Early Stage In Vitro Bioprofiling of Potential Low-Molecular-Weight Organoboron Compounds for Boron Neutron Capture Therapy (BNCT)—Proposal for a Guide
Abstract
1. Introduction
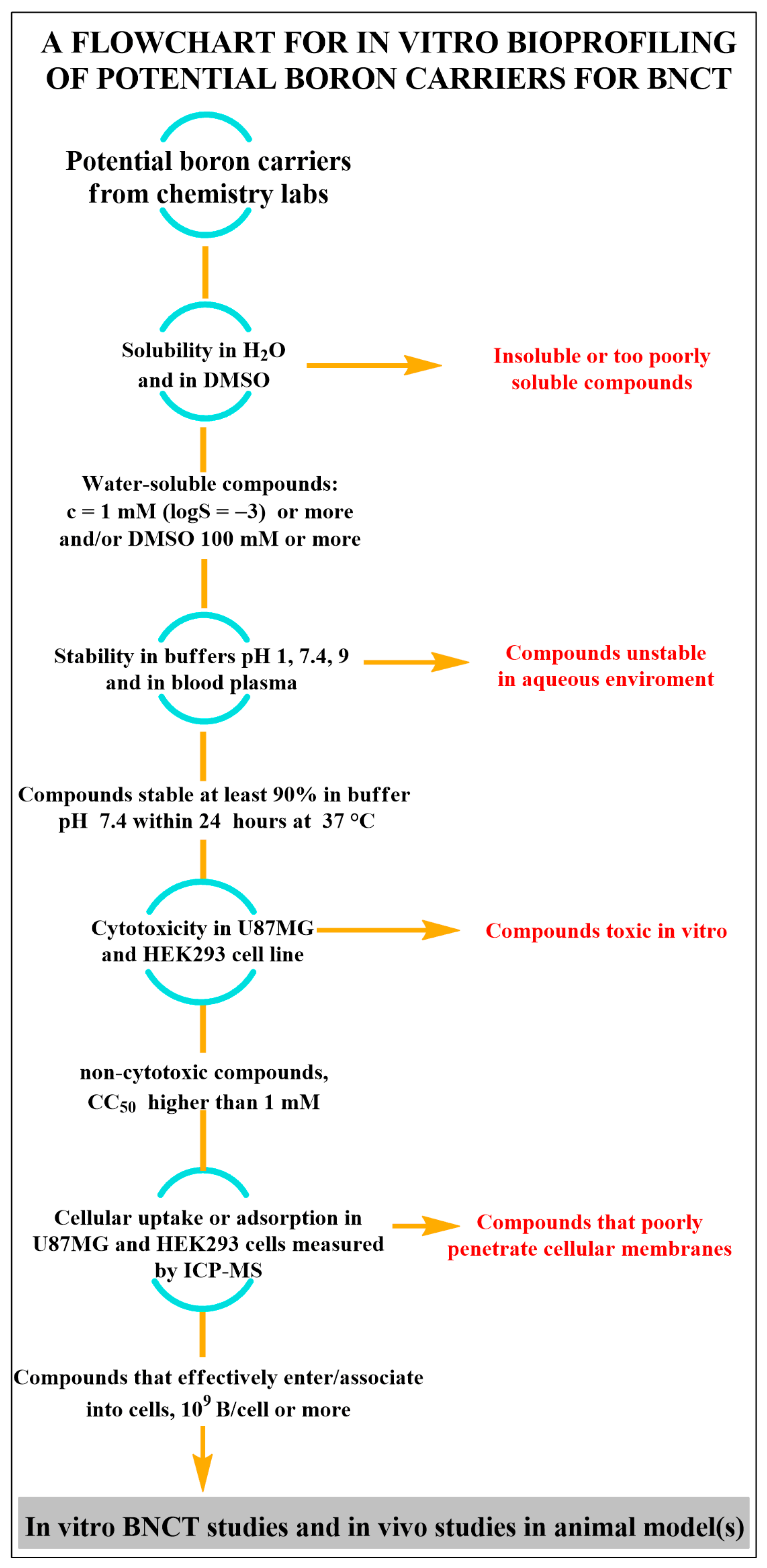
- solubility measurement in H2O and estimation of solubility in DMSO (soluble or insoluble).
- pKa, and log P/D determination.
- stability of the compounds at pH 1, 7.4, 9, and human plasma.
- cytotoxicity in vitro, in human glioblastoma multiforme cells U87MG, and/or squamous cell carcinoma, SAS, related to head and neck cancer or A375 for melanoma, as an example of cancer cells, and in HEK293 as an example of “normal” tissue cells. Of course, there is no obstacle to determining cytotoxicity in a larger number of cell lines, but these four should always be taken into account. As a criterion of cytotoxicity, we propose a concentration that reduces the number of viable cells by 50% (CC50) compared to untreated cells, lower than 100 mM.
- cellular uptake measured by ICP-AES in U87MG and HEK293c ells used in cytotoxicity studies.
2. Protocols for Testing BNCT Compounds
2.1. Principles
2.1.1. Solubility in H2O
2.1.2. pKa Determination
2.1.3. Log P/D Determination
2.1.4. Evaluation of a Compound’s Stability at Various pH Levels and in Human Plasma
2.1.5. Cellular Toxicity
2.1.6. Cellular Uptake
2.2. Protocols Suggested
2.2.1. Solubility Determinations
2.2.2. pKa Determination
2.2.3. Log P/D Determination
2.2.4. Stability of the Compounds in Human Plasma
2.2.5. Cellular Toxicity by Using the Neutral Red Assay
2.2.6. Boron Cellular Uptake Using the ICP-AES Method
3. Discussion
4. List of Example Laboratories Where Preliminary Bioprofiling Tests of Potential Boron Carriers Can Be Carried Out
4.1. Solubility in H2O
- Faculty of Pharmacy ULisboa, Portugal, https://imed.ulisboa.pt/members/rui-moreira/ (accessed on 4 March 2024), kinetic solubility, HPLC, Shimadzu LC-2050 (Shimadzu Europa GmbH, Duisburg, Germany).
- INNOpharma Platform for Drug Screening, University of Santiago de Compostela, Spain, ES-Openscreen, https://www.usc.es/biofarma/ (accessed on 4 March 2024), nephelometric method.
- Latvian Institute of Organic Synthesis, Latvia, https://www.osi.lv/en/services/analytical-chemistry/ (accessed on 4 March 2024), both kinetic and thermodynamic solubility assessment by direct concentration measurement in selected buffer solutions by HPLC/UV, Waters Alliance Separation module (Waters Co., Milford, MA, USA).
- LC-MS Metabolomics Center, School of Pharmacy, University of Eastern Finland, Finland, https://uefconnect.uef.fi/en/group/lc-ms-metabolomics-center/#information, (accessed on 4 March 2024), equilibrium shake flask thermodynamic solubility method, HPLC-UV/LC-MS (Agilent QQQ 6495) methods.
- National Library of Chemical Compounds POL-OPENSCREEN, Institute of Medicinal Biology PAS, Poland, https://pol-openscreen.pl (accessed on 4 March 2024), nephelometric method.
4.2. pKa Determination
- INNOpharma Platform for Drug Screening, University of Santiago de Compostela, SpainES-Openscreen, https://www.usc.es/biofarma/ (accessed on 4 March 2024), simultaneous determination of the UV spectra as a function of pH, EnVision Multilabel Reader.
- Latvian Institute of Organic Synthesis, Latvia, https://www.osi.lv/en/research/research-areas/physical-organic-chemistry/ (accessed on 4 March 2024), Nuclear Magnetic Resonance (NMR), 400 MHz or 600 MHz NMR system equipped with cryoprobes for better sensitivity (Bruker, Billerica, MA, USA).
- National Library of Chemical Compounds POL-OPENSCREEN, Institute of Medicinal Biology PAS, Poland, Poland, https://pol-openscreen.pl (accessed on 4 March 2024), potentiometric and spectrophotometric method, Pion SiriusT3 (Pion Inc. Ltd., Forest Row, UK).
4.3. Log P/D Determination
- Faculty of Pharmacy ULisboa, Portugal, https://imed.ulisboa.pt/members/rui-moreira/ (accessed on 4 March 2024), shake flask method, HPLC, Shimadzu LC-2050 (Shimadzu Europa GmbH, Duisburg, Germany).
- Latvian Institute of Organic Synthesis, Latvia, https://www.osi.lv/en/services/analytical-chemistry/ (accessed on 4 March 2024), shake flask method with HPLC/UV concentration measurements, Waters Alliance separation module (Waters, Milford, MA, USA).
- LC-MS Metabolomics Center, School of Pharmacy, University of Eastern Finland, Finland (https://uefconnect.uef.fi/en/group/lc-ms-metabolomics-center/#information (accessed on 4 March 2024), shake flask method for lipophilicity, HPLC-UV/LC-MS (Agilent QQQ 6495) methods.
- National Library of Chemical Compounds POL-OPENSCREEN, Institute of Medicinal Biology PAS, Poland, https://pol-openscreen.pl (accessed on 4 March 2024), potentiometric titration method, Pion SiriusT3 (Pion Inc. Ltd., Forest Row, UK).
- POL-OPENSCREEN, Institute of Biochemistry and Biophysics PAS, Poland, https://pol-openscreen.pl (accessed on 4 March 2024), hydrophobicity, reverse phase HPLC analysis (Knauer, Berlin, Germany).
4.4. Stability of Compounds in Buffers and Human Plasma
- Faculty of Pharmacy ULisboa, Portugal, https://imed.ulisboa.pt/facilities/ (accessed on 4 March 2024), HPLC and LC-MS, triple Quadrupole Micromass Quattro Micro API (Waters, Milford, MA, USA).
- INNOpharma Platform for Drug Screening, University of Santiago de Compostela, Spain (ES-Openscreen, https://www.usc.es/biofarma/ (accessed on 4 March 2024)), UPLC-MSMS/DAD.
- Latvian Institute of Organic Synthesis, Latvia, https://www.osi.lv/en/services/analytical-chemistry/ (accessed on 4 March 2024), LC/MS/MS, Waters Xevo-TQS (Waters, Milford, MA, USA).
- LC-MS Metabolomics Center, School of Pharmacy, University of Eastern Finland, Finland (https://uefconnect.uef.fi/en/group/lc-ms-metabolomics-center/#information (accessed on 4 March 2024)), HPLC-UV/LC-MS (Agilent QQQ 6495) methods.
- National Library of Chemical Compounds POL-OPENSCREEN, Institute of Medicinal Biology PAS, Poland, https://pol-openscreen.pl (accessed on 4 March 2024), LC-MS method, Agilent 6546 LC/Q-TOF (Santa Clara, CA, USA).
- POL-OPENSCREEN, Institute of Biochemistry and Biophysics PAS, Poland, https://pol-openscreen.pl (accessed on 4 March 2024), stability in buffers of various pH, LC/MS/MS quantitative method, mass spectrometer: Xevo TQ-S with standard EIS ion source (Waters), chromatograph: Acquity M-class (Waters).
4.5. Cellular Toxicity
- Faculty of Pharmacy ULisboa, Portugal, https://imed.ulisboa.pt/facilities/ (accessed on 4 March 2024), cell viability MTT assay, GloMax®-Multi+Microplate Reader (Promega Co., Madison, WI, USA).
- INNOpharma Platform for Drug Screening, University of Santiago de Compostela, Spain (ES-Openscreen, https://www.usc.es/biofarma/ (accessed on 4 March 2024)), cellular cytotoxicity measured using one healthy, and two cancer cell lines. Cell viability assays: MTS, MTT, CellTiter Glo.
- Screening Laboratory POL-OPENSCREEN, Institute of Medicinal Biology PAS, Poland, https://pol-openscreen.pl (accessed on 4 March 2024), xCELLigence RTCA system (Agilent Technologies, Santa Clara, CA, USA), real-time cell viability method or neutral red method.
4.6. Boron Cellular Uptake
- Biological and Chemical Research Centre, University of Warsaw, Poland, ICP-MS method, NexION 300D, PerkinElmer (Waltham, MA, USA).
- Laboratory of Chemistry of the Institute of Food Safety, Animal Health and Environment “BIOR”, Latvia, https://bior.lv/en (accessed on 4 March 2024), ICP-MS method, Thermo Scientific ICAP™ RQ ICP-MS (Waltham, MA, USA) and Agilent 7700 ×ICP-MS ((Santa Clara, CA, USA).
- LC-MS Metabolomics Center, School of Pharmacy, University of Eastern Finland, Finland (https://uefconnect.uef.fi/en/group/lc-ms-metabolomics-center/#information (accessed on 4 March 2024)), ICP-MS method, NeXION 350D (PerkinElmer Inc., Waltham, MA, USA).
- Dipartimento di Scienze e Innovazione Tecnologica, Università del Piemonte Orientale, Alessandria, Italy, ICP-MS method, Thermo Scientific iCAP RQ ICP_MS (Waltham, MA, USA); ICP-OES method: Spectro Genesis (AMETEK, Berwyn, PA, USA).
- Graduate School of Interdisciplinary Science and Engineering in Health Systems, Okayama University, Okayama, Japan. Contact info: eijimatu.01@gmail.com, ICP-AES method.
- Open Facility Center, Tokyo Institute of Technology, Japan, https://www.ofc.titech.ac.jp/en/ (accessed on 4 March 2024), ICP-MS method, ICP-MS ELAN-DRC-es (PerkinElmer Inc., Waltham, MA, USA).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sauerwein, W. Principles and roots of neutron capture therapy. In Neutron Capture Therapy. Principles and Applications; Sauerwein, W., Wittig, A., Moss, R., Nakagawa, Y., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2012; pp. 1–16. [Google Scholar] [CrossRef]
- IAEAL 23-01601; IAEA: Advances in Boron Neutron Capture Therapy. International Atomic Energy Agency: Vienna, Austria, 2023.
- Kumada, H.; Sakae, T.; Sakurai, H. Current development status of accelerator-based neutron source for boron neutron capture therapy. EPJ Tech. Instrum. 2023, 10, 18. [Google Scholar] [CrossRef]
- Safavi-Naeini, M.; Chacon, A.; Guatelli, S.; Franklin, D.R.; Bambery, K.; Gregoire, M.-C.; Rosenfeld, A. Opportunistic dose amplifcation for proton and carbon ion therapy via capture of internally generated thermal neutrons. Sci. Rep. 2018, 8, 16257. [Google Scholar] [CrossRef] [PubMed]
- Howell, N.; Middleton, R.J.; Sierro, F.; Wyatt, N.A.; Chacon, A.; HFraser, B.H.; Bambery, K.; Livio, E.; Dobie, C.; Bevitt, J.J.; et al. Neutron capture enhances dose and reduces cancer cell viability in and out of beam during helium and carbon ion therapy. Int. J. Radiat. Oncol. Biol. Phys. 2024. accepted. [Google Scholar] [CrossRef]
- Sauerwein, W.; Ulcar, M. Overview of Boron Neutron Capture Therapy in 2022. Cancer Biother. Radiopharm. 2023, 38, 143–147. [Google Scholar] [CrossRef]
- Laurenția, G.N.; Rodica, A.M. Boron neutron capture therapy: Delivery agents used in boron administration. Ther. Pharmacol. Clin. Toxicol. 2016, 20, 25–32. [Google Scholar]
- Sauerwein, W.; Bet, P.; Wittig, A. Drugs for BNCT: BSH and BPA. In Neutron Capture Therapy. Principles and Applications; Sauerwein, W., Wittig, A., Moss, R., Nakagawa, Y., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2012; pp. 117–160. [Google Scholar] [CrossRef]
- Kang, S.; Morrison, K.; Torgov, M.; Morrison, K. Boronotyrosine, a Borylated Amino Acid Mimetic with Enhanced Solubility, Tumor Boron Delivery, and Retention for the Reemerging Boron Neutron Capture Therapy Field. J. Med. Chem. 2023, 66, 13809–13820. [Google Scholar]
- Nishimura, K.; Kashiwagi, H.; Morita, T.; Fukuo, Y.; Okada, S.; Miura, K.; Matsumoto, Y.; Sugawara, Y.; Enomoto, T.; Suzuki, M.; et al. Efficient neutron capture therapy of glioblastoma with pteroyl-closo-dodecaborate-conjugated 4-(p-iodophenyl)butyric acid (PBC-IP). J. Control. Rel. 2023, 360, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Hirano, F.; Temma, T. Evaluation of 3-Borono-l-Phenylalanine as a Water-Soluble Boron Neutron Capture Therapy Agent. Pharmaceutics 2022, 14, 1106. [Google Scholar] [CrossRef]
- Oloo, S.O.; Smith, K.M.; Vicente, M.d.G.H. Multi-Functional Boron-Delivery Agents for Boron Neutron Capture Therapy of Cancers. Cancers 2023, 15, 3277. [Google Scholar] [CrossRef] [PubMed]
- DGBNCT-Boron Agents. Available online: https://dgbnct.com/index.php/boron-agents/ (accessed on 1 May 2024).
- Sauerwein, W.A.G.; Sancey, L.; Hey-Hawkins, E.; Kellert, M.; Panza, L.; Imperio, D.; Balcerzyk, M.; Rizzo, G.; Scalco, E.; Herrmann, K.; et al. Theranostics in Boron Neutron Capture Therapy. Life 2021, 11, 330. [Google Scholar] [CrossRef]
- Hattori, Y.; Andoh, T.; Kawabata, S.; Hu, N.; Michiue, H.; Nakamura, H.; Nomoto, T.; Suzuki, M.; Takata, T.; Tanaka, H.; et al. Proposal of recommended experimental protocols for in vitro and in vivo evaluation methods of boron agents for neutron capture therapy. J. Radiat. Res. 2023, 64, 859–869. [Google Scholar] [CrossRef] [PubMed]
- OECD Guidelines for the Testing of Chemicals; OECD iLibrary: Paris, France, 1995. [CrossRef]
- Hermans, A.; Milsmann, J.; Li, H.; Jede, C.; Moir, A.; Hens, B.; Morgado, J.; Wu, T.; Cohen, M. Challenges and Strategies for Solubility Measurements and Dissolution Method Development for Amorphous Solid Dispersion Formulations. AAPS J. 2023, 25, 11. [Google Scholar] [CrossRef] [PubMed]
- Massari, S.; Bertagnin, C.; Pismataro, M.C.; Donnadio, A.; Nannetti, G.; Felicetti, T.; Di Bona, S.; Nizi, M.G.; Tensi, L.; Manfroni, G.; et al. Synthesis and characterization of 1,2,4-triazolo [1,5-a]pyrimidine-2-carboxamide-based compounds targeting the PA-PB1 interface of influenza A virus polymerase. Eur. J. Med. Chem. 2021, 209, 112944. [Google Scholar] [CrossRef] [PubMed]
- Manallack, D.T. The pKa Distribution of Drugs: Application to Drug Discovery. Perspect. Medicin. Chem. 2007, 1, 25–38. [Google Scholar] [CrossRef]
- Han, G.E.; Priefer, R. A systematic review of various pKa determination techniques. Inter. J. Pharm. 2023, 635, 122783. [Google Scholar] [CrossRef] [PubMed]
- Andres, A.; Roses, M.; Rafolsa, C.; Boscha, E.; Espinosab, S.; Segarrab, V.; Huerta, J.M. Setup and validation of shake-flask procedures for the determination of partition coefficients (log D) from low drug amounts. Eur. J. Pharm. Sci. 2015, 76, 181–191. [Google Scholar] [CrossRef]
- Avdeef, A. Weighting scheme for regression analysis using pH data from acid-base titrations. Anal. Chim. Acta 1983, 148, 237–244. [Google Scholar] [CrossRef]
- Takács-Novák, K.; Avdeef, A. Interlaboratory study of logP determination by shake-flask and potentiometric methods. J. Pharm. Biomed. Anal. 1996, 14, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.Y.; Takacs-Novak, K. Multi-wavelength spectrophotometric determination of acid dissociation constants: A validation study. Anal. Chim. Acta 2001, 434, 157–167. [Google Scholar] [CrossRef]
- Sirius T3 User Manual, v1.1; Sirius Analytical Instruments Ltd.: East Sussex, UK, 2008.
- Patra, M.; Ingram, K.; Pierroz, V.; Ferrari, S.; Spingler, B.; Keiser, J.; Gasser, G. Ferrocenyl derivatives of the anthelmintic praziquantel: Design, synthesis, and biological evaluation. J. Med. Chem. 2012, 55, 8790–8798. [Google Scholar] [CrossRef]
- Keller, S.; Ching Ong, Y.; Lin, Y.; Cariou, K.; Gasser, G. A tutorial for the assessment of the stability of organometallic complexes in biological media. J. Organomet. Chem. 2019, 906, 121059. [Google Scholar] [CrossRef]
- Raitano, A.; Martin, T.; Zhang, C.; Malinao, M.-C.; Capo, L.; Ikeura, M.; Carroll, R.; Quintana, J.C.; Dlamini, S.; Kulenovic, L.; et al. Determination of pH Stability by UPLC/MS/MS, Application Note 2008; Waters Corporation: Milford, MA, USA, 2008. [Google Scholar]
- Justus, E.; Awad, D.; Hohnholt, M.; Schaffran, T.; Edwards, K.; Karlsson, G.; Damian, L.; Gabel, D. Synthesis, Liposomal Preparation, and in Vitro Toxicity of Two Novel Dodecaborate Cluster Lipids for Boron Neutron Capture Therapy. Bioconj. Chem. 2007, 18, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Kusaka, S.; Mukumoto, M.; Uehara, K.; Asano, T.; Suzuki, M.; Masunaga, S.; Ono, K.; Tanimori, S.; Kirihata, M. Biological Evaluation of Dodecaborate-Containing L-Amino Acids for Boron Neutron Capture Therapy. J. Med. Chem. 2012, 55, 6980–6984. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Verlinden, B.; Van Hoecke, K.; Aerts, A.; Daems, N.; Dobney, A.; Janssens, K.; Cardinaels, T. Quantification of boron in cells for evaluation of drug agents used in boron neutron capture therapy. J. Anal. At. Spectrom. 2021, 36, 598–606. [Google Scholar] [CrossRef]
- Dehring, K.A.; Workman, H.L.; Miller, K.D.; Mandagere, A.; Poole, S.K. Automated robotic liquid handling/laser-based nephelometry system for high throughput measurement of kinetic aqueous solubility. J. Pharmaceut. Biomed. Anal. 2004, 36, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Avdeef, A. pH-Metric log P.II. Refinement of partition coefficients and ionization constants of multiprotic substances. J. Pharm. Sci. 1993, 82, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Isik, M.; Levorse, D.; Rustenburg, A.S.; Ndukwe, I.E.; Wang, H.; Wang, X.; Reibarkh, M.; Martin, G.E.; Makarov, A.A.; Mobley, D.L.; et al. pKa measurements for the SAMPL6 2 prediction challenge for a set of kinase 3 inhibitor-like fragments. J. Comput. Aided Mol. Des. 2018, 32, 1117–1138. [Google Scholar] [CrossRef]
- Al-Sheddi, E.S.; Al-Oqail, M.M.; Saquib, Q.; Siddiqui, M.A.; Musarrat, J.; Al-Khedhairy, A.A.; Farshori, N.N. Novel All Trans-Retinoic Acid Derivatives: Cytotoxicity, Inhibition of Cell Cycle Progression and Induction of Apoptosis in Human Cancer Cell Lines. Molecules 2015, 20, 8181–8197. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protocol. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Ates, G.; Vanhaecke, T.; Rogiers, V.; Rodrigues, R.M. Assaying Cellular Viability Using the Neutral Red Uptake Assay. In Cell Viability Assays: Methods and Protocols; Methods in Molecular Biology; Gilbert, D.F., Friedrich, O., Eds.; Springer Science+Business Media: New York, NY, USA, 2017; Volume 1601, Chapter 2; pp. 19–26. [Google Scholar] [CrossRef]
- Białek-Pietras, M.; Olejniczak, A.B.; Tachikawa, S.; Nakamura, H.; Leśnikowski, Z.J. Towards new boron carriers for boron neutron capture therapy: Metallacarboranes bearing cobalt, iron and chromium and their cholesterol conjugates. Bioorg. Med. Chem. 2013, 21, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Balcer, E.; Joanna Giebułtowicz, J.; Sochacka, M.; Ruszczynska, A.; Muszynska, M.; Bulska, E. Investigation of the impact of L-phenylalanine and L-tyrosine pre-treatment on the uptake of 4-borono-L-phenylalanine in cancerous and normal cells using an analytical approach based on SC-ICP-MS. Molecules 2023, 28, 6552. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.E.; Issa, F.; Bhadbhade, M.; Gröebler, L.; Witting, P.K.; Kassiou, M.; Rutledge, P.J.; Rendina, L.M. Boronated phosphonium salts containing arylboronic acid, closo- or nido-carborane: Synthesis, X-ray diffraction, in vitro cytotoxicity and cellular uptake. J. Biol. Inorg. Chem. 2010, 15, 1305–1318. [Google Scholar] [CrossRef] [PubMed]
- Soloway, A.H.; Hatanaka, H.; Davis, M. Penetration of brain and brain tumor. VII. Tumor binding sulfhydryl compounds. J. Med. Chem. 1967, 10, 714–717. [Google Scholar] [CrossRef]
- Snyder, H.R.; Reedy, A.J.; Lennarj, W.J. Synthesis of aromatic boronic acids. Aldehyde boronic acids and a boronic acid analog of tyrosine. J. Am. Chem. Soc. 1958, 80, 835–838. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leśnikowski, Z.J.; Ekholm, F.; Hosmane, N.S.; Kellert, M.; Matsuura, E.; Nakamura, H.; Olejniczak, A.B.; Panza, L.; Rendina, L.M.; Sauerwein, W.A.G. Early Stage In Vitro Bioprofiling of Potential Low-Molecular-Weight Organoboron Compounds for Boron Neutron Capture Therapy (BNCT)—Proposal for a Guide. Cells 2024, 13, 798. https://doi.org/10.3390/cells13100798
Leśnikowski ZJ, Ekholm F, Hosmane NS, Kellert M, Matsuura E, Nakamura H, Olejniczak AB, Panza L, Rendina LM, Sauerwein WAG. Early Stage In Vitro Bioprofiling of Potential Low-Molecular-Weight Organoboron Compounds for Boron Neutron Capture Therapy (BNCT)—Proposal for a Guide. Cells. 2024; 13(10):798. https://doi.org/10.3390/cells13100798
Chicago/Turabian StyleLeśnikowski, Zbigniew J., Filip Ekholm, Narayan S. Hosmane, Martin Kellert, Eiji Matsuura, Hiroyuki Nakamura, Agnieszka B. Olejniczak, Luigi Panza, Louis M. Rendina, and Wolfgang A. G. Sauerwein. 2024. "Early Stage In Vitro Bioprofiling of Potential Low-Molecular-Weight Organoboron Compounds for Boron Neutron Capture Therapy (BNCT)—Proposal for a Guide" Cells 13, no. 10: 798. https://doi.org/10.3390/cells13100798
APA StyleLeśnikowski, Z. J., Ekholm, F., Hosmane, N. S., Kellert, M., Matsuura, E., Nakamura, H., Olejniczak, A. B., Panza, L., Rendina, L. M., & Sauerwein, W. A. G. (2024). Early Stage In Vitro Bioprofiling of Potential Low-Molecular-Weight Organoboron Compounds for Boron Neutron Capture Therapy (BNCT)—Proposal for a Guide. Cells, 13(10), 798. https://doi.org/10.3390/cells13100798










