Inhibition of Toll-like Receptor 4 Using Small Molecule, TAK-242, Protects Islets from Innate Immune Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Sample Collection
2.2. Islet Isolation
2.3. Surface Modification of Islets
2.4. In Vitro IBMIR
2.5. Clotting Assay
2.6. One-Way Mixed Lymphocyte Reaction
2.7. CD8+ T Cell Activation Assay
2.8. In Vitro Co-Culture of Allogenic PBMC and Islets
2.9. Cytokine Quantification
2.10. RT-qPCR for Secreted Stress and Damage miRNA
2.11. Flow Cytometry
2.12. Statistical Analysis
3. Results
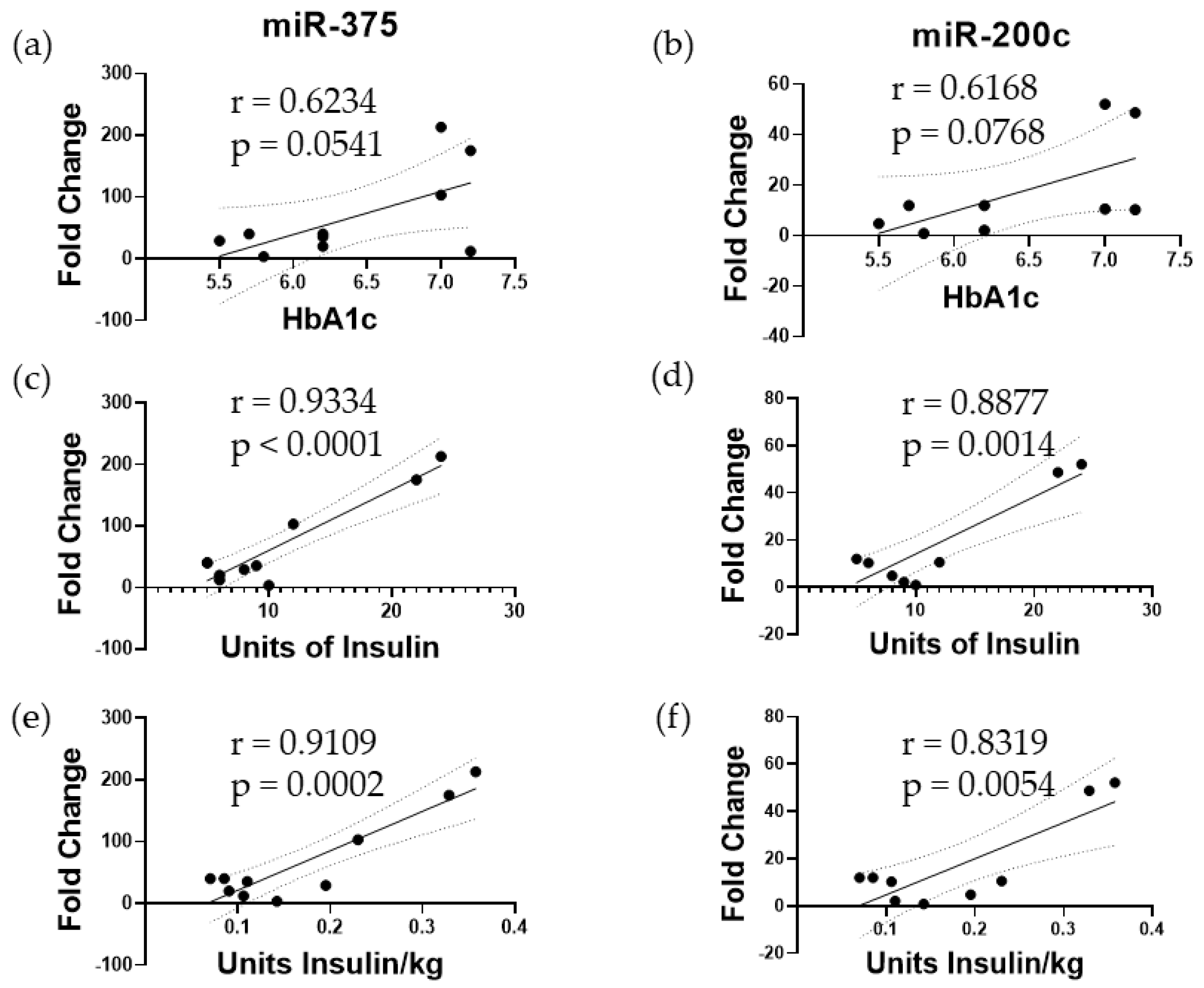
3.1. Elevated miR-375 and miR-200c Correspond to Poor Islet Function following TPIAT
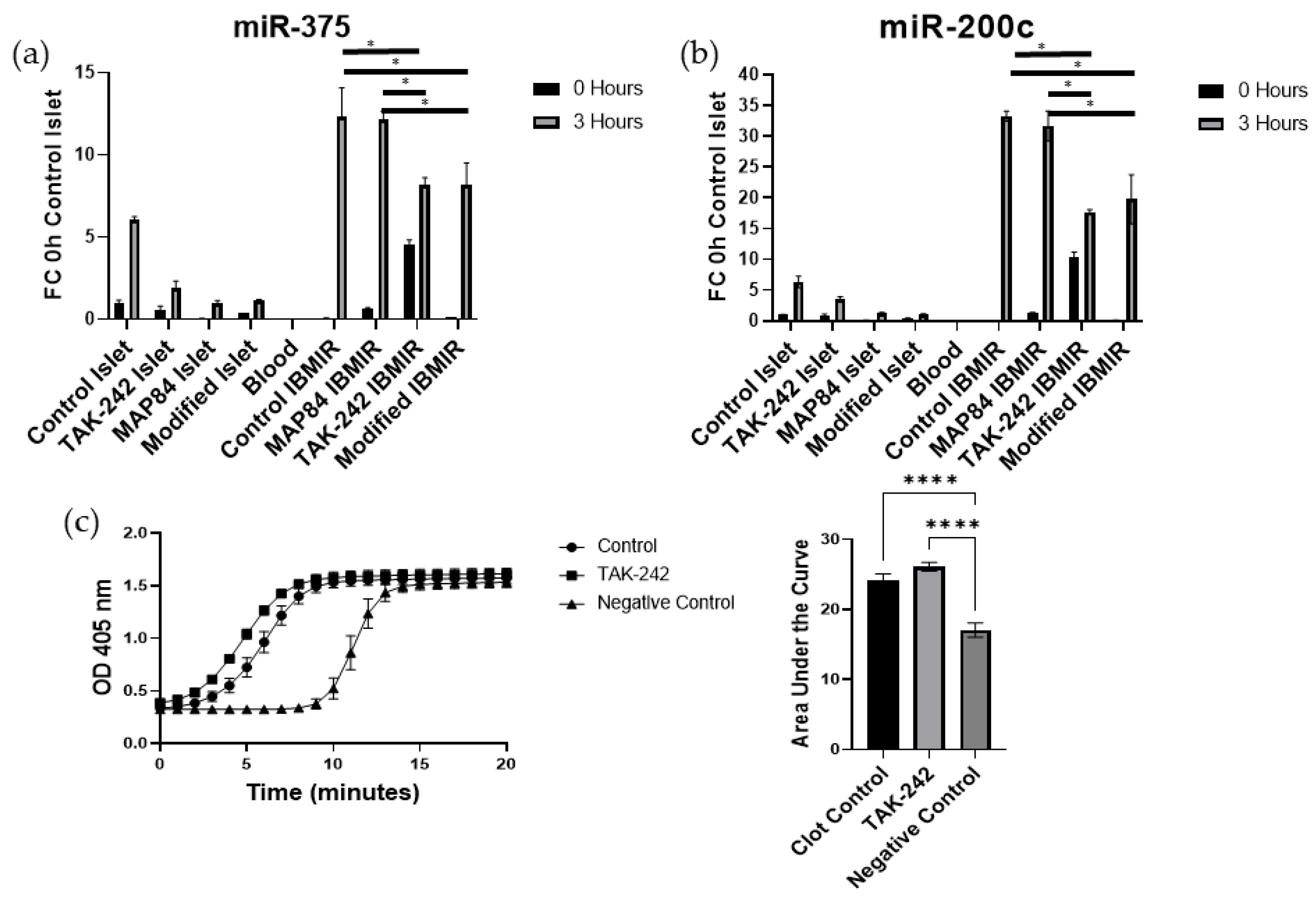
3.2. TAK-242 Reduces Damage to Islets Exposed to IBMIR In Vitro
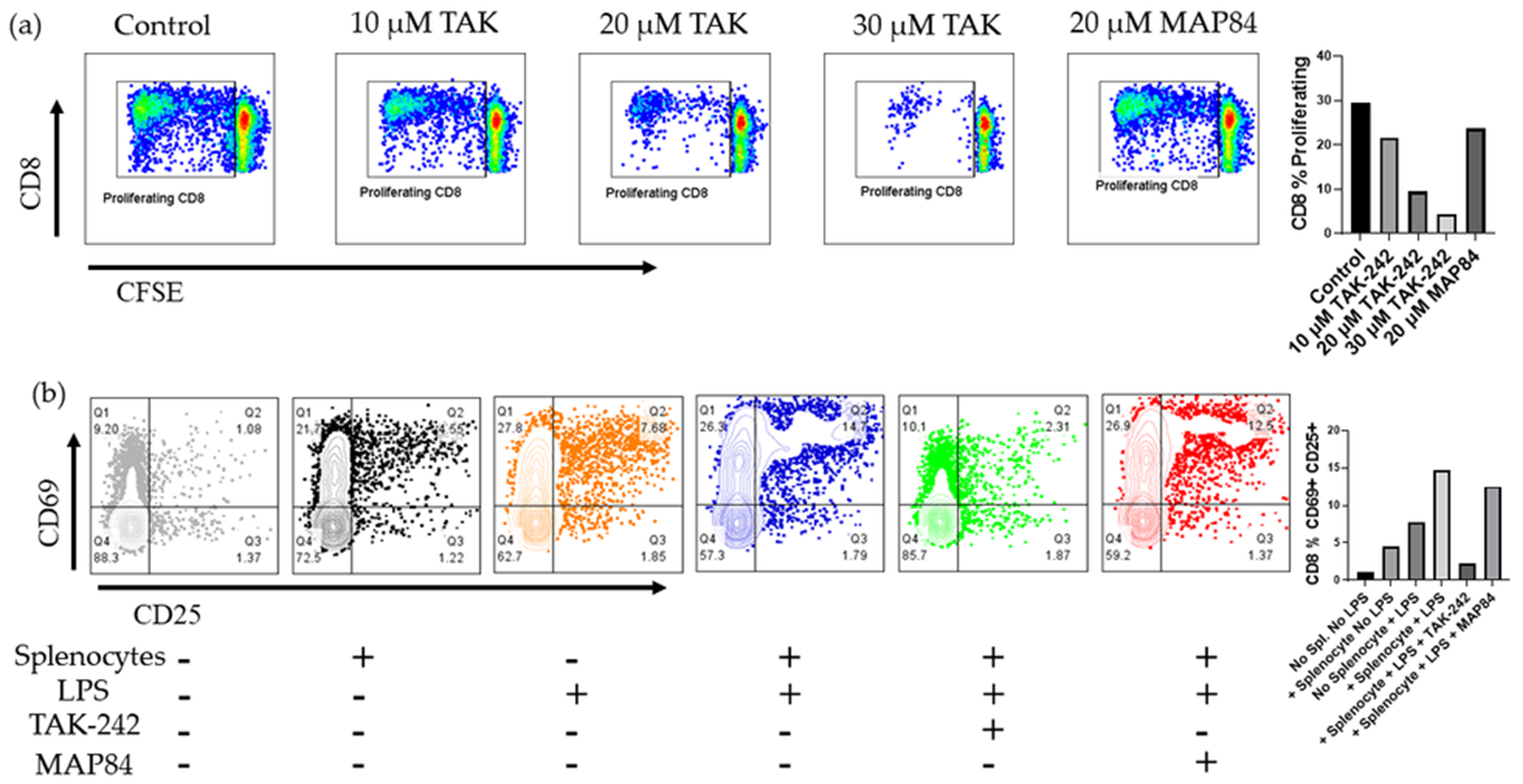
3.3. TAK-242 Inhibits T Cell Activation and Proliferation in a One-Way Mixed Lymphocyte Reaction with Splenocytes
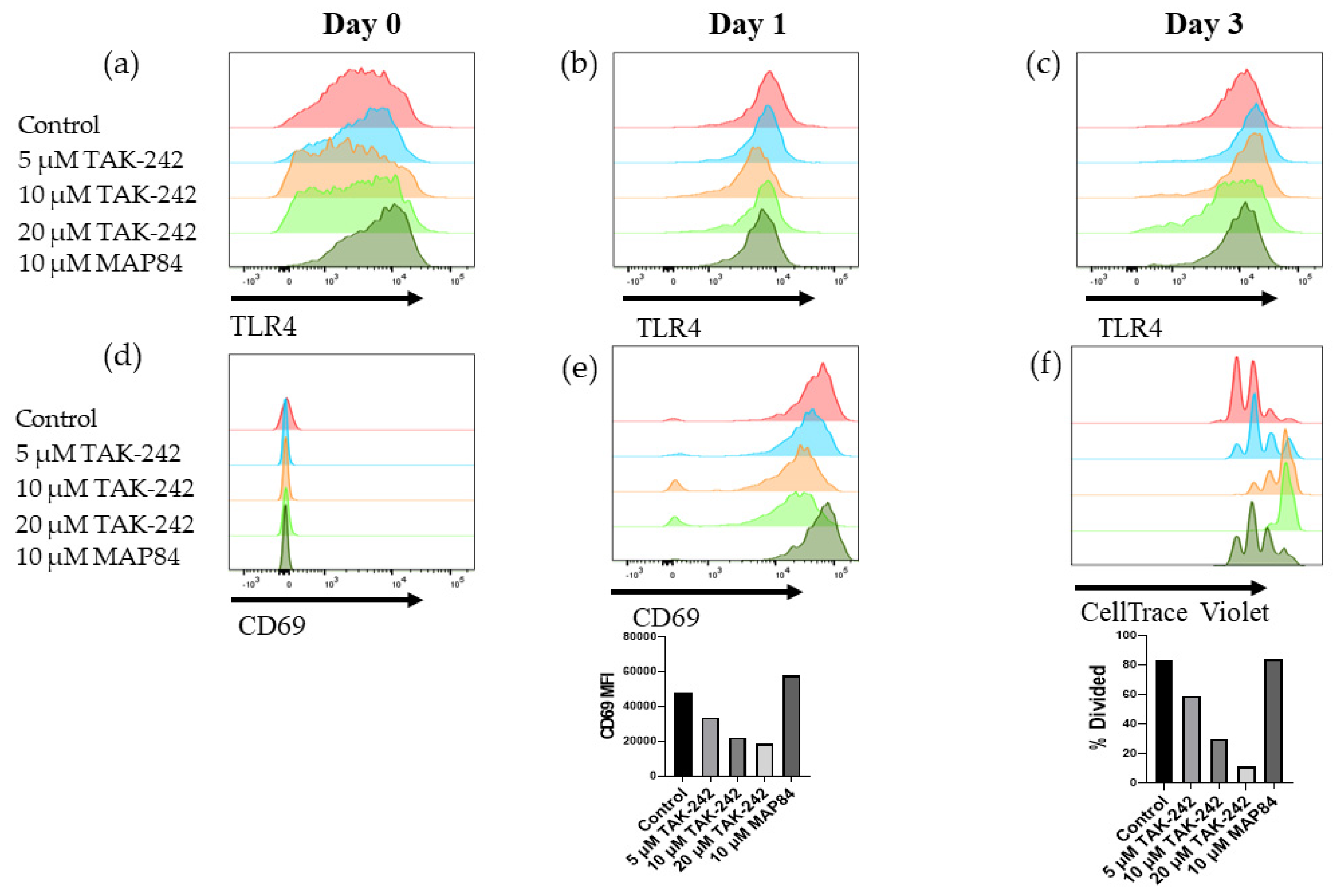
3.4. TAK-242 Directly Inhibits the Activation of CD8+ T Cells
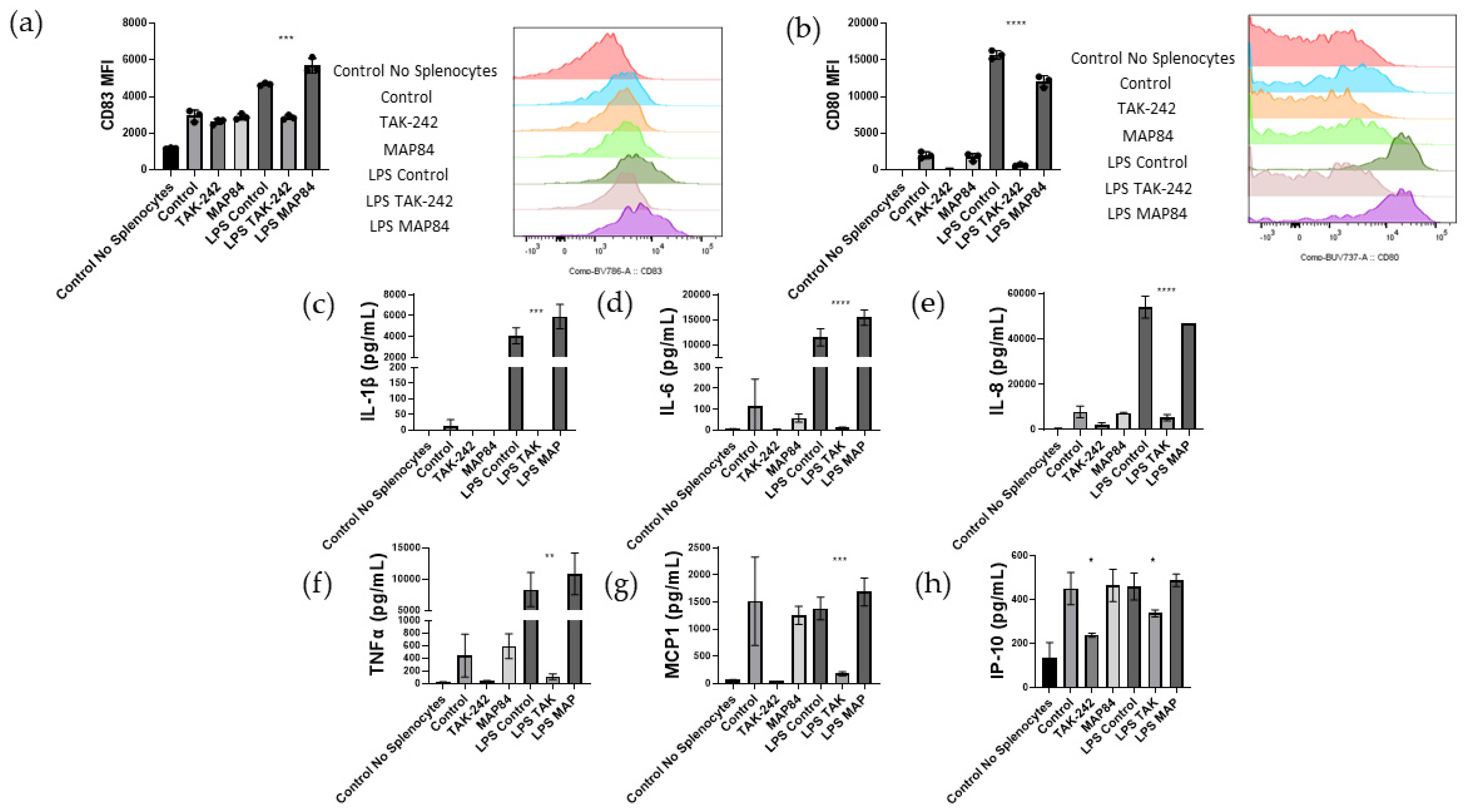
3.5. T Cell Activation and Islet Damage Is Inhibited by the Presence of TAK-242
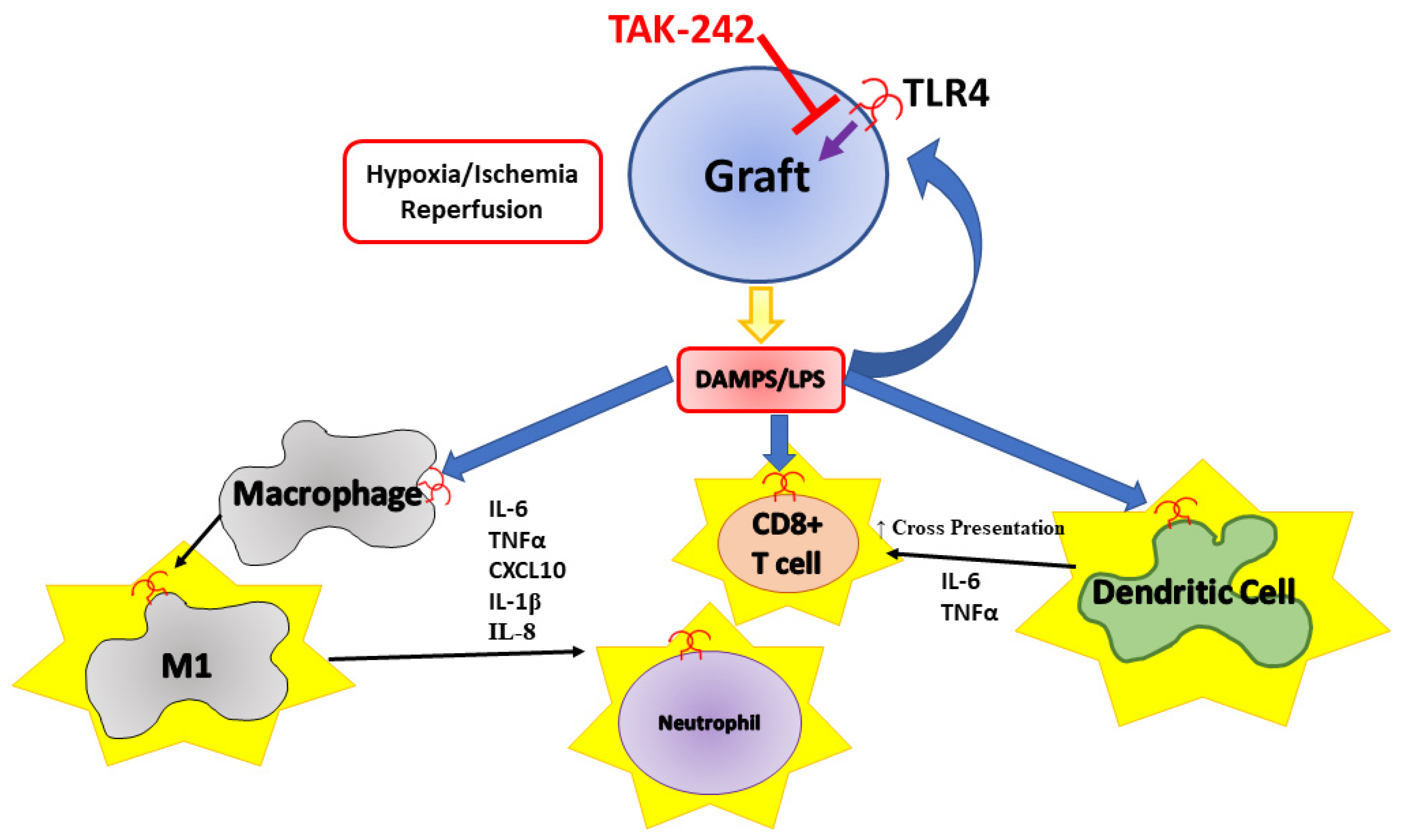
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, Å. Type 1 Diabetes Mellitus. Nat. Rev. Dis. Primer 2017, 3, 17016. [Google Scholar] [CrossRef]
- Shapiro, A.M.J.; Pokrywczynska, M.; Ricordi, C. Clinical Pancreatic Islet Transplantation. Nat. Rev. Endocrinol. 2017, 13, 268–277. [Google Scholar] [CrossRef]
- Sutherland, D.E.R.; Radosevich, D.M.; Bellin, M.D.; Hering, B.J.; Beilman, G.J.; Dunn, T.B.; Chinnakotla, S.; Vickers, S.M.; Bland, B.; Balamurugan, A.N.; et al. Total Pancreatectomy and Islet Autotransplantation for Chronic Pancreatitis. J. Am. Coll. Surg. 2012, 214, 409–424. [Google Scholar] [CrossRef]
- Shapiro, A.M.J.; Lakey, J.R.T.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet Transplantation in Seven Patients with Type 1 Diabetes Mellitus Using a Glucocorticoid-Free Immunosuppressive Regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef]
- Toso, C.; Shapiro, A.M.J.; Bowker, S.; Dinyari, P.; Paty, B.; Ryan, E.A.; Senior, P.; Johnson, J.A. Quality of Life After Islet Transplant: Impact of the Number of Islet Infusions and Metabolic Outcome. Transplantation 2007, 84, 664–666. [Google Scholar] [CrossRef]
- Foster, E.D.; Bridges, N.D.; Feurer, I.D.; Eggerman, T.L.; Hunsicker, L.G.; Alejandro, R. Clinical Islet Transplantation Consortium Improved Health-Related Quality of Life in a Phase 3 Islet Transplantation Trial in Type 1 Diabetes Complicated by Severe Hypoglycemia. Diabetes Care 2018, 41, 1001–1008. [Google Scholar] [CrossRef]
- Delaune, V.; Berney, T.; Lacotte, S.; Toso, C. Intraportal Islet Transplantation: The Impact of the Liver Microenvironment. Transpl. Int. 2017, 30, 227–238. [Google Scholar] [CrossRef]
- Bennet, W.; Groth, C.-G.; Larsson, R.; Nilsson, B.; Korsgren, O. Isolated Human Islets Trigger an Instant Blood Mediated Inflammatory Reaction: Implications for Intraportal Islet Transplantation as a Treatment for Patients with Type 1 Diabetes. Upsala J. Med Sci. 2000, 105, 125–133. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 Signal Transduction Pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular Mechanisms That Influence the Macrophage M1–M2 Polarization Balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef]
- Mok, D.; Black, M.; Gupta, N.; Arefanian, H.; Tredget, E.; Rayat, G.R. Early Immune Mechanisms of Neonatal Porcine Islet Xenograft Rejection. Xenotransplantation 2019, 26, e12546. [Google Scholar] [CrossRef]
- Wu, T.-T.; Chen, T.-L.; Chen, R.-M. Lipopolysaccharide Triggers Macrophage Activation of Inflammatory Cytokine Expression, Chemotaxis, Phagocytosis, and Oxidative Ability via a Toll-like Receptor 4-Dependent Pathway: Validated by RNA Interference. Toxicol. Lett. 2009, 191, 195–202. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, Y.; Song, L.; Kim, D.-S.; Wu, H.; Yang, L.; Li, S.; Morgan, K.A.; Adams, D.B.; Wang, H. Cell-Permeable Peptide Blocks TLR4 Signaling and Improves Islet Allograft Survival. Cell Transplant. 2016, 25, 1319–1329. [Google Scholar] [CrossRef]
- Shen, H.; Tesar, B.M.; Walker, W.E.; Goldstein, D.R. Dual Signaling of MyD88 and TRIF Is Critical for Maximal TLR4-Induced Dendritic Cell Maturation1. J. Immunol. 2008, 181, 1849–1858. [Google Scholar] [CrossRef]
- Alloatti, A.; Kotsias, F.; Pauwels, A.-M.; Carpier, J.-M.; Jouve, M.; Timmerman, E.; Pace, L.; Vargas, P.; Maurin, M.; Gehrmann, U.; et al. Toll-like Receptor 4 Engagement on Dendritic Cells Restrains Phago-Lysosome Fusion and Promotes Cross-Presentation of Antigens. Immunity 2015, 43, 1087–1100. [Google Scholar] [CrossRef]
- Weimershaus, M.; Mauvais, F.-X.; Saveanu, L.; Adiko, C.; Babdor, J.; Abramova, A.; Montealegre, S.; Lawand, M.; Evnouchidou, I.; Huber, K.J.; et al. Innate Immune Signals Induce Anterograde Endosome Transport Promoting MHC Class I Cross-Presentation. Cell Rep. 2018, 24, 3568–3581. [Google Scholar] [CrossRef]
- Goldberg, A.; Parolini, M.; Chin, B.Y.; Czismadia, E.; Otterbein, L.E.; Bach, F.H.; Wang, H. Toll-like Receptor 4 Suppression Leads to Islet Allograft Survival. FASEB J. 2007, 21, 2840–2848. [Google Scholar] [CrossRef]
- Giovannoni, L.; Muller, Y.D.; Lacotte, S.; Parnaud, G.; Borot, S.; Meier, R.P.H.; Lavallard, V.; Bédat, B.; Toso, C.; Daubeuf, B.; et al. Enhancement of Islet Engraftment and Achievement of Long-Term Islet Allograft Survival by Toll-like Receptor 4 Blockade. Transplantation 2015, 99, 29–35. [Google Scholar] [CrossRef]
- Krüger, B.; Yin, N.; Zhang, N.; Yadav, A.; Coward, W.; Lal, G.; Zang, W.; Heeger, P.S.; Bromberg, J.S.; Murphy, B.; et al. Islet-Expressed TLR2 and TLR4 Sense Injury and Mediate Early Graft Failure after Transplantation. Eur. J. Immunol. 2010, 40, 2914–2924. [Google Scholar] [CrossRef]
- Matsunaga, N.; Tsuchimori, N.; Matsumoto, T. Masayuki Ii TAK-242 (Resatorvid), a Small-Molecule Inhibitor of Toll-like Receptor (TLR) 4 Signaling, Binds Selectively to TLR4 and Interferes with Interactions between TLR4 and Its Adaptor Molecules. Mol. Pharmacol. 2011, 79, 34–41. [Google Scholar] [CrossRef]
- Rice, T.W.; Wheeler, A.P.; Bernard, G.R.; Vincent, J.-L.; Angus, D.C.; Aikawa, N.; Demeyer, I.; Sainati, S.; Amlot, N.; Cao, C.; et al. A Randomized, Double-Blind, Placebo-Controlled Trial of TAK-242 for the Treatment of Severe Sepsis*. Crit. Care Med. 2010, 38, 1685–1694. [Google Scholar] [CrossRef]
- Gao, Q.; Ma, L.L.; Gao, X.; Yan, W.; Williams, P.; Yin, D.P. TLR4 Mediates Early Graft Failure After Intraportal Islet Transplantation. Am. J. Transplant. 2010, 10, 1588–1596. [Google Scholar] [CrossRef]
- Chang, C.A.; Akinbobuyi, B.; Quintana, J.M.; Yoshimatsu, G.; Naziruddin, B.; Kane, R.R. Ex-Vivo Generation of Drug-Eluting Islets Improves Transplant Outcomes by Inhibiting TLR4-Mediated NFkB Upregulation. Biomaterials 2018, 159, 13–24. [Google Scholar] [CrossRef]
- Chinnakotla, S.; Beilman, G.J.; Dunn, T.B.; Bellin, M.D.; Freeman, M.L.; Radosevich, D.M.; Arain, M.; Amateau, S.K.; Mallery, J.S.; Schwarzenberg, S.J.; et al. Factors Predicting Outcomes after a Total Pancreatectomy and Islet Autotransplantation Lessons Learned From over 500 Cases. Ann. Surg. 2015, 262, 610–622. [Google Scholar] [CrossRef]
- Kin, T. Islet Isolation for Clinical Transplantation. In The Islets of Langerhans; Islam, M.S., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 683–710. ISBN 978-90-481-3271-3. [Google Scholar]
- Saravanan, P.B.; Vasu, S.; Yoshimatsu, G.; Darden, C.M.; Wang, X.; Gu, J.; Lawrence, M.C.; Naziruddin, B. Differential Expression and Release of Exosomal miRNAs by Human Islets under Inflammatory and Hypoxic Stress. Diabetologia 2019, 62, 1901–1914. [Google Scholar] [CrossRef]
- Kanak, M.A.; Takita, M.; Itoh, T.; SoRelle, J.A.; Murali, S.; Kunnathodi, F.; Shahbazov, R.; Lawrence, M.C.; Levy, M.F.; Naziruddin, B. Alleviation of Instant Blood-Mediated Inflammatory Reaction in Autologous Conditions through Treatment of Human Islets with NF-κB Inhibitors. Transplantation 2014, 98, 578. [Google Scholar] [CrossRef]
- Yoshimatsu, G.; Kunnathodi, F.; Saravanan, P.B.; Shahbazov, R.; Chang, C.; Darden, C.M.; Zurawski, S.; Boyuk, G.; Kanak, M.A.; Levy, M.F.; et al. Pancreatic β-Cell–Derived IP-10/CXCL10 Isletokine Mediates Early Loss of Graft Function in Islet Cell Transplantation. Diabetes 2017, 66, 2857–2867. [Google Scholar] [CrossRef]
- Wienhöfer, L.; Marker, M.; Antoni, A.-C.; Sutter, K.; Sander, A.; Dudda, M.; Flohé, S.B. TLR4 Transactivates CD8+ T Lymphocytes upon Acute Sterile Tissue Injury. ImmunoHorizons 2021, 5, 298–306. [Google Scholar] [CrossRef]
- GILLA, R.G. Antigen Presentation Pathways for Immunity to Islet Transplants: Relevance to Immunoisolation. Ann. N. Y. Acad. Sci. 1999, 875, 255–260. [Google Scholar] [CrossRef]
- Hughes, A.D.; Zhao, D.; Dai, H.; Abou-Daya, K.I.; Tieu, R.; Rammal, R.; Williams, A.L.; Landsittel, D.P.; Shlomchik, W.D.; Morelli, A.E.; et al. Cross-Dressed Dendritic Cells Sustain Effector T Cell Responses in Islet and Kidney Allografts. J. Clin. Investig. 2020, 130, 287–294. [Google Scholar] [CrossRef]
- Tripathy, A.; Khanna, S.; Padhan, P.; Smita, S.; Raghav, S.; Gupta, B. Direct Recognition of LPS Drive TLR4 Expressing CD8+ T Cell Activation in Patients with Rheumatoid Arthritis. Sci. Rep. 2017, 7, 933. [Google Scholar] [CrossRef]
- Sha, T.; Sunamoto, M.; Kitazaki, T.; Sato, J.; Ii, M.; Iizawa, Y. Therapeutic Effects of TAK-242, a Novel Selective Toll-like Receptor 4 Signal Transduction Inhibitor, in Mouse Endotoxin Shock Model. Eur. J. Pharmacol. 2007, 571, 231–239. [Google Scholar] [CrossRef]
- Liu, Y.; Pu, X.; Qin, X.; Gong, J.; Huang, Z.; Luo, Y.; Mou, T.; Zhou, B.; Shen, A.; Wu, Z. Neutrophil Extracellular Traps Regulate HMGB1 Translocation and Kupffer Cell M1 Polarization During Acute Liver Transplantation Rejection. Front. Immunol. 2022, 13, 823511. [Google Scholar] [CrossRef]
- Leopold Wager, C.M.; Wormley, F.L. Classical versus Alternative Macrophage Activation: The Ying and the Yang in Host Defense against Pulmonary Fungal Infections. Mucosal Immunol. 2014, 7, 1023–1035. [Google Scholar] [CrossRef]
- Kunkel, S.L.; Standiford, T.; Kasahara, K.; Strieter, R.M. Interleukin-8 (IL-8): The Major Neutrophil Chemotactic Factor in the Lung. Exp. Lung Res. 1991, 17, 17–23. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Montolio, M.; Biarnés, M.; Téllez, N.; Escoriza, J.; Soler, J.; Montanya, E. Interleukin-1β and Inducible Form of Nitric Oxide Synthase Expression in Early Syngeneic Islet Transplantation. J. Endocrinol. 2007, 192, 169–177. [Google Scholar] [CrossRef]
- Naziruddin, B.; Iwahashi, S.; Kanak, M.A.; Takita, M.; Itoh, T.; Levy, M.F. Evidence for Instant Blood-Mediated Inflammatory Reaction in Clinical Autologous Islet Transplantation. Am. J. Transplant. 2014, 14, 428–437. [Google Scholar] [CrossRef]
- Naziruddin, B.; Kanak, M.A.; Chang, C.A.; Takita, M.; Lawrence, M.C.; Dennison, A.R.; Onaca, N.; Levy, M.F. Improved Outcomes of Islet Autotransplant after Total Pancreatectomy by Combined Blockade of IL-1β and TNFα. Am. J. Transplant. 2018, 18, 2322–2329. [Google Scholar] [CrossRef]
- Baker, M.S.; Chen, X.; Rotramel, A.R.; Nelson, J.J.; Lu, B.; Gerard, C.; Kanwar, Y.; Kaufman, D.B. Genetic Deletion of Chemokine Receptor CXCR3 or Antibody Blockade of Its Ligand IP-10 Modulates Posttransplantation Graft-Site Lymphocytic Infiltrates and Prolongs Functional Graft Survival in Pancreatic Islet Allograft Recipients. Surgery 2003, 134, 126–133. [Google Scholar] [CrossRef]
- Piemonti, L.; Leone, B.E.; Nano, R.; Saccani, A.; Monti, P.; Maffi, P.; Bianchi, G.; Sica, A.; Peri, G.; Melzi, R.; et al. Human Pancreatic Islets Produce and Secrete MCP-1/CCL2: Relevance in Human Islet Transplantation. Diabetes 2002, 51, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 Activates Neutrophil Extracellular Traps to Ensnare Bacteria in Septic Blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Krüger, B.; Lal, G.; Luan, Y.; Yadav, A.; Zang, W.; Grimm, M.; Waaga-Gasser, A.M.; Murphy, B.; Bromberg, J.S.; et al. Inhibition of TLR4 Signaling Prolongs Treg-Dependent Murine Islet Allograft Survival. Immunol. Lett. 2010, 127, 119–125. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Variable | Value (n = 10) |
|---|---|
| Gender (female:male) | 8:2 |
| Age (years) | 40 ± 11.7 |
| Height (cm) | 165 ± 9.88 |
| Weight (kg) | 70.2 ± 11.82 |
| Body mass index (kg/m2) | 25.75 ± 3.71 |
| Disease duration (years) | 9.4 ± 5.9 |
| Fasting blood glucose (mg/dL) | 91.1 ± 12.3 |
| Stimulated blood glucose (mg/dL) | 153.5 ± 54.5 |
| Basal C-peptide (ng/mL) | 1.91 ± 1.39 |
| Stimulated C-peptide (ng/mL) | 7.39 ± 4.48 |
| ∆ C-peptide (ng/mL) | 5.48 ± 3.47 |
| Initial trimmed pancreas weight (g) | 134.6 ± 25.3 |
| Pancreas weight processed (g) | 88.0 ± 23.3 |
| Total islet yield (IEQ) | 560,973 ± 125,830 |
| Islet particle number (IN) | 335,700 ± 96,743 |
| Islet yield (IEQ/g pancreas) | 5986 ± 2012 |
| Dose (IEQ/kg patient) | 8147 ± 2040 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattke, J.; Darden, C.M.; Vasu, S.; Lawrence, M.C.; Kirkland, J.; Kane, R.R.; Naziruddin, B. Inhibition of Toll-like Receptor 4 Using Small Molecule, TAK-242, Protects Islets from Innate Immune Responses. Cells 2024, 13, 416. https://doi.org/10.3390/cells13050416
Mattke J, Darden CM, Vasu S, Lawrence MC, Kirkland J, Kane RR, Naziruddin B. Inhibition of Toll-like Receptor 4 Using Small Molecule, TAK-242, Protects Islets from Innate Immune Responses. Cells. 2024; 13(5):416. https://doi.org/10.3390/cells13050416
Chicago/Turabian StyleMattke, Jordan, Carly M. Darden, Srividya Vasu, Michael C. Lawrence, Jeffrey Kirkland, Robert R. Kane, and Bashoo Naziruddin. 2024. "Inhibition of Toll-like Receptor 4 Using Small Molecule, TAK-242, Protects Islets from Innate Immune Responses" Cells 13, no. 5: 416. https://doi.org/10.3390/cells13050416
APA StyleMattke, J., Darden, C. M., Vasu, S., Lawrence, M. C., Kirkland, J., Kane, R. R., & Naziruddin, B. (2024). Inhibition of Toll-like Receptor 4 Using Small Molecule, TAK-242, Protects Islets from Innate Immune Responses. Cells, 13(5), 416. https://doi.org/10.3390/cells13050416






