The Calprotectin Fragment, CPa9-HNE, Is a Plasma Biomarker of Mild Chronic Obstructive Pulmonary Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics and Study Participants
2.1.1. COPDGene Cohort
2.1.2. HOLLAND (Histopathology of Lung Aging and COPD) Cohort
2.2. Fragment Measurements
2.3. Spirometry and High-Resolution Computed Tomography Scans
2.4. SOMAscan Assay
2.5. Immunohistochemistry
2.6. Statistics
3. Results
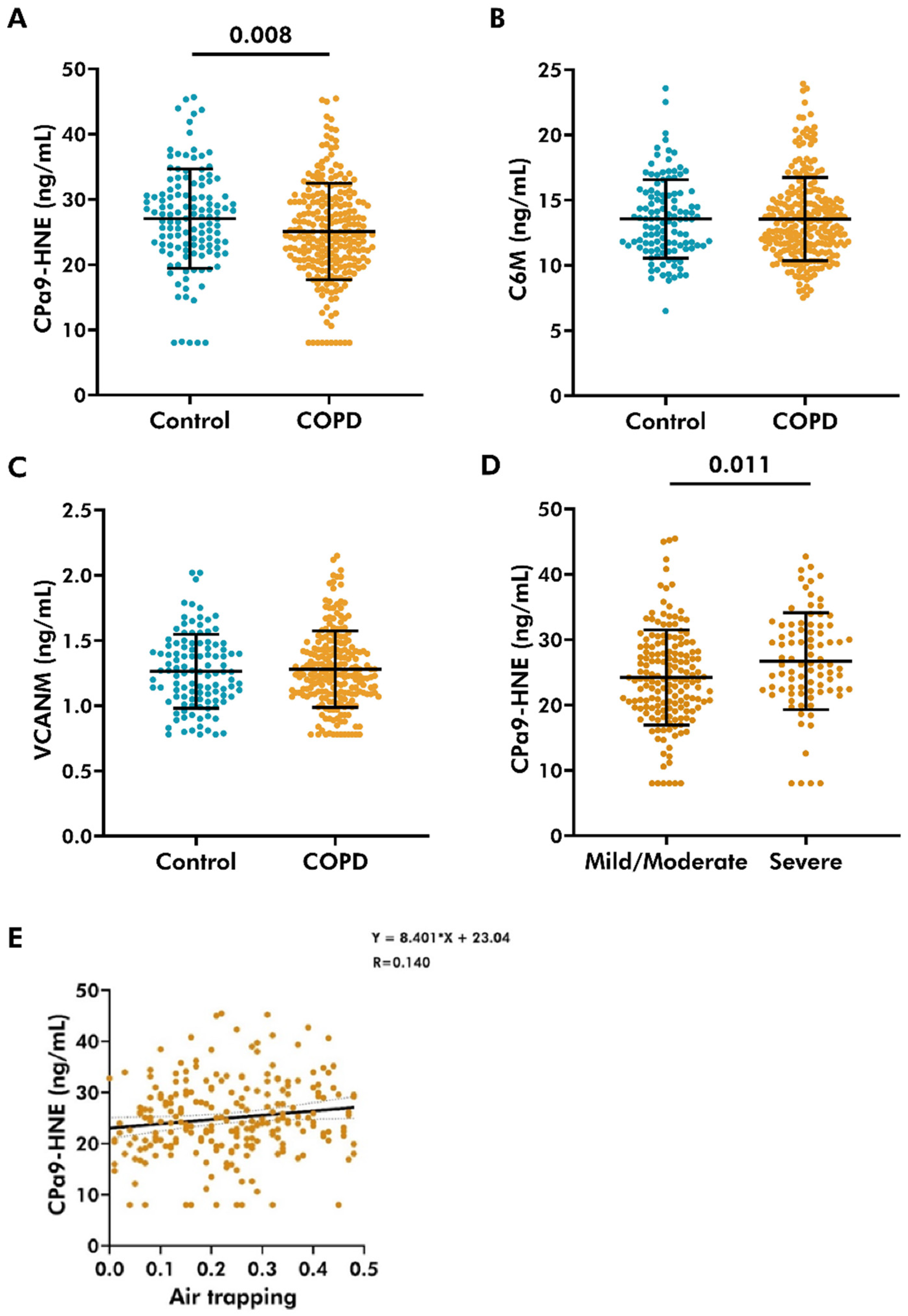
3.1. Lower Levels of CPa9-HNE Detected in the Plasma of COPD Patients
3.2. Plasma Levels of CPa9-HNE Did Not Associate with Lung Function
3.3. Plasma Levels of CPa9-HNE Did Not Associate with Markers of Chronic Bronchitis
3.4. Plasma Level of CPa9-HNE Was Positively Associated with Air-Trapping in COPD
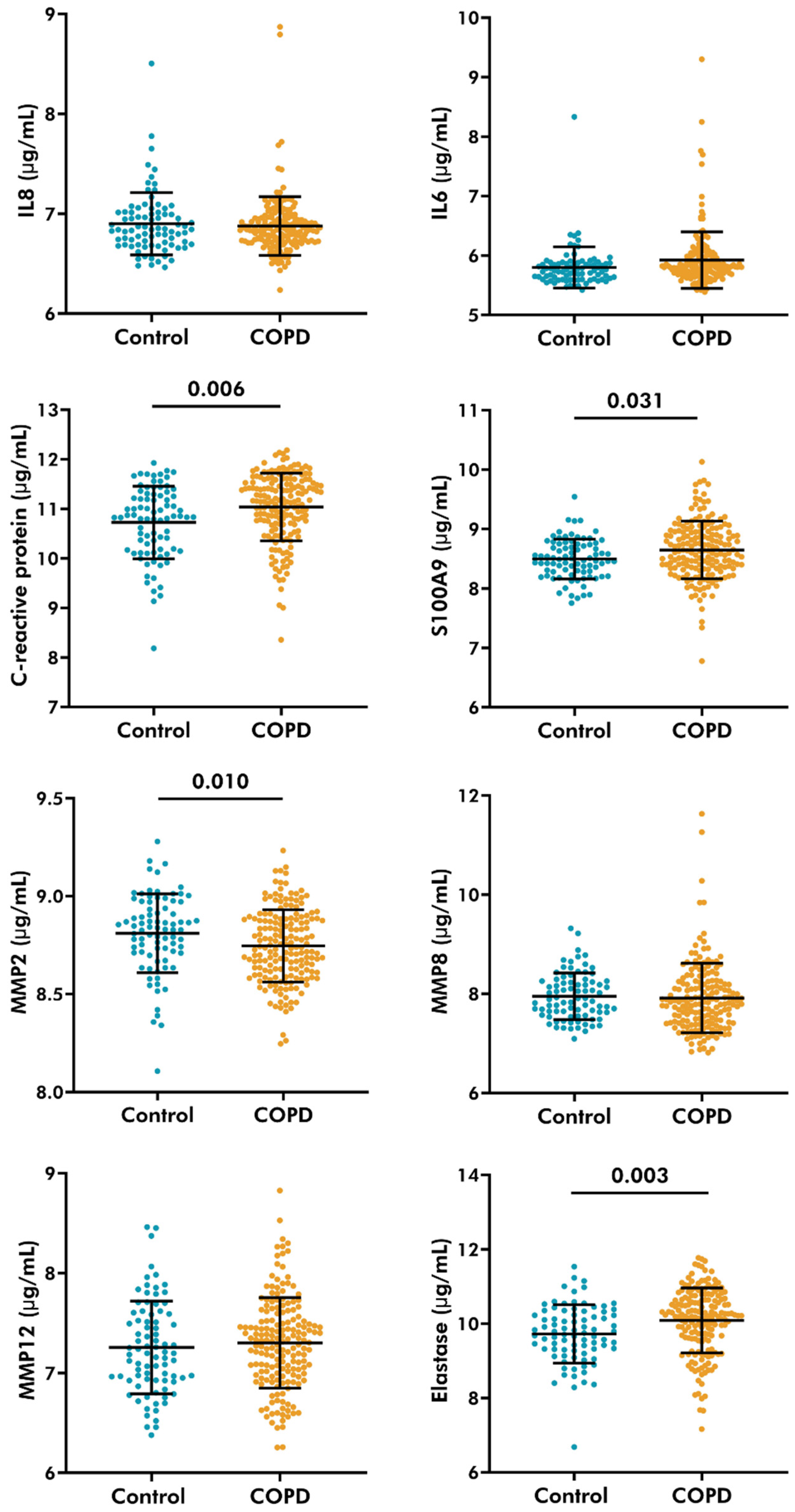
3.5. Serological Levels of C-Reactive Protein, S100A9, and Neutrophil Elastase Increased, While MMP-2 Decreased in COPD Patients
3.6. Plasma Levels of CPa9-HNE Were Associated with Serological Levels of MMP-2
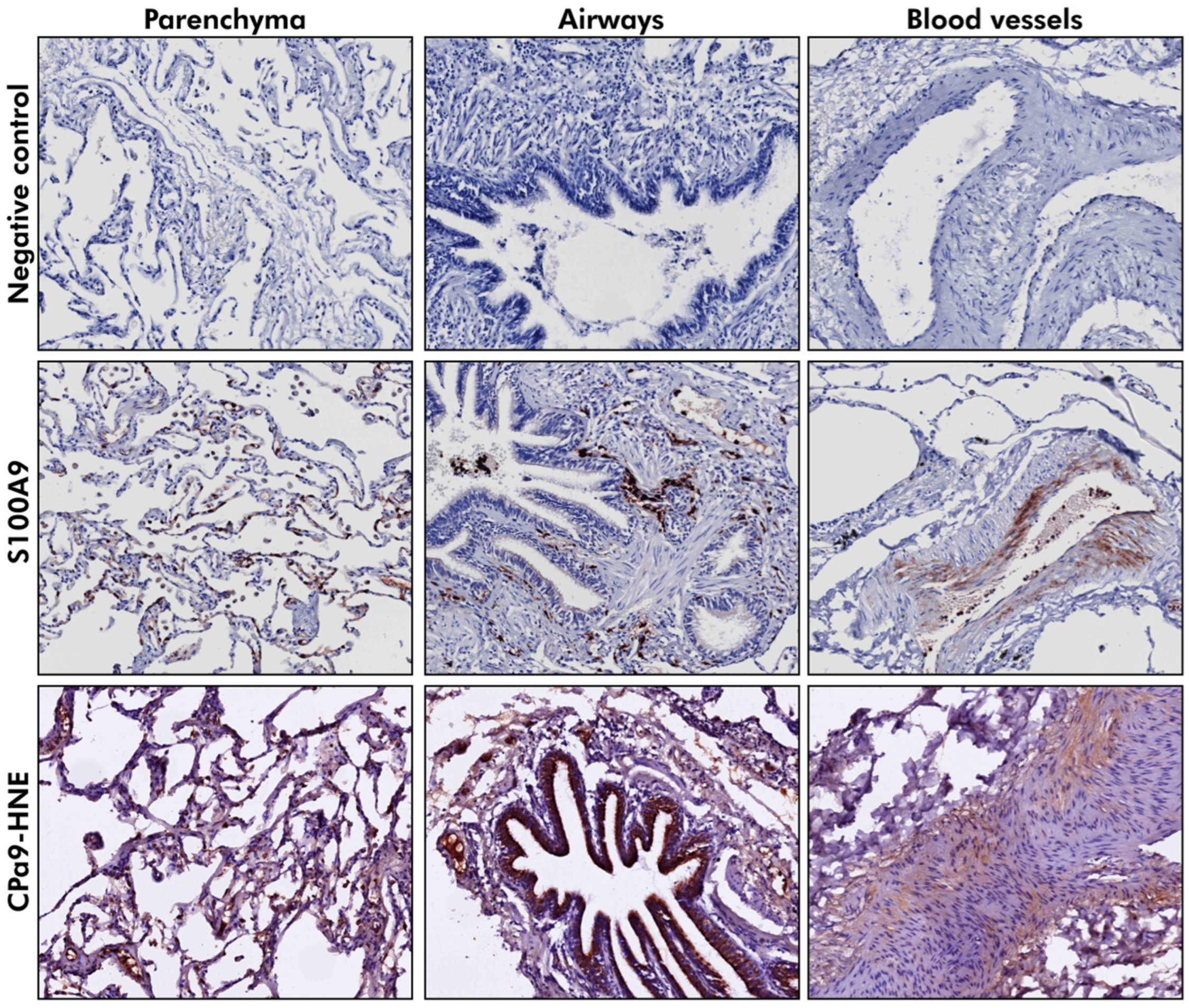
3.7. S100A9 Was Localized to the Parenchyma and the Airway Wall in Lung Tissue
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hogg, J.C.; Timens, W. The pathology of chronic obstructive pulmonary disease. Annu. Rev. Pathol. 2009, 4, 435–459. [Google Scholar] [CrossRef]
- Joglekar, M.M.; Nizamoglu, M.; Fan, Y.; Nemani, S.S.P.; Weckmann, M.; Pouwels, S.D.; Heijink, I.H.; Melgert, B.N.; Pillay, J.; Burgess, J.K. Highway to heal: Influence of altered extracellular matrix on infiltrating immune cells during acute and chronic lung diseases. Front. Pharmacol. 2022, 13, 995051. [Google Scholar] [CrossRef]
- Voynow, J.A.; Shinbashi, M. Neutrophil Elastase and Chronic Lung Disease. Biomolecules 2021, 11, 1065. [Google Scholar] [CrossRef]
- Shao, M.X.; Nadel, J.A. Neutrophil elastase induces MUC5AC mucin production in human airway epithelial cells via a cascade involving protein kinase C, reactive oxygen species, and TNF-alpha-converting enzyme. J. Immunol. 2005, 175, 4009–4016. [Google Scholar] [CrossRef]
- Jukic, A.; Bakiri, L.; Wagner, E.F.; Tilg, H.; Adolph, T.E. Calprotectin: From biomarker to biological function. Gut 2021, 70, 1978–1988. [Google Scholar] [CrossRef]
- Burgess, J.K.; Harmsen, M.C. Chronic lung diseases: Entangled in extracellular matrix. Eur. Respir. Rev. 2022, 31, 210202. [Google Scholar] [CrossRef]
- Joglekar, M.M.; Bekker, N.J.; Koloko Ngassie, M.L.; Vonk, J.M.; Borghuis, T.; Reinders-Luinge, M.; Bakker, J.; Woldhuis, R.R.; Pouwels, S.D.; Melgert, B.N.; et al. The lung extracellular matrix protein landscape in severe early-onset and moderate chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2024, 327, L304–L318. [Google Scholar] [CrossRef]
- Sand, J.M.B.; Knox, A.J.; Lange, P.; Sun, S.; Kristensen, J.H.; Leeming, D.J.; Karsdal, M.A.; Bolton, C.E.; Johnson, S.R. Accelerated extracellular matrix turnover during exacerbations of COPD. Respir. Res. 2015, 16, 69. [Google Scholar] [CrossRef]
- Bihlet, A.R.; Karsdal, M.A.; Sand, J.M.; Leeming, D.J.; Roberts, M.; White, W.; Bowler, R. Biomarkers of extracellular matrix turnover are associated with emphysema and eosinophilic-bronchitis in COPD. Respir. Res. 2017, 18, 22. [Google Scholar] [CrossRef]
- Sand, J.M.; Martinez, G.; Midjord, A.K.; Karsdal, M.A.; Leeming, D.J.; Lange, P. Characterization of serological neo-epitope biomarkers reflecting collagen remodeling in clinically stable chronic obstructive pulmonary disease. Clin. Biochem. 2016, 49, 1144–1151. [Google Scholar] [CrossRef]
- Stolz, D.; Leeming, D.J.; Kristensen, J.H.E.; Karsdal, M.A.; Boersma, W.; Louis, R.; Milenkovic, B.; Kostikas, K.; Blasi, F.; Aerts, J.; et al. Systemic Biomarkers of Collagen and Elastin Turnover Are Associated With Clinically Relevant Outcomes in COPD. Chest 2017, 151, 47–59. [Google Scholar] [CrossRef]
- Schumann, D.M.; Leeming, D.; Papakonstantinou, E.; Blasi, F.; Kostikas, K.; Boersma, W.; Louis, R.; Milenkovic, B.; Aerts, J.; Sand, J.M.B.; et al. Collagen Degradation and Formation Are Elevated in Exacerbated COPD Compared With Stable Disease. Chest 2018, 154, 798–807. [Google Scholar] [CrossRef]
- Dhiren, F.P.; Robert, J.S. The multifaceted roles of the matrikine Pro-Gly-Pro in pulmonary health and disease. Eur. Respir. Rev. 2018, 27, 180017. [Google Scholar] [CrossRef]
- Bale, S.; Verma, P.; Varga, J.; Bhattacharyya, S. Extracellular Matrix-Derived Damage-Associated Molecular Patterns (DAMP): Implications in Systemic Sclerosis and Fibrosis. J. Investative Dermatol. 2023, 143, 1877–1885. [Google Scholar] [CrossRef]
- Frevert, C.W.; Felgenhauer, J.; Wygrecka, M.; Nastase, M.V.; Schaefer, L. Danger-Associated Molecular Patterns Derived From the Extracellular Matrix Provide Temporal Control of Innate Immunity. J. Histochem. Cytochem. 2018, 66, 213–227. [Google Scholar] [CrossRef]
- Pouwels, S.D.; Heijink, I.H.; Hacken, N.H.T.; Vandenabeele, P.; Krysko, D.V.; Nawijn, M.C.; van Oosterhout, A.J. DAMPs activating innate and adaptive immune responses in COPD. Mucosal Immunol. 2014, 7, 215–226. [Google Scholar] [CrossRef]
- Dagher, R.; Fogel, P.; Wang, J.; Soussan, D.; Chiang, C.C.; Kearley, J.; Muthas, D.; Taillé, C.; Berger, P.; Bourdin, A.; et al. Proteomic profiling of serum identifies a molecular signature that correlates with clinical outcomes in COPD. PLoS ONE 2022, 17, e0277357. [Google Scholar] [CrossRef]
- Mormile, M.; Mormile, I.; Fuschillo, S.; Rossi, F.W.; Lamagna, L.; Ambrosino, P.; de Paulis, A.; Maniscalco, M. Eosinophilic Airway Diseases: From Pathophysiological Mechanisms to Clinical Practice. Int. J. Mol. Sci. 2023, 24, 7254. [Google Scholar] [CrossRef]
- Mulvanny, A.; Pattwell, C.; Beech, A.; Southworth, T.; Singh, D. Validation of Sputum Biomarker Immunoassays and Cytokine Expression Profiles in COPD. Biomedicines 2022, 10, 1949. [Google Scholar] [CrossRef]
- Tirelli, C.; Mira, S.; Belmonte, L.A.; De Filippi, F.; De Grassi, M.; Italia, M.; Maggioni, S.; Guido, G.; Mondoni, M.; Canonica, G.W.; et al. Exploring the Potential Role of Metabolomics in COPD: A Concise Review. Cells 2024, 13, 475. [Google Scholar] [CrossRef]
- Holmgaard, D.B.; Mygind, L.H.; Titlestad, I.; Madsen, H.; Pedersen, S.S.; Mortensen, O.H.; Pedersen, C. Calprotectin—A Marker of Mortality in COPD? Results from a Prospective Cohort Study. COPD J. Chronic Obstr. Pulm. Dis. 2013, 10, 581–587. [Google Scholar] [CrossRef]
- Pouwels, S.D.; Nawijn, M.C.; Bathoorn, E.; Riezebos-Brilman, A.; van Oosterhout, A.J.; Kerstjens, H.A.; Heijink, I.H. Increased serum levels of LL37, HMGB1 and S100A9 during exacerbation in COPD patients. Eur. Respir. J. 2015, 45, 1482–1485. [Google Scholar] [CrossRef]
- Huang, S.J.; Ding, Z.N.; Xiang, H.X.; Fu, L.; Fei, J. Association Between Serum S100A8/S100A9 Heterodimer and Pulmonary Function in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Lung 2020, 98, 645–652. [Google Scholar] [CrossRef]
- Rønnow, S.R.; Langholm, L.L.; Sand, J.M.B.; Thorlacius-Ussing, J.; Leeming, D.J.; Manon-Jensen, T.; Tal-Singer, R.; Miller, B.E.; Karsdal, M.A.; Vestbo, J. Specific elastin degradation products are associated with poor outcome in the ECLIPSE COPD cohort. Sci. Rep. 2019, 9, 4064. [Google Scholar] [CrossRef]
- Nissen, N.I.; Karsdal, M.; Willumsen, N. Post-translational modifications of vimentin reflect different pathological processes associated with non-small cell lung cancer and chronic obstructive pulmonary disease. Oncotarget 2019, 10, 6829–6841. [Google Scholar] [CrossRef]
- Klont, F.; Horvatovich, P.; Bowler, R.P.; van Rikxoort, E.; Charbonnier, J.P.; Kwiatkowski, M.; Lynch, D.A.; Humphries, S.; Bischoff, R.; Hacken, N.H.T.; et al. Plasma sRAGE levels strongly associate with centrilobular emphysema assessed by HRCT scans. Respir. Res. 2022, 23, 15. [Google Scholar] [CrossRef]
- Koloko Ngassie, M.L.; De Vries, M.; Borghuis, T.; Timens, W.; Sin, D.D.; Nickle, D.; Joubert, P.; Horvatovich, P.; Marko-Varga, G.; Teske, J.J.; et al. Age-associated differences in the human lung extracellular matrix. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 324, L799–L814. [Google Scholar] [CrossRef]
- Mortensen, J.H.; Sinkeviciute, D.; Manon-Jensen, T.; Domislović, V.; McCall, K.; Thudium, C.S.; Brinar, M.; Önnerfjord, P.; Goodyear, C.S.; Krznarić, Ž.; et al. A Specific Calprotectin Neo-epitope [CPa9-HNE] in Serum from Inflammatory Bowel Disease Patients Is Associated with Neutrophil Activity and Endoscopic Severity. J. Crohn’s Colitis 2022, 16, 1447–1460. [Google Scholar] [CrossRef]
- Veidal, S.S.; Karsdal, M.A.; Vassiliadis, E.; Nawrocki, A.; Larsen, M.R.; Nguyen, Q.H.; Hägglund, P.; Luo, Y.; Zheng, Q.; Vainer, B.; et al. MMP mediated degradation of type VI collagen is highly associated with liver fibrosis--identification and validation of a novel biochemical marker assay. PLoS ONE 2011, 6, e24753. [Google Scholar] [CrossRef]
- Barascuk, N.; Genovese, F.; Larsen, L.; Byrjalsen, I.; Zheng, Q.; Sun, S.; Hosbond, S.; Poulsen, T.S.; Diederichsen, A.; Jensen, J.M.; et al. A MMP derived versican neo-epitope is elevated in plasma from patients with atherosclerotic heart disease. Int. J. Clin. Exp. Med. 2013, 6, 174–184. [Google Scholar]
- Regan, E.A.; Hokanson, J.E.; Murphy, J.R.; Make, B.; Lynch, D.A.; Beaty, T.H.; Curran-Everett, D.; Silverman, E.K.; Crapo, J.D. Genetic epidemiology of COPD (COPDGene) study design. COPD J. Chronic Obstr. Pulm. Dis. 2010, 7, 32–43. [Google Scholar] [CrossRef]
- Miller, E.R.; Putman, R.K.; Diaz, A.A.; Xu, H.; San José Estépar, R.; Araki, T.; Nishino, M.; Poli de Frías, S.; Hida, T.; Ross, J.; et al. Increased Airway Wall Thickness in Interstitial Lung Abnormalities and Idiopathic Pulmonary Fibrosis. Ann. Am. Thorac. Soc. 2019, 16, 447–454. [Google Scholar] [CrossRef]
- Pompe, E.; van Rikxoort, E.M.; Schmidt, M.; Rühaak, J.; Estrella, L.G.; Vliegenthart, R.; Oudkerk, M.; de Koning, H.J.; van Ginneken, B.; de Jong, P.A.; et al. Parametric Response Mapping Adds Value to Current Computed Tomography Biomarkers in Diagnosing Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2015, 191, 1084–1086. [Google Scholar] [CrossRef]
- Pratte, K.A.; Curtis, J.L.; Kechris, K.; Couper, D.; Cho, M.H.; Silverman, E.K.; DeMeo, D.L.; Sciurba, F.C.; Zhang, Y.; Ortega, V.E.; et al. Soluble receptor for advanced glycation end products (sRAGE) as a biomarker of COPD. Respir. Res. 2021, 22, 127. [Google Scholar] [CrossRef]
- Galbán, C.J.; Han, M.K.; Boes, J.L.; Chughtai, K.A.; Meyer, C.R.; Johnson, T.D.; Galbán, S.; Rehemtulla, A.; Kazerooni, E.A.; Martinez, F.J.; et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat. Med. 2012, 18, 1711–1715. [Google Scholar] [CrossRef]
- Hansen, A.H.; Mortensen, J.H.; Rønnow, S.R.; Karsdal, M.A.; Leeming, D.J.; Sand, J.M.B. A Serological Neoepitope Biomarker of Neutrophil Elastase-Degraded Calprotectin, Associated with Neutrophil Activity, Identifies Idiopathic Pulmonary Fibrosis and Chronic Obstructive Pulmonary Disease More Effectively Than Total Calprotectin. J. Clin. Med. 2023, 12, 7589. [Google Scholar] [CrossRef]
- Oshagbemi, O.A.; Franssen, F.M.E.; Wouters, E.F.M.; Maitland-van der Zee, A.H.; Driessen, J.H.M.; de Boer, A.; de Vries, F. C-reactive protein as a biomarker of response to inhaled corticosteroids among patients with COPD. Pulm. Pharmacol. Ther. 2020, 60, 101870. [Google Scholar] [CrossRef]
- Mahor, D.; Kumari, V.; Vashisht, K.; Galgalekar, R.; Samarth, R.M.; Mishra, P.K.; Banerjee, N.; Dixit, R.; Saluja, R.; De, S.; et al. Elevated serum matrix metalloprotease (MMP-2) as a candidate biomarker for stable COPD. BMC Pulm. Med. 2020, 20, 302. [Google Scholar] [CrossRef]
- Isaksen, B.; Fagerhol, M.K. Calprotectin inhibits matrix metalloproteinases by sequestration of zinc. Mol. Pathol. 2001, 54, 289–292. [Google Scholar] [CrossRef]
- Sand, J.M.; Leeming, D.J.; Byrjalsen, I.; Bihlet, A.R.; Lange, P.; Tal-Singer, R.; Miller, B.E.; Karsdal, M.A.; Vestbo, J. High levels of biomarkers of collagen remodeling are associated with increased mortality in COPD—Results from the ECLIPSE study. Respir. Res. 2016, 17, 125. [Google Scholar] [CrossRef]
| Beta | p-Value | Lower Bound | Upper Bound | R | |
|---|---|---|---|---|---|
| FEV1 (% predicted) | |||||
| All donors | 0.014 | 0.373 | −0.016 | 0.044 | 0.092 |
| COPD donors | −0.029 | 0.175 | −0.070 | 0.013 | 0.085 |
| FVC (L) | |||||
| All donors | −0.080 | 0.872 | −1.051 | 0.892 | 0.080 |
| COPD donors | −0.709 | 0.110 | −1.578 | 0.161 | 0.101 |
| Smoking | |||||
| All donors | 0.074 | 0.934 | −1.703 | 1.852 | 0.080 |
| COPD donors | 1.138 | 0.291 | −0.981 | 3.256 | 0.066 |
| Pi10 | |||||
| All donors | 0.134 | 0.858 | −1.330 | 1.597 | 0.075 |
| COPD donors | 1.483 | 0.112 | −0.350 | 3.317 | 0.101 |
| LAA < 950 HU | |||||
| All donors | −0.008 | 0.840 | −0.086 | 0.070 | 0.075 |
| COPD donors | 0.033 | 0.440 | −0.051 | 0.116 | 0.049 |
| LAA < 910 HU | |||||
| All donors | −0.010 | 0.663 | −0.056 | 0.036 | 0.078 |
| COPD donors | 0.015 | 0.590 | −0.039 | 0.069 | 0.034 |
| LAA < 856 HU | |||||
| All donors | 0.004 | 0.855 | −0.039 | 0.047 | 0.076 |
| COPD donors | 0.041 | 0.099 | −0.008 | 0.091 | 0.109 |
| Micro-mapping (Normal tissue) | |||||
| All donors | −0.511 | 0.798 | −4.433 | 3.411 | 0.072 |
| COPD donors | −4.028 | 0.081 | −8.555 | 0.449 | 0.115 |
| Micro-mapping (Air-trapping) | |||||
| All donors | 2.084 | 0.534 | −4.506 | 8.673 | 0.079 |
| COPD donors | 8.525 | 0.031 | 0.778 | 16.272 | 0.142 |
| Micro-mapping (Emphysema) | |||||
| All donors | −0.682 | 0.870 | −8.868 | 7.503 | 0.072 |
| COPD donors | 4.248 | 0.339 | −4.481 | 12.977 | 0.063 |
| Severity centrilobular score | |||||
| All donors | −0.180 | 0.507 | −0.712 | 0.353 | 0.082 |
| COPD donors | 0.265 | 0.400 | −0.354 | 0.883 | 0.054 |
| Severity of paraseptal emphysema | |||||
| All donors | 0.186 | 0.722 | −0.841 | 1.213 | 0.076 |
| COPD donors | 0.216 | 0.705 | −0.910 | 1.342 | 0.024 |
| Beta | p-Value | Lower Bound | Upper Bound | R | |
|---|---|---|---|---|---|
| Elastase | |||||
| All donors | 0.085 | 0.878 | −1.005 | 1.175 | 0.100 |
| COPD donors | −0.052 | 0.937 | −1.368 | 1.263 | 0.006 |
| S100A9 | |||||
| All donors | −0.646 | 0.546 | −2.752 | 1.459 | 0.106 |
| COPD donors | −0.547 | 0.650 | −2.921 | 1.827 | 0.035 |
| IL8 | |||||
| All donors | 0.011 | 0.994 | −3.138 | 3.160 | 0.099 |
| COPD donors | −0.329 | 0.869 | −4.250 | 3.250 | 0.013 |
| MMP2 | |||||
| All donors | 4.989 | 0.044 | 0.135 | 0.062 | 0.160 |
| COPD donors | 6.339 | 0.044 | 0.164 | 12.515 | 0.153 |
| MMP8 | |||||
| All donors | 0.149 | 0.844 | −1.341 | 1.639 | 0.100 |
| COPD donors | 0.591 | 0.480 | −1.057 | 2.240 | 0.054 |
| MMP12 | |||||
| All donors | −1.119 | 0.307 | −3.274 | 1.036 | 0.118 |
| COPD donors | −0.683 | 0.609 | −3.311 | 1.945 | 0.039 |
| Compartment | Staining Intensity | Control (% Donors) | COPD Moderate (% Donors) | COPD Severe (% Donors) |
|---|---|---|---|---|
| S100A9 | ||||
| Parenchyma | No-weak | 47.06 | 69.23 | 72.73 |
| Moderate | 29.41 | 0 | 9.10 | |
| Strong | 23.53 | 30.77 | 18.18 | |
| Airway wall | No-weak | 47.06 | 38.46 | 36.36 |
| Moderate | 23.52 | 23.08 | 9.10 | |
| Strong | 29.41 | 38.46 | 54.55 | |
| Blood vessels | No-weak | 100 | 92.3 | 100 |
| Moderate | 0 | 0 | 0 | |
| Strong | 0 | 7.70 | 0 | |
| CPa9-HNE | ||||
| Parenchyma | No-weak | 70.59 | 92.31 | 83.33 |
| Moderate | 5.88 | 7.69 | 8.33 | |
| Strong | 23.53 | 0.00 | 8.33 | |
| Airway wall | No-weak | 52.94 | 84.62 | 83.33 |
| Moderate | 29.41 | 7.69 | 16.67 | |
| Strong | 11.76 | 7.69 | 0.00 | |
| Blood vessels | No-weak | 76.47 | 69.23 | 91.67 |
| Moderate | 17.65 | 30.77 | 8.33 | |
| Strong | 5.88 | 0.00 | 0.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joglekar, M.M.; Sand, J.M.B.; Borghuis, T.; Leeming, D.J.; Karsdal, M.; Klont, F.; Bowler, R.P.; Melgert, B.N.; Burgess, J.K.; Pouwels, S.D. The Calprotectin Fragment, CPa9-HNE, Is a Plasma Biomarker of Mild Chronic Obstructive Pulmonary Disease. Cells 2025, 14, 1155. https://doi.org/10.3390/cells14151155
Joglekar MM, Sand JMB, Borghuis T, Leeming DJ, Karsdal M, Klont F, Bowler RP, Melgert BN, Burgess JK, Pouwels SD. The Calprotectin Fragment, CPa9-HNE, Is a Plasma Biomarker of Mild Chronic Obstructive Pulmonary Disease. Cells. 2025; 14(15):1155. https://doi.org/10.3390/cells14151155
Chicago/Turabian StyleJoglekar, Mugdha M., Jannie M. B. Sand, Theo Borghuis, Diana J. Leeming, Morten Karsdal, Frank Klont, Russell P. Bowler, Barbro N. Melgert, Janette K. Burgess, and Simon D. Pouwels. 2025. "The Calprotectin Fragment, CPa9-HNE, Is a Plasma Biomarker of Mild Chronic Obstructive Pulmonary Disease" Cells 14, no. 15: 1155. https://doi.org/10.3390/cells14151155
APA StyleJoglekar, M. M., Sand, J. M. B., Borghuis, T., Leeming, D. J., Karsdal, M., Klont, F., Bowler, R. P., Melgert, B. N., Burgess, J. K., & Pouwels, S. D. (2025). The Calprotectin Fragment, CPa9-HNE, Is a Plasma Biomarker of Mild Chronic Obstructive Pulmonary Disease. Cells, 14(15), 1155. https://doi.org/10.3390/cells14151155








