Inter-Organellar Ca2+ Homeostasis in Plant and Animal Systems
Abstract
1. Introduction
2. Key Players in Intracellular Ca2+ Regulation in Animal Cells
2.1. Inositol Trisphosphate Receptors
2.2. Ryanodine Receptors
2.3. Two-Pore Channels
2.4. Stromal Interaction Molecules, Orai Proteins, and Store-Operated Calcium Entry
2.5. Mitochondrial Calcium Uniporters
2.6. Transient Receptor Potential Channels
2.7. P2X Receptors
2.8. Sarco/Endoplasmic Reticulum Ca2+-ATPase
2.9. Plasma Membrane Calcium ATPase
2.10. Sodium–Calcium Exchanger
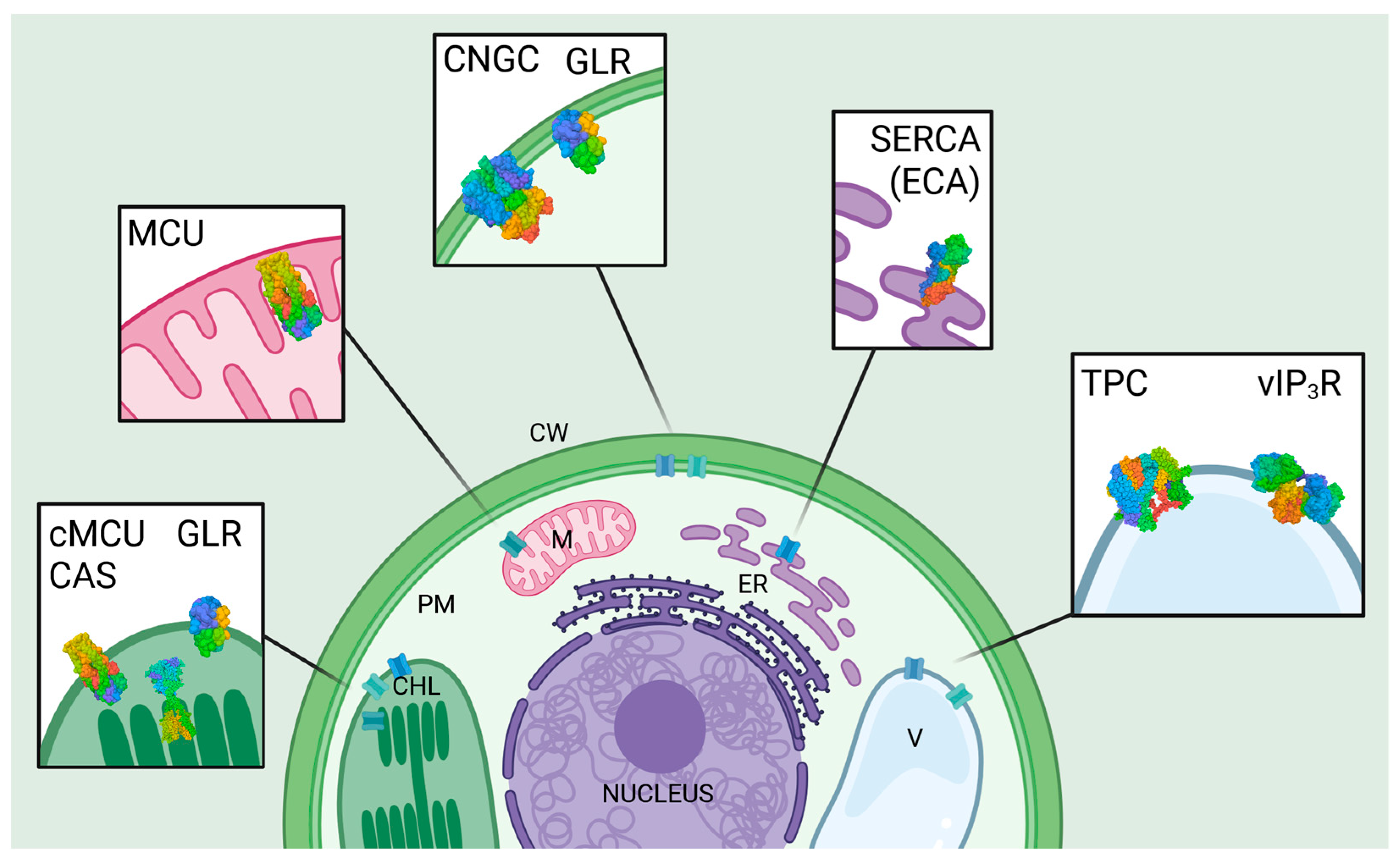
3. Key Players in Intracellular Ca2+ Regulation in Plant Cells
3.1. Vacuolar Inositol Trisphosphate Receptor
3.2. Cyclic Nucleotide-Gated Channels
3.3. Two-Pore Channels
3.4. Mitochondrial Calcium Uniporters
3.5. SERCA-like Transporters/ER-Type Ca2+ ATPases
3.6. Glutamate Receptor-like Channels
3.7. Chloroplast
4. Similarities of Inter-Organellar Ca2+ Homeostasis in Plants and Animals
5. Differences in Inter-Organellar Ca2+ Homeostasis in Plants and Animals
6. Inter-Organellar Ca2+ Regulation in Animal and Plant Cells
7. Excursus: Intracellular Ca2+ Regulation in Bacteria
8. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Islam, M.S. Calcium Signaling: From Basic to Bedside. Adv. Exp. Med. Biol. 2020, 1131, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ding, P. Calcium signaling in plant immunity: A spatiotemporally controlled symphony. Trends Plant Sci. 2023, 28, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Tang, Y.; Law, B.Y.K. Targeting calcium signaling in Alzheimer’s disease: Challenges and promising therapeutic avenues. Neural Regen. Res. 2024, 19, 501–502. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Burgoyne, T.; Patel, S.; Eden, E.R. Calcium signaling at ER membrane contact sites. Biochim. Biophys. Acta 2015, 1853, 2012–2017. [Google Scholar] [CrossRef]
- Villalobo, A.; Gonzalez-Munoz, M.; Berchtold, M.W. Proteins with calmodulin-like domains: Structures and functional roles. Cell. Mol. Life Sci. 2019, 76, 2299–2328. [Google Scholar] [CrossRef]
- Perochon, A.; Aldon, D.; Galaud, J.P.; Ranty, B. Calmodulin and calmodulin-like proteins in plant calcium signaling. Biochimie 2011, 93, 2048–2053. [Google Scholar] [CrossRef] [PubMed]
- Astegno, A.; Bonza, M.C.; Vallone, R.; La Verde, V.; D’Onofrio, M.; Luoni, L.; Molesini, B.; Dominici, P. Arabidopsis calmodulin-like protein CML36 is a calcium (Ca2+) sensor that interacts with the plasma membrane Ca2+-ATPase isoform ACA8 and stimulates its activity. J. Biol. Chem. 2017, 292, 15049–15061. [Google Scholar] [CrossRef]
- La Verde, V.; Dominici, P.; Astegno, A. Towards Understanding Plant Calcium Signaling through Calmodulin-Like Proteins: A Biochemical and Structural Perspective. Int. J. Mol. Sci. 2018, 19, 1331. [Google Scholar] [CrossRef]
- Yadav, M.; Pandey, J.; Chakraborty, A.; Hassan, M.I.; Kundu, J.K.; Roy, A.; Singh, I.K.; Singh, A. A Comprehensive Analysis of Calmodulin-Like Proteins of Glycine max Indicates Their Role in Calcium Signaling and Plant Defense Against Insect Attack. Front. Plant Sci. 2022, 13, 817950. [Google Scholar] [CrossRef] [PubMed]
- Heyer, M.; Scholz, S.S.; Reichelt, M.; Kunert, G.; Oelmuller, R.; Mithofer, A. The Ca2+ sensor proteins CML37 and CML42 antagonistically regulate plant stress responses by altering phytohormone signals. Plant Mol. Biol. 2022, 109, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Ogunrinde, A.; Munro, K.; Davidson, A.; Ubaid, M.; Snedden, W.A. Arabidopsis Calmodulin-Like Proteins, CML15 and CML16 Possess Biochemical Properties Distinct from Calmodulin and Show Non-overlapping Tissue Expression Patterns. Front. Plant Sci. 2017, 8, 2175. [Google Scholar] [CrossRef] [PubMed]
- Sevrieva, I.R.; Kampourakis, T.; Irving, M. Structural changes in troponin during activation of skeletal and heart muscle determined in situ by polarised fluorescence. Biophys. Rev. 2024, 16, 753–772. [Google Scholar] [CrossRef]
- Boudsocq, M.; Droillard, M.J.; Regad, L.; Lauriere, C. Characterization of Arabidopsis calcium-dependent protein kinases: Activated or not by calcium? Biochem. J. 2012, 447, 291–299. [Google Scholar] [CrossRef]
- Dekomah, S.D.; Bi, Z.; Dormatey, R.; Wang, Y.; Haider, F.U.; Sun, C.; Yao, P.; Bai, J. The role of CDPKs in plant development, nutrient and stress signaling. Front. Genet. 2022, 13, 996203. [Google Scholar] [CrossRef]
- Schulz, P.; Herde, M.; Romeis, T. Calcium-dependent protein kinases: Hubs in plant stress signaling and development. Plant Physiol. 2013, 163, 523–530. [Google Scholar] [CrossRef]
- An, L.; Fang, H.; Zhang, X.; Tang, J.; Gong, J.; Yi, Y.; Tang, M. Genome-Wide Identification and Characterization of the CDPK Family of Genes and Their Response to High-Calcium Stress in Yinshania henryi. Genes 2025, 16, 109. [Google Scholar] [CrossRef]
- Moccia, F.; Fiorio Pla, A.; Lim, D.; Lodola, F.; Gerbino, A. Intracellular Ca2+ signalling: Unexpected new roles for the usual suspect. Front. Physiol. 2023, 14, 1210085. [Google Scholar] [CrossRef]
- Pirayesh, N.; Giridhar, M.; Ben Khedher, A.; Vothknecht, U.C.; Chigri, F. Organellar calcium signaling in plants: An update. Biochim. Biophys. Acta 2021, 1868, 118948. [Google Scholar] [CrossRef]
- Hou, Y.; Bai, J.; Shen, X.; de Langen, O.; Li, A.; Lal, S.; Dos Remedios, C.G.; Baddeley, D.; Ruygrok, P.N.; Soeller, C.; et al. Nanoscale Organisation of Ryanodine Receptors and Junctophilin-2 in the Failing Human Heart. Front. Physiol. 2021, 12, 724372. [Google Scholar] [CrossRef]
- Maltsev, A.V.; Ventura Subirachs, V.; Monfredi, O.; Juhaszova, M.; Ajay Warrier, P.; Rakshit, S.; Tagirova, S.; Maltsev, A.V.; Stern, M.D.; Lakatta, E.G.; et al. Structure-Function Relationship of the Ryanodine Receptor Cluster Network in Sinoatrial Node Cells. Cells 2024, 13, 1885. [Google Scholar] [CrossRef]
- Schmiege, P.; Fine, M.; Li, X. The regulatory mechanism of mammalian TRPMLs revealed by cryo-EM. FEBS J. 2018, 285, 2579–2585. [Google Scholar] [CrossRef]
- Loncke, J.; Kaasik, A.; Bezprozvanny, I.; Parys, J.B.; Kerkhofs, M.; Bultynck, G. Balancing ER-Mitochondrial Ca2+ Fluxes in Health and Disease. Trends Cell Biol. 2021, 31, 598–612. [Google Scholar] [CrossRef]
- Kovacs, G.; Reimer, L.; Jensen, P.H. Endoplasmic Reticulum-Based Calcium Dysfunctions in Synucleinopathies. Front. Neurol. 2021, 12, 742625. [Google Scholar] [CrossRef]
- Garcia Bossi, J.; Kumar, K.; Barberini, M.L.; Dominguez, G.D.; Rondon Guerrero, Y.D.C.; Marino-Buslje, C.; Obertello, M.; Muschietti, J.P.; Estevez, J.M. The role of P-type IIA and P-type IIB Ca2+-ATPases in plant development and growth. J. Exp. Bot. 2020, 71, 1239–1248. [Google Scholar] [CrossRef]
- Furuichi, T.; Cunningham, K.W.; Muto, S. A putative two pore channel AtTPC1 mediates Ca2+ flux in Arabidopsis leaf cells. Plant Cell Physiol. 2001, 42, 900–905. [Google Scholar] [CrossRef]
- Hedrich, R.; Muller, T.D.; Marten, I.; Becker, D. TPC1 vacuole SV channel gains further shape—Voltage priming of calcium-dependent gating. Trends Plant Sci. 2023, 28, 673–684. [Google Scholar] [CrossRef]
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential. Pharmacol. Rev. 2015, 67, 821–870. [Google Scholar] [CrossRef]
- Simms, B.A.; Zamponi, G.W. Neuronal voltage-gated calcium channels: Structure, function, and dysfunction. Neuron 2014, 82, 24–45. [Google Scholar] [CrossRef]
- Dolphin, A.C. Voltage-gated calcium channels and their auxiliary subunits: Physiology and pathophysiology and pharmacology. J. Physiol. 2016, 594, 5369–5390. [Google Scholar] [CrossRef]
- Hasan, P.; Berezhnaya, E.; Rodriguez-Prados, M.; Weaver, D.; Bekeova, C.; Cartes-Saavedra, B.; Birch, E.; Beyer, A.M.; Santos, J.H.; Seifert, E.L.; et al. MICU1 and MICU2 control mitochondrial calcium signaling in the mammalian heart. Proc. Natl. Acad. Sci. USA 2024, 121, e2402491121. [Google Scholar] [CrossRef]
- Ruberti, C.; Feitosa-Araujo, E.; Xu, Z.; Wagner, S.; Grenzi, M.; Darwish, E.; Lichtenauer, S.; Fuchs, P.; Parmagnani, A.S.; Balcerowicz, D.; et al. MCU proteins dominate in vivo mitochondrial Ca2+ uptake in Arabidopsis roots. Plant Cell 2022, 34, 4428–4452. [Google Scholar] [CrossRef]
- Poggio, E.; Vallese, F.; Hartel, A.J.W.; Morgenstern, T.J.; Kanner, S.A.; Rauh, O.; Giamogante, F.; Barazzuol, L.; Shepard, K.L.; Colecraft, H.M.; et al. Perturbation of the host cell Ca2+ homeostasis and ER-mitochondria contact sites by the SARS-CoV-2 structural proteins E and M. Cell Death Dis. 2023, 14, 297. [Google Scholar] [CrossRef]
- Sassano, M.L.; Felipe-Abrio, B.; Agostinis, P. ER-mitochondria contact sites; a multifaceted factory for Ca2+ signaling and lipid transport. Front. Cell Dev. Biol. 2022, 10, 988014. [Google Scholar] [CrossRef]
- Patel, S.; Brailoiu, E. Triggering of Ca2+ signals by NAADP-gated two-pore channels: A role for membrane contact sites? Biochem. Soc. Trans. 2012, 40, 153–157. [Google Scholar] [CrossRef]
- Kilpatrick, B.S.; Eden, E.R.; Hockey, L.N.; Yates, E.; Futter, C.E.; Patel, S. An endosomal NAADP-sensitive two-pore Ca2+ channel regulates ER-endosome membrane contact sites to control growth factor signaling. Cell Rep. 2017, 18, 1636–1645. [Google Scholar] [CrossRef]
- Arlt, E.; Fraticelli, M.; Tsvilovskyy, V.; Nadolni, W.; Breit, A.; O’Neill, T.J.; Resenberger, S.; Wennemuth, G.; Wahl-Schott, C.; Biel, M.; et al. TPC1 deficiency or blockade augments systemic anaphylaxis and mast cell activity. Proc. Natl. Acad. Sci. USA 2020, 117, 18068–18078. [Google Scholar] [CrossRef]
- Steiner, P.; Arlt, E.; Boekhoff, I.; Gudermann, T.; Zierler, S. Two-pore channels regulate inter-organellar Ca2+ homeostasis in immune cells. Cells 2022, 11, 1465. [Google Scholar] [CrossRef]
- Hirabayashi, Y.; Kwon, S.K.; Paek, H.; Pernice, W.M.; Paul, M.A.; Lee, J.; Erfani, P.; Raczkowski, A.; Petrey, D.S.; Pon, L.A.; et al. ER-mitochondria tethering by PDZD8 regulates Ca2+ dynamics in mammalian neurons. Science 2017, 358, 623–630. [Google Scholar] [CrossRef]
- Moccia, F.; Zuccolo, E.; Soda, T.; Tanzi, F.; Guerra, G.; Mapelli, L.; Lodola, F.; D’Angelo, E. Stim and Orai proteins in neuronal Ca2+ signaling and excitability. Front. Cell. Neurosci. 2015, 9, 153. [Google Scholar] [CrossRef]
- Demaurex, N.; Nunes, P. The role of STIM and ORAI proteins in phagocytic immune cells. Am. J. Physiol. Cell Physiol. 2016, 310, C496–C508. [Google Scholar] [CrossRef]
- Navazio, L.; Formentin, E.; Cendron, L.; Szabo, I. Chloroplast Calcium Signaling in the Spotlight. Front. Plant Sci. 2020, 11, 186. [Google Scholar] [CrossRef]
- Wang, T.; Chen, X.; Ju, C.; Wang, C. Calcium signaling in plant mineral nutrition: From uptake to transport. Plant Commun. 2023, 4, 100678. [Google Scholar] [CrossRef]
- Xu, T.; Niu, J.; Jiang, Z. Sensing Mechanisms: Calcium Signaling Mediated Abiotic Stress in Plants. Front. Plant Sci. 2022, 13, 925863. [Google Scholar] [CrossRef]
- Prole, D.L.; Taylor, C.W. Structure and Function of IP3 Receptors. Cold Spring Harb. Perspect. Biol. 2019, 11, a035063. [Google Scholar] [CrossRef]
- Thillaiappan, N.B.; Chavda, A.P.; Tovey, S.C.; Prole, D.L.; Taylor, C.W. Ca2+ signals initiate at immobile IP3 receptors adjacent to ER-plasma membrane junctions. Nat. Commun. 2017, 8, 1505. [Google Scholar] [CrossRef]
- Yuan, Y.; Arige, V.; Saito, R.; Mu, Q.; Brailoiu, G.C.; Pereira, G.J.S.; Bolsover, S.R.; Keller, M.; Bracher, F.; Grimm, C.; et al. Two-pore channel-2 and inositol trisphosphate receptors coordinate Ca2+ signals between lysosomes and the endoplasmic reticulum. Cell Rep. 2024, 43, 113628. [Google Scholar] [CrossRef]
- Parys, J.B.; Lemos, F.O. The interplay between associated proteins, redox state and Ca2+ in the intraluminal ER compartment regulates the IP3 receptor. Cell Calcium 2024, 117, 102823. [Google Scholar] [CrossRef]
- Narayanan, D.; Adebiyi, A.; Jaggar, J.H. Inositol trisphosphate receptors in smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H2190–H2210. [Google Scholar] [CrossRef]
- Rezuchova, I.; Hudecova, S.; Soltysova, A.; Matuskova, M.; Durinikova, E.; Chovancova, B.; Zuzcak, M.; Cihova, M.; Burikova, M.; Penesova, A.; et al. Type 3 inositol 1,4,5-trisphosphate receptor has antiapoptotic and proliferative role in cancer cells. Cell Death Dis. 2019, 10, 186. [Google Scholar] [CrossRef]
- Lock, J.T.; Smith, I.F.; Parker, I. Spatial-temporal patterning of Ca2+ signals by the subcellular distribution of IP3 and IP3 receptors. Semin. Cell Dev. Biol. 2019, 94, 3–10. [Google Scholar] [CrossRef]
- Meissner, G. The structural basis of ryanodine receptor ion channel function. J. Gen. Physiol. 2017, 149, 1065–1089. [Google Scholar] [CrossRef]
- Diercks, B.P.; Werner, R.; Weidemuller, P.; Czarniak, F.; Hernandez, L.; Lehmann, C.; Rosche, A.; Kruger, A.; Kaufmann, U.; Vaeth, M.; et al. ORAI1, STIM1/2, and RYR1 shape subsecond Ca2+ microdomains upon T cell activation. Sci. Signal. 2018, 11, eaat0358. [Google Scholar] [CrossRef]
- Pessah, I.N. Ryanodine receptor acts as a sensor for redox stress. Pest. Manag. Sci. 2001, 57, 941–945. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kurebayashi, N.; Murayama, T. The Ryanodine Receptor as a Sensor for Intracellular Environments in Muscles. Int. J. Mol. Sci. 2021, 22, 10795. [Google Scholar] [CrossRef]
- Lalo, U.; Pankratov, Y. Astrocyte ryanodine receptors facilitate gliotransmission and astroglial modulation of synaptic plasticity. Front. Cell. Neurosci. 2024, 18, 1382010. [Google Scholar] [CrossRef]
- Xu, J.; Liao, C.; Yin, C.C.; Li, G.; Zhu, Y.; Sun, F. In situ structural insights into the excitation-contraction coupling mechanism of skeletal muscle. Sci. Adv. 2024, 10, eadl1126. [Google Scholar] [CrossRef]
- Luo, S.; Benitah, J.P.; Gomez, A.M. Can cardiomyocytes bypass the ‘Ca2+-induced Ca2+-release’ mechanism? Cardiovasc. Res. 2024, 120, 6–9. [Google Scholar] [CrossRef]
- Porretta, A.P.; Pruvot, E.; Bhuiyan, Z.A. Calcium Release Deficiency Syndrome (CRDS): Rethinking “Atypical” Catecholaminergic Polymorphic Ventricular Tachycardia. Cardiogenetics 2024, 14, 211–220. [Google Scholar] [CrossRef]
- Nikolaienko, R.; Bovo, E.; Zima, A.V. Expression level of cardiac ryanodine receptors dictates properties of Ca2+-induced Ca2+ release. Biophys. Rep. 2024, 4, 100183. [Google Scholar] [CrossRef]
- Chiantia, G.; Hidisoglu, E.; Marcantoni, A. The Role of Ryanodine Receptors in Regulating Neuronal Activity and Its Connection to the Development of Alzheimer’s Disease. Cells 2023, 12, 1236. [Google Scholar] [CrossRef] [PubMed]
- Steiner, P.; Arlt, E.; Boekhoff, I.; Gudermann, T.; Zierler, S. TPC Functions in the Immune System. Handb. Exp. Pharmacol. 2023, 278, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Calcraft, P.J.; Ruas, M.; Pan, Z.; Cheng, X.; Arredouani, A.; Hao, X.; Tang, J.; Rietdorf, K.; Teboul, L.; Chuang, K.T.; et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 2009, 459, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.X.; Ma, J.; Parrington, J.; Calcraft, P.J.; Galione, A.; Evans, A.M. Calcium signaling via two-pore channels: Local or global, that is the question. Am. J. Physiol. Cell Physiol. 2010, 298, C430–C441. [Google Scholar] [CrossRef]
- Rahman, T.; Cai, X.; Brailoiu, G.C.; Abood, M.E.; Brailoiu, E.; Patel, S. Two-pore channels provide insight into the evolution of voltage-gated Ca2+ and Na+ channels. Sci. Signal. 2014, 7, ra109. [Google Scholar] [CrossRef]
- Cai, X.; Patel, S. Degeneration of an intracellular ion channel in the primate lineage by relaxation of selective constraints. Mol. Biol. Evol. 2010, 27, 2352–2359. [Google Scholar] [CrossRef]
- Roggenkamp, H.G.; Khansahib, I.; Hernandez, C.L.; Zhang, Y.; Lodygin, D.; Kruger, A.; Gu, F.; Mockl, F.; Lohndorf, A.; Wolters, V.; et al. HN1L/JPT2: A signaling protein that connects NAADP generation to Ca2+ microdomain formation. Sci. Signal. 2021, 14, eabd5647. [Google Scholar] [CrossRef]
- Gunaratne, G.S.; Brailoiu, E.; He, S.; Unterwald, E.M.; Patel, S.; Slama, J.T.; Walseth, T.F.; Marchant, J.S. Essential requirement for JPT2 in NAADP-evoked Ca2+ signaling. Sci. Signal. 2021, 14, eabd5605. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, X.; Shah, K.; Yan, J. Lsm12 is an NAADP receptor and a two-pore channel regulatory protein required for calcium mobilization from acidic organelles. Nat. Commun. 2021, 12, 4739. [Google Scholar] [CrossRef]
- Li, Z.H.; King, T.P.; Ayong, L.; Asady, B.; Cai, X.; Rahman, T.; Vella, S.A.; Coppens, I.; Patel, S.; Moreno, S.N.J. A plastid two-pore channel essential for inter-organelle communication and growth of Toxoplasma gondii. Nat. Commun. 2021, 12, 5802. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Kilpatrick, B.S. Two-pore channels and disease. Biochim. Biophys. Acta 2018, 1865, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Butorac, C.; Krizova, A.; Derler, I. Review: Structure and Activation Mechanisms of CRAC Channels. Adv. Exp. Med. Biol. 2020, 1131, 547–604. [Google Scholar] [CrossRef] [PubMed]
- Derler, I.; Jardin, I.; Romanin, C. Molecular mechanisms of STIM/Orai communication. Am. J. Physiol. Cell Physiol. 2016, 310, C643–C662. [Google Scholar] [CrossRef]
- Zhang, S.L.; Yu, Y.; Roos, J.; Kozak, J.A.; Deerinck, T.J.; Ellisman, M.H.; Stauderman, K.A.; Cahalan, M.D. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 2005, 437, 902–905. [Google Scholar] [CrossRef]
- Vig, M.; Peinelt, C.; Beck, A.; Koomoa, D.L.; Rabah, D.; Koblan-Huberson, M.; Kraft, S.; Turner, H.; Fleig, A.; Penner, R.; et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 2006, 312, 1220–1223. [Google Scholar] [CrossRef]
- Prakriya, M.; Feske, S.; Gwack, Y.; Srikanth, S.; Rao, A.; Hogan, P.G. Orai1 is an essential pore subunit of the CRAC channel. Nature 2006, 443, 230–233. [Google Scholar] [CrossRef]
- Tedeschi, V.; La Russa, D.; Franco, C.; Vinciguerra, A.; Amantea, D.; Secondo, A. Plasma Membrane and Organellar Targets of STIM1 for Intracellular Calcium Handling in Health and Neurodegenerative Diseases. Cells 2021, 10, 2518. [Google Scholar] [CrossRef]
- Li, Z.; Liu, L.; Deng, Y.; Ji, W.; Du, W.; Xu, P.; Chen, L.; Xu, T. Graded activation of CRAC channel by binding of different numbers of STIM1 to Orai1 subunits. Cell Res. 2011, 21, 305–315. [Google Scholar] [CrossRef]
- Park, C.Y.; Hoover, P.J.; Mullins, F.M.; Bachhawat, P.; Covington, E.D.; Raunser, S.; Walz, T.; Garcia, K.C.; Dolmetsch, R.E.; Lewis, R.S. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 2009, 136, 876–890. [Google Scholar] [CrossRef]
- Berlansky, S.; Humer, C.; Sallinger, M.; Frischauf, I. More Than Just Simple Interaction between STIM and Orai Proteins: CRAC Channel Function Enabled by a Network of Interactions with Regulatory Proteins. Int. J. Mol. Sci. 2021, 22, 471. [Google Scholar] [CrossRef]
- Emrich, S.M.; Yoast, R.E.; Trebak, M. Physiological Functions of CRAC Channels. Annu. Rev. Physiol. 2022, 84, 355–379. [Google Scholar] [CrossRef]
- Vaeth, M.; Kahlfuss, S.; Feske, S. CRAC Channels and Calcium Signaling in T Cell-Mediated Immunity. Trends Immunol. 2020, 41, 878–901. [Google Scholar] [CrossRef]
- Fahrner, M.; Schindl, R.; Romanin, C. Studies of Structure-Function and Subunit Composition of Orai/STIM Channel. In Calcium Entry Channels in Non-Excitable Cells; Kozak, J.A., Putney, J.W., Jr., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 25–50. [Google Scholar]
- Pathak, T.; Trebak, M. Mitochondrial Ca2+ signaling. Pharmacol. Ther. 2018, 192, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Balderas, E.; Eberhardt, D.R.; Lee, S.; Pleinis, J.M.; Sommakia, S.; Balynas, A.M.; Yin, X.; Parker, M.C.; Maguire, C.T.; Cho, S.; et al. Mitochondrial calcium uniporter stabilization preserves energetic homeostasis during Complex I impairment. Nat. Commun. 2022, 13, 2769. [Google Scholar] [CrossRef] [PubMed]
- Kamer, K.J.; Mootha, V.K. The molecular era of the mitochondrial calcium uniporter. Nat. Rev. Mol. Cell Biol. 2015, 16, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Zhang, J.; Tsai, C.W.; Orlando, B.J.; Rodriguez, M.; Xu, Y.; Liao, M.; Tsai, M.F.; Feng, L. Structure and mechanism of the mitochondrial Ca2+ uniporter holocomplex. Nature 2020, 582, 129–133. [Google Scholar] [CrossRef]
- Gibhardt, C.S.; Riemer, J.; Bogeski, I. Calcium and redox signals at mitochondrial interfaces: A nanoview perspective. Cell Calcium 2022, 103, 102550. [Google Scholar] [CrossRef]
- Fieni, F.; Lee, S.B.; Jan, Y.N.; Kirichok, Y. Activity of the mitochondrial calcium uniporter varies greatly between tissues. Nat. Commun. 2012, 3, 1317. [Google Scholar] [CrossRef]
- Paupe, V.; Prudent, J. New insights into the role of mitochondrial calcium homeostasis in cell migration. Biochem. Biophys. Res. Commun. 2018, 500, 75–86. [Google Scholar] [CrossRef]
- Shumanska, M.; Lodygin, D.; Gibhardt, C.S.; Ickes, C.; Stejerean-Todoran, I.; Krause, L.C.M.; Pahl, K.; Jacobs, L.; Paluschkiwitz, A.; Liu, S.; et al. Mitochondrial calcium uniporter complex controls T-cell-mediated immune responses. EMBO Rep. 2024, 26, 407–442. [Google Scholar] [CrossRef]
- Stejerean-Todoran, I.; Zimmermann, K.; Gibhardt, C.S.; Vultur, A.; Ickes, C.; Shannan, B.; Bonilla Del Rio, Z.; Wolling, A.; Cappello, S.; Sung, H.M.; et al. MCU controls melanoma progression through a redox-controlled phenotype switch. EMBO Rep. 2022, 23, e54746. [Google Scholar] [CrossRef]
- Flockerzi, V.; Nilius, B. TRPs: Truly remarkable proteins. Handb. Exp. Pharmacol. 2014, 222, 1–12. [Google Scholar] [CrossRef]
- Santoni, G.; Maggi, F.; Amantini, C.; Marinelli, O.; Nabissi, M.; Morelli, M.B. Pathophysiological Role of Transient Receptor Potential Mucolipin Channel 1 in Calcium-Mediated Stress-Induced Neurodegenerative Diseases. Front. Physiol. 2020, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Ouologuem, L.; Bartel, K. Endolysosomal transient receptor potential mucolipins and two-pore channels: Implications for cancer immunity. Front. Immunol. 2024, 15, 1389194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, X.; Yu, L.; Yang, J.; Calvo, R.; Patnaik, S.; Hu, X.; Gao, Q.; Yang, M.; Lawas, M.; et al. MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat. Commun. 2016, 7, 12109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fang, Y.; Zhao, X.; Zheng, Y.; Ma, Y.; Li, S.; Huang, Z.; Li, L. miR-204 silencing reduces mitochondrial autophagy and ROS production in a murine AD model via the TRPML1-activated STAT3 pathway. Mol. Ther. Nucleic Acids 2021, 24, 822–831. [Google Scholar] [CrossRef]
- Wu, L.-K.; Agarwal, S.; Kuo, C.-H.; Kung, Y.-L.; Day, C.H.; Lin, P.-Y.; Lin, S.-Z.; Hsieh, D.J.; Huang, C.-Y.; Chiang, C.-Y. Artemisia Leaf Extract protects against neuron toxicity by TRPML1 activation and promoting autophagy/mitophagy clearance in both in vitro and in vivo models of MPP+/MPTP-induced Parkinson’s disease. Phytomedicine 2022, 104, 154250. [Google Scholar] [CrossRef]
- Pan, Y.; Zhao, Q.; He, H.; Qi, Y.; Bai, Y.; Zhao, J.; Yang, Y. TRPML1 as a potential therapeutic target for triple-negative breast cancer: A review. Front. Oncol. 2023, 13, 1326023. [Google Scholar] [CrossRef]
- Krogsaeter, E.; Rosato, A.S.; Grimm, C. TRPMLs and TPCs: Targets for lysosomal storage and neurodegenerative disease therapy? Cell Calcium 2022, 103, 102553. [Google Scholar] [CrossRef]
- Spix, B.; Castiglioni, A.J.; Remis, N.N.; Flores, E.N.; Wartenberg, P.; Wyatt, A.; Boehm, U.; Gudermann, T.; Biel, M.; Garcia-Anoveros, J.; et al. Whole-body analysis of TRPML3 (MCOLN3) expression using a GFP-reporter mouse model reveals widespread expression in secretory cells and endocrine glands. PLoS ONE 2022, 17, e0278848. [Google Scholar] [CrossRef]
- Spix, B.; Butz, E.S.; Chen, C.C.; Rosato, A.S.; Tang, R.; Jeridi, A.; Kudrina, V.; Plesch, E.; Wartenberg, P.; Arlt, E.; et al. Lung emphysema and impaired macrophage elastase clearance in mucolipin 3 deficient mice. Nat. Commun. 2022, 13, 318. [Google Scholar] [CrossRef]
- Spix, B.; Chao, Y.K.; Abrahamian, C.; Chen, C.C.; Grimm, C. TRPML Cation Channels in Inflammation and Immunity. Front. Immunol. 2020, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Rautenberg, S.; Keller, M.; Leser, C.; Chen, C.C.; Bracher, F.; Grimm, C. Expanding the Toolbox: Novel Modulators of Endolysosomal Cation Channels. Handb. Exp. Pharmacol. 2023, 278, 249–276. [Google Scholar] [CrossRef] [PubMed]
- Lange, I.; Yamamoto, S.; Partida-Sanchez, S.; Mori, Y.; Fleig, A.; Penner, R. TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci. Signal. 2009, 2, ra23. [Google Scholar] [CrossRef] [PubMed]
- Sumoza-Toledo, A.; Lange, I.; Cortado, H.; Bhagat, H.; Mori, Y.; Fleig, A.; Penner, R.; Partida-Sanchez, S. Dendritic cell maturation and chemotaxis is regulated by TRPM2-mediated lysosomal Ca2+ release. FASEB J. 2011, 25, 3529–3542. [Google Scholar] [CrossRef]
- Abiria, S.A.; Krapivinsky, G.; Sah, R.; Santa-Cruz, A.G.; Chaudhuri, D.; Zhang, J.; Adstamongkonkul, P.; DeCaen, P.G.; Clapham, D.E. TRPM7 senses oxidative stress to release Zn2+ from unique intracellular vesicles. Proc. Natl. Acad. Sci. USA 2017, 114, E6079–E6088. [Google Scholar] [CrossRef]
- Doyle, C.A.; Busey, G.W.; Iobst, W.H.; Kiessling, V.; Renken, C.; Doppalapudi, H.; Stremska, M.E.; Manjegowda, M.C.; Arish, M.; Wang, W.; et al. Endosomal fusion of pH-dependent enveloped viruses requires ion channel TRPM7. Nat. Commun. 2024, 15, 8479. [Google Scholar] [CrossRef]
- Krapivinsky, G.; Mochida, S.; Krapivinsky, L.; Cibulsky, S.M.; Clapham, D.E. The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron 2006, 52, 485–496. [Google Scholar] [CrossRef]
- Zierler, S.; Sumoza-Toledo, A.; Suzuki, S.; Duill, F.O.; Ryazanova, L.V.; Penner, R.; Ryazanov, A.G.; Fleig, A. TRPM7 kinase activity regulates murine mast cell degranulation. J. Physiol. 2016, 594, 2957–2970. [Google Scholar] [CrossRef]
- Khajavi, N.; Beck, A.; Ricku, K.; Beyerle, P.; Jacob, K.; Syamsul, S.F.; Belkacemi, A.; Reinach, P.S.; Schreier, P.C.; Salah, H.; et al. TRPM7 kinase is required for insulin production and compensatory islet responses during obesity. JCI Insight 2023, 8, e163397. [Google Scholar] [CrossRef]
- Nadolni, W.; Immler, R.; Hoelting, K.; Fraticelli, M.; Ripphahn, M.; Rothmiller, S.; Matsushita, M.; Boekhoff, I.; Gudermann, T.; Sperandio, M.; et al. TRPM7 Kinase Is Essential for Neutrophil Recruitment and Function via Regulation of Akt/mTOR Signaling. Front. Immunol. 2020, 11, 606893. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, A.; Vettore, V.; Rezzonico-Jost, T.; Hampe, S.; Rottoli, E.; Nadolni, W.; Perotti, M.; Meier, M.A.; Hermanns, C.; Geiger, S.; et al. TRPM7 kinase activity is essential for T cell colonization and alloreactivity in the gut. Nat. Commun. 2017, 8, 1917. [Google Scholar] [CrossRef]
- Oken, A.C.; Lisi, N.E.; Krishnamurthy, I.; McCarthy, A.E.; Godsey, M.H.; Glasfeld, A.; Mansoor, S.E. High-affinity agonism at the P2X(7) receptor is mediated by three residues outside the orthosteric pocket. Nat. Commun. 2024, 15, 6662. [Google Scholar] [CrossRef] [PubMed]
- Sander, S.; Muller, I.; Garcia-Alai, M.M.; Nicke, A.; Tidow, H. New insights into P2X7 receptor regulation: Ca2+-calmodulin and GDP bind to the soluble P2X7 ballast domain. J. Biol. Chem. 2022, 298, 102495. [Google Scholar] [CrossRef] [PubMed]
- Durner, A.; Durner, E.; Nicke, A. Improved ANAP incorporation and VCF analysis reveal details of P2X7 current facilitation and a limited conformational interplay between ATP binding and the intracellular ballast domain. eLife 2023, 12, e82479. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, Q.; Zhou, S.; Luo, H.; Zhang, W. Role and therapeutic targets of P2X7 receptors in neurodegenerative diseases. Front. Immunol. 2024, 15, 1345625. [Google Scholar] [CrossRef]
- Alves, M.; Gil, B.; Villegas-Salmeron, J.; Salari, V.; Martins-Ferreira, R.; Arribas Blazquez, M.; Menendez Mendez, A.; Da Rosa Gerbatin, R.; Smith, J.; de Diego-Garcia, L.; et al. Opposing effects of the purinergic P2X7 receptor on seizures in neurons and microglia in male mice. Brain Behav. Immun. 2024, 120, 121–140. [Google Scholar] [CrossRef]
- Bianchi, C.; Alvarez-Castelao, B.; Sebastian-Serrano, A.; Di Lauro, C.; Soria-Tobar, L.; Nicke, A.; Engel, T.; Diaz-Hernandez, M. P2X7 receptor inhibition ameliorates ubiquitin-proteasome system dysfunction associated with Alzheimer’s disease. Alzheimers Res. Ther. 2023, 15, 105. [Google Scholar] [CrossRef]
- Engel, T.; Nicke, A.; Deussing, J.M.; Sperlagh, B.; Diaz-Hernandez, M. Editorial: P2X7 as Common Therapeutic Target in Brain Diseases. Front. Mol. Neurosci. 2021, 14, 656011. [Google Scholar] [CrossRef]
- Tewari, M.; Michalski, S.; Egan, T.M. Modulation of Microglial Function by ATP-Gated P2X7 Receptors: Studies in Rat, Mice and Human. Cells 2024, 13, 161. [Google Scholar] [CrossRef] [PubMed]
- Jooss, T.; Zhang, J.; Zimmer, B.; Rezzonico-Jost, T.; Rissiek, B.; Felipe Pelczar, P.; Seehusen, F.; Koch-Nolte, F.; Magnus, T.; Zierler, S.; et al. Macrophages and glia are the dominant P2X7-expressing cell types in the gut nervous system-No evidence for the role of neuronal P2X7 receptors in colitis. Mucosal Immunol. 2023, 16, 180–193. [Google Scholar] [CrossRef]
- Sierra-Marquez, J.; Schaller, L.; Sassenbach, L.; Ramirez-Fernandez, A.; Alt, P.; Rissiek, B.; Zimmer, B.; Schredelseker, J.; Hector, J.; Stahler, T.; et al. Different localization of P2X4 and P2X7 receptors in native mouse lung—Lack of evidence for a direct P2X4-P2X7 receptor interaction. Front. Immunol. 2024, 15, 1425938. [Google Scholar] [CrossRef]
- Sluyter, R.; Adriouch, S.; Fuller, S.J.; Nicke, A.; Sophocleous, R.A.; Watson, D. Animal Models for the Investigation of P2X7 Receptors. Int. J. Mol. Sci. 2023, 24, 8225. [Google Scholar] [CrossRef]
- Dunker, C.; Vinnenberg, L.; Isaak, A.; Karabatak, E.; Hundehege, P.; Budde, T.; Murakami, K.; Junker, A. Exploring P2X receptor activity: A journey from cellular impact to electrophysiological profiling. Biochem. Pharmacol. 2024, 229, 116543. [Google Scholar] [CrossRef]
- Cabral-Garcia, G.A.; Cruz-Munoz, J.R.; Valdez-Morales, E.E.; Barajas-Espinosa, A.; Linan-Rico, A.; Guerrero-Alba, R. Pharmacology of P2X Receptors and Their Possible Therapeutic Potential in Obesity and Diabetes. Pharmaceuticals 2024, 17, 1291. [Google Scholar] [CrossRef]
- Poejo, J.; Gumerova, N.I.; Rompel, A.; Mata, A.M.; Aureliano, M.; Gutierrez-Merino, C. Unveiling the agonistic properties of Preyssler-type Polyoxotungstates on purinergic P2 receptors. J. Inorg. Biochem. 2024, 259, 112640. [Google Scholar] [CrossRef]
- Illes, P.; Muller, C.E.; Jacobson, K.A.; Grutter, T.; Nicke, A.; Fountain, S.J.; Kennedy, C.; Schmalzing, G.; Jarvis, M.F.; Stojilkovic, S.S.; et al. Update of P2X receptor properties and their pharmacology: IUPHAR Review 30. Br. J. Pharmacol. 2021, 178, 489–514. [Google Scholar] [CrossRef]
- Primeau, J.O.; Armanious, G.P.; Fisher, M.E.; Young, H.S. The SarcoEndoplasmic Reticulum Calcium ATPase. Subcell. Biochem. 2018, 87, 229–258. [Google Scholar] [CrossRef]
- del Monte, F.; Williams, E.; Lebeche, D.; Schmidt, U.; Rosenzweig, A.; Gwathmey, J.K.; Lewandowski, E.D.; Hajjar, R.J. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca2+-ATPase in a rat model of heart failure. Circulation 2001, 104, 1424–1429. [Google Scholar] [CrossRef]
- Britzolaki, A.; Saurine, J.; Klocke, B.; Pitychoutis, P.M. A Role for SERCA Pumps in the Neurobiology of Neuropsychiatric and Neurodegenerative Disorders. Adv. Exp. Med. Biol. 2020, 1131, 131–161. [Google Scholar] [CrossRef]
- Gorski, P.A.; Ceholski, D.K.; Young, H.S. Structure-Function Relationship of the SERCA Pump and Its Regulation by Phospholamban and Sarcolipin. Adv. Exp. Med. Biol. 2017, 981, 77–119. [Google Scholar] [CrossRef] [PubMed]
- Khurram, I.; Khan, M.U.; Ibrahim, S.; Ghani, M.U.; Amin, I.; Falzone, L.; Herrera-Bravo, J.; Setzer, W.N.; Sharifi-Rad, J.; Calina, D. Thapsigargin and its prodrug derivatives: Exploring novel approaches for targeted cancer therapy through calcium signaling disruption. Med. Oncol. 2024, 42, 7. [Google Scholar] [CrossRef]
- Kim, J.; Chang, H.S.; Yun, H.J.; Chang, H.J.; Park, K.C. New Small-Molecule SERCA Inhibitors Enhance Treatment Efficacy in Lenvatinib-Resistant Papillary Thyroid Cancer. Int. J. Mol. Sci. 2024, 25, 10646. [Google Scholar] [CrossRef]
- Kim, S.M.; Park, K.; Yun, H.J.; Kim, J.M.; Choi, K.H.; Park, K.C. Identification of new small molecules for selective inhibition of SERCA 1 in patient-derived metastatic papillary thyroid cancer. Br. J. Pharmacol. 2025, 182, 2392–2408. [Google Scholar] [CrossRef]
- Steiner, P.; Melek, K.; Andosch, A.; Wiesbauer, L.; Madlmayr, A.; Duggan, M.; Kerschbaum, H.H.; Zierler, S. Thapsigargin triggers a non-apoptotic, caspase-independent programmed cell death in basophilic leukaemia cells. Cell Death Discov. 2025, 11, 313. [Google Scholar] [CrossRef]
- Aureliano, M.; Fraqueza, G.; Berrocal, M.; Cordoba-Granados, J.J.; Gumerova, N.I.; Rompel, A.; Gutierrez-Merino, C.; Mata, A.M. Inhibition of SERCA and PMCA Ca2+-ATPase activities by polyoxotungstates. J. Inorg. Biochem. 2022, 236, 111952. [Google Scholar] [CrossRef]
- Aguayo-Ortiz, R.; Espinoza-Fonseca, L.M. Linking Biochemical and Structural States of SERCA: Achievements, Challenges, and New Opportunities. Int. J. Mol. Sci. 2020, 21, 4146. [Google Scholar] [CrossRef]
- Rashid, H.O.; Yadav, R.K.; Kim, H.R.; Chae, H.J. ER stress: Autophagy induction, inhibition and selection. Autophagy 2015, 11, 1956–1977. [Google Scholar] [CrossRef]
- Strehler, E.E.; Caride, A.J.; Filoteo, A.G.; Xiong, Y.; Penniston, J.T.; Enyedi, A. Plasma membrane Ca2+ ATPases as dynamic regulators of cellular calcium handling. Ann. N. Y. Acad. Sci. 2007, 1099, 226–236. [Google Scholar] [CrossRef]
- Stafford, N.; Wilson, C.; Oceandy, D.; Neyses, L.; Cartwright, E.J. The Plasma Membrane Calcium ATPases and Their Role as Major New Players in Human Disease. Physiol. Rev. 2017, 97, 1089–1125. [Google Scholar] [CrossRef]
- Krebs, J. Structure, Function and Regulation of the Plasma Membrane Calcium Pump in Health and Disease. Int. J. Mol. Sci. 2022, 23, 1027. [Google Scholar] [CrossRef] [PubMed]
- Themistocleous, S.C.; Yiallouris, A.; Tsioutis, C.; Zaravinos, A.; Johnson, E.O.; Patrikios, I. Clinical significance of P-class pumps in cancer. Oncol. Lett. 2021, 22, 658. [Google Scholar] [CrossRef]
- Berrocal, M.; Cordoba-Granados, J.J.; Carabineiro, S.A.C.; Gutierrez-Merino, C.; Aureliano, M.; Mata, A.M. Gold Compounds Inhibit the Ca-ATPase Activity of Brain PMCA and Human Neuroblastoma SH-SY5Y Cells and Decrease Cell Viability. Metals 2021, 11, 1934. [Google Scholar] [CrossRef]
- Yu, S.P.; Choi, D.W. Na+-Ca2+ exchange currents in cortical neurons: Concomitant forward and reverse operation and effect of glutamate. Eur. J. Neurosci. 1997, 9, 1273–1281. [Google Scholar] [CrossRef]
- Scranton, K.; John, S.; Angelini, M.; Steccanella, F.; Umar, S.; Zhang, R.; Goldhaber, J.I.; Olcese, R.; Ottolia, M. Cardiac function is regulated by the sodium-dependent inhibition of the sodium-calcium exchanger NCX1. Nat. Commun. 2024, 15, 3831. [Google Scholar] [CrossRef]
- Dong, Y.; Yu, Z.; Li, Y.; Huang, B.; Bai, Q.; Gao, Y.; Chen, Q.; Li, N.; He, L.; Zhao, Y. Structural insight into the allosteric inhibition of human sodium-calcium exchanger NCX1 by XIP and SEA0400. EMBO J. 2024, 43, 14–31. [Google Scholar] [CrossRef]
- Ren, X.; Philipson, K.D. The topology of the cardiac Na+/Ca2+ exchanger, NCX1. J. Mol. Cell. Cardiol. 2013, 57, 68–71. [Google Scholar] [CrossRef]
- Liao, J.; Li, H.; Zeng, W.; Sauer, D.B.; Belmares, R.; Jiang, Y. Structural insight into the ion-exchange mechanism of the sodium/calcium exchanger. Science 2012, 335, 686–690. [Google Scholar] [CrossRef]
- Palty, R.; Silverman, W.F.; Hershfinkel, M.; Caporale, T.; Sensi, S.L.; Parnis, J.; Nolte, C.; Fishman, D.; Shoshan-Barmatz, V.; Herrmann, S.; et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl. Acad. Sci. USA 2010, 107, 436–441. [Google Scholar] [CrossRef]
- Gobbi, P.; Castaldo, P.; Minelli, A.; Salucci, S.; Magi, S.; Corcione, E.; Amoroso, S. Mitochondrial localization of Na+/Ca2+ exchangers NCX1-3 in neurons and astrocytes of adult rat brain in situ. Pharmacol. Res. 2007, 56, 556–565. [Google Scholar] [CrossRef]
- Xue, J.; Zeng, W.; Han, Y.; John, S.; Ottolia, M.; Jiang, Y. Structural mechanisms of the human cardiac sodium-calcium exchanger NCX1. Nat. Commun. 2023, 14, 6181. [Google Scholar] [CrossRef]
- Pottosin, I.I.; Schonknecht, G. Vacuolar calcium channels. J. Exp. Bot. 2007, 58, 1559–1569. [Google Scholar] [CrossRef]
- Krinke, O.; Novotna, Z.; Valentova, O.; Martinec, J. Inositol trisphosphate receptor in higher plants: Is it real? J. Exp. Bot. 2007, 58, 361–376. [Google Scholar] [CrossRef]
- Brosnan, J.M.; Sanders, D. Identification and Characterization of High-Affinity Binding Sites for Inositol Trisphosphate in Red Beet. Plant Cell 1993, 5, 931–940. [Google Scholar] [CrossRef][Green Version]
- Schonknecht, G. Calcium Signals from the Vacuole. Plants 2013, 2, 589–614. [Google Scholar] [CrossRef]
- Randall, S.K. Characterization of vacuolar calcium-binding proteins. Plant Physiol. 1992, 100, 859–867. [Google Scholar] [CrossRef][Green Version]
- Peiter, E. The plant vacuole: Emitter and receiver of calcium signals. Cell Calcium 2011, 50, 120–128. [Google Scholar] [CrossRef]
- Ren, H.; Zhao, X.; Li, W.; Hussain, J.; Qi, G.; Liu, S. Calcium Signaling in Plant Programmed Cell Death. Cells 2021, 10, 1089. [Google Scholar] [CrossRef]
- Costa, A.; Navazio, L.; Szabo, I. The contribution of organelles to plant intracellular Calcium signalling. J. Exp. Bot. 2018, 69, 4175–4193. [Google Scholar] [CrossRef]
- Jarratt-Barnham, E.; Wang, L.; Ning, Y.; Davies, J.M. The Complex Story of Plant Cyclic Nucleotide-Gated Channels. Int. J. Mol. Sci. 2021, 22, 874. [Google Scholar] [CrossRef]
- Swiezawska-Boniecka, B.; Duszyn, M.; Kwiatkowski, M.; Szmidt-Jaworska, A.; Jaworski, K. Cross Talk Between Cyclic Nucleotides and Calcium Signaling Pathways in Plants-Achievements and Prospects. Front. Plant Sci. 2021, 12, 643560. [Google Scholar] [CrossRef]
- Dietrich, P.; Moeder, W.; Yoshioka, K. Plant Cyclic Nucleotide-Gated Channels: New Insights on Their Functions and Regulation. Plant Physiol. 2020, 184, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Berkowitz, G.A. Ca2+ conduction by plant cyclic nucleotide gated channels and associated signaling components in pathogen defense signal transduction cascades. New Phytol. 2011, 190, 566–572. [Google Scholar] [CrossRef]
- Pan, Y.; Chai, X.; Gao, Q.; Zhou, L.; Zhang, S.; Li, L.; Luan, S. Dynamic Interactions of Plant CNGC Subunits and Calmodulins Drive Oscillatory Ca2+ Channel Activities. Dev. Cell 2019, 48, 710–725.e5. [Google Scholar] [CrossRef] [PubMed]
- Carpaneto, A.; Gradogna, A. Modulation of calcium and potassium permeation in plant TPC channels. Biophys. Chem. 2018, 236, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hedrich, R.; Marten, I. TPC1-SV channels gain shape. Mol. Plant 2011, 4, 428–441. [Google Scholar] [CrossRef]
- Tong, T.; Li, Q.; Jiang, W.; Chen, G.; Xue, D.; Deng, F.; Zeng, F.; Chen, Z.H. Molecular Evolution of Calcium Signaling and Transport in Plant Adaptation to Abiotic Stress. Int. J. Mol. Sci. 2021, 22, 12308. [Google Scholar] [CrossRef]
- Negi, N.P.; Prakash, G.; Narwal, P.; Panwar, R.; Kumar, D.; Chaudhry, B.; Rustagi, A. The calcium connection: Exploring the intricacies of calcium signaling in plant-microbe interactions. Front. Plant Sci. 2023, 14, 1248648. [Google Scholar] [CrossRef]
- Patel, S.; Penny, C.J.; Rahman, T. Two-pore Channels Enter the Atomic Era: Structure of Plant TPC Revealed. Trends Biochem. Sci. 2016, 41, 475–477. [Google Scholar] [CrossRef]
- Guo, J.T.; Zeng, W.Z.; Chen, Q.F.; Lee, C.; Chen, L.P.; Yang, Y.; Cang, C.L.; Ren, D.J.; Jiang, Y.X. Structure of the voltage-gated two-pore channel TPC1 from. Nature 2016, 531, 196–201. [Google Scholar] [CrossRef]
- Kintzer, A.F.; Stroud, R.M. Structure, inhibition and regulation of two-pore channel TPC1 from Arabidopsis thaliana. Nature 2016, 531, 258–262. [Google Scholar] [CrossRef]
- Guo, J.; Zeng, W.; Jiang, Y. Tuning the ion selectivity of two-pore channels. Proc. Natl. Acad. Sci. USA 2017, 114, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Teardo, E.; Carraretto, L.; Wagner, S.; Formentin, E.; Behera, S.; De Bortoli, S.; Larosa, V.; Fuchs, P.; Lo Schiavo, F.; Raffaello, A.; et al. Physiological Characterization of a Plant Mitochondrial Calcium Uniporter in Vitro and in Vivo. Plant Physiol. 2017, 173, 1355–1370. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.F.; Phillips, C.B.; Ranaghan, M.; Tsai, C.W.; Wu, Y.; Willliams, C.; Miller, C. Dual functions of a small regulatory subunit in the mitochondrial calcium uniporter complex. eLife 2016, 5, e15545. [Google Scholar] [CrossRef]
- Liang, F.; Cunningham, K.W.; Harper, J.F.; Sze, H. ECA1 complements yeast mutants defective in Ca2+ pumps and encodes an endoplasmic reticulum-type Ca2+-ATPase in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1997, 94, 8579–8584. [Google Scholar] [CrossRef]
- Wimmers, L.E.; Ewing, N.N.; Bennett, A.B. Higher plant Ca2+-ATPase: Primary structure and regulation of mRNA abundance by salt. Proc. Natl. Acad. Sci. USA 1992, 89, 9205–9209. [Google Scholar] [CrossRef]
- He, J.; Rossner, N.; Hoang, M.T.T.; Alejandro, S.; Peiter, E. Transport, functions, and interaction of calcium and manganese in plant organellar compartments. Plant Physiol. 2021, 187, 1940–1972. [Google Scholar] [CrossRef]
- Hwang, I.; Ratterman, D.M.; Sze, H. Distinction between Endoplasmic Reticulum-Type and Plasma Membrane-Type Ca2+ Pumps (Partial Purification of a 120-Kilodalton Ca2+-ATPase from Endomembranes). Plant Physiol. 1997, 113, 535–548. [Google Scholar] [CrossRef]
- Wang, C.; Tang, R.J.; Kou, S.; Xu, X.; Lu, Y.; Rauscher, K.; Voelker, A.; Luan, S. Mechanisms of calcium homeostasis orchestrate plant growth and immunity. Nature 2024, 627, 382–388. [Google Scholar] [CrossRef]
- Rahmati Ishka, M.; Brown, E.; Rosenberg, A.; Romanowsky, S.; Davis, J.A.; Choi, W.G.; Harper, J.F. Arabidopsis Ca2+-ATPases 1, 2, and 7 in the endoplasmic reticulum contribute to growth and pollen fitness. Plant Physiol. 2021, 185, 1966–1985. [Google Scholar] [CrossRef]
- Periasamy, M.; Kalyanasundaram, A. SERCA pump isoforms: Their role in calcium transport and disease. Muscle Nerve 2007, 35, 430–442. [Google Scholar] [CrossRef]
- Grenzi, M.; Bonza, M.C.; Costa, A. Signaling by plant glutamate receptor-like channels: What else! Curr. Opin. Plant Biol. 2022, 68, 102253. [Google Scholar] [CrossRef]
- Simon, A.A.; Navarro-Retamal, C.; Feijo, J.A. Merging Signaling with Structure: Functions and Mechanisms of Plant Glutamate Receptor Ion Channels. Annu. Rev. Plant Biol. 2023, 74, 415–452. [Google Scholar] [CrossRef]
- Kang, X.; Zhao, L.; Liu, X. Calcium Signaling and the Response to Heat Shock in Crop Plants. Int. J. Mol. Sci. 2023, 25, 324. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Liu, N.; Tang, S.; Qin, T.; Huang, J. Roles of Glutamate Receptor-Like Channels (GLRs) in Plant Growth and Response to Environmental Stimuli. Plants 2022, 11, 3450. [Google Scholar] [CrossRef] [PubMed]
- Corti, F.; Festa, M.; Stein, F.; Stevanato, P.; Siroka, J.; Navazio, L.; Vothknecht, U.C.; Alboresi, A.; Novak, O.; Formentin, E.; et al. Comparative analysis of wild-type and chloroplast MCU-deficient plants reveals multiple consequences of chloroplast calcium handling under drought stress. Front. Plant Sci. 2023, 14, 1228060. [Google Scholar] [CrossRef] [PubMed]
- Nomura, H.; Komori, T.; Uemura, S.; Kanda, Y.; Shimotani, K.; Nakai, K.; Furuichi, T.; Takebayashi, K.; Sugimoto, T.; Sano, S.; et al. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat. Commun. 2012, 3, 926. [Google Scholar] [CrossRef]
- Teardo, E.; Segalla, A.; Formentin, E.; Zanetti, M.; Marin, O.; Giacometti, G.M.; Lo Schiavo, F.; Zoratti, M.; Szabo, I. Characterization of a plant glutamate receptor activity. Cell. Physiol. Biochem. 2010, 26, 253–262. [Google Scholar] [CrossRef]
- Frank, J.; Happeck, R.; Meier, B.; Hoang, M.T.T.; Stribny, J.; Hause, G.; Ding, H.; Morsomme, P.; Baginsky, S.; Peiter, E. Chloroplast-localized BICAT proteins shape stromal calcium signals and are required for efficient photosynthesis. New Phytol. 2019, 221, 866–880. [Google Scholar] [CrossRef]
- Resentini, F.; Grenzi, M.; Ancora, D.; Cademartori, M.; Luoni, L.; Franco, M.; Bassi, A.; Bonza, M.C.; Costa, A. Simultaneous imaging of ER and cytosolic Ca2+ dynamics reveals long-distance ER Ca2+ waves in plants. Plant Physiol. 2021, 187, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Duckney, P.; Zhang, T.; Fu, Y.; Li, X.; Kroon, J.; De Jaeger, G.; Cheng, Y.; Hussey, P.J.; Wang, P. TraB family proteins are components of ER-mitochondrial contact sites and regulate ER-mitochondrial interactions and mitophagy. Nat. Commun. 2022, 13, 5658. [Google Scholar] [CrossRef] [PubMed]
- Arruda, A.P.; Hotamisligil, G.S. Calcium Homeostasis and Organelle Function in the Pathogenesis of Obesity and Diabetes. Cell Metab. 2015, 22, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Resentini, F.; Ruberti, C.; Grenzi, M.; Bonza, M.C.; Costa, A. The signatures of organellar calcium. Plant Physiol. 2021, 187, 1985–2004. [Google Scholar] [CrossRef]
- Deutsch, R.; Kudrina, V.; Freichel, M.; Grimm, C. Two-pore channel regulators—Who is in control? Front. Physiol. 2024, 15, 1534071. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, Y.; Alharbi, A.; Hanbashi, A.; Alhoshani, A.; Parrington, J. Targeting Two-Pore Channels: Current Progress and Future Challenges. Trends Pharmacol. Sci. 2020, 41, 582–594. [Google Scholar] [CrossRef]
- Lagostena, L.; Festa, M.; Pusch, M.; Carpaneto, A. The human two-pore channel 1 is modulated by cytosolic and luminal calcium. Sci. Rep. 2017, 7, 43900. [Google Scholar] [CrossRef]
- Nagata, T.; Iizumi, S.; Satoh, K.; Ooka, H.; Kawai, J.; Carninci, P.; Hayashizaki, Y.; Otomo, Y.; Murakami, K.; Matsubara, K.; et al. Comparative analysis of plant and animal calcium signal transduction element using plant full-length cDNA data. Mol. Biol. Evol. 2004, 21, 1855–1870. [Google Scholar] [CrossRef]
- Edel, K.H.; Marchadier, E.; Brownlee, C.; Kudla, J.; Hetherington, A.M. The Evolution of Calcium-Based Signalling in Plants. Curr. Biol. 2017, 27, R667–R679. [Google Scholar] [CrossRef]
- Xiao, X.H.; Yang, M.; Sui, J.L.; Qi, J.Y.; Fang, Y.J.; Hu, S.N.; Tang, C.R. The calcium-dependent protein kinase (CDPK) and CDPK-related kinase gene families in Hevea brasiliensis-comparison with five other plant species in structure, evolution, and expression. FEBS Open Bio 2017, 7, 4–24. [Google Scholar] [CrossRef]
- Patel, S.; Marchant, J.S.; Brailoiu, E. Two-pore channels: Regulation by NAADP and customized roles in triggering calcium signals. Cell Calcium 2010, 47, 480–490. [Google Scholar] [CrossRef]
- Dematteis, G.; Tapella, L.; Casali, C.; Talmon, M.; Tonelli, E.; Reano, S.; Ariotti, A.; Pessolano, E.; Malecka, J.; Chrostek, G.; et al. ER-mitochondria distance is a critical parameter for efficient mitochondrial Ca2+ uptake and oxidative metabolism. Commun. Biol. 2024, 7, 1294. [Google Scholar] [CrossRef]
- Stael, S.; Wurzinger, B.; Mair, A.; Mehlmer, N.; Vothknecht, U.C.; Teige, M. Plant organellar calcium signalling: An emerging field. J. Exp. Bot. 2012, 63, 1525–1542. [Google Scholar] [CrossRef]
- Carraretto, L.; Checchetto, V.; De Bortoli, S.; Formentin, E.; Costa, A.; Szabo, I.; Teardo, E. Calcium Flux across Plant Mitochondrial Membranes: Possible Molecular Players. Front. Plant Sci. 2016, 7, 354. [Google Scholar] [CrossRef] [PubMed]
- Cortese, E.; Moscatiello, R.; Pettiti, F.; Carraretto, L.; Baldan, B.; Frigerio, L.; Vothknecht, U.C.; Szabo, I.; De Stefani, D.; Brini, M.; et al. Monitoring calcium handling by the plant endoplasmic reticulum with a low-Ca2+-affinity targeted aequorin reporter. Plant J. 2022, 109, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Ramazanov, B.R.; Parchure, A.; Di Martino, R.; Kumar, A.; Chung, M.; Kim, Y.; Griesbeck, O.; Schwartz, M.A.; Luini, A.; von Blume, J. Calcium flow at ER-TGN contact sites facilitates secretory cargo export. Mol. Biol. Cell 2024, 35, ar50. [Google Scholar] [CrossRef] [PubMed]
- Rayl, M.; Truitt, M.; Held, A.; Sargeant, J.; Thorsen, K.; Hay, J.C. Penta-EF-Hand Protein Peflin Is a Negative Regulator of ER-To-Golgi Transport. PLoS ONE 2016, 11, e0157227. [Google Scholar] [CrossRef]
- Malek, M.; Wawrzyniak, A.M.; Koch, P.; Luchtenborg, C.; Hessenberger, M.; Sachsenheimer, T.; Jang, W.; Brugger, B.; Haucke, V. Inositol triphosphate-triggered calcium release blocks lipid exchange at endoplasmic reticulum-Golgi contact sites. Nat. Commun. 2021, 12, 2673. [Google Scholar] [CrossRef]
- Yang, Y.; Valencia, L.A.; Lu, C.H.; Nakamoto, M.L.; Tsai, C.T.; Liu, C.; Yang, H.; Zhang, W.; Jahed, Z.; Lee, W.R.; et al. Plasma membrane curvature regulates the formation of contacts with the endoplasmic reticulum. Nat. Cell Biol. 2024, 26, 1878–1891. [Google Scholar] [CrossRef]
- Gil, D.; Guse, A.H.; Dupont, G. Three-Dimensional Model of Sub-Plasmalemmal Ca2+ Microdomains Evoked by the Interplay Between ORAI1 and InsP(3) Receptors. Front. Immunol. 2021, 12, 659790. [Google Scholar] [CrossRef]
- Liu, L.; Li, J. Communications Between the Endoplasmic Reticulum and Other Organelles During Abiotic Stress Response in Plants. Front. Plant Sci. 2019, 10, 749. [Google Scholar] [CrossRef] [PubMed]
- Saheki, Y.; De Camilli, P. Endoplasmic Reticulum-Plasma Membrane Contact Sites. Annu. Rev. Biochem. 2017, 86, 659–684. [Google Scholar] [CrossRef]
- Pottosin, I.; Dobrovinskaya, O. Major vacuolar TPC1 channel in stress signaling: What matters, K+, Ca2+ conductance or an ion-flux independent mechanism? Stress Biol. 2022, 2, 31. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Wong, Y.C.; Krainc, D. Mitochondria-lysosome contacts regulate mitochondrial Ca2+ dynamics via lysosomal TRPML1. Proc. Natl. Acad. Sci. USA 2020, 117, 19266–19275. [Google Scholar] [CrossRef] [PubMed]
- Rosencrans, W.M.; Rajendran, M.; Bezrukov, S.M.; Rostovtseva, T.K. VDAC regulation of mitochondrial calcium flux: From channel biophysics to disease. Cell Calcium 2021, 94, 102356. [Google Scholar] [CrossRef]
- Sargsyan, Y.; Bickmeyer, U.; Gibhardt, C.S.; Streckfuss-Bomeke, K.; Bogeski, I.; Thoms, S. Peroxisomes contribute to intracellular calcium dynamics in cardiomyocytes and non-excitable cells. Life Sci. Alliance 2021, 4, e202000987. [Google Scholar] [CrossRef]
- Sargsyan, Y.; Kalinowski, J.; Thoms, S. Calcium in peroxisomes: An essential messenger in an essential cell organelle. Front. Cell Dev. Biol. 2022, 10, 992235. [Google Scholar] [CrossRef]
- Valm, A.M.; Cohen, S.; Legant, W.R.; Melunis, J.; Hershberg, U.; Wait, E.; Cohen, A.R.; Davidson, M.W.; Betzig, E.; Lippincott-Schwartz, J. Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature 2017, 546, 162–167. [Google Scholar] [CrossRef]
- Faxen, K.; Andersen, J.L.; Gourdon, P.; Fedosova, N.; Morth, J.P.; Nissen, P.; Moller, J.V. Characterization of a Listeria monocytogenes Ca2+ pump: A SERCA-type ATPase with only one Ca2+-binding site. J. Biol. Chem. 2011, 286, 1609–1617. [Google Scholar] [CrossRef]
- Prabudiansyah, I.; Oradd, F.; Magkakis, K.; Pounot, K.; Levantino, M.; Andersson, M. Dephosphorylation and ion binding in prokaryotic calcium transport. Sci. Adv. 2024, 10, eadp2916. [Google Scholar] [CrossRef]
- Suarez-Delgado, E.; Islas, L.D. A novel origin for calcium selectivity. eLife 2020, 9, e55216. [Google Scholar] [CrossRef]
- Kolodkin-Gal, I.; Parsek, M.R.; Patrauchan, M.A. The roles of calcium signaling and calcium deposition in microbial multicellularity. Trends Microbiol. 2023, 31, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- King, M.M.; Kayastha, B.B.; Franklin, M.J.; Patrauchan, M.A. Calcium Regulation of Bacterial Virulence. Adv. Exp. Med. Biol. 2020, 1131, 827–855. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hu, Y.-Y.; Tang, Z.-X.; Jiang, Z.-B.; Huang, J.; Zhang, T.; Shen, H.-Y.; Ye, X.-P.; Huang, X.-Y.; Wang, X.; et al. Calcium Transport and Enrichment in Microorganisms: A Review. Foods 2024, 13, 3612. [Google Scholar] [CrossRef]
- Steiner, P.; Buchner, O.; Andosch, A.; Wanner, G.; Neuner, G.; Lutz-Meindl, U. Fusion of Mitochondria to 3-D Networks, Autophagy and Increased Organelle Contacts are Important Subcellular Hallmarks during Cold Stress in Plants. Int. J. Mol. Sci. 2020, 21, 8753. [Google Scholar] [CrossRef]
- Steiner, P.; Luckner, M.; Kerschbaum, H.; Wanner, G.; Lutz-Meindl, U. Ionic stress induces fusion of mitochondria to 3-D networks: An electron tomography study. J. Struct. Biol. 2018, 204, 52–63. [Google Scholar] [CrossRef]
- Edel, K.H.; Kudla, J. Increasing complexity and versatility: How the calcium signaling toolkit was shaped during plant land colonization. Cell Calcium 2015, 57, 231–246. [Google Scholar] [CrossRef]
- Swarbreck, S.M.; Colaco, R.; Davies, J.M. Plant calcium-permeable channels. Plant Physiol. 2013, 163, 514–522. [Google Scholar] [CrossRef]
- Kudla, J.; Becker, D.; Grill, E.; Hedrich, R.; Hippler, M.; Kummer, U.; Parniske, M.; Romeis, T.; Schumacher, K. Advances and current challenges in calcium signaling. New Phytol. 2018, 218, 414–431. [Google Scholar] [CrossRef]
- Plieth, C. Plant calcium signaling and monitoring: Pros and cons and recent experimental approaches. Protoplasma 2001, 218, 1–23. [Google Scholar] [CrossRef]

| Kingdom | Organelles | Functionality | References |
|---|---|---|---|
| Animal | ER—mitochondria | Spatial Ca2+ transfer (IP3R) | [203] |
| Animal | Plasma membrane—ER | Lipid- and Ca2+ homeostasis (ORAI, IP3R) | [210,211] |
| Animal | Endolysosomes—ER | Spatial Ca2+ transfer (TPC) | [37,39,48] |
| Animal | Endolysosomes—mitochondria | Intracellular and mitochondrial Ca2+ dynamics (TRPML) | [215,216] |
| Animal | ER—Golgi | Interorganellar Ca2+ regulation (IP3R) | [207,208,209] |
| Plant | Chloroplast—mitochondria | Ca2+ signaling (MCU) | [204,205] |
| Plant | Plasma membrane—ER | Lipid- and Ca2+ homeostasis | [212,213] |
| Plant | Chloroplast—ER | Intracellular Ca2+ regulation | [206] |
| Plant | Vacuole—ER | Vacuolar Ca2+ buffering and spatial Ca2+ transfer (TPC) | [214,216] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steiner, P.; Zierler, S. Inter-Organellar Ca2+ Homeostasis in Plant and Animal Systems. Cells 2025, 14, 1204. https://doi.org/10.3390/cells14151204
Steiner P, Zierler S. Inter-Organellar Ca2+ Homeostasis in Plant and Animal Systems. Cells. 2025; 14(15):1204. https://doi.org/10.3390/cells14151204
Chicago/Turabian StyleSteiner, Philip, and Susanna Zierler. 2025. "Inter-Organellar Ca2+ Homeostasis in Plant and Animal Systems" Cells 14, no. 15: 1204. https://doi.org/10.3390/cells14151204
APA StyleSteiner, P., & Zierler, S. (2025). Inter-Organellar Ca2+ Homeostasis in Plant and Animal Systems. Cells, 14(15), 1204. https://doi.org/10.3390/cells14151204






