Purified Cornel Iridoid Glycosides Attenuated Oxidative Stress Induced by Cerebral Ischemia-Reperfusion Injury via Morroniside and Loganin Targeting Nrf2/NQO-1/HO-1 Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
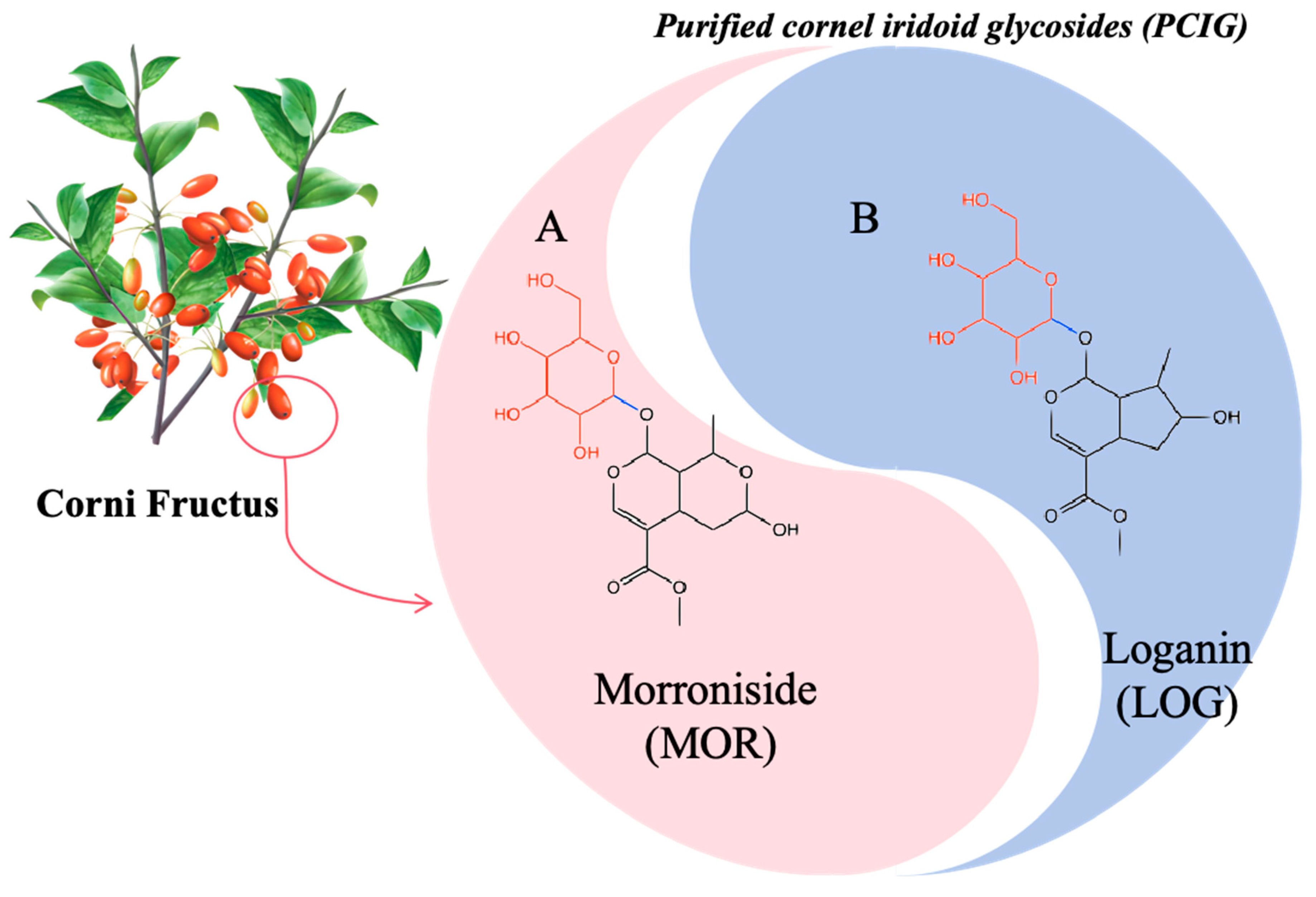
2.1. Drugs
2.2. Animal, Middle Cerebral Artery Occlusion Injury (MCAO/R) and Drug Treatment
2.3. Neurological Deficit
2.4. 2,3,5-Triphenyltetrazolium Hydrochloride (TTC) Staining
2.5. Brain Water Content Measurement
2.6. H&E and TUNEL Staining
2.7. Cell Culture and Treatment
2.8. MTT Assays
2.9. Determination of ROS Levels
2.10. Determination of Mitochondrial Function
2.11. Immunofluorescence Detection
2.12. Western Blotting
2.13. Biochemical Analysis
2.14. Molecular Docking
2.15. Statistical Analysis
3. Results
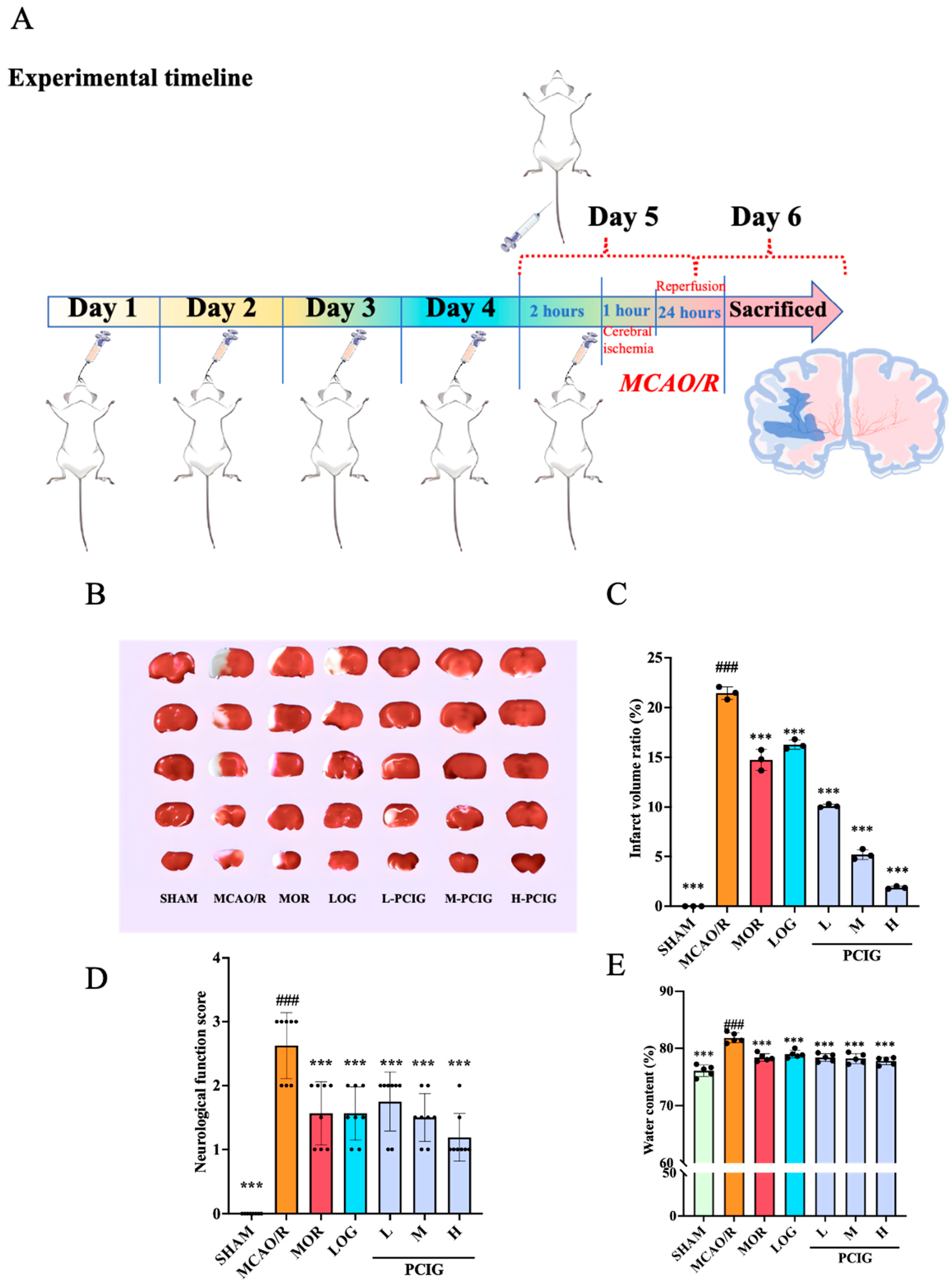
3.1. Protected the Rats Against MCAO/R-Induced Brain Damages
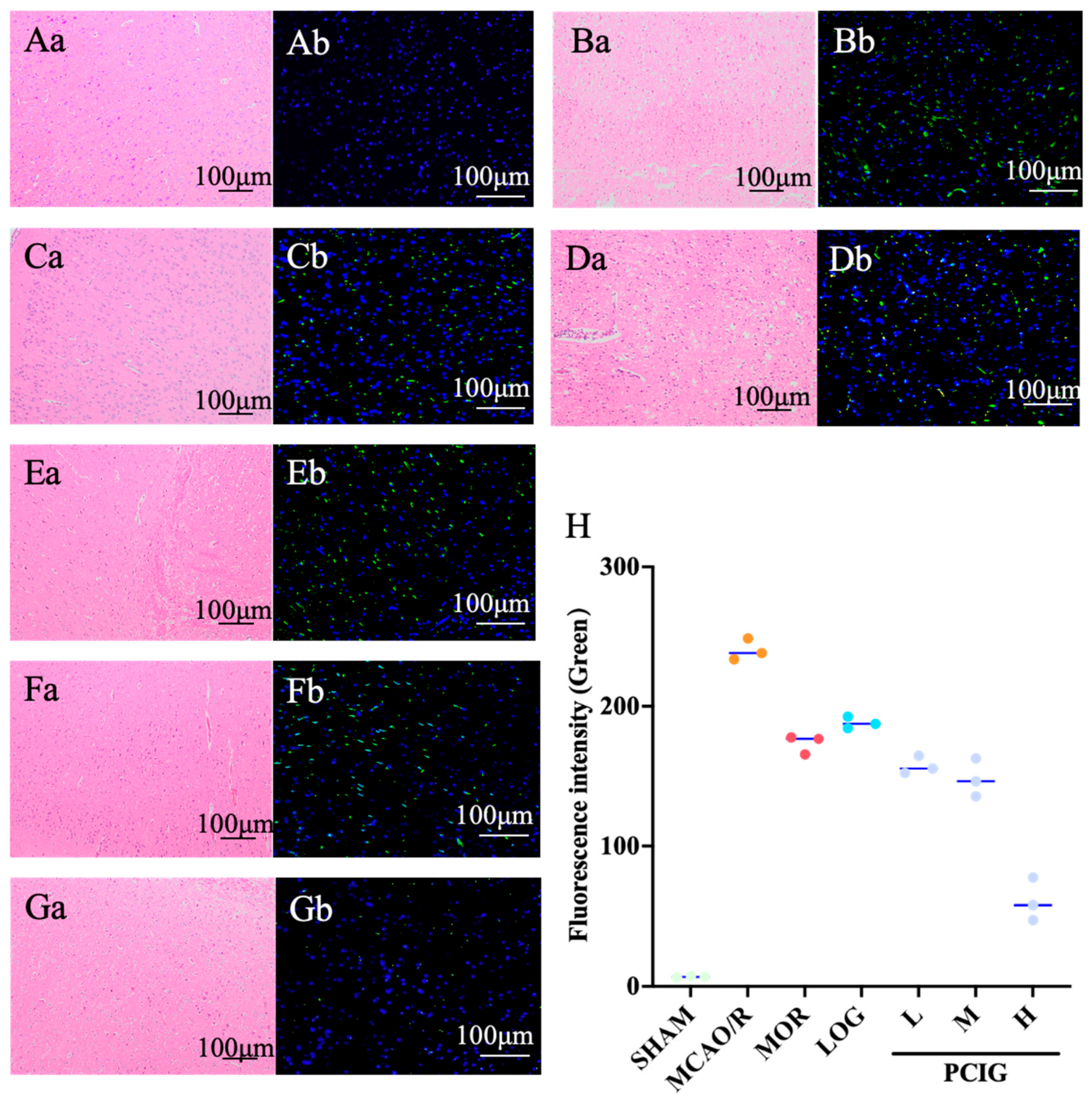
3.2. Improvement on Histopathological Changes in MCAO/R Rats
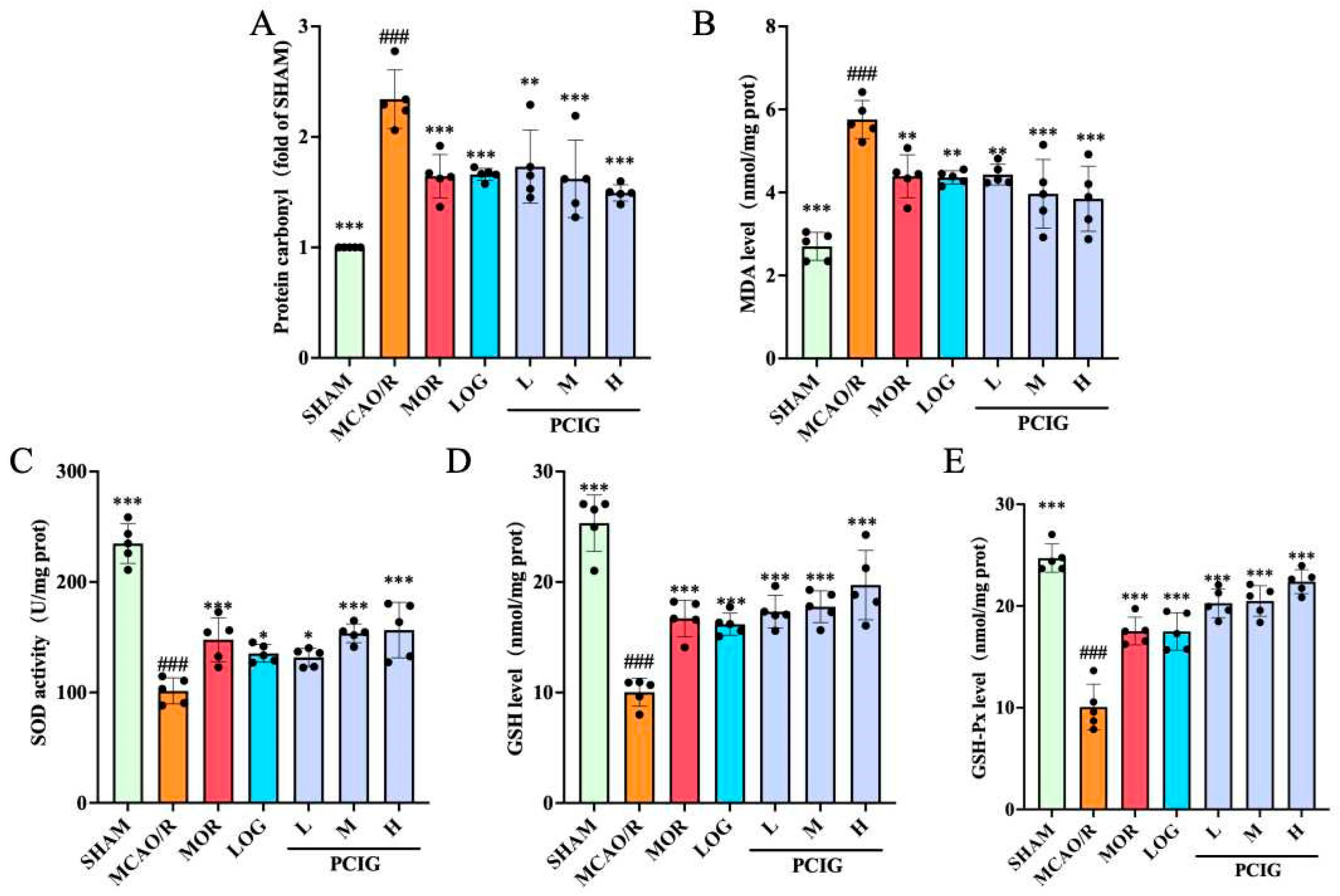
3.3. Enhanced the Antioxidant Capacity of MCAO/R Rats
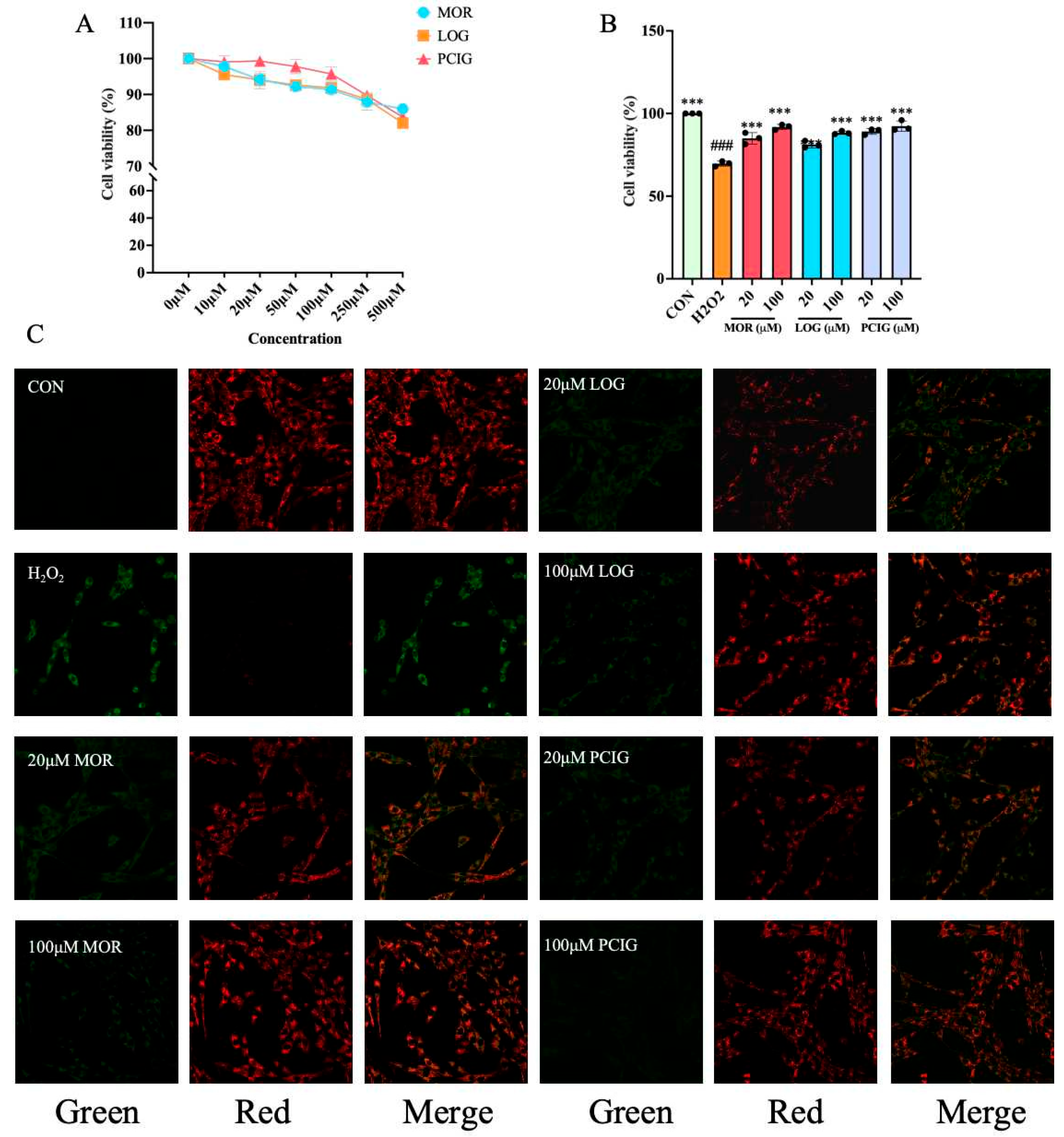
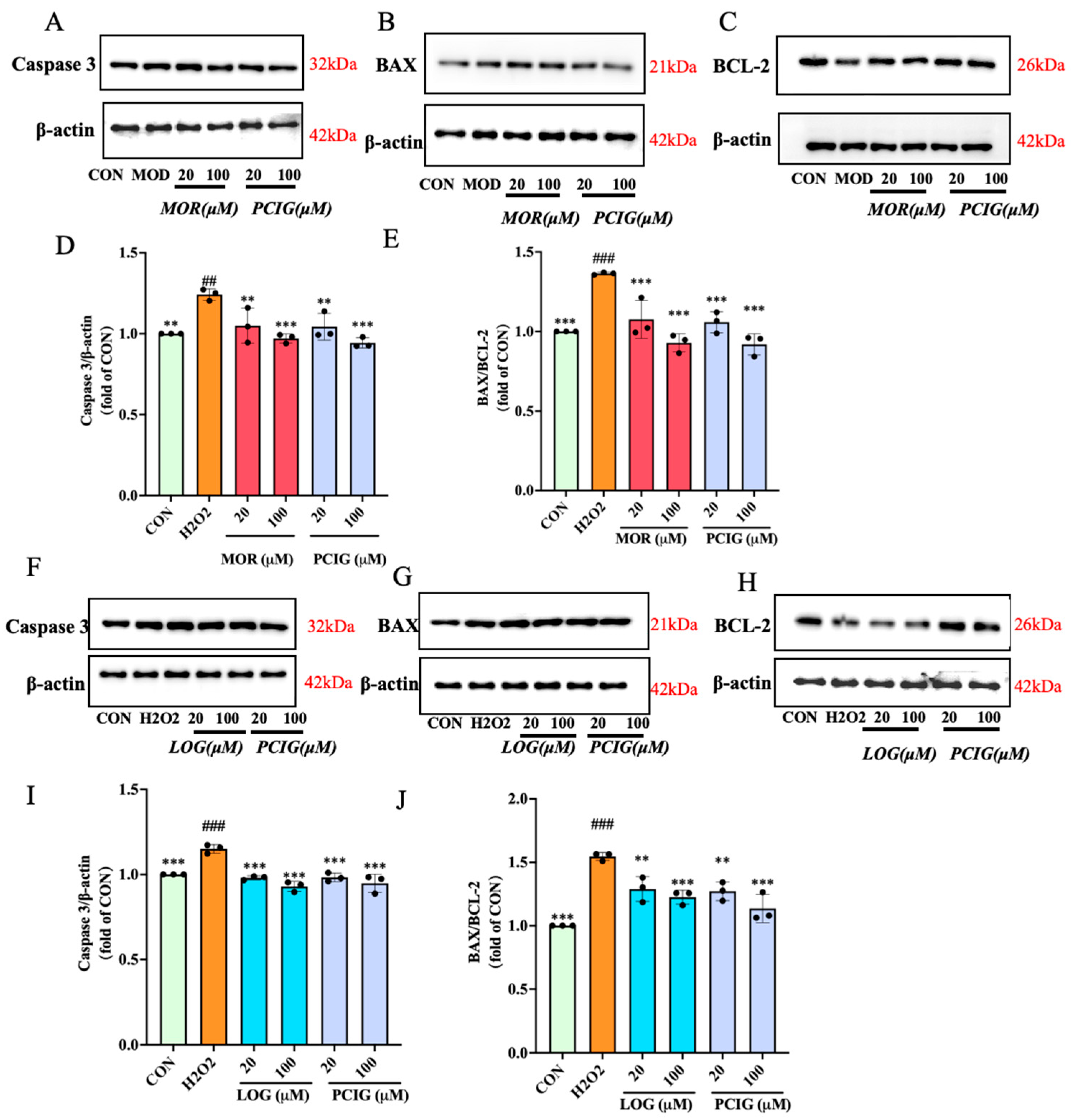
3.4. Protected PC12 Cells Against H2O2-Induced Cell Apoptosis
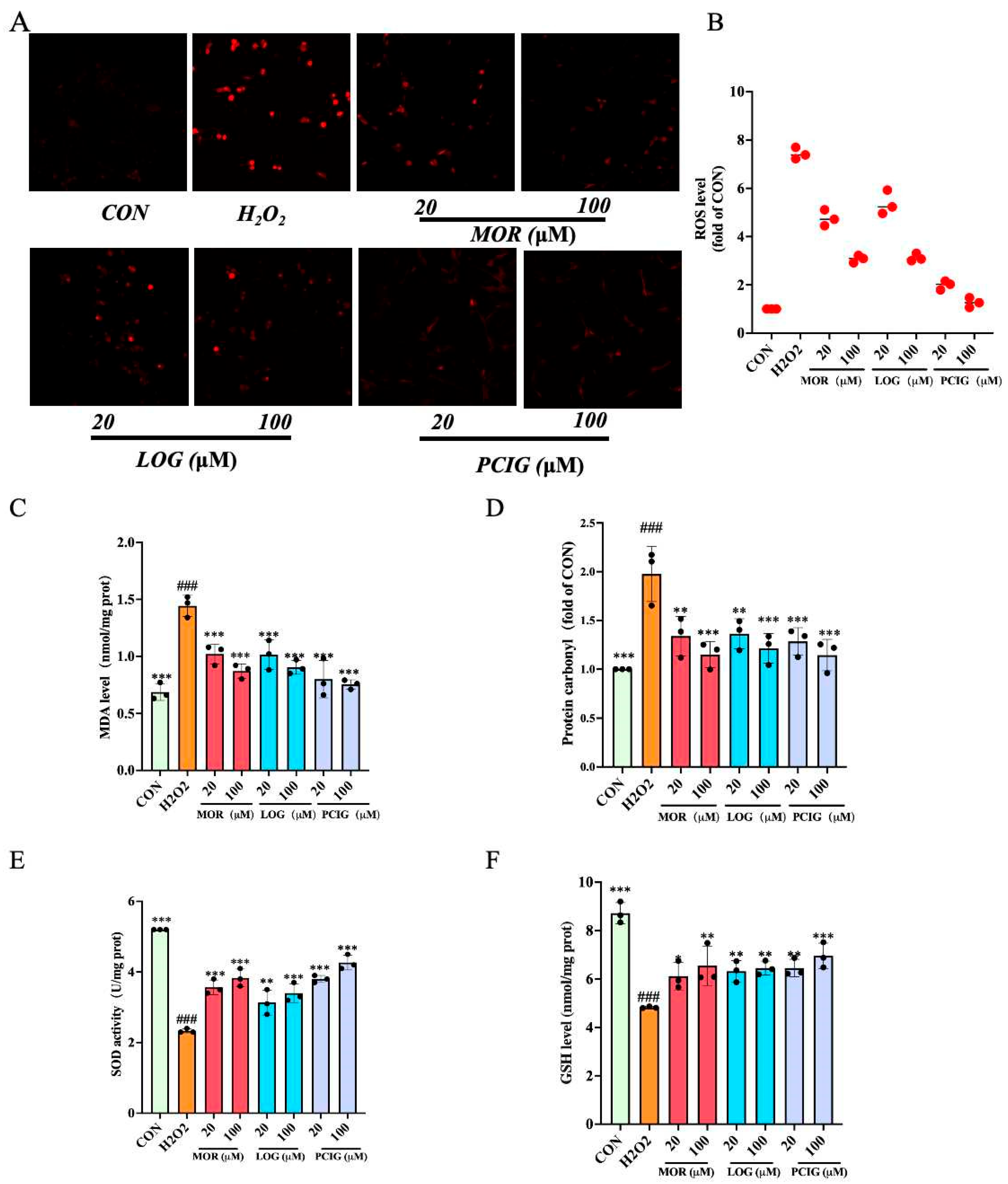
3.5. Enhanced Activities on Scavenging ROS
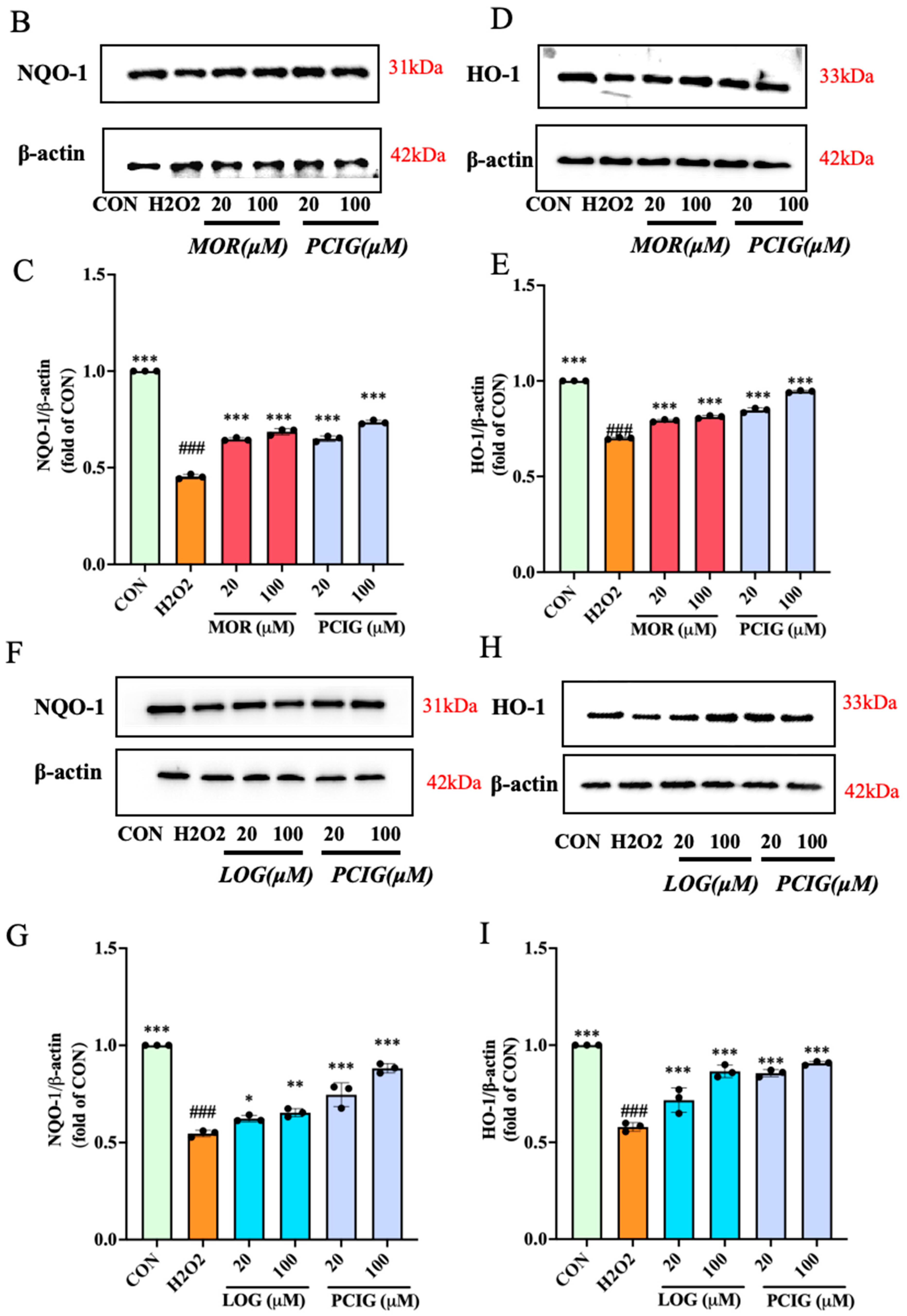
3.6. Activated Signaling Pathways of Nrf2/NQO-1 and Nrf2/HO-1
3.7. Molecular Docking Studies of Morroniside and Loganin with Keap1
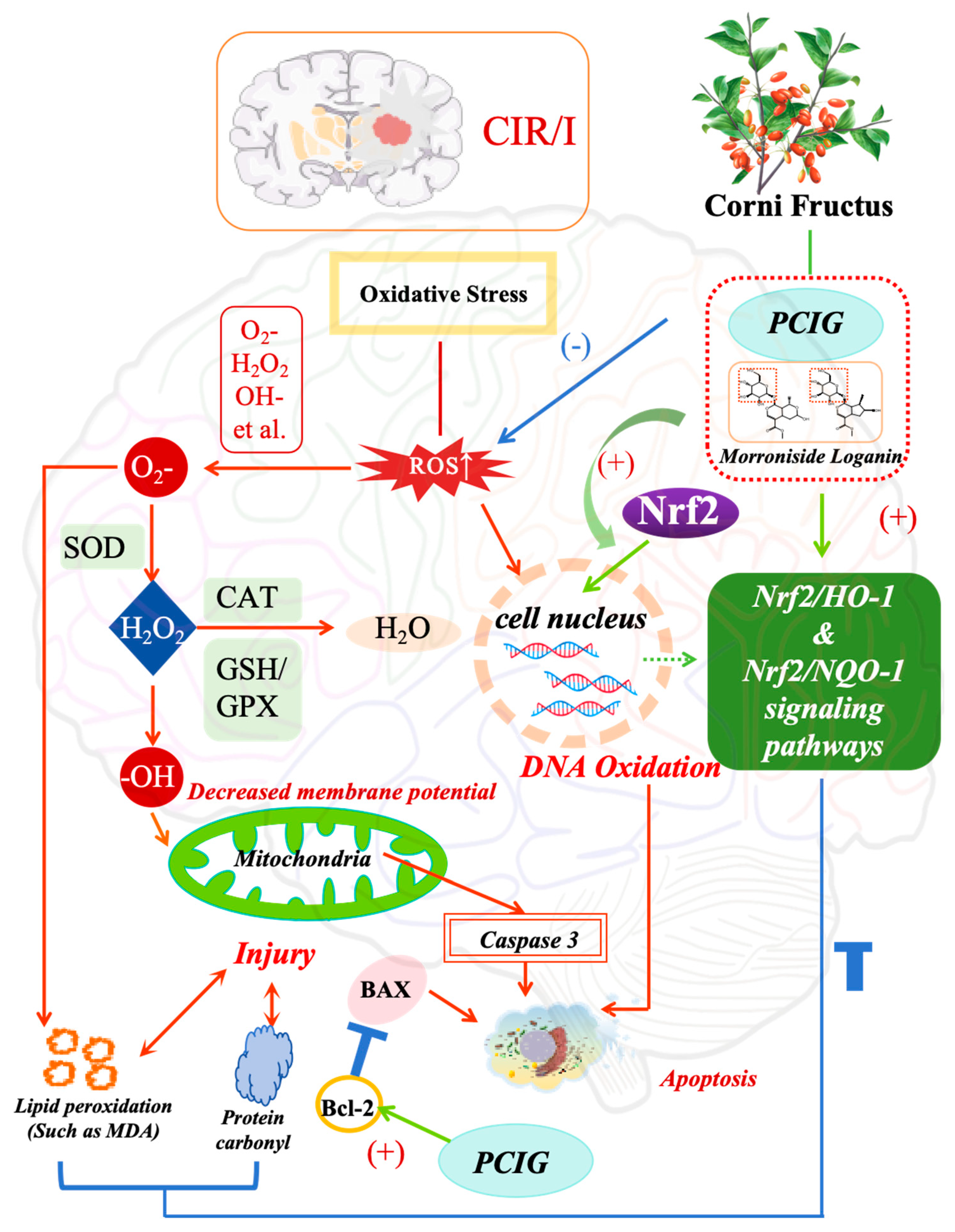
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bevers, M.B.; Battey, T.W.K.; Ostwaldt, A.C.; Jahan, R.; Saver, J.L.; Kimberly, W.T.; Kidwell, C.S. Apparent diffusion coefficient signal intensity ratio predicts the effect of revascularization on ischemic cerebral edema. Cerebrovasc. Dis. 2018, 45, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Jolugbo, P.; Ariëns, R.A.S. Thrombus composition and efficacy of thrombolysis and thrombectomy in acute ischemic stroke. Stroke 2021, 52, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Saini, V.; Guada, L.; Yavagal, D.R. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology 2021, 97, S6–S16. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wang, L.; Song, G.; Cai, X.; Wang, W.; Chen, J.; Wang, G. Physcion protects rats against cerebral ischemia-repertusion injury via inhibition of TLR4/NF-κB sigaling ratbway. Drug Des. Dev. Ther. 2021, 15, 277–287. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, D. Research progress on related mechanisms of cerebral ischemia-reperfusion injury. Acta Neuropharmacol. 2020, 10, 60–63. [Google Scholar]
- Zhang, H.; Feng, Y.; Si, Y.; Lu, C.; Wang, J.; Wang, S.; Li, L.; Xie, W.; Yue, Z.; Yong, J.; et al. Shank3 ameliorates neuronal injury after cerebral ischemia/reperfusion via inhibiting oxidative stress and inflammation. Redox Biol. 2024, 69, 102983. [Google Scholar] [CrossRef]
- Liang, G.; Shi, B.; Luo, W.; Yang, J. The protective effect of caffeic acid on global cerebral ischemia-reperfusion injury in rats. Behav. Brain Funct. 2015, 11, 18. [Google Scholar] [CrossRef]
- Lim, C.; Zhen, A.X.; Ok, S.; Fernando, P.D.S.M.; Herath, H.M.U.L.; Piao, M.J.; Hyun, J.W. Hesperidin Protects SH-SY5Y Neuronal Cells against High Glucose-Induced Apoptosis via Regulation of MAPK Signaling. Antioxidants 2022, 11, 1707. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, 453–462. [Google Scholar] [CrossRef]
- Wang, L.; Wei, Y.; Sun, Z.; Tai, W.; Li, H.; Yin, Y.; Jiang, L.H.; Wang, J.Z. Effectiveness and mechanisms of combined use of antioxidant nutrients in protecting against oxidative stress-induced neuronal loss and related neurological deficits. CNS Neurosci. Ther. 2024, 30, e14886. [Google Scholar] [CrossRef]
- Li, Q.; Fadoul, G.; Ikonomovic, M.; Yang, T.; Zhang, F. Sulforaphane promotes white matter plasticity and improves long-term neurological outcomes after ischemic stroke via the Nrf2 pathway. Free Radic. Biol. Med. 2022, 193, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Li, Z.; Gao, X.; Wu, X.; Pi, J.; Wang, X. Melatonin attenuates brain edema via the PI3K/Akt/Nrf2 pathway in rats with cerebral ischemia-reperfusion injury. J. Stroke Cerebrovasc. Dis. 2025, 34, 108299. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, Y.; Zhou, H.; Chen, H.; Wu, X.; Wu, Z.; Zheng, Z. Synthesis, target analysis, and cerebroprotective effects of novel imide antioxidants via the Nrf2/HO-1 pathway in cerebral ischemia-reperfusion injury. Front. Pharmacol. 2025, 16, 1552717. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Wang, Q.; Liu, Y.; You, Q.; Wei, W.; Zhu, C.; Liu, N. Isoliquiritigenin alleviates cerebral ischemia-reperfusion injury by reducing oxidative stress and ameliorating mitochondrial dysfunction via activating the Nrf2 pathway. Redox Biol. 2024, 77, 103406. [Google Scholar] [CrossRef]
- Guo, R.; Xue, F.; Zhang, J.; Li, J.; Li, H.; Qiao, B. Cornel iridoid glycosides exerted neuroprotective effects against cerebral ischemia/reperfusion injury in rats via inhibiting TLR4/MyD88/NF-κB pathway. Eur. J. Pharmacol. 2025, 1001, 177742. [Google Scholar] [CrossRef]
- National Pharmacopoeia Committee. Pharmacopoeia of People’s Republic of China; Chemical Industry Press: Beijing, China, 2020. [Google Scholar]
- Cao, L.; Wu, Y.; Li, W.; Zhang, Z.; Niu, Y.; Li, C.; Gu, S. Cornus officinalis vinegar reduces body weight and attenuates hepatic steatosis in mouse model of nonalcoholic fatty liver disease. J. Food Sci. 2022, 87, 3248–3259. [Google Scholar] [CrossRef]
- Jang, J.; Yang, G.; Seok, J.; Kang, H.; Cho, Y.; Lee, H.; Lee, J. Loganin prevents hepatic steatosis by blocking NLRP3 inflammasome activation. Biomol. Ther. 2023, 31, 40–47. [Google Scholar] [CrossRef]
- Hyun, H.; Cheol, P.; EunJin, B.; Hyuk, S.K.; Sung, J.B.; Eunjeong, K.; Yung, H.C. Morroniside protects C2C12 myoblasts from oxidative damage caused by ROS-mediated mitochondrial damage and induction of endoplasmic reticulum stress. Biomol. Ther. 2024, 32, 349–360. [Google Scholar]
- Wang, Z.; Xue, F.; Zhang, J.; Wang, Y.; Hu, E.; Zheng, Y.; Luo, X.; Li, H.; Qiao, B. The cornel Iridoid glycoside attenuated brain edema of the cerebral ischemia/reperfusion rats by modulating the polarized aquaporin 4. Fitoterapia 2024, 177, 106098. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, Y.; Su, W.; Zhang, H.; Li, C.; Routledge, M.N.; Gong, Y.; Qiao, B. Organ specific differences in alteration of aquaporin expression in rats treated with sennoside A, senna anthraquinones and rhubarb anthraquinones. Int. J. Mol. Sci. 2021, 22, 8026. [Google Scholar] [CrossRef]
- Mi, W.; Yu, M.; Yin, S.; Ji, Y.; Shi, T.; Li, N. Analysis of the Renal Protection and Antioxidative Stress Effects of Panax notoginseng Saponins in Diabetic Nephropathy Mice. J. Immunol. Res. 2022, 2022, 3610935. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Gao, J.; Liu, P.; Shen, Z.; Xu, K.; Wu, C.; Tian, F.; Chen, M.; Wang, L.; Li, P. Morroniside promotes PGC-1α-mediated cholesterol efflux in sodium palmitate or high glucose-induced mouse renal tubular epithelial cells. Biomed. Res. Int. 2021, 2021, 9942152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jin, B.; Liu, L.; Li, H.; Zheng, X.; Li, M.; Wang, K. Glutathione might attenuate arsenic-induced liver injury by modulating the Foxa2-XIAP axis to reduce oxidative stress and mitochondrial apoptosis. Biol. Trace Elem. Res. 2023, 201, 5201–5212. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, Y.; Yang, S.; Zhang, L.; Liu, B.; Zhang, J.; Yu, X.; Wei, X.; Li, S.; Wang, J.; et al. Targeting antioxidant factor Nrf2 by raffinose ameliorates lipid dysmetabolism-induced pyroptosis, inflammation and fibrosis in NAFLD. Phytomedicine 2024, 130, 155756. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xiao, J. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxid. Med. Cell. Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef]
- Hu, Y.; Cao, X.; Zhao, Y.; Jin, Y.; Li, F.; Xu, B.; Zhang, L. The function of Spag6 to repair brain edema damage after Cerebral Ischemic stroke-reperfusion. Neuroscience 2023, 522, 132–149. [Google Scholar] [CrossRef]
- Lee, Y.; Kao, S.; Cheng, C. Acorus tatarinowii Schott extract reduces cerebral edema caused by ischemia–reperfusion injury in rats: Involvement in regulation of astrocytic NKCC1/AQP4 and JNK/iNOS-mediated signaling. BMC Complement. Med. Ther. 2020, 20, 374. [Google Scholar] [CrossRef]
- Zou, R.; Liu, Z.; Wang, P.; Liu, Y. Ginkgolide B binds to GPX4 and FSP1 to alleviate cerebral ischemia/reperfusion injury in rats. Toxicol. Appl. Pharmacol. 2025, 495, 117237. [Google Scholar] [CrossRef]
- Jin, J.; Wang, H.; Zheng, X.; Xie, S.; Zheng, L.; Zhan, R. Inhibition of LncRNA MALAT1 attenuates cerebral ischemic reperfusion injury via regulating AQP4 expression. Eur. Neurol. 2020, 83, 581–590. [Google Scholar] [CrossRef]
- Liu, W.; Liu, X.; Liu, M.; Zhao, R.; Zhao, Z.; Xiao, J.; Xu, R. Oxyglutamate Carrier Alleviates Cerebral Ischaemia–Reperfusion Injury by Regulating Mitochondrial Function. Eur. J. Neurosci. 2025, 61, e16659. [Google Scholar] [CrossRef]
- Xu, X.; Duan, Z.; Zhou, X.; Zhao, R.; Xu, J.; Zhang, Z.; Cui, Y. SFXN1 reduction alleviates cerebral ischemia-reperfusion injury by promoting neuronal survival and reducing neuroinflammation. CNS Neurosci. Ther. 2025, 31, e70457. [Google Scholar] [CrossRef]
- Themistoklis, K.M.; Papasilekas, T.I.; Melanis, K.S.; Boviatsis, K.A.; Korfias, S.I.; Vekrellis, K.; Sakas, D.E. Transient intraluminal filament middle cerebral artery occlusion stroke model in rats: A step-by-step guide and technical considerations. World Neurosurg. 2022, 168, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.; Tran, M.; Linninger, A. Dynamic regulation of aquaporin-4 water channels in neurological disorders. Croat. Med. J. 2015, 56, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Q.; Zhang, X.; Sheng, X.; Zhou, Y.; Li, M.; Zhang, L. HPLC study of pharmacokinetics and tissue distribution of morroniside in rats. J. Pharm. Biomed. Anal. 2007, 45, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yan, Y.; Ding, K.; Lian, W.; Li, L.; Wang, W.; Xu, J. Development and validation of a simple and rapid UPLC-MS/MS method for loganin and its application in pharmacokinetic and tissue distribution studies. J. Ethnopharmacol. 2023, 319, 117130. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, L.; Wen, J. Studies on pharmacokinetics of loganin and morroniside in Cornus officinalis injection in mice. China J. Chin. Mater. Med. 2003, 28, 509–512. [Google Scholar]
- Ming, S.; Tian, J.; Ma, K.; Pei, C.; Li, L.; Wang, Z.; Fang, Z.; Liu, M.; Dong, H.; Li, W.; et al. Oxalate-induced apoptosis through ERS-ROS-NF-κB signalling pathway in renal tubular epithelial cell. Mol. Med. 2022, 28, 88. [Google Scholar] [CrossRef]
- Guo, J.; Yang, N.; Zhang, J.; Huang, Y.; Xiang, Q.; Wen, J.; Chen, Y.; Hu, T.; Liu, Q.; Rao, C. Neurotoxicity study of ethyl acetate extract of Zanthoxylum armatum DC. on SH-SY5Y based on ROS mediated mitochondrial apoptosis pathway. J. Ethnopharmacol. 2024, 319, 117321. [Google Scholar] [CrossRef]
- Madhuri, B.; Omme, F.S.; Md, A.I.; Alvir, R.; P Hemachandra, R. Rlip76 in ageing and Alzheimer’s disease: Focus on oxidative stress and mitochondrial mechanisms. Ageing Res. Rev. 2024, 103, 102600. [Google Scholar] [CrossRef]
- Lazina, H.; Karina, P.G.; Yang, X.; Emily, L.; Jacques, D.T.; Pierre, Y.W.; Spencer, B.G. Vascular Endothelial Growth Factor C (VEGF-C) sensitizes lymphatic endothelial cells to oxidative-stress-induced apoptosis through DNA damage and mitochondrial dysfunction: Implications for lymphedema. Int. J. Mol. Sci. 2024, 25, 7828. [Google Scholar]
- Jongwachirachai, P.; Ruankham, W.; Apiraksattayakul, S.; Intharakham, S.; Prachayasittikul, V.; Suwanjang, W.; Prachayasittikul, S.; Phopin, K. Neuroprotective Properties of Coriander-Derived Compounds on Neuronal Cell Damage under Oxidative Stress-Induced SH-SY5Y Neuroblastoma and in Silico ADMET Analysis. Neurochem. Res. 2024, 49, 3308–3325. [Google Scholar] [CrossRef]
- Zhang, R.; Teng, L.; Zhong, Y.; Ma, P.; Xu, L.; Xiao, P. Neuroprotection of isookanin against MPTP-induced cell death of SH-SY5Y cells via BCL2/BAX and PI3K/AKT pathways. Psychopharmacology 2023, 240, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Qadri, R.; Namdeo, M.; Behari, M.; Goyal, V.; Sharma, S.; Mukhopadhyay, A.K. Alterations in mitochondrial membrane potential in peripheral blood mononuclear cells in Parkinson’s Disease: Potential for a novel biomarker. Restor. Neurol. Neurosci. 2018, 36, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Alruhaimi, R.S.; Hassanein, E.H.M.; Ahmeda, A.F.; Alnasser, S.M.; Atwa, A.M.; Sabry, M.; Alzoghaibi, M.A.; Mahmoud, A.M. Attenuation of inflammation, oxidative stress and TGF-β1/Smad3 signaling and upregulation of Nrf2/HO-1 signaling mediate the protective effect of diallyl disulfide against cadmium nephrotoxicity. Tissue Cell 2024, 91, 102576. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Yuan, P.; Zhu, Y.; Hu, P.; Song, T.; Liu, R.; Liu, H.; Cai, D. Time-restricted feeding relieves high temperature-induced impairment on meat quality by activating the Nrf2/HO-1 pathway, modification of muscle fiber composition, and enriching the polyunsaturated fatty acids in pigs. Stress Biol. 2024, 4, 39. [Google Scholar] [CrossRef]
- Bansal, S.; Biswas, G.; Avadhani, N.G. Mitochondria-targeted heme oxygenase-1 induces oxidative stress and mitochondrial dysfunction in macrophages, kidney fibroblasts and in chronic alcohol hepatotoxicity. Redox Biol. 2014, 2, 273–283. [Google Scholar] [CrossRef]
- Wang, M.; Sun, J.; Yu, T.; Wang, M.; Jin, L.; Liang, S.; Liang, G. Diacerein protects liver against APAP-induced injury via targeting JNK and inhibiting JNK-mediated oxidative stress and apoptosis. Biomed. Pharmacother. 2022, 149, 112917. [Google Scholar] [CrossRef]
- He, Y.; Jiang, K.; Zhao, X. Taraxasterol protects hippocampal neurons from oxygen-glucose deprivation-induced injury through activation of Nrf2 signalling pathway. Artif. Cells Nanomed. Biotechnol. 2020, 48, 252–258. [Google Scholar] [CrossRef]
- Forman, H.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Liu, M.; Guan, G.; Wang, Y.; Lu, X.; Duan, X.; Xu, X. p-Hydroxy benzaldehyde, a phenolic compound from Nostoc commune, ameliorates DSS-induced colitis against oxidative stress via the Nrf2/HO-1/NQO-1/NF-κB/AP-1 pathway. Phytomedicine 2024, 133, 155941. [Google Scholar] [CrossRef]
- Huang, H.; Kuang, X.; Zhao, X.; Cheng, H.; Zou, Y.; Du, H.; Tang, H.; Zhou, L.; Zeng, Z.; Liu, H. Maintaining blood retinal barrier homeostasis to attenuate retinal ischemia-reperfusion injury by targeting the KEAP1/NRF2/ARE pathway with lycopene. Cell. Signal. 2021, 88, 110153. [Google Scholar] [CrossRef] [PubMed]
- Alkhalifah, E.A.R.; Alobaid, A.A.; Almajed, M.A.; Alomair, M.K.; Alabduladheem, L.S.; Al-Subaie, S.F.; Akbar, A.; Attimarad, M.V.; Younis, N.S.; Mohamed, M.E. Cardamom extract alleviates the oxidative stress, inflammation and apoptosis induced during acetaminophen-induced hepatic toxicity via modulating Nrf2/HO-1/NQO-1 pathway. Curr. Issues Mol. Biol. 2022, 44, 5390–5404. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Li, J.; Meng, C.; Li, Y.; Tang, H.; Wang, J.; Deng, P.; Lu, Y. Diet-derived circulating antioxidants and risk of stroke: A mendelian randomization study. Oxid. Med. Cell. Longev. 2022, 2022, 6457318. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, F.; Wen, F.; Raghu, G.; Marenzi, G.; Jaeschke, H.; Richeldi, L.; Calverley, P.M.A. The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disorders Characterized by Oxidative Stress. Curr. Neuropharma 2021, 19, 1202–1224. [Google Scholar]
- Shang, Y.; Xue, W.; Kong, J.; Chen, Y.; Qiu, X.; An, X.; An, J. Ultrafine black carbon caused mitochondrial oxidative stress, mitochondrial dysfunction and mitophagy in SH-SY5Y cells. Sci. Total Environ. 2021, 813, 151899. [Google Scholar] [CrossRef]
- Komakula, S.; Bhatia, R.; Sahib, A.; Upadhyay, A.; Leve, J.; Garg, A.; Vishnu, V.; Pandit, A.; Vibha, D.; Singh, M. Safety and efficacy of n-acetylcysteine (nac) as an adjunct to standard treatment in patients with acute ischemic stroke: A randomized controlled pilot trial (nactlys). Sci. Rep. 2024, 14, 9. [Google Scholar] [CrossRef]
- Rachel, S.; Emily, O.; Lorraine, W. Oxidative stress and the use of antioxidants in stroke. Antioxidants 2014, 3, 472–501. [Google Scholar] [CrossRef]
- Briones-Valdivieso, C.; Briones, F.; Sofía, O.; Chichiarelli, S.; Saso, L.; Ramón, R. Novel multi-antioxidant approach for ischemic stroke therapy targeting the role of oxidative stress. Biomedicines 2024, 12, 23. [Google Scholar] [CrossRef]

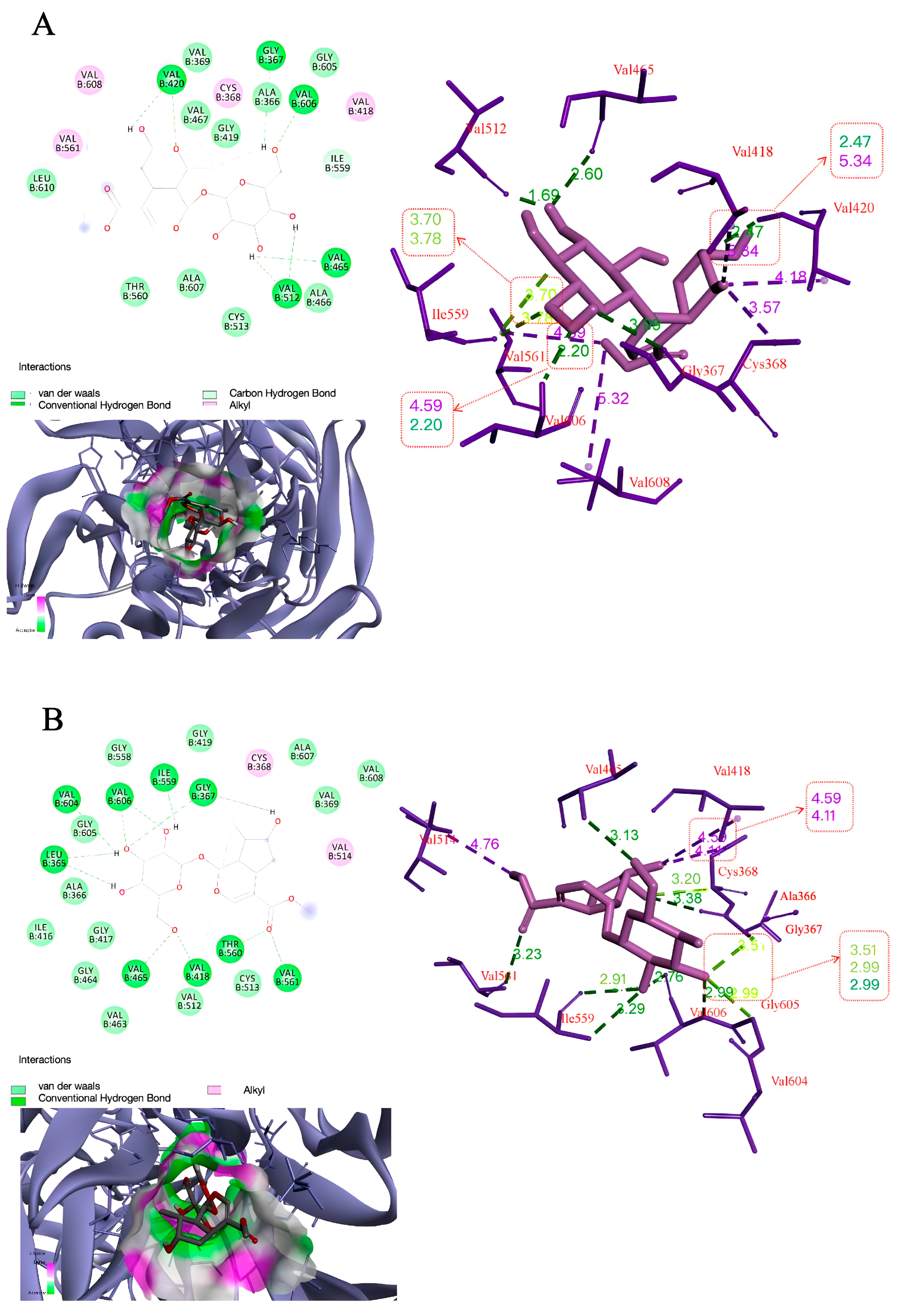
| Binding Energy (kcal/mol) | Hydrogen Bonding Interactions | Distance (A°) | |
|---|---|---|---|
| Morroniside | −5.31 | GLY367 | 3.09 |
| VAL420 | 2.47, 2.20 | ||
| VAL465 | 2.60 | ||
| VAL512 | 2.22, 1.69 | ||
| VAL606 | 2.20 | ||
| Loganin | −6.54 | GLY367 | 2.42, 2.56 |
| ILE559 | 1.96 | ||
| LEU365 | 2.83, 2.82 | ||
| THR560 | 2.72 | ||
| VAL418 | 2.65 | ||
| VAL465 | 2.23 | ||
| VAL561 | 2.75 | ||
| VAL604 | 2.96 | ||
| VAL606 | 2.06, 2.44 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Xue, F.; Hu, E.; Wang, Y.; Li, H.; Qiao, B. Purified Cornel Iridoid Glycosides Attenuated Oxidative Stress Induced by Cerebral Ischemia-Reperfusion Injury via Morroniside and Loganin Targeting Nrf2/NQO-1/HO-1 Signaling Pathway. Cells 2025, 14, 1205. https://doi.org/10.3390/cells14151205
Wang Z, Xue F, Hu E, Wang Y, Li H, Qiao B. Purified Cornel Iridoid Glycosides Attenuated Oxidative Stress Induced by Cerebral Ischemia-Reperfusion Injury via Morroniside and Loganin Targeting Nrf2/NQO-1/HO-1 Signaling Pathway. Cells. 2025; 14(15):1205. https://doi.org/10.3390/cells14151205
Chicago/Turabian StyleWang, Zhaoyang, Fangli Xue, Enjie Hu, Yourui Wang, Huiliang Li, and Boling Qiao. 2025. "Purified Cornel Iridoid Glycosides Attenuated Oxidative Stress Induced by Cerebral Ischemia-Reperfusion Injury via Morroniside and Loganin Targeting Nrf2/NQO-1/HO-1 Signaling Pathway" Cells 14, no. 15: 1205. https://doi.org/10.3390/cells14151205
APA StyleWang, Z., Xue, F., Hu, E., Wang, Y., Li, H., & Qiao, B. (2025). Purified Cornel Iridoid Glycosides Attenuated Oxidative Stress Induced by Cerebral Ischemia-Reperfusion Injury via Morroniside and Loganin Targeting Nrf2/NQO-1/HO-1 Signaling Pathway. Cells, 14(15), 1205. https://doi.org/10.3390/cells14151205






