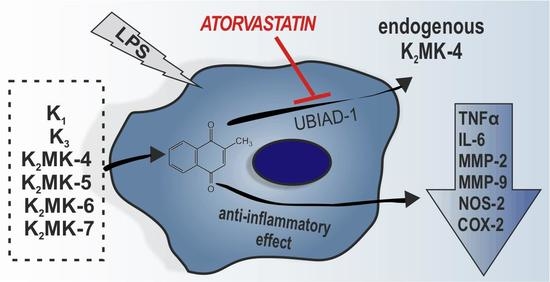
Exogenous Vitamins K Exert Anti-Inflammatory Effects Dissociated from Their Role as Substrates for Synthesis of Endogenous MK-4 in Murine Macrophages Cell Line
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. Synthesis of Various Forms of Vitamins K
2.3. Anti-Inflammatory Effect of Vitamins K in RAW 264.7 Cells
2.4. Measurements of Cytokines Production by RAW 264.7 Cells
2.5. Measurements of NO Production in RAW 264.7 Cells
2.6. UHPLC-MS/MS Measurements of Eicosanoid Production by RAW 264.7 Cells
2.7. Measurements of Expression of NOS-2, COX-2 and MMPs in RAW 264.7 Cells
2.8. Measurements of Mitochondrial Function in RAW 264.7 Cells
2.9. Measurements of Endogenous Content of Vitamins K in RAW 264.7 Cells
2.10. UHPLC-MS/MS Quantification of Vitamins K
2.11. Statistical Analysis
3. Results
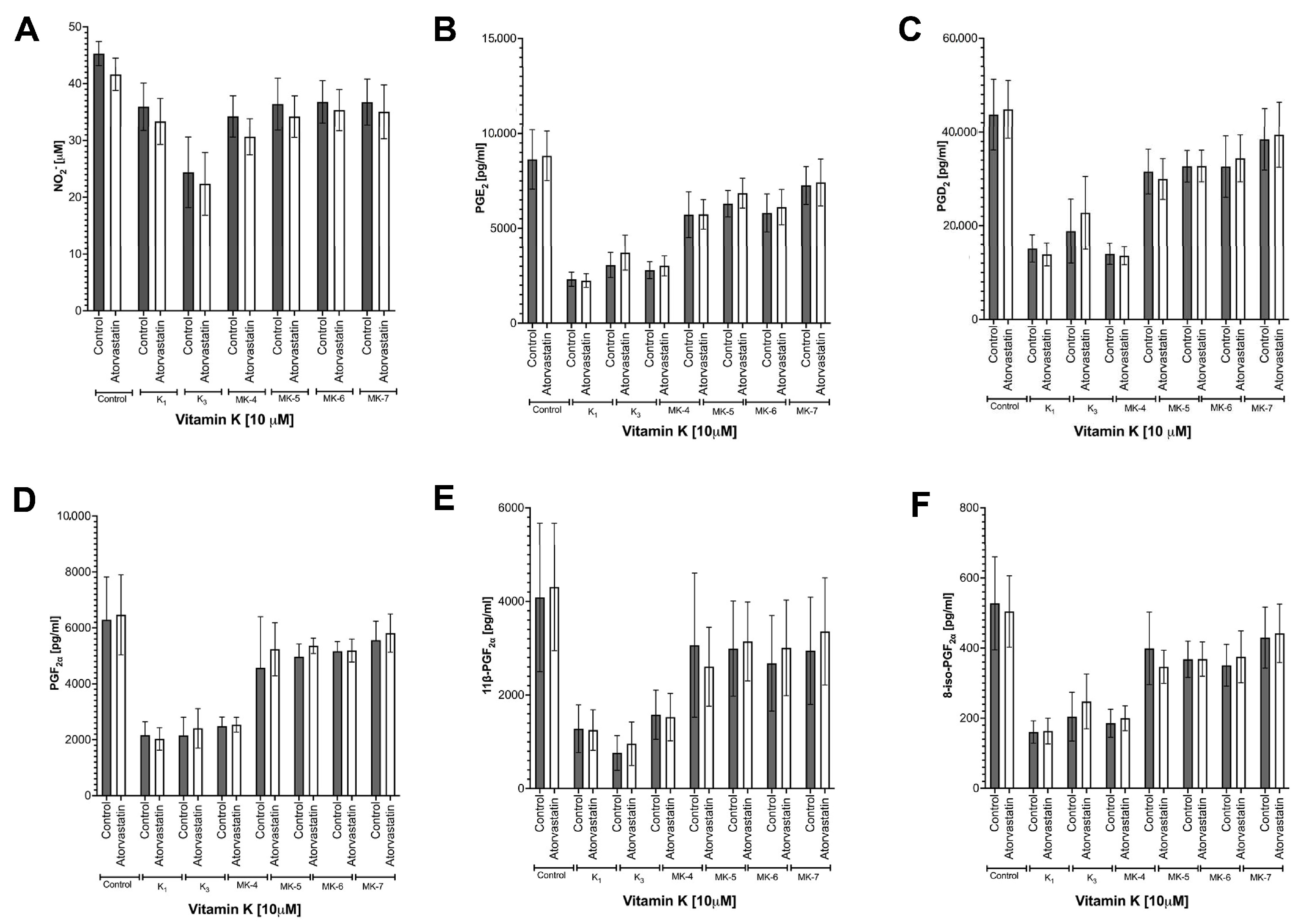
3.1. Effects of Vitamins K on LPS-Induced NO Production and Induction of NOS-2 in RAW 264.7 Cells
3.2. Effects of Vitamins K on LPS-Induced Cytokines Production in RAW 264.7 Cells
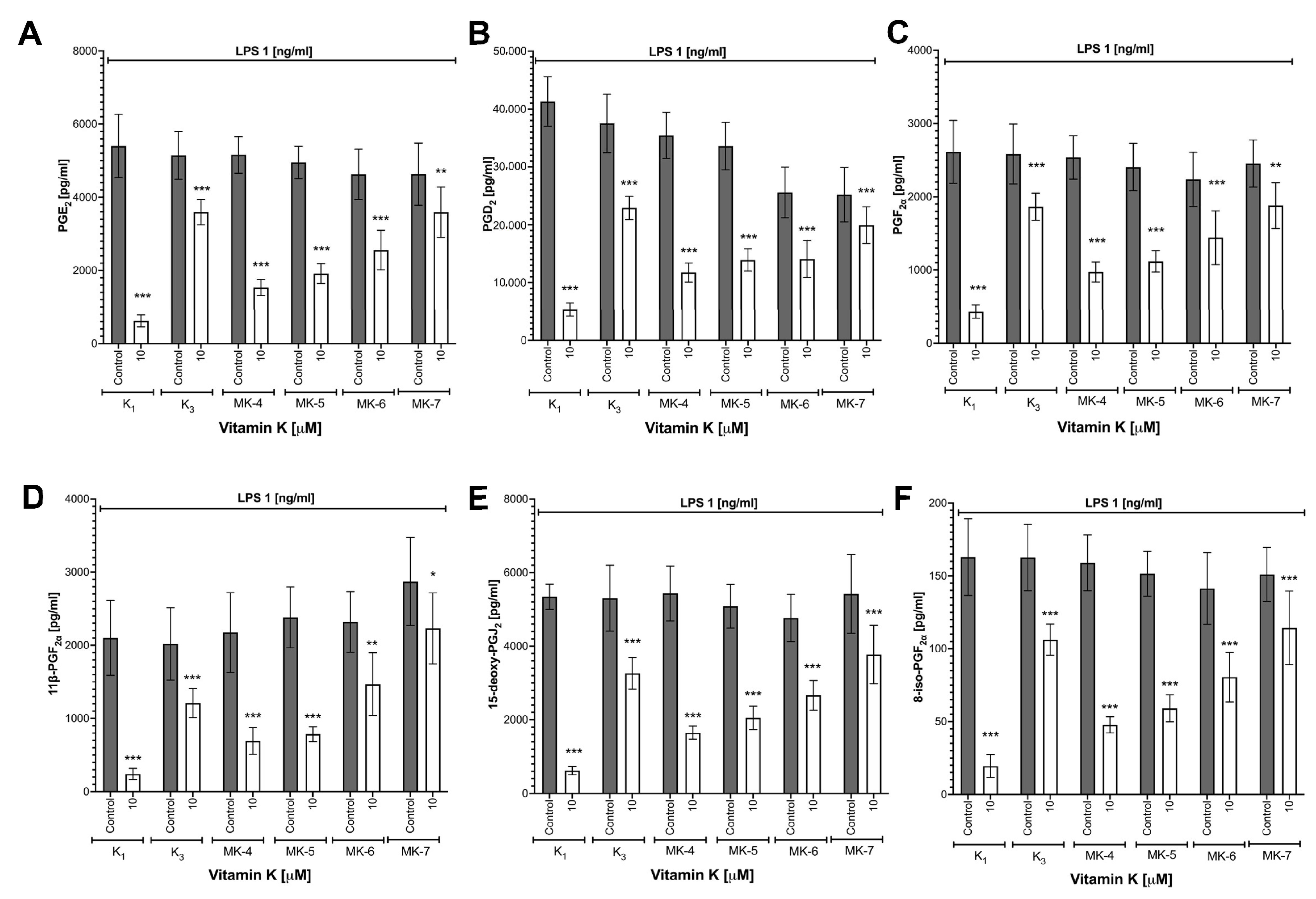
3.3. Effects of Vitamins K on LPS-Induced Eicosanoid Production and Induction of COX-2 in RAW 264.7 Cells
3.4. Effects of Vitamins K on LPS-Induced MMP-2 and MMP-9 Expression in RAW 264.7 Cells
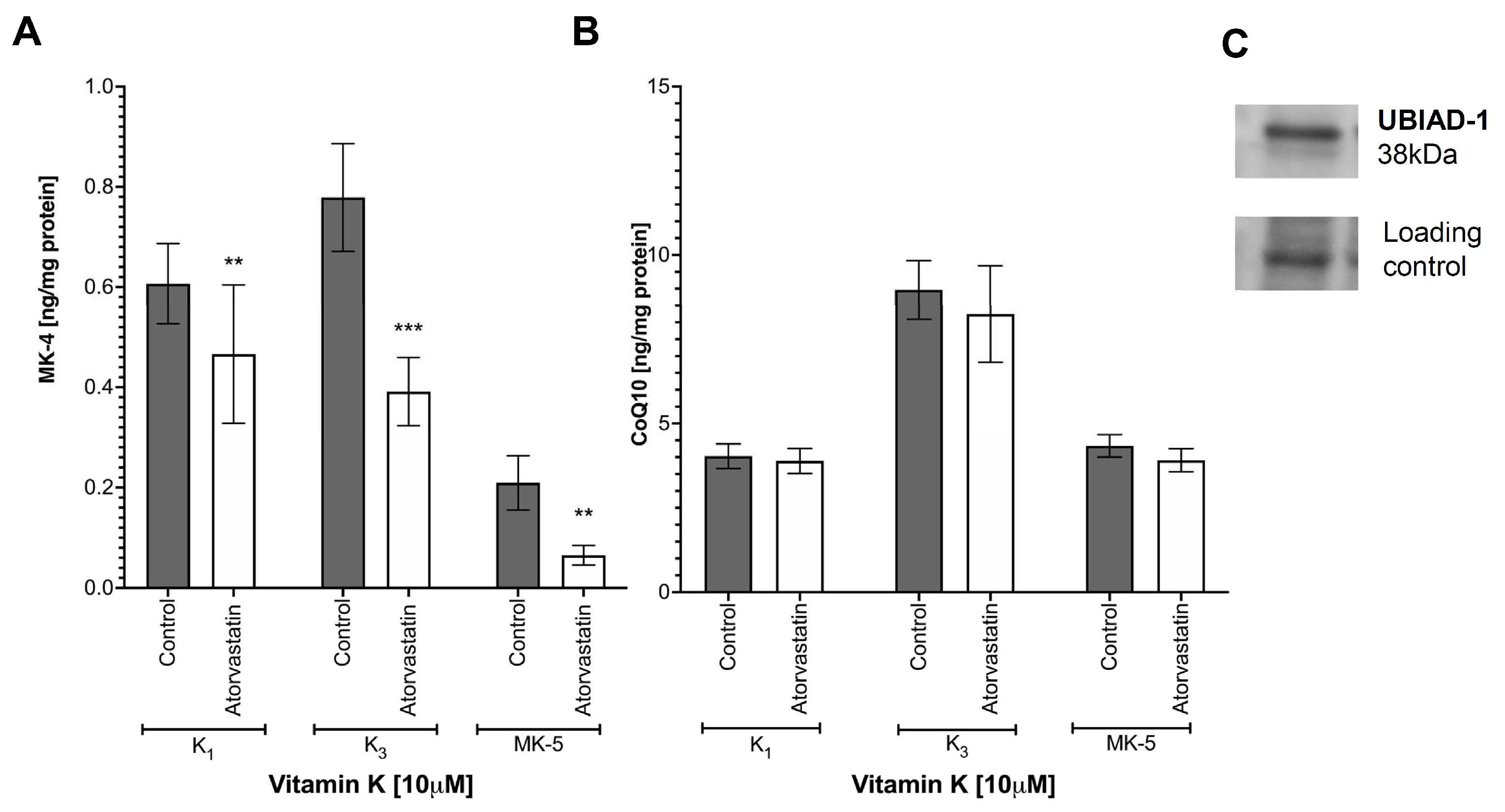
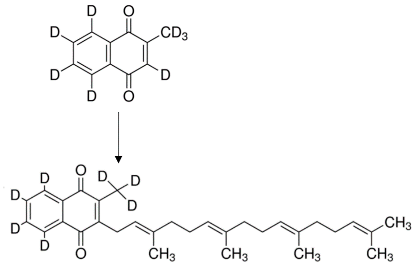
3.5. Synthesis of Endogenous MK-4 from Exogenous Vitamins K
3.6. Effects of Atorvastatin on Endogenous Production of MK-4 from Exogenous Vitamins K and on Anti-Inflammatory Effect of Vitamins K
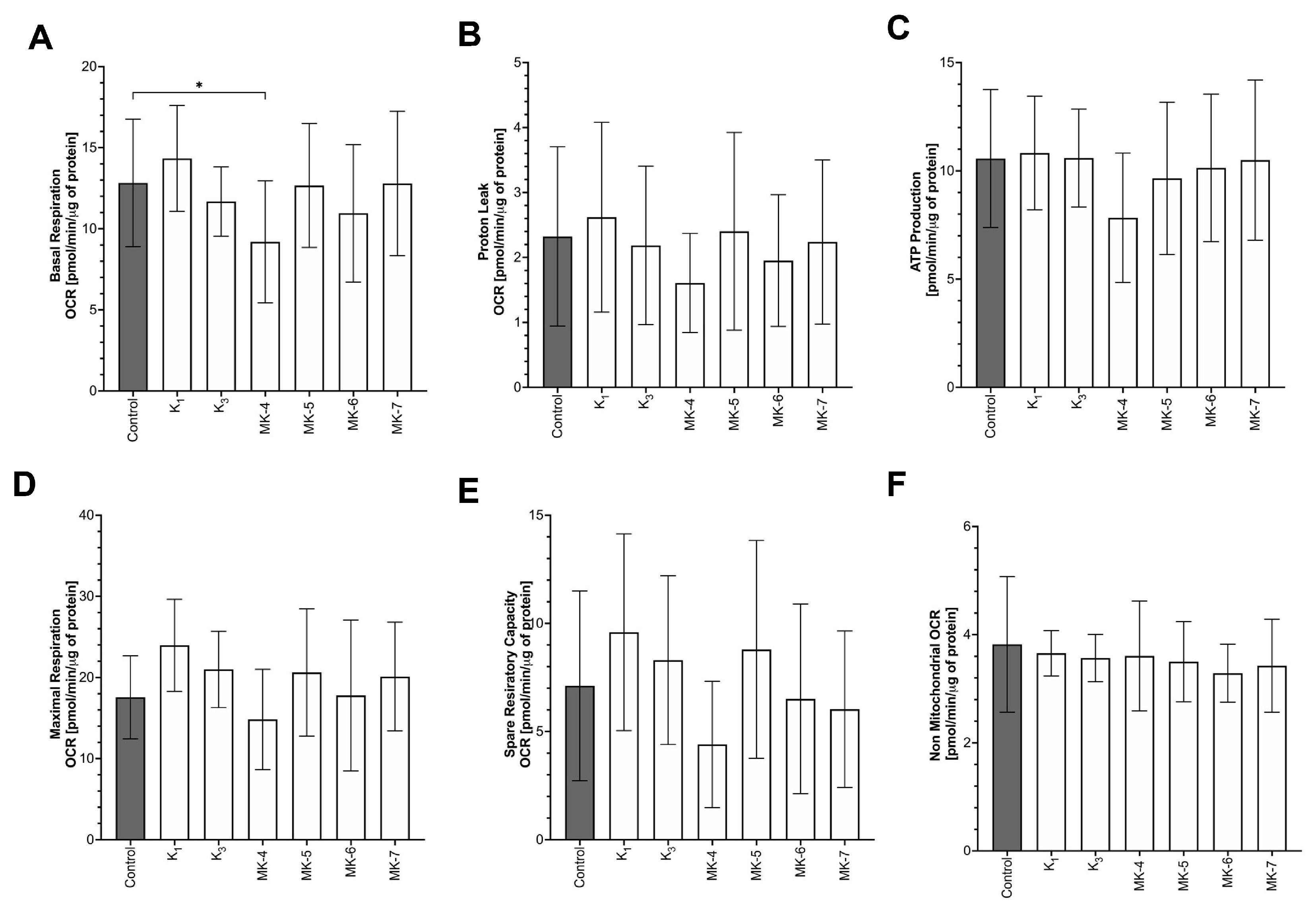
3.7. Effects of Vitamins K on Mitochondrial Respiration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferland, G. Vitamins K, an emerging nutrient in brain function. BioFactors 2012, 38, 151–157. [Google Scholar] [CrossRef]
- Tie, J.-K.; Jin, D.-Y.; Straight, D.L.; Stafford, D.W. Functional study of the vitamins K cycle in mammalian cells. Blood 2011, 117, 2967–2974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, L.; Feil, S.; Wolters, M.; Thunemann, M.; Regler, F.; Schmidt, K.; Friebe, A.; Olbrich, M.; Langer, H.; Gawaz, M.; et al. A shear-dependent NO-cGMP-cGKI cascade in platelets acts as an auto-regulatory brake of thrombosis. Nat. Commun. 2018, 1–11. [Google Scholar] [CrossRef]
- Booth, S.L. Roles for vitamins K beyond coagulation. Annu. Rev. Nutr. 2009, 29, 89–110. [Google Scholar] [CrossRef] [PubMed]
- Schwalfenberg, G.K. Vitamins K and K2: The Emerging Group of Vitamins Required for Human Health. J. Nutr. Metab. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M.J.; Fu, X.; Booth, S.L. Vitamins K nutrition, metabolism, and requirements: Current concepts and future research. Adv. Nutr. 2012, 3, 182–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shearer, M.J.; Newman, P. Metabolism and cell biology of vitamins K. Thromb. Haemost. 2008. [Google Scholar] [CrossRef]
- Siltari, A.; Vapaatalo, H. Vascular Calcification, Vitamins K and Warfarin Therapy-Possible or Plausible Connection? Basic Clin. Pharmacol. Toxicol. 2018, 122, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Shea, M.K.; Booth, S.L.; Massaro, J.M.; Jacques, P.F.; D’Agostino, R.B.; Dawson-Hughes, B.; Ordovas, J.M.; O’Donnell, C.J.; Kathiresan, S.; Keaney, J.F.; et al. Vitamins K and vitamin D status: Associations with inflammatory markers in the Framingham offspring study. Am. J. Epidemiol. 2008. [Google Scholar] [CrossRef] [Green Version]
- Pan, M.H.; Maresz, K.; Lee, P.S.; Wu, J.C.; Ho, C.T.; Popko, J.; Mehta, D.S.; Stohs, S.J.; Badmaev, V. Inhibition of TNF-α, IL-1α, and IL-1β by Pretreatment of Human Monocyte-Derived Macrophages with Menaquinone-7 and Cell Activation with TLR Agonists in Vitro. J. Med. Food 2016, 19, 663–669. [Google Scholar] [CrossRef]
- Cavaco, S.; Viegas, C.S.B.; Rafael, M.S.; Ramos, A.; Magalhães, J.; Blanco, F.J.; Vermeer, C.; Simes, D.C. Gla-rich protein is involved in the cross-talk between calcification and inflammation in osteoarthritis. Cell. Mol. Life Sci. 2016, 73, 1051–1065. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, Y.; Shirakawa, H.; Hiwatashi, K.; Furukawa, Y.; Mizutani, T.; Komai, M. Vitamins K suppresses lipopolysaccharide-induced inflammation in the rat. Biosci. Biotechnol. Biochem. 2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohsaki, Y.; Shirakawa, H.; Miura, A.; Giriwono, P.E.; Sato, S.; Ohashi, A.; Iribe, M.; Goto, T.; Komai, M. Vitamins K suppresses the lipopolysaccharide-induced expression of inflammatory cytokines in cultured macrophage-like cells via the inhibition of the activation of nuclear factor αB through the repression of IKKα/β phosphorylation. J. Nutr. Biochem. 2010, 21, 1120–1126. [Google Scholar] [CrossRef]
- Viegas, C.S.B.; Costa, R.M.; Santos, L.; Videira, P.A.; Silva, Z.; Araújo, N.; Macedo, A.L.; Matos, A.P.; Vermeer, C.; Simes, D.C. Gla-rich protein function as an anti-inflammatory agent in monocytes/macrophages: Implications for calcification-related chronic inflammatory diseases. PLoS ONE 2017, 12, e0177829. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.; Stevenson, D.; Meganathan, R.; Hudspeth, M.E.S. Menaquinone (vitamins K2) biosynthesis: Localization and characterization of the menA gene from Escherichia coli. J. Bacteriol. 1998, 180, 2782–2787. [Google Scholar] [CrossRef] [Green Version]
- Vos, M.; Esposito, G.; Edirisinghe, J.N.; Vilain, S.; Haddad, D.M.; Slabbaert, J.R.; Van Meensel, S.; Schaap, O.; De Strooper, B.; Meganathan, R.; et al. Vitamins K is a mitochondrial electron carrier that rescues pink1 deficiency. Science 2012. [Google Scholar] [CrossRef] [Green Version]
- Cerqua, C.; Casar, A.; Pierrel, F.; Fonseca, L.V.; Viola, G.; Salviati, L.; Trevisson, E. Vitamins K cannot substitute Coenzyme Q 10 as electron carrier in the mitochondrial respiratory chain of mammalian cells. Sci. Rep. 2019, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Jedynak, Ł.; Jedynak, M.; Kossykowska, M.; Zagrodzka, J. A novel method for the determination of chemical purity and assay of menaquinone-7. Comparison with the methods from the official USP monograph. J. Pharm. Biomed. Anal. 2017. [Google Scholar] [CrossRef]
- Przyborowski, K.; Proniewski, B.; Czarny, J.; Smeda, M.; Sitek, B.; Zakrzewska, A.; Zoladz, J.A.; Chlopicki, S. Vascular Nitric Oxide-Superoxide Balance and Thrombus Formation after Acute Exercise. Med. Sci. Sports Exerc. 2018. [Google Scholar] [CrossRef]
- Kij, A.; Kus, K.; Czyzynska-Cichon, I.; Chlopicki, S.; Walczak, M. Development and validation of a rapid, specific and sensitive LC-MS/MS bioanalytical method for eicosanoid quantification-assessment of arachidonic acid metabolic pathway activity in hypertensive rats. Biochimie 2020. [Google Scholar] [CrossRef]
- Rivero-Gutiérrez, B.; Anzola, A.; Martínez-Augustin, O.; De Medina, F.S. Stain-free detection as loading control alternative to Ponceau and housekeeping protein immunodetection in Western blotting. Anal. Biochem. 2014. [Google Scholar] [CrossRef] [Green Version]
- Kaczara, P.; Motterlini, R.; Rosen, G.M.; Augustynek, B.; Bednarczyk, P.; Szewczyk, A.; Foresti, R.; Chlopicki, S. Carbon monoxide released by CORM-401 uncouples mitochondrial respiration and inhibits glycolysis in endothelial cells: A role for mitoBKCa channels. Biochim. Biophys. Acta-Bioenerg. 2015. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.Y.; Li, H.; Tang, J.J.; Wang, J.; Luo, J.; Liu, B.; Wang, J.K.; Shi, X.J.; Cui, H.W.; Tang, J.; et al. Discovery of a potent HMG-CoA reductase degrader that eliminates statin-induced reductase accumulation and lowers cholesterol. Nat. Commun. 2018. [Google Scholar] [CrossRef] [Green Version]
- Bar, A.; Kus, K.; Manterys, A.; Proniewski, B.; Sternak, M.; Przyborowski, K.; Moorlag, M.; Sitek, B.; Marczyk, B.; Jasztal, A.; et al. Vitamins K-MK-7 improves nitric oxide-dependent endothelial function in ApoE/LDLR−/− mice. Vasc. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Checker, R.; Sharma, D.; Sandur, S.K.; Khan, N.M.; Patwardhan, R.S.; Kohli, V.; Sainis, K.B. Vitamins K suppressed inflammatory and immune responses in a redox-dependent manner. Free Radic. Res. 2011, 45, 975–985. [Google Scholar] [CrossRef]
- Cirilli, I.; Orlando, P.; Marcheggiani, F.; Dludla, P.V.; Silvestri, S.; Damiani, E.; Tiano, L. The protective role of bioactive quinones in stress-induced senescence phenotype of endothelial cells exposed to cigarette smoke extract. Antioxidants 2020, 9, 1008. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U.; Reichrath, J.; Holick, M.F.; Kisters, K. Vitamins K: An old vitamin in a new perspective. Dermato-Endocrinology 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatron, N.; Hammed, A.; Benoît, E.; Lattard, V. Structural insights into phylloquinone (Vitamins K), menaquinone (MK4, MK7), and menadione (vitamins K3) binding to VKORC1. Nutrients 2019, 11, 67. [Google Scholar] [CrossRef] [Green Version]
- Conly, J.M.; Stein, K. The production of menaquinones (vitamins K2) by intestinal bacteria and their role in maintaining coagulation homeostasis. Prog. Food Nutr. Sci. 1992, 16, 307–343. [Google Scholar] [PubMed]
- Harshman, S.G.; Kyla Shea, M.; Fu, X.; Grusak, M.A.; Smith, D.; Lamon-Fava, S.; Kuliopulos, A.; Greenberg, A.; Booth, S.L. Atorvastatin decreases renal menaquinone-4 formation in C57BL/6 Male mice. J. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okano, T.; Shimomura, Y.; Yamane, M.; Suhara, Y.; Kamao, M.; Sugiura, M.; Nakagawa, K. Conversion of phylloquinone (vitamins K1) into menaquinone-4 (vitamins K2) in mice: Two possible routes for menaquinone-4 accumulation in cerebra of mice. J. Biol. Chem. 2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirota, Y.; Tsugawa, N.; Nakagawa, K.; Suhara, Y.; Tanaka, K.; Uchino, Y.; Takeuchi, A.; Sawada, N.; Kamao, M.; Wada, A.; et al. Menadione (vitamins K3) is a catabolic product of oral phylloquinone (vitamins K1) in the intestine and a circulating precursor of tissue menaquinone-4 (vitamins K2) in rats. J. Biol. Chem. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegarty, J.M.; Yang, H.; Chi, N.C. UBIAD1-mediated vitamins K2 synthesis is required for vascular endothelial cell survival and development. Development 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broniarek, I.; Dominiak, K.; Galganski, L.; Jarmuszkiewicz, W. The influence of statins on the aerobic metabolism of endothelial cells. Int. J. Mol. Sci. 2020, 21, 1485. [Google Scholar] [CrossRef] [Green Version]
- Schurgers, L.J.; Teunissen, K.J.F.; Hamulyák, K.; Knapen, M.H.J.; Vik, H.; Vermeer, C. Vitamins K-containing dietary supplements: Comparison of synthetic vitamins K1 and natto-derived menaquinone-7. Blood 2007. [Google Scholar] [CrossRef] [Green Version]
- Conly, J.M.; Stein, K.; Worobetz, L.; Rutledge-Harding, S. The Contribution of Vitamins K (Menaquinones) Produced by the Intestinal Microflora to Human Nutritional Requirements for Vitamins K. Am. J. Gastroenterol. 1994. [Google Scholar] [CrossRef]
- Presse, N.; Belleville, S.; Gaudreau, P.; Greenwood, C.E.; Kergoat, M.J.; Morais, J.A.; Payette, H.; Shatenstein, B.; Ferland, G. Vitamins K status and cognitive function in healthy older adults. Neurobiol. Aging 2013. [Google Scholar] [CrossRef]
- Kimura, S.; Satoh, H.K.M. The roles of intestinal flora and intestinal function on vitamins K metabolism. J. Nutr. Sci. Vitaminol. 1992, 38, 425–428. [Google Scholar] [CrossRef]
- Nakagawa, K.; Hirota, Y.; Sawada, N.; Yuge, N.; Watanabe, M.; Uchino, Y.; Okuda, N.; Shimomura, Y.; Suhara, Y.; Okano, T. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature 2010. [Google Scholar] [CrossRef]
- Thijssen, H.H.W.; Vervoort, L.M.T.; Schurgers, L.J.; Shearer, M.J. Menadione is a metabolite of oral vitamins K. Br. J. Nutr. 2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, S.; Hanzawa, F.; Takahashi, S.; Suzuki, N.; Sano, K.; Oda, H.; Uchida, T. Tissue distribution of menaquinone-7 and the effect of a-tocopherol intake on menaquinone-7 concentration in rats. J. Nutr. Sci. Vitaminol. 2018. [Google Scholar] [CrossRef]
- Miyazawa, K.; Yaguchi, M.; Funato, K.; Gotoh, A.; Kawanishi, Y.; Nishizawa, Y.; Yuo, A.; Ohyashiki, K. Apoptosis/differentiation-inducing effects of vitamins K2 on HL-60 cells: Dichotomous nature of vitamins K2 in leukemia cells. Leukemia 2001. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, T.; Miyazawa, K.; Naito, M.; Toyotake, J.; Tauchi, T.; Itoh, M.; Yuo, A.; Hayashi, Y.; Georgescu, M.M.; Kondo, Y.; et al. Vitamins K induces autophagy and apoptosis simultaneously in leukemia cells. Autophagy 2008. [Google Scholar] [CrossRef]
- Iguchi, T.; Miyazawa, K.; Asada, M.; Gotoh, A.; Mizutani, S.; Ohyashiki, K. Combined treatment of leukemia cells with vitamins K2 and 1α,25-dihydroxy vitamin D3 enhances monocytic differentiation along with becoming resistant to apoptosis by induction of cytoplasmic p21 CIP1. Int. J. Oncol. 2005. [Google Scholar] [CrossRef]
- Mandatori, D.; Penolazzi, L.; Pipino, C.; Di Tomo, P.; Di Silvestre, S.; Di Pietro, N.; Trevisani, S.; Angelozzi, M.; Ucci, M.; Piva, R.; et al. Menaquinone-4 enhances osteogenic potential of human amniotic fluid mesenchymal stem cells cultured in 2D and 3D dynamic culture systems. J. Tissue Eng. Regen. Med. 2018. [Google Scholar] [CrossRef]
- Willems, B.A.G.; Vermeer, C.; Reutelingsperger, C.P.M.; Schurgers, L.J. The realm of vitamins K dependent proteins: Shifting from coagulation toward calcification. Mol. Nutr. Food Res. 2014. [Google Scholar] [CrossRef]
- Hoffmann, R.F.; Jonker, M.R.; Brandenburg, S.M.; de Bruin, H.G.; ten Hacken, N.H.T.; van Oosterhout, A.J.M.; Heijink, I.H. Mitochondrial dysfunction increases pro-inflammatory cytokine production and impairs repair and corticosteroid responsiveness in lung epithelium. Sci. Rep. 2019. [Google Scholar] [CrossRef] [PubMed]
- Tabb, M.M.; Sun, A.; Zhou, C.; Grün, F.; Errandi, J.; Romero, K.; Pham, H.; Inoue, S.; Mallick, S.; Lin, M.; et al. Vitamins K Regulation of Bone Homeostasis Is Mediated by the Steroid and Xenobiotic Receptor SXR. J. Biol. Chem. 2003. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, T.; Horie-Inoue, K.; Ikeda, K.; Blumberg, B.; Inoue, S. Steroid and xenobiotic receptor SXR mediates vitamins K2- activated transcription of extracellular matrix-related genes and collagen accumulation in osteoblastic cells. J. Biol. Chem. 2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horie-Inoue, K.; Inoue, S. Steroid and xenobiotic receptor mediates a novel vitamins K2 signaling pathway in osteoblastic cells. J. Bone Miner. Metab. 2008. [Google Scholar] [CrossRef]
- Sultana, H.; Kato, A.; Ohashi, A.; Takashima, R.; Katsurai, T.; Sato, S.; Monma, M.; Ohsaki, Y.; Goto, T.; Komai, M.; et al. Effect of Vitamins K-Mediated PXR Activation on Drug-Metabolizing Gene Expression in Human Intestinal Carcinoma LS180 Cell Line. Nutrients 2021, 13, 1709. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.H.; Lin-Shiau, S.Y.; Lin, J.K. Suppression of nitric oxide synthase and the down-regulation of the activation of NFκB in macrophages by resveratrol. Br. J. Pharmacol. 1999. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.B.; Han, A.R.; Park, E.Y.; Kim, J.Y.; Cho, W.; Lee, J.; Seo, E.K.; Lee, K.T. Inhibition of LPS-induced iNOS, COX-2 and cytokines expression by poncirin through the NF-κB inactivation in RAW 264.7 macrophage cells. Biol. Pharm. Bull. 2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jae, W.R.; Lee, K.W.; Kim, D.; Lee, Y.; Jeon, O.H.; Kwon, H.J.; Kim, D.S. NF-κB-dependent regulation of matrix metalloproteinase-9 gene expression by lipopolysaccharide in a macrophage cell line RAW 264.7. J. Biochem. Mol. Biol. 2007. [Google Scholar] [CrossRef] [Green Version]
- Halder, M.; Petsophonsakul, P.; Akbulut, A.C.; Pavlic, A.; Bohan, F.; Anderson, E.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamins K: Double bonds beyond coagulation insights into differences between vitamins K1 and K2 in health and disease. Int. J. Mol. Sci. 2019, 20, 896. [Google Scholar] [CrossRef] [Green Version]
- Rasmi, R.R.; Sakthivel, K.M.; Guruvayoorappan, C. NF-κB inhibitors in treatment and prevention of lung cancer. Biomed. Pharmacother. 2020. [Google Scholar] [CrossRef] [PubMed]
- Baeuerle, P.A.; Baichwal, V.R. NF-κB as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv. Immunol. 1997, 65, 111–137. [Google Scholar]
- Biswas, R.; Bagchi, A. NFkB pathway and inhibition: An overview. Comput. Mol. Biol. 2016. [Google Scholar] [CrossRef]
- Dofferhoff, A.S.M.; Piscaer, I.; Schurgers, L.J.; Visser, M.P.J.; van den Ouweland, J.M.W.; de Jong, P.A.; Gosens, R.; Hackeng, T.M.; van Daal, H.; Lux, P.; et al. Reduced Vitamins K Status as a Potentially Modifiable Risk Factor of Severe Coronavirus Disease 2019. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Varsha, M.K.N.S.; Thiagarajan, R.; Manikandan, R.; Dhanasekaran, G. Vitamins K alleviates streptozotocin-induced type 1 diabetes by mitigating free radical stress, as well as inhibiting NF-κB activation and iNOS expression in rat pancreas. Nutrition 2015. [Google Scholar] [CrossRef]
- Van Den Heuvel, E.G.H.M.; Van Schoor, N.M.; Lips, P.; Magdeleyns, E.J.P.; Deeg, D.J.H.; Vermeer, C.; Heijer, M. Den Circulating uncarboxylated matrix Gla protein, a marker of vitamins K status, as a risk factor of cardiovascular disease. Maturitas 2014. [Google Scholar] [CrossRef]
- Shea, M.K.; Kritchevsky, S.B.; Hsu, F.C.; Nevitt, M.; Booth, S.L.; Kwoh, C.K.; McAlindon, T.E.; Vermeer, C.; Drummen, N.; Harris, T.B.; et al. The association between vitamins K status and knee osteoarthritis features in older adults: The Health, Aging and Body Composition Study. Osteoarthr. Cartil. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, B.A.; Thompson, P.D. Muscle-related side-effects of statins: From mechanisms to evidence-based solutions. Curr. Opin. Pediatr. 2015. [Google Scholar] [CrossRef]
- Golomb, B.A.; McGraw, J.J.; Evans, M.A.; Dimsdale, J.E. Physician response to patient reports of adverse drug effects: Implications for patient-targeted adverse effect surveillance. Drug Saf. 2007. [Google Scholar] [CrossRef] [PubMed]
- Aiman, U.; Najmi, A.; Khan, R.A. Statin induced diabetes and its clinical implications. J. Pharmacol. Pharmacother. 2014. [Google Scholar] [CrossRef] [Green Version]


| Exogenous Vitamins K | Endogenous MK-4 [ng/mg Protein] | Endogenous MK-4-d7 [ng/mg Protein] |
|---|---|---|
| K1 | 0.78 ± 0.13 | - |
| K1-d7 | - | 0.65 ± 0.11 |
| K3 | 0.53 ± 0.13 | - |
| K3-d8 | - | 1.14 ± 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kieronska-Rudek, A.; Kij, A.; Kaczara, P.; Tworzydlo, A.; Napiorkowski, M.; Sidoryk, K.; Chlopicki, S. Exogenous Vitamins K Exert Anti-Inflammatory Effects Dissociated from Their Role as Substrates for Synthesis of Endogenous MK-4 in Murine Macrophages Cell Line. Cells 2021, 10, 1571. https://doi.org/10.3390/cells10071571
Kieronska-Rudek A, Kij A, Kaczara P, Tworzydlo A, Napiorkowski M, Sidoryk K, Chlopicki S. Exogenous Vitamins K Exert Anti-Inflammatory Effects Dissociated from Their Role as Substrates for Synthesis of Endogenous MK-4 in Murine Macrophages Cell Line. Cells. 2021; 10(7):1571. https://doi.org/10.3390/cells10071571
Chicago/Turabian StyleKieronska-Rudek, Anna, Agnieszka Kij, Patrycja Kaczara, Anna Tworzydlo, Marek Napiorkowski, Katarzyna Sidoryk, and Stefan Chlopicki. 2021. "Exogenous Vitamins K Exert Anti-Inflammatory Effects Dissociated from Their Role as Substrates for Synthesis of Endogenous MK-4 in Murine Macrophages Cell Line" Cells 10, no. 7: 1571. https://doi.org/10.3390/cells10071571
APA StyleKieronska-Rudek, A., Kij, A., Kaczara, P., Tworzydlo, A., Napiorkowski, M., Sidoryk, K., & Chlopicki, S. (2021). Exogenous Vitamins K Exert Anti-Inflammatory Effects Dissociated from Their Role as Substrates for Synthesis of Endogenous MK-4 in Murine Macrophages Cell Line. Cells, 10(7), 1571. https://doi.org/10.3390/cells10071571