Impaired Long-Term Quantitative Cellular Response to SARS-CoV-2 Vaccine in Thiopurine-Treated IBD Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Assessment of Humoral Immune Response
2.3. Assessment of Cellular Immune Response
2.4. Clinical Variables in Inflammatory Bowel Disease, Vaccine Effect, and COVID-19 Infection
2.5. Statistical Analysis
3. Results
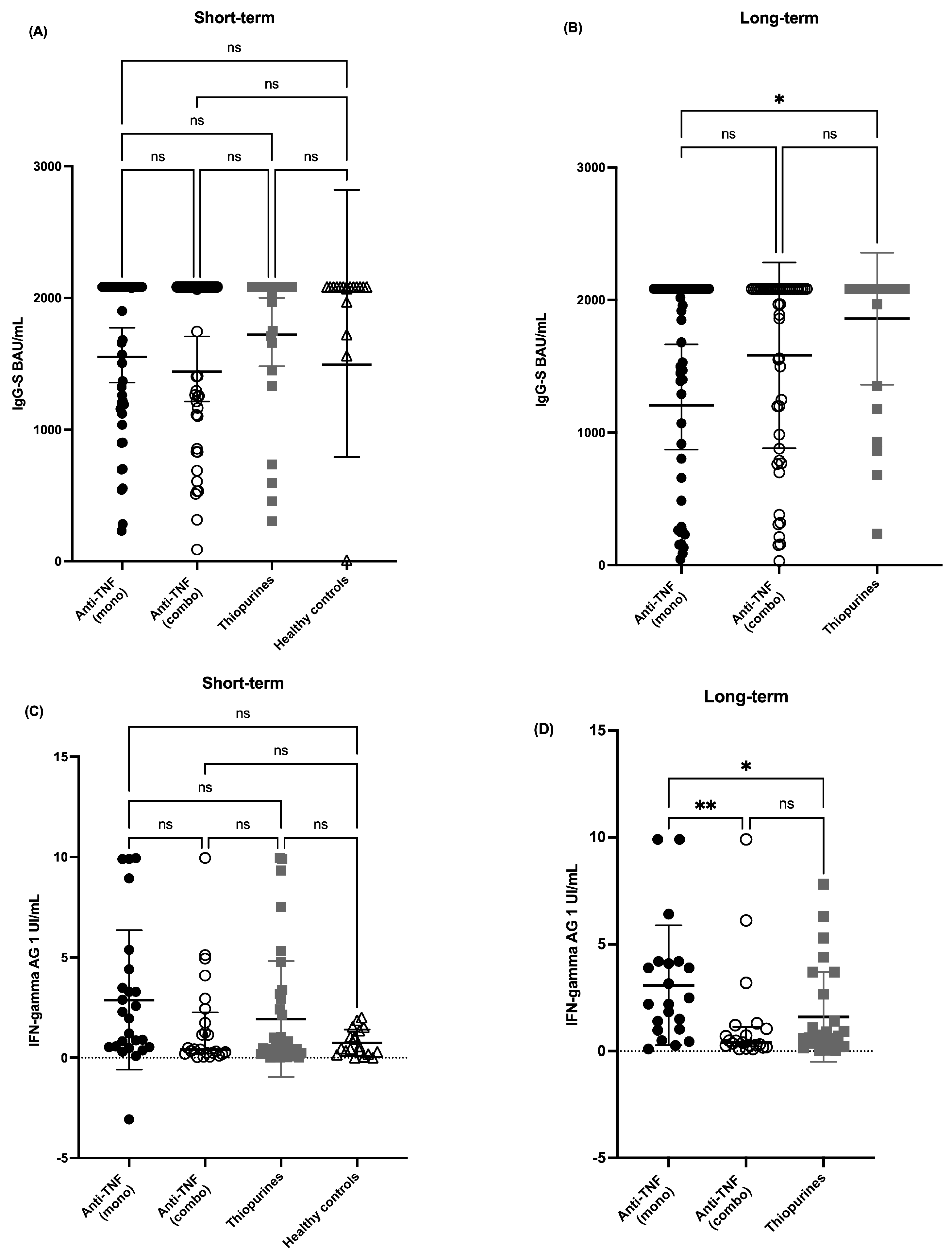
3.1. Humoral Immune Response
3.2. Cellular Immune Response
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AE | Adverse Event |
| AG1/AG2 | Antigen Tube 1/Antigen Tube 2 |
| BAU/mL | Binding Antibody Units per Milliliter |
| CD | Crohn’s Disease |
| CEIm | Comité de Ética de Investigación con Medicamentos |
| CLIA | Chemiluminescence Immunoassay |
| COVID-19 | Coronavirus Disease 2019 |
| HC | Healthy Controls |
| HBI | Harvey-Bradshaw Index |
| IBD | Inflammatory Bowel Disease |
| IFN-γ | Interferon Gamma |
| IGRA | Interferon Gamma Release Assay |
| IgG-N | Immunoglobulin G against Nucleocapsid |
| IgG-S | Immunoglobulin G against Spike protein |
| IU/mL | International Units per Milliliter |
| mRNA | Messenger Ribonucleic Acid |
| PCR | Polymerase Chain Reaction |
| RBD | Receptor Binding Domain |
| RN | Registered Nurse |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SCCAI | Simple Clinical Colitis Activity Index |
| SD | Standard Deviation |
| TNF | Tumor Necrosis Factor |
| UC | Ulcerative Colitis |
| VHIR | Vall d’Hebron Institut de Recerca |
Appendix A
| Anti-TNF mono | Anti-TNF combo | Thiopurine | Controls | ||
|---|---|---|---|---|---|
| IgG-S BAU/mL Short-term | 2082 [1261–2082] | 2082 [1189–2082] | 2082 [1915–2082] | 2082 [2040–2082] | p = 0.26 |
| IgG-N BAU/mL Short-term | 0.09 [0.08–0.13] | 0.09 [0.08–0.09] | 0.09 [0.08–0.4] | 0.07 [0.07–1.36] | p = 0.07 |
| IgG-S BAU/mL Long-term | 2050 [887–2082] * | 2038 [985–2082] | 2082 [1240–2082] * | NA | * p < 0.05 |
| IgG-N BAU/mL Long-term | 0.31 [0.08–5.26] | 0.47 [0.09–9.01] | 0.35 [0.1–6.20] | NA | p = 0.25 |
References
- Jena, A.; James, D.; Singh, A.K.; Dutta, U.; Sebastian, S.; Sharma, V. Effectiveness and Durability of COVID-19 Vaccination in 9447 Patients With IBD: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 1456–1479.e18. [Google Scholar] [CrossRef]
- Alexander, J.L.; A Kennedy, N.; Ibraheim, H.; Anandabaskaran, S.; Saifuddin, A.; Seoane, R.C.; Liu, Z.; Nice, R.; Bewshea, C.; D’MEllo, A.; et al. COVID-19 vaccine-induced antibody responses in immunosuppressed patients with inflammatory bowel disease (VIP): A multicentre, prospective, case-control study. Lancet Gastroenterol. Hepatol. 2022, 7, 342–352. [Google Scholar] [CrossRef]
- Kennedy, N.A.; Lin, S.; Goodhand, J.R.; Chanchlani, N.; Hamilton, B.; Bewshea, C.; Nice, R.; Chee, D.; Cummings, J.F.; Fraser, A.; et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut 2021, 70, 1884–1893. [Google Scholar] [CrossRef]
- Alexander, J.L.; Liu, Z.; Sandoval, D.M.; Reynolds, C.; Ibraheim, H.; Anandabaskaran, S.; Saifuddin, A.; Seoane, R.C.; Anand, N.; Nice, R.; et al. COVID-19 vaccine-induced antibody and T-cell responses in immunosuppressed patients with inflammatory bowel disease after the third vaccine dose (VIP): A multicentre, prospective, case-control study. Lancet Gastroenterol. Hepatol. 2022, 7, 1005–1015. [Google Scholar] [CrossRef]
- Lin, S.; Kennedy, N.A.; Saifuddin, A.; Sandoval, D.M.; Reynolds, C.J.; Seoane, R.C.; Kottoor, S.H.; Pieper, F.P.; Lin, K.-M.; Butler, D.K.; et al. Antibody decay, T cell immunity and breakthrough infections following two SARS-CoV-2 vaccine doses in inflammatory bowel disease patients treated with infliximab and vedolizumab. Nat. Commun. 2022, 13, 1–14. [Google Scholar] [CrossRef]
- Kennedy, N.A.; Janjua, M.; Chanchlani, N.; Lin, S.; Bewshea, C.; Nice, R.; McDonald, T.J.; Auckland, C.; Harries, L.W.; Davies, M.; et al. Vaccine escape, increased breakthrough and reinfection in infliximab-treated patients with IBD during the Omicron wave of the SARS-CoV-2 pandemic. Gut 2022, 72, 295–305. [Google Scholar] [CrossRef]
- Liu, Z.; Le, K.; Zhou, X.; Alexander, J.L.; Lin, S.; Bewshea, C.; Chanchlani, N.; Nice, R.; McDonald, T.J.; Lamb, C.A.; et al. Neutralising antibody potency against SARS-CoV-2 wild-type and omicron BA.1 and BA.4/5 variants in patients with inflammatory bowel disease treated with infliximab and vedolizumab after three doses of COVID-19 vaccine (CLARITY IBD): An analysis of a prospective multicentre cohort study. Lancet Gastroenterol. Hepatol. 2023, 8, 145–156. [Google Scholar]
- Connolly, C.M.; Teles, M.; Frey, S.; Boyarsky, B.J.; Alejo, J.L.; A Werbel, W.; Albayda, J.; Christopher-Stine, L.; Garonzik-Wang, J.; Segev, D.L.; et al. Booster-dose SARS-CoV-2 vaccination in patients with autoimmune disease: A case series. Ann. Rheum. Dis. 2022, 81, 291–293. [Google Scholar] [CrossRef]
- Apostolidis, S.A.; Kakara, M.; Painter, M.M.; Goel, R.R.; Mathew, D.; Lenzi, K.; Rezk, A.; Patterson, K.R.; Espinoza, D.A.; Kadri, J.C.; et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021, 27, 1990–2001. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Geisen, U.M.; Rose, R.; Neumann, F.; Ciripoi, M.; Vullriede, L.; Reid, H.M.; Berner, D.K.; Bertoglio, F.; Hoff, P.; Hust, M.; et al. The long term vaccine-induced anti-SARS-CoV-2 immune response is impaired in quantity and quality under TNFalpha blockade. J. Med. Virol. 2022, 94, 5780–5789. [Google Scholar] [CrossRef]
- Ramos, L.; Hernández-Porto, M.; Carrillo-Palau, M.; Alonso-Abreu, I.; Reygosa, C.; Hernandez-Guerra, M. Impact of Biologic Agents on the Immune Response Induced by the Additional Dose of SARS-CoV-2 Vaccine in Inflammatory Bowel Disease Patients. Inflamm. Bowel Dis. 2022, 29, 1165–1169. [Google Scholar] [CrossRef]
- Cerna, K.; Duricova, D.; Hindos, M.; Hrebackova, J.H.; Lukas, M.; Machkova, N.; Hruba, V.; Mitrova, K.; Kubickova, K.; Kastylova, K.; et al. Cellular and Humoral Immune Responses to SARS-CoV-2 Vaccination in Inflammatory Bowel Disease Patients. J. Crohn’s Colitis 2022, 16, 1347–1353. [Google Scholar] [CrossRef]
- Woelfel, S.; Dütschler, J.; König, M.; Graf, N.; Oikonomou, V.; Krieger, C.; Truniger, S.; Franke, A.; Eckhold, A.; Forsch, K.; et al. Systemic and T cell-associated responses to SARS-CoV-2 immunisation in gut inflammation (STAR SIGN study): Effects of biologics on vaccination efficacy of the third dose of mRNA vaccines against SARS-CoV-2. Aliment. Pharmacol. Ther. 2022, 57, 103–116. [Google Scholar] [CrossRef]
- Caldera, F.; A Farraye, F.; Necela, B.M.; Cogen, D.; Saha, S.; Wald, A.; Daoud, N.D.; Chun, K.; Grimes, I.; Lutz, M.; et al. Higher Cell-Mediated Immune Responses in Patients With Inflammatory Bowel Disease on Anti-TNF Therapy After COVID-19 Vaccination. Inflamm. Bowel Dis. 2022, 29, 1202–1209. [Google Scholar] [CrossRef]
- Li, D.; Xu, A.; Mengesha, E.; Elyanow, R.; Gittelman, R.M.; Chapman, H.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Pozdnyakova, V.; et al. The T-Cell Response to SARS-CoV-2 Vaccination in Inflammatory Bowel Disease is Augmented with Anti-TNF Therapy. Inflamm. Bowel Dis. 2022, 28, 1130–1133. [Google Scholar] [CrossRef]
- Qui, M.; Le Bert, N.; Chan, W.P.W.; Tan, M.; Hang, S.K.; Hariharaputran, S.; Sim, J.X.Y.; Low, J.G.H.; Ng, W.; Wan, W.Y.; et al. Favorable vaccine-induced SARS-CoV-2–specific T cell response profile in patients undergoing immune-modifying therapies. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef]
- Boland, B.S.; Goodwin, B.; Zhang, Z.; Bloom, N.; Kato, Y.; Neill, J.; Le, H.; Tysl, T.; Collins, A.E.; Dulai, P.S.; et al. Preserved SARS-CoV-2 Vaccine Cell-Mediated Immunogenicity in Patients With Inflammatory Bowel Disease on Immune-Modulating Therapies. Clin. Transl. Gastroenterol. 2022, 13, e00484. [Google Scholar] [CrossRef]
- Zhang, E.; O Nguyen, T.H.; Allen, L.F.; Kedzierski, L.; Rowntree, L.C.; Chang, S.Y.; Zhang, W.; Habel, J.R.; Foo, I.J.; Menon, T.; et al. Robust SARS-CoV-2 antibody and T cell immunity following three COVID-19 vaccine doses in inflammatory bowel disease patients receiving anti-TNF or alternative treatments. Gut 2023, 73, 712–714. [Google Scholar] [CrossRef]
- van den Dijssel, J.; Duurland, M.C.; Konijn, V.A.; Kummer, L.Y.; Hagen, R.R.; Kuijper, L.H.; Wieske, L.; van Dam, K.P.; Stalman, E.W.; Steenhuis, M.; et al. mRNA-1273 vaccinated inflammatory bowel disease patients receiving TNF inhibitors develop broad and robust SARS-CoV-2-specific CD8+ T cell responses. J. Autoimmun. 2024, 144, 103175. [Google Scholar] [CrossRef]
- Kristiansen, P.A.; Page, M.; Bernasconi, V.; Mattiuzzo, G.; Dull, P.; Makar, K.; Plotkin, S.; Knezevic, I. WHO International Standard for anti-SARS-CoV-2 immunoglobulin. Lancet 2021, 397, 1347–1348. [Google Scholar] [CrossRef]
- Martinez-Gallo, M.; Esperalba, J.; Pujol-Borrell, R.; Sandá, V.; Arrese-Muñoz, I.; Fernández-Naval, C.; Antón, A.; Cardona, V.; Labrador-Horrillo, M.; Pumarola, T.; et al. Commercialized kits to assess T-cell responses against SARS-CoV-2 S peptides. A pilot study in health care workers. Med. Clin. 2022, 159, 116–123. [Google Scholar] [CrossRef]
- Barnes, E.; Goodyear, C.S.; Willicombe, M.; Gaskell, C.; Siebert, S.; I de Silva, T.; Murray, S.M.; Rea, D.; Snowden, J.A.; Carroll, M.; et al. SARS-CoV-2-specific immune responses and clinical outcomes after COVID-19 vaccination in patients with immune-suppressive disease. Nat. Med. 2023, 29, 1760–1774. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e1415. [Google Scholar] [CrossRef]
- Moderbacher, C.R.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef]
- Krüttgen, A.; Cornelissen, C.G.; Dreher, M.; Hornef, M.; Imöhl, M.; Kleines, M. Comparison of four new commercial serologic assays for determination of SARS-CoV-2 IgG. J. Clin. Virol. 2020, 128, 104394. [Google Scholar] [CrossRef]
- Murugesan, K.; Jagannathan, P.; Pham, T.D.; Pandey, S.; Bonilla, H.F.; Jacobson, K.; Parsonnet, J.; Andrews, J.R.; Weiskopf, D.; Sette, A.; et al. Interferon-γ Release Assay for Accurate Detection of Severe Acute Respiratory Syndrome Coronavirus 2 T-Cell Response. Clin. Infect. Dis. 2020, 73, e3130–e3132. [Google Scholar] [CrossRef]
- Painter, M.M.; Johnston, T.S.; Lundgreen, K.A.; Santos, J.J.S.; Qin, J.S.; Goel, R.R.; Apostolidis, S.A.; Mathew, D.; Fulmer, B.; Williams, J.C.; et al. Prior vaccination promotes early activation of memory T cells and enhances immune responses during SARS-CoV-2 breakthrough infection. Nat. Immunol. 2023, 24, 1711–1724. [Google Scholar] [CrossRef]
- Caldera, F.; Rolak, S.; Farraye, F.A.; Necela, B.M.; Cogen, D.; Zona, E.E.; Schell, T.L.; Ramirez, O.R.; Almasry, M.; Chun, K.; et al. Higher and Sustained Cell-Mediated Immune Responses After 3 Doses of mRNA COVID-19 Vaccine in Patients With Inflammatory Bowel Disease on Anti-Tumor Necrosis Factor Therapy. Clin. Transl. Gastroenterol. 2024, 15, e00688. [Google Scholar] [CrossRef]
- Suresh, M.; Singh, A.; Fischer, C. Role of Tumor Necrosis Factor Receptors in Regulating CD8 T-Cell Responses during Acute Lymphocytic Choriomeningitis Virus Infection. J. Virol. 2005, 79, 202–213. [Google Scholar] [CrossRef]
- Tiede, I.; Fritz, G.; Strand, S.; Poppe, D.; Dvorsky, R.; Strand, D.; Lehr, H.A.; Wirtz, S.; Becker, C.; Atreya, R.; et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J. Clin. Investig. 2003, 111, 1133–1145. [Google Scholar] [CrossRef]
- Ben-Horin, S.; Goldstein, I.; Fudim, E.; Picard, O.; Yerushalmi, Z.; Barshack, I.; Bank, I.; Goldschmid, Y.; Meir, S.B.; Mayer, L.; et al. Early preservation of effector functions followed by eventual T cell memory depletion: A model for the delayed onset of the effect of thiopurines. Gut 2008, 58, 396–403. [Google Scholar] [CrossRef]
- Macaluso, F.S.; Principi, M.; Facciotti, F.; Contaldo, A.; Todeschini, A.; Saibeni, S.; Bezzio, C.; Castiglione, F.; Nardone, O.M.; Spagnuolo, R.; et al. Reduced humoral response to two doses of COVID-19 vaccine in patients with inflammatory bowel disease: Data from ESCAPE-IBD, an IG-IBD study. Dig. Liver Dis. 2022, 55, 154–159. [Google Scholar] [CrossRef]
n = 166 | Anti-TNF mono n = 57 | Anti-TNF combo n = 53 | Thiopurines n = 38 | Controls n = 18 | p |
|---|---|---|---|---|---|
| Age (mean ± SD) | 45 ± 13.96 | 43 ± 11.26 | 40 ± 10.77 | 51 ± 11.87 | p = 0.06 |
| Gender n (% men) | 32 (56) | 31 (58) | 26 (68) | 5 (28) | p = 0.025 |
| Years of diagnosis (mean ± SD) | 12 ± 8.77 | 13 ± 8.23 | 10,5 ± 9.5 | — | p = 0.43 |
| Crohn’s disease/Ulcerative colitis (n) | 47/10 | 39/14 | 17/21 | — | p < 0.01 |
| Anti-TNF n (%) Infliximab Adalimumab Golimumab | 18 (32) 37 (65) 2 (3) | 32 (60) 20 (38) 1 (2) | — | — | p < 0.05 |
| Months of treatment (mean ± SD) | 64 ± 53.93 | 77 ± 51.73 | 105 ± 74.86 | — | p = 0.07 |
| Escalated dose (%) | 27 (47) | 23 (43) | — | — | p = 0.35 |
| Simple Colitis Activity Index (mean ± SD) | 2 ± 3.53 | 0 ± 1.94 | 1 ± 1.16 | — | p = 0.18 |
| Harvey-Bradshaw (mean ± SD) | 0 ± 1.88 | 1 ± 1.58 | 0 ± 1.77 | — | p = 0.72 |
| Anti-TNF mono | Anti-TNF combo | Thiopurine | Controls | ||
|---|---|---|---|---|---|
| AG1 UI/mL Short-term | 1.36 (0.53–3.95) | 0.51 (0.20–2.26) | 0.66 (0.24–2.68) | 0.56 (0.18–1.35) | p = 0.051 |
| AG2 UI/mL Short-term | 1.03 (0.36–2.79) | 0.39 (0.14–1.39) | 0.45 (0.15–1.45) | 0.61 (0.17–1.97) | p = 0.26 |
| AG1 UI/mL Long-term | 2.2 (1.0–4.15) *# | 0.4 (0.22–1.13) * | 0.7 (0.26–2.35) # | — | * p < 0.01 # p < 0.05 |
| AG2 UI/mL Long-term | 1.51 (0.66–4.27) | 0.23 (0.11–0.59) | 0.51 (0.16–1.09) | — | p = 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayorga Ayala, L.; Herrera-deGuise, C.; Esperalba, J.; Martinez-Gomez, X.; Céspedes Martinez, E.; Serra Ruiz, X.; Robles, V.; Lastiri, E.; Perez, Z.; Oller, E.; et al. Impaired Long-Term Quantitative Cellular Response to SARS-CoV-2 Vaccine in Thiopurine-Treated IBD Patients. Cells 2025, 14, 1156. https://doi.org/10.3390/cells14151156
Mayorga Ayala L, Herrera-deGuise C, Esperalba J, Martinez-Gomez X, Céspedes Martinez E, Serra Ruiz X, Robles V, Lastiri E, Perez Z, Oller E, et al. Impaired Long-Term Quantitative Cellular Response to SARS-CoV-2 Vaccine in Thiopurine-Treated IBD Patients. Cells. 2025; 14(15):1156. https://doi.org/10.3390/cells14151156
Chicago/Turabian StyleMayorga Ayala, Luis, Claudia Herrera-deGuise, Juliana Esperalba, Xavier Martinez-Gomez, Elena Céspedes Martinez, Xavier Serra Ruiz, Virginia Robles, Ernesto Lastiri, Zahira Perez, Elena Oller, and et al. 2025. "Impaired Long-Term Quantitative Cellular Response to SARS-CoV-2 Vaccine in Thiopurine-Treated IBD Patients" Cells 14, no. 15: 1156. https://doi.org/10.3390/cells14151156
APA StyleMayorga Ayala, L., Herrera-deGuise, C., Esperalba, J., Martinez-Gomez, X., Céspedes Martinez, E., Serra Ruiz, X., Robles, V., Lastiri, E., Perez, Z., Oller, E., Fernandez-Naval, C., Martinez-Gallo, M., Casellas, F., & Borruel, N. (2025). Impaired Long-Term Quantitative Cellular Response to SARS-CoV-2 Vaccine in Thiopurine-Treated IBD Patients. Cells, 14(15), 1156. https://doi.org/10.3390/cells14151156






