Pilot Study of [11C]HY-2-15: A Mixed Alpha-Synuclein and Tau PET Radiotracer
Abstract
1. Introduction
2. Materials and Methods
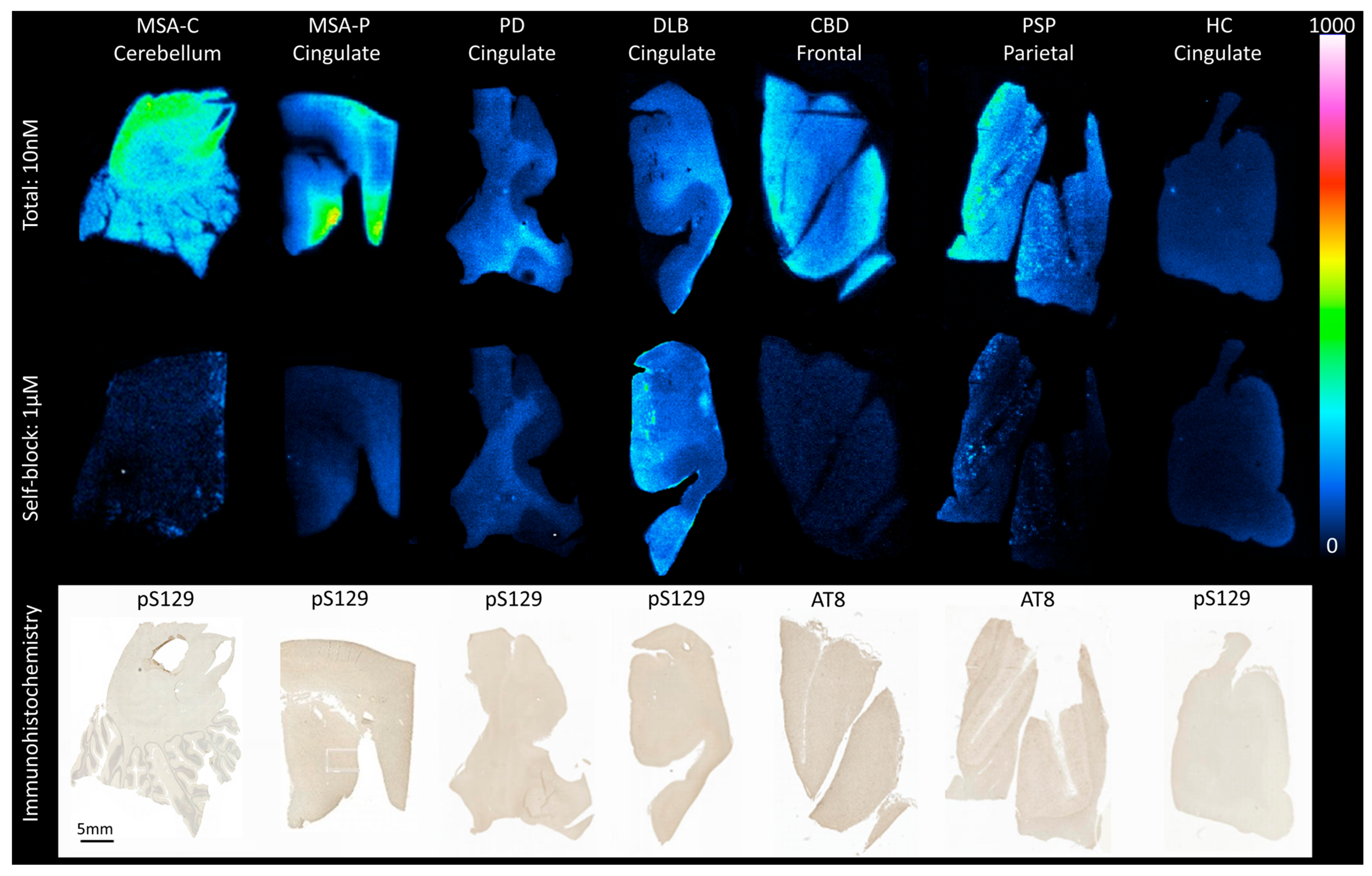
2.1. In Vitro Real-Time Autoradiography and Immunohistochemistry (IHC)
2.2. Participants
2.3. Preparation of [11C]HY-2-15
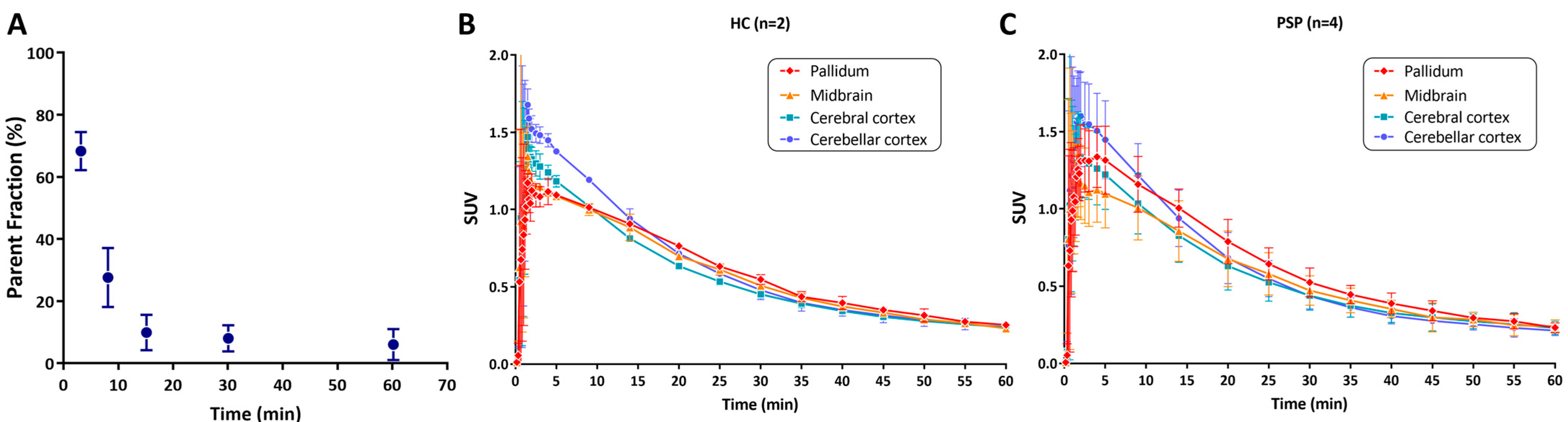
2.4. Radiometabolism Study of [11C]HY-2-15
2.5. PET Data Acquisition
2.5.1. University of Pennsylvania
2.5.2. Washington University in Saint Louis
2.6. Image Analysis
3. Results
3.1. In Vitro Autoradiography
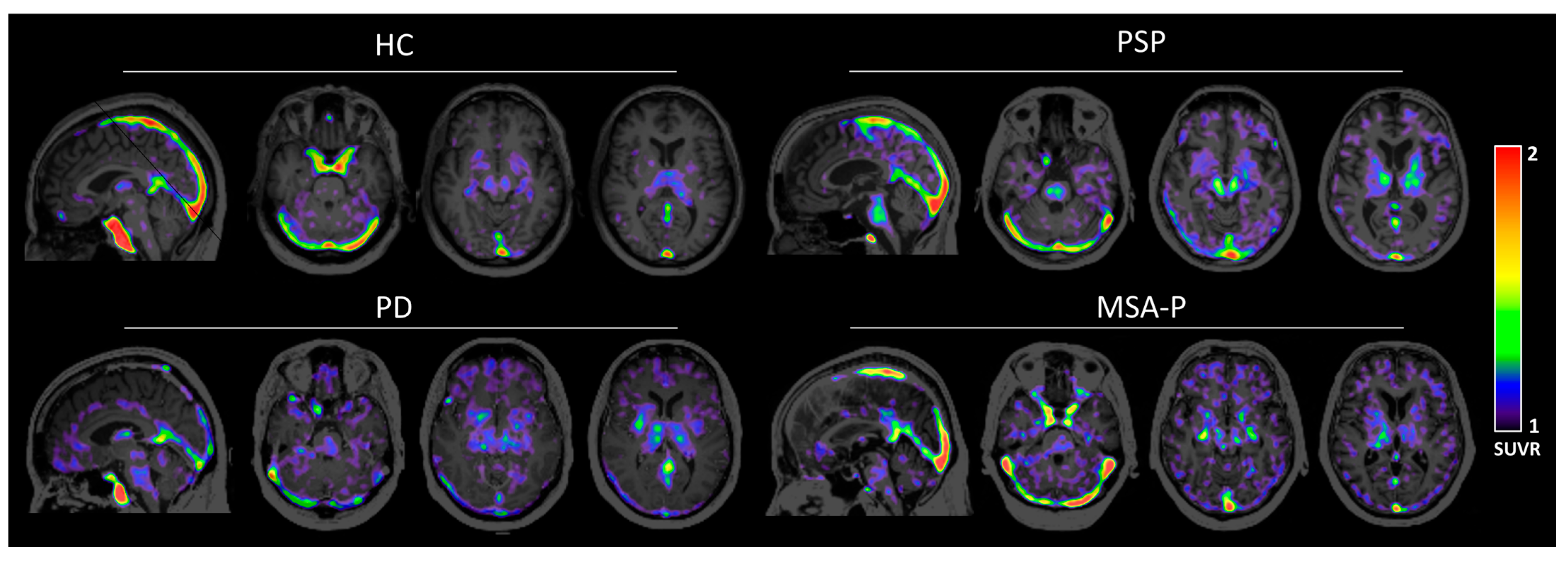
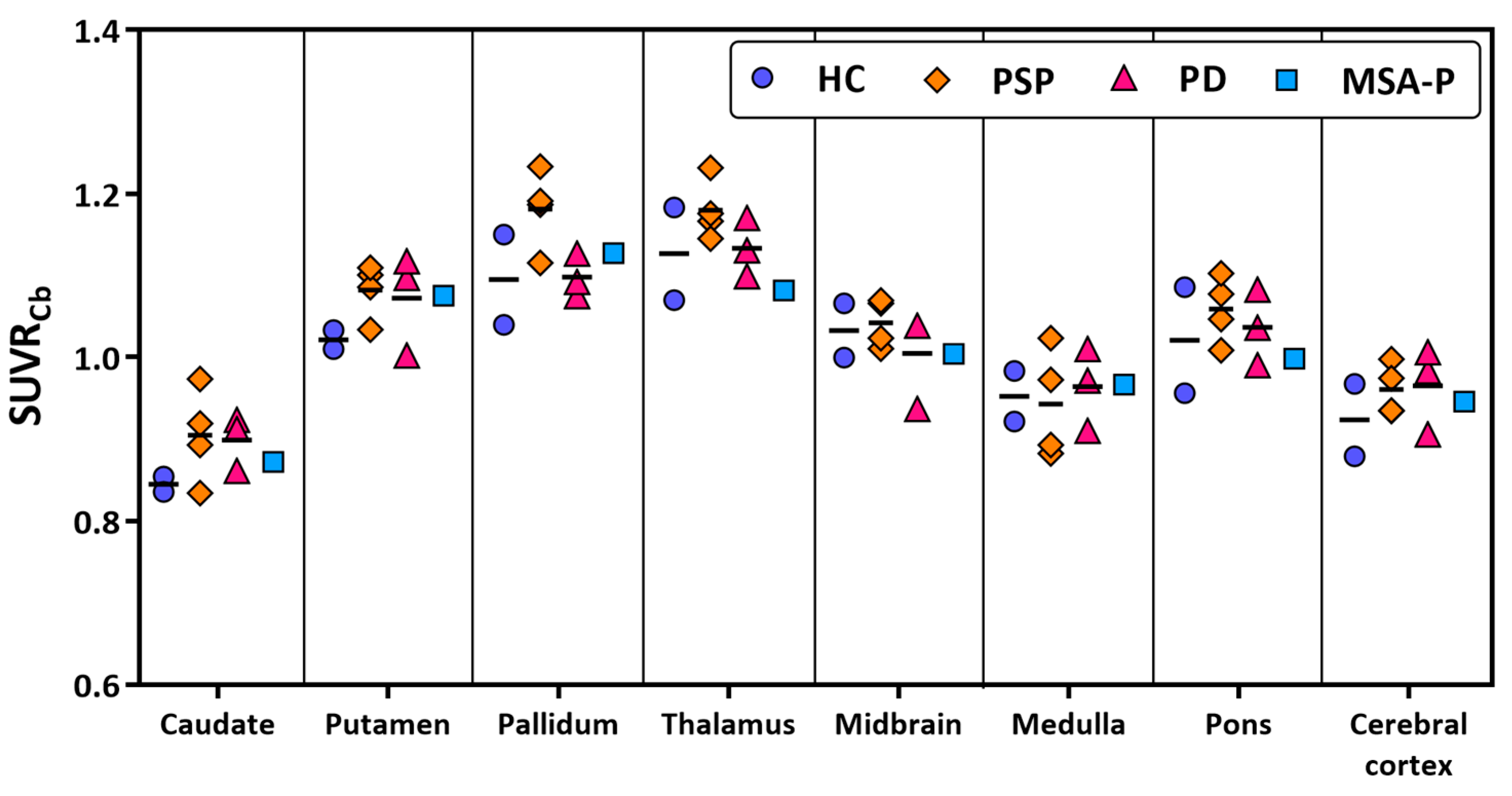
3.2. Human Brain Images of [11C]HY-2-15
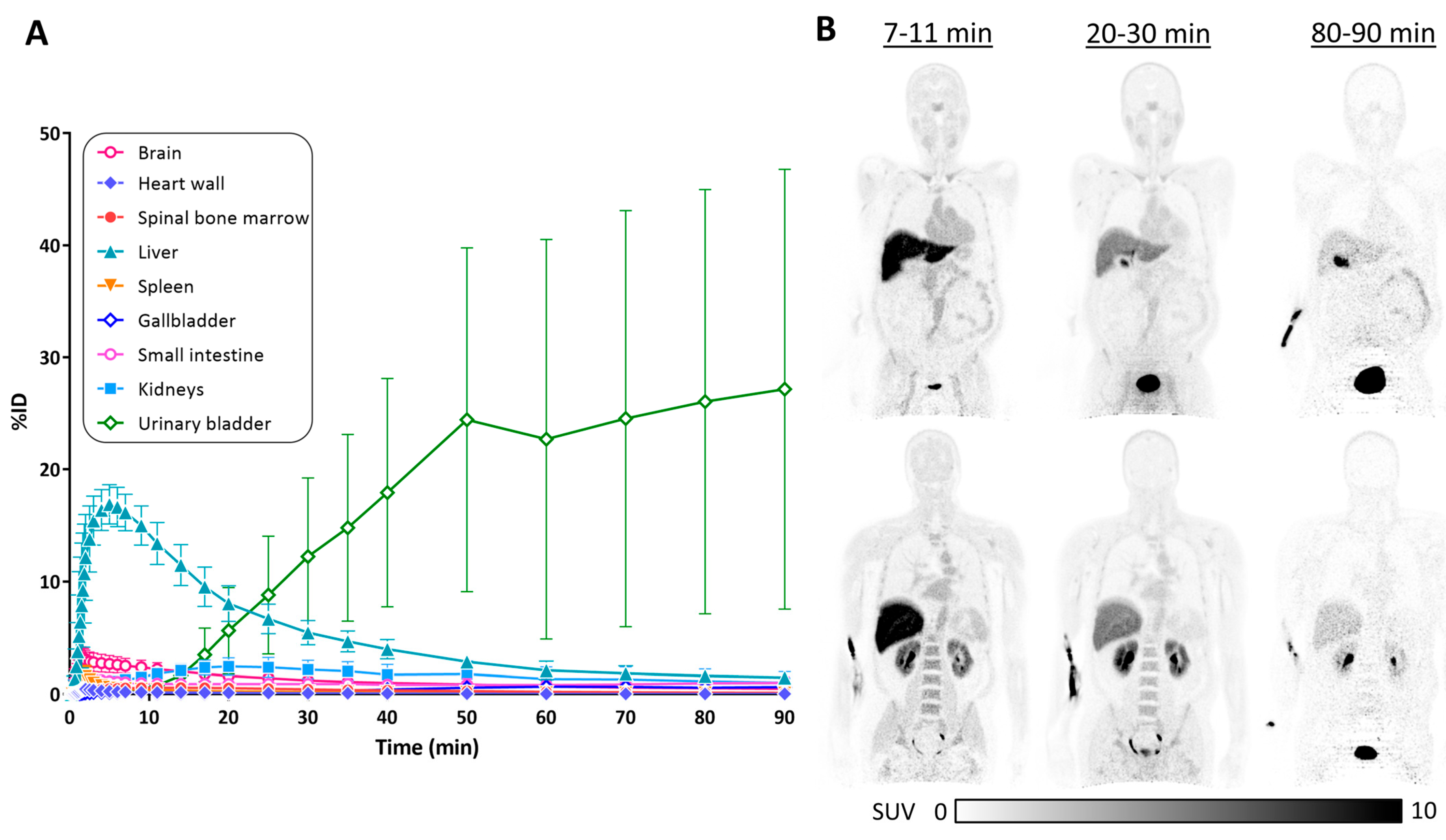
3.3. Biodistribution of [11C]HY-2-15
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Parkinson’s disease |
| MSA | Multiple system atrophy |
| α-syn | Alpha-synuclein |
| GCI | Glial cytoplasmic inclusion |
| PSP | Progressive supranuclear palsy |
| CBD | Corticobasal degeneration |
| 4R | 4-repeat |
| PET | Positron emission tomography |
| AD | Alzheimer’s disease |
| HC | Healthy control |
| IHC | Immunohistochemistry |
| DLB | Dementia with Lewy bodies |
| HPLC | High-performance liquid chromatography |
| MR | Magnetic resonance |
| VOI | Volume of interest |
| CT | Computed tomography |
| MNI | Montreal Neurologic Institute |
| AAL | Automated anatomical labeling |
| SUVR | Standardized uptake value ratio |
| %ID | Percent injected dose |
References
- Hayes, M.T. Parkinson’s disease and parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef]
- Poewe, W.; Stankovic, I.; Halliday, G.; Meissner, W.G.; Wenning, G.K.; Pellecchia, M.T.; Seppi, K.; Palma, J.-A.; Kaufmann, H. Multiple system atrophy. Nat. Rev. Dis. Primers 2022, 8, 56. [Google Scholar] [CrossRef]
- Saranza, G.M.; Whitwell, J.L.; Kovacs, G.G.; Lang, A.E. Corticobasal degeneration. Int. Rev. Neurobiol. 2019, 149, 87–136. [Google Scholar] [CrossRef]
- Kovacs, G.G.; Lukic, M.J.; Irwin, D.J.; Arzberger, T.; Respondek, G.; Lee, E.B.; Coughlin, D.; Giese, A.; Grossman, M.; Kurz, C. Distribution patterns of tau pathology in progressive supranuclear palsy. Acta Neuropathol. 2020, 140, 99–119. [Google Scholar] [CrossRef]
- Parmera, J.B.; Oliveira, M.C.B.d.; Rodrigues, R.D.; Coutinho, A.M. Progressive supranuclear palsy and corticobasal degeneration: Novel clinical concepts and advances in biomarkers. Arq. Neuro-Psiquiatr. 2022, 80, 126–136. [Google Scholar] [CrossRef]
- Smith, R.; Capotosti, F.; Schain, M.; Ohlsson, T.; Vokali, E.; Molette, J.; Touilloux, T.; Hliva, V.; Dimitrakopoulos, I.K.; Puschmann, A. The α-synuclein PET tracer [18F] ACI-12589 distinguishes multiple system atrophy from other neurodegenerative diseases. Nat. Commun. 2023, 14, 6750. [Google Scholar] [CrossRef]
- Endo, H.; Ono, M.; Takado, Y.; Matsuoka, K.; Takahashi, M.; Tagai, K.; Kataoka, Y.; Hirata, K.; Takahata, K.; Seki, C. Imaging α-synuclein pathologies in animal models and patients with Parkinson’s and related diseases. Neuron 2024, 112, 2540–2557.e8. [Google Scholar] [CrossRef]
- Okamura, N.; Harada, R.; Ishiki, A.; Kikuchi, A.; Nakamura, T.; Kudo, Y. The development and validation of tau PET tracers: Current status and future directions. Clin. Transl. Imaging 2018, 6, 305–316. [Google Scholar] [CrossRef]
- Li, L.; Liu, F.T.; Li, M.; Lu, J.Y.; Sun, Y.M.; Liang, X.; Bao, W.; Chen, Q.S.; Li, X.Y.; Zhou, X.Y. Clinical Utility of 18F-APN-1607 Tau PET Imaging in Patients with Progressive Supranuclear Palsy. Mov. Disord. 2021, 36, 2314–2323. [Google Scholar] [CrossRef]
- Tagai, K.; Ono, M.; Kubota, M.; Kitamura, S.; Takahata, K.; Seki, C.; Takado, Y.; Shinotoh, H.; Sano, Y.; Yamamoto, Y. High-contrast in vivo imaging of tau pathologies in Alzheimer’s and non-Alzheimer’s disease tauopathies. Neuron 2021, 109, 42–58. [Google Scholar] [CrossRef]
- Kim, H.Y.; Chia, W.K.; Hsieh, C.-J.; Saturnino Guarino, D.; Graham, T.J.; Lengyel-Zhand, Z.; Schneider, M.; Tomita, C.; Lougee, M.G.; Kim, H.J. A novel brain PET radiotracer for imaging alpha synuclein fibrils in multiple system atrophy. J. Med. Chem. 2023, 66, 12185–12202. [Google Scholar] [CrossRef]
- Pantel, A.R.; Viswanath, V.; Daube-Witherspoon, M.E.; Dubroff, J.G.; Muehllehner, G.; Parma, M.J.; Pryma, D.A.; Schubert, E.K.; Mankoff, D.A.; Karp, J.S. PennPET Explorer: Human imaging on a whole-body imager. J. Nucl. Med. 2020, 61, 144–151. [Google Scholar] [CrossRef]
- Kovacs, G.G.; Xie, S.X.; Robinson, J.L.; Lee, E.B.; Smith, D.H.; Schuck, T.; Lee, V.M.-Y.; Trojanowski, J.Q. Sequential stages and distribution patterns of aging-related tau astrogliopathy (ARTAG) in the human brain. Acta Neuropathol. Commun. 2018, 6, 1–19. [Google Scholar] [CrossRef]
- Passamonti, L.; Vázquez Rodríguez, P.; Hong, Y.T.; Allinson, K.S.; Williamson, D.; Borchert, R.J.; Sami, S.; Cope, T.E.; Bevan-Jones, W.R.; Jones, P.S. 18F-AV-1451 positron emission tomography in Alzheimer’s disease and progressive supranuclear palsy. Brain 2017, 140, 781–791. [Google Scholar] [CrossRef]
- Martinez-Valbuena, I.; Visanji, N.P.; Kim, A.; Lau, H.H.; So, R.W.; Alshimemeri, S.; Gao, A.; Seidman, M.A.; Luquin, M.R.; Watts, J.C. Alpha-synuclein seeding shows a wide heterogeneity in multiple system atrophy. Transl. Neurodegener. 2022, 11, 7. [Google Scholar] [CrossRef]
- Lin, K.-J.; Hsu, W.-C.; Hsiao, T.; Wey, S.-P.; Jin, L.-W.; Skovronsky, D.; Wai, Y.-Y.; Chang, H.-P.; Lo, C.-W.; Yao, C.H. Whole-body biodistribution and brain PET imaging with [18F] AV-45, a novel amyloid imaging agent—A pilot study. Nucl. Med. Biol. 2010, 37, 497–508. [Google Scholar] [CrossRef]
- Akamatsu, G.; Nishio, T.; Adachi, K.; Ikari, Y.; Senda, M. Whole-body biodistribution and the influence of body activity on brain kinetic analysis of the 11C-PiB PET scan. Radiol. Phys. Technol. 2017, 10, 464–474. [Google Scholar] [CrossRef]
- Bullich, S.; Barret, O.; Constantinescu, C.; Sandiego, C.; Mueller, A.; Berndt, M.; Papin, C.; Perrotin, A.; Koglin, N.; Kroth, H. Evaluation of dosimetry, quantitative methods, and test–retest variability of 18F-PI-2620 PET for the assessment of tau deposits in the human brain. J. Nucl. Med. 2020, 61, 920–927. [Google Scholar] [CrossRef]
- Lin, K.-J.; Huang, S.-Y.; Huang, K.-L.; Huang, C.-C.; Hsiao, I.-T. Human biodistribution and radiation dosimetry for the tau tracer [18F]Florzolotau in healthy subjects. EJNMMI Radiopharm. Chem. 2024, 9, 27. [Google Scholar] [CrossRef]
| HC | PSP | PD | MSA-P | |
|---|---|---|---|---|
| Sample size | 2 | 4 | 3 | 1 |
| Sex (Female/Male) | 1/1 | 1/3 | 1/2 | 0/1 |
| Age (Year) | 71.5 ± 3.5 | 62.8 ± 5.0 | 57.0 ± 6.9 | 72 |
| Hoehn–Yahr Stage | - | 3.5 ± 0.6 | 2.7 ± 1.2 | 2 |
| UPDRS III | 4.0 ± 5.7 | 42.5 ± 24.4 | 58.7 ± 22.1 | 43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, C.-J.; Saturnino Guarino, D.; Young, A.J.; Siderowf, A.D.; Nasrallah, I.; Schmitz, A.; Garcia, C.; Kim, H.Y.; Schubert, E.K.; Lee, H.; et al. Pilot Study of [11C]HY-2-15: A Mixed Alpha-Synuclein and Tau PET Radiotracer. Cells 2025, 14, 1157. https://doi.org/10.3390/cells14151157
Hsieh C-J, Saturnino Guarino D, Young AJ, Siderowf AD, Nasrallah I, Schmitz A, Garcia C, Kim HY, Schubert EK, Lee H, et al. Pilot Study of [11C]HY-2-15: A Mixed Alpha-Synuclein and Tau PET Radiotracer. Cells. 2025; 14(15):1157. https://doi.org/10.3390/cells14151157
Chicago/Turabian StyleHsieh, Chia-Ju, Dinahlee Saturnino Guarino, Anthony J. Young, Andrew D. Siderowf, Ilya Nasrallah, Alexander Schmitz, Carol Garcia, Ho Young Kim, Erin K. Schubert, Hsiaoju Lee, and et al. 2025. "Pilot Study of [11C]HY-2-15: A Mixed Alpha-Synuclein and Tau PET Radiotracer" Cells 14, no. 15: 1157. https://doi.org/10.3390/cells14151157
APA StyleHsieh, C.-J., Saturnino Guarino, D., Young, A. J., Siderowf, A. D., Nasrallah, I., Schmitz, A., Garcia, C., Kim, H. Y., Schubert, E. K., Lee, H., Perlmutter, J. S., & Mach, R. H. (2025). Pilot Study of [11C]HY-2-15: A Mixed Alpha-Synuclein and Tau PET Radiotracer. Cells, 14(15), 1157. https://doi.org/10.3390/cells14151157








