Abstract
The pathogenesis of Friedreich’s ataxia (FRDA) remains poorly understood. The most important event is the deficiency of frataxin, a protein related to iron metabolism and, therefore, involved in oxidative stress. Studies on oxidative stress markers and gene expression in FRDA patients have yielded inconclusive results. This is largely due to the limited number of studies, small sample sizes, and methodological differences. A notable finding is the decreased activity of mitochondrial respiratory chain complexes I, II, and III, as well as aconitase, in endomyocardial tissue. In contrast, numerous studies in experimental models of FRDA (characterized by frataxin deficiency) have shown evidence of the involvement of oxidative stress in cellular degeneration. These findings include increased iron concentration, mitochondrial dysfunction (with reduced respiratory chain complex activity and membrane potential), and decreased aconitase activity. Additionally, there is the induction of antioxidant enzymes, reduced glutathione levels, elevated markers of lipoperoxidation, and DNA and carbonyl protein oxidation. The expression of NRF2 is decreased, along with the downregulation of PGC-1α. Therefore, it is plausible that antioxidant treatment may help improve symptoms and slow the progression of FRDA. Among the antioxidant treatments tested in FRDA patients, only omaveloxolone and, to a lesser extent, idebenone (particularly for cardiac hypertrophy) have shown some efficacy. However, many antioxidant drugs have shown the ability to reduce oxidative stress in experimental models of FRDA. Therefore, these drugs may be useful in treating FRDA and are likely candidates for future clinical trials. Future studies investigating oxidative stress and antioxidant therapies in FRDA should adopt a prospective, multicenter, long-term, double-blind design.
1. Introduction
Between 1863 and 1877, Nicolas Friedreich described a clinical syndrome in nine patients from five families. The condition began at puberty and included progressive gait and limb ataxia, along with dysarthria. Over time, additional symptoms appeared, such as areflexia, sensory deficits, nystagmus, muscle weakness, scoliosis, diabetes, and tachycardia. In four of the patients, autopsy studies showed degeneration of the posterior funiculus, posterior spinal roots, Clarke’s spine, and lateral funiculus, and three presented evidence of cardiomyopathy [1]. Therefore, Friedreich is considered to be the first author to describe the concept of hereditary ataxia and to carry out a clinical–pathological study of a form of spinocerebellar degeneration.
Friedreich’s ataxia (FRDA), with a prevalence of approximately 0.5–1 per 100,000, is the most common hereditary recessive ataxia. Clinically, FRDA presents with gait and limb ataxia, dysarthria, areflexia, and loss of vibratory and proprioceptive sensation. Additional features include scoliosis (70–75%), pes cavus (55–75%), cardiomyopathy with atrial arrhythmias and heart failure (40–50%), vision loss due to optic neuropathy (40–50%), hearing loss from auditory nerve axonopathy (15–20%), and diabetes mellitus (5–10%) [2].
FRDA is caused by a heterozygous autosomal recessive mutation. This involves a pathogenic expansion of the GAA trinucleotide repeat in intron 1 of the frataxin gene (FXN, FA, X25, CyaY, FARR, or FRDA; chromosome 9q21.11; gene ID 2395; MIM 606829. https://www.ncbi.nlm.nih.gov/gene/2395 (accessed on 30 July 2025). This mutation is found in 98% of cases. [3]. FXN is a nuclear gene that encodes a mitochondrial protein belonging to the FXN family, which is implicated in the regulation of iron transport in the mitochondria (likely related to iron–sulfur protein production and storage and iron chaperone activity), although its presence in the cytosol has also been described [4]. The GAA mutation leads to a marked decrease in the synthesis of FXN. FXN is highly expressed in the spinal cord and heart, and, to a lesser extent, in the cerebellum, liver, pancreas, skeletal muscles, and cerebral cortex [4].
The primary histopathological hallmark of FRDA is the degeneration of dorsal root ganglia. This leads to the secondary degeneration of the dorsal spinal roots, posterior spinal cord columns, gracilis and cuneate nuclei, spinocerebellar tracts, dentate nuclei of the cerebellum, corticospinal tracts, and the heart [4,5]. Although the pathogenic mechanism of FRDA is not fully understood, the marked deficiency of FXN and its role in iron metabolism suggest that oxidative stress plays a central role. Oxidative stress refers to an imbalance between the production of reactive oxygen species (ROS) and the body’s antioxidant defenses, leading to the oxidation of lipids, proteins, and DNA.
This narrative review aims to summarize the current evidence on the role of oxidative stress in the pathogenesis of FRDA. These studies cover several areas: oxidative stress marker concentrations in different tissues from patients diagnosed with FRDA (including proteomic, metabolomic, lipidomic, and multi-omic studies), case-control studies on the possible association of genes related to oxidative stress with the risk for FRDA, studies showing the presence of oxidative stress in experimental models of FRDA, and studies addressing the possible efficacy of antioxidant drugs in the treatment of FRDA—both clinical trials in patients with FRDA or in experimental models of this disease. For this purpose, we performed a literature search encompassing the terms “Friedreich’s ataxia” and “oxidative stress” using the PubMed Database from 1966 to 25 July 2025. We analyzed manually, one by one, the 435 references retrieved by the whole search, and selected only those strictly related to the topic, as described above.
2. Oxidative Stress Markers in Patients with FRDA
The main results, methods used for biochemical determinations, and participating subjects in each of the studies addressing oxidative stress markers in different tissues (including brain/spinal cord, plasma, serum, whole blood, erythrocytes, lymphocytes/lymphoblasts, fibroblasts, endomyocardial and skeletal muscle tissues, and urine) from patients with FRDA compared to controls are described in detail in Supplementary Table S1 [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38] and summarized in Table 1.

Table 1.
Summary of the results of studies addressing concentrations of oxidative stress markers in patients with FRDA compared to HC.
Markers of lipid peroxidation, mainly malonyldialdehyde (MDA) and/or thiobarbituric acid (TBA) reactive substances (TBARS), have been found to be similar between FRDA patients and healthy controls (HC) in the cerebellum, brainstem, and cortex, although they have been described as having a decreased susceptibility to in vitro lipid peroxidation in FRDA patients [6]. Plasma MDA levels were found to be increased in FRDA patients in a single study [11], and 4-hydroxynonenal (4-HNE) levels increased in another [30]. However, a lipidomic analysis in the erythrocytes of patients with FRDA and controls did not find significant differences in lipid peroxidation in the two study groups [12]. Variations in study outcomes may be attributed to differences in sample size, assay methodologies, and the disease stage of the participants.
Plasma levels of the marker of hydroxyl radical attack dihydroxybenzoic acid (DHBA) [13] and susceptibility to oxidative stress [11] have been reported to be similar in FRDA patients and HC in two studies, while another described a significant increase in the free radical superoxide in FRDA [33]. 8-hydroxy-2′-deoxyguanosine (8-OHdG), a marker of DNA oxidation, is increased in the urine of FRDA patients [13]. Carbonyl proteins have been found to be increased, and total antioxidant capacity (TAC) decreased in patients with FRDA [12]. However, the sample sizes of most of these studies are relatively small.
Studies addressing mitochondrial respiratory chain complex enzymatic activities or expression in the cerebellum [8], dorsal root ganglia [8], or spinal cord [7]; lymphocytes/lymphoblasts [27]; peripheral blood mononuclear cells (PBMC) [29]; platelets [29]; skin fibroblasts [27]; and skeletal muscle [8,27] showed similar values in FRDA patients and HC. However, studies performed with endomyocardial biopsy samples showed a significant decrease in the activity of mitochondrial respiratory chain complexes I, II, and III [8,27], and there was similar activity in complexes IV [8,27] and V [27] in patients with FRDA compared to HC.
Despite the important role of FXN in iron metabolism, concentrations of iron in the cerebellum [8,9], spinal cord [8], dorsal root ganglia [8], peripheral nerves [8], erythrocytes [23], and skeletal muscle [8] have been reported to be similar to that of HC, although iron levels were decreased in plasma [14], PBMC [29], platelets [29], and skin fibroblasts [31,33,34]. Concentrations or activity of the enzyme aconitase (an important iron–sulfur protein) in FRDA patients, in comparison to HC, have been found to be similar in the cerebellum and dorsal root [8], skin fibroblasts [11,27], and skeletal muscle [8,27], while these were reported as decreased in endomyocardial tissue from FRDA patients [8,27]. Concentrations of other iron-related proteins, such as hemoglobin [26], protoporphyrin IX [26], and ferrochelatase [26] in the erythrocytes of FRDA patients, have been reported to be similar to those of controls, but glutathionyl–hemoglobin was increased [22].
Alpha-tocopherol (vitamin E) concentrations were found to be similar in the cerebellum, brainstem, and cortex in both FRDA patients and HC [6]. Two studies described non-significant differences in serum vitamin E levels between FRDA patients and controls [16,17], while two others described decreased serum vitamin E in FRDA patients [18,19]. Once again, the sample size, assay method, and disease stage could be related to the differences in the results of these studies. Serum coenzyme Q10 concentrations are decreased [19], and serum uric acid levels increased [20] in FRDA patients.
Glutathione bound to proteins has been found to be increased in the spinal cord [7] and fibroblasts [32] in FRDA patients. Total glutathione levels were similar in the plasma [12], whole blood [22,23], lymphocytes [28], and fibroblasts [23,32] in FRDA patients and HC, except in one study that showed decreased levels of fibroblasts in FRDA patients [31]. However, free glutathione concentrations were found to be decreased in whole blood [22], and the reduced glutathione–oxidized glutathione (GSH–GSSG) ratio led to a decrease in plasma [12], lymphocytes [28], and fibroblasts [32] in FRDA patients.
Glutathione peroxidase (GPx) activity was found to be decreased in the whole blood of FRDA patients [24] but was similar to that of HC in erythrocytes [25] and fibroblasts [33]. Total superoxide dismutase (SOD) and glutathione-S-transferase (GST) activities were increased in erythrocytes [23], total SOD was decreased in fibroblasts [34], manganese-SOD (Mn-SOD or SOD2) activity and mRNA increased [33] or decreased [34] in the fibroblasts of FRDA patients, copper–zinc SOD (Cu–Zn-SOD or SOD1) activity and mRNA decreased [34] or were similar to that of HC [33], and catalase (CAT) activity [33] was similar to that of HC in fibroblasts. Recently, Quatrana et al. [30] reported a significant upregulation of the antioxidant enzymes thioredoxin 1 (TRX1) and glutaredoxin 1 (GLRX1) in FRDA patients compared to HC, which was associated with the activation of the nuclear factor kappa B (NF-κB) p65 subunit (NF-κB p65) in fibroblasts.
One study using proton magnetic resonance spectroscopy (1H-MRS) described decreased N-acetylaspartate (NAA neuroaxonal marker) and choline (a membrane marker) and increased mean diffusivity in the FRDA cerebellum [10]. Lodi et al. [36], using 31phosphorus magnetic resonance spectroscopy (31P-MRS), showed a decreased phosphocreatine–adenosine triphosphate (ATP) ratio in heart and skeletal muscle—suggesting mitochondrial dysfunction—in FRDA patients compared to HC. Finally, Swarup et al. [15] described the downregulation of seven proteins and the upregulation of four proteins in the plasma of FRDA patients—some of these are related to oxidative stress processes.
Castaldo et al. [39] described a significant telomere shortening (oxidative stress being a modulator of this parameter), assessed by real-time polymerase chain reaction quantitative analysis (RT-qPCR) in leukocytes from 37 patients with FRDA compared to 36 age- and sex-matched HC, as well as an inverse correlation between this value and disease duration. Anjomani Virmouni [40], using RT-qPCR, also described telomere shortening in leukocytes from 18 FRDA patients and 12 age- and sex-matched controls, which was not correlated with GAA repeat size, as well as in autopsy cerebellar tissues (age and sex not provided). Further, they found significantly higher (but dysfunctional) telomeres in fibroblasts from seven FRDA patients compared to three HC. Finally, Scarabini et al. [41], in a cohort of 61 FRDA biallelic patients, 29 heterozygous FRDA carriers, and 87 age-matched HC, using RT-qPCR, showed non-significant differences in leukocyte telomere length (LTL) between FRDA patients and HC (although LTL was longer in patients aged less than 35 years and higher in those aged more than 36 years), while FRDA carriers showed longer LTL than HC. In addition, they described a positive correlation of LTL with GAA repeat length and a negative correlation with age, as well as an association between shorter leukocyte telomeres and the presence of cardiomyopathy.
3. Genetic Variants of Genes Related to Oxidative Stress in Patients with Friedreich’s Ataxia
Haugen et al. [42], in a microarray analysis involving 28 FRDA patients (M:F 16:12, mean age 13.5 ± 2.3 years, mean disease duration 6.0 ± 3.5 years) and 10 controls (M:F 8:2, mean age 20.3 ± 1.4 years) in peripheral blood samples, found the downregulation of 899 genes and upregulation of 471 genes, including those related to apoptosis signaling, transcription/RNA processing, cell–cell signaling, the cell cycle, the ubiquitin cycle, proteolysis–protein catabolism, response to stimuli, and fatty acid beta-oxidation.
Hayashi et al. [43] investigated the expression of 84 oxidative stress genes in cellular cultures of B-lymphoblasts from patients with FRDA and HC using real-time quantitative polymerase chain reaction (RT-qPCR) followed by Western blot analysis, and found increased mRNA expression of PDZ and LIM domain 1 (PDLIM1), eosinophil peroxidase (EPX), glutathione peroxidase 2 (GPX2), phosphatidylinositol-3,4,5-trisphosphate-dependent Rac exchange factor 1 (PREX1), as well as decreased mRNA expression for periredoxin 5 (PRDX5), ring finger protein 7 (RNF7), dual-specificity phosphatase 1 (DUSP1), periredoxin 2 (PRDX2), neutrophil cytosolic factor 2 (NCF2), and surfactant protein D (SFTPD) genes using primary qPCR. Western blot analysis confirmed the changes in mRNA expression of PDLIM1, PRDX5, NCF2, and SFTPD genes.
Steinkellner et al. [26], in a study using cDNA-based microarray (IronChip)-containing genes involved in iron and copper metabolism in three FRDA patients and six matched HC, found the upregulation of mRNA for endothelial PAS domain protein 1 (EPAS1) and amyloid beta (A4) precursor protein (APP), and the downregulation of FXN, tumor necrosis factor, alpha-induced protein 3 (TNFAIP3), vascular endothelial growth factor A (VEGF), Toll-like receptor 4 (TLR4), FBJ murine osteosarcoma viral oncogene homolog (FOX), N-myc downstream-regulated 1 (NDRG1), selectin P (granule membrane protein 140kDa, antigen CD62, SELP), selenoprotein P plasma 1 (SEPP1), and spermatogenesis-associated 1 (SPAP1) genes.
Kelly et al. [44], in a study involving 79 FRDA patients and 170 controls, showed an association between the rs5186 single-nucleotide polymorphism (SNP) in the angiotensin-II type-1 receptor (AGTR1) gene and FRDA, with this SNP also being related to an increase in interventricular septal wall thickness and left ventricular mass (LVM), while other SNPs in the same gene and in angiotensin-converting enzyme (ACE) and ACE2 genes showed a lack of association with FRDA.
Singh et al. [45], in a PCR amplification and sequencing analysis of the D-loop, NADH (nicotin–adenin–dinucleotide) dehydrogenase subunits 1-6 (ND1-6), and ATP regions of the mitochondrial genome (comprising 50% of the latter) involving 30 FRDA patients and 62 controls, showed a significantly higher load of mitochondrial variations per individual, and an over-representation of the non-synonymous variation p.L237M in ND2 in FRDA cases compared to controls, this variation being associated with longevity and myocardial infarction.
Bolotta et al. [12], in a study on leukocytes from seven FRDA patients and seven HC using qPCR, found overexpression of thioredoxin reductase 1 (TXNRD1) and nuclear factor erythroid 2-like 2 (NRF2) genes in FRDA patients, while SOD1, SOD2, CAT, GPX1, and GSH S-reductase (GSR) gene expressions were similar in FRDA patients and HC. Tozzi et al. [25] found non-significant differences in the distribution of GSTP1-1 genotypes and allelic variants between 14 FRDA patients and 21 age- and sex-matched HC.
McMackin et al. [24], in a study on lymphocytes and skin fibroblasts from FRDA patients and histological dorsal rood ganglion from a mouse model of FRDA, described overlapping gene expression changes primarily affecting antioxidant genes related to selenium metabolism and GPx activity (selenoprotein W1-SEPW1-, GPX7, and TXNRD1), ten genes related to the positive regulation of the apoptotic process, and nine genes related to mitochondrial translation and transcription.
Tiano et al. [46], in a comparative microarray study on lymphoblastoid cells from a FRDA patient with GM15850 transfected cells, found ninety-one differentially expressed genes, seven of them downregulated and eighty-four upregulated, the more significant being chromosome 19 open reading frame 12 (C19orf12, downregulated, which causes a form of neurodegeneration with brain iron accumulation) and HCLS1-associated X-1 (HAX-1, upregulated; HAX-1 mRNA expression and HAX-1 protein levels were correlated with FXN expression). Subsequently, they analyzed FXN and HAX-1 mRNA and protein expression in peripheral blood from 39 FRDA patients and 28 age- and sex-matched controls and described a decrease in these values in FRDA patients and a correlation between them.
Petrillo et al. [23], in an analysis (by using RT-qPCR) of the GSH-related genes glutamate–cysteine ligase (GCL, which encodes the step-limiting enzyme of the GSH synthesis) and GSR (responsible for the re-cycling of GSH from its oxidized form GSSG) and NRF2 gene expression involving a FRDA patient, his mother and sister (both asymptomatic carriers), his father, and three age- and sex-matched controls, found a significant upregulation of the GCL gene in the leukocytes and fibroblasts of the asymptomatic mother and similar expression levels in the proband, the sister, and the father compared to controls, while the expression levels of GSR and NRF2 were significantly higher in the proband, the mother, and the sister, and similar to those of controls and the father. The same group, in a study performed on skin fibroblasts from three FRDA patients compared to three HC using RT-qPCR and Western blotting, showed a significant decrease in mRNA expression and protein levels of NRF2, NAD(P)H quinone oxidoreductase 1 (NQO1), Heme Oxygenase-1 (HO-1), and GCL in FRDA patients [23].
Finally, Quesada et al. [47] described an overexpression of iron–sulfur cluster genes and other oxidative stress-related genes, active-caspase 3, and other apoptosis-related genes, as well as the upregulation of brain-derived neurotrophic factor (BDNF), neuregulin 1, and miR-132 in periodontal ligament cells from patients with FRDA compared to HC.
Most of these studies, performed with different methods, need to be confirmed by replication studies.
4. Oxidative Stress in Experimental Models of FRDA
Several experimental models of FRDA have been developed in animals, including Saccharomyces cerevisiae yeast, Drosophila flies, the nematode Caenorhabditis elegans, and mice with a reduced expression of FXN [48,49].
A yeast gene (yeast FXN homolog, YFH1) encodes a mitochondrial protein involved in iron homeostasis and respiratory function that has a high sequence similarity with the human FXN [50]. Radisky et al. [51] showed that yeast strains with the absence of the YHF1 protein suffered from mitochondrial damage that was closely related to the concentration of iron, suggesting that the accumulation of iron, leading to oxidative stress, was responsible for this mitochondrial damage. The reintroduction of the FXN homolog reversed these alterations [51].
Based on these data, Wong et al. [52] showed that the incubation of human fibroblasts from FRDA patients with iron or with hydrogen peroxide caused oxidant-induced death, which was partially reversed by iron chelators (deferoxamine), intracellular Ca2+ chelators, and apoptosis inhibitors. In contrast, other authors described that oligomycin was able to induce oxidative stress, inducing SOD1 and SOD2, and the loss of mitochondrial membrane iron-containing enzyme activity in fibroblasts from controls but not in fibroblasts from FRDA patients, as well as the lack of induction of SOD activity in the heart of knock-out mice for the FXN gene, suggesting that oxidative damage to iron–sulfur clusters was responsible for the mitochondrial deficiency and long-term mitochondrial iron overload in FRDA [53]. Consistent with this finding, Jiralerspong et al. [54] showed a lack of the normal upregulation of the stress defense protein SOD2 and the absence of increased activation of the redox-sensitive factor NF-κB in FRDA fibroblasts exposed to iron.
Bulteau et al. [55] showed, in a yeast model of FRDA, that cells lacking FXN (Deltayfh1) had neither growth defects nor aconitase functional deficits when cultured anaerobically but, under aerobic conditions, showed a high degradation of aconitase, an accumulation of carbonylated proteins in the cytosol and the mitochondria, and a decrease in the cytosolic activity of the 20S proteasome compared to wild-type cells. Also, in the yeast model of FXN deficiency, Auchère et al. [56] described a 5-fold decrease in total glutathione (GSH + GSSG), an imbalance in GSH–GSSG pools, higher GPx activity, and a 3-fold increase in glucose-6-phosphate dehydrogenase activity (suggesting a stimulation of the pentose phosphate pathway) compared to wild-type cells. These findings suggest an important role of oxidative stress in this FRDA model.
Llorens et al. [57], using RNA interference (RNAi) in Drosophila flies to suppress FXN expression, showed in this model a shortened life span, reduced climbing abilities, and enhanced sensitivity to oxidative stress that was parallel to the situation of FRDA patients. They found an important decrease in aconitase activity and an impairment of mitochondrial respiration (suggesting an important role of oxidative stress), while activities of mitochondrial respiratory chain complexes I to IV were normal [57]. The same group described increased iron, zinc, copper, manganese, and aluminum levels [58], as well as increased levels of lipid peroxidation markers, such as MDA + 4-hydroxyalkenals (HAE) [58,59], and of GSSG [59] in this model compared to control animals [58]. In the same model, Navarro et al. [60] described the accumulation of lipid-like droplets composed of high levels of free fatty acids and a marked increase of lipoperoxides in glial cells, which were related to a shortening in lifespan.
Rodríguez et al. [61] showed, in cultures from the human SH-SY5Y neuroblastoma cell line and in a RNAi Drosophila fly model of FRDA, a compromise of the function of Endoplasmic Reticulum mitochondria-associated membranes (MAMs, involved in the regulation of essential cellular processes such as lipid metabolism and calcium signaling), which improved with treatment with antioxidants and the promotion of mitochondrial calcium uptake.
Shidhara et al. [62], in Drosophila larvae with reduced FXN expression (DfhIR), showed a decrease in mitochondrial membrane potential in cell bodies, axons, and neuromuscular junctions (NMJs) of segmental nerves, as well as defects in axonal transport in mitochondria without a basal increase in reactive oxygen substances in any neuronal region, although this was notably increased after exposure to antimycin A.
Calmels et al. [63] developed a murine cellular model of FRDA using a combination of cell lines carrying a FXN conditional allele with an EGFP-Cre recombinase to create murine cellular models depleted for endogenous FXN and expressing missense-mutated human FXN. In this model, the absence of FXN was lethal, but cells expressing the mutated FXN showed affected mitochondrial, cytosolic, and nuclear iron–sulfur cluster enzyme activities; mitochondrial iron accumulation; and an increased sensitivity to oxidative stress (similarly to FRDA patients) that were correlated with disease severity.
Wagner et al. [64], using two conditional mouse models of FRDA (neuron-specific enolase NSE and muscle creatine kinase MCK–Cre knock-out mice), characterized by the development of a fatal cardiomyopathy and impaired activity of iron–sulfur cluster-dependent respiratory complexes, showed a marked hyperacetylation of many proteins that was related to the inhibition of the NAD(+)-dependent sirtuin 3 (SIRT3) deacetylase and caused by an important decrease in the mitochondrial NAD(+)–NADH ratio and direct carbonyl group modification of SIRT3, which was reversed by incubating with excess SIRT3 and NAD+.
Sanz-Alcázar et al. [65] showed that FXN deficiency in primary cultures of dorsal root ganglia neurons and dorsal root ganglia from the FXNI151F mouse model and the decreased enzymatic activity of mitochondrial respiratory chain complexes (specifically, I and II) caused alterations in mitochondrial morphology, decreased the NAD+–NADH ratio and SIRT3 activity, increased the acetylation of alpha-tubulin and SOD2 (leading to their inactivation), and increased mitochondrial levels of superoxide anion, Fe2+, and 4-HNE. In the same models, this group described the upregulation of transferrin receptor 1 and decreased ferritin and the impaired activation of NRF2 (which caused the downregulation of SLC7A11—important for glutathione synthesis—and GPx4—increasing increased lipid peroxidation), which seemed to be due to an increase in Kelch-like ECH-associated protein 1 (KEAP1), the activation of Glycogen synthase kinase-3β (GSK3β), and deficiency in the Liver Kinase B1–AMP-activated protein kinase (LKB1–AMPK) pathway (involving the reduction of levels of the kinase LKB1 and pAMPK), as well as a decrease of SIRT1 (activator of LKB1), suggesting the presence of ferroptosis [66].
In the MCK–Cre model, increased iron levels have been shown in the heart, liver, spleen, and kidneys (an increase of iron in the liver, spleen, and kidneys seemed to be a “protective” mechanism to increase systemic iron levels and iron loading in tissues where FXN expression was intact), related to mitochondrial aggregates of iron, phosphorus, and sulfur that likely contribute to oxidative stress, as well as iron supplementation, which improved cardiac hypertrophy without affecting FXN levels [67].
Shan et al. [68], in a YG8R hemizygous mouse model of FRDA (which showed defects in movement and growth defects in dorsal root ganglia neurites) using a microarray and q-RT-PCR, showed an important decrease in transcripts encoding antioxidants (peroxiredoxins, glutaredoxins, and GST), a significant decrease of Nrf2 in the cerebellum and dorsal root ganglia that was correlated with FXN expression, and a reduction in total GSH levels. Sandi et al. [69] showed, in fibroblast and neural stem cells from YG8R mice (compared to control Y47R mouse cells), decreased FXN expression, increased DNA methylation, increased sensitivity to oxidative stress, decreased aconitase activity, the downregulation of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and antioxidant gene expression levels (especially SOD2 and GPx1), and a significant reduction in the expression of several DNA mismatch repair (MMR) genes.
Abeti et al. [70,71], in cultured fibroblasts of knock-in–knock-out (KIKO; B6.Cg-Fxntm1MknFxntm1Pand/J) and YG8R FRDA mouse models, compared with their appropriate controls (wild-type and Y47R), showed increased lipid peroxidation and decreased mitochondrial membrane potential (the latter due to an inhibition of complex I, which is partially compensated for by an overactivation of complex II).
Codazzi et al. [72], in induced pluripotent stem cell (iPSC)-derived neurons from FRDA patients, when compared to those of controls, showed a decrease in iron–sulfur (Fe–S) and lipoic acid-containing protein concentrations; an increase in labile iron pool (LIP), ROS, and SOD2 expression; and a decrease in GSH concentrations.
Igoillo-Esteve et al. [73] showed that in pancreatic β-cell death in cultures of the clonal rat insulinoma cell line (INS-1E), primary rat β-cells, dispersed rat and human islets, and iPSC-derived neurons from FRDA patients, FXN deficiency was a consequence of the oxidative stress-mediated activation of the intrinsic pathway of apoptosis with the participation of the pro-apoptotic Bcl-2 family members Bad, DP5, and Bim.
Moreno-Lorite et al. [74], in a human neural cell line with doxycycline-induced FXN knockdown derived from the human neuroblastoma SH-SY5Y (iFKD-SY), which can differentiate into mature neuron-like cells, showed that the induction of FXN deficiency was accompanied by increases in oxidative stress, DNA oxidative damage (with concomitant transcriptional deregulation in many of the genes implicated in DNA repair pathways), decreased aconitase enzyme activity, increased levels of p53 and p21, the activation of caspase-3, and apoptosis.
Hackett et al. [75] showed that in yeast plasmids, the Lon protease Pim1 is responsible for FXN degradation and the increased turnover of Yfh1 under situations of mitochondrial oxidative stress. They also showed decreased ferritin levels in cultures of fibroblasts from FRDA patients (compared to those of controls) incubated in media with elevated iron and in cultures of rat H9C2 cardiomyocytes treated with doxorubicin.
Interestingly, Navarro et al. [76], in transgenic Drosophila flies that overexpressed human or fly FXN, showed the presence of oxidative stress (including a reduction of the level of aconitase and a decrease in the level of subunit S3 of the complex I of the mitochondrial respiratory chain), the impairment of the normal embryonic development of muscle and the peripheral nervous system, a reduction of life span, and impairment in locomotor ability and brain degeneration. Edenharter et al. [77], also in a Drosophila model, described that overexpression of FXN led to an increase in oxidative phosphorylation, a modification of mitochondrial morphology, an alteration in iron homeostasis, and the triggering of oxidative stress-dependent cell death, with cell survival being improved with genetic manipulation of mitochondrial iron metabolism by silencing mitoferrin. Similarly, Vannocci et al. [78] showed, in a human cellular model with an overexpression of FXN (HEK-cFXN, a biallelic knock-out of the endogenous FXN gene with the presence of an exogenous, inducible cDNA FXN cassette), a significant increase of oxidative stress and LIP levels, mitochondrial dysfunction, and decreased ATP production, which was similar to that found in FXN deficiency. Moreover, the transfection of the FXN gene into lymphoblasts of FRDA compound heterozygotes (FRDA-CH) with deficient FXN expression (which show high sensitivity to iron and hydrogen peroxide-induced oxidative stress) rescued fully or partially the increase in mitochondrial iron levels, the decrease in aconitase and isocitrate dehydrogenase (enzymes related to the support of mitochondrial membrane potential), and the decrease in mitochondrial membrane potential [79].
5. Data from Omics (Proteomics, Metabolomics) Studies
5.1. Omics Studies in Patients with FRDA Compared to HC
A proteomic study performed on plasma from 42 FRDA patients and 20 age- and sex-matched HC showed 13 differentially expressed proteins (DEPs) between the two groups, with albumin (considered as an oxidative stress marker) being upregulated in FRDA patients [15]. Another proteomic study in PBMC from 25 FRDA patients and 24 age- and sex-matched HC showed eight DEPs related to neuroinflammation, cardiomyopathy, altered glucose metabolism, and iron transport, and among them, decreased pyruvate dehydrogenase subunit E1 (PDHE1) and increased serotransferrin [80].
A proteomic analysis of cultured B-lymphocytes from a patient diagnosed with FRDA and his asymptomatic brother showed 278 DEPs, and among them, a significant decrease in FXN; mitochondrial respiratory chain complexes I, II, and III; and enzymes involved in mitochondrial metabolism and in defense against oxidative stress [81].
Napierala et al. [82], using a new proteomic technique—reverse-phase protein array—in 44 samples of fibroblasts from FRDA patients and 18 HC, found 30 DEPs (20 upregulated and 10 downregulated), including decreased FXN and the increased expression of the enzymes aldehyde dehydrogenase 1 family member 3 (ALDH1A3) and aldehyde-oxidase 1 (AOX1), associated with oxidative stress and the regulation of retinoic acid levels.
Indelicato et al. [83], in a proteomic analysis in skeletal muscle of five FRDA patients and four age-matched HC, identified 228 DEPs (227 downregulated), with most of them related to oxidative phosphorylation, ribosomal elements, mitochondrial architecture control, and fission–fusion pathways and 74% of them being targeted to NRF2.
A proteomic study performed in the cerebrospinal fluid (CSF) of five FRDA patients and nineteen controls found thirty-four DEPs (twenty-eight upregulated and six downregulated) with a high significance between the two study groups. Most of these DEPs were related to markers of neuroinflammation and neurodegeneration, with GPX3 (overexpressed) being the only one related to oxidative stress [84].
O’Connell et al. [85] performed a metabolomic analysis of plasma from 10 FRDA patients and 11 age- and sex-matched HC, including 540 metabolites, using a combination of two techniques and found 59 metabolites that were significantly different between the two groups, mainly associated with one-carbon (1C) metabolism, composed of formate, sarcosine, hypoxanthine (a precursor of uric acid, related to oxidative stress), and homocysteine.
In a metabolomic and lipidomic analysis of fibroblasts from nine FRDA patients and nine age- and sex-matched HC by Wang et al. [86], the main findings were an increase in 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) and β-hydroxybutyrate-CoA levels, increased levels of several ceramides (especially long-chain fatty acid ceramides related to oxidative stress; these were correlated with the GAA repeat length and the frataxin protein levels), and increased levels of PUFA-containing triglycerides and phosphatidylglycerols. As we discussed previously, another lipidomic analysis did not show differences in lipid peroxidation in erythrocytes from patients with FRDA compared to HC [12].
5.2. Omics Studies in Experimental Models of FRDA
A proteomic analysis of heart tissue from 9-week-old MCK–Cre conditional frataxin KO mice compared to wild-type mice showed a decreased expression of complexes I and II of the mitochondrial electron transport chain and enzymes involved in ATP synthesis, as well as an increased expression of enzymes involved in the citric acid cycle, pyruvate decarboxylation, and oxidative stress protection [87].
Purroy et al. [88], in a proteomic analysis performed for a cardiac cellular model of FRDA based on neonatal rat cardiac myocytes and lentivirus-mediated frataxin RNA interference, described decreased content of the PDHA1 subunit from pyruvate dehydrogenase. The same group, using selected reaction monitoring-based targeted proteomics (including 21 proteins related to oxidative stress) in mice carrying the FXN I151F mutation and wild-type mice, showed a marked decrease in mitochondrial aconitase (ACO2) and the two components of the oxidative phosphorylation (OXPHOS) complex II in the cerebrum and cerebellum at 21 and 39 weeks, as well as for complex II at 21 weeks in the heart, as the most relevant changes, although SOD2 was also overexpressed in the cerebrum at 21 and 39 weeks and underexpressed in the heart at 21 weeks and overexpressed at 39 weeks [89].
A target metabolomic analysis of the cerebellum of the KIKO mouse model of FRDA showed a shift towards glycolysis and the production of itaconate (related to the Nrf2 and GSH pathway) and, therefore, to oxidative stress in microglia [90].
Finally, a multi-omic (metabolomic and transcriptomic) analysis of cardiac mitochondrial stress in three mouse models with different degrees of frataxin deficiency (YG8-800, KIKO-700, and FXNG127V) showed an increase in metabolites related to glutathione and purine metabolism, the citric acid cycle (tricarboxylic acid cycle), and several amino acid-related metabolic pathways in female KIKO-700 hearts, as well as changes in only six metabolites (4-pyridoxic acid, NADH, kynurenic acid, glutathione, deoxyribose 5-phosphate, and guanosine triphosphate) in YG8-800 hearts. However, differentially expressed genes consistent with cardiomyopathy and mitochondria-integrated stress responses were only identified in FXNG127V hearts [91].
6. Antioxidant Therapies Tested in FRDA Patients or in Experimental Models of FRDAs
Supplementary Table S2 summarizes the design and main results of clinical trials of different antioxidant drugs tested in FRDA patients [10,12,13,36,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120].
6.1. Idebenone Alone or Associated with Deferiprone
Idebenone is a quinone derivative and synthetic analog of CoQ10 that acts as a transporter of the mitochondrial respiratory chain, increasing the production of ATP. Moreover, this compound is a potent free-radical scavenger and has important effects against lipoperoxidation. To date, idebenone is the drug for which the highest number of clinical trials have been conducted, including randomized clinical trials, in patients diagnosed with FRDA. Idebenone is well-tolerated even at high doses, and its administration increases plasma levels of the substance in a dose-proportional manner [121,122], although this linearity can be lost at the highest doses [122].
The pyridone derivative deferiprone (3-hydroxy-1,2-dimethylpyridin-4(1H)-one) is a potent iron chelator. To date, three clinical trials addressed its possible role in the therapy of FRDA—all of them in association with idebenone [108,109,110]—and, in one of them, in association with riboflavin as well [110].
6.1.1. Studies in Patients with FRDA
Rustin et al. [92] reported for the first time a significant improvement in several echocardiographic parameters in three patients with FRDA who were administered low doses of idebenone without observing remarkable changes in neurological evaluation. These changes were accompanied by the demonstration of the improved activity of mitochondrial complex II and reduced lipid peroxidation in endomyocardial tissue samples obtained from the same patients [92]. Furthermore, another later study showed a decrease in urinary concentrations of 8-OHdG after administration of idebenone at low doses in patients with FRDA [13], suggesting a possible action of idebenone on a marker of oxidative stress in patients with that disease. However, other authors did not find any change in this parameter in patients treated with different doses of idebenone in comparison to a placebo [100].
Since then, results from several clinical trials have been published, including seven open-label studies [94,95,96,98,99,101,102], one placebo-controlled crossover study [93], and six double-blind placebo-controlled studies [97,100,104,105,106,107], whose results are summarized in Supplementary Table S2. In most of these studies, no significant improvement was shown in the scores of the clinical scales used to evaluate neurological deterioration [93,97,101,104,107], and some even describe the worsening of these scales [98,99,103]. Some have reported improvement in these clinical scales [96], at least in patients treated with intermediate or high doses of idebenone [100,106], and others only showed improvement in speech capability tests [107].
Regarding the effect of idebenone on echocardiographic or neuroimaging parameters, most studies showed improvement in some of them (the specific details are summarized in Supplementary Table S2) [20,94,95,97,98,99], while others did not find significant changes [93,96,101,102,105].
Two systematic reviews of the literature concluded that idebenone had a positive effect on cardiac hypertrophy and had a non-significant effect on neurological function in adults [123,124] but can induce a modest improvement in pediatric patients with FRDA [123].
The possible efficacy of idebenone in association with deferiprone in the treatment of FRDA has been tested in two open-label studies [108,110] (one of them used riboflavin, as well [110]) and in a randomized double-blind placebo-controlled study [109] (Supplementary Table S2). These three studies showed improvement in several echocardiographic parameters. However, regarding neurological function, one of them described improvement in three out of five patients [109], another described a lack of improvement [108], and another had a better annual worsening rate compared with an untreated FRDA cohort [110]. Velasco-Sánchez et al. [108] described improvement in iron deposits in the dentate nucleus, assessed using MRI.
6.1.2. Studies in Human Cell Cultures
García-Giménez et al. [33] described that idebenone was able to inhibit mitochondriogenic responses in skin fibroblast cultures from three patients with FRDA by reversing the increased expression of PGC-1α. Similarly, idebenone has shown the capacity of preventing cell death due to endogenous oxidative stress (related to the block of glutathione synthesis) in cultured fibroblasts from FRDA patients [31,125,126,127].
Quesada et al. [47] reported that cocultures of periodontal ligament cells from three FRDA patients with idebenone and deferiprone decreased caspase expression and increased antioxidant gene and FXN expression.
Lee et al. [128], in a human iPSCl (hiPSC)-derived cardiomyocyte model from a skin biopsy of a FRDA patient, showed improvement in ROS production (related to reduced iron accumulation) and in the decay velocity of calcium-handling kinetics with treatment with deferiprone, but not with idebenone administration.
6.1.3. Studies in Experimental Models of FRDA
Seznec et al. [129], in a FXN-deficient mouse model of FRDA with isolated cardiac disease confirmed by echocardiographic, biochemical, and histological studies, showed that the administration of idebenone (and not of a placebo) was able to delay cardiac disease onset, progression, and death.
Idebenone prevented oxidative stress and reduced lipid peroxidation in two cellular models of FRDA (a yeast strain lacking the yeast FXN homolog Yfh1 gene and, in the murine fibroblast, I154F missense-mutation model) [130].
Soriano et al., in an experimental model of FRDA in knockdown flies with 30% FXN, described improvement in life span and in motor abilities after the administration of idebenone [131] (which caused the recovery of the reduction of aconitase activity) or deferiprone [58,131] (which was related to a reduction of labile iron in the mitochondria) and with copper and zinc chelators [58] without inducing changes in SOD1 activity [58].
6.2. Coenzyme Q10, Vitamin E, and Vitamin E Derivatives
Coenzyme Q10 is a powerful antioxidant that plays an important role in mitochondrial oxidative phosphorylation. Vitamin E, which exists in eight forms—four tocopherols and four tocotrienols—also has an important role as an antioxidant. The results of clinical trials with coenzyme Q10, vitamin E, and vitamin E derivatives are summarized in Supplementary Table S2.
Three clinical trials, two of them open-label [37,111] and another double-blind and placebo-controlled [19], addressed the effect of the coadministration of CoQ10 and vitamin E in patients with FRDA (Supplementary Table S2). None of these studies showed significant improvement in echocardiographic measures or global clinical scales of ataxia, although two reported a modest benefit in posture and gait subscales [37,111]. Interestingly, an increase in the cardiac and skeletal muscle phosphocreatine–ATP ratio and ATP synthesis induced by this treatment was demonstrated by 31P-MRS [37,111].
One short-term double-blind placebo-controlled study with the vitamin derivative alpha-tocopheryl–quinone showed improvement in the Friedreich’s Ataxia Rating Scale (FARS) and in the Global Impression of Clinical Severity (CGIS) [67]. Another study showed that prolonged therapy (two years) with alpha-tocotrienol–quinone improved FARS scores when compared to a matched cohort without any treatment [113]. Finally, an open-label study involving only seven patients under therapy with idebenone failed to show any improvement with a tocotrienol mixture as add-on therapy in clinical neurological scales, echocardiographic measures, and neuroimaging studies, despite improvement in some biochemical parameters [12].
Both coenzyme Q10 and vitamin E were able to prevent cell death due to endogenous oxidative stress (related to the block of glutathione synthesis) in cultured fibroblasts from FRDA patients [125]. Petrillo et al. [31] described that the vitamin E analog alpha-tocotrienolquinone (as well as idebenone) increased mRNA FXN levels and reversed the increased levels of endogenous lipid peroxidation and mitochondrial ROS (mROS), as well as the decreased GSH, in human skin fibroblasts from FRDA patients. The administration of tocotrienol to PBMC in FRDA patients caused a significant increase in FXN-3 (but not in FXNs 1 and 2) isoform expression, which seemed to be independent of Peroxisome Proliferator-Activated Receptor Gamma (PPARG) expression [132]. In addition, MitoVit E (a vitamin E analog that is taken up and accumulated into mitochondria) reduced ROS production in a yeast FXN knock-out model of FRDA [133].
6.3. Recombinant Human Erythropoietin
Boesch et al. [114], in an open-label study, showed significant improvement in neurological clinical scales and in biochemical parameters (serum FXN, peroxide, and urinary 8-OHdG levels) in a short series of patients with FRDA treated with recombinant human erythropoietin. However, these results were not confirmed in two further double-blind placebo-controlled trials [115,116].
6.4. Resveratrol
Resveratrol, produced by several plants, is a stilbenoid derivative, a type of natural phenol, and a phytoalexin with important antioxidant actions. An open-label study involving 24 FRDA patients with low or high doses of resveratrol showed improvement in FARS, International Cooperative Ataxia Rating Scale (ICARS), and pitch perception, but not in quality-of-life scales or echocardiographic parameters [117]. This study showed a decrease in plasma F2-isoprostane levels with a high dose of resveratrol, but not in PBMC FXN or in urinary 8-OHdG concentrations [117].
Resveratrol increased FXN gene expression and FXN levels in lymphoblast and fibroblast cell lines derived from individuals with FRDA and in a humanized GAA repeat expansion mouse model of FRDA [134], but not in pluripotent stem cell-derived neurons in FRDA patients [135].
6.5. Omaveloxolone
Omaveloxolone is a synthetic oleanane triterpenoid compound that can activate the antioxidative transcription factor (Nrf2) and inhibit the pro-inflammatory transcription factor NF-κB-light-chain-enhancer of activated B cells. Omaveloxolone improved FARS and the scores of a quality-of-life scale in a double-blind, randomized, placebo-controlled, parallel-group trial involving 103 adult FRDA patients [118]. This drug was indicated for patients with FRDA.
Moreover, omaveloxolone restored mitochondrial complex I deficiency in cerebellar granular cells in two FRDA mouse models (KIKO and YG8R) [136] and in human fibroblasts in FRDA patients [31,136]. It also increased mRNA FXN levels [31] and reversed the increased level of endogenous lipid peroxidation and mROS in the former model and the decreased GSH in the latter [31,136]. A recent study showed that omaveloxolone (but not other Nrf2 activators, such as dimethyl fumarate) was able to improve cardiomyopathy in the conditional Fxnflox/null::MCK–Cre knock-out (FXN-cKO) mouse model of FRDA [137].
6.6. Other Drugs Tested in FRDA Patients
The results of clinical trials with other drugs tested in FRDA patients are summarized in Supplementary Table S2.
Treatment with (+)-epicatechin, a drug that induces mitochondrial biogenesis and antioxidant metabolism in muscle fibers and neurons, showed improvement in cardiac function, a non-significant reduction in FARS and modified FARS (mFARS) scores, and upregulation of a muscle-regeneration biomarker in an open-label study involving 10 FRDA patients [119].
An open-label pilot study involving seven patients with FRDA showed that recombinant human granulocyte-colony stimulating factor (G-CSF) was able to improve many biochemical parameters. However, it did not investigate the effect of that drug on the clinical parameters of that disease [120].
6.7. Other Drugs Tested in Experimental Models of FRDA
17β-estradiol (E2) [138] and other estrogen-like compounds, such as phenolic estrogens (which are independent of estrogen receptors) [139], the soy-derived estrogen receptor β agonist S-equol, and its estrogen receptor α-preferring enantiomer, R-equol [140], have shown a cytoprotective effect in FRDA skin fibroblasts from induced oxidative insults, causing the inhibition of de novo glutathione (GSH) synthesis.
Marmolino et al. [35] described that treatment with a PGC-1alpha activator and PPARG agonist (Pioglitazone) or with a cAMP-dependent protein kinase (AMPK) agonist (AICAR) restored PGC-1alpha levels—and Mn-SOD decreased mRNA expression and protein levels—in skin fibroblasts in FRDA patients and in the cerebellum and spinal cord of a mouse KIKO model of FRDA. Rodríguez-Pascau et al. [141] showed that treatment with leriglitazone (another PGC-1alpha activator and PPARG agonist) increased FXN protein levels, reduced neurite degeneration and α-fodrin cleavage mediated by calpain and caspase 3, improved mitochondrial functions and calcium homeostasis, and increased survival in FXN-deficient dorsal root ganglia neurons. Further, it prevented lipid droplet accumulation in FXN-deficient primary neonatal cardiomyocytes and improved a motor function deficit in the YG8sR FRDA mouse model.
Marobbio et al. [133] showed a decrease in ROS production in a yeast FXN knock-out model of FRDA with pretreatment with N-acetylcysteine or with the inhibitor of TOR kinases, rapamycin. The latter was also able to produce a decrease in the mitochondrial mass in mutant cells in this model. Calap-Quintana et al. [59] showed in a Drosophila RNAi-based model of FRDA that a genetic reduction in TOR complex 1 (TORC1) signaling or pharmacologic inhibition of TORC1 signaling by rapamycin (which increased the transcription of antioxidant genes, including Nrf2 as well) improved the impaired motor performance phenotype of FRDA model flies and reversed the increased lipid peroxidation markers MDA and 4-HAE and the increased total glutathione. Petrillo et al. [31] reported that pretreatment with N-acetylcysteine, sulforaphane, or dimethyl fumarate increased mRNA FXN levels and reversed the increased level of endogenous lipid peroxidation and mROS, as well as the decrease of GSH in human skin fibroblasts in FRDA patients.
N-acetylcysteine improved the survival, locomotor function, resistance to oxidative stress, and aconitase activity in a Drosophila model of FRDA with CRISPR/Cas9 insertion of approximately 200 GAA in the intron of the fly FXN gene fh [142]. Despite N-acetylcysteine being able to increase GSH levels and attenuate the induction of the NRF2, KEAP1, and BTB domain and CNC homolog 1 (BACH1) induced by sulforaphane in cultures from the human SK-N-MC neuroepithelioma cell type, its administration to MCK–Cre conditional FXN knock-out (MCK KO) mouse models did not lead to improvement in the cardiac hypertrophy suffered by these animals [143]. The administration of N-acetylcysteine or the pyruvate dehydrogenase cofactors thiamine and lipoic acid to cultures of FXN-deficient neonatal rat cardiac myocytes did not prevent accumulation of lipid droplets in these cells, while the pyruvate dehydrogenase activator dichloroacetate and the superoxide-scavenger mitochondria-targeted Tiron did have a preventive effect [89].
Jones et al. [144] described that the addition of adipose stem cells from healthy humans to cultures of periodontal ligament cells from FRDA patients had a protective effect on oxidative stress (increasing cell survival) on them, which was related to the upregulation of oxidative stress-related genes and the FXN gene and the expression of several—especially brain-derived—neurotrophic factors (BDNFs). Similarly, treatment of fibroblasts derived from FRDA patients [34] or the siRNA-induced knockdown of FXN in SH-SY5Y cells (an experimental model of FRDA) [145] with conditioned bone marrow-derived mesenchymal stem cells (MSCs) decreases oxidative stress by restoring the decreased expression of SOD1 and SOD2 and the FXN deficiency.
The addition of mesenchymal stem cells from YG8 mice (a model of FRDA) or wild-type mice to cultures of dorsal root ganglia primary cultures isolated from YG8 mice exposed to oxidative stress resulted in the increased survival of these cells, which was related to an increase in oxidative stress-related markers and FXN expression levels, BDNF, neurotrophin 3 (NT3), and neurotrophin 4 (NT4) trophic factors [146].
Cotticelli et al. [130] described that treatment with the polyunsaturated fatty acids (PUFA) linoleic and α-linolenic, deuterated at peroxidation-prone bis-allylic positions, was able to rescue oxidative stress and reduce lipid peroxidation in a yeast strain lacking the Yfh1 gene and murine fibroblast in a I154F missense-mutation model in a similar way to treatment with idebenone.
Methylene blue, which acts as an alternative electron carrier that bypasses mitochondrial complexes I-III, shows the ability to prevent heart dysfunction in a Drosophila model of FRDA [147]. Moreover, some methylene blue analogs increased FXN levels and mitochondrial biogenesis, improved aconitase activity, acted as reactive oxygen scavengers, inhibited complex I and glutathione depletion, preserved mitochondrial membrane potential, and increased ATP production, resulting in an important neuroprotective effect in cultured lymphocytes from FRDA patients [148].
Liver growth factor (LGF), administered intraperitoneally to transgenic mice of the FXNtm1MknTg (FXN)YG8Pook strain, restored motor coordination and showed a neuroprotective effect on neurons of the lumbar spinal cord and improved cardiac hypertrophy, with these events being related to an increase in FXN expression in the spinal cord and heart, increased mitochondrial chain complex IV expression in the spinal cord, and the reduction of the GSSG–GSH ratio in skeletal muscle [149].
The administration of phosphodiesterase inhibitors to primary cultures of sensory neurons from dorsal root ganglia of the YG8R FRDA mouse model was able to restore improper cytosolic Ca2+ levels and revert the axonal dystrophy found in this model [150].
Nicotinamide increased FXN gene expression and FXN levels in lymphoblast and fibroblast cell lines derived from individuals with FRDA but failed to do so in pluripotent stem cell-derived neurons from FRDA patients [135].
Villa et al. [151] showed that the systemic delivery of the catalyst activity of gold cluster superstructures (Au8-pXs) was able to improve the cell response to mitochondrial ROS and FRDA-related pathology in mesenchymal stem cells from patients with FRDA and ameliorate motor function and cardiac contractility in a YG8sR mouse model of FRDA.
A derivative of curcumin (Cur@SF NPs), which acts as a Nrf2 activator and iron chelator, has shown the ability to improve FRDA manifestation in cultures of lymphoblasts from FRDA patients and after systemic administration in a YG8R mouse FRDA model (150 mg/kg/5 days), which was related to the removal of iron from the heart, a decrease in oxidative stress, the improvement of mitochondrial function, and the compensation of FXN deficiency [152]. Abeti et al. [70] showed that other Nrf2 activators, such as sulforaphane and tricyclic bis (cyanoenone, TBE-31), protected kidney fibroblasts in KIKO and YG8R mouse models in cultures from lipid peroxidation and decreased mitochondrial membrane potential. Sulforaphane has shown the ability to improve the viability of FRDA sensory neurons generated from FRDA patient-induced pluripotent stem cells up to 61% versus the untreated control and also caused an increase of the GSH–GSSG ratio and the expression of FXN and redox markers, while omaveloxolone and dimethyl fumarate had only modest effects [153].
The administration of adenosine to a skin fibroblast culture from an FRDA patient showed a protective effect against oxidative stress and mitochondrial dysfunction (increasing cell viability) caused by an inhibitor of de novo glutathione (GSH) synthesis, which was related to the promotion of ATP production and mitochondrial biogenesis, as well as the modulation of the expression of several key metabolic genes [154].
Luffarelli et al. [155] showed a reduction of the sensitivity to hydrogen peroxide-induced cell death in FRDA fibroblasts after treatment with interferon-γ (IFN-γ), which was related to the induction of a rapid expression of Nrf2 and SOD2, leading to a potentiation of antioxidant responses.
Zhao et al. [156] showed a beneficial effect of treatment of lymphoblasts and fibroblasts derived from FRDA patients with the mitochondrion-targeted peptide SS-31, which consisted of a dose-dependent upregulation of FXN protein concentration, an increase in activities of iron–sulfur enzymes (including aconitase and complexes II and III of the respiratory chain), and improvement of mitochondrial membrane potential, ATP concentrations, and the NAD+–NADH ratio, as well as the morphology of mitochondria.
Selenium and small organoselenium compounds that act as mimics of GPx, such as ebselen, can prevent cell death due to endogenous oxidative stress (related to the block of glutathione synthesis) in cultured fibroblasts from FRDA patients [125]. In the same model, Hackett et al. [75] showed that the antihypertensive 4-hydroxychalcone and dibenzoylmethane were able to increase mRNA levels of NRF2 and FXN levels and to downregulate the antioxidants TRX, GR, and SOD2, as well as decrease ROS production.
Igoillo-Esteve et al. [157] showed that the glucagon-like peptide-1 (GLP-1) exenatide was able to improve glucose homeostasis (by enhancing the insulin content and secretion in pancreatic β cells), induce FXN and iron–sulfur cluster-containing proteins in β cells and the brain, and protect sensory neurons in dorsal root ganglia in a FXN-deficient KIKO mouse model. This drug also induced FXN expression, reduced oxidative stress, and improved mitochondrial function in FRDA patients’ iPSC-derived β cells and iPCS-derived sensory neurons. The administration of exenatide over 5 weeks to five FRDA patients showed a modest increase in FXN protein levels in platelets but not in PBMC, and increased mRNA FXN expression both in platelets and in PBMC.
A recent study by Edzemeay et al. [158] showed that the combined treatment of FRDA patient-derived fibroblasts and 2D sensory neurons with L-ascorbic acid, N-acetylcysteine, and dimethyl fumarate caused a significant decrease in mitochondrial and cellular ROS and an increase in aconitase–citrate synthase activity, GSH–GSSG ratios, and mitochondrial membrane potential.
Finally, the transduction of human FRDA fibroblasts with recombinant adeno-associated viral and lentiviral vectors encoding the human FXN cDNA partially reversed FXN deficiency and the increased mitochondrial iron and sensitivity to oxidative stress [159].
7. Discussion and Conclusions
The results of studies of oxidative stress markers in different tissues of patients diagnosed with FRDA compared to those of HC have shown controversial or inconclusive results. This could be due to the scarcity of publications on the subject, the fact that many of them have a small sample size, and the differences in the assay methods and disease stage of the individuals involved in the studies. The same facts have also occurred with studies on the expression of genes related to oxidative stress in patients diagnosed with FRDA.
Among the findings of these studies, it is worth mentioning that there is (1) a decrease in the activity of mitochondrial respiratory chain complexes I, II, and III [8,27] and aconitase restricted to endomyocardial tissue [8,27] found in patients with FRDA, and (2) there is a description of the association between SNP and the AGTR1 gene and FRDA, as well as its relation to the increased interventricular septal wall thickness and left ventricular mass in a case-control association study [44]. However, the sample sizes are too small, and the results should be confirmed by replication studies.
In contrast to the results obtained in biological samples from patients with FRDA, the data obtained from different experimental models of FRDA, based on FXN deficiency, are highly suggestive of the probable role of oxidative stress in this disease. However, as we discussed earlier, the results obtained in experimental models are not always able to be extrapolated to the disease in humans. In most of these models, the deficiency of FXN induces oxidative stress related to increased iron concentration, which causes damage and mitochondrial dysfunction; a decrease in mitochondrial respiratory chain complexes and mitochondrial membrane potential; a decrease in aconitase activity, such as the induction of antioxidant enzymes such as SOD1, SOD2, GPx, peroxiredoxins, glutaredoxins, and GST (among others); a decrease in GSH concentrations and the GHS–GSSG ratio; an increase in markers of lipoperoxidation and oxidation of DNA, as well as carbonyl proteins; and the decreased expression of NRF2 and downregulation of PGC-1α. Paradoxically, experimental situations of FXN overexpression can also cause oxidative stress [76,79].
Drawing from key findings in both patient studies and experimental models, Figure 1 illustrates the proposed interactions among the various mechanisms of oxidative stress contributing to FRDA pathogenesis. FXN mutation or the induction of FXN deficiency would lead to a loss of Fe–S clusters in various mitochondrial enzymes, which would increase iron. This, in turn, would cause mitochondrial dysfunction and an increase in ROS production, inducing oxidative stress, the induction of antioxidant enzymes, and a decrease in GSH, NRF2, and PGC1-alpha. Mitochondrial dysfunction would also cause glutamatergic disturbances and alterations in calcium homeostasis (which would, in turn, lead to an increase in ROS production). As a consequence of oxidative stress, there would be an increase in the oxidation of lipids, proteins, and DNA, and an alteration in oxidative phosphorylation (with a decrease in ATP synthesis). All these mechanisms would ultimately lead to cell death. However, we must keep in mind that the results obtained from experimental models have significant limitations. One of the most important facts is that the heterogeneity and pathophysiological complexity of human diseases mean that the results of the experimental studies cannot be fully extrapolated to what happens in them [160,161,162].
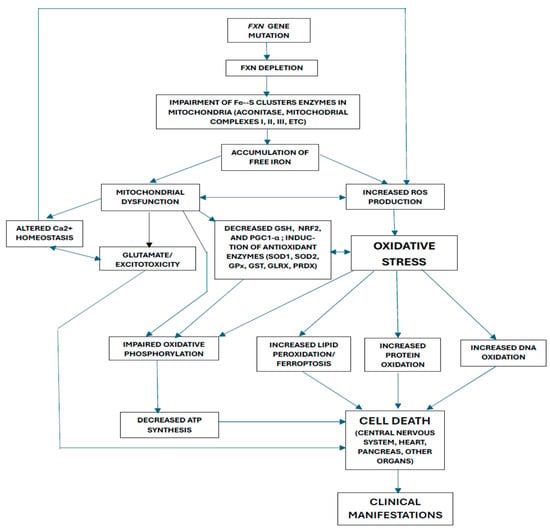
Figure 1.
Possible interactions between the different pathogenic mechanisms proposed for Friedreich’s ataxia.
Although idebenone, CoQ10, and vitamin E derivatives have shown benefits in experimental models, clinical trials with idebenone in FRDA patients have demonstrated only modest improvement in cardiac hypertrophy and minimal neurological benefit. These effects may be slightly enhanced when combined with deferiprone, though evidence is limited to three studies, with only one double-blind trial involving five patients. Combinations of vitamin E and CoQ10 and vitamin E on its own have not shown beneficial effects, while vitamin E derivatives such as alpha-tocopheryl–quinone and alpha-tocotrienol–quinone caused a modest improvement in a short series of patients with FRDA (although studies with these treatments were double-blind, randomized, and placebo-controlled, and, therefore, had a high level of evidence).
The activator of Nrf2, omaveloxolone has been the only drug that has shown a clear, although modest, improvement of neurological symptoms in patients with FRDA in a double-blind, placebo-controlled trial involving a significant number of patients (and, therefore, has a high level of evidence) [118], and as of today, it is indicated as a treatment for this disease. In addition, this drug showed improvement in oxidative stress parameters [31,136] and in cardiac hypertrophy [138] in experimental models.
Resveratrol [118] and epicatechin [119] showed a modest improvement of certain clinical symptoms and G-CSF [120] of several biochemical parameters in a short series of FRDA patients (although the clinical efficacy was not assessed), but the studies with these drugs were short-term, open-label, and involved a low number of patients, so they had a low level of evidence. The promising beneficial effects of treatment with recombinant human erythropoietin in an open-label study [114] were not confirmed in double-blind placebo-controlled trials [115,116].
Many drugs have demonstrated significant benefits in reducing oxidative stress parameters in various experimental models of FRDA. These include, among others, estrogen derivatives [138,139,140], PGC-1alpha activators acting as PPARG agonists [35,141], AMPK agonists [35], TOR kinase inhibitors [59,133], N-acetylcysteine [31,133,142], sulforaphane [31,143], dimethyl fumarate [31], PUFAs [130], methylene blue [147,148], LGF [149], phosphodiesterase inhibitors [150], NRF2 activators [70,152,153], adenosine [154], IFN-γ [155], and the combination of L-ascorbic acid, N-acetylcysteine, and dimethyl fumarate [158]. To our knowledge, the possible efficacy and safety of these drugs in patients with FRDA has not yet been explored, except in the case of the NRF2 activator omaveloxolene [118].
8. Future Directions
Further data from studies in different FRDA models suggest the possibility of an important role of oxidative stress mechanisms in this disease, which have been described with sufficient detail in a previous section. However, data on FRDA patients are insufficient due to the scarcity of studies published on this topic. It is striking that no data have been published on studies of oxidative stress markers in CSF (except for a proteomic study with a low sample size), despite its easy accessibility.
Although the data on the contribution of oxidative stress to the pathogenesis of FRDA inferred from experimental models are available, it would be desirable to increase the number of studies and the sample size regarding oxidative stress markers in patients with FRDA compared to HC. For future studies related to this topic, we propose that it would be advisable to meet the following conditions:
- Design prospective and multicenter studies with a long-term follow-up period.
- Ensure the participation of an important number of patients, both symptomatic and non-symptomatic, with a genetic-molecular diagnosis of FRDA, along with a comparable number of healthy age- and sex-matched individuals.
- Exclude subjects (both in the FRDA and HC groups) exposed to any factors that may alter oxidative stress markers, such as medication use (antioxidant vitamins, calcium or mineral supplements, diuretics, bisphosphonates), atypical dietary habits, pregnancy, some comorbidities (undernutrition, obesity, oncologic and acute infectious diseases, and thyroid, parathyroid, kidney, or liver disorders), as well as recent trauma or surgical interventions. Some other possible confounding variables, such as diet, physical activity, alcohol consumption and tobacco smoking, exposure to toxic substances, history of arterial hypertension, diabetes mellitus, and hypercholesterolemia, should be controlled.
- Collect plasma/serum, blood cells, and CSF to analyze oxidative stress biomarkers at baseline and after a long-term follow-up, both in FRDA patients and HC.
- Conduct periodic clinical evaluations (every 3–6 months) of patients with FRDA using standardized scales for FRDA [163,164,165,166,167,168] and cardiological assessments to monitor disease severity and progression.
- Finally, in the event of death, brain donation from FRDA patients and HC should be encouraged to enable examination of oxidative stress markers in both groups.
Regarding the use of antioxidant treatments, as previously described, many of them have shown efficacy in experimental models of FRDA, while in clinical trials in patients with this disease, only omaveloxolone and, to a lesser extent, idebenone (at least in the treatment of cardiac hypertrophy) have shown some degree of effectiveness. To better assess the effectiveness of antioxidant therapies in FRDA, future research should include large-scale, prospective, multicenter, randomized, placebo-controlled trials testing compounds that have shown promise in experimental models. However, we must keep in mind that the results obtained in experimental models with different drugs would not always be able to be extrapolated to those expected in clinical trials with patients.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cells14181406/s1: Supplementary Table S1. Results of studies reported on oxidative stress markers in different tissues from Friedrich’s ataxia (FRDA) patients compared to those of healthy controls (HC); Supplementary Table S2. Results of clinical trials with antioxidant compounds tested in patients with FRDA. Refs. [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120] have been cited in the Supplementary Materials.
Author Contributions
F.J.J.-J.: Conceptualization, Methodology, Investigation, Validation, Formal analysis, Investigation, Writing—original draft, Writing—review and editing, Project administration. H.A.-N.: Conceptualization, Methodology, Investigation, Validation, Formal analysis, Investigation, Writing—original draft, Writing—review and editing, Project administration. E.G.-M.: Conceptualization, Methodology, Investigation, Validation, Formal analysis, Investigation, Writing—original draft, Writing—review and editing, Project administration, Funding acquisition. A.C.-F.: Conceptualization, Methodology, Investigation, Validation, Formal analysis, Investigation, Writing—original draft, Writing—review and editing, Project administration. M.A.M.-G.: Conceptualization, Methodology, Investigation, Validation, Formal analysis, Investigation, Writing—original draft, Writing—review and editing, Project administration. J.A.G.A.: Conceptualization, Methodology, Investigation, Validation, Formal analysis, Investigation, Writing—original draft, Writing—review and editing, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work at the authors’ laboratory is supported in part by Grants PI24/01358 and PI21/01683 from the Fondo de Investigación Sanitaria, Instituto de Salud Carlos III, Madrid, Spain, and was partially funded with FEDER funds.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We recognize the effort of José Manuel Lorenzo Estrada (personnel of the Library of Hospital Universitario del Sureste, Arganda del Rey), who retrieved an important number of papers for us.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
AAS, atomic absorption spectrophotometry; ACE, angiotensin-converting enzyme (ACE); ADLS, Activities of Daily Living Scale; AGTR1, angiotensin-II type-1 receptor; ALDH1A3, aldehyde dehydrogenase 1 family member 3; AMP, adenosin monophosphate; AMPK, cAMP-dependent protein kinase; AOX1, aldehyde–oxidase 1; APP, amyloid beta (A4) precursor protein; ATP, adenosin–triphosphate; BACH1, BTB domain and CNC homolog 1; BDNF, brain-derived neurotrophic factor; CAT, catalase; CGIC, clinical global impression of change; Cho, choline; CoQ10, coenzyme Q10; C19Orf12, chromosome 19 open reading frame 12; Cr, creatinine; 2-D-DIGE, two-dimensional difference in gel electrophoresis; DEP, differentially expressed protein (DEP); DHBD, dihydroxybenzoic acid; DI, Disposition Index; DNA, deoxyribonucleic acid; DTI, diffusion tensor imaging; DUSP1, dual-specificity phosphatase 1; DWI, diffusion-weighted imaging; ECLIA, Electrochemiluminescence immunoassay; ELISA, Enzyme-Linked ImmunoSorbent Assay; EPAS1, endothelial PAS domain protein 1; EPI, (+)-Epicatechin; EPI-743, alpha-tocotrienolol quinone or vatiquinone; EPO, erythropoietin; EPX, eosinophil peroxidase; ESI–MS, electrospray ionization–mass spectrometry; F, female; FAIS, Friedreich’s Ataxia Impact Scale; FARS, Friedreich’s Ataxia Rating Scale; FOX, FBJ murine osteosarcoma viral oncogene homolog; FRDA, Friedreich’s Ataxia; fsIVGTT modified, frequently sampled intravenous glucose tolerance test; FXN, frataxin; GAA, guanine–adenosine–adenosine; GCL, glutamate–cysteine ligase; G-CSF, granulocyte colony-stimulating factor; GICS, Global Impression of Clinical Severity; GLP-1, glucagon-like peptide 1; GLRX1, Glutaredoxin 1; GPx, glutathione peroxidase; GSH, reduced glutathione; GSK3β, Glycogen synthase kinase-3β; GSR, glutathione disulfide reductase; GSSG, oxidized glutathione; GST, glutathione transferase; HAE, 4-hydroxyalkenals; HAX-1, HCLS1-associated X-1; HC, healthy controls; 1H-MRS, proton magnetic resonance spectroscopy; HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme A; 4-HNE, 4-hydroxy-2-nonenal; M, male; HO-1, Heme Oxygenase-1 (HO-1); HPLC, high-performance liquid chromatography; 9HPT, Nine-Hole Peg Test; hiPSC, human-induced pluripotent stem cell; iPSC, induced pluripotent stem cell; ICARS, International Cooperative Ataxia Rating Scale; IVS, interventricular septal; KEAP1, Kelch-like ECH-associated protein 1; KIKO, knock-in–knock-out; LCSLA, Low-Contrast Sloan Letter Acuity; LGF, liver growth factor; LKB1/AMPK, Liver Kinase B1/AMP-activated protein kinase; LVEF, left ventricular ejection fraction; LVM, left ventricular mass; LVMI, left ventricular mass index; LVPV, left ventricular posterior wall; MCK–Cre, muscle creatine kinase; MDA, malonyldialdehyde; mFARS, modified FARS; MFIS, modified Fatigue Impact Scale; MRI, magnetic resonance imaging; mRNA, messenger ribonucleic acid; MSC, mesenchymal stem cells; NAA, N-acetyl-aspartate; NAD, nicotin–adenin–dinucleotide, NADH, reduced nicotin–adenin–dinucleotide; NCF2, neutrophil cytosolic factor 2; NDRG1, N-myc downstream-regulated 1; NFκB, nuclear factor kappa B; NMJs, neuromuscular junctions; NO, nitric oxide; NQO1, NAD(P)H quinone oxidoreductase 1; NRF1, nuclear respiratory factor 1; Nrf2 or NRF2, nuclear factor erythroid 2-related factor 2; NSE, neuronal specific enolase; NT3/NT4, Neurotrophins 3 and 4; OH-dG, 8-hydroxy-deoxyguanosine; OXPHOS, oxidative phosphorylation; PBMC, peripheral blood mononuclear cells; PDLIM1, PDZ and LIM domain 1; PDEH1, pyruvate dehydrogenase subunit E1; PedsQL, Pediatric Quality of Life Inventory 4.0; PGC1α, peroxisome proliferator-activated receptor-gamma coactivator 1 alpha; PGIC, patient global impression of change; 31P-MRS, 31phosphorus magnetic resonance imaging; PPARG, Peroxisome Proliferator-Activated Receptor Gamma; PRDX, periredoxin; PREX1, phosphatidylinositol-3,4,5-trisphosphate-dependent Rac exchange factor 1; PWT, posterior wall thickness; PUFA, polyunsaturated fatty acids; RNAi, ribonucleic acid interference; RNF7, ring finger protein 7; ROS, reactive oxygen species; RT-PCR, real-time polymerase chain + reaction; RT-qPCR, real-time quantitative PCR; RWT, relative wall thickness; SARA, Scale for the Assessment and Rating of Ataxia; SELP, selectin P (granule membrane protein 140kDa, antigen CD62, SEPP1 selenoprotein P plasma 1); SEPW1, selenoprotein W1; SF36, 36-Item Short Form Health Survey; SFTPD, surfactant protein D; SIRT, sirtuin; SLC7A11, solute carrier family 7 member 11; SNP, single-nucleotide polymorphism; SOD1, superoxide–dismutase 1; SOD1, superoxide–dismutase 2; SPAP1, spermatogenesis-associated 1 SWI susceptibility-weighted imaging; SWT, septal wall thickness; TAC, total antioxidant capacity; TBA, thiobarbituric acid; TBARS, thiobarbituric acid-reactive substances; T25FW, Timed 25 Foot Walk (T25FW); TLR4, Toll-like receptor 4; TNFAIP3, tumor necrosis factor alpha-induced protein 3; TOR1, TOR complex 1; TRX1, thioredoxin 1; TXNRD1, thioredoxin reductase 1; VEGF, vascular endothelial growth factor A; YFH1, yeast FXN homolog 1.
References
- Berciano, J. Historia de las ataxias hereditarias y comentario sobre el legado de Hans Joachim Scherer. Neurosci. His. 2018, 6, 85–100. Available online: https://nah.sen.es/vmfiles/vol6/NAHV6N3201885_100_ES.pdf (accessed on 30 July 2025).
- Keita, M.; McIntyre, K.; Rodden, L.-N.; Schadt, K.; Lynch, D.R. Friedreich ataxia: Clinical features and new developments. Neurodegener. Dis. Manag. 2022, 12, 267–283. [Google Scholar] [CrossRef]
- Campuzano, V.; Montermini, L.; Moltò, M.D.; Pianese, L.; Cossée, M.; Cavalcanti, F.; Monros, E.; Rodius, F.; Duclos, F.; Monticelli, A.; et al. Friedreich’s ataxia: Autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 1996, 271, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Corben, L.-A.; Georgiou-Karistianis, N.; Bradshaw, J.L.; Evans-Galea, M.V.; Churchyard, A.J.; Delatycki, M.B. Characterising the neuropathology and neurobehavioural phenotype in Friedreich ataxia: A systematic review. Adv. Exp. Med. Biol. 2012, 769, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Koeppen, A.H.; Mazurkiewicz, J.E. Friedreich ataxia: Neuropathology revised. J. Neuropathol. Exp. Neurol. 2013, 72, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Macevilly, C.J.; Muller, D.P. Oxidative stress does not appear to be involved in the aetiology of Friedreich’s ataxia. Restor. Neurol. Neurosci. 1997, 11, 131–137. [Google Scholar] [CrossRef]
- Sparaco, M.; Gaeta, L.M.; Santorelli, F.M.; Passarelli, C.; Tozzi, G.; Bertini, E.; Simonati, A.; Scaravilli, F.; Taroni, F.; Duyckaerts, C.; et al. Friedreich’s ataxia: Oxidative stress and cytoskeletal abnormalities. J. Neurol. Sci. 2009, 287, 111–118. [Google Scholar] [CrossRef]
- Bradley, J.L.; Blake, J.C.; Chamberlain, S.; Thomas, P.K.; Cooper, J.M.; Schapira, A.H. Clinical, biochemical and molecular genetic correlations in Friedreich’s ataxia. Hum. Mol. Genet. 2000, 9, 275–282. [Google Scholar] [CrossRef]
- Solbach, K.; Kraff, O.; Minnerop, M.; Beck, A.; Schöls, L.; Gizewski, E.R.; Ladd, M.E.; Timmann, D. Cerebellar pathology in Friedreich’s ataxia: Atrophied dentate nuclei with normal iron content. Neuroimage Clin. 2014, 6, 93–99. [Google Scholar] [CrossRef]
- Gramegna, L.L.; Tonon, C.; Manners, D.N.; Pini, A.; Rinaldi, R.; Zanigni, S.; Bianchini, C.; Evangelisti, S.; Fortuna, F.; Carelli, V.; et al. Combined Cerebellar Proton MR Spectroscopy and DWI Study of Patients with Friedreich’s Ataxia. Cerebellum 2017, 16, 82–88. [Google Scholar] [CrossRef]
- Bradley, J.L.; Homayoun, S.; Hart, P.E.; Schapira, A.H.; Cooper, J.M. Role of oxidative damage in Friedreich’s ataxia. Neurochem. Res. 2004, 29, 561–567. [Google Scholar] [CrossRef]
- Bolotta, A.; Pini, A.; Abruzzo, P.M.; Ghezzo, A.; Modesti, A.; Gamberi, T.; Ferreri, C.; Bugamelli, F.; Fortuna, F.; Vertuani, S.; et al. Effects of tocotrienol supplementation in Friedreich’s ataxia: A model of oxidative stress pathology. Exp. Biol. Med. 2020, 245, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.B.; Dehmer, T.; Schöls, L.; Mende, H.; Hardt, C.; Vorgerd, M.; Bürk, K.; Matson, W.; Dichgans, J.; Beal, M.F.; et al. Oxidative stress in patients with Friedreich ataxia. Neurology 2000, 55, 1719–1721. [Google Scholar] [CrossRef] [PubMed]
- Pathak, D.; Srivastava, A.K.; Gulati, S.; Rajeswari, M.R. Assessment of cell-free levels of iron and copper in patients with Friedreich’s ataxia. Biometals 2019, 32, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Swarup, V.; Srivastava, A.K.; Padma, M.V.; Rajeswari, M.R. Quantitative profiling and identification of differentially expressed plasma proteins in Friedreich’s ataxia. J. Neurosci. Res. 2013, 91, 1483–1491. [Google Scholar] [CrossRef]
- Barbeau, A.; Melançon, S.B.; Lemieux, B.; Roy, M. Biochemical markers in Friedreich’s ataxia. Ital. J. Neurol. Sci. 1984, 13–26. [Google Scholar]
- Muller, D.P.; Matthews, S.; Harding, A.E. Serum vitamin E concentrations are normal in Friedreich’s ataxia. J. Neurol. Neurosurg. Psychiatry 1987, 50, 625–627. [Google Scholar] [CrossRef]
- Eusebi, M.P.; Battisti, C.; De Stefano, N.; De Michele, G.; Filla, A.; Federico, A. Serum vitamin E in inherited ataxias. Acta Neurol. 1990, 12, 147–150. [Google Scholar]
- Cooper, J.M.; Korlipara, L.V.; Hart, P.E.; Bradley, J.L.; Schapira, A.H. Coenzyme Q10 and vitamin E deficiency in Friedreich’s ataxia: Predictor of efficacy of vitamin E and coenzyme Q10 therapy. Eur. J. Neurol. 2008, 15, 1371–1379. [Google Scholar] [CrossRef]
- Schirinzi, T.; Vasco, G.; Zanni, G.; Petrillo, S.; Piemonte, F.; Castelli, E.; Bertini, E.S. Serum uric acid in Friedreich Ataxia. Clin. Biochem. 2018, 54, 139–141. [Google Scholar] [CrossRef]
- Lazaropoulos, M.; Dong, Y.; Clark, E.; Greeley, N.R.; Seyer, L.A.; Brigatti, K.W.; Christie, C.; Perlman, S.L.; Wilmot, G.R.; Gomez, C.M.; et al. Frataxin levels in peripheral tissue in Friedreich ataxia. Ann. Clin. Transl. Neurol. 2015, 2, 831–842. [Google Scholar] [CrossRef]
- Piemonte, F.; Pastore, A.; Tozzi, G.; Tagliacozzi, D.; Santorelli, F.M.; Carrozzo, R.; Casali, C.; Damiano, M.; Federici, G.; Bertini, E. Glutathione in blood of patients with Friedreich’s ataxia. Eur. J. Clin. Investig. 2001, 31, 1007–1011. [Google Scholar] [CrossRef]
- Petrillo, S.; Santoro, M.; La Rosa, P.; Perna, A.; Gallo, M.G.; Bertini, E.S.; Silvestri, G.; Piemonte, F. Nuclear Factor Erythroid 2-Related Factor 2 Activation Might Mitigate Clinical Symptoms in Friedreich’s Ataxia: Clues of an “Out-Brain Origin” of the Disease From a Family Study. Front. Neurosci. 2021, 15, 638810. [Google Scholar] [CrossRef]
- McMackin, M.Z.; Durbin-Johnson, B.; Napierala, M.; Napierala, J.S.; Ruiz, L.; Napoli, E.; Perlman, S.; Giulivi, C.; Cortopassi, G.A. Potential biomarker identification for Friedreich’s ataxia using overlapping gene expression patterns in patient cells and mouse dorsal root ganglion. PLoS ONE 2019, 14, e0223209. [Google Scholar] [CrossRef]
- Tozzi, G.; Nuccetelli, M.; Lo Bello, M.; Bernardini, S.; Bellincampi, L.; Ballerini, S.; Gaeta, L.M.; Casali, C.; Pastore, A.; Federici, G.; et al. Antioxidant enzymes in blood of patients with Friedreich’s ataxia. Arch. Dis. Child. 2002, 86, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Steinkellner, H.; Singh, H.N.; Muckenthaler, M.U.; Goldenberg, H.; Moganty, R.R.; Scheiber-Mojdehkar, B.; Sturm, B. No changes in heme synthesis in human Friedreich’s ataxia erythroid progenitor cells. Gene 2017, 621, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Rötig, A.; de Lonlay, P.; Chretien, D.; Foury, F.; Koenig, M.; Sidi, D.; Munnich, A.; Rustin, P. Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nat. Genet. 1997, 17, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Bulteau, A.L.; Planamente, S.; Jornea, L.; Dur, A.; Lesuisse, E.; Camadro, J.M.; Auchère, F. Changes in mitochondrial glutathione levels and protein thiol oxidation in Δyfh1 yeast cells and the lymphoblasts of patients with Friedreich’s ataxia. Biochim. Biophys. Acta 2012, 1822, 212–225. [Google Scholar] [CrossRef]
- Selak, M.A.; Lyver, E.; Micklow, E.; Deutsch, E.C.; Onder, O.; Selamoglu, N.; Yager, C.; Knight, S.; Carroll, M.; Daldal, F.; et al. Blood cells from Friedreich ataxia patients harbor frataxin deficiency without a loss of mitochondrial function. Mitochondrion 2011, 11, 342–350. [Google Scholar] [CrossRef]
- Quatrana, A.; Petrillo, S.; Torda, C.; De Santis, E.; Bertini, E.; Piemonte, F. Redox homeostasis and inflammation in fibroblasts of patients with Friedreich Ataxia: A possible cross talk. Front. Mol. Neurosci. 2025, 18, 1571402. [Google Scholar] [CrossRef]
- Petrillo, S.; D’Amico, J.; La Rosa, P.; Bertini, E.S.; Piemonte, F. Targeting NRF2 for the Treatment of Friedreich’s Ataxia: A Comparison among Drugs. Int. J. Mol. Sci. 2019, 20, 5211. [Google Scholar] [CrossRef]
- Pastore, A.; Tozzi, G.; Gaeta, L.M.; Bertini, E.; Serafini, V.; Di Cesare, S.; Bonetto, V.; Casoni, F.; Carrozzo, R.; Federici, G.; et al. Actin glutathionylation increases in fibroblasts of patients with Friedreich’s ataxia: A potential role in the pathogenesis of the disease. J. Biol. Chem. 2003, 278, 42588–42595. [Google Scholar] [CrossRef] [PubMed]
- García-Giménez, J.L.; Gimeno, A.; Gonzalez-Cabo, P.; Dasí, F.; Bolinches-Amorós, A.; Mollá, B.; Palau, F.; Pallardó, F.V. Differential expression of PGC-1α and metabolic sensors suggest age-dependent induction of mitochondrial biogenesis in Friedreich ataxia fibroblasts. PLoS ONE 2011, 6, e20666. [Google Scholar] [CrossRef] [PubMed]
- Dey, R.; Kemp, K.; Gray, E.; Rice, C.; Scolding, N.; Wilkins, A. Human mesenchymal stem cells increase anti-oxidant defences in cells derived from patients with Friedreich’s ataxia. Cerebellum 2012, 11, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Marmolino, D.; Manto, M.; Acquaviva, F.; Vergara, P.; Ravella, A.; Monticelli, A.; Pandolfo, M. PGC-1alpha down-regulation affects the antioxidant response in Friedreich’s ataxia. PLoS ONE 2010, 5, e10025. [Google Scholar] [CrossRef]
- Lodi, R.; Hart, P.E.; Rajagopalan, B.; Taylor, D.J.; Crilley, J.G.; Bradley, J.L.; Blamire, A.M.; Manners, D.; Styles, P.; Schapira, A.H.; et al. Antioxidant treatment improves in vivo cardiac and skeletal muscle bioenergetics in patients with Friedreich’s ataxia. Ann. Neurol. 2001, 49, 590–596. [Google Scholar] [CrossRef]
- Lodi, R.; Rajagopalan, B.; Bradley, J.L.; Taylor, D.J.; Crilley, J.G.; Hart, P.E.; Blamire, A.M.; Manners, D.; Styles, P.; Schapira, A.H.; et al. Mitochondrial dysfunction in Friedreich’s ataxia: From pathogenesis to treatment perspectives. Free Radic. Res. 2002, 36, 461–466. [Google Scholar] [CrossRef]
- Nachbauer, W.; Wanschitz, J.; Steinkellner, H.; Eigentler, A.; Sturm, B.; Hufler, K.; Scheiber-Mojdehkar, B.; Poewe, W.; Reindl, M.; Boesch, S. Correlation of frataxin content in blood and skeletal muscle endorses frataxin as a biomarker in Friedreich ataxia. Mov. Disord. 2011, 26, 1935–1938. [Google Scholar] [CrossRef]
- Castaldo, I.; Vergara, P.; Pinelli, M.; Filla, A.; De Michele, G.; Cocozza, S.; Monticelli, A. Can telomere shortening in human peripheral blood leukocytes serve as a disease biomarker of Friedreich’s ataxia? Antioxid. Redox Signal. 2013, 18, 1303–1306. [Google Scholar] [CrossRef]
- Anjomani Virmouni, S.; Al-Mahdawi, S.; Sandi, C.; Yasaei, H.; Giunti, P.; Slijepcevic, P.; Pook, M.A. Identification of telomere dysfunction in Friedreich ataxia. Mol. Neurodegener. 2015, 10, 22. [Google Scholar] [CrossRef]
- Scarabino, D.; Veneziano, L.; Nethisinghe, S.; Mantuano, E.; Fiore, A.; Granata, G.; Solanky, N.; Zanni, G.; Cavalcanti, F.; Corbo, R.M.; et al. Unusual Age-Dependent Behavior of Leukocytes Telomere Length in Friedreich’s Ataxia. Mov. Disord. 2024, 39, 2058–2066. [Google Scholar] [CrossRef]
- Haugen, A.C.; Di Prospero, N.A.; Parker, J.S.; Fannin, R.D.; Chou, J.; Meyer, J.N.; Halweg, C.; Collins, J.B.; Durr, A.; Fischbeck, K.; et al. Altered gene expression and DNA damage in peripheral blood cells from Friedreich’s ataxia patients: Cellular model of pathology. PLoS Genet. 2010, 6, e1000812. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, G.; Cortopassi, G. Lymphoblast Oxidative Stress Genes as Potential Biomarkers of Disease Severity and Drug Effect in Friedreich’s Ataxia. PLoS ONE 2016, 11, e0153574. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.; Bagnall, R.D.; Peverill, R.E.; Donelan, L.; Corben, L.; Delatycki, M.B.; Semsarian, C. A polymorphic miR-155 binding site in AGTR1 is associated with cardiac hypertrophy in Friedreich ataxia. J. Mol. Cell. Cardiol. 2011, 51, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Faruq, M.; Padma, M.V.; Goyal, V.; Behari, M.; Grover, A.; Mukerji, M.; Srivastava, A.K. Investigation of mitochondrial DNA variations among Indian Friedreich’s ataxia (FRDA) patients. Mitochondrion 2015, 25, 1–5. [Google Scholar] [CrossRef]
- Tiano, F.; Amati, F.; Cherubini, F.; Morini, E.; Vancheri, C.; Maletta, S.; Fortuni, S.; Serio, D.; Quatrana, A.; Luffarelli, R.; et al. Frataxin deficiency in Friedreich’s ataxia is associated with reduced levels of HAX-1, a regulator of cardiomyocyte death and survival. Hum. Mol. Genet. 2020, 29, 471–482. [Google Scholar] [CrossRef]
- Quesada, M.P.; Jones, J.; Rodríguez-Lozano, F.J.; Moraleda, J.M.; Martinez, S. Novel aberrant genetic and epigenetic events in Friedreich’s ataxia. Exp. Cell Res. 2015, 335, 51–61. [Google Scholar] [CrossRef]
- González-Cabo, P.; Llorens, J.V.; Palau, F.; Moltó, M.D. Friedreich ataxia: An update on animal models, frataxin function and therapies. Adv. Exp. Med. Biol. 2009, 652, 247–261. [Google Scholar] [CrossRef]
- Llorens, J.V.; Soriano, S.; Calap-Quintana, P.; Gonzalez-Cabo, P.; Moltó, M.D. The Role of Iron in Friedreich’s Ataxia: Insights From Studies in Human Tissues and Cellular and Animal Models. Front. Neurosci. 2019, 13, 75. [Google Scholar] [CrossRef]
- Babcock, M.; de Silva, D.; Oaks, R.; Davis-Kaplan, S.A.C.; Jiralerspong, S.; Montermini, L.; Pandolfo, M.; Kaplan, J. Regulation of mitochondrial iron accumulation by Yfh1p, a putative homolog of frataxin. Science 1997, 276, 1709–1712. [Google Scholar] [CrossRef]
- Radisky, D.C.; Babcock, M.C.; Kaplan, J. The yeast frataxin homologue mediates mitochondrial iron efflux. Evidence for a mitochondrial iron cycle. J. Biol. Chem. 1999, 274, 4497–4499. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Yang, J.; Cavadini, P.; Gellera, C.; Lonnerdal, B.; Taroni, F.; Cortopassi, G. The Friedreich’s ataxia mutation confers cellular sensitivity to oxidant stress which is rescued by chelators of iron and calcium and inhibitors of apoptosis. Hum. Mol. Genet. 1999, 8, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Chantrel-Groussard, K.; Geromel, V.; Puccio, H.; Koenig, M.; Munnich, A.; Rötig, A.; Rustin, P. Disabled early recruitment of antioxidant defenses in Friedreich’s ataxia. Hum. Mol. Genet. 2001, 10, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Jiralerspong, S.; Ge, B.; Hudson, T.J.; Pandolfo, M. Manganese superoxide dismutase induction by iron is impaired in Friedreich ataxia cells. FEBS Lett. 2001, 509, 101–105. [Google Scholar] [CrossRef]
- Bulteau, A.L.; Dancis, A.; Gareil, M.; Montagne, J.J.; Camadro, J.M.; Lesuisse, E. Oxidative stress and protease dysfunction in the yeast model of Friedreich ataxia. Free Radic. Biol. Med. 2007, 42, 1561–1570. [Google Scholar] [CrossRef]
- Auchère, F.; Santos, R.; Planamente, S.; Lesuisse, E.; Camadro, J.M. Glutathione-dependent redox status of frataxin-deficient cells in a yeast model of Friedreich’s ataxia. Hum. Mol. Genet. 2008, 17, 2790–2802. [Google Scholar] [CrossRef]
- Llorens, J.V.; Navarro, J.A.; Martínez-Sebastián, M.J.; Baylies, M.K.; Schneuwly, S.; Botella, J.A.; Moltó, M.D. Causative role of oxidative stress in a Drosophila model of Friedreich ataxia. FASEB J. 2007, 21, 333–344. [Google Scholar] [CrossRef]
- Soriano, S.; Calap-Quintana, P.; Llorens, J.V.; Al-Ramahi, I.; Gutiérrez, L.; Martínez-Sebastián, M.J.; Botas, J.; Moltó, M.D. Metal Homeostasis Regulators Suppress FRDA Phenotypes in a Drosophila Model of the Disease. PLoS ONE 2016, 11, e0159209. [Google Scholar] [CrossRef]
- Calap-Quintana, P.; Soriano, S.; Llorens, J.V.; Al-Ramahi, I.; Botas, J.; Moltó, M.D.; Martínez-Sebastián, M.J. TORC1 Inhibition by Rapamycin Promotes Antioxidant Defences in a Drosophila Model of Friedreich’s Ataxia. PLoS ONE 2015, 10, e0132376. [Google Scholar] [CrossRef]
- Navarro, J.A.; Ohmann, E.; Sanchez, D.; Botella, J.A.; Liebisch, G.; Moltó, M.D.; Ganfornina, M.D.; Schmitz, G.; Schneuwly, S. Altered lipid metabolism in a Drosophila model of Friedreich’s ataxia. Hum. Mol. Genet. 2010, 19, 2828–2840. [Google Scholar] [CrossRef]
- Rodríguez, L.R.; Calap-Quintana, P.; Lapeña-Luzón, T.; Pallardó, F.V.; Schneuwly, S.; Navarro, J.A.; Gonzalez-Cabo, P. Oxidative stress modulates rearrangement of endoplasmic reticulum-mitochondria contacts and calcium dysregulation in a Friedreich’s ataxia model. Redox Biol. 2020, 37, 101762. [Google Scholar] [CrossRef]
- Shidara, Y.; Hollenbeck, P.J. Defects in mitochondrial axonal transport and membrane potential without increased reactive oxygen species production in a Drosophila model of Friedreich ataxia. J. Neurosci. 2010, 30, 11369–11378. [Google Scholar] [CrossRef]
- Calmels, N.; Schmucker, S.; Wattenhofer-Donzé, M.; Martelli, A.; Vaucamps, N.; Reutenauer, L.; Messaddeq, N.; Bouton, C.; Koenig, M.; Puccio, H. The first cellular models based on frataxin missense mutations that reproduce spontaneously the defects associated with Friedreich ataxia. PLoS ONE 2009, 4, e6379. [Google Scholar] [CrossRef]
- Wagner, G.R.; Pride, P.M.; Babbey, C.M.; Payne, R.M. Friedreich’s ataxia reveals a mechanism for coordinate regulation of oxidative metabolism via feedback inhibition of the SIRT3 deacetylase. Hum. Mol. Genet. 2012, 21, 2688–2697. [Google Scholar] [CrossRef]
- Sanz-Alcázar, A.; Britti, E.; Delaspre, F.; Medina-Carbonero, M.; Pazos-Gil, M.; Tamarit, J.; Ros, J.; Cabiscol, E. Mitochondrial impairment, decreased sirtuin activity and protein acetylation in dorsal root ganglia in Friedreich Ataxia models. Cell. Mol. Life Sci. 2023, 81, 12. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Alcázar, A.; Portillo-Carrasquer, M.; Delaspre, F.; Pazos-Gil, M.; Tamarit, J.; Ros, J.; Cabiscol, E. Deciphering the ferroptosis pathways in dorsal root ganglia of Friedreich ataxia models. The role of LKB1/AMPK, KEAP1, and GSK3β in the impairment of the NRF2 response. Redox Biol. 2024, 76, 103339. [Google Scholar] [CrossRef]
- Whitnall, M.; Suryo Rahmanto, Y.; Huang, M.L.; Saletta, F.; Lok, H.C.; Gutiérrez, L.; Lázaro, F.J.; Fleming, A.J.; St Pierre, T.G.; Mikhael, M.R.; et al. Identification of nonferritin mitochondrial iron deposits in a mouse model of Friedreich ataxia. Proc. Natl. Acad. Sci. USA 2012, 109, 20590–20595. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Schoenfeld, R.A.; Hayashi, G.; Napoli, E.; Akiyama, T.; Iodi Carstens, M.; Carstens, E.E.; Pook, M.A.; Cortopassi, G.A. Frataxin deficiency leads to defects in expression of antioxidants and Nrf2 expression in dorsal root ganglia of the Friedreich’s ataxia YG8R mouse model. Antioxid. Redox Signal. 2013, 19, 1481–1493. [Google Scholar] [CrossRef]
- Sandi, C.; Sandi, M.; Jassal, H.; Ezzatizadeh, V.; Anjomani-Virmouni, S.; Al-Mahdawi, S.; Pook, M.A. Generation and characterisation of Friedreich ataxia YG8R mouse fibroblast and neural stem cell models. PLoS ONE 2014, 9, e89488. [Google Scholar] [CrossRef]
- Abeti, R.; Uzun, E.; Renganathan, I.; Honda, T.; Pook, M.A.; Giunti, P. Targeting lipid peroxidation and mitochondrial imbalance in Friedreich’s ataxia. Pharmacol. Res. 2015, 99, 344–350. [Google Scholar] [CrossRef]
- Abeti, R.; Parkinson, M.H.; Hargreaves, I.P.; Angelova, P.R.; Sandi, C.; Pook, M.A.; Giunti, P.; Abramov, A.Y. Mitochondrial energy imbalance and lipid peroxidation cause cell death in Friedreich’s ataxia. Cell Death Dis. 2016, 7, e2237. [Google Scholar] [CrossRef]
- Codazzi, F.; Hu, A.; Rai, M.; Donatello, S.; Salerno Scarzella, F.; Mangiameli, E.; Pelizzoni, I.; Grohovaz, F.; Pandolfo, M. Friedreich ataxia-induced pluripotent stem cell-derived neurons show a cellular phenotype that is corrected by a benzamide HDAC inhibitor. Hum. Mol. Genet. 2016, 25, 4847–4855. [Google Scholar] [CrossRef]
- Igoillo-Esteve, M.; Gurgul-Convey, E.; Hu, A.; Dos Santos, L.R.B.; Abdulkarim, B.; Chintawar, S.; Marselli, L.; Marchetti, P.; Jonas, J.C.; Eizirik, D.L.; et al. Unveiling a common mechanism of apoptosis in β-cells and neurons in Friedreich’s ataxia. Hum. Mol. Genet. 2015, 24, 2274–2286. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Lorite, J.; Pérez-Luz, S.; Katsu-Jiménez, Y.; Oberdoerfer, D.; Díaz-Nido, J. DNA repair pathways are altered in neural cell models of frataxin deficiency. Mol. Cell. Neurosci. 2021, 111, 103587. [Google Scholar] [CrossRef] [PubMed]
- Hackett, P.T.; Jia, X.; Li, L.; Ward, D.M. Posttranslational regulation of mitochondrial frataxin and identification of compounds that increase frataxin levels in Friedreich’s ataxia. J. Biol. Chem. 2022, 298, 101982. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.A.; Llorens, J.V.; Soriano, S.; Botella, J.A.; Schneuwly, S.; Martínez-Sebastián, M.J.; Moltó, M.D. Overexpression of human and fly frataxins in Drosophila provokes deleterious effects at biochemical, physiological and developmental levels. PLoS ONE 2011, 6, e21017. [Google Scholar] [CrossRef]
- Edenharter, O.; Clement, J.; Schneuwly, S.; Navarro, J.A. Overexpression of Drosophila frataxin triggers cell death in an iron-dependent manner. J. Neurogenet. 2017, 31, 189–202. [Google Scholar] [CrossRef]
- Vannocci, T.; Notario Manzano, R.; Beccalli, O.; Bettegazzi, B.; Grohovaz, F.; Cinque, G.; de Riso, A.; Quaroni, L.; Codazzi, F.; Pastore, A. Adding a temporal dimension to the study of Friedreich’s ataxia: The effect of frataxin overexpression in a human cell model. Dis. Model. Mech. 2018, 11, dmm032706. [Google Scholar] [CrossRef]
- Tan, G.; Chen, L.S.; Lonnerdal, B.; Gellera, C.; Taroni, F.A.; Cortopassi, G.A. Frataxin expression rescues mitochondrial dysfunctions in FRDA cells. Hum. Mol. Genet. 2001, 10, 2099–2107. [Google Scholar] [CrossRef]
- Pathak, D.; Srivastava, A.K.; Padma, M.V.; Gulati, S.; Rajeswari, M.R. Quantitative Proteomic and Network Analysis of Differentially Expressed Proteins in PBMC of Friedreich’s Ataxia (FRDA) Patients. Front. Neurosci. 2019, 13, 1054. [Google Scholar] [CrossRef]
- Télot, L.; Rousseau, E.; Lesuisse, E.; Garcia, C.; Morlet, B.; Léger, T.; Camadro, J.-M.; Serre, V. Quantitative Proteomics in Friedreich’s Ataxia B-Lymphocytes: A Valuable Approach to Decipher the Biochemical Events Responsible for Pathogenesis. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 997–1009. [Google Scholar] [CrossRef]
- Napierala, J.S.; Rajapakshe, K.; Clark, A.; Chen, Y.-Y.; Huang, S.; Mesaros, C.; Xu, P.; Blair, I.A.; Hauser, L.A.; Farmer, J.; et al. Reverse Phase Protein Array Reveals Correlation of Retinoic Acid Metabolism with Cardiomyopathy in Friedreich’s Ataxia. Mol. Cell. Proteomics 2021, 20, 100094. [Google Scholar] [CrossRef]
- Indelicato, E.; Faserl, K.; Amprosi, M.; Nachbauer, W.; Schneider, R.; Wanschitz, J.; Sarg, B.; Boesch, S. Skeletal Muscle Proteome Analysis Underpins Multifaceted Mitochondrial Dysfunction in Friedreich’s Ataxia. Front. Neurosci. 2023, 17, 1289027. [Google Scholar] [CrossRef] [PubMed]
- Imbault, V.; Dionisi, C.; Naeije, G.; Communi, D.; Pandolfo, M. Cerebrospinal Fluid Proteomics in Friedreich Ataxia Reveals Markers of Neurodegeneration and Neuroinflammation. Front. Neurosci. 2022, 16, 885313. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, T.M.; Logsdon, D.L.; Payne, R.M. Metabolomics Analysis Reveals Dysregulation in One Carbon Metabolism in Friedreich Ataxia. Mol. Genet. Metab. 2022, 136, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ho, E.S.; Cotticelli, M.G.; Xu, P.; Napierala, J.S.; Hauser, L.A.; Napierala, M.; Himes, B.E.; Wilson, R.B.; Lynch, D.R.; et al. Skin Fibroblast Metabolomic Profiling Reveals That Lipid Dysfunction Predicts the Severity of Friedreich’s Ataxia. J. Lipid Res. 2022, 63, 100255. [Google Scholar] [CrossRef]
- Sutak, R.; Xu, X.; Whitnall, M.; Kashem, M.A.; Vyoral, D.; Richardson, D.R. Proteomic Analysis of Hearts from Frataxin Knockout Mice: Marked Rearrangement of Energy Metabolism, a Response to Cellular Stress and Altered Expression of Proteins Involved in Cell Structure, Motility and Metabolism. Proteomics 2008, 8, 1731–1741. [Google Scholar] [CrossRef]
- Purroy, R.; Medina-Carbonero, M.; Ros, J.; Tamarit, J. Frataxin-Deficient Cardiomyocytes Present an Altered Thiol-Redox State Which Targets Actin and Pyruvate Dehydrogenase. Redox Biol. 2020, 32, 101520. [Google Scholar] [CrossRef]
- Medina-Carbonero, M.; Sanz-Alcázar, A.; Britti, E.; Delaspre, F.; Cabiscol, E.; Ros, J.; Tamarit, J. Mice Harboring the FXN I151F Pathological Point Mutation Present Decreased Frataxin Levels, a Friedreich Ataxia-like Phenotype, and Mitochondrial Alterations. Cell. Mol. Life Sci. 2022, 79, 74. [Google Scholar] [CrossRef]
- Sciarretta, F.; Zaccaria, F.; Ninni, A.; Ceci, V.; Turchi, R.; Apolloni, S.; Milani, M.; Della Valle, I.; Tiberi, M.; Chiurchiù, V.; et al. Frataxin Deficiency Shifts Metabolism to Promote Reactive Microglia via Glucose Catabolism. Life Sci. Alliance 2024, 7, e202402609. [Google Scholar] [CrossRef]
- Sayles, N.M.; Napierala, J.S.; Anrather, J.; Diedhiou, N.; Li, J.; Napierala, M.; Puccio, H.; Manfredi, G. Comparative Multi-Omic Analyses of Cardiac Mitochondrial Stress in Three Mouse Models of Frataxin Deficiency. Dis. Model. Mech. 2023, 16, dmm050114. [Google Scholar] [CrossRef]
- Rustin, P.; von Kleist-Retzow, J.C.; Chantrel-Groussard, K.; Sidi, D.; Munnich, A.; Rötig, A. Effect of idebenone on cardiomyopathy in Friedreich’s ataxia: A preliminary study. Lancet 1999, 354, 477–479. [Google Scholar] [CrossRef]
- Schöls, L.; Vorgerd, M.; Schillings, M.; Skipka, G.; Zange, J. Idebenone in patients with Friedreich ataxia. Neurosci. Lett. 2001, 306, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Hausse, A.O.; Aggoun, Y.; Bonnet, D.; Sidi, D.; Munnich, A.; Rötig, A.; Rustin, P. Idebenone and reduced cardiac hypertrophy in Friedreich’s ataxia. Heart 2002, 87, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Rustin, P.; Rötig, A.; Munnich, A.; Sidi, D. Heart hypertrophy and function are improved by idebenone in Friedreich’s ataxia. Free Radic. Res. 2002, 36, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Artuch, R.; Aracil, A.; Mas, A.; Colomé, C.; Rissech, M.; Monrós, E.; Pineda, M. Friedreich’s ataxia: Idebenone treatment in early stage patients. Neuropediatrics 2002, 33, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, C.; Solari, A.; Torta, D.; Marano, L.; Fiorentini, C.; Di Donato, S. Idebenone treatment in Friedreich patients: One-year-long randomized placebo-controlled trial. Neurology 2003, 60, 1676–1679. [Google Scholar] [CrossRef]
- Buyse, G.; Mertens, L.; Di Salvo, G.; Matthijs, I.; Weidemann, F.; Eyskens, B.; Goossens, W.; Goemans, N.; Sutherland, G.R.; Van Hove, J.L. Idebenone treatment in Friedreich’s ataxia: Neurological, cardiac, and biochemical monitoring. Neurology 2003, 60, 1679–1681. [Google Scholar] [CrossRef]
- Ribaï, P.; Pousset, F.; Tanguy, M.L.; Rivaud-Pechoux, S.; Le Ber, I.; Gasparini, F.; Charles, P.; Béraud, A.S.; Schmitt, M.; Koenig, M.; et al. Neurological, cardiological, and oculomotor progression in 104 patients with Friedreich ataxia during long-term follow-up. Arch. Neurol. 2007, 64, 558–564. [Google Scholar] [CrossRef]
- Di Prospero, N.A.; Baker, A.; Jeffries, N.; Fischbeck, K.H. Neurological effects of high-dose idebenone in patients with Friedreich’s ataxia: A randomised, placebo-controlled trial. Lancet Neurol. 2007, 6, 878–886. [Google Scholar] [CrossRef]
- Pineda, M.; Arpa, J.; Montero, R.; Aracil, A.; Domínguez, F.; Galván, M.; Mas, A.; Martorell, L.; Sierra, C.; Brandi, N.; et al. Idebenone treatment in paediatric and adult patients with Friedreich ataxia: Long-term follow-up. Eur. J. Paediatr. Neurol. 2008, 12, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, C.; Tucci, T.; Maione, S.; Giunta, A.; De Michele, G.; Filla, A. Low-dose idebenone treatment in Friedreich’s ataxia with and without cardiac hypertrophy. J. Neurol. 2009, 256, 1434–1437. [Google Scholar] [CrossRef] [PubMed]
- Brandsema, J.F.; Stephens, D.; Hartley, J.; Yoon, G. Intermediate-dose idebenone and quality of life in Friedreich ataxia. Pediatr. Neurol. 2010, 42, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.R.; Perlman, S.L.; Meier, T. A phase 3, double-blind, placebo-controlled trial of idebenone in Friedreich ataxia. Arch. Neurol. 2010, 67, 941–947. [Google Scholar] [CrossRef]
- Lagedrost, S.J.; Sutton, M.S.; Cohen, M.S.; Satou, G.M.; Kaufman, B.D.; Perlman, S.L.; Rummey, C.; Meier, T.; Lynch, D.R. Idebenone in Friedreich ataxia cardiomyopathy—Results from a 6-month phase III study (IONIA). Am. Heart J. 2011, 161, 639–645.e1. [Google Scholar] [CrossRef]
- Meier, T.; Perlman, S.L.; Rummey, C.; Coppard, N.J.; Lynch, D.R. Assessment of neurological efficacy of idebenone in pediatric patients with Friedreich’s ataxia: Data from a 6-month controlled study followed by a 12-month open-label extension study. J. Neurol. 2012, 259, 284–291. [Google Scholar] [CrossRef]
- Cook, A.; Boesch, S.; Heck, S.; Brunt, E.; Klockgether, T.; Schöls, L.; Schulz, A.; Giunti, P. Patient-reported outcomes in Friedreich’s ataxia after withdrawal from idebenone. Acta Neurol. Scand. 2019, 139, 533–539. [Google Scholar] [CrossRef]
- Velasco-Sánchez, D.; Aracil, A.; Montero, R.; Mas, A.; Jiménez, L.; O’Callaghan, M.; Tondo, M.; Capdevila, A.; Blanch, J.; Artuch, R.; et al. Combined therapy with idebenone and deferiprone in patients with Friedreich’s ataxia. Cerebellum 2011, 10, 1–8. [Google Scholar] [CrossRef]
- Elincx-Benizri, S.; Glik, A.; Merkel, D.; Arad, M.; Freimark, D.; Kozlova, E.; Cabantchik, I.; Hassin-Baer, S. Clinical Experience With Deferiprone Treatment for Friedreich Ataxia. J. Child Neurol. 2016, 31, 1036–1040. [Google Scholar] [CrossRef]
- Arpa, J.; Sanz-Gallego, I.; Rodríguez-de-Rivera, F.J.; Domínguez-Melcón, F.J.; Prefasi, D.; Oliva-Navarro, J.; Moreno-Yangüela, M. Triple therapy with deferiprone, idebenone and riboflavin in Friedreich’s ataxia—Open-label trial. Acta Neurol. Scand. 2014, 129, 32–40. [Google Scholar] [CrossRef]
- Hart, P.E.; Lodi, R.; Rajagopalan, B.; Bradley, J.L.; Crilley, J.G.; Turner, C.; Blamire, A.M.; Manners, D.; Styles, P.; Schapira, A.H.; et al. Antioxidant treatment of patients with Friedreich ataxia: Four-year follow-up. Arch. Neurol. 2005, 62, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.R.; Willi, S.M.; Wilson, R.B.; Cotticelli, M.G.; Brigatti, K.W.; Deutsch, E.C.; Kucheruk, O.; Shrader, W.; Rioux, P.; Miller, G.; et al. A0001 in Friedreich ataxia: Biochemical characterization and effects in a clinical trial. Mov. Disord. 2012, 27, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Zesiewicz, T.; Salemi, J.L.; Perlman, S.; Sullivan, K.L.; Shaw, J.D.; Huang, Y.; Isaacs, C.; Gooch, C.; Lynch, D.R.; Klein, M.B. Double-blind, randomized and controlled trial of EPI-743 in Friedreich’s ataxia. Neurodegener. Dis. Manag. 2018, 8, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Boesch, S.; Sturm, B.; Hering, S.; Scheiber-Mojdehkar, B.; Steinkellner, H.; Goldenberg, H.; Poewe, W. Neurological effects of recombinant human erythropoietin in Friedreich’s ataxia: A clinical pilot trial. Mov. Disord. 2008, 23, 1940–1944. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, C.; Fancellu, R.; Caldarazzo, S.; Nanetti, L.; Di Bella, D.; Plumari, M.; Lauria, G.; Cappellini, M.D.; Duca, L.; Solari, A.; et al. Erythropoietin in Friedreich ataxia: No effect on frataxin in a randomized controlled trial. Mov. Disord. 2012, 27, 446–449. [Google Scholar] [CrossRef]
- Boesch, S.; Nachbauer, W.; Mariotti, C.; Sacca, F.; Filla, A.; Klockgether, T.; Klopstock, T.; Schöls, L.; Jacobi, H.; Büchner, B.; et al. Safety and tolerability of carbamylated erythropoietin in Friedreich’s ataxia. Mov. Disord. 2014, 29, 935–939. [Google Scholar] [CrossRef]
- Yiu, E.M.; Tai, G.; Peverill, R.E.; Lee, K.J.; Croft, K.D.; Mori, T.A.; Scheiber-Mojdehkar, B.; Sturm, B.; Praschberger, M.; Vogel, A.P.; et al. An open-label trial in Friedreich ataxia suggests clinical benefit with high-dose resveratrol, without effect on frataxin levels. J. Neurol. 2015, 262, 1344–1353. [Google Scholar] [CrossRef]
- Lynch, D.R.; Chin, M.P.; Delatycki, M.B.; Subramony, S.H.; Corti, M.; Hoyle, J.C.; Boesch, S.; Nachbauer, W.; Mariotti, C.; Mathews, K.D.; et al. Safety and Efficacy of Omaveloxolone in Friedreich Ataxia (MOXIe Study). Ann. Neurol. 2021, 89, 212–225. [Google Scholar] [CrossRef]
- Qureshi, M.Y.; Patterson, M.C.; Clark, V.; Johnson, J.N.; Moutvic, M.A.; Driscoll, S.W.; Kemppainen, J.L.; Huston, J., III; Anderson, J.R.; Badley, A.D.; et al. Safety and efficacy of (+)-epicatechin in subjects with Friedreich’s ataxia: A phase II, open-label, prospective study. J. Inherit. Metab. Dis. 2021, 44, 502–514. [Google Scholar] [CrossRef]
- Kemp, K.C.; Georgievskaya, A.; Hares, K.; Redondo, J.; Bailey, S.; Rice, C.M.; Scolding, N.J.; Metcalfe, C.; Wilkins, A. An open-label pilot study of recombinant granulocyte-colony stimulating factor in Friedreich’s ataxia. Nat. Commun. 2022, 13, 4655. [Google Scholar] [CrossRef]
- Di Prospero, N.A.; Sumner, C.J.; Penzak, S.R.; Ravina, B.; Fischbeck, K.H.; Taylor, J.P. Safety, tolerability, and pharmacokinetics of high-dose idebenone in patients with Friedreich ataxia. Arch. Neurol. 2007, 64, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Fuentes, A.J.; Cesar, S.; Montero, R.; Latre, C.; Genovès, J.; Martorell, L.; Cuadras, D.; Colom, H.; Pineda, M.; O’Callaghan, M. del M.; et al. Plasma idebenone monitoring in Friedreich’s ataxia patients during a long-term follow-up. Biomed. Pharmacother. 2021, 143, 112143. [Google Scholar] [CrossRef]
- Tonon, C.; Lodi, R. Idebenone in Friedreich’s ataxia. Expert Opin. Pharmacother. 2008, 9, 2327–2337. [Google Scholar] [CrossRef] [PubMed]
- Kearney, M.; Orrell, R.W.; Fahey, M.; Pandolfo, M. Antioxidants and other pharmacological treatments for Friedreich ataxia. Cochrane Database Syst. Rev 2009, CD007791, Update in: Cochrane Database Syst. Rev. 2012, CD007791. [Google Scholar] [CrossRef]
- Jauslin, M.L.; Wirth, T.; Meier, T.; Schoumacher, F. A cellular model for Friedreich Ataxia reveals small-molecule glutathione peroxidase mimetics as novel treatment strategy. Hum. Mol. Genet. 2002, 11, 3055–3063. [Google Scholar] [CrossRef] [PubMed]
- Jauslin, M.L.; Meier, T.; Smith, R.A.; Murphy, M.P. Mitochondria-targeted antioxidants protect Friedreich Ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J. 2003, 17, 1972–1974. [Google Scholar] [CrossRef]
- Jauslin, M.L.; Vertuani, S.; Durini, E.; Buzzoni, L.; Ciliberti, N.; Verdecchia, S.; Palozza, P.; Meier, T.; Manfredini, S. Protective effects of Fe-Aox29, a novel antioxidant derived from a molecular combination of Idebenone and vitamin E, in immortalized fibroblasts and fibroblasts from patients with Friedreich Ataxia. Mol. Cell. Biochem. 2007, 30, 79–85. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lau, Y.M.; Ng, K.M.; Lai, W.H.; Ho, S.L.; Tse, H.F.; Siu, C.W.; Ho, P.W. Efficient attenuation of Friedreich’s ataxia (FRDA) cardiomyopathy by modulation of iron homeostasis-human induced pluripotent stem cell (hiPSC) as a drug screening platform for FRDA. Int. J. Cardiol. 2016, 203, 964–971. [Google Scholar] [CrossRef]
- Seznec, H.; Simon, D.; Monassier, L.; Criqui-Filipe, P.; Gansmuller, A.; Rustin, P.; Koenig, M.; Puccio, H. Idebenone delays the onset of cardiac functional alteration without correction of Fe-S enzymes deficit in a mouse model for Friedreich ataxia. Hum. Mol. Genet. 2004, 13, 1017–1024. [Google Scholar] [CrossRef]
- Cotticelli, M.G.; Crabbe, A.M.; Wilson, R.B.; Shchepinov, M.S. Insights into the role of oxidative stress in the pathology of Friedreich ataxia using peroxidation resistant polyunsaturated fatty acids. Redox Biol. 2013, 1, 398–404. [Google Scholar] [CrossRef]
- Soriano, S.; Llorens, J.V.; Blanco-Sobero, L.; Gutiérrez, L.; Calap-Quintana, P.; Morales, M.P.; Moltó, M.D.; Martínez-Sebastián, M.J. Deferiprone and idebenone rescue frataxin depletion phenotypes in a Drosophila model of Friedreich’s ataxia. Gene 2013, 521, 274–281. [Google Scholar] [CrossRef]
- Abruzzo, P.M.; Marini, M.; Bolotta, A.; Malisardi, G.; Manfredini, S.; Ghezzo, A.; Pini, A.; Tasco, G.; Casadio, R. Frataxin mRNA isoforms in FRDA patients and normal subjects: Effect of tocotrienol supplementation. Biomed Res. Int. 2013, 2013, 276808. [Google Scholar] [CrossRef]
- Marobbio, C.M.; Pisano, I.; Porcelli, V.; Lasorsa, F.M.; Palmieri, L. Rapamycin reduces oxidative stress in frataxin-deficient yeast cells. Mitochondrion 2012, 12, 156–161. [Google Scholar] [CrossRef]
- Li, L.; Voullaire, L.; Sandi, C.; Pook, M.A.; Ioannou, P.A.; Delatycki, M.B.; Sarsero, J.P. Pharmacological screening using an FXN-EGFP cellular genomic reporter assay for the therapy of Friedreich ataxia. PLoS ONE 2013, 8, e55940. [Google Scholar] [CrossRef]
- Georges, P.; Boza-Moran, M.G.; Gide, J.; Pêche, G.A.; Forêt, B.; Bayot, A.; Rustin, P.; Peschanski, M.; Martinat, C.; Aubry, L. Induced pluripotent stem cells-derived neurons from patients with Friedreich ataxia exhibit differential sensitivity to resveratrol and nicotinamide. Sci. Rep. 2019, 9, 14568. [Google Scholar] [CrossRef] [PubMed]
- Abeti, R.; Baccaro, A.; Esteras, N.; Giunti, P. Novel Nrf2-Inducer Prevents Mitochondrial Defects and Oxidative Stress in Friedreich’s Ataxia Models. Front. Cell. Neurosci. 2018, 12, 188. [Google Scholar] [CrossRef] [PubMed]
- Salinas, L.; Figueroa, F.; Montgomery, C.B.; Thai, P.N.; Chiamvimonvat, N.; Cortopassi, G.; Dedkova, E.N. Omaveloxolone, But Not Dimethyl Fumarate, Improves Cardiac Function in Friedreich’s Ataxia Mice With Severe Cardiomyopathy. J. Am. Heart Assoc. 2025, 14, e038505. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.E.; Yang, S.H.; Wen, Y.; Simpkins, J.W. Estrogen protection in Friedreich’s ataxia skin fibroblasts. Endocrinology 2011, 152, 2742–2749. [Google Scholar] [CrossRef][Green Version]
- Richardson, T.E.; Yu, A.E.; Wen, Y.; Yang, S.H.; Simpkins, J.W. Estrogen prevents oxidative damage to the mitochondria in Friedreich’s ataxia skin fibroblasts. PLoS ONE 2012, 7, e34600. [Google Scholar] [CrossRef]
- Richardson, T.E.; Simpkins, J.W. R- and S-equol have equivalent cytoprotective effects in Friedreich’s ataxia. BMC Pharmacol. Toxicol. 2012, 13, 12. [Google Scholar] [CrossRef]
- Rodríguez-Pascau, L.; Britti, E.; Calap-Quintana, P.; Dong, Y.N.; Vergara, C.; Delaspre, F.; Medina-Carbonero, M.; Tamarit, J.; Pallardó, F.V.; Gonzalez-Cabo, P.; et al. PPAR gamma agonist leriglitazone improves frataxin-loss impairments in cellular and animal models of Friedreich Ataxia. Neurobiol. Dis. 2021, 148, 105162. [Google Scholar] [CrossRef]
- Russi, M.; Martin, E.; D’Autréaux, B.; Tixier, L.; Tricoire, H.; Monnier, V. A Drosophila model of Friedreich ataxia with CRISPR/Cas9 insertion of GAA repeats in the frataxin gene reveals in vivo protection by N-acetyl cysteine. Hum. Mol. Genet. 2020, 29, 2831–2844. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.; Huang, M.L.H.; Richardson, D.R. Treatment of dilated cardiomyopathy in a mouse model of Friedreich’s ataxia using N-acetylcysteine and identification of alterations in microRNA expression that could be involved in its pathogenesis. Pharmacol. Res. 2020, 159, 104994. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Estirado, A.; Redondo, C.; Bueno, C.; Martínez, S. Human adipose stem cell-conditioned medium increases survival of Friedreich’s ataxia cells submitted to oxidative stress. Stem Cells Dev. 2012, 21, 2817–2826. [Google Scholar] [CrossRef] [PubMed]
- Kemp, K.; Dey, R.; Cook, A.; Scolding, N.; Wilkins, A. Mesenchymal Stem Cell-Derived Factors Restore Function to Human Frataxin-Deficient Cells. Cerebellum 2017, 16, 840–851. [Google Scholar] [CrossRef]
- Jones, J.; Estirado, A.; Redondo, C.; Martinez, S. Stem cells from wildtype and Friedreich’s ataxia mice present similar neuroprotective properties in dorsal root ganglia cells. PLoS ONE 2013, 8, e62807. [Google Scholar] [CrossRef]
- Tricoire, H.; Palandri, A.; Bourdais, A.; Camadro, J.M.; Monnier, V. Methylene blue rescues heart defects in a Drosophila model of Friedreich’s ataxia. Hum. Mol. Genet. 2014, 23, 968–979. [Google Scholar] [CrossRef]
- Khdour, O.M.; Bandyopadhyay, I.; Chowdhury, S.R.; Visavadiya, N.P.; Hecht, S.M. Lipophilic methylene blue analogues enhance mitochondrial function and increase frataxin levels in a cellular model of Friedreich’s ataxia. Bioorg. Med. Chem. 2018, 26, 3359–3369. [Google Scholar] [CrossRef]
- Calatrava-Ferreras, L.; Gonzalo-Gobernado, R.; Reimers, D.; Herranz, A.S.; Casarejos, M.J.; Jiménez-Escrig, A.; Regadera, J.; Velasco-Martín, J.; Vallejo-Muñoz, M.; Díaz-Gil, J.J.; et al. Liver Growth Factor (LGF) Upregulates Frataxin Protein Expression and Reduces Oxidative Stress in Friedreich’s Ataxia Transgenic Mice. Int. J. Mol. Sci. 2016, 17, 2066. [Google Scholar] [CrossRef]
- Mollá, B.; Muñoz-Lasso, D.C.; Calap, P.; Fernandez-Vilata, A.; de la Iglesia-Vaya, M.; Pallardó, F.V.; Moltó, M.D.; Palau, F.; Gonzalez-Cabo, P. Phosphodiesterase Inhibitors Revert Axonal Dystrophy in Friedreich’s Ataxia Mouse Model. Neurotherapeutics 2019, 16, 432–449. [Google Scholar] [CrossRef]
- Villa, C.; Legato, M.; Umbach, A.; Riganti, C.; Jones, R.; Martini, B.; Boido, M.; Medana, C.; Facchinetti, I.; Barni, D.; et al. Treatment with ROS detoxifying gold quantum clusters alleviates the functional decline in a mouse model of Friedreich ataxia. Sci. Transl. Med. 2021, 13, eabe1633. [Google Scholar] [CrossRef]
- Xu, L.; Sun, Z.; Xing, Z.; Liu, Y.; Zhao, H.; Tang, Z.; Luo, Y.; Hao, S.; Li, K. Cur@SF NPs alleviate Friedreich’s ataxia in a mouse model through synergistic iron chelation and antioxidation. J. Nanobiotechnol. 2022, 20, 118. [Google Scholar] [CrossRef]
- Yang, W.; Thompson, B.; Miellet, S.; Maddock, M.; Napierala, M.; Dottori, M.; Kwa, F. Sulforaphane Targets Multiple Pathological Processes in Friedreich Ataxia Patient-Induced Pluripotent Stem Cell-Derived Sensory Neurons. Antioxid. Redox Signal. 2025, 43, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Lew, S.Y.; Mohd Hisam, N.S.; Phang, M.W.L.; Syed Abdul Rahman, S.N.; Poh, R.Y.Y.; Lim, S.H.; Kamaruzzaman, M.A.; Chau, S.C.; Tsui, K.C.; Lim, L.W.; et al. Adenosine Improves Mitochondrial Function and Biogenesis in Friedreich’s Ataxia Fibroblasts Following L-Buthionine Sulfoximine-Induced Oxidative Stress. Biology 2023, 12, 559. [Google Scholar] [CrossRef] [PubMed]
- Luffarelli, R.; Panarello, L.; Quatrana, A.; Tiano, F.; Fortuni, S.; Rufini, A.; Malisan, F.; Testi, R.; Condò, I. Interferon Gamma Enhances Cytoprotective Pathways via Nrf2 and MnSOD Induction in Friedreich’s Ataxia Cells. Int. J. Mol. Sci. 2023, 24, 12687. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, H.; Hao, S.; Chen, J.; Wu, J.; Song, C.; Zhang, M.; Qiao, T.; Li, K. Peptide SS-31 Upregulates Frataxin Expression and Improves the Quality of Mitochondria: Implications in the Treatment of Friedreich Ataxia. Sci. Rep. 2017, 7, 9840. [Google Scholar] [CrossRef]
- Igoillo-Esteve, M.; Oliveira, A.F.; Cosentino, C.; Fantuzzi, F.; Demarez, C.; Toivonen, S.; Hu, A.; Chintawar, S.; Lopes, M.; Pachera, N.; et al. Exenatide Induces Frataxin Expression and Improves Mitochondrial Function in Friedreich Ataxia. JCI Insight 2020, 5, e134221. [Google Scholar] [CrossRef]
- Edzeamey, F.J.; Ramchunder, Z.; Valle Gómez, A.; Ge, H.; Marobbio, C.M.T.; Pourzand, C.; Virmouni, S.A. Therapeutic Combination of L-Ascorbic Acid, N-Acetylcysteine, and Dimethyl Fumarate in Friedreich’s Ataxia: Insights from In Vitro Models. Redox Rep. 2025, 30, 2505303. [Google Scholar] [CrossRef]
- Fleming, J.; Spinoulas, A.; Zheng, M.; Cunningham, S.C.; Ginn, S.L.; McQuilty, R.C.; Rowe, P.B.; Alexander, I.E. Partial Correction of Sensitivity to Oxidant Stress in Friedreich Ataxia Patient Fibroblasts by Frataxin-Encoding Adeno-Associated Virus and Lentivirus Vectors. Hum. Gene Ther. 2005, 16, 947–956. [Google Scholar] [CrossRef]
- Brubaker, D.K.; Lauffenburger, D.A. Translating Preclinical Models to Humans. Science 2020, 367, 742–743. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Why Animal Model Studies Are Lost in Translation. J. Cardiovasc. Aging 2022, 2, 22. [Google Scholar] [CrossRef]
- Frühwein, H.; Paul, N.W. “Lost in Translation?” Animal Research in the Era of Precision Medicine. J. Transl. Med. 2025, 23, 152. [Google Scholar] [CrossRef]
- Trouillas, P.; Takayanagi, T.; Hallett, M.; Currier, R.D.; Subramony, S.H.; Wessel, K.; Bryer, A.; Diener, H.C.; Massaquoi, S.; Gomez, C.M.; et al. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. International Cooperative Ataxia Rating Scale for Pharmacological Assessment of the Cerebellar Syndrome. J. Neurol. Sci. 1997, 145, 205–211. [Google Scholar] [CrossRef]
- Schmitz-Hübsch, T.; du Montcel, S.T.; Baliko, L.; Berciano, J.; Boesch, S.; Depondt, C.; Giunti, P.; Globas, C.; Infante, J.; Kang, J.S.; et al. Scale for the Assessment and Rating of Ataxia: Development of a New Clinical Scale. Neurology 2006, 66, 1717–1720. [Google Scholar] [CrossRef]
- Lynch, D.R.; Farmer, J.M.; Tsou, A.Y.; Perlman, S.; Subramony, S.H.; Gomez, C.M.; Ashizawa, T.; Wilmot, G.R.; Wilson, R.B.; Balcer, L.J. Measuring Friedreich Ataxia: Complementary Features of Examination and Performance Measures. Neurology 2006, 66, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Tai, G.; Corben, L.A.; Gurrin, L.; Yiu, E.M.; Churchyard, A.; Fahey, M.; Hoare, B.; Downie, S.; Delatycki, M.B. A Study of up to 12 Years of Follow-Up of Friedreich Ataxia Utilising Four Measurement Tools. J. Neurol. Neurosurg. Psychiatry 2015, 86, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Burk, K.; Schulz, S.R.; Schulz, J.B. Monitoring Progression in Friedreich Ataxia (FRDA): The Use of Clinical Scales. J. Neurochem. 2013, 126 (Suppl. S1), 118–124. [Google Scholar] [CrossRef] [PubMed]
- Rummey, C.; Bushara, K.; Ravina, B.; Periman, S. Psychometric Properties of the Friedreich Ataxia Rating Scale. Neurol. Genet. 2019, 5, e371. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).