Regulation Networks of Non-Coding RNA-Associated ceRNAs in Cisplatin-Induced Acute Kidney Injury
Abstract
1. Introduction
2. Materials and Methods
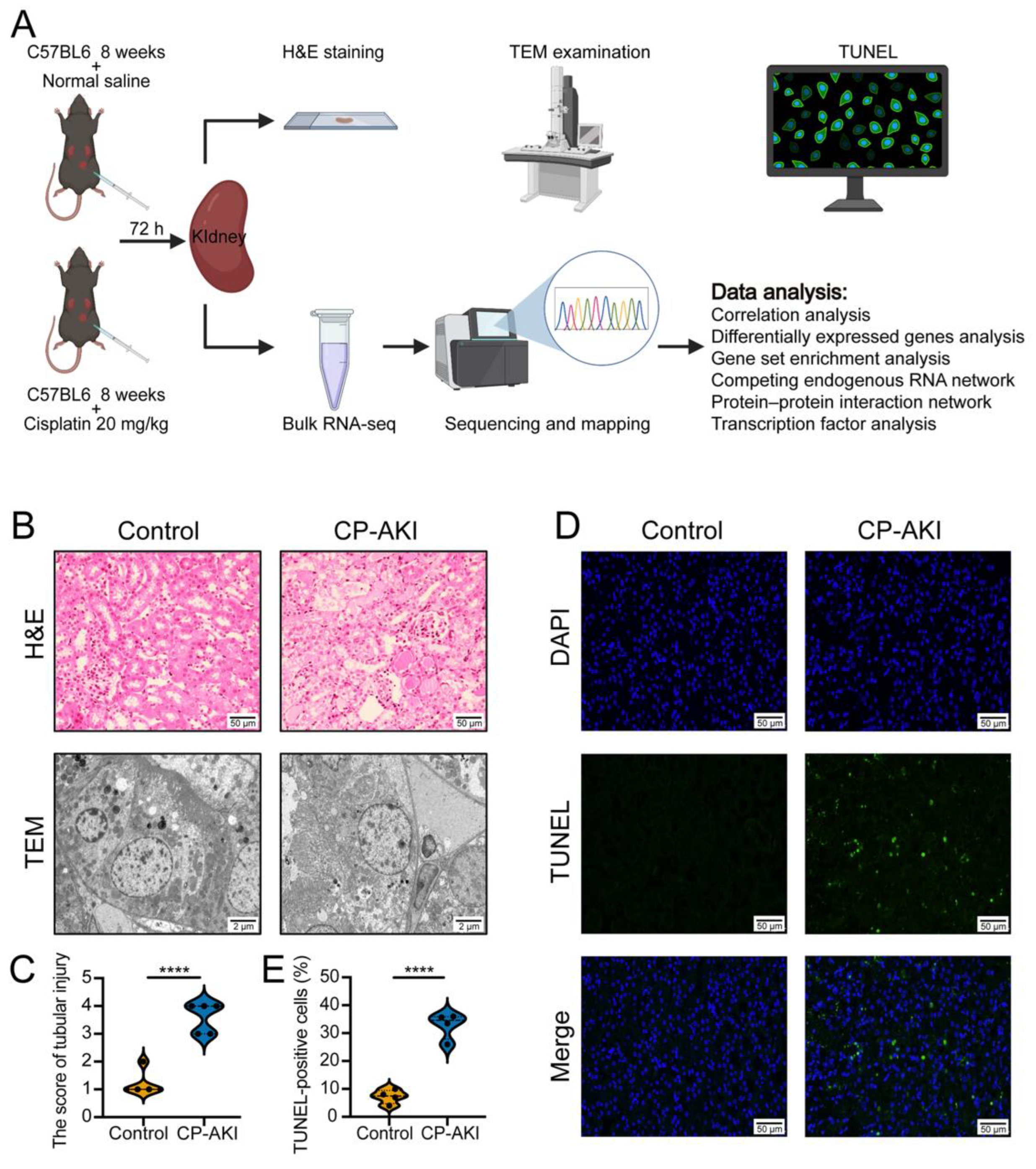
2.1. CP-AKI Mouse Model
2.2. Blood Biomarker Assay
2.3. Hematoxylin and Eosin (H&E) Staining
2.4. Transmission Electron Microscopy (TEM) Examination
2.5. Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling (TUNEL) Assay
2.6. RNA-Seq Analysis
2.7. Gene Expression Analysis
2.8. Gene Set Enrichment Analysis (GSEA)
2.9. Differential Gene Expression Analysis
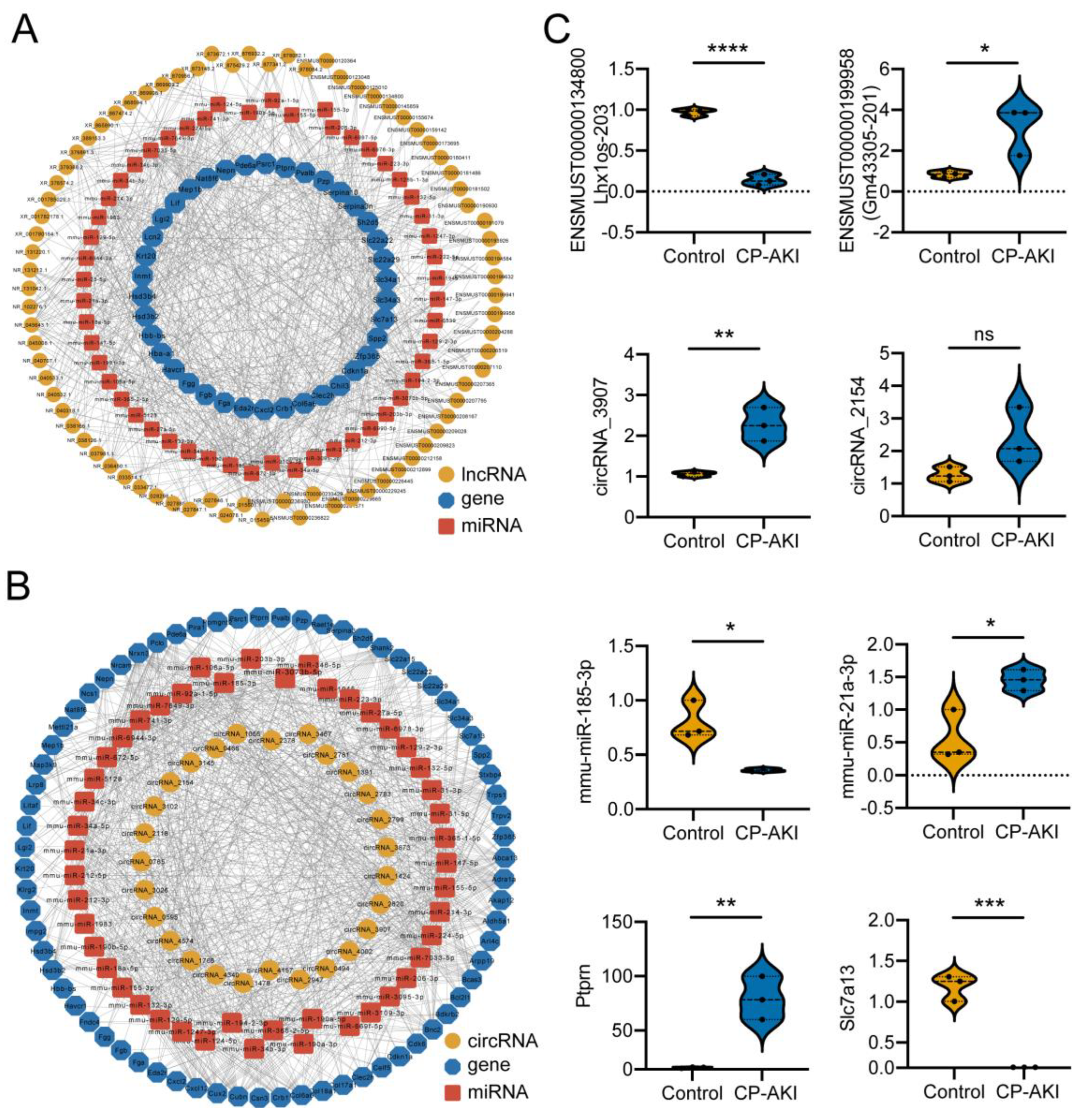
2.10. ceRNA Network Analysis
2.11. Protein–Protein Interaction (PPI) Network Analysis and Hub Gene Identification
2.12. Transcription Factor (TF) Analysis
2.13. RT-qPCR Validation
2.14. Statistical Analysis
3. Results
3.1. Evaluation of the CP-AKI Mouse Model
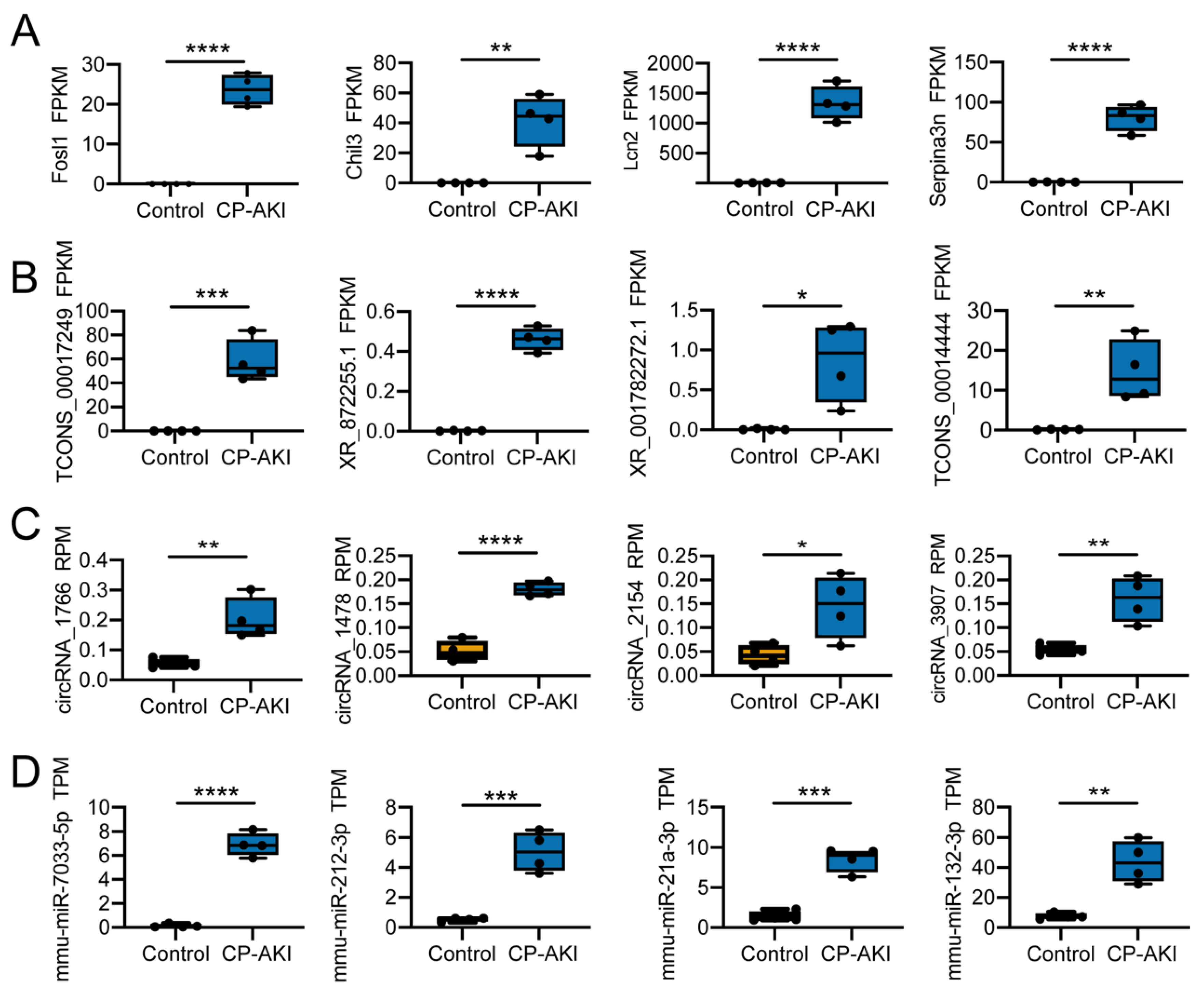
3.2. Biological Processes of CP-AKI Revealed by RNA-Seq
3.3. Potential Regulation Mechanisms in CP-AKI Mice
3.4. Construction and Validation of ncRNA-Associated ceRNA Networks in CP-AKI Mice
3.5. Construction and Analysis of the PPI Network and Hub Genes
3.6. Identification of Activated TFs in CP-AKI Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Fu, H.; Zhou, D.; Zhu, H.; Liao, J.; Lin, L.; Hong, X.; Hou, F.F.; Liu, Y. Matrix metalloproteinase-7 protects against acute kidney injury by priming renal tubules for survival and regeneration. Kidney Int. 2019, 95, 1167–1180. [Google Scholar] [CrossRef]
- Holditch, S.J.; Brown, C.N.; Lombardi, A.M.; Nguyen, K.N.; Edelstein, C.L. Recent Advances in Models, Mechanisms, Biomarkers, and Interventions in Cisplatin-Induced Acute Kidney Injury. Int. J. Mol. Sci. 2019, 20, 3011. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Radu, S.; Costea, C.F.; Ciocoiu, M.; Carauleanu, A.; Lacatusu, C.M.; Maranduca, M.A.; Floria, M.; Rezus, C. The Predictive Role of the Biomarker Kidney Molecule-1 (KIM-1) in Acute Kidney Injury (AKI) Cisplatin-Induced Nephrotoxicity. Int. J. Mol. Sci. 2019, 20, 5238. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Lameire, N.; Group, K.A.G.W. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit. Care 2013, 17, 204. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.J.; Kellum, J.A.; Selby, N.M.; Zarbock, A.; Palevsky, P.M.; Bagshaw, S.M.; Goldstein, S.L.; Cerda, J.; Chawla, L.S. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 2018, 14, 607–625. [Google Scholar] [CrossRef]
- Ranganathan, P.; Hamad, R.; Mohamed, R.; Jayakumar, C.; Muthusamy, T.; Ramesh, G. Histone deacetylase-mediated silencing of AMWAP expression contributes to cisplatin nephrotoxicity. Kidney Int. 2016, 89, 317–326. [Google Scholar] [CrossRef]
- Li, C.M.; Li, M.; Ye, Z.C.; Huang, J.Y.; Li, Y.; Yao, Z.Y.; Peng, H.; Lou, T.Q. Circular RNA expression profiles in cisplatin-induced acute kidney injury in mice. Epigenomics 2019, 11, 1191–1207. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Ping, Y.; Yang, L.; Duan, X.; Resegofetse Maimela, N.; Li, B.; Li, X.; Chen, J.; Zhang, K.; Wang, L.; et al. Characterization of a non-coding RNA-associated ceRNA network in metastatic lung adenocarcinoma. J. Cell Mol. Med. 2020, 24, 11680–11690. [Google Scholar] [CrossRef]
- Li, Y.; Ashraf, U.; Chen, Z.; Zhou, D.; Imran, M.; Ye, J.; Chen, H.; Cao, S. Genome-wide profiling of host-encoded circular RNAs highlights their potential role during the Japanese encephalitis virus-induced neuroinflammatory response. BMC Genom. 2020, 21, 409. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, D.D.; Li, J.Y.; Yin, Y.C.; Li, P.C.; Qiu, L.; Chen, L.M. Identification of microRNA-mRNA networks involved in cisplatin-induced renal tubular epithelial cells injury. Eur. J. Pharmacol. 2019, 851, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fasolo, F.; Di Gregoli, K.; Maegdefessel, L.; Johnson, J.L. Non-coding RNAs in cardiovascular cell biology and atherosclerosis. Cardiovasc. Res. 2019, 115, 1732–1756. [Google Scholar] [CrossRef] [PubMed]
- Panni, S.; Lovering, R.C.; Porras, P.; Orchard, S. Non-coding RNA regulatory networks. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2020, 1863, 194417. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Li, X.W.; Xiao, Y.Q.; Duan, S.B. Non-coding RNA-Associated ceRNA Networks in a New Contrast-Induced Acute Kidney Injury Rat Model. Mol. Ther. Nucleic Acids 2019, 17, 102–112. [Google Scholar] [CrossRef]
- Matsui, M.; Corey, D.R. Non-coding RNAs as drug targets. Nat. Rev. Drug Discov. 2017, 16, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.L.; Zhu, J.; Li, J.; Meng, X.M. Noncoding RNAs in acute kidney injury. J. Cell Physiol. 2019, 234, 2266–2276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, P.M.; Niu, Y.; Garcia Cordoba, C.A.; Huang, X.R.; Yu, C.; Lan, H.Y. Long Non-coding RNA LRNA9884 Promotes Acute Kidney Injury via Regulating NF-kB-Mediated Transcriptional Activation of MIF. Front. Physiol. 2020, 11, 590027. [Google Scholar] [CrossRef] [PubMed]
- Brandenburger, T.; Salgado Somoza, A.; Devaux, Y.; Lorenzen, J.M. Noncoding RNAs in acute kidney injury. Kidney Int. 2018, 94, 870–881. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, C.F.; Ge, W.H.; Du, Y.P.; Hu, N.B. Circular RNA VMA21 ameliorates sepsis-associated acute kidney injury by regulating miR-9-3p/SMG1/inflammation axis and oxidative stress. J. Cell Mol. Med. 2020, 24, 11397–11408. [Google Scholar] [CrossRef] [PubMed]
- Ledeganck, K.J.; Gielis, E.M.; Abramowicz, D.; Stenvinkel, P.; Shiels, P.G.; Van Craenenbroeck, A.H. MicroRNAs in AKI and Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2019, 14, 454–468. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Wang, J.; Miao, H. MiR-107 induces TNF-alpha secretion in endothelial cells causing tubular cell injury in patients with septic acute kidney injury. Biochem. Biophys. Res. Commun. 2017, 483, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Lan, Y.F.; Li, H.F.; Cheng, C.F.; Lai, P.F.; Li, W.H.; Lin, H. Urinary miR-16 transactivated by C/EBPbeta reduces kidney function after ischemia/reperfusion-induced injury. Sci. Rep. 2016, 6, 27945. [Google Scholar] [CrossRef]
- Ge, Q.M.; Huang, C.M.; Zhu, X.Y.; Bian, F.; Pan, S.M. Differentially expressed miRNAs in sepsis-induced acute kidney injury target oxidative stress and mitochondrial dysfunction pathways. PLoS ONE 2017, 12, e0173292. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, H.; Yi, B.; Yang, S.; Liu, J.; Hu, J.; Wang, J.; Cao, K.; Zhang, W. VDR activation attenuate cisplatin induced AKI by inhibiting ferroptosis. Cell Death Dis. 2020, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Pachter, L. Streaming fragment assignment for real-time analysis of sequencing experiments. Nat. Methods 2013, 10, 71–73. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression of RNA-Seq data at the gene level–the DESeq package. Heidelb. Ger. Eur. Mol. Biol. Lab. (EMBL) 2012, 10, f1000research. [Google Scholar]
- Langmead, B. Aligning short sequencing reads with Bowtie. Curr. Protoc. Bioinform. 2010, 32, 11.7.1–11.7.14. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Jones, S.; Saini, H.K.; van Dongen, S.; Enright, A.J. miRBase: Tools for microRNA genomics. Nucleic Acids Res. 2008, 36, D154–D158. [Google Scholar] [CrossRef] [PubMed]
- Enright, A.J.; John, B.; Gaul, U.; Tuschl, T.; Sander, C.; Marks, D.S. MicroRNA targets in Drosophila. Genome Biol. 2003, 5, R1. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.T.; Grimes, M.; Kutlu, B.; Bot, J.J.; Galas, D.J. RCytoscape: Tools for exploratory network analysis. BMC Bioinform. 2013, 14, 217. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alonso, L.; Holland, C.H.; Ibrahim, M.M.; Turei, D.; Saez-Rodriguez, J. Benchmark and integration of resources for the estimation of human transcription factor activities. Genome Res. 2019, 29, 1363–1375. [Google Scholar] [CrossRef]
- Kolde, R. Package ‘pheatmap’. R Package 2015, 1, 790. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Levey, A.S.; James, M.T. Acute Kidney Injury. Ann. Intern. Med. 2017, 167, ITC66–ITC80. [Google Scholar] [CrossRef]
- Perazella, M.A. Pharmacology behind Common Drug Nephrotoxicities. Clin. J. Am. Soc. Nephrol. 2018, 13, 1897–1908. [Google Scholar] [CrossRef]
- Cortazar, F.B.; Marrone, K.A.; Troxell, M.L.; Ralto, K.M.; Hoenig, M.P.; Brahmer, J.R.; Le, D.T.; Lipson, E.J.; Glezerman, I.G.; Wolchok, J.; et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016, 90, 638–647. [Google Scholar] [CrossRef]
- Vaz, M.; Rajasekaran, S.; Potteti, H.R.; Reddy, S.P. Myeloid-specific Fos-related antigen-1 regulates cigarette smoke-induced lung inflammation, not emphysema, in mice. Am. J. Respir. Cell Mol. Biol. 2015, 53, 125–134. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, E.; Ilyas, G.; Lalazar, G.; Lin, Y.; Haseeb, M.; Tanaka, K.E.; Czaja, M.J. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy 2015, 11, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Sadtler, K.; Wolf, M.T.; Ganguly, S.; Moad, C.A.; Chung, L.; Majumdar, S.; Housseau, F.; Pardoll, D.M.; Elisseeff, J.H. Divergent immune responses to synthetic and biological scaffolds. Biomaterials 2019, 192, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Rudman-Melnick, V.; Adam, M.; Potter, A.; Chokshi, S.M.; Ma, Q.; Drake, K.A.; Schuh, M.P.; Kofron, J.M.; Devarajan, P.; Potter, S.S. Single-Cell Profiling of AKI in a Murine Model Reveals Novel Transcriptional Signatures, Profibrotic Phenotype, and Epithelial-to-Stromal Crosstalk. J. Am. Soc. Nephrol. 2020, 31, 2793–2814. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.T.; Shimbo, T.; Wijaya, E.; Kitayama, T.; Takaki, S.; Ikegami, K.; Miyashita, K.; Ouchi, Y.; Takaki, E.; Yamamoto, R.; et al. Longitudinal Single-Cell Transcriptomics Reveals a Role for Serpina3n-Mediated Resolution of Inflammation in a Mouse Colitis Model. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 547–566. [Google Scholar] [CrossRef] [PubMed]
- Tiemin, P.; Fanzheng, M.; Peng, X.; Jihua, H.; Ruipeng, S.; Yaliang, L.; Yan, W.; Junlin, X.; Qingfu, L.; Zhefeng, H.; et al. MUC13 promotes intrahepatic cholangiocarcinoma progression via EGFR/PI3K/AKT pathways. J. Hepatol. 2020, 72, 761–773. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, X.; Li, B.; Sha, J.; Wang, C.; Yang, T.; Cui, H.; Fan, H. Dexmedetomidine Protects Against Lipopolysaccharide-Induced Acute Kidney Injury by Enhancing Autophagy Through Inhibition of the PI3K/AKT/mTOR Pathway. Front. Pharmacol. 2020, 11, 128. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Y.; Liang, T.; Hu, Y.; Tang, H.; Song, D.; Fang, H. The regulatory effect of microRNA-21a-3p on the promotion of telocyte angiogenesis mediated by PI3K (p110alpha)/AKT/mTOR in LPS induced mice ARDS. J. Transl. Med. 2019, 17, 427. [Google Scholar] [CrossRef]
- Nagamori, S.; Wiriyasermkul, P.; Guarch, M.E.; Okuyama, H.; Nakagomi, S.; Tadagaki, K.; Nishinaka, Y.; Bodoy, S.; Takafuji, K.; Okuda, S.; et al. Novel cystine transporter in renal proximal tubule identified as a missing partner of cystinuria-related plasma membrane protein rBAT/SLC3A1. Proc. Natl. Acad. Sci. USA 2016, 113, 775–780. [Google Scholar] [CrossRef]
- Suckale, J.; Solimena, M. The insulin secretory granule as a signaling hub. Trends Endocrinol. Metab. 2010, 21, 599–609. [Google Scholar] [CrossRef]
- Xue, X.X.; Lei, H.Q.; Zhao, L.; Wang, X.Y.; Wang, Z.; Xie, L.Y.; Jia, J.H. MiR-185-3p downregulates advanced glycosylation end product receptor expression and improves renal function in diabetic nephropathy mice. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5018–5027. [Google Scholar] [CrossRef]
- Liu, P.; Li, X.; Lv, W.; Xu, Z. Inhibition of CXCL1-CXCR2 axis ameliorates cisplatin-induced acute kidney injury by mediating inflammatory response. Biomed. Pharmacother. 2020, 122, 109693. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, H.Y.; Zhao, X.; Duan, H.; Cheng, B.; Liu, Y.; Zhao, M.; Shu, W.; Mei, Y.; Wen, Z.; et al. A methylation-phosphorylation switch determines Plk1 kinase activity and function in DNA damage repair. Sci. Adv. 2019, 5, eaau7566. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yuan, H.; Cao, W.; Wang, T.; Chen, W.; Yu, H.; Fu, Y.; Jiang, B.; Zhou, H.; Guo, H.; et al. Blocking interleukin-6 trans-signaling protects against renal fibrosis by suppressing STAT3 activation. Theranostics 2019, 9, 3980–3991. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.T.; Su, H.; Ye, C.; Tang, H.; Gao, P.; Wan, C.; He, F.F.; Wang, Y.M.; Zhang, C. The classic signalling and trans-signalling of interleukin-6 are both injurious in podocyte under high glucose exposure. J. Cell Mol. Med. 2018, 22, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Lee, A.S.; Nguyen-Thanh, T.; Kim, D.; Kang, K.P.; Lee, S.; Park, S.K.; Kim, W. SIRT2 Regulates LPS-Induced Renal Tubular CXCL2 and CCL2 Expression. J. Am. Soc. Nephrol. 2015, 26, 1549–1560. [Google Scholar] [CrossRef]
- De Filippo, K.; Dudeck, A.; Hasenberg, M.; Nye, E.; van Rooijen, N.; Hartmann, K.; Gunzer, M.; Roers, A.; Hogg, N. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 2013, 121, 4930–4937. [Google Scholar] [CrossRef]
- De Blasio, C.; Zonfrilli, A.; Franchitto, M.; Mariano, G.; Cialfi, S.; Verma, N.; Checquolo, S.; Bellavia, D.; Palermo, R.; Benelli, D.; et al. PLK1 targets NOTCH1 during DNA damage and mitotic progression. J. Biol. Chem. 2019, 294, 17941–17950. [Google Scholar] [CrossRef]
- Ajay, A.K.; Kim, T.M.; Ramirez-Gonzalez, V.; Park, P.J.; Frank, D.A.; Vaidya, V.S. A bioinformatics approach identifies signal transducer and activator of transcription-3 and checkpoint kinase 1 as upstream regulators of kidney injury molecule-1 after kidney injury. J. Am. Soc. Nephrol. 2014, 25, 105–118. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, X.; Wang, X.; Liu, B.; Yuan, Y.; Zuo, X. Curcumin attenuates inflammation and cell apoptosis through regulating NF-kappaB and JAK2/STAT3 signaling pathway against acute kidney injury. Cell Cycle 2020, 19, 1941–1951. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, P.; Guo, X.; Liu, T.; Luo, X.; Zhu, Y.T. Inhibition of JAK2/STAT3 signaling pathway protects mice from the DDP-induced acute kidney injury in lung cancer. Inflamm. Res. 2019, 68, 751–760. [Google Scholar] [CrossRef]
- Chang-Panesso, M.; Kadyrov, F.F.; Lalli, M.; Wu, H.; Ikeda, S.; Kefaloyianni, E.; Abdelmageed, M.M.; Herrlich, A.; Kobayashi, A.; Humphreys, B.D. FOXM1 drives proximal tubule proliferation during repair from acute ischemic kidney injury. J. Clin. Investig. 2019, 129, 5501–5517. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Dwivedi, N.; Woodgett, J.; Tao, S.; Howard, C.; Fields, T.A.; Jamadar, A.; Rao, R. Glycogen synthase kinase-3beta inhibits tubular regeneration in acute kidney injury by a FoxM1-dependent mechanism. FASEB J. 2020, 34, 13597–13608. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, G.P.; Kaushal, V.; Herzog, C.; Yang, C. Autophagy delays apoptosis in renal tubular epithelial cells in cisplatin cytotoxicity. Autophagy 2008, 4, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wang, H.; Weng, C.; Jiang, H.; Chen, J. Caspase 3/GSDME-dependent pyroptosis contributes to chemotherapy drug-induced nephrotoxicity. Cell Death Dis. 2021, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.S.; Martinez, M.R.; Komissarova, E.V.; Llobet-Navas, D.; Bansal, M.; Paull, E.O.; Silva, J.; Yang, X.; Sumazin, P.; Califano, A. The number of titrated microRNA species dictates ceRNA regulation. Nucleic Acids Res. 2018, 46, 4354–4369. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Torisu, K.; Tomita, K.; Kawai, Y.; Tsuruya, K.; Nakano, T.; Kitazono, T. Arginase 2 is a mediator of ischemia-reperfusion injury in the kidney through regulation of nitrosative stress. Kidney Int. 2020, 98, 673–685. [Google Scholar] [CrossRef]
- Mallipattu, S.K.; Horne, S.J.; D'Agati, V.; Narla, G.; Liu, R.; Frohman, M.A.; Dickman, K.; Chen, E.Y.; Ma'ayan, A.; Bialkowska, A.B.; et al. Kruppel-like factor 6 regulates mitochondrial function in the kidney. J. Clin. Investig. 2015, 125, 1347–1361. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Li, J.; Zhang, W.; Zheng, L.; Yang, C.; Zhu, T.; Rong, R. The mTOR signal regulates myeloid-derived suppressor cells differentiation and immunosuppressive function in acute kidney injury. Cell Death Dis. 2017, 8, e2695. [Google Scholar] [CrossRef]
- Herzog, C.; Seth, R.; Shah, S.V.; Kaushal, G.P. Role of meprin A in renal tubular epithelial cell injury. Kidney Int. 2007, 71, 1009–1018. [Google Scholar] [CrossRef]
- Marchese, F.P.; Grossi, E.; Marin-Bejar, O.; Bharti, S.K.; Raimondi, I.; Gonzalez, J.; Martinez-Herrera, D.J.; Athie, A.; Amadoz, A.; Brosh, R.M., Jr.; et al. A Long Noncoding RNA Regulates Sister Chromatid Cohesion. Mol. Cell 2016, 63, 397–407. [Google Scholar] [CrossRef]
- Haddad, G.; Kolling, M.; Wegmann, U.A.; Dettling, A.; Seeger, H.; Schmitt, R.; Soerensen-Zender, I.; Haller, H.; Kistler, A.D.; Dueck, A.; et al. Renal AAV2-Mediated Overexpression of Long Non-Coding RNA H19 Attenuates Ischemic Acute Kidney Injury Through Sponging of microRNA-30a-5p. J. Am. Soc. Nephrol. 2021, 32, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Jia, H.; Ji, P.; He, Y.; Chen, L. Identification of the candidate lncRNA biomarkers for acute kidney injury: A systematic review and meta-analysis. Expert Rev. Mol. Diagn. 2021, 21, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.Y.; Wang, B.; Tang, T.T.; Wen, Y.; Li, Z.L.; Feng, S.T.; Wu, M.; Liu, D.; Yin, D.; Ma, K.L.; et al. Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury. Theranostics 2021, 11, 5248–5266. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.L.; Feng, Y.; Wu, M.; Wang, B.; Li, Z.L.; Zhong, X.; Wu, W.J.; Chen, J.; Ni, H.F.; Tang, T.T.; et al. Exosomal miRNA-19b-3p of tubular epithelial cells promotes M1 macrophage activation in kidney injury. Cell Death Differ. 2020, 27, 210–226. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Wan, S.; Liu, W.; Lu, Y.; Xu, Q.; Gan, Y.; Yan, L.; Gu, Y.; Liu, Z.; Hu, Y.; et al. Regulation Networks of Non-Coding RNA-Associated ceRNAs in Cisplatin-Induced Acute Kidney Injury. Cells 2022, 11, 2971. https://doi.org/10.3390/cells11192971
Ding Y, Wan S, Liu W, Lu Y, Xu Q, Gan Y, Yan L, Gu Y, Liu Z, Hu Y, et al. Regulation Networks of Non-Coding RNA-Associated ceRNAs in Cisplatin-Induced Acute Kidney Injury. Cells. 2022; 11(19):2971. https://doi.org/10.3390/cells11192971
Chicago/Turabian StyleDing, Yun, Shengfeng Wan, Wenna Liu, Yanfang Lu, Qin Xu, Yujin Gan, Lei Yan, Yue Gu, Ziyang Liu, Yifeng Hu, and et al. 2022. "Regulation Networks of Non-Coding RNA-Associated ceRNAs in Cisplatin-Induced Acute Kidney Injury" Cells 11, no. 19: 2971. https://doi.org/10.3390/cells11192971
APA StyleDing, Y., Wan, S., Liu, W., Lu, Y., Xu, Q., Gan, Y., Yan, L., Gu, Y., Liu, Z., Hu, Y., Cao, H., & Shao, F. (2022). Regulation Networks of Non-Coding RNA-Associated ceRNAs in Cisplatin-Induced Acute Kidney Injury. Cells, 11(19), 2971. https://doi.org/10.3390/cells11192971




