Nanomedicine in the Face of Parkinson’s Disease: From Drug Delivery Systems to Nanozymes
Abstract
1. Introduction
2. Current Knowledge of Parkinson’s Disease’s Neuropathology
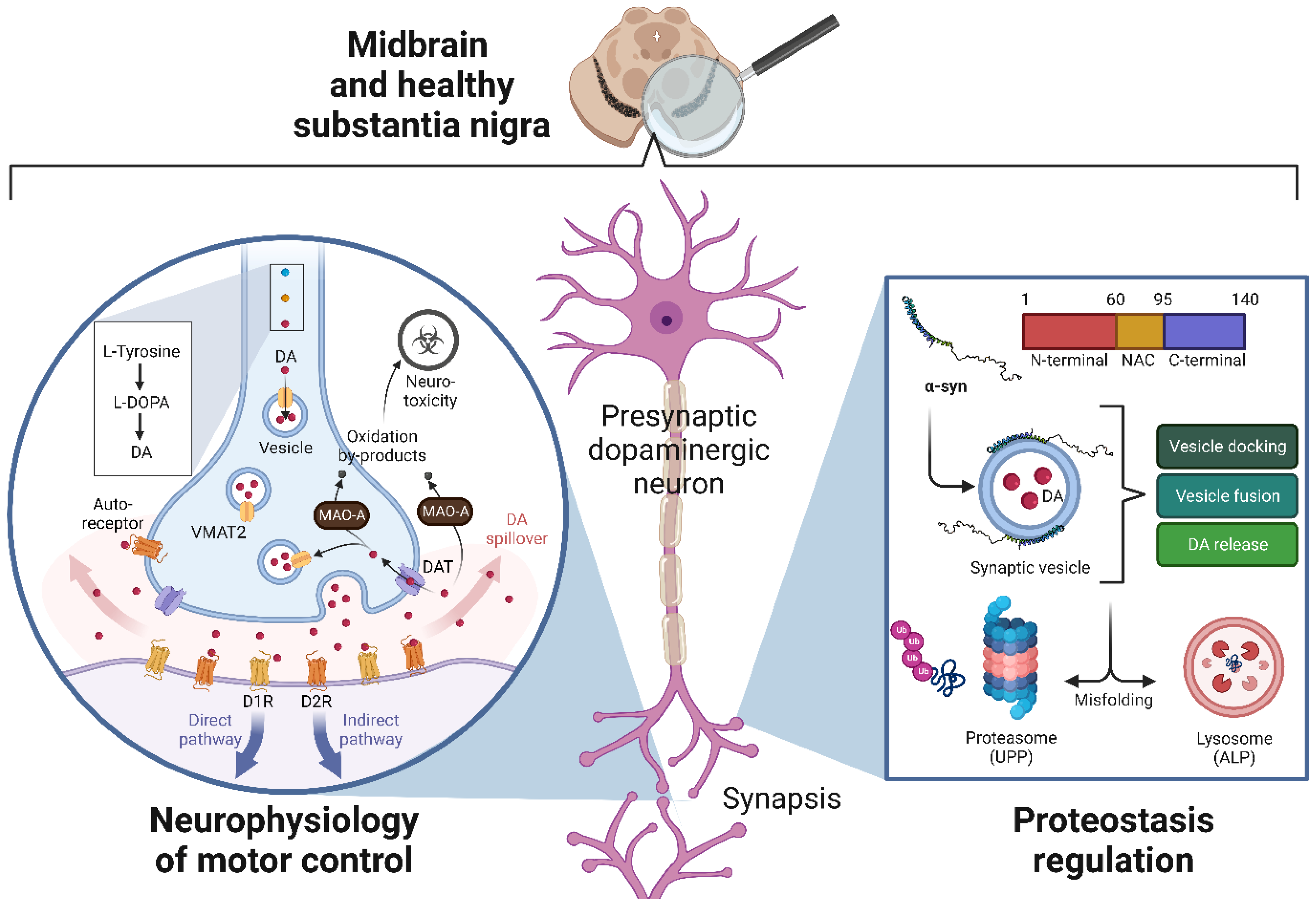
2.1. Normal Function of the Dopaminergic Neurons
2.1.1. Neurophysiology of Motor Control
2.1.2. Physiological Regulation of Proteostasis and Alpha-Synuclein
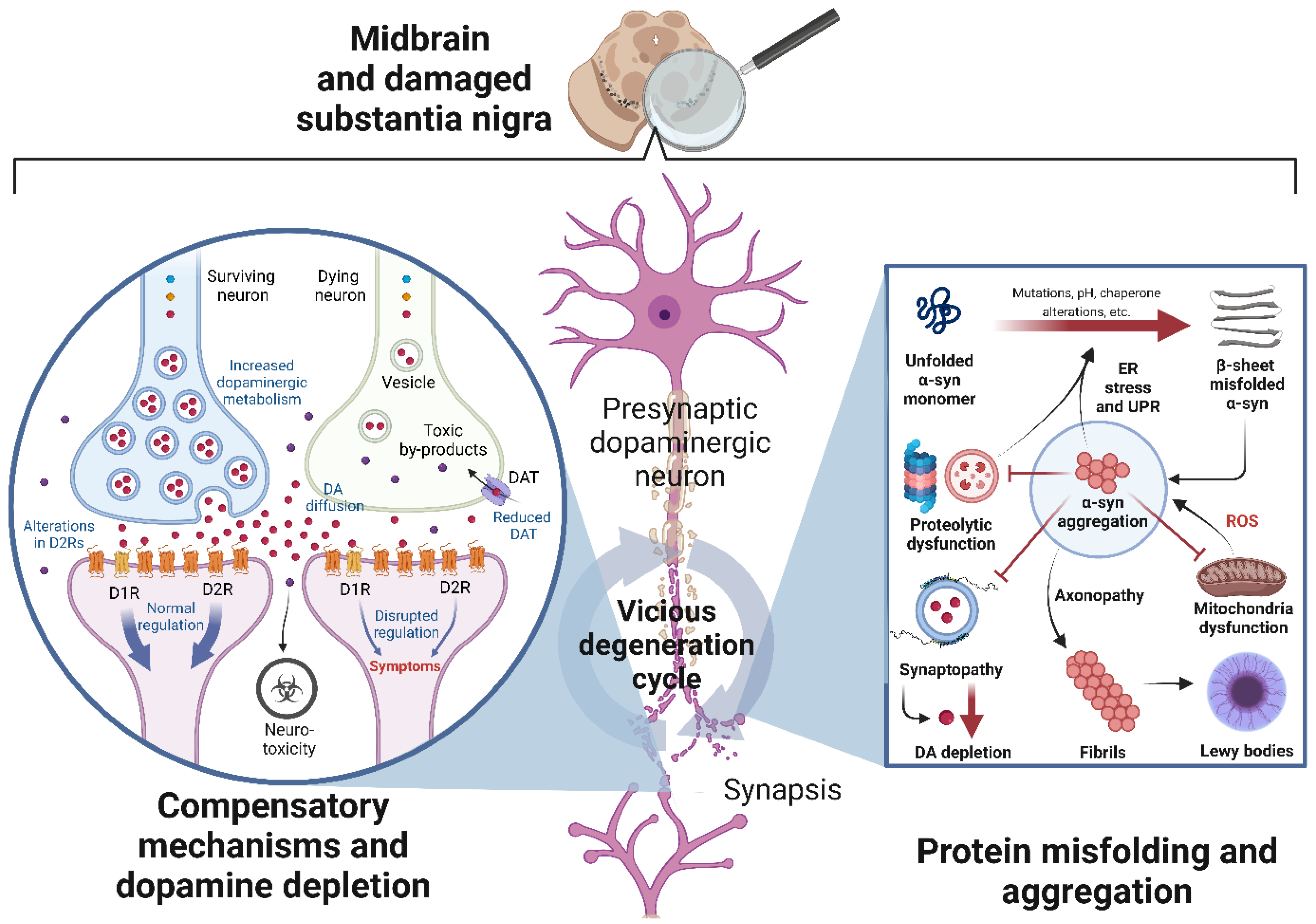
2.2. Pathophysiological Mechanisms of Neurodegeneration in PD
2.2.1. Loss of Proteostasis
Alpha-Synuclein Misfolding, Aggregation, and Propagation
Proteolytic Dysfunction, Endoplasmic Reticulum Stress, and the Unfolded Protein Response
Consequences of Alpha-Synuclein Misfolding and Aggregation
2.2.2. Loss of Dopaminergic Neurons and Their Projections
Compensatory Mechanisms upon Dopaminergic Neuron Death
Consequences of Dopamine Depletion in the Dorsal Striatum
Consequences of Dopamine Depletion in the Ventral Striatum
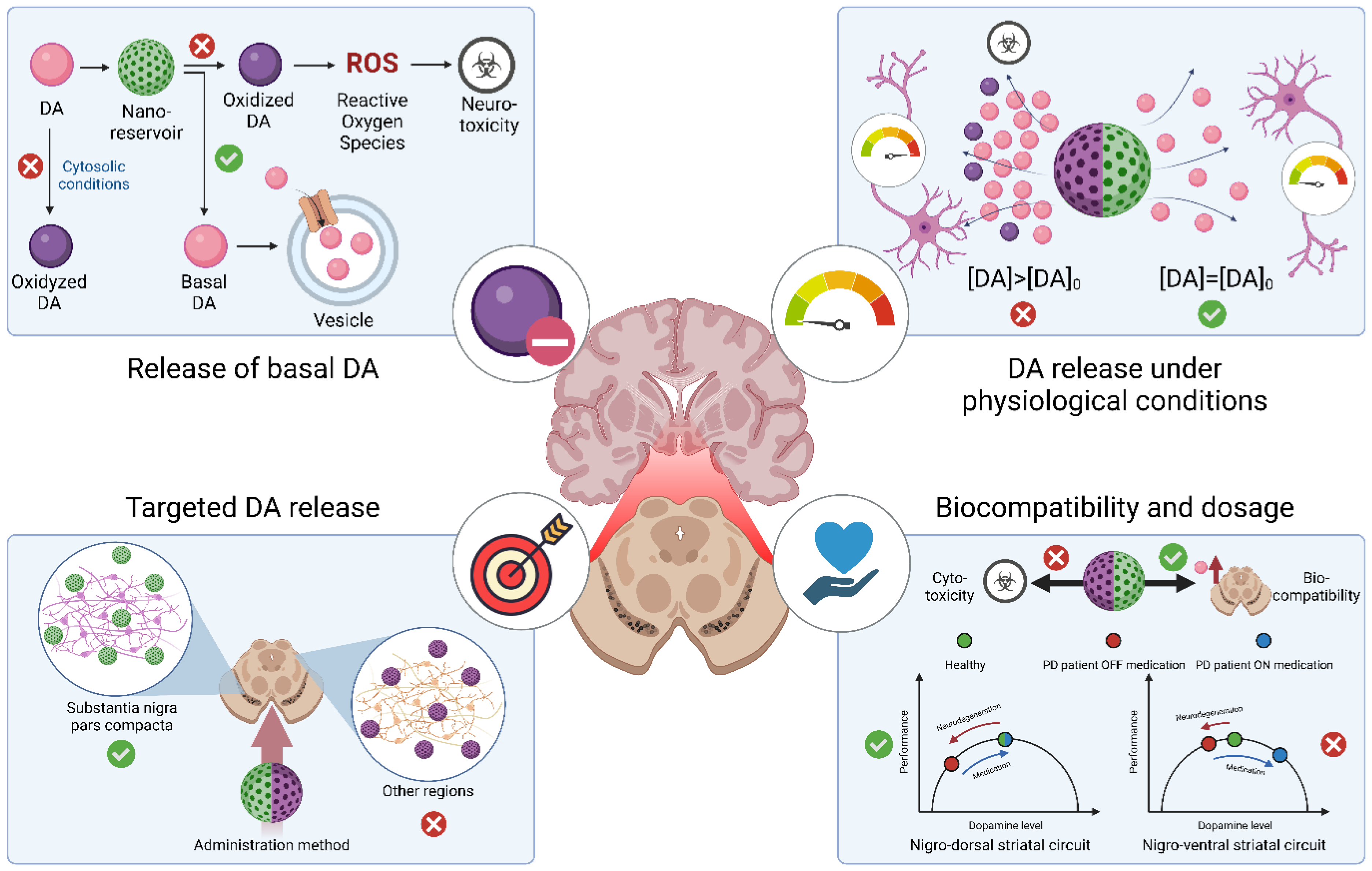
3. Controlled Drug Delivery on Parkinson’s Disease
3.1. Dopamine Administration as A Physiological Approach
3.2. Nanocarriers for Controlled Release of Dopamine
3.2.1. Polymers and Derivatives
3.2.2. Liposomes and Solid-Lipid NPs
3.2.3. Metal Oxide NPs
3.2.4. Inorganic NPs
| Type of Nanostructure | Nanocarrier + Functionalizing Agent | Administration Pathway | Ref. |
|---|---|---|---|
| Polymeric | Cellulose acetate phthalate | Stereotaxic surgery | [115] |
| Chitosan | Intraperitoneal | [117] | |
| Chitosan + esters/amides | Intranasal | [118,119,120] | |
| Poly(lactic-co-glycolic acid) | Intravenous | [123] | |
| Poly(lactic-co-glycolic acid) + albumin | [124] | ||
| Poly(lactic-co-glycolic acid) + borneol/lactoferrin | Intranasal | [125] | |
| Polyvinylpyrrolidone/Polyacrylic acid nanogel | Intraperitoneal | [126] | |
| Hydrogel | ‡ | [128] | |
| Oxidized alginate | Intraperitoneal | [129] | |
| 1,4-bis(imidazole-1-ylmethyl) benzene | Intranasal | [130] | |
| Lipidic | Liposome | Stereotaxic surgery | [137] |
| Liposome + stearylamine | Intraperitoneal | [138] | |
| Liposome | Intraperitoneal | [139] | |
| Liposome + thiolated chitosan | ‡ | [140] | |
| Liposome + glutamate | Intraperitoneal | [141] | |
| Liposome + transferrin | ‡ | [142] | |
| Liposome + amyloid precursor protein | Intraperitoneal | [144] | |
| Liposome + virus glycoproteins | Intravenous | [145] | |
| Solid lipids | ‡ | [149,150] | |
| Metal oxide | Titanium dioxide | Stereotaxic surgery | [157] |
| Titanium dioxide + chitosan | Oral | [162] | |
| Silicon dioxide | Stereotaxic surgery | [169] | |
| Inorganic | Gold NPs | ‡ | [175,176] |
| Selenide/cadmium quantum dots + Polyethylene glycol | ‡ | [178] | |
| Carbon quantum dots | Intravenous | [179] | |
| Carbon quantum dots + chitosan | ‡ | [180] | |
| Copper sulfide + chitosan | ‡ | [181] |
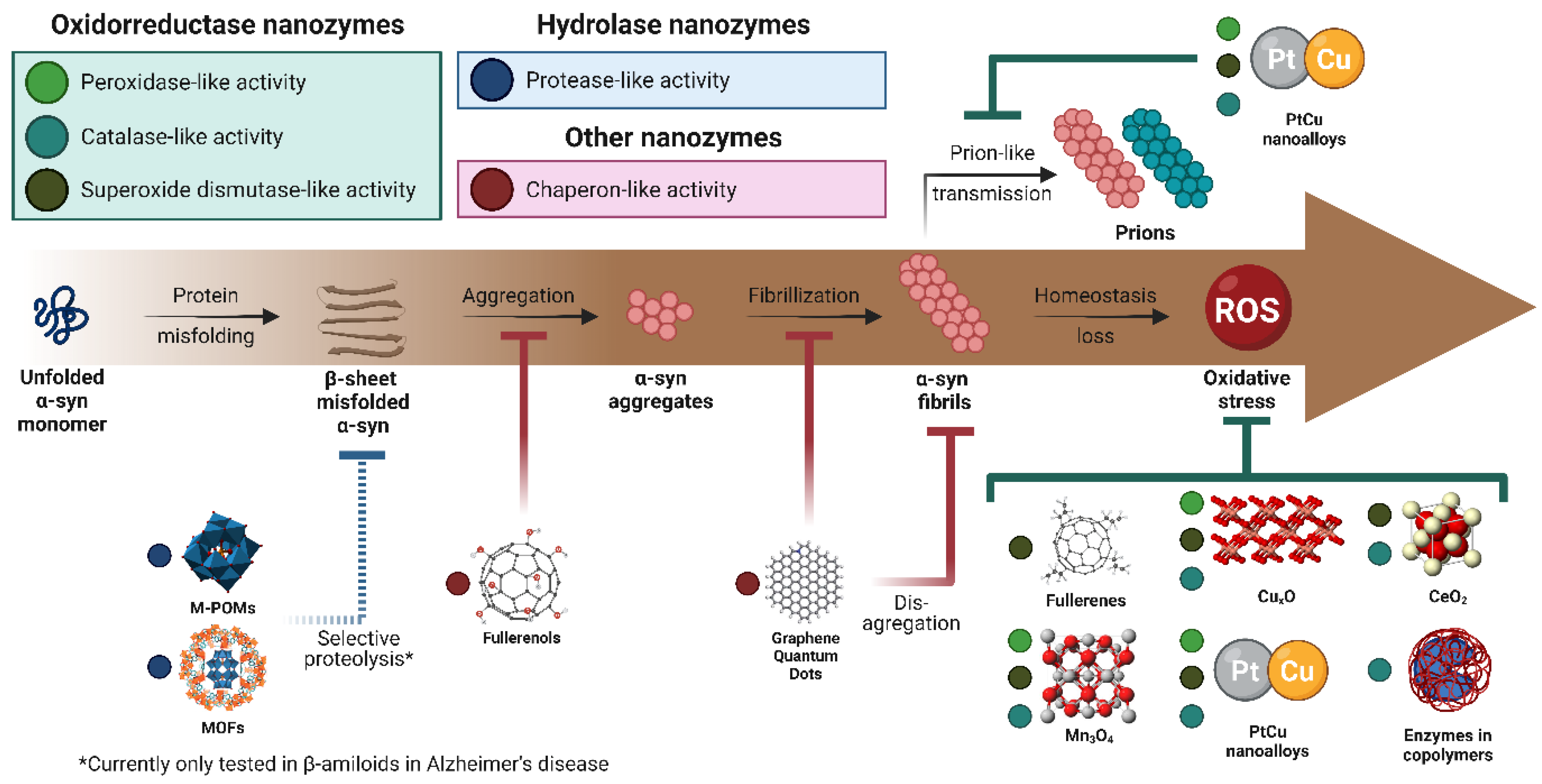
4. Oxidative Stress Reduction, Protein Aggregation Inhibition, and Selective Proteolysis on Parkinson’s Disease
4.1. Nanozymes as A Potential Treatment of Parkinson’s Disease
4.2. Nanozymes for Oxidative Stress Reduction
4.2.1. Fullerene-Based Antioxidant Nanozymes
4.2.2. Metal-Oxide-Based Antioxidant Nanozymes
4.2.3. Metal-Based Antioxidant Nanozymes
4.2.4. Polymer-Enzyme-Based Antioxidant Nanozymes
4.3. Nanozymes for Protein Aggregation Inhibition
4.4. Nanozymes for Selective Proteolysis
4.4.1. Polyoxometalate (POM)-Based Nanozymes
4.4.2. Metal-Organic Framework-Based Nanozymes
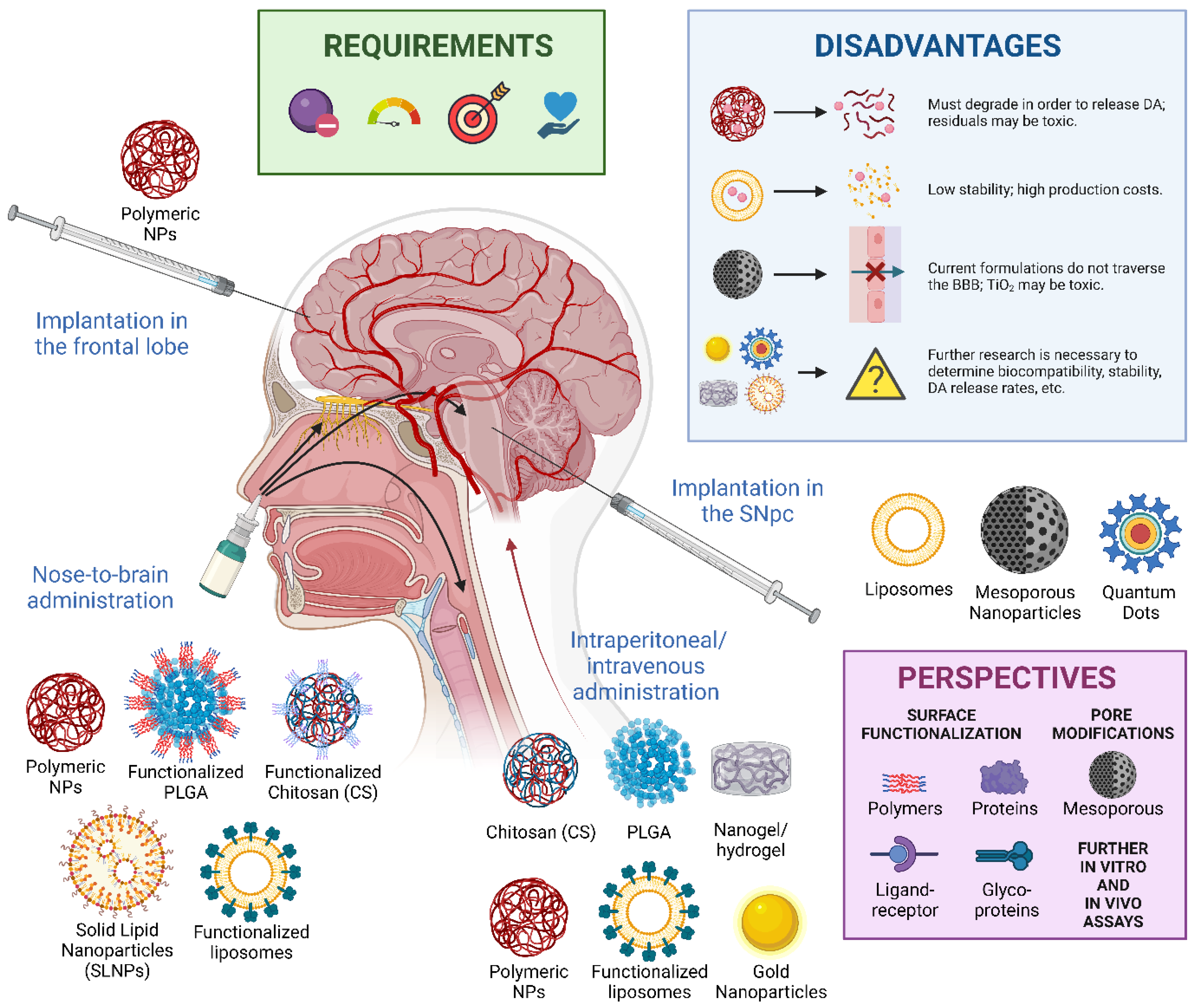
5. Perspectives on Nanomedicine-Based Therapies for Parkinson’s Disease
5.1. Non-Invasive Administration Methods for NPs
5.1.1. Overcoming the Blood–Brain Barrier
5.1.2. Nose-to-Brain Administration of NPs
5.2. Improved Selectivity for NPs
5.2.1. Surface Functionalization for Optimized Selectivity
5.2.2. Towards a Specific Enzyme-Like Activity for Misfolded Alpha-Synuclein
5.3. Limitations and Safety Considerations for Nanomedicines in Parkinson’s Disease
5.3.1. The Limits of Surface Modification in NPs
5.3.2. Nano-Derived Oxidative Stress
5.3.3. Nano-Derived Autophagy and Lysosomal Dysfunction
5.4. Towards Clinical Translation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obeso, J.A.; Stamelou, M.; Goetz, C.G.; Poewe, W.; Lang, A.E.; Weintraub, D.; Burn, D.; Halliday, G.M.; Bezard, E.; Przedborski, S.; et al. Past, Present, and Future of Parkinson’s Disease: A Special Essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 2017, 32, 1264–1310. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, R.; Schapira, A.H.V. Parkinson Disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s Disease: Mechanisms and Models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Lee, T.K.; Yankee, E.L. A Review on Parkinson’s Disease Treatment. Neuroimmunol. Neuroinflamm. 2021, 8, 222–244. [Google Scholar] [CrossRef]
- Olanow, C.W.; Stocchi, F. Levodopa: A New Look at an Old Friend. Mov. Disord. 2017, 33, 859–866. [Google Scholar] [CrossRef]
- Calabrese, V.; Santoro, A.; Monti, D.; Crupi, R.; Di Paola, R.; Latteri, S.; Cuzzocrea, S.; Zappia, M.; Giordano, J.; Calabrese, E.J.; et al. Aging and Parkinson’s Disease: Inflammaging, Neuroinflammation and Biological Remodeling as Key Factors in Pathogenesis. Free. Radic. Biol. Med. 2018, 115, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W. Neuropathology of Parkinson Disease. Park. Relat. Disord. 2018, 46, S30–S33. [Google Scholar] [CrossRef]
- Freitas, R.A., Jr. Nanomedicine: Basic Capabilities; Landes Bioscience: Georgetown, TX, USA, 1999; Volume 1, ISBN 978-1-57059-645-2. [Google Scholar]
- Haber, S.N.; Johnson, G.M. The Basal Ganglia. In The Human Nervous System; Elsevier Academic Press: San Diego, CA, USA, 2004. [Google Scholar]
- Prensa, L.; Giménez-Amaya, J.M.; Parent, A.; Bernácer, J.; Cebrián, C. The Nigrostriatal Pathway: Axonal Collateralization and Compartmental Specificity. In Birth, Life and Death of Dopaminergic Neurons in the Substantia Nigra; Springer: Vienna, Austria, 2009; Volume 73, pp. 49–58. [Google Scholar]
- German, D.C.; Manaye, K.F. Midbrain Dopaminergic Neurons (Nuclei A8, A9, and A10): Three-Dimensional Reconstruction in the Rat. J. Comp. Neurol. 1993, 331, 297–309. [Google Scholar] [CrossRef]
- Cebrián, C.; Parent, A.; Prensa, L. Patterns of Axonal Branching of Neurons of the Substantia Nigra Pars Reticulata and Pars Lateralis in the Rat. J. Comp. Neurol. 2005, 492, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, D.E.; Langley, J.; Sedlacik, J.; Boelmans, K.; Factor, S.A.; Hu, X.P. In Vivo Detection of Lateral–Ventral Tier Nigral Degeneration in Parkinson’s Disease. Hum. Brain Mapp. 2017, 38, 2627–2634. [Google Scholar] [CrossRef]
- Bergquist, F.; Shahabi, H.N.; Nissbrandt, H. Somatodendritic Dopamine Release in Rat Substantia Nigra Influences Motor Performance on the Accelerating Rod. Brain Res. 2003, 973, 81–91. [Google Scholar] [CrossRef]
- Sulzer, D.; Cragg, S.J.; Rice, M.E. Striatal Dopamine Neurotransmission: Regulation of Release and Uptake. Basal Ganglia 2016, 6, 123–148. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S. Noradrenergic Neurotransmission. In Primer on the Autonomic Nervous System; Elsevier Academic Press: Cambridge, MA, USA, 2012; pp. 37–43. ISBN 978-0-12-386525-0. [Google Scholar]
- Guillot, T.S.; Miller, G.W. Protective Actions of the Vesicular Monoamine Transporter 2 (VMAT2) in Monoaminergic Neurons. Mol. Neurobiol. 2009, 39, 149–170. [Google Scholar] [CrossRef] [PubMed]
- Palade, G.E.; Palay, S.L. Electron Microscope Observations of Intraneuronal and Neuromuscular Synapses. Anat. Rec. 1954, 118, 335. [Google Scholar]
- Mosharov, E.V.; Gong, L.-W.; Khanna, B.; Sulzer, D.; Lindau, M. Intracellular Patch Electrochemistry: Regulation of Cytosolic Catecholamines in Chromaffin Cells. J. Neurosci. 2003, 23, 5835–5845. [Google Scholar] [CrossRef]
- Miyazaki, I.; Asanuma, M. Dopaminergic Neuron-Specific Oxidative Stress Caused by Dopamine Itself. Acta Med. Okayama 2008, 62, 141–150. [Google Scholar] [CrossRef]
- Burbulla, L.F.; Song, P.; Mazzulli, J.R.; Zampese, E.; Wong, Y.C.; Jeon, S.; Santos, D.P.; Blanz, J.; Obermaier, C.D.; Strojny, C.; et al. Dopamine Oxidation Mediates Mitochondrial and Lysosomal Dysfunction in Parkinson’s Disease. Science 2017, 357, 1255–1261. [Google Scholar] [CrossRef]
- Rice, M.E.; Cragg, S.J.; Greenfield, S.A. Characteristics of Electrically Evoked Somatodendritic Dopamine Release in Substantia Nigra and Ventral Tegmental Area in Vitro. J. Neurophysiol. 1997, 77, 853–862. [Google Scholar] [CrossRef]
- Zhang, H.; Sulzer, D. Regulation of Striatal Dopamine Release by Presynaptic Auto- and Heteroreceptors. Basal Ganglia 2012, 2, 5–13. [Google Scholar] [CrossRef]
- Albillos, A.; Dernick, G.; Horstmann, H.; Almers, W.; de Alvarez Toledo, G.; Lindau, M. The Exocytotic Event in Chromaffin Cells Revealed by Patch Amperometry. Nature 1997, 389, 509–512. [Google Scholar] [CrossRef]
- Omiatek, D.M.; Bressler, A.J.; Cans, A.-S.; Andrews, A.M.; Heien, M.L.; Erwing, A.G. The Real Catecholamine Content of Secretory Vesicles in the CNS Revealed by Electrochemical Cytometry. Sci. Rep. 2013, 3, 1447. [Google Scholar] [CrossRef] [PubMed]
- Descarriers, L.; Watkins, K.C.; Garcia, S.; Bosler, O.; Doucet, G. Dual Character, Asynaptic and Synaptic, of the Dopamine Innervation in Adult Rat Neostriatum: A Quantitative Autoradiographic and Immunocytochemical Analysis. J. Comp. Neurol. 1996, 375, 167–186. [Google Scholar] [CrossRef]
- Cragg, S.J.; Rice, M.E. DAncing Past the DAT at a DA Synapse. Trends Neurosci. 2004, 27, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Arbuthnott, G.W.; Wickens, J. Space, Time and Dopamine. Trends Neurosci. 2007, 30, 62–69. [Google Scholar] [CrossRef]
- Khan, Z.U.; Mrzljak, L.; Gutierrez, A.; de la Calle, A.; Goldman-Rakic, P.S. Prominence of the Dopamine D2 Short Isoform in Dopaminergic Pathways. Proc. Natl. Acad. Sci. USA 1998, 95, 7731–7736. [Google Scholar] [CrossRef]
- Young, C.B.; Reddy, V.; Sonne, J. Neuroanatomy, Basal Ganglia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Wightmann, R.M.; Zimmerman, J.B. Control of Dopamine Extracellular Concentration in Rat Striatum by Impulse Flow and Uptake. Brian Res. Rev. 1990, 15, 135–144. [Google Scholar] [CrossRef]
- Nirenberg, M.J.; Chan, J.; Pohorille, A.; Vaughan, R.A.; Uhl, G.R.; Kuhar, M.J.; Pickel, V.M. The Dopamine Transporter: Comparative Ultrastructure of Dopaminergic Axons in Limbic and Motor Compartments of the Nucleus Accumbens. J. Neurosci. 1997, 17, 6899–6907. [Google Scholar] [CrossRef]
- Nirenberg, M.J.; Vaughan, R.A.; Uhl, G.R.; Kuhar, M.J.; Pickel, V.M. The Dopamine Transporter Is Localized to Dendritic and Axonal Plasma Membranes of Nigrostriatal Dopaminergic Neurons. J. Neurosci. 1996, 16, 436–447. [Google Scholar] [CrossRef]
- Rice, M.E.; Cragg, S.J. Dopamine Spillover after Quantal Release: Rethinking Dopamine Transmission in the Nigrostriatal Pathway. Brain Res. Rev. 2008, 58, 303–313. [Google Scholar] [CrossRef]
- Martel, J.C.; McArthur, S.G. Dopamine Receptor Subtypes, Physiology and Pharmacology: New Ligands and Concepts in Schizophrenia. Front. Pharmacol. 2020, 11, 1003. [Google Scholar] [CrossRef]
- Lehtonen, Š.; Sonninen, T.-M.; Wojciechowski, S.; Goldsteins, G.; Koistinaho, J. Dysfunction of Cellular Proteostasis in Parkinson’s Disease. Front. Neurosci. 2019, 13, 457. [Google Scholar] [CrossRef] [PubMed]
- Glickman, M.H.; Ciechanover, A. The Ubiquitin-Proteasome Proteolytic Pathway: Destruction for the Sake of Construction. Physiol. Rev. 2002, 82, 373–428. [Google Scholar] [CrossRef] [PubMed]
- Parzych, K.R.; Klionsky, D.J. An Overview of Autophagy: Morphology, Mechanism, and Regulation. Antoxidants Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Witt, S.N. Molecular Chaperones, Alpha-Synuclein and Neurodegeneration. Mol. Neurobiol. 2012, 47, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Lashuel, H.A.; Overk, C.R.; Oueslati, A.; Masliah, E. The Many Faces of α-Synuclein: From Structure and Toxicity to Therapeutic Target. Nat. Rev. Neurosci. 2013, 14, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Ghiglieri, V.; Calabrese, V.; Calabresi, P. Alpha-Synuclein: From Early Synaptic Dysfunction to Neurodegeneration. Front. Neurol. 2018, 9, 295. [Google Scholar] [CrossRef] [PubMed]
- Zaltieri, M.; Grigoletto, J.; Longhena, F.; Navarria, L.; Favero, G.; Castrezzati, S.; Colivicchi, M.A.; Corte, L.D.; Rezzani, R.; Pizzi, M.; et al. α-Synuclein and Synapsin III Cooperatively Regulate Synapticfunction in Dopamine Neurons. J. Cell Sci. 2015, 128, 2231–2243. [Google Scholar] [CrossRef]
- Rutherford Fields, C.; Bengoa-Vergniory, N.; Wade-Martins, R. Targeting Alpha-Synuclein as a Therapy for Parkinson’s Disease. Front. Mol. Neurosci. 2019, 12, 299. [Google Scholar] [CrossRef] [PubMed]
- Samuel, F.; Flavin, W.P.; Iqbal, S.; Pacelli, C.; Sri Renganathan, S.D.; Trudeau, L.-É.; Campbell, E.M.; Fraser, P.E.; Tandon, A. Effects of Serine 129 Phosphorylation on α-Synuclein Aggregation, Membrane Association, and Internalization. J. Biol. Chem. 2016, 291, 4374–4385. [Google Scholar] [CrossRef]
- Wong, Y.C.; Krainc, D. α-Synuclein Toxicity in Neurodegeneration: Mechanism and Therapeutic Strategies. Nat. Med. 2017, 23, 1–13. [Google Scholar] [CrossRef]
- Bengoa-Vergniory, N.; Roberts, R.F.; Wade-Martins, R.; Alegre-Abarrategui, J. Alpha-Synuclein Oligomers: A New Hope. Acta Neuropathol. 2017, 134, 819–838. [Google Scholar] [CrossRef]
- Erecińska, M.; Silver, I.A. ATP and Brain Function. J. Cereb. Blood Flow Metab. 1989, 9, 2–19. [Google Scholar] [CrossRef]
- Squier, T.C. Oxidative Stress and Protein Aggregation during Biological Aging. Exp. Gerontol. 2001, 36, 1539–1550. [Google Scholar] [CrossRef]
- Olzscha, H.; Schermann, S.M.; Woerner, A.C.; Hayer-Hartl, M.; Hartl, F.U.; Vabulas, R.M. Amyloid-like Aggregates Sequester Numerous Metastable Proteins with Essential Cellular Functions. Cell 2011, 144, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Witt, S.N. Protein Chaperones and Protection from Neurodegenerative Diseases; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Breydo, L.; Wu, J.W.; Uversky, V.N. A-Synuclein Misfolding and Parkinson’s Disease. Biochim. Et Biophys. Acta Mol. Basis Dis. 2012, 1822, 261–285. [Google Scholar] [CrossRef]
- Mehra, S.; Sahay, S.; Maji, S.K. α-Synuclein Misfolding and Aggregation: Implications in Parkinson’s Disease Pathogenesis. Biochim. Et Biophys. Acta BBA Proteins Proteom. 2019, 1867, 890–908. [Google Scholar] [CrossRef] [PubMed]
- Desplats, P.; Lee, H.J.; Bae, E.J.; Patrick, C.; Rockenstein, E.; Crews, L.; Spencer, B.; Masliah, E.; Lee, S.J. Inclusion Formation and Neuronal Cell Death through Neuron-to-Neuron Transmission of α-Synuclein. Proc. Natl. Acad. Sci. USA 2009, 106, 13010–13015. [Google Scholar] [CrossRef] [PubMed]
- Prusiner, S.B.; Woerman, A.L.; Mordes, D.A.; Watts, J.C.; Rampersaud, R.; Berry, D.B.; Patel, S.; Oehler, A.; Lowe, J.K.; Kravitz, S.N.; et al. Evidence for α-Synuclein Prions Causing Multiple System Atrophy in Humans with Parkinsonism. Proc. Natl. Acad. Sci. USA 2015, 112, E5308–E5317. [Google Scholar] [CrossRef]
- Lindersson, E.; Beedholm, R.; Højrup, P.; Moos, T.; Gai, W.; Hendil, K.B.; Jensen, P.H. Proteasomal Inhibition by Alpha-Synuclein Filaments and Oligomers. J. Biol. Chem. 2004, 279, 12924–12934. [Google Scholar] [CrossRef]
- Ghee, M.; Fournier, A.; Mallet, J. Rat α-Synuclein Interacts with Tat Binding Protein 1, a Component of the 26S Proteasomal Complex. J. Neurochem. 2002, 75, 2221–2224. [Google Scholar] [CrossRef]
- Snyder, H.; Mensah, K.; Theisler, C.; Lee, J.; Matouschek, A.; Wolozin, B. Aggregated and Monomeric Alpha-Synuclein Bind to the S6’ Proteasomal Protein and Inhibit Proteasomal Function. J. Biol. Chem. 2003, 278, 11753–11759. [Google Scholar] [CrossRef] [PubMed]
- Malkus, K.A.; Ischiropoulos, H. Regional Deficiencies in Chaperone-Mediated Autophagy Underlie α-Synuclein Aggregation and Neurodegeneration. Neurobiol. Dis. 2012, 46, 732–744. [Google Scholar] [CrossRef]
- Winslow, A.R.; Chen, C.-W.; Corrochano, S.; Acevedo-Arozena, A.; Gordon, D.E.; Peden, A.A.; Lichtenberg, M.; Menzies, F.M.; Ravikumar, B.; Imarisio, S.; et al. α-Synuclein Impairs Macroautophagy: Implications for Parkinson’s Disease. J. Cell Biol. 2010, 190, 1023–1027. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal Integration in the Endoplasmic Reticulum Unfolded Protein Response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Padilla-Godínez, F.J.; Ramos-Acevedo, R.; Martínez-Becerril, H.A.; Bernal-Conde, L.D.; Garrido-Figueroa, J.F.; Hiriart, M.; Hernández-López, A.; Argüero-Sánchez, R.; Callea, F.; Guerra-Crespo, M. Protein Misfolding and Aggregation: The Relatedness between Parkinson’s Disease and Hepatic Endoplasmic Reticulum Storage Disorders. Int. J. Mol. Sci. 2021, 22, 12467. [Google Scholar] [CrossRef]
- Chung, C.Y.; Khurana, V.; Auluck, P.K.; Tardiff, D.F.; Mazzulli, J.R.; Soldner, F.; Baru, V.; Lou, Y.; Freyzon, Y.; Cho, S.; et al. Identification and Rescue of α-Synuclein Toxicity in Parkinson Patient-Derived Neurons. Science 2013, 342, 983–987. [Google Scholar] [CrossRef]
- Heman-Ackah, S.; Manzano, R.; Hoozemans, J.J.M.; Scheper, W.; Flynn, R.; Haerty, W.; Cowley, S.A.; Bassett, A.R.; Wood, M.J.A. Alpha-Synuclein Induces the Unfolded Protein Response in Parkinson’s Disease SNCA Triplication IPSC-Derived Neurons. Hum. Mol. Genet. 2017, 26, 4441–4450. [Google Scholar] [CrossRef] [PubMed]
- Diederich, N.J.; Uchihara, T.; Grillner, S.; Goetz, C.G. The Evolution-Driven Signature of Parkinson’s Disease. Trends Neurosci. 2020, 43, 475–492. [Google Scholar] [CrossRef]
- Burré, J. The Synaptic Function of α-Synuclein. J. Park. Dis. 2015, 5, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Bridi, J.C.; Hirth, F. Mechanisms of α-Synuclein Induced Synaptopathy in Parkinson’s Disease. Front. Neurosci. 2018, 12, 80. [Google Scholar] [CrossRef]
- Imbriani, P.; Schirinzi, T.; Meringolo, M.; Mercuri, N.B.; Pisani, A. Centrality of Early Synaptopathy in Parkinson’s Disease. Front. Neurol. 2018, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Morfini, G.A.; Langhamer, L.B.; He, Y.; Brady, S.T.; Kordower, J.H. Alteration in Axonal Transport Motor Proteins in Sporadic and Experimental PD. Brain 2012, 135, 2058–2073. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.E.; Paek, S.H. Mitochondrial Dysfunction in Parkinson’s Disease. Exp. Neurol. 2015, 24, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.; Raghavendran, V.; Prabhu, B.M.; Avadhani, N.G.; Anandatheerthavarada, H.K. Mitochondrial Import and Accumulation of α-Synuclein Impair Complex I in Human Dopaminergic Neuronal Cultures and Parkinson Disease Brain. J. Biol. Chem. 2008, 283, 9089–9100. [Google Scholar] [CrossRef]
- Luth, E.S.; Stavrovskaya, I.G.; Bartels, T.; Kristal, B.S.; Selkoe, D.J. Soluble, Prefibrillar α-Synuclein Oligomers Promote Complex I-Dependent, Ca2+ -Induced Mitochondrial Dysfunction. J. Biol. Chem. 2014, 289, 21490–21507. [Google Scholar] [CrossRef]
- Maio, R.D.; Barrett, P.J.; Hoffman, E.K.; Barrett, C.W.; Zharikov, A.; Borah, A.; Hu, X.; McCoy, J.; Chu, C.T.; Burton, E.A.; et al. α-Synuclein Binds to TOM20 and Inhibits Mitochondrial Protein Import in Parkinson’s Disease. Sci. Transl. Med. 2016, 8, 342ra78. [Google Scholar] [CrossRef] [PubMed]
- Kouli, A.; Torsney, K.M.; Kuan, W.-L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Codon Publications: Brisbane, Australia, 2018. [Google Scholar]
- Zigmond, M.J. Do Compensatory Processes Underlie the Preclinical Phase of Neurodegenerative Disease? Insights from an Animal Model of Parkinsonism. Neurobiol. Dis. 1997, 4, 247–253. [Google Scholar] [CrossRef]
- Bernheimer, H.; Hornykiewicz, O. Decreased Homovanillic Acid Concentration in the Brain in Parkinsonian Subjects as an Expression of a Disorder of Central Dopamine Metabolism. Klin. Wochenschr. 1965, 43, 711–715. [Google Scholar] [CrossRef]
- Pifl, C.; Hornykiewicz, O. Dopamine Turnover Is Upregulated in the Caudate/Putamen of Asymptomatic MPTP-Treated Rhesus Monkeys. Neurochem. Int. 2006, 49, 519–524. [Google Scholar] [CrossRef]
- Lee, C.S.; Samii, A.; Sossi, V.; Ruth, T.J.; Schulzer, M.; Holden, J.E.; Wudel, J.; Pal, P.K.; de la Fuente-Fernandez, R.; Calne, D.B.; et al. In Vivo Positron Emission Tomographic Evidence for Compensatory Changes in Presynaptic Dopaminergic Nerve Terminals in Parkinson’s Disease. Ann. Neurol. 2000, 47, 493–503. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J. Regulation of Dopamine Presynaptic Markers and Receptors in the Striatum of DJ-1 and Pink1 Knockout Rats. Neurosci. Lett. 2013, 557, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Chefer, S.I.; Kimes, A.S.; Matochik, J.A.; Horti, A.G.; Kurian, V.; Shumway, D.; Domino, E.F.; London, E.D.; Mukhin, A.G. Estimation of D2-like Receptor Occupancy by Dopamine in the Putamen of Hemiparkinsonian Monkeys. Neuropsychopharmacology 2008, 33, 270–278. [Google Scholar] [CrossRef]
- Adams, J.R.; van Netten, H.; Schulzer, M.; Mak, E.; Mckenzie, J.; Strongosky, A.; Sossi, V.; Ruth, T.J.; Lee, C.S.; Farrer, M.; et al. PET in LRRK2 Mutations: Comparison to Sporadic Parkinson’s Disease and Evidence for Presymptomatic Compensation. Brain 2005, 128, 2777–2785. [Google Scholar] [CrossRef] [PubMed]
- Stoessl, A.J. Positron Emission Tomography in Premotor Parkinson’s Disease. Park. Relat. Disord. 2007, 13, S421–S424. [Google Scholar] [CrossRef]
- Wile, D.J.; Stoessl, A.J. Serotonin and Dopamine Transporter PET Changes in the Premotor Phase of LRRK2 Parkinsonism: Cross-Sectional Studies. Lancet Neurol. 2017, 16, 351–359. [Google Scholar] [CrossRef]
- Picconi, B.; Piccoli, G.; Calabresi, P. Synaptic Dysfunction in Parkinson’s Disease. In Synaptic Plasticity; Advances in Experimental Medicine and Biology; Springer: Vienna, Austria, 2012; Volume 970, pp. 553–572. ISBN 978-3-7091-0932-8. [Google Scholar]
- Bolam, J.P.; Pissadaki, E.K. Living on the Edge with Too Many Mouths to Feed: Why Dopamine Neurons Die. Mov. Disord. 2012, 27, 1478–1483. [Google Scholar] [CrossRef]
- Matsuda, W.; Furuta, T.; Nakamura, K.C.; Hioki, H.; Fujiyama, F.; Arai, R.; Kaneko, T. Single Nigrostriatal Dopaminergic Neurons Form Widely Spread and Highly Dense Axonal Arborizations in the Neostriatum. J. Neurosci. 2009, 29, 444–453. [Google Scholar] [CrossRef]
- Blesa, J.; Trigo-Damas, I.; Dileone, M.; del Re Lopez-Gonzalez, N.; Hernandez, L.F.; Obeso, J.A. Compensatory Mechanisms in Parkinson’s Disease: Circuits Adaptations and Role in Disease Modification. Exp. Neurol. 2017, 298, 148–161. [Google Scholar] [CrossRef]
- Vigerhoets, F.J.G.; Schulzer, M.; Calne, D.B.; Snow, B.J. Which Clinical Sign of Parkinson’s Disease Best Reflects the Nigrostriatal Lesion? Ann. Neurol. 2004, 41, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.A. Levodopa: Past, Present, and Future. Eur. Neurol. 2009, 62, 1–8. [Google Scholar] [CrossRef]
- Edwards, M.; Quinn, N.; Bhatia, K. Parkinson’s Disease and Other Movement Disorders; Oxford Specialist Handbooks in Neurology; Oxford University Press: Oxford, UK, 2008; ISBN 978-0-19-856984-8. [Google Scholar]
- Cenci, M.A.; Lundblad, M. Post- versus Presynaptic Plasticity in L-DOPA-Induced Dyskinesia. J. Neurochem. 2006, 99, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Troiano, A.R.; de la Fuente-Fernandez, R.; Sossi, V.; Schulzer, M.; Mark, E.; Ruth, T.J.; Stoessl, A.J. PET Demonstrates Reduced Dopamine Transporter Expression in PD with Dyskinesias. Neurology 2009, 72, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M.; Khoshbin, S. A Physiological Mechanism of Bradykinesia. Brain 1980, 103, 301–314. [Google Scholar] [CrossRef]
- Ingvarsson, P.E.; Gordon, A.M.; Forssberg, H. Coordination of Manipulative Forces in Parkinson’s Disease. Exp. Neurol. 1997, 145, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Wenzelburger, R.; Zhang, B.-R.; Pohle, S.; Klebe, S.; Lorenz, D.; Herzog, J.; Wilms, H.; Deuschl, G.; Krack, P. Force Overflow and Levodopa-induced Dyskinesias in Parkinson’s Disease. Brain 2002, 125, 871–879. [Google Scholar] [CrossRef]
- Meder, D.; Herz, D.M.; Rowe, J.B.; Lehéricy, S.; Siebner, H.R. The Role of Dopamine in the Brain—Lessons Learned from Parkinson’s Disease. NeuroImage 2019, 190, 79–93. [Google Scholar] [CrossRef]
- Luo, S.X.; Huang, E.J. Dopaminergic Neurons and Brain Reward Pathways. Am. J. Pathol. 2016, 186, 478–488. [Google Scholar] [CrossRef]
- Kish, S.J.; Shannak, K.; Hornykiewicz, O. Uneven Pattern of Dopamine Loss in the Striatum of Patients with Idiopathic Parkinson’s Disease. N. Engl. J. Med. 1988, 318, 876–880. [Google Scholar] [CrossRef]
- Robbins, T.W.; Cools, R. Cognitive Deficits in Parkinson’s Disease: A Cognitive Neuroscience Perspective. Mov. Disord. 2014, 29, 597–607. [Google Scholar] [CrossRef]
- Schultz, W. Reward Prediction Error. Curr. Biol. 2017, 27, R369–R371. [Google Scholar] [CrossRef]
- Schultz, W. Neuronal Reward and Decision Signals: From Theories to Data. Physiol. Rev. 2015, 95, 853–951. [Google Scholar] [CrossRef]
- Christopoulos, G.I.; Tobler, P.N.; Bossaerts, P.; Doland, R.J.; Schultz, W. Neural Correlates of Value, Risk, and Risk Aversion Contributing to Decision Making under Risk. J. Neurosci. 2009, 29, 12574–12583. [Google Scholar] [CrossRef] [PubMed]
- Cools, R.; Lewis, S.J.G.; Clark, L.; Barker, R.A.; Robbins, T.W. L-DOPA Disrupts Activity in the Nucleus Accumbens during Reversal Learning in Parkinson’s Disease. Neuropsychopharmacology 2007, 32, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Cools, R. Dopaminergic Modulation of Cognitive Function-Implications for L-DOPA Treatment in Parkinson’s Disease. Neurosci. Biobehav. Rev. 2006, 30, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Cools, R.; Barker, R.A.; Sahakian, B.J.; Robbins, T.W. Enhanced or Impaired Cognitive Function in Parkinson’s Disease as a Function of Dopaminergic Medication and Task Demands. Cereb. Cortex 2001, 11, 1136–1143. [Google Scholar] [CrossRef]
- Sternin, J.; Choo, R. The Power of Positive Deviancy. An Effort to Reduce Malnutrition in Vietnam Offers an Important Lesson about Managing Change. Harv. Bus. Rev. 2000, 78, 14–15. [Google Scholar] [PubMed]
- Marsh, D.R.; Schroeder, D.G.; Dearden, K.A.; Sternin, J.; Sternin, M. The Power of Positive Deviance. BMJ 2004, 329, 1177–1179. [Google Scholar] [CrossRef]
- Muñoz, P.; Huenchuguala, S.; Segura-Aguilar, J. Dopamine Oxidation and Autophagy. Park. Dis. 2012, 2012, 920953. [Google Scholar] [CrossRef]
- Segura-Aguilar, J.; Paris, I.; Muñoz, P.; Ferrari, E.; Zecca, L.; Zucca, F.A. Protective and Toxic Roles of Dopamine in Parkinson’s Disease. J. Neurochem. 2014, 129, 898–915. [Google Scholar] [CrossRef]
- Biosa, A.; Arduini, I.; Soriano, M.E.; Giorgio, V.; Bernardi, P.; Bisaglia, M.; Bubacco, L. Dopamine Oxidation Products as Mitochondrial Endotoxins, a Potential Molecular Mechanism for Preferential Neurodegeneration in Parkinson’s Disease. ACS Chem. Neurosci. 2018, 9, 2849–2858. [Google Scholar] [CrossRef]
- Zhang, S.; Song, Y.; Xiao, G.; Gao, F.; Li, Z.; Tao, G.; Zhuang, P.; Yue, F.; Cai, X. Real-Time Simultaneous Recording of Electrophysiological Activities and Dopamine Overflow in the Deep Brain Nuclei of a Non-Human Primate with Parkinson’s Disease Using Nano-Based Microelectrode Arrays. Microsyst. Nanoeng. 2018, 4, 17070. [Google Scholar] [CrossRef]
- Struzyna, L.A.; Browne, K.D.; Brodnik, Z.D.; Burrell, J.C.; Harris, J.P.; Chen, H.I.; Wolf, J.A.; Panzer, K.V.; Lim, J.; Duda, J.E.; et al. Tissue Engineered Nigrostriatal Pathway for Treatment of Parkinson’s Disease. J. Tissue Eng. Regen. Med. 2018, 12, 1702–1716. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, X.; Shen, H.; He, Q.; Wu, Z.; Liao, W.; Yuan, M. Application of the Nano-Drug Delivery System in Treatment of Cardiovascular Diseases. Front. Bioeng. Biotechnol. 2020, 7, 489. [Google Scholar] [CrossRef]
- Natarajan, J.V.; Nugraha, C.; Ng, X.W.; Venkatraman, S. Sustained-Release from Nanocarriers: A Review. J. Control. Release 2014, 193, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Pillay, S.; Pillay, V.; Choonara, Y.E.; Naidoo, D.; Khan, R.A.; du Toit, L.C.; Ndesendo, V.M.K.; Modi, G.; Danckwerts, M.P.; Iyuke, S.E. Design, Biometric Simulation and Optimization of a Nano-Enabled Scaffold Device for Enhanced Delivery of Dopamine to the Brain. Int. J. Pharm. 2009, 382, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Divya, K.; Jisha, M.S. Chitosan Nanoparticles Preparation and Applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Trapani, A.; Giglio, E.D.; Cafagna, D.; Denora, N.; Agrimi, G.; Cassano, T.; Gaetani, S.; Cuomo, V.; Trapani, G. Characterization and Evaluation of Chitosan Nanoparticles for Dopamine Brain Delivery. Int. J. Pharm. 2011, 419, 296–307. [Google Scholar] [CrossRef]
- Di Gioia, S.; Trapani, A.; Cassano, R.; Di Gioia, M.L.; Trombino, S.; Cellamare, S.; Bolognino, I.; Hossain, M.N.; Sanna, E.; Trapani, G.; et al. Nose-to-Brain Delivery: A Comparative Study between Carboxymethyl Chitosan Based Conjugates of Dopamine. Int. J. Pharm. 2021, 599, 120453. [Google Scholar] [CrossRef]
- Cassano, R.; Trapani, A.; Di Gioia, M.L.; Mandracchia, D.; Pellitteri, R.; Tripodo, G.; Trombino, S.; Di Gioia, S.; Conese, M. Synthesis and Characterization of Novel Chitosan-Dopamine or Chitosan-Tyrosine Conjugates for Potential Nose-to-Brain Delivery. Int. J. Pharm. 2020, 589, 119829. [Google Scholar] [CrossRef]
- Ragusa, A.; Priore, P.; Giudetti, A.M.; Ciccarella, G.; Gaballo, A. Neuroprotective Investigation of Chitosan Nanoparticles for Dopamine Delivery. Appl. Sci. Switz. 2018, 8, 474. [Google Scholar] [CrossRef]
- Wohlfart, S.; Gelperina, S.; Kreuter, J. Transport of Drugs across the Blood–Brain Barrier by Nanoparticles. J. Control. Release 2012, 161, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Kim, H.K.; Lee, H. Dopamine-Loaded Poly(D,L-Lactic-Co-Glycolic Acid) Microspheres: New Strategy for Encapsulating Small Hydrophilic Drugs with High Efficiency. Biotechnol. Prog. 2014, 30, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Pahuja, R.; Seth, K.; Shukla, R.K.; Bhatnagar, P.; Chauhan, B.P.S.; Patel, D.K.; Singh, S.P.; Shukla, R.; Khanna, V.K.; Kumar, P.; et al. Trans-Blood Brain Barrier Delivery of Dopamine-Loaded Nanoparticles Reverses Functional Deficits in Parkinsonian Rats. ACS Nano 2015, 9, 4850–4871. [Google Scholar] [CrossRef] [PubMed]
- Monge-Fuentes, V.; Mayer, A.B.; Lima, M.R.; Geraldes, L.R.; Zanotto, L.N.; Moreira, K.G.; Martins, O.P.; Piva, H.L.; Soares Felipe, M.S.; Amaral, A.C.; et al. Dopamine-Loaded Nanoparticle Systems Circumvent the Blood–Brain Barrier Restoring Motor Function in Mouse Model for Parkinson’s Disease. Sci. Rep. 2021, 11, 15185. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Yan, X.; Yang, X.; Song, Y.; Sun, K.; Yu, X.; Liu, R.; Wu, Z.; Xue, P. Brain-Targeted Intranasal Delivery of Dopamine with Borneol and Lactoferrin Co-Modified Nanoparticles for Treating Parkinson’s Disease. Drug Deliv. 2019, 26, 700–707. [Google Scholar] [CrossRef]
- Rashed, E.R.; El-Rehim, H.A.A.; El-Ghazaly, M.A. Potential Efficacy of Dopamine Loaded-PVP/PAA Nanogel in Experimental Models of Parkinsonism: Possible Disease Modifying Activity. J. Biomed. Mater. Res. Part A 2014, 103, 1713–1720. [Google Scholar] [CrossRef]
- Pinelli, F.; Ortolà, Ó.F.; Makvandi, P.; Perale, G.; Rossi, F. In Vivo Drug Delivery Applications of Nanogels: A Review. Nanomedicine 2020, 15, 27. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, X.; Liang, X.; Ma, P.X.; Guo, B. Injectable Hydrogel Based on Quaternized Chitosan, Gelatin and Dopamine as Localized Drug Delivery System to Treat Parkinson’s Disease. Int. J. Biol. Macromol. 2017, 105, 1079–1087. [Google Scholar] [CrossRef]
- Trapani, A.; Corbo, F.; Agrimi, G.; Ditaranto, N.; Cioffi, N.; Perna, F.; Quivelli, A.; Stefàno, E.; Lunetti, P.; Muscella, A.; et al. Oxidized Alginate Dopamine Conjugate: In Vitro Characterization for Nose-to-Brain Delivery Application. Materials 2021, 14, 3495. [Google Scholar] [CrossRef]
- García-Pardo, J.; Novio, F.; Nador, F.; Cavaliere, I.; Suárez-García, S.; Lope-Piedrafita, S.; Candiota, A.P.; Romero-Gimenez, J.; Rodríguez-Galván, B.; Bové, J.; et al. Bioinspired Theranostic Coordination Polymer Nanoparticles for Intranasal Dopamine Replacement in Parkinson’s Disease. ACS Nano 2021, 15, 8592–8609. [Google Scholar] [CrossRef]
- Lockman, P.R.; Mumper, R.J.; Khan, M.A.; Allen, D.D. Nanoparticle Technology for Drug Delivery Across the Blood-Brain Barrier. Drug Dev. Ind. Pharm. 2002, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Joshi, A.; Toor, A.P.; Verma, G. Chapter 27—Drug Delivery: Advancements and Challenges. In Nanostructures for Drug Delivery: Micro and Nano Technologies; Elsevier Academic Press: Cambridge, MA, USA, 2017; pp. 865–886. ISBN 978-0-323-46143-6. [Google Scholar]
- Gao, W.; Hu, C.-M.J.; Fang, R.H.; Zhang, L. Liposome-like Nanostructures for Drug Delivery. J. Mater. Chem. B 2013, 1, 6569–6585. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Choi, J.S. Liposomes: Versatile and Biocompatible Nanovesicles for Efficient Biomolecules Delivery. J. Nanosci. Nanotechnol. 2014, 14, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.A.; Augood, S.J.; Kingsbury, A.E.; Foster, O.J.F.; Emson, P.C. Dopamine Transporter (DAT) and Synaptic Vesicle Amine Transporter (VMAT2) Gene Expression in the Substantia Nigra of Control and Parkinson’s Disease. Mol. Brain Res. 1996, 36, 157–162. [Google Scholar] [CrossRef]
- Vieira, D.B.; Gamarra, L.F. Getting into the Brain: Liposome-Based Strategies for Effective Drug Delivery across the Blood–Brain Barrier. Int. J. Nanomed. 2016, 11, 5381–5414. [Google Scholar] [CrossRef]
- During, M.J.; Freese, A.; Deutch, A.Y.; Kibat, P.G.; Sabel, B.A.; Langer, R.; Roth, R.H. Biochemical and Behavioral Recovery in a Rodent Model of Parkinson’s Disease Following Stereotactic Implantation of Dopamine-Containing Liposomes. Exp. Neurol. 1992, 115, 193–199. [Google Scholar] [CrossRef]
- Jain, N.K.; Rana, A.C.; Jain, S.K. Brain Drug Delivery System Bearing Dopamine Hydrochloride for Effective Management of Parkinsonism. Drug Dev. Ind. Pharm. 1998, 24, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Zhigaltsev, I.V.; Kaplun, A.P.; Kucheryanu, V.G.; Kryzhanovsky, G.N.; Kolomeichuk, S.N.; Shvets, V.I.; Yurasov, V.V. Liposomes Containing Dopamine Entrapped in Response to the Transmembrane Ammonium Sulfate Gradient as Carrier System for Dopamine Delivery into the Brain of Parkinsonian Mice. J. Liposome Res. 2001, 11, 55–71. [Google Scholar] [CrossRef]
- Trapani, A.; Mandracchia, D.; Tripodo, G.; Cometa, S.; Cellamare, S.; De Giglio, E.; Klepetsanis, P.; Antimisiaris, S.G. Protection of Dopamine towards Autoxidation Reaction by Encapsulation into Non-Coated- or Chitosan- or Thiolated Chitosan-Coated-Liposomes. Colloids Surf. B Biointerfaces 2018, 170, 11–19. [Google Scholar] [CrossRef]
- Khare, P.; Jain, A.; Jain, N.K.; Soni, V.; Jain, S.K. Glutamate-Conjugated Liposomes of Dopamine Hydrochloride for Effective Management of Parkinsonism’s. PDA J. Pharm. Sci. Technol. 2009, 63, 372–379. [Google Scholar] [PubMed]
- Lopalco, A.; Cutrignelli, A.; Denora, N.; Lopedota, A.; Franco, M.; Laquintana, V. Transferrin Functionalized Liposomes Loading Dopamine HCl: Development and Permeability Studies across an In Vitro Model of Human Blood-Brain Barrier. Nanomaterials 2018, 8, 178. [Google Scholar] [CrossRef]
- Ulbrich, K.; Hekmatara, T.; Herbert, E.; Kreuter, J. Transferrin- and Transferrin-Receptor-Antibody-Modified Nanoparticles Enable Drug Delivery across the Blood-Brain Barrier (BBB). Eur. J. Pharm. Biopharm. 2009, 71, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Kahana, M.; Weizman, A.; Gabay, M.; Loboda, Y.; Segal-Gavish, H.; Gavish, A.; Barhum, Y.; Offen, D.; Finberg, J.; Allon, N.; et al. Liposome-Based Targeting of Dopamine to the Brain: A Novel Approach for the Treatment of Parkinson’s Disease. Mol. Psychiatry 2020, 26, 2626–2632. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Lin, Q.; He, S.; Wang, L.; Fu, Y.; Zhang, Z.; Zhang, L. A Brain Targeting Functionalized Liposomes of the Dopamine Derivative N-3,4-Bis(Pivaloyloxy)-Dopamine for Treatment of Parkinson’s Disease. J. Control. Release 2018, 277, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Karthivashan, G.; Ganesan, P.; Park, S.-Y.; Lee, H.-W.; Choi, D.-K. Lipid-Based Nanodelivery Approaches for Dopamine-Replacement Therapies in Parkinson’s Disease: From Preclinical to Translational Studies. Biomaterials 2020, 232, 119704. [Google Scholar] [CrossRef]
- Schwarz, C.; Mehnert, W.; Lucks, J.S.; Müller, R.H. Solid Lipid Nanoparticles (SLN) for Controlled Drug Delivery. I. Production, Characterization and Sterilization. J. Control. Release 1994, 30, 83–96. [Google Scholar] [CrossRef]
- Cacciatore, I.; Ciulla, M.; Fornasari, E.; Marinelli, L.; Di Stefano, A. Solid Lipid Nanoparticles as a Drug Delivery System for the Treatment of Neurodegenerative Diseases. Expert Opin. Drug Deliv. 2016, 13, 1121–1131. [Google Scholar] [CrossRef]
- Cometa, S.; Bonifacio, M.A.; Trapani, G.; Di Gioia, S.; Dazzi, L.; De Giglio, E.; Trapani, A. In Vitro Investigations on Dopamine Loaded Solid Lipid Nanoparticles. J. Pharm. Biomed. Anal. 2020, 185, 113257. [Google Scholar] [CrossRef]
- Ortega, E.; Blanco, S.; Ruiz, A.; Peinado, M.Á.; Peralta, S.; Morales, M.E. Lipid Nanoparticles for the Transport of Drugs like Dopamine through the Blood-Brain Barrier. J. Nanoparticle Res. 2021, 23, 106. [Google Scholar] [CrossRef]
- Alipour, N.; Namazi, H. Chelating ZnO-Dopamine on the Surface of Graphene Oxide and Its Application as PH-Responsive and Antibacterial Nanohybrid Delivery Agent for Doxorubicin. Mater. Sci. Eng. C 2020, 108, 110459. [Google Scholar] [CrossRef] [PubMed]
- Yegappan, R.; Selvaprithiviraj, V.; Mohandas, A.; Jayakumar, R. Nano Polydopamine Crosslinked Thiol-Functionalized Hyaluronic Acid Hydrogel for Angiogenic Drug Delivery. Biointerfaces 2019, 177, 41–49. [Google Scholar] [CrossRef]
- Masoudipour, E.; Kashanian, S.; Maleki, N. A Targeted Drug Delivery System Based on Dopamine Functionalized Nano Graphene Oxide. Chem. Phys. Lett. 2017, 668, 56–63. [Google Scholar] [CrossRef]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, J.-Y.; Li, H.-Q.; Chen, Z.; Zhao, A.Z.-J.; Wang, Y.; Zhang, K.-Q.; Sun, H.-T.; Al-Deyab, S.S.; Lai, Y.-K. TiO2 Nanotube Platforms for Smart Drug Delivery: A Review. Int. J. Nanomed. 2021, 11, 4819–4834. [Google Scholar] [CrossRef]
- López-Goerne, T.; Rodríguez-Reinoso, F.; Padilla-Godínez, F.J.; Silvestre-Albero, A.M. Atomic and Electronic Composition Study of Different Anticonvulsants Stabilized on Titania-Nanoreservoirs. Spectrosc. Lett. 2022, 55, 58–64. [Google Scholar] [CrossRef]
- Vergara-Aragón, P.; Domínguez-Marrufo, L.E.; Ibarra-Guerrero, P.; Hernandez-Ramírez, H.; Hernández-Téllez, B.; López-Martínez, I.E.; Sánchez-Cervantes, I.; Santiago-Jacinto, P.; García-Macedo, J.A.; Valverde-Aguilar, G.; et al. Tio2-Dopamine Complex Implanted Unilaterally in the Caudate Nucleus Improves Motor Activity and Behavior Function of Rats with Induced Hemiparkinsonism. Proc. West. Pharmacol. Soc. 2011, 54, 15–20. [Google Scholar] [PubMed]
- Valverde-Aguilar, G.; Prado-Ponce, G.; Vergara-Aragón, P.; Garcia-Macedo, J.; Santiago, P.; Rendón, L. Photoconductivity Studies on Nanoporous TiO2/Dopamine Films Prepared by Sol–Gel Method. Appl. Phys. A 2014, 116, 1075–1084. [Google Scholar] [CrossRef]
- Prado-Ponce, G.; Valverde-Aguilar, G.; García-Macedo, J.; Vergara-Aragón, P. Photoconductivity and Stabilization of Dopamine Embedded in Sol-Gel TiO2 Matrix. In Proceedings of the Nanophotonic Materials IX, San Diego, CA, USA, 15–16 August 2012; Volume 8456, p. 84560. [Google Scholar]
- Gómez-Chavarín, M.; Prado-Prone, G.; Padilla, P.; Ramírez Santos, J.; Gutiérrez-Ospina, G.; García-Macedo, J. Dopamine Released from TiO2 Semicrystalline Lattice Implants Attenuates Motor Symptoms in Rats Treated with 6-Hydroxydopamine. ACS Omega 2019, 4, 7953–7962. [Google Scholar] [CrossRef]
- Velázquez-Paniagua, M.; Vázquez-Álvarez, A.M.; Valverde-Aguilar, G.; Vergara-Aragón, P. Current Treatments in Parkinson’s Including the Proposal of an Innovative Dopamine Microimplant. Rev. Méd. Hosp. Gen. Méx. 2016, 79, 79–87. [Google Scholar] [CrossRef]
- Safari, M.; Ghiaci, M.; Jafari-Asl, M.; Ensafi, A.A. Nanohybrid Organic-Inorganic Chitosan/Dopamine/TiO2 Composites with Controlled Drug-Delivery Properties. Appl. Surf. Sci. 2015, 342, 26–33. [Google Scholar] [CrossRef]
- Long, T.C.; Tajuba, J.; Sama, P.; Saleh, N.; Swartz, C.; Parker, J.; Hester, S.; Lowry, G.V.; Veronesi, B. Nanosize Titanium Dioxide Stimulates Reactive Oxygen Species in Brain Microglia and Damages Neurons in Vitro. Environ. Health Perspect. 2007, 115, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Heidari, Z.; Mohammadipour, A.; Haeri, P.; Ebrahimzadeh-bideskan, A. The Effect of Titanium Dioxide Nanoparticles on Mice Midbrain Substantia Nigra. Iran. J. Basic Med. Sci. 2019, 22, 745–751. [Google Scholar] [CrossRef]
- Czajka, M.; Sawicki, K.; Sikorska, K.; Popek, S.; Kruszewski, M.; Kapka-Skrzypczak, L. Toxicity of Titanium Dioxide Nanoparticles in Central Nervous System. Toxicol. Vitr. 2015, 29, 1042–1052. [Google Scholar] [CrossRef]
- Wu, J.; Xie, H. Effects of Titanium Dioxide Nanoparticles on α-Synuclein Aggregation and the Ubiquitin-Proteasome System in Dopaminergic Neurons. Artif. CellsNanomed. Biotechnol. 2016, 44, 690–694. [Google Scholar] [CrossRef]
- Mohammadi, S.; Nikkhah, M. TiO2 Nanoparticles as Potential Promoting Agents of Fibrillation of α-Synuclein, a Parkinson’s Disease-Related Protein. Iran. J. Biotechnol. 2017, 15, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous Silica Nanoparticles in Target Drug Delivery System: A Review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef] [PubMed]
- López, T.; Quintana, P.; Martínez, J.M.; Esquivel, D. Stabilization of Dopamine in Nanosilica Sol-Gel Matrix to Be Used as a Controlled Drug Delivery System. J. Non Cryst. Solids 2007, 353, 987–989. [Google Scholar] [CrossRef]
- López, T.; Bata-García, J.L.; Esquivel, D.; Ortiz-Islas, E.; Gonzalez, R.; Ascencio, J.; Quintana, P.; Oskam, G.; Álvarez-Cervera, F.J.; Heredia-López, F.J.; et al. Treatment of Parkinson’s Disease: Nanostructured Sol-Gel Silica-Dopamine Reservoirs for Controlled Drug Release in the Central Nervous System. Int. J. Nanomed. 2011, 6, 19–31. [Google Scholar] [CrossRef]
- López, T.; Ortiz, E.; Kozina, A.; Esquivel, D.; Espinoza, K. In Situ Controlled Release of Dopamine for Treatment of Parkinson’s Disease. In Nanopharmaceutics: The Potential Application of Nanomaterials; World Scientific Publishing: Singapore, 2012; pp. 445–466. ISBN 10.1142/9789814368674_0016. [Google Scholar]
- Stocchi, F.; Vacca, L.; Ruggieri, S.; Olanow, C.W. Intermittent vs Continuous Levodopa Administration in Patients with Advanced Parkinson Disease: A Clinical and Pharmacokinetic Study. Arch. Neurol. 2005, 62, 905–910. [Google Scholar] [CrossRef]
- De, D.; Upadhyay, P.; Das, A.; Ghosh, A.; Adhikary, A.; Goswami, M.M. Studies on Cancer Cell Death through Delivery of Dopamine as Anti-Cancer Drug by a Newly Functionalized Cobalt Ferrite Nano-Carrier. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127202. [Google Scholar] [CrossRef]
- Xu, Z.P.; Zeng, Q.H.; Lu, G.Q.; Yu, A.B. Inorganic Nanoparticles as Carriers for Efficient Cellular Delivery. Chem. Eng. Sci. 2006, 61, 1027–1040. [Google Scholar] [CrossRef]
- Rout, J.; Swain, B.C.; Mishra, P.P.; Tripathy, U. Spectroscopic Insight into the Interaction of Dopamine with Spherical Gold Nanoparticles. J. Photochem. Photobiol. B Biol. 2020, 203, 111770. [Google Scholar] [CrossRef] [PubMed]
- Kalčec, N.; Peranić, N.; Barbir, R.; Hall, C.R.; Smith, T.A.; Sani, M.A.; Frkanec, R.; Separovic, F.; Vrček, I.V. Spectroscopic Study of L-DOPA and Dopamine Binding on Novel Gold Nanoparticles towards More Efficient Drug-Delivery System for Parkinson’s Disease. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 268, 120707. [Google Scholar] [CrossRef]
- Wagner, A.M.; Knipe, J.M.; Orive, G.; Peppas, N.A. Quantum Dots in Biomedical Applications. Acta Biomater. 2019, 94, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Malvindi, M.A.; Di Corato, R.; Curcio, A.; Melisi, D.; Rimoli, M.G.; Tortiglione, C.; Tino, A.; George, C.; Brunetti, V.; Cingolani, R.; et al. Multiple Functionalization of Fluorescent Nanoparticles for Specific Biolabeling and Drug Delivery of Dopamine. Nanoscale 2011, 3, 5110–5119. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Pandey, S.; Talib, A.; Bhaisare, M.L.; Wu, H.-F. Controlled Delivery of Dopamine Hydrochloride Using Surface Modified Carbon Dots for Neuro Diseases. Colloids Surf. B Biointerfaces 2015, 134, 140–146. [Google Scholar] [CrossRef]
- Mathew, S.A.; Praveena, P.; Dhanavel, S.; Manikandan, R.; Senthilkumar, S.; Stephen, A. Luminescent Chitosan/Carbon Dots as an Effective Nano-Drug Carrier for Neurodegenerative Diseases. R. Soc. Chem. Adv. 2020, 10, 24386–24396. [Google Scholar] [CrossRef]
- Mathew, S.A.; Prakash, P.A.; Jaabir, M.S.M.; Dhanavel, S.; Manikandan, R.; Stephen, A. Dopamine-Conjugated CuS/Chitosan Nanocomposite for Targeted Photothermal Drug Delivery: In Vitro Cytotoxicity Study to Establish Bio-Compatibility. J. Drug Deliv. Sci. Technol. 2021, 61, 102193. [Google Scholar] [CrossRef]
- Manea, F.; Houillon, F.B.; Pasquato, L.; Scrimin, P. Nanozymes: Gold-Nanoparticle-Based Transphosphorylation Catalysts. Angew. Chem. Int. Ed. Engl. 2004, 43, 6165–6169. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; Yan, X. Intrinsic Peroxidase-like Activity of Ferromagnetic Nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yang, X.; Zhu, L.; Chu, H.; Li, X.; Xu, W. Nanozymes: Activity Origin, Catalytic Mechanism, and Biological Application. Coord. Chem. Rev. 2021, 448, 214170. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Wei, H. Nanomaterials with Enzyme-like Characteristics (Nanozymes): Next-Generation Artificial Enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Wu, H.; Liao, H.; Li, F.; Lee, J.; Hu, P.; Shao, W.; Li, X.; Ling, D. Bioactive ROS-Scavenging Nanozymes for Regenerative Medicine: Reestablishing the Antioxidant Firewall. Nano Sel. 2020, 1, 285–297. [Google Scholar] [CrossRef]
- Dugan, L.L.; Tian, L.; Quick, K.L.; Hardt, J.I.; Karimi, M.; Brown, C.; Loftin, S.; Flores, H.; Morlein, S.M.; Polich, J.; et al. Carboxyfullerene Neuroprotection Postinjury in Parkinsonian Nonhuman Primates. Ann. Neurol. 2014, 76, 393–402. [Google Scholar] [CrossRef]
- Singh, N.; Savanur, M.A.; Srivastava, S.; D’Silva, P.; Mugesh, G. A Redox Modulatory Mn3O4 Nanozyme with Multi-Enzyme Activity Provides Efficient Cytoprotection to Human Cells in a Parkinson’s Disease Model. Angew. Chem. Int. Ed. 2017, 56, 14267–14271. [Google Scholar] [CrossRef]
- Xu, Z.; Qu, A.; Wang, W.; Lu, M.; Shi, B.; Chen, C.; Hao, C.; Xu, L.; Sun, M.; Xu, C.; et al. Facet-Dependent Biodegradable Mn3O4 Nanoparticles for Ameliorating Parkinson’s Disease. Adv. Healthc. Mater. 2021, 10, 2101316. [Google Scholar] [CrossRef]
- Kwon, H.J.; Cha, M.-Y.; Kim, D.; Kim, D.K.; Soh, M.; Shin, K.; Hyeon, T.; Mook-Jung, I. Mitochondria-Targeting Ceria Nanoparticles as Antioxidants for Alzheimer’s Disease. ACS Nano 2016, 10, 2860–2870. [Google Scholar] [CrossRef]
- Kwon, H.J.; Kim, D.; Seo, K.; Kim, Y.G.; Han, S.I.; Kang, T.; Soh, M.; Hyeon, T. Ceria Nanoparticle Systems for Selective Scavenging of Mitochondrial, Intracellular, and Extracellular Reactive Oxygen Species in Parkinson’s Disease. Angew. Chem. Int. Ed. 2018, 57, 9408–9412. [Google Scholar] [CrossRef]
- Hao, C.; Qu, A.; Xu, L.; Sun, M.; Zhang, H.; Xu, C.; Kuang, H. Chiral Molecule-Mediated Porous CuxO Nanoparticle Clusters with Antioxidation Activity for Ameliorating Parkinson’s Disease. J. Am. Chem. Soc. 2018, 141, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Q.; Mao, Y.; Xu, E.; Jia, H.; Zhang, S.; Dawson, V.L.; Dawson, T.M.; Li, Y.-M.; Zheng, Z.; He, W.; et al. Nanozyme Scavenging ROS for Prevention of Pathologic α-Synuclein Transmission in Parkinson’s Disease. Nano Today 2021, 36, 101027. [Google Scholar] [CrossRef]
- Chung, H.K.; Ho, H.-A.; Pérez-Acuña, D.; Lee, S.-J. Modeling α-Synuclein Propagation with Preformed Fibril Injections. J. Mov. Disord. 2019, 12, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; Zeng, C.; Okyere, S.K.; Chen, Z.; Hu, Y. Glycine Nano-selenium Prevents Brain Oxidative Stress and Neurobehavioral Abnormalities Caused by MPTP in Rats. J. Trace Elem. Med. Biol. 2021, 64, 126680. [Google Scholar] [CrossRef]
- Klyachko, N.L.; Haney, M.J.; Zhao, Y.; Manickam, D.S.; Mahajan, V.; Suresh, P.; Hingtgen, S.D.; Mosley, R.L.; Gendelman, H.E.; Kabanov, A.V.; et al. Macrophages Offer a Paradigm Switch for CNS Delivery of Therapeutic Proteins. Nanomedicine 2014, 9, 1403–1422. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Zhao, Y.; Li, S.; Higginbotham, S.M.; Booth, S.L.; Han, H.-Y.; Vetro, J.A.; Mosley, R.L.; Kabanov, A.V.; Gendelman, H.E.; et al. Cell-Mediated Transfer of Catalase Nanoparticles from Macrophages to Brain Endothelial, Glial and Neuronal Cells. Nanomedicine 2011, 6, 1215–1230. [Google Scholar] [CrossRef] [PubMed]
- Brynskikh, A.M.; Zhao, Y.; Mosley, R.L.; Li, S.; Boska, M.D.; Klyachko, N.L.; Kabanov, A.V.; Gendelman, H.E.; Batrakova, E.V. Macrophage Delivery of Therapeutic Nanozymes in a Murine Model of Parkinson’s Disease. Nanomedicine 2010, 5, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Stefanis, L. Alpha-Synuclein in Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef]
- Brundin, P.; Dave, K.D.; Kordower, J.H. Therapeutic Approaches to Target Alpha-Synuclein Pathology. Exp. Neurol. 2017, 298, 225–235. [Google Scholar] [CrossRef]
- Tira, R.; De Cecco, E.; Rigamonti, V.; Santambrogio, C.; Barracchia, C.G.; Munari, F.; Romero, A.; Legname, G.; Prosperi, D.; Grandori, R.; et al. Dynamic Molecular Exchange and Conformational Transitions of Alpha-Synuclein at the Nano-Bio Interface. Int. J. Biol. Macromol. 2020, 154, 206–216. [Google Scholar] [CrossRef]
- Mohammadi, S.; Nikkhah, M.; Hosseinkhani, S. Investigation of the Effects of Carbon-Based Nanomaterials on A53T Alpha-Synuclein Aggregation Using a Whole-Cell Recombinant Biosensor. Int. J. Nanomed. 2017, 12, 8831–8840. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yoo, J.M.; Hwang, H.; Lee, J.; Lee, S.H.; Yun, S.P.; Park, M.J.; Lee, M.; Choi, S.; Kwon, Y.J.; et al. Graphene Quantum Dots Prevent α-Synucleinopathy in Parkinson’s Disease. Nat. Nanotechnol. 2018, 13, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Ysselstein, D.; Krainc, D. Untangling Alpha Synuclein Fibrils by Graphene Quantum Dots. Mov. Disord. 2018, 33, 1673. [Google Scholar] [CrossRef]
- Sun, Y.; Kakinen, A.; Zhang, C.; Yang, Y.; Faridi, A.; Davis, T.P.; Cao, W.; Ke, P.C.; Ding, F. Amphiphilic Surface Chemistry of Fullerenols Is Necessary for Inhibiting the Amyloid Aggregation of Alpha-Synuclein NACore. Nanoscale 2019, 11, 11933–11945. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with Enzyme-like Characteristics (Nanozymes): Next-Generation Artificial Enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, R.; Scrimin, P.; Mancin, F. Phosphatediesters Cleavage Mediated by Ce(Iv) Complexes Self-Assembled on Gold Nanoparticles. Org. Biomol. Chem. 2010, 8, 2622–2626. [Google Scholar] [CrossRef]
- Diez-Castellnou, M.; Mancin, F.; Scrimin, P. Efficient Phosphodiester Cleaving Nanozymes Resulting from Multivalency and Local Medium Polarity Control. J. Am. Chem. Soc. 2014, 136, 1158–1161. [Google Scholar] [CrossRef]
- Wang, S.-S.; Yang, G.-Y. Recent Advances in Polyoxometalate-Catalyzed Reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef]
- de Azambuja, F.; Moons, J.; Parac-Vogt, T.N. The Dawn of Metal-Oxo Clusters as Artificial Proteases: From Discovery to the Present and Beyond. Acc. Chem. Res. 2021, 54, 1673–1684. [Google Scholar] [CrossRef]
- Miras, H.N.; Yan, J.; Long, D.-L.; Cronin, L. Engineering Polyoxometalates with Emergent Properties. Chem. Soc. Rev. 2012, 41, 7403–7430. [Google Scholar] [CrossRef]
- Van Rompuy, L.S.; Savić, N.D.; Rodriguez, A.; Parac-Vogt, T.N. Selective Hydrolysis of Transferrin Promoted by Zr-Substituted Polyoxometalates. Molecules 2020, 25, 3472. [Google Scholar] [CrossRef] [PubMed]
- Moons, J.; de Azambuja, F.; Mihailovic, J.; Kozma, K.; Smiljanic, K.; Amiri, M.; Velickovic, T.C.; Nyman, M.; Parac-Vogt, T.N. Discrete Hf18 Metal-Oxo Cluster as a Heterogeneous Nanozyme for Site-Specific Proteolysis. Angew. Chem. Int. Ed. 2020, 59, 9094–9100. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, S.A.M.; Vanderbroek, L.; de Azambuja, F.; Parac-Vogt, T.N. Redox Activity of Ce(IV)-Substituted Polyoxometalates toward Amino Acids and Peptides. Inorg. Chem. 2020, 59, 10569–10577. [Google Scholar] [CrossRef]
- Ly, H.G.T.; Mihaylov, T.T.; Proost, P.; Pierloot, K.; Harvey, J.N.; Parac-Vogt, T.N. Chemical Mimics of Aspartate-Directed Proteases: Predictive and Strictly Specific Hydrolysis of a Globular Protein at Asp-X Sequence Promoted by Polyoxometalate Complexes Rationalized by a Combined Experimental and Theoretical Approach. Chemistry 2019, 25, 14370–14381. [Google Scholar] [CrossRef] [PubMed]
- Ly, H.G.T.; Mihaylov, T.; Absillis, G.; Pierloot, K.; Parac-Vogt, T.N. Reactivity of Dimeric Tetrazirconium(IV) Wells–Dawson Polyoxometalate toward Dipeptide Hydrolysis Studied by a Combined Experimental and Density Functional Theory Approach. Inorg. Chem. 2015, 54, 11477–11492. [Google Scholar] [CrossRef] [PubMed]
- Paul, T.J.; Parac-Vogt, T.N.; Quiñonero, D.; Prabhakar, R. Investigating Polyoxometalate-Protein Interactions at Chemically Distinct Binding Sites. J. Phys. Chem. B 2018, 122, 7219–7232. [Google Scholar] [CrossRef]
- Van Rompuy, L.S.; Parac-Vogt, T.N. Interactions between Polyoxometalates and Biological Systems: From Drug Design to Artificial Enzymes. Curr. Opin. Biotechnol. 2019, 58, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Ly, H.G.T.; Parac-Vogt, T.N. Spectroscopic Study of the Interaction between Horse Heart Myoglobin and Zirconium(IV)-Substituted Polyoxometalates as Artificial Proteases. ChemPhysChem 2017, 18, 2451–2458. [Google Scholar] [CrossRef]
- Gao, N.; Zhao, A.; Sun, H.; Wang, Y.; Ren, J.; Qu, X. Polyoxometalate-Based Nanozyme: Design of a Multifunctional Enzyme for Multi-Faceted Treatment of Alzheimer’s Disease. Nano Res. 2016, 9, 1079–1090. [Google Scholar] [CrossRef]
- Gao, N.; Sun, H.; Ren, J.; Duan, T.; Xu, C.; Qu, X. Transition-Metal-Substituted Polyoxometalate Derivatives as Functional Anti-Amyloid Agents for Alzheimer’s Disease. Nat. Commun. 2014, 5, 3422. [Google Scholar] [CrossRef]
- Hedstrom, L. Serine Protease Mechanism and Specificity. Chem. Rev. 2002, 102, 4501–4524. [Google Scholar] [CrossRef]
- Guan, Y.; Li, M.; Dong, K.; Gao, N.; Ren, J.; Zheng, Y.; Qu, X. Ceria/POMs Hybrid Nanoparticles as a Mimicking Metallopeptidase for Treatment of Neurotoxicity of Amyloid-β Peptide. Biomaterials 2016, 98, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Ma, S. Gas Adsorption Applications of Porous Metal–Organic Frameworks. Pure Appl. Chem. 2009, 81, 2235–2251. [Google Scholar] [CrossRef]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal–Organic Framework Materials as Catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.H.; Lee, B.J.; Kim, H.; Suh, J. Artificial Metalloprotease with Active Site Comprising Aldehyde Group and Cu(II)Cyclen Complex. J. Am. Chem. Soc. 2005, 127, 9593–9602. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Dai, L.; Liu, D.; Du, W.; Wang, Y. Progress & Prospect of Metal-Organic Frameworks (MOFs) for Enzyme Immobilization (Enzyme/MOFs). Renew. Sustain. Energy Rev. 2018, 91, 793–801. [Google Scholar] [CrossRef]
- Feng, D.; Gu, Z.-Y.; Li, J.-R.; Jiang, H.-L.; Wei, Z.; Zhou, H.-C. Zirconium-Metalloporphyrin PCN-222: Mesoporous Metal–Organic Frameworks with Ultrahigh Stability as Biomimetic Catalysts. Angew. Chem. Int. Ed. 2012, 51, 10307–10310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-W.; Zhang, H.-T.; Du, Z.-Y.; Wang, X.; Yu, S.-H.; Jiang, H.-L. Water-Stable Metal–Organic Frameworks with Intrinsic Peroxidase-like Catalytic Activity as a Colorimetric Biosensing Platform. Chem. Commun. 2014, 50, 1092–1094. [Google Scholar] [CrossRef]
- Ly, H.G.T.; Fu, G.; Kondinski, A.; Bueken, B.; De Vos, D.; Parac-Vogt, T.N. Superactivity of MOF-808 toward Peptide Bond Hydrolysis. J. Am. Chem. Soc. 2018, 140, 6325–6335. [Google Scholar] [CrossRef]
- Ly, H.G.T.; Fu, G.; de Azambuja, F.; De Vos, D.; Parac-Vogt, T.N. Nanozymatic Activity of UiO-66 Metal–Organic Frameworks: Tuning the Nanopore Environment Enhances Hydrolytic Activity toward Peptide Bonds. ACS Appl. Nano Mater. 2020, 3, 8931–8938. [Google Scholar] [CrossRef]
- Loosen, A.; de Azambuja, F.; Smolders, S.; Moons, J.; Simms, C.; De Vos, D.; Parac-Vogt, T.N. Interplay between Structural Parameters and Reactivity of Zr6-Based MOFs as Artificial Proteases. Chem. Sci. 2020, 11, 6662–6669. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, J.; Yan, Z.; Jiang, L.; Duan, D.; He, J.; Luo, Z.; Zhang, J.; Yuan, F. MOFzyme: Intrinsic Protease-like Activity of Cu-MOF. Sci. Rep. 2014, 4, 6759. [Google Scholar] [CrossRef] [PubMed]
- Pulgar, V.M. Transcytosis to Cross the Blood Brain Barrier, New Advancements and Challenges. Front. Neurosci. 2019, 12, 1019. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Ali, A.; Md, S.; Baboota, S.; Sahni, J.K.; Ali, J. Insights into Direct Nose to Brain Delivery: Current Status and Future Perspective. Drug Deliv. 2014, 21, 75–86. [Google Scholar] [CrossRef]
- Geiger, B.M.; Frank, L.E.; Caldera-Siu, A.D.; Pothos, E.N. Survivable Stereotaxic Surgery in Rodents. J. Vis. Exp. 2008, 20, 880. [Google Scholar] [CrossRef]
- Sandoval, K.E.; Witt, K.A. Blood-Brain Barrier Tight Junction Permeability and Ischemic Stroke. Neurobiol. Dis. 2008, 32, 200–219. [Google Scholar] [CrossRef]
- Grabrucker, A.M.; Chhabra, R.; Belletti, D.; Forni, F.; Vandelli, M.A.; Ruozi, B.; Tosi, G. Nanoparticles as Blood–Brain Barrier Permeable CNS Targeted Drug Delivery Systems. In The Blood Brain Barrier (BBB); Topics in Medicinal Chemistry; Springer: Berlin/Heidelberg, Germany, 2013; Volume 10, pp. 71–89. ISBN 978-3-662-43786-5. [Google Scholar]
- Zheng, W.; Aschner, M.; Ghersi-Egea, J.-F. Brain Barrier Systems: A New Frontier in Metal Neurotoxicological Research. Toxicol. Appl. Pharmacol. 2003, 192, 1–11. [Google Scholar] [CrossRef]
- Gumerlock, M.K.; Belshe, B.D.; Madsen, R.; Watts, C. Osmotic Blood-Brain Barrier Disruption and Chemotherapy in the Treatment of High Grade Malignant Glioma: Patient Series and Literature Review. J. Neurooncol. 1992, 12, 33–46. [Google Scholar] [CrossRef]
- Wu, S.-K.; Chu, P.-C.; Chai, W.-Y.; Kang, S.-T.; Tsai, C.-H.; Fan, C.-H.; Yeh, C.-K.; Liu, H.-L. Characterization of Different Microbubbles in Assisting Focused Ultrasound-Induced Blood-Brain Barrier Opening. Sci. Rep. 2017, 7, 46689. [Google Scholar] [CrossRef]
- Chu, P.-C.; Chai, W.-Y.; Tsai, C.-H.; Kang, S.-T.; Yeh, C.-K.; Liu, H.-L. Focused Ultrasound-Induced Blood-Brain Barrier Opening: Association with Mechanical Index and Cavitation Index Analyzed by Dynamic Contrast-Enhanced Magnetic-Resonance Imaging. Sci. Rep. 2016, 6, 33264. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Drug Delivery Systems, CNS Protection, and the Blood Brain Barrier. BioMed. Res. Int. 2014, 2014, 869269. [Google Scholar] [CrossRef]
- Hu, J.-J.; Xiao, D.; Zhang, X.-Z. Advances in Peptide Functionalization on Mesoporous Silica Nanoparticles for Controlled Drug Release. Small 2016, 12, 3344–3359. [Google Scholar] [CrossRef]
- Velasco-Aguirre, C.; Morales, F.; Gallardo-Toledo, E.; Guerrero, S.; Giralt, E.; Araya, E.; Kogan, M.J. Peptides and Proteins Used to Enhance Gold Nanoparticle Delivery to the Brain: Preclinical Approaches. Int. J. Nanomed. 2015, 10, 4919–4936. [Google Scholar] [CrossRef]
- Gonzalez-Carter, D.; Liu, X.; Tockary, T.A.; Dirisala, A.; Toh, K.; Anraku, Y.; Kataoka, K. Targeting Nanoparticles to the Brain by Exploiting the Blood–Brain Barrier Impermeability to Selectively Label the Brain Endothelium. Proc. Natl. Acad. Sci. USA 2020, 117, 19141–19150. [Google Scholar] [CrossRef] [PubMed]
- Peluffo, H.; Unzueta, U.; Negro-Demontel, M.L.; Xu, Z.; Váquez, E.; Ferrer-Miralles, N.; Villaverde, A. BBB-Targeting, Protein-Based Nanomedicines for Drug and Nucleic Acid Delivery to the CNS. Biotechnol. Adv. 2015, 33, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, S. Functional Nucleic-Acid-Decorated Spherical Nanoparticles: Preparation Strategies and Current Applications in Cancer Therapy. Small Sci. 2021, 1, 2000056. [Google Scholar] [CrossRef]
- Loureiro, J.A.; Gomes, B.; Coelho, M.A.N.; do Carmo Pereira, M.; Rocha, S. Targeting Nanoparticles across the Blood–Brain Barrier with Monoclonal Antibodies. Nanomedicine 2014, 9, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, K.B.; Bak, M.; Kempen, P.J.; Melander, F.; Burkhart, A.; Thomsen, M.S.; Nielsen, M.S.; Moos, T.; Andresen, T.L. Antibody Affinity and Valency Impact Brain Uptake of Transferrin Receptor-Targeted Gold Nanoparticles. Theranostics 2018, 8, 3416–3436. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.A.; Vanhecke, D.; Michen, B.; Blank, F.; Gehr, P.; Petri-Fink, A.; Rothern-Rutishauser, B. Different Endocytotic Uptake Mechanisms for Nanoparticles in Epithelial Cells and Macrophages. Beilstein J. Nanotechnol. 2014, 5, 1625–1636. [Google Scholar] [CrossRef]
- Smith, N.M.; Gachulincova, I.; Ho, D.; Bailey, C.; Bartlett, C.A.; Norret, M.; Murphy, J.; Buckley, A.; Rigby, P.J.; House, M.J.; et al. An Unexpected Transient Breakdown of the Blood Brain Barrier Triggers Passage of Large Intravenously Administered Nanoparticles. Sci. Rep. 2016, 6, 22595. [Google Scholar] [CrossRef]
- Patching, S.G. Glucose Transporters at the Blood-Brain Barrier: Function, Regulation and Gateways for Drug Delivery. Mol. Neurobiol. 2017, 54, 1046–1077. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–Endothelial Interactions at the Blood–Brain Barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Cutler, E.G.; Cho, H. Therapeutic Nanoplatforms and Delivery Strategies for Neurological Disorders. Nano Converg. 2018, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Gan, L.-S. Receptor-Mediated Endocytosis and Brain Delivery of Therapeutic Biologics. Int. J. Cell Biol. 2013, 2013, 703545. [Google Scholar] [CrossRef]
- Lam, F.C.; Morton, S.W.; Wyckoff, J.; Han, T.-L.V.; Hwang, M.K.; Maffa, A.; Balkanska-Sinclair, E.; Yaffe, M.B.; Flyod, S.R.; Hammond, P.T. Enhanced Efficacy of Combined Temozolomide and Bromodomain Inhibitor Therapy for Gliomas Using Targeted Nanoparticles. Nat. Commun. 2018, 9, 1991. [Google Scholar] [CrossRef] [PubMed]
- Wiley, D.T.; Webster, P.; Gale, A.; Davis, M.E. Transcytosis and Brain Uptake of Transferrin-Containing Nanoparticles by Tuning Avidity to Transferrin Receptor. Proc. Natl. Acad. Sci. USA 2013, 110, 8662–8667. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Ke, W.; Liu, Y.; Wu, D.; Feng, L.; Jiang, C.; Pei, Y. Gene Therapy Using Lactoferrin-Modified Nanoparticles in a Rotenone-Induced Chronic Parkinson Model. J. Neurol. Sci. 2010, 290, 123–130. [Google Scholar] [CrossRef]
- Yan, X.; Xu, L.; Bi, C.; Duan, D.; Chu, L.; Yu, X.; Wu, Z.; Wang, A.; Sun, K. Lactoferrin-Modified Rotigotine Nanoparticles for Enhanced Nose-to-Brain Delivery: LESA-MS/MS-Based Drug Biodistribution, Pharmacodynamics, and Neuroprotective Effects. Int. J. Nanomed. 2018, 13, 273–281. [Google Scholar] [CrossRef]
- Mathias, N.R.; Hussain, M.A. Non-Invasive Systemic Drug Delivery: Developability Considerations for Alternate Routes of Administration. J. Pharm. Sci. 2010, 99, 1–20. [Google Scholar] [CrossRef]
- Alam, M.I.; Baboota, S.; Ahuja, A.; Ali, M.; Ali, J.; Sahni, J.K.; Bhatnagar, A. Pharmacoscintigraphic Evaluation of Potential of Lipid Nanocarriers for Nose-to-Brain Delivery of Antidepressant Drug. Int. J. Pharm. 2014, 470, 99–106. [Google Scholar] [CrossRef]
- Erdő, F.; Bors, L.A.; Karkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of Intranasal Delivery Route of Drug Administration for Brain Targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Md, S.; Mustafa, G.; Baboota, S.; Ali, J. Nanoneurotherapeutics Approach Intended for Direct Nose to Brain Delivery. Drug Dev. Ind. Pharm. 2015, 41, 1922–1934. [Google Scholar] [CrossRef] [PubMed]
- Md, S.; Bhattmisra, S.K.; Zeeshan, F.; Shahzad, N.; Mujtaba, M.A.; Meka, V.S.; Radhakrishnan, A.; Kesharwani, P.; Baboota, S.; Ali, J. Nano-Carrier Enabled Drug Delivery Systems for Nose to Brain Targeting for the Treatment of Neurodegenerative Disorders. J. Drug Deliv. Sci. Technol. 2018, 43, 295–310. [Google Scholar] [CrossRef]
- Rehman, S.; Nabi, B.; Zafar, A.; Baboota, S.; Ali, J. Intranasal Delivery of Mucoadhesive Nanocarriers: A Viable Option for Parkinson’s Disease Treatment? Expert Opin. Drug Deliv. 2019, 16, 1355–1366. [Google Scholar] [CrossRef]
- Sonvico, F.; Clementino, A.; Buttini, F.; Colombo, G.; Pescina, S.; Guterres, S.S.; Pohlmann, A.R.; Nicoli, S. Surface-Modified Nanocarriers for Nose-to-Brain Delivery: From Bioadhesion to Targeting. Pharmaceutics 2018, 10, 34. [Google Scholar] [CrossRef]
- Bourganis, V.; Kammona, O.; Alexopoulos, A.; Kiparissides, C. Recent Advances in Carrier Mediated Nose-to-Brain Delivery of Pharmaceutics. Eur. J. Pharm. Biopharm. 2018, 128, 337–362. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H., III. Intranasal Delivery to the Central Nervous System: Mechanisms and Experimental Considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef]
- Pires, P.C.; Santos, A.O. Nanosystems in Nose-to-Brain Drug Delivery: A Review of Non-Clinical Brain Targeting Studies. J. Control. Release 2018, 270, 89–100. [Google Scholar] [CrossRef]
- Crowe, T.P.; West Greenlee, M.H.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of Intranasal Drug Delivery Directly to the Brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Islam, S.U.; Shehzad, A.; Ahmed, M.B.; Lee, Y.S. Intranasal Delivery of Nanoformulations: A Potential Way of Treatment for Neurological Disorders. Molecules 2020, 25, 1929. [Google Scholar] [CrossRef]
- Wang, H.; Wan, K.; Shi, X. Recent Advances in Nanozyme Research. Adv. Mater. 2019, 31, 1805368. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Lien, C.-W.; Wang, C.-W.; Harroun, S.G.; Huang, C.-C.; Chang, H.-T. Immobilization of Aptamer-Modified Gold Nanoparticles on BiOCl Nanosheets: Tunable Peroxidase-like Activity by Protein Recognition. Biosens. Bioelectron. 2016, 75, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lu, N.; Zhang, J.; Yan, R.; Li, J.; Wang, L.; Wang, N.; Lv, M.; Zhang, M. Ultrasensitive Aptamer-Based Protein Assays Based on One-Dimensional Core-Shell Nanozymes. Biosens. Bioelectron. 2020, 150, 111881. [Google Scholar] [CrossRef]
- You, C.-C.; Agasti, S.S.; Rotello, V.M. Isomeric Control of Protein Recognition with Amino Acid- and Dipeptide-Functionalized Gold Nanoparticles. Chemistry 2008, 14, 143–150. [Google Scholar] [CrossRef]
- Bizzarri, A.R.; Cannistraro, S. SERS Detection of Thrombin by Protein Recognition Using Functionalized Gold Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 306–310. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Wang, J.; Liu, T.; Zhang, Y.; Han, X.; Wang, T.; Guo, S.; Dong, T.; Xu, J.; Anderson, G.J.; et al. Targeted Brain Delivery of Rabies Virus Glycoprotein 29-Modified Deferoxamine-Loaded Nanoparticles Reverses Functional Deficits in Parkinsonian Mice. ACS Nano 2018, 12, 4123–4139. [Google Scholar] [CrossRef]
- Nutt, J.G.; Carter, J.H.; Sexton, G.J. The Dopamine Transporter: Importance in Parkinson’s Disease. Ann. Neurol. 2004, 55, 766–773. [Google Scholar] [CrossRef]
- Ambrosini, S.; Beyazit, S.; Haupt, K.; Bui, B.T.S. Solid-Phase Synthesis of Molecularly Imprinted Nanoparticles for Protein Recognition. Chem. Commun. 2013, 49, 6746. [Google Scholar] [CrossRef]
- Zhou, W.-H.; Lu, C.-H.; Guo, X.-C.; Chen, F.-R.; Yang, H.-H.; Wang, X.-R. Mussel-Inspired Molecularly Imprinted Polymer Coating Superparamagnetic Nanoparticles for Protein Recognition. J. Mater. Chem. 2010, 20, 880–883. [Google Scholar] [CrossRef]
- Xia, Z.; Lin, Z.; Xiao, Y.; Wang, L.; Zheng, J.; Yang, H.; Chen, G. Facile Synthesis of Polydopamine-Coated Molecularly Imprinted Silica Nanoparticles for Protein Recognition and Separation. Biosens. Bioelectron. 2013, 47, 120–126. [Google Scholar] [CrossRef]
- Han, W.; Han, X.; Liu, Z.; Zhang, S.; Li, Y.; Lu, J.; Chen, J.; Ou, L.; Fu, G. Facile Modification of Protein-Imprinted Polydopamine Coatings over Nanoparticles with Enhanced Binding Selectivity. Chem. Eng. J. 2020, 385, 123463. [Google Scholar] [CrossRef]
- Li, A.; Chen, Y.; Zhang, L. Hybrid Nanozyme: More Than One Plus One. In Nanozymology: Connecting Biology and Nanotechnology; Nanostructure Science and Technology; Springer: New York, NY, USA, 2020; pp. 367–391. ISBN 978-9811514890. [Google Scholar]
- Sullivan, M.V.; Clay, O.; Moazami, M.P.; Watts, J.K.; Turner, N.W. Hybrid Aptamer-Molecularly Imprinted Polymer (AptaMIP) Nanoparticles from Protein Recognition—A Trypsin Model. Macromol. Biosci. 2021, 21, 2100002. [Google Scholar] [CrossRef]
- Shoghi, E.; Mirahmadi-Zare, S.Z.; Ghasemi, R.; Asghari, M.; Poorebrahim, M.; Nasr-Esfahani, M.-H. Nanosized Aptameric Cavities Imprinted on the Surface of Magnetic Nanoparticles for High-Throughput Protein Recognition. Microchim. Acta 2018, 185, 241. [Google Scholar] [CrossRef]
- Sun, K.; Xia, N.; Zhao, L.; Liu, K.; Hou, W.; Liu, L. Aptasensors for the Selective Detection of Alpha-Synuclein Oligomer by Colorimetry, Surface Plasmon Resonance and Electrochemical Impedance Spectroscopy. Sens. Actuators B Chem. 2017, 245, 87–94. [Google Scholar] [CrossRef]
- You, X.; Gopinath, S.C.B.; Lakshmipriya, T.; Li, D. High-Affinity Detection of Alpha-Synuclein by Aptamer-Gold Conjugates on an Amine-Modified Dielectric Surface. J. Anal. Methods Chem. 2019, 2019, 6526850. [Google Scholar] [CrossRef]
- Guo, C.; Hu, M.; Li, Z.; Duan, F.; He, L.; Zhang, Z.; Marchetti, F.; Du, M. Structural Hybridization of Bimetallic Zeolitic Imidazolate Framework (ZIF) Nanosheets and Carbon Nanofibers for Efficiently Sensing α-Synuclein Oligomers. Sens. Actuators B Chem. 2020, 309, 127821. [Google Scholar] [CrossRef]
- An, Y.; Tang, L.; Jiang, X.; Chen, H.; Yang, M.; Jin, L.; Zhang, S.; Wang, C.; Zhang, W. A Photoelectrochemical Immunosensor Based on Au-Doped TiO2 Nanotube Arrays for the Detection of α-Synuclein. Chem. A Eur. J. 2010, 16, 14439–14446. [Google Scholar] [CrossRef] [PubMed]
- Karaboğa, M.N.S.; Sezgintürk, M.K. Cerebrospinal Fluid Levels of Alpha-Synuclein Measured Using a Poly-Glutamic Acid-Modified Gold Nanoparticle-Doped Disposable Neuro-Biosensor System. Analyst 2019, 144, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hu, Q.; Liu, C.; Wang, L. A Nanospherical Conjugated Microporous Polymer-Graphene Nanosheets Modified Molecularly Imprinted Electrochemical Sensor for High Sensitivity Detection of α-Synuclein. J. Electroanal. Chem. 2020, 862, 113994. [Google Scholar] [CrossRef]
- Chauhan, N.; Soni, S.; Jain, U. Recent Advances in Nanosensors Development for Biomarker Alpha-Synuclein Protein Detection. Process Biochem. 2021, 111, 105–113. [Google Scholar] [CrossRef]
- Catalan-Figueroa, J.; Palma-Florez, S.; Alvarez, G.; Fritz, H.F.; Jara, M.O.; Morales, J.O. Nanomedicine and Nanotoxicology: The Pros and Cons for Neurodegeneration and Brain Cancer. Nanomedicine 2016, 11, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Zern, B.J.; Chacko, A.-M.; Liu, J.; Greineder, C.F.; Blankemeyer, E.R.; Radhakrishnan, R.; Muzykantov, V. Reduction of Nanoparticle Avidity Enhances the Selectivity of Vascular Targeting and PET Detection of Pulmonary Inflammation. ACS Nano 2013, 7, 2461–2469. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chu, D.S.; Schellinger, J.G.; Bocek, M.J.; Johnson, R.N.; Pun, S.H. Optimization of Tet1 Ligand Density in HPMA-Co-Oligolysine Copolymers for Targeted Neuronal Gene Delivery. Biomaterials 2013, 34, 9632–9637. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.S.; Song, K.S.; Ryu, H.R.; Sung, J.H.; Park, J.D.; Park, H.M.; Song, N.W.; Shin, B.S.; Marshak, D.; et al. Biopersistence of Silver Nanoparticles in Tissues from Sprague-Dawley Rats. Part. Fibre Toxicol. 2013, 10, 36. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Jiao, F.; Lao, F.; Li, W.; Gu, Y.; Li, Y.; Ge, C.; Zhou, G.; Li, B.; et al. Time-Dependent Translocation and Potential Impairment on Central Nervous System by Intranasally Instilled TiO2 Nanoparticles. Toxicology 2008, 254, 82–90. [Google Scholar] [CrossRef]
- Huerta-García, E.; Pérez-Arizti, J.A.; Márquez-Ramírez, S.G.; Delgado-Buenrostro, N.L.; Chirino, Y.I.; Gutiérrez Iglesias, G.; López-Marure, R. Titanium Dioxide Nanoparticles Induce Strong Oxidative Stress and Mitochondrial Damage in Glial Cells. Free. Radic. Biol. Med. 2014, 73, 84–94. [Google Scholar] [CrossRef]
- Siddiqi, N.J.; Abdelhalim, M.A.K.; El-Ansary, A.K.; Alhomida, A.S.; Ong, W.Y. Identification of Potential Biomarkers of Gold Nanoparticle Toxicity in Rat Brains. J. Neuroinflamm. 2012, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Paris, I.; Segura-Aguilar, J. The Role of Metal Ions in Dopaminergic Neuron Degeneration in Parkinsonism and Parkinson’s Disease. Mon. Chem. Chem. Mon. 2011, 142, 365–374. [Google Scholar] [CrossRef]
- Stern, S.T.; Adiseshaiah, P.P.; Crist, R.M. Autophagy and Lysosomal Dysfunction as Emerging Mechanisms of Nanomaterial Toxicity. Part. Fibre Toxicol. 2012, 9, 20. [Google Scholar] [CrossRef]
- Bellettato, C.M.; Scarpa, M. Pathophysiology of Neuropathic Lysosomal Storage Disorders. J. Inherit. Metab. Dis. 2010, 33, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding Biophysicochemical Interactions at the Nano–Bio Interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Berry, C.C.; Wells, S.; Charles, S.; Curtis, A.S.G. Dextran and Albumin Derivatised Iron Oxide Nanoparticles: Influence on Fibroblasts in Vitro. Biomaterials 2003, 24, 4551–4557. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M.; Yarwood, S.J.; Curtis, A.S.G. Effect of Cellular Uptake of Gelatin Nanoparticles on Adhesion, Morphology and Cytoskeleton Organisation of Human Fibroblasts. J. Control. Release 2004, 95, 197–207. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, N.; Matarasso, A.; Heck, I.; Li, H.; Lu, W.; Phaup, J.G.; Schneider, M.J.; Wu, Y.; Weng, Z.; et al. Implantable Aptamer-Graphene Microtransistors for Real-Time Monitoring of Neurochemical Release in Vivo. Nano Lett. 2022, 22, 3668–3677. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Guardado, A.; Barkam, S.; Peppler, M.; Biswas, A.; Dennis, W.; Das, S.; Seal, S.; Chanda, D. Enzyme-Free Plasmonic Biosensor for Direct Detection of Neurotransmitter Dopamine from Whole Blood. Nano Lett. 2019, 19, 449–454. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padilla-Godínez, F.J.; Ruiz-Ortega, L.I.; Guerra-Crespo, M. Nanomedicine in the Face of Parkinson’s Disease: From Drug Delivery Systems to Nanozymes. Cells 2022, 11, 3445. https://doi.org/10.3390/cells11213445
Padilla-Godínez FJ, Ruiz-Ortega LI, Guerra-Crespo M. Nanomedicine in the Face of Parkinson’s Disease: From Drug Delivery Systems to Nanozymes. Cells. 2022; 11(21):3445. https://doi.org/10.3390/cells11213445
Chicago/Turabian StylePadilla-Godínez, Francisco J., Leonardo I. Ruiz-Ortega, and Magdalena Guerra-Crespo. 2022. "Nanomedicine in the Face of Parkinson’s Disease: From Drug Delivery Systems to Nanozymes" Cells 11, no. 21: 3445. https://doi.org/10.3390/cells11213445
APA StylePadilla-Godínez, F. J., Ruiz-Ortega, L. I., & Guerra-Crespo, M. (2022). Nanomedicine in the Face of Parkinson’s Disease: From Drug Delivery Systems to Nanozymes. Cells, 11(21), 3445. https://doi.org/10.3390/cells11213445






