargreaves’s Test
2.5. Behavioral Spectrometer
2.6. Toe-Spread Assessment
2.7. Tumor Size Measurement
2.8. Multiplex Immunohistochemistry and Immunofluorescence (mIHC/IF) Analysis
2.9. RNA Sequencing
2.10. Statistical Analysis
3. Results
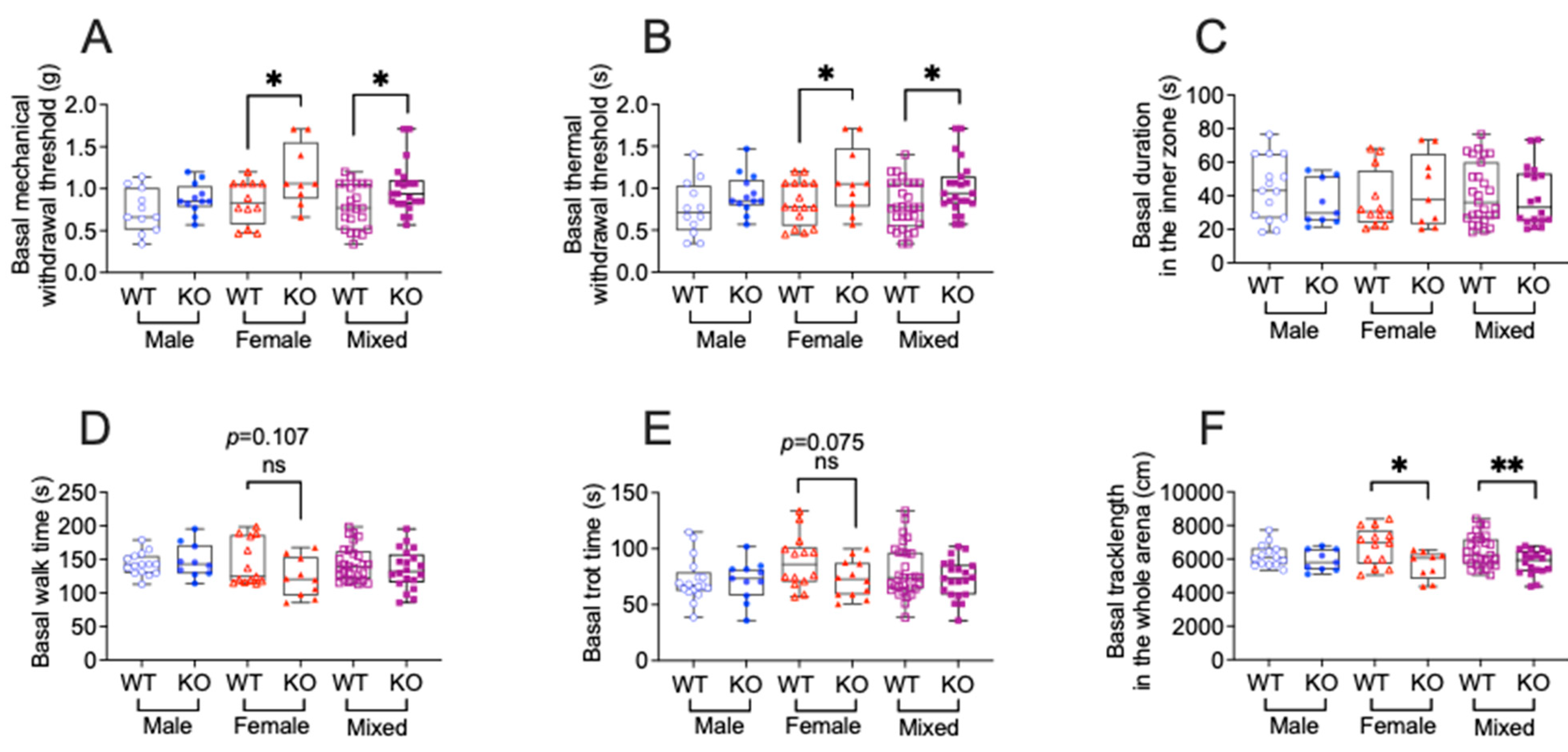
3.1. TNFR1 Gene Deletion Affects Sensory and Motor Function in a Sex Dependent Manner in Tumor-Free Mice
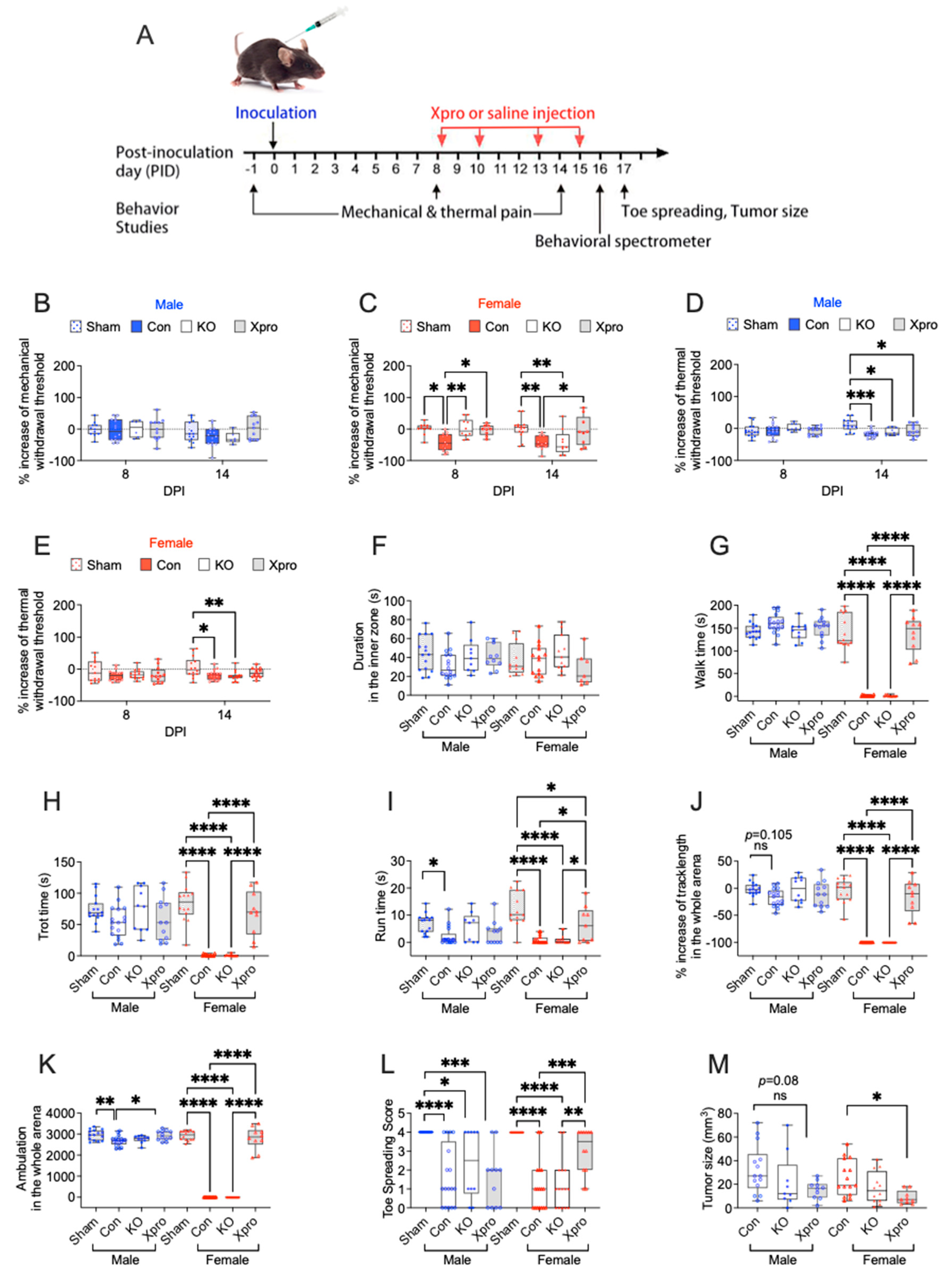
3.2. TNFR1 Gene Deletion or XPro1595 Treatment Affects Sensory and Motor Function in a Sex Dependent Manner in Mice with PNI
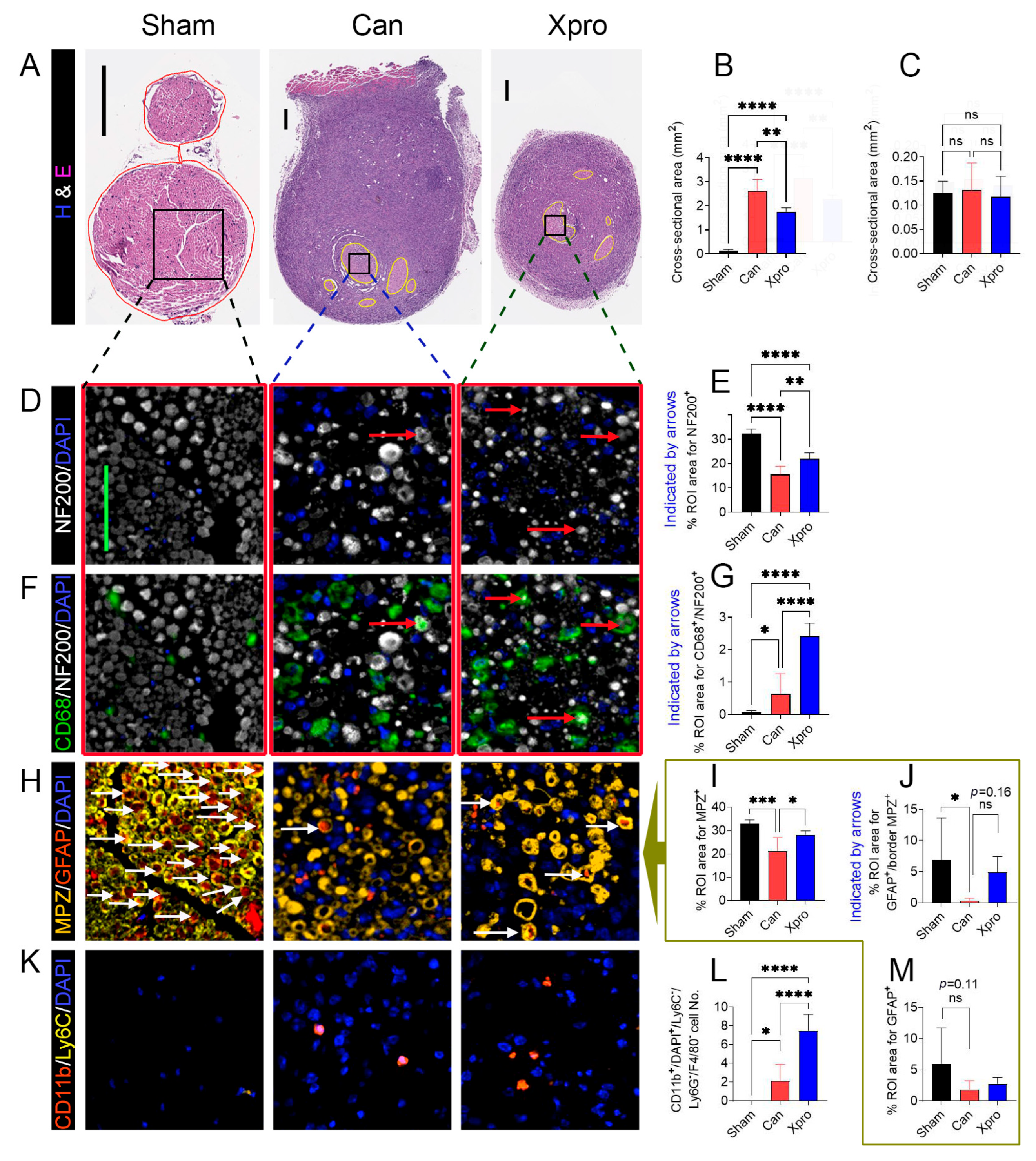
3.3. Xpro1595 Reduced Nerve Damage, Neuroinflammation, and Tumor Size
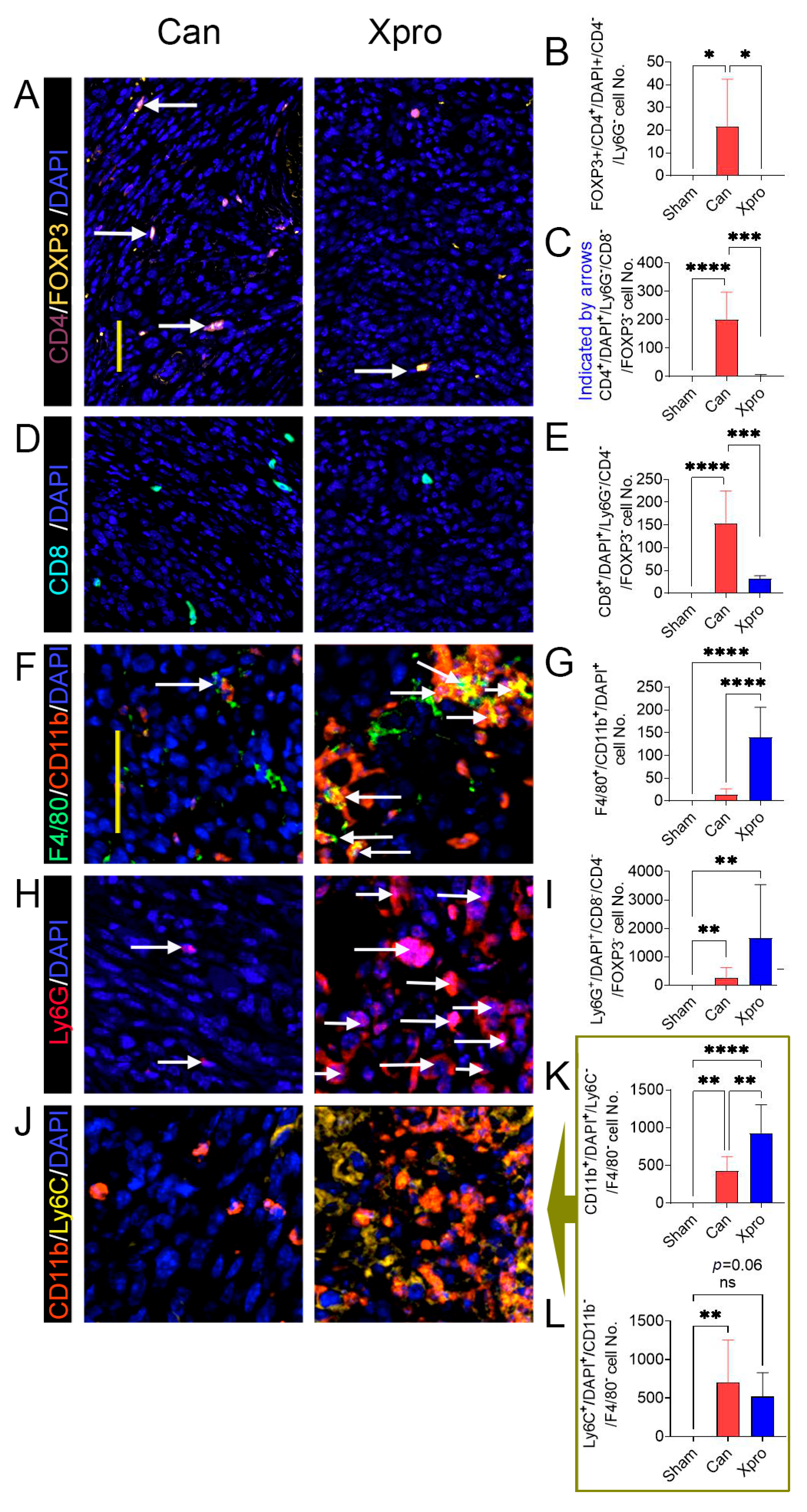
3.4. XPro1595 Treatment Altered Tumor Immune Environment
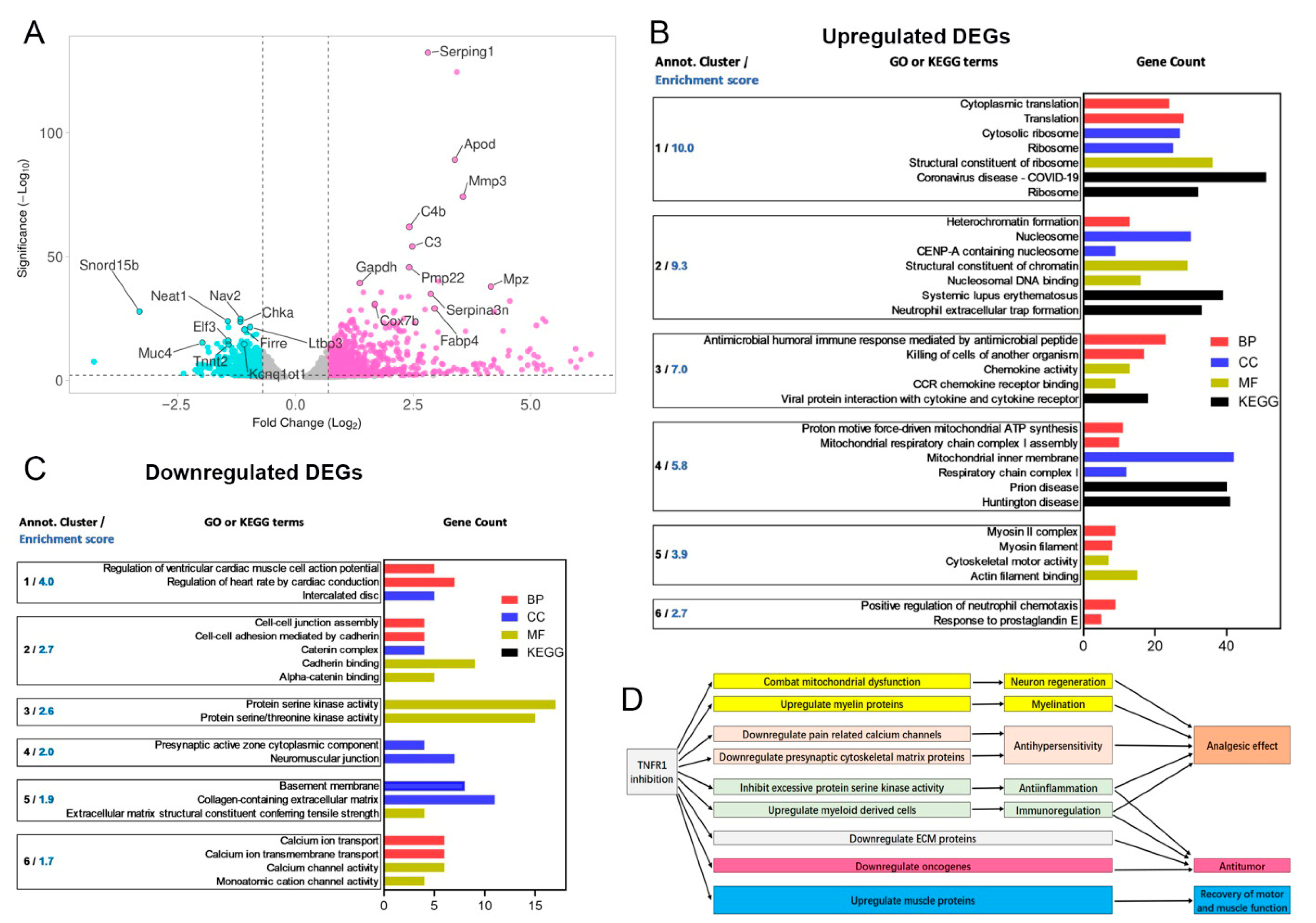
3.5. Transcriptomic Insights on the Analgesic Mechanism of XPro1595
3.5.1. Upregulated DEGs and Functional Clusters
3.5.2. Downregulated DEGs and Their Functional Clusters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PNI | Perineural invasion |
| TNFα | Tumor necrosis factor α |
| solTNFα | Soluble tumor necrosis factor α |
| tmTNFα | Transmembrane tumor necrosis factor α |
| TNFR1 | Tumor necrosis factor receptor 1 |
| HNC | Head and neck cancer |
| ECM | Extracellular matrix |
| SCs | Schwann cells |
| DEGs | Differentially expressed genes |
| KO | Knockout |
| WT | Wide-type |
| MOC2 | Mouse oral cancer |
| PID | Post-inoculation day |
| ROIs | Regions of interest |
| EASE | Expression Analysis Systematic Explorer |
| BPs | Biological processes |
| CCs | Cellular components |
| MFs | Molecular functions |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| ANOVA | Analysis of variance |
| SEM | Standard error of the mean |
| GABA | Gamma-aminobutyric acid |
| NMDA | N-methyl-D-aspartate |
| CGRP | Calcitonin gene-related peptide |
| CCI | Spared nerve injury |
References
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural invasion in cancer: A review of the literature. Cancer 2009, 115, 3379–3391. [Google Scholar] [CrossRef]
- Frunza, A.; Slavescu, D.; Lascar, I. Perineural invasion in head and neck cancers–A review. J. Med. Life 2014, 7, 121–123. [Google Scholar]
- Santi, M.D.; Zhang, M.; Asam, K.; Yu, G.; Dong, P.M.; Sheehan, D.H.; Aouizerat, B.E.; Thomas, C.M.; Viet, C.T.; Ye, Y. Perineural Invasion Is Associated With Function-evoked Pain and Altered Extracellular Matrix in Patients With Head and Neck Squamous Cell Carcinoma. J. Pain 2024, 25, 104615. [Google Scholar] [CrossRef]
- Zhang, M.; Yuan, M.; Asam, K.; Gong, Z.; Xie, T.; Gleber-Netto, F.; Santi, M.D.; Kobayashi, Y.; Shimizu, E.; Aouizerat, B.; et al. Perineural Invasion Exhibits Traits of Neurodegeneration. J. Dent. Res. 2025, 104, 1352–1360. [Google Scholar] [CrossRef]
- Gutierrez, S.; Parker, R.A.; Zhang, M.; Santi, M.D.; Ye, Y.; Boada, M.D. Advanced cancer perineural invasion induces profound peripheral neuronal plasticity, pain, and somatosensory mechanical deactivation, unmitigated by the lack of TNFR1. Part. 1: Behavior and single-cell in vivo electrophysiology. Mol. Pain 2025, 21, 17448069251314738. [Google Scholar] [CrossRef]
- Gutierrez, S.; Parker, R.A.; Zhang, M.; Santi, M.D.; Ye, Y.; Boada, M.D. Advanced cancer perineural invasion induces profound peripheral neuronal plasticity, pain, and somatosensory mechanical deactivation, unmitigated by the lack of TNFR1. Part 2. Biophysics and gene expression. Mol. Pain 2025, 21, 17448069251323666. [Google Scholar] [CrossRef]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Greenwel, P.; Tanaka, S.; Penkov, D.; Zhang, W.; Olive, M.; Moll, J.; Vinson, C.; Di Liberto, M.; Ramirez, F. Tumor Necrosis Factor Alpha Inhibits Type I Collagen Synthesis through Repressive CCAAT/Enhancer-Binding Proteins. Mol. Cell. Biol. 2000, 20, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Fresegna, D.; Bullitta, S.; Musella, A.; Rizzo, F.R.; De Vito, F.; Guadalupi, L.; Caioli, S.; Balletta, S.; Sanna, K.; Dolcetti, E.; et al. Re-Examining the Role of TNF in MS Pathogenesis and Therapy. Cells 2020, 9, 2290. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, N.; Soorappan, R.N.; Haque, M.; Sriramula, S.; Francis, J. TNF-α-induced mitochondrial oxidative stress and cardiac dysfunction: Restoration by superoxide dismutase mimetic Tempol. Am. J. Physiol. Circ. Physiol. 2007, 293, H2726–H2737. [Google Scholar] [CrossRef] [PubMed]
- Fazzi, F.; Njah, J.; Di Giuseppe, M.; Winnica, D.E.; Go, K.; Sala, E.; Croix, C.M.S.; Watkins, S.C.; Tyurin, V.A.; Phinney, D.G.; et al. TNFR1/Phox Interaction and TNFR1 Mitochondrial Translocation Thwart Silica-Induced Pulmonary Fibrosis. J. Immunol. 2014, 192, 3837–3846. [Google Scholar] [CrossRef]
- Andrade, P.; Visser-Vandewalle, V.; Hoffmann, C.; Steinbusch, H.W.M.; Daemen, M.A.; Hoogland, G. Role of TNF-alpha during central sensitization in preclinical studies. Neurol. Sci. 2011, 32, 757–771. [Google Scholar] [CrossRef]
- Durham, Z.L.; Hawkins, J.L.; Durham, P.L. Tumor necrosis factor-Alpha stimulates cytokine expression and transient sensitization of trigeminal nociceptive neurons. Arch. Oral Biol. 2017, 75, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Higerd-Rusli, G.P.; Ghovanloo, M.-R.; Dib-Hajj, F.; Zhao, P.; Liu, S.; Kim, D.-H.; Shim, J.S.; Park, K.-S.; Waxman, S.G.; et al. Compartment-specific regulation of NaV1.7 in sensory neurons after acute exposure to TNF-α. Cell Rep. 2024, 43, 113685. [Google Scholar] [CrossRef] [PubMed]
- Leo, M.; Argalski, S.; Schäfers, M.; Hagenacker, T. Modulation of Voltage-Gated Sodium Channels by Activation of Tumor Necrosis Factor Receptor-1 and Receptor-2 in Small DRG Neurons of Rats. Mediat. Inflamm. 2015, 2015, 124942. [Google Scholar] [CrossRef]
- Mercogliano, M.F.; Bruni, S.; Mauro, F.; Elizalde, P.V.; Schillaci, R. Harnessing Tumor Necrosis Factor Alpha to Achieve Effective Cancer Immunotherapy. Cancers 2021, 13, 564. [Google Scholar] [CrossRef]
- Goertzen, C.; Mahdi, H.; Laliberte, C.; Meirson, T.; Eymael, D.; Gil-Henn, H.; Magalhaes, M. Oral inflammation promotes oral squamous cell carcinoma invasion. Oncotarget 2018, 9, 29047–29063. [Google Scholar] [CrossRef]
- Leung, L.; Cahill, C.M. TNF-alpha and neuropathic pain—A review. J. Neuroinflammation 2010, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Scheff, N.N.; Ye, Y.; Bhattacharya, A.; MacRae, J.; Hickman, D.N.; Sharma, A.K.; Dolan, J.C.; Schmidt, B.L. Tumor necrosis factor alpha secreted from oral squamous cell carcinoma contributes to cancer pain and associated inflammation. Pain 2017, 158, 2396–2409. [Google Scholar] [CrossRef]
- Salvo, E.; Tu, N.H.; Scheff, N.N.; Dubeykovskaya, Z.A.; Chavan, S.A.; Aouizerat, B.E.; Ye, Y. TNFα promotes oral cancer growth, pain, and Schwann cell activation. Sci. Rep. 2021, 11, 1840. [Google Scholar] [CrossRef]
- Atherton, M.A.; Park, S.; Horan, N.L.; Nicholson, S.; Dolan, J.C.; Schmidt, B.L.; Scheff, N.N. Sympathetic modulation of tumor necrosis factor alpha-induced nociception in the presence of oral squamous cell carcinoma. Pain 2022, 164, 27–42. [Google Scholar] [CrossRef]
- Watts, A.D.; Hunt, N.H.; Madigan, M.C.; Chaudhri, G. Soluble TNF-α receptors bind and neutralize over-expressed transmembrane TNF-α on macrophages, but do not inhibit its processing. J. Leukoc. Biol. 1999, 66, 1005–1013. [Google Scholar] [CrossRef]
- Ruiz, A.; Palacios, Y.; Garcia, I.; Chavez-Galan, L. Transmembrane TNF and Its Receptors TNFR1 and TNFR2 in Mycobacterial Infections. Int. J. Mol. Sci. 2021, 22, 5461. [Google Scholar] [CrossRef] [PubMed]
- Papazian, I.; Tsoukala, E.; Boutou, A.; Karamita, M.; Kambas, K.; Iliopoulou, L.; Fischer, R.; Kontermann, R.E.; Denis, M.C.; Kollias, G.; et al. Fundamentally different roles of neuronal TNF receptors in CNS pathology: TNFR1 and IKKβ promote microglial responses and tissue injury in demyelination while TNFR2 protects against excitotoxicity in mice. J. Neuroinflamm. 2021, 18, 222. [Google Scholar] [CrossRef] [PubMed]
- Kartikasari, A.E.R.; Cassar, E.; Razqan, M.A.M.; Szydzik, C.; Huertas, C.S.; Mitchell, A.; Plebanski, M. Elevation of circulating TNF receptor 2 in cancer: A systematic meta-analysis for its potential as a diagnostic cancer biomarker. Front. Immunol. 2022, 13, 918254. [Google Scholar] [CrossRef]
- Alim, L.F.; Keane, C.; Souza-Fonseca-Guimaraes, F. Molecular mechanisms of tumour necrosis factor signalling via TNF receptor 1 and TNF receptor 2 in the tumour microenvironment. Curr. Opin. Immunol. 2023, 86, 102409. [Google Scholar] [CrossRef]
- García-Domínguez, M. The Role of TNF-α in Neuropathic Pain: An Immunotherapeutic Perspective. Life 2025, 15, 785. [Google Scholar] [CrossRef] [PubMed]
- del Rivero, T.; Fischer, R.; Yang, F.; Swanson, K.A.; Bethea, J.R. Tumor necrosis factor receptor 1 inhibition is therapeutic for neuropathic pain in males but not in females. Pain 2018, 160, 922–931. [Google Scholar] [CrossRef]
- Camara, M.L.; Corrigan, F.; Jaehne, E.J.; Jawahar, M.C.; Anscomb, H.; Baune, B.T. Tumor necrosis factor alpha and its receptors in behaviour and neurobiology of adult mice, in the absence of an immune challenge. Behav. Brain Res. 2015, 290, 51–60. [Google Scholar] [CrossRef]
- Patel, A.; Siegel, A.; Zalcman, S.S. Lack of aggression and anxiolytic-like behavior in TNF receptor (TNF-R1 and TNF-R2) deficient mice. Brain Behav. Immun. 2010, 24, 1276–1280. [Google Scholar] [CrossRef]
- Dellarole, A.; Morton, P.; Brambilla, R.; Walters, W.; Summers, S.; Bernardes, D.; Grilli, M.; Bethea, J.R. Neuropathic pain-induced depressive-like behavior and hippocampal neurogenesis and plasticity are dependent on TNFR1 signaling. Brain Behav. Immun. 2014, 41, 65–81. [Google Scholar] [CrossRef]
- Swanson, K.A.; Nguyen, K.L.; Gupta, S.; Ricard, J.; Bethea, J.R. TNFR1/p38αMAPK signaling in Nex+ supraspinal neurons regulates sex-specific chronic neuropathic pain. Res. Sq. 2023; preprint. [Google Scholar]
- Salvo, E.; Campana, W.M.; Scheff, N.N.; Nguyen, T.H.; Jeong, S.-H.; Wall, I.; Wu, A.K.; Zhang, S.; Kim, H.; Bhattacharya, A.; et al. Peripheral nerve injury and sensitization underlie pain associated with oral cancer perineural invasion. Pain 2020, 161, 2592–2602. [Google Scholar] [CrossRef]
- Deborde, S.; Yu, Y.; Marcadis, A.; Chen, C.H.; Fan, N.; Bakst, R.L.; Wong, R.J. An In Vivo Murine Sciatic Nerve Model of Perineural Invasion. J. Vis. Exp. 2018, 134, 56857. [Google Scholar]
- Lis, K.; Kuzawińska, O.; Bałkowiec-Iskra, E. Tumor necrosis factor inhibitors—State of knowledge. Arch. Med. Sci. 2014, 10, 1175–1185. [Google Scholar] [CrossRef]
- Steed, P.M.; Tansey, M.G.; Zalevsky, J.; Zhukovsky, E.A.; Desjarlais, J.R.; Szymkowski, D.E.; Abbott, C.; Carmichael, D.; Chan, C.; Cherry, L.; et al. Inactivation of TNF Signaling by Rationally Designed Dominant-Negative TNF Variants. Science 2003, 301, 1895–1898. [Google Scholar] [CrossRef] [PubMed]
- Barnum, C.J.; Chen, X.; Chung, J.; Chang, J.; Williams, M.; Grigoryan, N.; Tesi, R.J.; Tansey, M.G. Peripheral Administration of the Selective Inhibitor of Soluble Tumor Necrosis Factor (TNF) XPro®1595 Attenuates Nigral Cell Loss and Glial Activation in 6-OHDA Hemiparkinsonian Rats. J. Park. Dis. 2014, 4, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Jensen, D.D.; Viet, C.T.; Pan, H.L.; Campana, W.M.; Amit, M.; Boada, M.D. Advances in Head and Neck Cancer Pain. J. Dent. Res. 2022, 101, 1025–1033. [Google Scholar] [CrossRef]
- Randhi, R.; Damon, M.; Dixon, K.J. Selective inhibition of soluble TNF using XPro1595 relieves pain and attenuates cerulein-induced pathology in mice. BMC Gastroenterol. 2021, 21, 243. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, M.; Alles, S.R.; Montera, M.A.; Adams, I.; Santi, M.D.; Inoue, K.; Tu, N.H.; Westlund, K.N.; Ye, Y. Peroxisome proliferator-activated receptor gamma agonist ELB00824 suppresses oxaliplatin-induced pain, neuronal hypersensitivity, and oxidative stress. Neuropharmacology 2022, 218, 109233. [Google Scholar] [CrossRef]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Brodkin, J.; Frank, D.; Grippo, R.; Hausfater, M.; Gulinello, M.; Achterholt, N.; Gutzen, C. Validation and implementation of a novel high-throughput behavioral phenotyping instrument for mice. J. Neurosci. Methods 2014, 224, 48–57. [Google Scholar] [CrossRef]
- Latorre, R.; Ramírez-Garcia, P.D.; Hegron, A.; Grace, J.L.; Retamal, J.S.; Shenoy, P.; Tran, M.; Aurelio, L.; Flynn, B.; Poole, D.P.; et al. Sustained endosomal release of a neurokinin-1 receptor antagonist from nanostars provides long-lasting relief of chronic pain. Biomaterials 2022, 285, 121536. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.H.E.; Omura, T.; Cobos, E.J.; Latrémolière, A.; Ghasemlou, N.; Brenner, G.J.; van Veen, E.; Barrett, L.; Sawada, T.; Gao, F.; et al. Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice. J. Clin. Investig. 2011, 121, 4332–4347. [Google Scholar] [CrossRef] [PubMed]
- Ehrenberg, A.J.; Morales, D.O.; Piergies, A.M.; Li, S.H.; Tejedor, J.S.; Mladinov, M.; Mulder, J.; Grinberg, L.T. A manual multiplex immunofluorescence method for investigating neurodegenerative diseases. J. Neurosci. Methods 2020, 339, 108708. [Google Scholar] [CrossRef] [PubMed]
- Forndran, T.; Große, S.; Weber, G.; Hausdorf, L.; Samsel, D.; Berndt, A.; Gaßler, N.; Groten, T. Protocol for the quantitative analysis of images retrieved by multiplex immunofluorescence staining to allow cell type-specific spatial phenotyping of markers of interest in the human placenta. Placenta 2024, 166, 139–144. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Zack, G.W.; ERogers, W.; ALatt, S. Automatic measurement of sister chromatid exchange frequency. J. Histochem. Cytochem. 1977, 25, 741–753. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Hong, G.; Zhang, W.; Li, H.; Shen, X.; Guo, Z. Separate enrichment analysis of pathways for up- and downregulated genes. J. R. Soc. Interface 2014, 11, 20130950. [Google Scholar] [CrossRef]
- Zhou, K.-X.; He, X.-T.; Hu, X.-F.; Zhao, W.-J.; Li, C.-X.; Zhang, C.; Zhang, T.; Gu, Z.-X.; Deng, J.-P.; Dong, Y.-L. XPro1595 ameliorates bone cancer pain in rats via inhibiting p38-mediated glial cell activation and neuroinflammation in the spinal dorsal horn. Brain Res. Bull. 2019, 149, 137–147. [Google Scholar] [CrossRef]
- Jamwal, S.; Blackburn, J.K.; Elsworth, J.D. PPARγ/PGC1α signaling as a potential therapeutic target for mitochondrial biogenesis in neurodegenerative disorders. Pharmacol. Ther. 2021, 219, 107705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Zhang, M.; Li, G.; Zhu, S.; Xie, K.; Xiao, B.; Li, L. Clinical Relevance and Drug Modulation of PPAR Signaling Pathway in Triple-Negative Breast Cancer: A Comprehensive Analysis. PPAR Res. 2024, 2024, 4164906. [Google Scholar] [CrossRef]
- Gan, L.; Liu, Z.; Cao, W.; Zhang, Z.; Sun, C. FABP4 reversed the regulation of leptin on mitochondrial fatty acid oxidation in mice adipocytes. Sci. Rep. 2015, 5, 13588. [Google Scholar] [CrossRef] [PubMed]
- Rassart, E.; Desmarais, F.; Najyb, O.; Bergeron, K.F.; Mounier, C. Apolipoprotein D. Gene 2020, 756, 144874. [Google Scholar] [CrossRef]
- Chen, J.T.-C.; Hu, X.; Otto, I.U.; Schürger, C.; von Bieberstein, B.R.; Doppler, K.; Krug, S.M.; Hankir, M.K.; Blasig, R.; Sommer, C.; et al. Myelin barrier breakdown, mechanical hypersensitivity, and painfulness in polyneuropathy with claudin-12 deficiency. Neurobiol. Dis. 2023, 185, 106246. [Google Scholar] [CrossRef]
- Wang, X.; Yang, W.; Wang, L.; Zheng, L.; Choi, W.S. Platinum-based chemotherapy induces demyelination of Schwann cells in oral squamous cell carcinoma treatment. Toxicol. Appl. Pharmacol. 2023, 481, 116751. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, A.; Ghasemi, S.; Zarbakhsh, S. Exercise induced myelin protein zero improvement in neuropathic pain rats. Somatosens. Mot. Res. 2023, 40, 141–146. [Google Scholar] [CrossRef]
- Hamzeh, O.; Rabiei, F.; Shakeri, M.; Parsian, H.; Saadat, P.; Rostami-Mansoor, S. Mitochondrial dysfunction and inflammasome activation in neurodegenerative diseases: Mechanisms and therapeutic implications. Mitochondrion 2023, 73, 72–83. [Google Scholar] [CrossRef]
- Tapias, V. Editorial: Mitochondrial Dysfunction and Neurodegeneration. Front. Neurosci. 2019, 13, 1372. [Google Scholar] [CrossRef]
- Johri, A.; Beal, M.F. Mitochondrial Dysfunction in Neurodegenerative Diseases. J. Pharmacol. Exp. Ther. 2012, 342, 619–630. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Zamel, R.; Bai, X.-H.; Liu, M. PKC Activation Induces Inflammatory Response and Cell Death in Human Bronchial Epithelial Cells. PLoS ONE 2013, 8, e64182. [Google Scholar] [CrossRef]
- Kouri, V.-P.; Olkkonen, J.; Nurmi, K.; Peled, N.; Ainola, M.; Mandelin, J.; Nordström, D.C.; Eklund, K.K. IL-17A and TNF synergistically drive expression of proinflammatory mediators in synovial fibroblasts via IκBζ-dependent induction of ELF3. Rheumatology 2022, 62, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Saloman, J.L.; Weiland, G.; Auh, Q.-S.; Chung, M.-K.; Ro, J.Y. Functional interactions between NMDA receptors and TRPV1 in trigeminal sensory neurons mediate mechanical hyperalgesia in the rat masseter muscle. Pain 2012, 153, 1514–1524. [Google Scholar] [CrossRef]
- Escobedo, G.; Rasband, M.N. Potassium channel clustering: Mechanisms shaping axonal excitability. Front. Cell. Neurosci. 2025, 19, 1627517. [Google Scholar] [CrossRef] [PubMed]
- Matta, C.; Takács, R.; Ducza, L.; Ebeid, R.A.; Choi, H.; Mobasheri, A. Ion channels involved in inflammation and pain in osteoarthritis and related musculoskeletal disorders. Am. J. Physiol. Physiol. 2023, 325, C257–C271. [Google Scholar] [CrossRef]
- Shen, L.; Lu, W.; Huang, Y.; He, J.; Wang, Q.; Zheng, X.; Wang, Z. SNORD15B and SNORA5C: Novel Diagnostic and Prognostic Biomarkers for Colorectal Cancer. BioMed Res. Int. 2022, 2022, 8260800. [Google Scholar] [CrossRef]
- Hu, W.; Li, X.; Cheng, R.; Ke, J.; Liu, Y.; Ma, M.; Cao, Y.; Liu, D. NAV2 facilitates invasion of cutaneous melanoma cells by targeting SNAI2 through the GSK-3β/β-catenin pathway. Arch. Dermatol. Res. 2019, 311, 399–410. [Google Scholar] [CrossRef]
- Yu, X.; Li, Z.; Zheng, H.; Chan, M.T.; Wu, W.K. NEAT1: A novel cancer-related long non-coding RNA. Cell Prolif. 2017, 50, e12329. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, T.; Xu, H.; Wang, Y.; Yang, T.; Liu, L. LncRNA FIRRE promotes the proliferation and metastasis of hepatocellular carcinoma by regulating the expression of PXN through interacting with MBNL3. Biochem. Biophys. Res. Commun. 2022, 625, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Meng, Z.; Niu, J.; Tian, L.; Chen, Y.; Meng, Q.; Liu, Y.; Zhou, Z. Cardiac tropoini T (TNNT2) plays a potential oncogenic role in colorectal carcinogenesis. Cancer Cell Int. 2023, 23, 146. [Google Scholar] [CrossRef]
- Li, Y.; Shi, B.; Dong, F.; Zhu, X.; Liu, B.; Liu, Y. LncRNA KCNQ1OT1 facilitates the progression of bladder cancer by targeting MiR-218-5p/HS3ST3B1. Cancer Gene Ther. 2020, 28, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Ortega, D.; De Molina, A.R.; Ramos, M.A.; Valdes-Mora, F.; Barderas, M.G.; Sarmentero-Estrada, J.; Lacal, J.C. Differential Role of Human Choline Kinase α and β Enzymes in Lipid Metabolism: Implications in Cancer Onset and Treatment. PLoS ONE 2009, 4, e7819. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, C.A.; VanderVorst, K.; Free, S.; Rowson-Hodel, A.; Carraway, K.L. The role of membrane mucin MUC4 in breast cancer metastasis. Endocr. Relat. Cancer 2021, 29, R17–R32. [Google Scholar] [CrossRef]
- Cunha, T.M.; Verri, W.A., Jr.; Silva, J.S.; Poole, S.; Cunha, F.Q.; Ferreira, S.H. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 1755–1760. [Google Scholar] [CrossRef]
- Junger, H.; Sorkin, L.S. Nociceptive and inflammatory effects of subcutaneous TNF α. Pain 2000, 85, 145–151. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, L.; Ji, R. Spinal injection of TNF-α-activated astrocytes produces persistent pain symptom mechanical allodynia by releasing monocyte chemoattractant protein-1. Glia 2010, 58, 1871–1880. [Google Scholar] [CrossRef]
- Zhang, H.; Dougherty, P.M. Acute inhibition of signalling phenotype of spinal GABAergic neurons by tumour necrosis factor-α. J. Physiol. 2011, 589, 4511–4526. [Google Scholar] [CrossRef]
- Yang, L.; Lindholm, K.; Konishi, Y.; Li, R.; Shen, Y. Target Depletion of Distinct Tumor Necrosis Factor Receptor Subtypes Reveals Hippocampal Neuron Death and Survival through Different Signal Transduction Pathways. J. Neurosci. 2002, 22, 3025–3032. [Google Scholar] [CrossRef]
- Zhang, L.; Berta, T.; Xu, Z.-Z.; Liu, T.; Park, J.Y.; Ji, R.-R. TNF-alpha contributes to spinal cord synaptic plasticity and inflammatory pain: Distinct role of TNF receptor subtypes 1 and 2. Pain 2011, 152, 419–427. [Google Scholar] [CrossRef]
- Westlund, K.N.; Zhang, L.; Ma, F.; Oz, H.S. Chronic inflammation and pain in a tumor necrosis factor receptor (TNFR) (p55/p75-/-) dual deficient murine model. Transl. Res. 2011, 160, 84–94. [Google Scholar] [CrossRef]
- Wheeler, M.A.; Heffner, D.L.; Kim, S.; Espy, S.M.; Spano, A.J.; Cleland, C.L.; Deppmann, C.D. TNF-α/TNFR1 Signaling Is Required for the Development and Function of Primary Nociceptors. Neuron 2014, 82, 587–602. [Google Scholar] [CrossRef]
- Li, Y.; Ye, R.; Dai, H.; Lin, J.; Cheng, Y.; Zhou, Y.; Lu, Y. Exploring TNFR1: From discovery to targeted therapy development. J. Transl. Med. 2025, 23, 71. [Google Scholar] [CrossRef]
- Preedy, M.K.; White, M.R.H.; Tergaonkar, V. Cellular heterogeneity in TNF/TNFR1 signalling: Live cell imaging of cell fate decisions in single cells. Cell Death Dis. 2024, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, D.; Song, Y.; Liu, S.; Long, Q.; Yao, L.; Li, W.; Duan, Z.; Wu, D.; Liu, L. HRG switches TNFR1-mediated cell survival to apoptosis in Hepatocellular Carcinoma. Theranostics 2020, 10, 10434–10447. [Google Scholar] [CrossRef]
- Debnath, S.; Sarkar, A.; Das Mukherjee, D.; Ray, S.; Mahata, B.; Mahata, T.; Parida, P.K.; Das, T.; Mukhopadhyay, R.; Ghosh, Z.; et al. Eriodictyol mediated selective targeting of the TNFR1/FADD/TRADD axis in cancer cells induce apoptosis and inhibit tumor progression and metastasis. Transl. Oncol. 2022, 21, 101433. [Google Scholar] [CrossRef] [PubMed]
- Lis, K.; Grygorowicz, T.; Cudna, A.; Szymkowski, D.E.; Bałkowiec-Iskra, E. Inhibition of TNF reduces mechanical orofacial hyperalgesia induced by Complete Freund’s Adjuvant by a TRPV1-dependent mechanism in mice. Pharmacol. Rep. 2017, 69, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. Publisher Correction: The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 662. [Google Scholar] [CrossRef]
- Jang, B.-K.; Shin, S.J.; Park, H.H.; Kumar, V.; Park, Y.H.; Kim, J.-Y.; Kang, H.-Y.; Park, S.; Kwon, Y.; Shin, S.-E.; et al. Investigation of Novel Aronia Bioactive Fraction-Alginic Acid Nanocomplex on the Enhanced Modulation of Neuroinflammation and Inhibition of Aβ Aggregation. Pharmaceutics 2024, 17, 13. [Google Scholar] [CrossRef]
- Reyes-Gibby, C.C.; Anderson, K.O.; Merriman, K.W.; Todd, K.H.; Shete, S.S.; Hanna, E.Y. Survival Patterns in Squamous Cell Carcinoma of the Head and Neck: Pain as an Independent Prognostic Factor for Survival. J. Pain 2014, 15, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S.; Parisien, M.; Esfahani, S.J.; Diatchenko, L. Sex differences in mechanisms of pain hypersensitivity. Neurosci. Biobehav. Rev. 2024, 163, 105749. [Google Scholar] [CrossRef]
- Ye, Y.; Cardoso, D.d.M.; Kayahara, G.M.; Bernabé, D.G. A pilot study to improve pain phenotyping in head and neck cancer patients. Front. Pain Res. 2023, 4, 1146667. [Google Scholar] [CrossRef] [PubMed]
- Zaldivar, V.; Magri, M.L.; Zárate, S.; Jaita, G.; Eijo, G.; Radl, D.; Ferraris, J.; Pisera, D.; Seilicovich, A. Estradiol Increases the Expression of TNF-α and TNF Receptor 1 in Lactotropes. Neuroendocrinology 2011, 93, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Sorge, R.E.; Mapplebeck, J.C.S.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.-S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef]
- Gregus, A.M.; Levine, I.S.; Eddinger, K.A.; Yaksh, T.L.; Buczynski, M.W. Sex differences in neuroimmune and glial mechanisms of pain. Pain 2021, 162, 2186–2200. [Google Scholar] [CrossRef]
- Deb, S.; Amin, S.; Imir, A.G.; Yilmaz, M.B.; Suzuki, T.; Sasano, H.; Bulun, S.E. Estrogen Regulates Expression of Tumor Necrosis Factor Receptors in Breast Adipose Fibroblasts. J. Clin. Endocrinol. Metab. 2004, 89, 4018–4024. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Liu, N.; Asam, K.; Meng, C.; Aouizerat, B.; Ye, Y. TNFR1 Suppression by XPro1595 Reduces Peripheral Neuropathies Associated with Perineural Invasion in Female Mice. Cells 2025, 14, 1749. https://doi.org/10.3390/cells14221749
Zhang M, Liu N, Asam K, Meng C, Aouizerat B, Ye Y. TNFR1 Suppression by XPro1595 Reduces Peripheral Neuropathies Associated with Perineural Invasion in Female Mice. Cells. 2025; 14(22):1749. https://doi.org/10.3390/cells14221749
Chicago/Turabian StyleZhang, Morgan, Naijiang Liu, Kesava Asam, Charles Meng, Bradley Aouizerat, and Yi Ye. 2025. "TNFR1 Suppression by XPro1595 Reduces Peripheral Neuropathies Associated with Perineural Invasion in Female Mice" Cells 14, no. 22: 1749. https://doi.org/10.3390/cells14221749
APA StyleZhang, M., Liu, N., Asam, K., Meng, C., Aouizerat, B., & Ye, Y. (2025). TNFR1 Suppression by XPro1595 Reduces Peripheral Neuropathies Associated with Perineural Invasion in Female Mice. Cells, 14(22), 1749. https://doi.org/10.3390/cells14221749





