Abstract
In type 2 diabetes, metabolic stress has a negative impact on pancreatic β-cell function and survival (T2D). Although the pathogenesis of metabolic stress is complex, an imbalance in redox homeostasis causes abnormal tissue damage and β-cell death due to low endogenous antioxidant expression levels in β-cells. Under diabetogenic conditions, the susceptibility of β-cells to oxidative damage by NADPH oxidase has been related to contributing to β-cell dysfunction. Here, we consider recent insights into how the redox response becomes deregulated under diabetic conditions by NADPH oxidase, as well as the therapeutic benefits of NOX inhibitors, which may provide clues for understanding the pathomechanisms and developing strategies aimed at the treatment or prevention of metabolic stress associated with β-cell failure.
1. Introduction
Type 2 diabetes (T2D) is a complicated metabolic condition marked by peripheral insulin resistance [1], obesity [1], hyperglycemia [2], and elevated levels of cytokines [3], all of which contribute to a lack of insulin and consequent β-cell failure. Further, considerable evidence has been presented that explains this metabolic dysfunction, including evidence that indicates the role of mitochondrial dysfunction, oxidative stress, endoplasmic reticulum (ER) stress, hyperglycemia (glucotoxicity), dyslipidemia, and the combination of both, glucolipotoxicity [4]. The activation of chronic inflammation is another way that this diabetogenic environment leads to the onset and/or progression of type 2 diabetes. Furthermore, there is strong evidence that suggests that chronically elevated levels of reactive oxygen species (ROS) lead to increased oxidative stress in β-cells. Given the ability of ROS to directly damage and oxidize DNA, proteins, and lipids, β-cell functioning is worsened in terms of insulin secretion and action [5,6]. NADPH oxidase (NOX) proteins are membrane-associated multiunit enzymes that play a physiological role in response to various factors, as well as pathophysiological roles in diabetic pancreatic β-cells. In this review of the current literature, we focus on the role of NOX enzymes in signal transduction in pancreatic β-cells, as well as discuss how these cells might contribute to the development of type 2 diabetes.
2. NADPH Oxidase Isoforms: An Overview
NOX enzymes are multi-subunit enzymes that transfer electrons across biological membranes and are essential for superoxide production. The prototypical NADPH oxidase NOX2, which catalyzes the NADPH-dependent reduction of oxygen to superoxide in phagocytes, was the first enzyme discovered [7]. The phagocytic NOX2 comprises six different subunits that interact to form an active enzyme complex. The catalytic gp91phox and p22phox subunits are located in the plasma membrane, and the regulatory (p47phox, p67phox, p40phox, and small G-protein Rac1/2) subunits are present in the cytosolic components and assembly of all NOX components responsible for the production of superoxide [8,9]. Under resting conditions, the multi-subunit enzyme is inactive. Upon stimulation, the entire cytosolic regulatory complex subsequently translocates to the plasma membrane and associates with catalytic subunits to form the active oxidase, generating superoxide by transferring an electron from NADPH in the cytosol to oxygen on the luminal space [10,11]. In parallel with the progress toward understanding the phagocytic NOX, a series of studies have identified six NOX2 homologs (NOX1, NOX3, NOX4, NOX5, DUOX (dual-oxidase) 1, and DUOX2) in mammalian systems [12,13,14,15]. There are indeed significant similarities and differences between the NOX homologs, for example, subunits that bind NOX1 are NOXO1 (NOX organizer 1) and NOXA1 (NOX activator 1) [16]. Notably, all members of the NOX family contain at least six transmembrane domains, and cytosolic FAD and NADPH-binding domains. However, NOX5 and DUOX1/2 contain extra functional domains that lack NOX1–4. NOX5 contains EF-hand Ca2+-binding domains, whereas DUXO1/2 has a seventh transmembrane domain at the NH2 terminus with an extracellular peroxidase-like domain in addition to the EF-hand and gp91phox homology domains [17,18] (Figure 1).
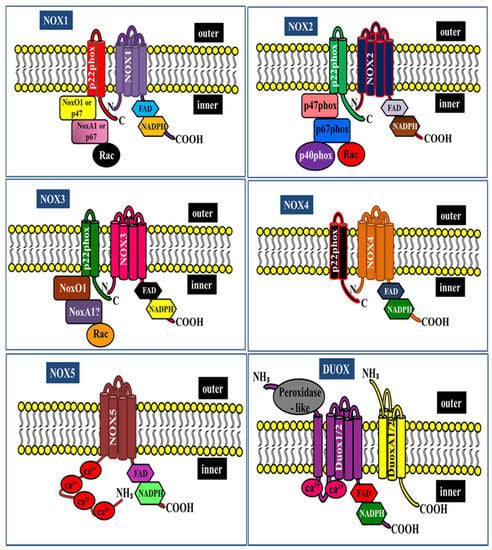
Figure 1.
NADPH oxidase family structure and its subunits.
3. The Role of NADPH Oxidase in Insulin Secretion
Pancreatic β-cells are specialized endocrine cells that secrete insulin to maintain normal fuel homeostasis in response to nutrients, as well as hormonal, neural, and pharmacological factors. Among these, glucose is the most important regulator of insulin secretion via the glycolytic and respiratory metabolism-mediated generation of reactive oxygen species (ROS), leading to accelerated ATP generation in β-cells. The plasmalemmal ATP-sensitive potassium channel (KATP) closes as the ATP:ADP ratio in the cytoplasm rises, resulting in plasma membrane depolarization and the opening of the voltage-gated Ca2+ channel. This causes an influx of Ca2+ into the cell, which causes insulin granules to exocytose [19,20]. Importantly, increasing evidence suggests that brief high-glucose exposure to isolated mouse islets and rat INS-1 (832/13) cells resulted in a significant accumulation of intracellular H2O2 at the same time as increased glucose-stimulated insulin secretion (GSIS) with decreased GSH-to-GSSG ratios. The ratio of GSH to GSSG has long been thought to be a good indication of oxidative stress and redox signaling. As a result, the observed drop in the GSH-to-GSSG ratio in response to glucose stimulation is consistent with the formation of ROS following high glucose, and could indicate the quenching of the H2O2 signal. Furthermore, H2O2-stimulated insulin secretion is a Ca2+-dependent extracellular activity, implying that H2O2 is involved in Ca2+ influx [21,22]. Although mitochondria remain the primary source of free radicals, emerging evidence implicates NOX as a major source of mitochondrial ROS in β-cells. Furthermore, there is considerable evidence regarding the expression of NOX1, NOX2, NOX4, and p22phox, as well as cytosolic regulators p40phox, p47phox, and p67phox, and their homologs Noxo1 and Noxa1 in pancreatic β-cells [23,24,25]. However, the pharmacological inhibition of NOX using diphenyleneiodonium, a non-selective flavoprotein inhibitor, impairs GSIS, which is attributed to the importance of hydrogen peroxide production in beta cells [24,26]. However, this drug also inhibits complex I of the electron transport chain and K+ current via a mechanism that does not involve the inhibition of H2O2 production by a NOX enzyme. In addition, the Ca2+ current might be attributed to poor specificity, thereby misleading the function of NOXs in β-cells [27]. In pancreatic rat islets, it was found that antisense blockage of p47phox subunits markedly reduced the stimulatory glucose-induced H2O2 production and GSIS, thus avoiding the poor specificity of NOX inhibitors [28]. In contrast, the individual deletion of NOX isoforms did not impair glucose-stimulated insulin secretion in mouse islets. Moreover, NOX2-null islets exhibited enhanced glucose-stimulated secretory responses through the reduction in ROS generation. In addition, NOX2-deficient islets showed elevated cAMP levels, suggesting that cAMP potentiates the secretory response. There is evidence regarding the inhibition of p47phox phosphorylation through the cAMP-mediated reduction in superoxide production and via the blocking of the respiratory burst in neutrophils [29]. Phosphodiesterase, the predominant enzyme that selectively hydrolyzes and inactivates cAMP, is present in the pancreatic beta cells and plays a role in insulin secretion [30]. In addition, the inhibition of phosphodiesterase has been shown to reverse the NADPH catalytic subunit gp91phox-mediated, oxidative stress-induced behavioral signature, that is, depression and anxiety by cAMP signaling [31]. However, the downstream mechanism by which NOX2-deficient islets lead to cAMP activation is unknown. With this functional characterization, we suggest that the phosphodiesterase–NOX2 axis may contribute to defects in cAMP activation as well as insulin secretion. Further investigations are needed to clarify these unexpected findings.
4. The Role of NADPH Oxidase in Hyperglycemia-Induced β-Cell Dysfunction
Chronic hyperglycemia has deleterious effects on pancreatic β-cell function and survival in patients with type 2 diabetes (T2D). Hyperglycemia stimulates increased glycolytic flux and the subsequent production of reducing equivalents, leading to the production of ROS, including superoxide, hydrogen peroxide, and hydroxyl radicals [32,33,34]. Recent studies have shown that superoxide radicals generated by NOXs could lead to both the induction of intracellular oxidative stress in pancreatic β-cell damage and apoptosis [25,35,36]. Several pieces of evidence support a key role of RAC1, a small guanosine triphosphate (GTP)-bound protein required for the assembly and catalytic activation of NOX2 [37,38,39]. In addition, RAC1 has long been recognized as a signaling molecule in cytoskeletal dynamics and in a variety of cellular processes, including cell polarization, morphogenesis, migration, apoptosis, vesicle trafficking, and cellular transformation [40]. Furthermore, pancreatic β-cell-specific RAC1 deficiency causes the polymerization of F-actin, which restricts the recruitment of insulin granules at the surface of the plasma membrane, leading to reduced insulin secretion [41]. However, guanine–nucleotide exchange factors facilitate the detachment of guanosine diphosphate, and the binding of GTP may control the conversion of inactive RAC1 to the active RAC1-GTP form [42,43]. Several guanine exchange factors (T-cell lymphoma invasion and metastasis (TIAM1), the oncogene F proto-oncogene (VAV2), and the triple functional domain protein (Trio)) are known to mediate RAC1 activation [44]. Activated RAC1 recruits p67phox, which associates with p47phox and increases superoxide production [45,46]. On the other hand, evidence suggests that TIAM1 triggers RAC1 activation to increase the dysfunction and apoptosis of pancreatic beta cells under high glucose stress via the activation of NOX. Moreover, it should be noted that the activation of JNK signaling lies upstream of mitochondrial dysfunction and caspase-3 activation in response to high glucose [36]. Therefore, it is likely that the inhibition of RAC1 activation reduces NOX-mediated oxidative stress and ROS-mediated JNK signal transduction. An additional consideration is how changes in the balance of antioxidant enzymes, and increases in ROS production by mitochondria, can alter the susceptibility to dysfunction by the activation of stress kinases. In addition, under hyperglycemic conditions, elevated levels of diacylglycerol activate PKC, which subsequently increases the levels of oxidants, such as H2O2 via the PKC-dependent activation of NOX [47]. Furthermore, mice deficient in NOX2 were shown to have attenuated β-cell destruction and preserved islet function in streptozotocin-induced diabetes, partially through the reduction in ROS generation [48]. In contrast, NOX2 deficiency increased GSIS and did not prevent high glucose-induced islet DNA fragmentation and β-cell apoptosis [49]. Alternatively, the pharmacological inhibition of NOX1–4 protected against the high glucose-induced inhibition of human islet insulin release and survival [50,51]. The reason for this inconsistency is unclear. Thus, NOX-induced oxidative stress and mitochondrial dysfunction contribute to impaired endogenous antioxidant defenses, leading to pancreatic β-cell dysfunction in diabetes.
An additional consideration is the upregulation of NOX enzymes in response to chronic hyperglycemia. This activates the angiotensin II type 1 receptor (AT1R) and increases superoxide production, as well as p47phox and p22phox expression in a rat insulin-producing cell line and human pancreatic islets [52,53]. Furthermore, hyperglycemia and angiotensin II type 1 receptor-induced proinflammatory cytokines in human islets cause impaired insulin secretion and inflammation [54,55]. The inhibition of AT1R selectively downregulates NOX; this, in turn, suppresses oxidative stress, thus improving β-cell insulin secretion and decreasing β-cell apoptosis [56]. Indeed, it has been observed that proinflammatory cytokines upregulate NOX1 in β-cells, leading to the loss of function and activation of oxidative stress linked to β-cell failure [57,58]. In addition, pro-inflammatory cytokines induce 12-lipoxygenase (12-LO) expression, which may be a mediator of NOX1-induced β-cell dysfunction by producing 12-hydroxyeicosatetraenoic acid from arachidonic acid. In addition, the inhibition of 12-LO activity blocked the induction of NOX1 by pro-inflammatory cytokines [59,60]. On the other hand, there is increasing evidence that cytokines activate RAC1, which subsequently increases the levels of oxidants via the activation of NOX2. This contributes to alterations in mitochondrial function, leading to caspase-3 activation and the metabolic dysfunction of β-cells. The inhibition of RAC1 activation significantly suppressed the cytokine-induced activation of iNOS and NOX2 activation and the alterations in downstream signaling events involved in cell dysfunction [61,62,63,64]. These results help integrate intracellular events with an elevation of ROS stimulated by pro-inflammatory cytokines, culminating in the onset of islet dysfunction and diabetes. Further investigation is needed to clarify these findings.
5. The Role of NADPH Oxidase in β-Cell Dysfunction Caused by Lipotoxicity
Several studies have shown that elevated levels of glucose, along with circulating free fatty acids (FFAs), constitute the major causes of insulin resistance and β-cell dysfunction during the development of type 2 diabetes [65,66,67,68]. The exposure of β-cells to high glucose induces an increased expression of CD36 in the plasma membrane, and may exacerbate disease aggravation over time by inducing fatty acid uptake, leading to metabolic and functional alterations [69,70]. Interestingly, this effect was mediated by a robust upregulation of the RAC1-mediated activation of NOX activity. Furthermore, the inhibition of RAC1-NOX activation blocked high glucose-induced CD36 expression as well as fatty acid uptake and the downstream signal transduction-mediated cell apoptosis [71]. Moreover, CD36 deficiency attenuates obesity-associated oxidative stress in the heart by reducing NOX activity [72]. Although evidence of direct interaction between NOX and CD36 is still lacking, we found that inhibitors of each target can abrogate the downstream signaling damage in response to high glucose [71,73]. CD36 modulates cellular FFA transfer, and studies with the Zucker diabetic fatty (ZDF) rat, an obesity-induced diabetic animal model, indicate that elevated levels of FFA cause ceramide accumulation, which destroys β-cells by apoptosis. In addition, the increased activation of RAC1 and NOX-associated oxidative stress, in turn, promotes the activation of JNK1/2 and mitochondrial dysregulation in ZDF rats [36]. Based on the existing information, we also found that ceramide-induced β-cell functional defects, through the engagement of Rac1-NOX signaling, coordinates JNK activation, contributing to insulin resistance, obesity, and the production of inflammatory cytokines [73,74,75,76]. However, the mechanism by which the NOX-JNK interlink promotes β-cell damage is not completely understood. It was recently shown that p66Shc mediates lipotoxicity-induced apoptosis in pancreatic β-cells, suggesting that p66Shc could sense the impaired metabolic changes in diabetes and promote cellular dysfunction [77]. p66Shc is a member of the ShcA family of adaptor proteins that consists of three members: p46Shc, p52Shc, and p66Shc, which are derived from the same transcript via an alternative splicing of two distinct ATG start codons. p52/p46Shc contains three phospho-tyrosine sites at Y239, Y240, and Y317. These are located in the CH1 region, enabling Shc proteins to recruit the Grb2-SOS complex that activates the GTPase Ras and the Grb2-Gab2-phosphoinositide-3-kinase (PI3K) complex, which is necessary for transmitting mitogenic and cell survival signals to downstream targets. Furthermore, the longest isoform, p66Shc, possesses an additional N-terminal CH2 region of 110 amino acids, containing an S36 phosphorylation site that has been implicated in mediating oxidative stress signaling [78,79,80]. Earlier studies have suggested that JNK-dependent p66Shc serine36 phosphorylation leads to ROS production and cell death [81]. Based on these results, we found that JNK-dependent p66Shc serine36 phosphorylation leads to ROS production and cell death by ceramide in a NOX-dependent manner [73]. These signaling events subsequently promote the translocation of p66Shc to mitochondria to drive reactive oxygen production and promote the activation of oxidized PRDX3 accumulation in the mitochondria, which would favor MPTP opening and mitochondrial swelling [73]. These observations were further supported by using NOX2 knockout mice, suggesting that NOX2-dependent H2O2 production is a likely cause of early palmitate-dependent impairment in insulin secretion and the induction of β-cell dysfunction [82]. However, experimental data suggest that palmitate triggers transient receptor potential melastatin (TRPM)-2 channels by activating NOX2. The induction of TRPM2 channels caused an increase in mitochondrial Zn2+, leading to mitochondrial membrane potential loss and mitochondrial fission [83]. Further understanding of this link should provide valuable insights for the identification of therapeutic targets to protect β-cell function and prevent T2D. It has also been demonstrated that oxidized LDL induces β-cell dysfunction via reactive oxygen species and radical lipid hydroperoxides [84,85]. However, it is not clear how OX-LDL regulates apoptotic stimuli in beta cells. Importantly, OX-LDL induces oxidative stress in neutrophils by activating NOX via TLR-PKC-IRAK-MAPK [86]. However, the downstream mechanism by which OX-LDL leads to apoptosis is not clear, and this may be because the crosslink between OX-LDL and NOX may determine cellular commitment to apoptosis through ROS production. In addition, ER stress is linked to oxidative stress in oxidized LDL-induced β-cell dysfunction and death [87]. There is also a connection between OX-LDL and ER stress via a robust upregulation of NOX4 in human umbilical vein endothelial cells [88,89,90]. Together, these findings suggest the possibility of a novel pathway involving the ER and mitochondria, through which these organelles orchestrate the regulation of death signals. Further understanding of this link should provide insights for the identification of therapeutic targets to protect β-cell function and prevent T2D (Figure 2).
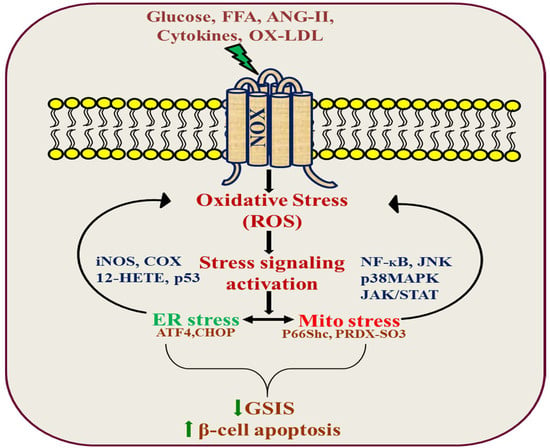
Figure 2.
NADPH oxidase signal transduction in pancreatic β-cell dysfunction.
6. NADPH Oxidase-Targeted Therapeutic Agents
NOX is associated with β-cell dysfunction, apoptosis, insulin resistance, and T2D. Therefore, agents that reduce NOX expression or activity are useful in treating obesity, peripheral insulin resistance, hyperglycemia, and T2D. The chemical inhibitor apocynin (4’-hydroxy-30methoxyacetophenone), a naturally occurring methoxy-substituted catechol, and DP1 diphenyleneiodonium, a non-selective flavoprotein inhibitor, are considered selective NOX inhibitors [24,26,36,48,61]. Importantly, these compounds have off-target effects and are not considered as selective NOX inhibitors because their specificity of action has been questioned in previous reports [91,92]. However, two nontoxic, pyrazolopyridine dione-based, structurally related compounds, GKT136901 and GKT137831, demonstrating a preferential inhibition of NOX1 and NOX4, have been identified and proposed as a novel therapy for NOX inhibition [93,94]. In addition, active phenothiazine (2-acetylphenothiazine) compounds have been shown to inhibit NOX1, and cell-permeable thiotriazolopyrimidine compounds are promising candidates for both exploring the role of NOX subunits in disease pathology, and to assess their inhibitory activity as a new therapeutic approach to disease [95,96,97]. There is a growing body of evidence that metformin ameliorates lipotoxic dysfunction by inhibiting oxidative stress and ER stress mediated by NOX in β-cells, as well as in other experimental diabetes models [98,99,100]. Furthermore, the activation of PPARγ by pioglitazone, a thiazolidinedione class of antidiabetic agents, was shown to inhibit ANG II-induced COX-2 expression, likely by interfering with downregulating ROS production via inhibiting NOX activity [101]. Recently, a novel DPP-4 inhibitor, teneligliptin, was found to exhibit a broad spectrum of bioactivities, alter the pro-inflammatory phenotype of adipocytes, and inhibit atherogenesis by reducing the expression of NOX4, a major NOX subunit in adipocytes [102]. Similarly, we found that teneligliptin treatment suppressed high glucose-mediated β-cell oxidative stress by increasing the stability and activity of SIRT1 [103]. In addition, it has been reported that the upregulation of NOX oxidase subunits p22phox and NOX4 eventually leads to endothelial dysfunction due to superoxide production via SIRT1 inhibition [104]. Plant-derived flavonoids have recently been discovered to have a diverse range of biochemical and pharmacological activities that may have a major impact on the functions of various cellular systems [105,106]. In this context, we have also shown that myricetin (3, 5, 7, 3′, 4′, 5′-hexahydroxyflavone) enhances β-cell function and survival after thapsigargin-induced endoplasmic reticulum stress via NOX inactivation [107]. Based on the results of these studies, we can conclude that compounds that inhibit the NOX subunits show promise as a means of exploring the role of NOX subunits in disease pathology, and that the assessment of their inhibition could potentially lead to the development of novel therapeutics to prevent T2D.
7. Conclusions
There is experimental evidence of the contribution of NOX to pancreatic β-cell dysfunction under diabetogenic conditions. Understanding this complex scenario and the function of NOX-activated redox-regulated pathways may lead to the improved treatment of β-cell failure and T2DM; however, due to low specificity and off-target effects, answers to several key questions remain elusive. Thus, isoform-specific inhibitors, or genetic models in pancreatic β-cells, are required to determine the link between NOX and β-cell failure. This could contribute to the development of novel therapeutics for the prevention and treatment of T2DM.
Author Contributions
Conceptualization, S.E., U.K.; resources, S.E., U.K., J.-S.M.; data curation, S.E., U.K.; writing—original draft preparation, S.E. and U.K.; writing—review and editing, U.K., S.E., J.-S.M. and K.-C.W.; visualization, S.E.; supervision, K.-C.W.; funding acquisition, K.-C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the 2019 Yeungnam University Research Grant and National Research Foundation of Korea (NRF) grants funded by the Korean government (NRF-2020R1A2C1003649 K.-C.W. and 2020R1A2C4002626 J.-S.M.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kahn, S.E. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 2003, 46, 3–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, R.P.; Harmon, J.; Tran, P.O.; Poitout, V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 2004, 53, S119–S124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cnop, M.; Welsh, N.; Jonas, J.C.; Jorns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: Many differences, few similarities. Diabetes 2005, 54, S97–S107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weir, G.C. Glucolipotoxicity, beta-Cells, and Diabetes: The Emperor Has No Clothes. Diabetes 2020, 69, 273–278. [Google Scholar] [CrossRef]
- Prentki, M.; Peyot, M.L.; Masiello, P.; Madiraju, S.R.M. Nutrient-Induced Metabolic Stress, Adaptation, Detoxification, and Toxicity in the Pancreatic beta-Cell. Diabetes 2020, 69, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Cerf, M.E. Beta Cell Physiological Dynamics and Dysfunctional Transitions in Response to Islet Inflammation in Obesity and Diabetes. Metabolites 2020, 10, 452. [Google Scholar] [CrossRef]
- Schrenzel, J.; Serrander, L.; Banfi, B.; Nusse, O.; Fouyouzi, R.; Lew, D.P.; Demaurex, N.; Krause, K.H. Electron currents generated by the human phagocyte NADPH oxidase. Nature 1998, 392, 734–737. [Google Scholar] [CrossRef]
- Babior, B.M.; Lambeth, J.D.; Nauseef, W. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 2002, 397, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Guichard, C.; Pedruzzi, E.; Dewas, C.; Fay, M.; Pouzet, C.; Bens, M.; Vandewalle, A.; Ogier-Denis, E.; Gougerot-Pocidalo, M.A.; Elbim, C. Interleukin-8-induced priming of neutrophil oxidative burst requires sequential recruitment of NADPH oxidase components into lipid rafts. J. Biol. Chem. 2005, 280, 37021–37032. [Google Scholar] [CrossRef] [Green Version]
- Groemping, Y.; Rittinger, K. Activation and assembly of the NADPH oxidase: A structural perspective. Biochem. J. 2005, 386, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Sumimoto, H.; Miyano, K.; Takeya, R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem. Biophys. Res. Commun. 2005, 338, 677–686. [Google Scholar] [CrossRef]
- Brown, D.I.; Griendling, K.K. Nox proteins in signal transduction. Free Radic. Biol. Med. 2009, 47, 1239–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedard, K.; Lardy, B.; Krause, K.H. NOX family NADPH oxidases: Not just in mammals. Biochimie 2007, 89, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, J.D. Nox enzymes, ROS, and chronic disease: An example of antagonistic pleiotropy. Free Radic. Biol. Med. 2007, 43, 332–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banfi, B.; Clark, R.A.; Steger, K.; Krause, K.H. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J. Biol. Chem. 2003, 278, 3510–3513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banfi, B.; Tirone, F.; Durussel, I.; Knisz, J.; Moskwa, P.; Molnar, G.Z.; Krause, K.H.; Cox, J.A. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5). J. Biol. Chem. 2004, 279, 18583–18591. [Google Scholar] [CrossRef] [Green Version]
- Ameziane-El-Hassani, R.; Morand, S.; Boucher, J.L.; Frapart, Y.M.; Apostolou, D.; Agnandji, D.; Gnidehou, S.; Ohayon, R.; Noel-Hudson, M.S.; Francon, J.; et al. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J. Biol. Chem. 2005, 280, 30046–30054. [Google Scholar] [CrossRef] [Green Version]
- Ashcroft, F.M.; Harrison, D.E.; Ashcroft, S.J. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature 1984, 312, 446–448. [Google Scholar] [CrossRef]
- Jitrapakdee, S.; Wutthisathapornchai, A.; Wallace, J.C.; MacDonald, M.J. Regulation of insulin secretion: Role of mitochondrial signalling. Diabetologia 2010, 53, 1019–1032. [Google Scholar] [CrossRef] [Green Version]
- Pi, J.; Bai, Y.; Zhang, Q.; Wong, V.; Floering, L.M.; Daniel, K.; Reece, J.M.; Deeney, J.T.; Andersen, M.E.; Corkey, B.E.; et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 2007, 56, 1783–1791. [Google Scholar] [CrossRef] [Green Version]
- Saadeh, M.; Ferrante, T.C.; Kane, A.; Shirihai, O.; Corkey, B.E.; Deeney, J.T. Reactive oxygen species stimulate insulin secretion in rat pancreatic islets: Studies using mono-oleoyl-glycerol. PLoS ONE 2012, 7, e30200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, H.R.; Verlengia, R.; Carvalho, C.R.; Britto, L.R.; Curi, R.; Carpinelli, A.R. Pancreatic beta-cells express phagocyte-like NAD(P)H oxidase. Diabetes 2003, 52, 1457–1463. [Google Scholar] [CrossRef] [Green Version]
- Uchizono, Y.; Takeya, R.; Iwase, M.; Sasaki, N.; Oku, M.; Imoto, H.; Iida, M.; Sumimoto, H. Expression of isoforms of NADPH oxidase components in rat pancreatic islets. Life Sci. 2006, 80, 133–139. [Google Scholar] [CrossRef]
- Morgan, D.; Oliveira-Emilio, H.R.; Keane, D.; Hirata, A.E.; Santos da Rocha, M.; Bordin, S.; Curi, R.; Newsholme, P.; Carpinelli, A.R. Glucose, palmitate and pro-inflammatory cytokines modulate production and activity of a phagocyte-like NADPH oxidase in rat pancreatic islets and a clonal beta cell line. Diabetologia 2007, 50, 359–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imoto, H.; Sasaki, N.; Iwase, M.; Nakamura, U.; Oku, M.; Sonoki, K.; Uchizono, Y.; Iida, M. Impaired insulin secretion by diphenyleneiodium associated with perturbation of cytosolic Ca2+ dynamics in pancreatic beta-cells. Endocrinology 2008, 149, 5391–5400. [Google Scholar] [CrossRef] [Green Version]
- Weir, E.K.; Wyatt, C.N.; Reeve, H.L.; Huang, J.; Archer, S.L.; Peers, C. Diphenyleneiodonium inhibits both potassium and calcium currents in isolated pulmonary artery smooth muscle cells. J. Appl. Physiol. 1994, 76, 2611–2615. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.; Rebelato, E.; Abdulkader, F.; Graciano, M.F.; Oliveira-Emilio, H.R.; Hirata, A.E.; Rocha, M.S.; Bordin, S.; Curi, R.; Carpinelli, A.R. Association of NAD(P)H oxidase with glucose-induced insulin secretion by pancreatic beta-cells. Endocrinology 2009, 150, 2197–2201. [Google Scholar] [CrossRef]
- Bengis-Garber, C.; Gruener, N. Protein kinase A downregulates the phosphorylation of p47 phox in human neutrophils: A possible pathway for inhibition of the respiratory burst. Cell. Signal. 1996, 8, 291–296. [Google Scholar] [CrossRef]
- Pyne, N.J.; Furman, B.L. Cyclic nucleotide phosphodiesterases in pancreatic islets. Diabetologia 2003, 46, 1179–1189. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Xiaokaiti, Y.; Yang, J.; Pan, J.; Li, Z.; Luria, V.; Li, Y.; Song, G.; Zhu, X.; Zhang, H.T.; et al. Inhibition of phosphodiesterase 2 reverses gp91phox oxidase-mediated depression- and anxiety-like behavior. Neuropharmacology 2018, 143, 176–185. [Google Scholar] [CrossRef]
- Bensellam, M.; Laybutt, D.R.; Jonas, J.C. The molecular mechanisms of pancreatic beta-cell glucotoxicity: Recent findings and future research directions. Mol. Cell. Endocrinol. 2012, 364, 1–27. [Google Scholar] [CrossRef]
- Jonas, J.C.; Bensellam, M.; Duprez, J.; Elouil, H.; Guiot, Y.; Pascal, S.M. Glucose regulation of islet stress responses and beta-cell failure in type 2 diabetes. Diabetes Obes. Metab. 2009, 11, 65–81. [Google Scholar] [CrossRef]
- Tang, C.; Koulajian, K.; Schuiki, I.; Zhang, L.; Desai, T.; Ivovic, A.; Wang, P.; Robson-Doucette, C.; Wheeler, M.B.; Minassian, B.; et al. Glucose-induced beta cell dysfunction in vivo in rats: Link between oxidative stress and endoplasmic reticulum stress. Diabetologia 2012, 55, 1366–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newsholme, P.; Morgan, D.; Rebelato, E.; Oliveira-Emilio, H.C.; Procopio, J.; Curi, R.; Carpinelli, A. Insights into the critical role of NADPH oxidase(s) in the normal and dysregulated pancreatic beta cell. Diabetologia 2009, 52, 2489–2498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syed, I.; Kyathanahalli, C.N.; Jayaram, B.; Govind, S.; Rhodes, C.J.; Kowluru, R.A.; Kowluru, A. Increased phagocyte-like NADPH oxidase and ROS generation in type 2 diabetic ZDF rat and human islets: Role of Rac1-JNK1/2 signaling pathway in mitochondrial dysregulation in the diabetic islet. Diabetes 2011, 60, 2843–2852. [Google Scholar] [CrossRef] [Green Version]
- Abo, A.; Pick, E.; Hall, A.; Totty, N.; Teahan, C.G.; Segal, A.W. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature 1991, 353, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Abo, A.; Webb, M.R.; Grogan, A.; Segal, A.W. Activation of NADPH oxidase involves the dissociation of p21rac from its inhibitory GDP/GTP exchange protein (rhoGDI) followed by its translocation to the plasma membrane. Biochem. J. 1994, 298, 585–591. [Google Scholar] [CrossRef] [Green Version]
- Sarfstein, R.; Gorzalczany, Y.; Mizrahi, A.; Berdichevsky, Y.; Molshanski-Mor, S.; Weinbaum, C.; Hirshberg, M.; Dagher, M.C.; Pick, E. Dual role of Rac in the assembly of NADPH oxidase, tethering to the membrane and activation of p67phox: A study based on mutagenesis of p67phox-Rac1 chimeras. J. Biol. Chem. 2004, 279, 16007–16016. [Google Scholar] [CrossRef] [Green Version]
- Chi, X.; Wang, S.; Huang, Y.; Stamnes, M.; Chen, J.L. Roles of rho GTPases in intracellular transport and cellular transformation. Int. J. Mol. Sci. 2013, 14, 7089–7108. [Google Scholar] [CrossRef]
- Asahara, S.; Shibutani, Y.; Teruyama, K.; Inoue, H.Y.; Kawada, Y.; Etoh, H.; Matsuda, T.; Kimura-Koyanagi, M.; Hashimoto, N.; Sakahara, M.; et al. Ras-related C3 botulinum toxin substrate 1 (RAC1) regulates glucose-stimulated insulin secretion via modulation of F-actin. Diabetologia 2013, 56, 1088–1097. [Google Scholar] [CrossRef] [Green Version]
- Marei, H.; Carpy, A.; Macek, B.; Malliri, A. Proteomic analysis of Rac1 signaling regulation by guanine nucleotide exchange factors. Cell Cycle 2016, 15, 1961–1974. [Google Scholar] [CrossRef] [Green Version]
- Marei, H.; Malliri, A. GEFs: Dual regulation of Rac1 signaling. Small GTPases 2017, 8, 90–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowluru, A. Small G proteins in islet beta-cell function. Endocr. Rev. 2010, 31, 52–78. [Google Scholar] [CrossRef] [Green Version]
- Dang, P.M.; Cross, A.R.; Babior, B.M. Assembly of the neutrophil respiratory burst oxidase: A direct interaction between p67PHOX and cytochrome b558. Proc. Natl. Acad. Sci. USA 2001, 98, 3001–3005. [Google Scholar] [CrossRef] [Green Version]
- Dang, P.M.; Johnson, J.L.; Babior, B.M. Binding of nicotinamide adenine dinucleotide phosphate to the tetratricopeptide repeat domains at the N-terminus of p67PHOX, a subunit of the leukocyte nicotinamide adenine dinucleotide phosphate oxidase. Biochemistry 2000, 39, 3069–3075. [Google Scholar] [CrossRef]
- Inoguchi, T.; Li, P.; Umeda, F.; Yu, H.Y.; Kakimoto, M.; Imamura, M.; Aoki, T.; Etoh, T.; Hashimoto, T.; Naruse, M.; et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C—Dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 2000, 49, 1939–1945. [Google Scholar] [CrossRef] [Green Version]
- Xiang, F.L.; Lu, X.; Strutt, B.; Hill, D.J.; Feng, Q. NOX2 deficiency protects against streptozotocin-induced beta-cell destruction and development of diabetes in mice. Diabetes 2010, 59, 2603–2611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, A.H.; Santos, L.R.B.; Roma, L.P.; Bensellam, M.; Carpinelli, A.R.; Jonas, J.C. NADPH oxidase-2 does not contribute to beta-cell glucotoxicity in cultured pancreatic islets from C57BL/6J mice. Mol. Cell. Endocrinol. 2017, 439, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Anvari, E.; Wikstrom, P.; Walum, E.; Welsh, N. The novel NADPH oxidase 4 inhibitor GLX351322 counteracts glucose intolerance in high-fat diet-treated C57BL/6 mice. Free Radic. Res. 2015, 49, 1308–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Elksnis, A.; Wikstrom, P.; Walum, E.; Welsh, N.; Carlsson, P.O. The novel NADPH oxidase 4 selective inhibitor GLX7013114 counteracts human islet cell death in vitro. PLoS ONE 2018, 13, e0204271. [Google Scholar] [CrossRef]
- Lastra, G.; Manrique, C. The expanding role of oxidative stress, renin angiotensin system, and beta-cell dysfunction in the cardiometabolic syndrome and Type 2 diabetes mellitus. Antioxid. Redox Signal. 2007, 9, 943–954. [Google Scholar] [CrossRef]
- Lupi, R.; Del Guerra, S.; Bugliani, M.; Boggi, U.; Mosca, F.; Torri, S.; Del Prato, S.; Marchetti, P. The direct effects of the angiotensin-converting enzyme inhibitors, zofenoprilat and enalaprilat, on isolated human pancreatic islets. Eur. J. Endocrinol. 2006, 154, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.Y.; Lau, T.; Carlsson, P.O.; Leung, P.S. Angiotensin II type 1 receptor blockade improves beta-cell function and glucose tolerance in a mouse model of type 2 diabetes. Diabetes 2006, 55, 367–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauter, N.S.; Thienel, C.; Plutino, Y.; Kampe, K.; Dror, E.; Traub, S.; Timper, K.; Bedat, B.; Pattou, F.; Kerr-Conte, J.; et al. Angiotensin II induces interleukin-1beta-mediated islet inflammation and beta-cell dysfunction independently of vasoconstrictive effects. Diabetes 2015, 64, 1273–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, K.Y.; Leung, P.S. Angiotensin II Type 1 receptor antagonism mediates uncoupling protein 2-driven oxidative stress and ameliorates pancreatic islet beta-cell function in young Type 2 diabetic mice. Antioxid. Redox Signal. 2007, 9, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.R.; Grzesik, W.; Taylor-Fishwick, D.A. Inhibition of NADPH oxidase-1 preserves beta cell function. Diabetologia 2015, 58, 113–121. [Google Scholar] [CrossRef]
- Weaver, J.R.; Taylor-Fishwick, D.A. Regulation of NOX-1 expression in beta cells: A positive feedback loop involving the Src-kinase signaling pathway. Mol. Cell. Endocrinol. 2013, 369, 35–41. [Google Scholar] [CrossRef]
- Weaver, J.; Taylor-Fishwick, D.A. Relationship of NADPH Oxidase-1 expression to beta cell dysfunction induced by inflammatory cytokines. Biochem. Biophys. Res. Commun. 2017, 485, 290–294. [Google Scholar] [CrossRef]
- Weaver, J.R.; Holman, T.R.; Imai, Y.; Jadhav, A.; Kenyon, V.; Maloney, D.J.; Nadler, J.L.; Rai, G.; Simeonov, A.; Taylor-Fishwick, D.A. Integration of pro-inflammatory cytokines, 12-lipoxygenase and NOX-1 in pancreatic islet beta cell dysfunction. Mol. Cell. Endocrinol. 2012, 358, 88–95. [Google Scholar] [CrossRef]
- Kowluru, A. Oxidative Stress in Cytokine-Induced Dysfunction of the Pancreatic Beta Cell: Known Knowns and Known Unknowns. Metabolites 2020, 10, 480. [Google Scholar] [CrossRef]
- Michalska, M.; Wolf, G.; Walther, R.; Newsholme, P. Effects of pharmacological inhibition of NADPH oxidase or iNOS on pro-inflammatory cytokine, palmitic acid or H2O2-induced mouse islet or clonal pancreatic beta-cell dysfunction. Biosci. Rep. 2010, 30, 445–453. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.M.; Syeda, K.; Hadden, T.; Kowluru, A. Upregulation of phagocyte-like NADPH oxidase by cytokines in pancreatic beta-cells: Attenuation of oxidative and nitrosative stress by 2-bromopalmitate. Biochem. Pharmacol. 2013, 85, 109–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subasinghe, W.; Syed, I.; Kowluru, A. Phagocyte-like NADPH oxidase promotes cytokine-induced mitochondrial dysfunction in pancreatic beta-cells: Evidence for regulation by Rac1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R12–R20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briaud, I.; Harmon, J.S.; Kelpe, C.L.; Segu, V.B.; Poitout, V. Lipotoxicity of the pancreatic beta-cell is associated with glucose-dependent esterification of fatty acids into neutral lipids. Diabetes 2001, 50, 315–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haber, E.P.; Ximenes, H.M.; Procopio, J.; Carvalho, C.R.; Curi, R.; Carpinelli, A.R. Pleiotropic effects of fatty acids on pancreatic beta-cells. J. Cell. Physiol. 2003, 194, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Prentki, M.; Nolan, C.J. Islet beta cell failure in type 2 diabetes. J. Clin. Investig. 2006, 116, 1802–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unger, R.H.; Zhou, Y.T. Lipotoxicity of beta-cells in obesity and in other causes of fatty acid spillover. Diabetes 2001, 50, S118–S121. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.W.; Moon, J.S.; Seo, Y.J.; Park, S.Y.; Kim, J.Y.; Yoon, J.S.; Lee, I.K.; Lee, H.W.; Won, K.C. Inhibition of fatty acid translocase cluster determinant 36 (CD36), stimulated by hyperglycemia, prevents glucotoxicity in INS-1 cells. Biochem. Biophys. Res. Commun. 2012, 420, 462–466. [Google Scholar] [CrossRef]
- Wallin, T.; Ma, Z.; Ogata, H.; Jorgensen, I.H.; Iezzi, M.; Wang, H.; Wollheim, C.B.; Bjorklund, A. Facilitation of fatty acid uptake by CD36 in insulin-producing cells reduces fatty-acid-induced insulin secretion and glucose regulation of fatty acid oxidation. Biochim. Biophys. Acta 2010, 1801, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Elumalai, S.; Karunakaran, U.; Lee, I.K.; Moon, J.S.; Won, K.C. Rac1-NADPH oxidase signaling promotes CD36 activation under glucotoxic conditions in pancreatic beta cells. Redox Biol. 2017, 11, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Gharib, M.; Tao, H.; Fungwe, T.V.; Hajri, T. Cluster Differentiating 36 (CD36) Deficiency Attenuates Obesity-Associated Oxidative Stress in the Heart. PLoS ONE 2016, 11, e0155611. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, U.; Elumalai, S.; Moon, J.S.; Won, K.C. CD36 dependent redoxosomes promotes ceramide-mediated pancreatic beta-cell failure via p66Shc activation. Free Radic. Biol. Med. 2019, 134, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Gorgun, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef]
- Holzer, R.G.; Park, E.J.; Li, N.; Tran, H.; Chen, M.; Choi, C.; Solinas, G.; Karin, M. Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell 2011, 147, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Kant, S.; Standen, C.L.; Morel, C.; Jung, D.Y.; Kim, J.K.; Swat, W.; Flavell, R.A.; Davis, R.J. A Protein Scaffold Coordinates SRC-Mediated JNK Activation in Response to Metabolic Stress. Cell Rep. 2017, 20, 2775–2783. [Google Scholar] [CrossRef] [Green Version]
- Natalicchio, A.; Tortosa, F.; Labarbuta, R.; Biondi, G.; Marrano, N.; Carchia, E.; Leonardini, A.; Cignarelli, A.; Bugliani, M.; Marchetti, P.; et al. The p66(Shc) redox adaptor protein is induced by saturated fatty acids and mediates lipotoxicity-induced apoptosis in pancreatic beta cells. Diabetologia 2015, 58, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Bonfini, L.; Migliaccio, E.; Pelicci, G.; Lanfrancone, L.; Pelicci, P.G. Not all Shc’s roads lead to Ras. Trends Biochem. Sci. 1996, 21, 257–261. [Google Scholar] [CrossRef]
- Ravichandran, K.S. Signaling via Shc family adapter proteins. Oncogene 2001, 20, 6322–6330. [Google Scholar] [CrossRef] [Green Version]
- van der Geer, P.; Wiley, S.; Gish, G.D.; Pawson, T. The Shc adaptor protein is highly phosphorylated at conserved, twin tyrosine residues (Y239/240) that mediate protein-protein interactions. Curr. Biol. 1996, 6, 1435–1444. [Google Scholar] [CrossRef] [Green Version]
- Khalid, S.; Drasche, A.; Thurner, M.; Hermann, M.; Ashraf, M.I.; Fresser, F.; Baier, G.; Kremser, L.; Lindner, H.; Troppmair, J. cJun N-terminal kinase (JNK) phosphorylation of serine 36 is critical for p66Shc activation. Sci. Rep. 2016, 6, 20930. [Google Scholar] [CrossRef] [Green Version]
- Vilas-Boas, E.A.; Nalbach, L.; Ampofo, E.; Lucena, C.F.; Naudet, L.; Ortis, F.; Carpinelli, A.R.; Morgan, B.; Roma, L.P. Transient NADPH oxidase 2-dependent H2O2 production drives early palmitate-induced lipotoxicity in pancreatic islets. Free Radic. Biol. Med. 2021, 162, 1–13. [Google Scholar] [CrossRef]
- Li, F.; Munsey, T.S.; Sivaprasadarao, A. TRPM2-mediated rise in mitochondrial Zn(2+) promotes palmitate-induced mitochondrial fission and pancreatic beta-cell death in rodents. Cell Death Differ. 2017, 24, 1999–2012. [Google Scholar] [CrossRef] [Green Version]
- Cnop, M.; Hannaert, J.C.; Grupping, A.Y.; Pipeleers, D.G. Low density lipoprotein can cause death of islet beta-cells by its cellular uptake and oxidative modification. Endocrinology 2002, 143, 3449–3453. [Google Scholar] [CrossRef] [Green Version]
- Grupping, A.Y.; Cnop, M.; Van Schravendijk, C.F.; Hannaert, J.C.; Van Berkel, T.J.; Pipeleers, D.G. Low density lipoprotein binding and uptake by human and rat islet beta cells. Endocrinology 1997, 138, 4064–4068. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, D.; Nagarkoti, S.; Kumar, A.; Dubey, M.; Singh, A.K.; Pathak, P.; Chandra, T.; Barthwal, M.K.; Dikshit, M. Oxidized LDL induced extracellular trap formation in human neutrophils via TLR-PKC-IRAK-MAPK and NADPH-oxidase activation. Free Radic. Biol. Med. 2016, 93, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Plaisance, V.; Brajkovic, S.; Tenenbaum, M.; Favre, D.; Ezanno, H.; Bonnefond, A.; Bonner, C.; Gmyr, V.; Kerr-Conte, J.; Gauthier, B.R.; et al. Endoplasmic Reticulum Stress Links Oxidative Stress to Impaired Pancreatic Beta-Cell Function Caused by Human Oxidized LDL. PLoS ONE 2016, 11, e0163046. [Google Scholar] [CrossRef] [Green Version]
- Honjo, T.; Otsui, K.; Shiraki, R.; Kawashima, S.; Sawamura, T.; Yokoyama, M.; Inoue, N. Essential role of NOXA1 in generation of reactive oxygen species induced by oxidized low-density lipoprotein in human vascular endothelial cells. Endothelium 2008, 15, 137–141. [Google Scholar] [CrossRef]
- Li, M.; Dou, L.; Jiao, J.; Lu, Y.; Guo, H.B.; Man, Y.; Wang, S.; Li, J. NADPH oxidase 2-derived reactive oxygen species are involved in dysfunction and apoptosis of pancreatic beta-cells induced by low density lipoprotein. Cell. Physiol. Biochem. 2012, 30, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Bai, Y.P.; Gao, H.C.; Wang, X.; Li, L.F.; Zhang, G.G.; Hu, C.P. Ox-LDL induces endothelial cell apoptosis via the LOX-1-dependent endoplasmic reticulum stress pathway. Atherosclerosis 2014, 235, 310–317. [Google Scholar] [CrossRef]
- Riganti, C.; Gazzano, E.; Polimeni, M.; Costamagna, C.; Bosia, A.; Ghigo, D. Diphenyleneiodonium inhibits the cell redox metabolism and induces oxidative stress. J. Biol. Chem. 2004, 279, 47726–47731. [Google Scholar] [CrossRef] [Green Version]
- Vejrazka, M.; Micek, R.; Stipek, S. Apocynin inhibits NADPH oxidase in phagocytes but stimulates ROS production in non-phagocytic cells. Biochim. Biophys. Acta 2005, 1722, 143–147. [Google Scholar] [CrossRef]
- Schildknecht, S.; Weber, A.; Gerding, H.R.; Pape, R.; Robotta, M.; Drescher, M.; Marquardt, A.; Daiber, A.; Ferger, B.; Leist, M. The NOX1/4 inhibitor GKT136901 as selective and direct scavenger of peroxynitrite. Curr. Med. Chem. 2014, 21, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, G.; Szyndralewiez, C.; Molango, S.; Carnesecchi, S.; Heitz, F.; Wiesel, P.; Wood, J.M. Therapeutic potential of NADPH oxidase 1/4 inhibitors. Br. J. Pharmacol. 2017, 174, 1647–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augsburger, F.; Filippova, A.; Rasti, D.; Seredenina, T.; Lam, M.; Maghzal, G.; Mahiout, Z.; Jansen-Durr, P.; Knaus, U.G.; Doroshow, J.; et al. Pharmacological characterization of the seven human NOX isoforms and their inhibitors. Redox Biol. 2019, 26, 101272. [Google Scholar] [CrossRef]
- Dao, V.T.; Elbatreek, M.H.; Altenhofer, S.; Casas, A.I.; Pachado, M.P.; Neullens, C.T.; Knaus, U.G.; Schmidt, H. Isoform-selective NADPH oxidase inhibitor panel for pharmacological target validation. Free Radic. Biol. Med. 2020, 148, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Massari, M.; Marchese, S.; Ceccon, M.; Aalbers, F.S.; Corana, F.; Valente, S.; Mai, A.; Magnani, F.; Mattevi, A. A closer look into NADPH oxidase inhibitors: Validation and insight into their mechanism of action. Redox Biol. 2020, 32, 101466. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Lee, J.S.; Kwak, B.K.; Hwang, W.M.; Kim, M.J.; Kim, Y.B.; Chung, S.S.; Park, K.S. Metformin Ameliorates Lipotoxic beta-Cell Dysfunction through a Concentration-Dependent Dual Mechanism of Action. Diabetes Metab. J. 2019, 43, 854–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Hua, N.; Fu, X.; Pan, Y.; Li, B.; Li, X. Metformin Regulates the Expression of SK2 and SK3 in the Atria of Rats With Type 2 Diabetes Mellitus Through the NOX4/p38MAPK Signaling Pathway. J. Cardiovasc. Pharmacol. 2018, 72, 205–213. [Google Scholar] [CrossRef]
- Takeno, A.; Kanazawa, I.; Tanaka, K.; Notsu, M.; Yokomoto, M.; Yamaguchi, T.; Sugimoto, T. Activation of AMP-activated protein kinase protects against homocysteine-induced apoptosis of osteocytic MLO-Y4 cells by regulating the expressions of NADPH oxidase 1 (Nox1) and Nox2. Bone 2015, 77, 135–141. [Google Scholar] [CrossRef]
- Palacios-Ramirez, R.; Hernanz, R.; Martin, A.; Perez-Giron, J.V.; Barrus, M.T.; Gonzalez-Carnicero, Z.; Aguado, A.; Jaisser, F.; Briones, A.M.; Salaices, M.; et al. Pioglitazone Modulates the Vascular Contractility in Hypertension by Interference with ET-1 Pathway. Sci. Rep. 2019, 9, 16461. [Google Scholar] [CrossRef] [Green Version]
- Salim, H.M.; Fukuda, D.; Higashikuni, Y.; Tanaka, K.; Hirata, Y.; Yagi, S.; Soeki, T.; Shimabukuro, M.; Sata, M. Teneligliptin, a dipeptidyl peptidase-4 inhibitor, attenuated pro-inflammatory phenotype of perivascular adipose tissue and inhibited atherogenesis in normoglycemic apolipoprotein-E-deficient mice. Vascul. Pharmacol. 2017, 96–98, 19–25. [Google Scholar] [CrossRef]
- Elumalai, S.; Karunakaran, U.; Moon, J.S.; Won, K.C. High glucose-induced PRDX3 acetylation contributes to glucotoxicity in pancreatic beta-cells: Prevention by Teneligliptin. Free Radic. Biol. Med. 2020, 160, 618–629. [Google Scholar] [CrossRef]
- Wosniak, J., Jr.; Santos, C.X.; Kowaltowski, A.J.; Laurindo, F.R. Cross-talk between mitochondria and NADPH oxidase: Effects of mild mitochondrial dysfunction on angiotensin II-mediated increase in Nox isoform expression and activity in vascular smooth muscle cells. Antioxid. Redox Signal. 2009, 11, 1265–1278. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousefian, M.; Shakour, N.; Hosseinzadeh, H.; Hayes, A.W.; Hadizadeh, F.; Karimi, G. The natural phenolic compounds as modulators of NADPH oxidases in hypertension. Phytomedicine 2019, 55, 200–213. [Google Scholar] [CrossRef]
- Karunakaran, U.; Lee, J.E.; Elumalai, S.; Moon, J.S.; Won, K.C. Myricetin prevents thapsigargin-induced CDK5-P66Shc signalosome mediated pancreatic beta-cell dysfunction. Free Radic. Biol. Med. 2019, 141, 59–66. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).