Abstract
Alfalfa (Medicago sativa L.) is a valuable forage legume, but its production is largely affected by high temperature. In this study, we investigated the effect of heat stress on 15 alfalfa cultivars to identify heat-tolerant and -sensitive cultivars. Seedlings were exposed to 38/35 °C day/night temperature for 7 days and various parameters were measured. Heat stress significantly reduced the biomass, relative water content (RWC), chlorophyll content, and increased the electrolyte leakage (EL) and malondialdehyde (MDA) content of heat-sensitive alfalfa cultivars. However, heat-tolerant cultivars showed higher soluble sugar (SS) and soluble protein (SP) content. The heat tolerance of each cultivar was comprehensively evaluated based on membership function value. Cultivars with higher mean membership function value of 0.86 (Bara310SC) and 0.80 (Magna995) were heat tolerant, and Gibraltar and WL712 with lower membership function value (0.24) were heat sensitive. The heat tolerance of the above four cultivars were further evaluated by chlorophyll a fluorescence analysis. Heat stress significantly affected the photosynthetic activity of heat-sensitive cultivars. The overall results indicate that Bara310SC and WL712 are heat-tolerant and heat-sensitive cultivars, respectively. This study provides basic information for understanding the effect of heat stress on growth and productivity of alfalfa.
1. Introduction
Heat stress is one of the major abiotic stresses limiting plant growth and development. When plants are exposed to high temperature, several cellular injuries, including cell death, may occur within minutes, which then leads to an appalling failure of cellular organization [,]. In addition, the rapid closure of stomata, reduction in cell size, an increase in stomatal, trachomatous densities, and xylem vessels of both root and shoot were reported to occur in response to heat stress []. However, different plant species may show different responses to heat stress []. Generally, heat stress triggers various morphological, physiological, biochemical, and molecular changes to inhibit plant growth and development. Heat stress inhibits seed germination; causes scorching; twigs and burning of leaves, branches, and stems; leaf senescence and abscission; shoot and root growth inhibition; fruit discoloration and damage; reduced yield; and finally plant death [,]. High temperature stress also affects shoot net assimilation and decreases the overall dry weight of the plant [].
It is well established that heat stress has detrimental impacts on various key physiological, biochemical, and metabolic processes of plants, and disrupts normal cellular homeostasis []. It promotes the overproduction and accumulation of reactive oxygen species (ROS), malondialdehyde (MDA) production due to lipid peroxidation, photoinhibition, protein denaturation, and accumulation of compatible solutes [,,]. The oxidative stress caused by heat stress further leads to cellular injury, membrane proteins breakage, lipid peroxidation, photosynthetic pigment degradation, and enzymes and nucleic acid denaturation [,,]. Furthermore, heat stress influences plant photosynthesis and respiration processes to curtail the life cycle and reduce plant productivity []. The heat stress sensitivity of plants varies with the plant genotype and the stage of plant development, but the effect is highly dependent on genotype and species, as well as with abundant inter- and intraspecific variations [].
Alfalfa (Medicago sativa L.) is one of the most important perennial forage legume species. Due to its outstanding nutritional quality, alfalfa is an excellent source of feed nutrient for animals. High temperature stress is a limiting factor for alfalfa cultivation [,]. Previous studies have shown that increasing temperature above optimal level markedly affects alfalfa’s morphological, physiological, and proteomic processes, and reduced photosynthetic rate, destroyed plasma membrane structure, and accelerated the process of aging [,,,]. Therefore, it is of a great significance to develop heat stress-tolerant alfalfa cultivar that withstand heat stress-induced growth inhibition and biomass reduction. Studying plants’ physiology in response to heat stress could be helpful to further understand the molecular tolerance traits [] and will provide fundamental knowledge to develop heat-tolerant cultivars. It is well reported that different genotypes of a single plant species demonstrate high degrees of variation for heat tolerance; therefore, the selection of varieties with high thermotolerance potential is crucial to further improve thermotolerance. The genetic variability present in alfalfa could be exploited to evaluate and screen for high-temperature tolerance. Moreover, compared with other abiotic stresses reports in alfalfa, studies about the effect of heat stress on alfalfa growth and physiology are very limited. Thus, the objectives of this study were to investigate the effect of heat stress on the growth and physiological traits of alfalfa, to evaluate for heat tolerance, and to identify heat-tolerant and heat-sensitive alfalfa cultivars.
2. Materials and Methods
2.1. Plant Materials, Growth Conditions, and Heat Treatment
In this study, fifteen alfalfa cultivars (Medicago sativa L.) were used and the details of the cultivars are presented in Table 1. Ten seeds of each cultivar were sown in each plastic pot filled with clay, sand, and loamy soil (1:1:2, v/v). The pots were then kept in a greenhouse with a temperature of 25 °C, relative humidity of approximately 60%, light intensity of 500–550 µmol m−2 s−1, and a photoperiod of 14 h/10 h light/dark []. There were five replications for each cultivar and treatment. The seedlings were watered daily to field capacity level and fertilized once a week with a half-strength Hoagland nutrient solution. The four-week-old seedlings were divided into two groups for heat treatment. The control group was kept in a greenhouse at 25 °C and the treatment group was transferred into a growth incubator and treated at 38/35 °C light/dark and light intensity of 500–550 µmol m−2 s−1 for 7 days.

Table 1.
Alfalfa cultivars used in the study.
2.2. Plant Biomass Measurement
After seven days of heat stress treatment, five plants for each of the treatment and control group were randomly harvested from each pot (25 plants for each cultivar and treatment). The roots of selected plants were washed with distilled water and separated from the shoot. The fresh weight of roots and shoots were measured separately using analytical balance (precision 0.0001 g). Dry weight was measured after drying the shoot and root in an oven at 80 °C for 24 h. Total fresh and dry biomasses were calculated using the following formula:
where TFW is the total fresh biomass, SFW is the shoot fresh weight, RFW is the root fresh weight, TDB is the total dry biomass, SDW is the shoot dry weight, and RDW is the root dry weight.
TFB = SFW + RFW
TDB = SDW + RDW
TDB = SDW + RDW
2.3. Physiological Traits Analysis
Fresh leaves were used to measure relative water content, electrolyte leakage, chlorophyll content, and soluble sugar content, while MDA and soluble protein content were determined from liquid nitrogen dried leaves which were stored at −80 °C freezer.
2.4. Relative Water Content (RWC) Measurement
For RWC determination, fresh weight (FW) was measured from the fully expanded top leaves, and Turgid weight (TW) was recorded after dipping the leaves in distilled water for 12 h. Dry weight (DW) was then measured after oven drying the leaves at 80 °C for 24 h and RWC was calculated using the following formula:
where FW is the fresh weight of leaves, DW is the dry weight of leaves, and TW is the turgid weight of leaves.
RWC% = ((FW − DW)/(TW − DW)) × 100
2.5. Chlorophyll Content Determination
For chlorophyll content determination, 0.1 g fresh alfalfa leaves were placed into a centrifuge tube containing 5 mL of 95% alcohol. The test tubes were wrapped with aluminum foil and incubated for 48 h at room temperature in the dark. The absorbance of the chlorophyll extract was read at 665 and 649 nm. The chlorophyll content was calculated according to the following formula:
where D is the absorbance of the chlorophyll extract and W is the fresh weight leaves (g).
Chl a (mg·g−1 FW) = (13.95 × D665 − 6.88 × D649) × 0.005 ÷ W
Chl b (mg·g−1 FW) = (24.96 × D649 − 7.32 × D665) × 0.005 ÷ W
Total Chl (mg·g−1 FW) = (18.08 × D649 + 6.63 × D665) × 0.005 ÷ W
2.6. Electrolyte Linkage (EL) Measurement
The electrolyte leakage was measured using the method described by Huang et al. []. Briefly, 0.5 g of fresh alfalfa leaves were collected and washed with deionized water three times and then transferred into a 50 mL plastic centrifuge tube that was filled with 15 mL of deionized water. The tubes were incubated at room temperature for 12 h on a conical shaker and initial conductivity (EL1) using a conductivity meter (JENCO-3173, Jenco Instruments, Inc., San Diego, CA, USA). To release all electrolytes, the leaves were killed by autoclaving at 121 °C for 30 min. The tubes were then cooled at room temperature and the second conductivity (EL2) was measured. The relative EL was calculated by using the formula:
Relative EL (%) = (EL1/EL2) × 100.
2.7. Soluble Sugar Content Determination
For soluble sugar content determination, 0.1 g fresh leaves were placed into a test tube containing 5 mL distilled water. The tubes were then sealed with plastic film and soaked in the boiling water bath for 30 min. The supernatant was collected and extraction continued for the second time using the residue. The supernatant from the primary and secondary extraction was mixed together, and 0.5 mL extract was taken and added to each tube containing 1.5 mL distilled water. Then 0.5 mL anthrone reagent (1 g anthrone dissolved in 50 mL ethyl acetate) and 5 mL 98% (W/V) H2SO4 (NA) was added to the test tubes and mixed gently, then placed in a boiling water bath (100 °C) for 10 min. The tubes were then cooled rapidly under running cold water and the absorbance was measured at 620 nm against the blank reagent. The concentration of total soluble sugar was obtained from the glucose standard curve. Finally, the total soluble sugar content was calculated using the equation:
where C is soluble sugar concentration from the standard curve (μg), and W is the fresh weight of leaves (g).
Soluble sugar content (%) = (C × 7.5)/(W × 104)
2.8. Preparation of Crude Enzyme Extract
For crude enzyme extraction, about 0.3 g of alfalfa leaves, which were dried with liquid nitrogen, were ground into powder in liquid nitrogen using prechilled mortar and pestle (4 °C). Then, 5 mL of 150 mM sodium phosphate buffer (PBS), pH 7.4, was added to the powder and the homogenate was centrifuged at 12,000 rpm for 20 min at 4 °C. The supernatant was collected and used as a crude extract to determine malondialdehyde (MDA) and soluble protein content.
2.9. Determination of MDA Content
The MDA content was determined by the thiobarbituric acid (TBA) method according to a previous report []. Briefly, previously prepared 1 mL crude enzyme extract was mixed with 2 mL of reaction mixture containing 20% (v/v) trichloroacetic acid and 0.5% (v/v) thiobarbituric acid. The mixture was then heated for 30 min in a 95 °C water bath and directly cooled to room temperature. The mixture was then centrifuged at 12,000 g for 10 min at 20 °C, and the absorbance of the supernatant was read at 450, 532, and 600 nm with a spectrophotometer (UV2600, UNIC, Shanghai, China). The MDA content was calculated using the following formula:
where Vt is the volume of extraction liquid (mL), Vs is the volume of extraction solution (mL), and FW is the fresh weight of samples (g).
MDA (mmol·g−1 FW) = [6.425 × (OD532 − OD600) − 0.559 × OD450] × Vt/(Vs × FW)
2.10. Soluble Protein Content Determination
Soluble protein content was determined following the Bradford assay method []. Briefly, 100 μL crude enzyme extract was added to a tube containing 3 mL Bradford working solution. After 10 min, the absorbance was measured at 595 nm using a spectrophotometer (UV2600, UNIC, Shanghai, China). The protein concentration of the crude extract was determined from the standard curve established by the reference solution of bovine serum albumin (BSA) and the OD595 value of the sample.
2.11. A Comprehensive Evaluation of Alfalfa for Heat Tolerance
The comprehensive evaluation was performed according to the previous report [] based on membership function value, which were calculated from the heat tolerance coefficients. The correlation of each biomass and physiological trait for all alfalfa cultivars was analyzed using heat tolerance coefficients. The heat tolerance coefficient of all biomass and physiological traits was calculated using the following equation:
where CK is the mean value of a single trait under the control treatment and HT is the mean value of a single trait under heat treatment.
Heat tolerance coefficient = (HT/CK) × 100
The membership function values of all biomass and physiological traits were calculated from the heat tolerance coefficient using the formula (F1 and F2). Formula F1 was used for the traits that are directly related to heat tolerance, and F2 was used for the traits that are inversely related traits.
where X is the i heat tolerance coefficient, max is the maximum heat tolerance coefficient value from all cultivars of the i trait, and Xmin is the minimum heat tolerance coefficient from all cultivars of the i trait.
F1 (Xi) = (Xi − Xmin)/(Xmax − Xmin)
F2 (Xi) = 1 − (Xi − Xmin)/(Xmax − Xmin)
2.12. Chlorophyll a (Chl a) Fluorescence Analysis
Based on the comprehensive heat tolerance evaluation, four alfalfa cultivars—two relatively heat tolerant (Bara310SC and Magna995) and two relatively heat sensitive (Gibraltar and WL712)—were selected and further evaluated by Chl a fluorescence analysis to select the most heat-tolerant and -sensitive alfalfa. Chl a fluorescence transient was measured using a pulse–amplitude modulation (PAM) fluorometer (PAM2500 Heinz Walz GmbH, Eichenring, Germany) according to the previous report []. Briefly, the leaves were kept in the dark for 30 min, and all measurements were taken using a saturating light intensity of 2000 µmol photons m−2 s−1. Five measurements were taken for each cultivar and treatment. The strong light pulses inducted Chl a fluorescence emission, which was subsequently measured and digitized between 10 µs and 300 ms. The chlorophyll fluorescence induction curve (OJIP) transients were analyzed using the JIP-test.
2.13. Statistical Analysis
Data were subjected to analysis of variance (ANOVA) using SPSS software version 22.0 (IBM Corporation, Chicago, IL, USA). All values were shown as mean ± SD (Standard Deviation) (n = 5). The independent sample t-test was employed to compare the control and the treatment groups using the least significant difference (LSD) test. All statistical results were considered significant at p ≤ 0.05. All figures were created by Origin 9.0 (Origin Lab, Inc., Hampton, MA, USA).
3. Results
3.1. Effect of Heat Stress on Alfalfa Plant Biomass
Heat stress affected the biomass of all alfalfa cultivars (Table 2). Compared with the control, heat stress significantly reduced the shoot fresh weight of Golden Queen (45.74%), 55V48 (34.07%), WL354HQ (33.47%), Gibraltar (31.55%), and WL712 (28.57%). In addition, significantly higher shoot dry weight reduction was noticed in WL354HQ (55.10%), Golden Queen (45.45%), WL712 (40.43%), and Gibraltar (35.14%) (p < 0.05).

Table 2.
Effect of heat stress on alfalfa plant biomass (g).
Furthermore, heat stress remarkably affected the fresh and dry root weights of most cultivars, with significant reductions recorded in SK3010, Sanditi, and Gibraltar (Table 2). Relative to the control, heat stress significantly decreased the total fresh biomass of Golden Queen, WL354HQ, 55V48, Gibraltar, and WL712 cultivars by 41.43%, 30.06%, 29.75%, 29.45%, and 24.04%, respectively (p < 0.05). Similarly, total dry biomass was significantly reduced by 48.48%, 43.55%, 41.54%, 39.74%, and 39.62% in WL354HQ, Golden Queen, WL712, Sanditi, and Gibraltar cultivars, respectively (Table 2). The biomass of Gibraltar, Golden Queen, and WL712 were highly affected by heat stress, which may indicate the sensitivity of the cultivars. By contrast, Bara310SC, Magna995, and WL363HQ cultivars were able to maintain their biomass under the heat stress condition, which may indicate their tolerance.
3.2. Heat Stress Reduced the Relative Water Content (RWC) of Alfalfa
Heat stress obviously decreased the leaf relative water content of all alfalfa cultivars compared to the control (Figure 1A). Of which, significantly higher decrement was noted in WL354HQ, WL712, Sanditi, WL440HQ, 55V48, and Siriver cultivars by 15.29%, 13.28%, 12.54%, 12.42%, 11.43%, and 10.01%, respectively (p < 0.05) (Figure 1A). Despite the reduction of RWC in Gibraltar, Golden Queen, and SK3010 cultivars being small (<10%), it was still significant compared to the control (Figure 1A). The result revealed that heat stress had a higher impact on the RWC of some alfalfa cultivars, especially on WL354HQ and WL712 cultivars, which showed higher water loss under heat stress. Bara310SC and Magna995 cultivars sustained their relative water content under heat stress and could be heat tolerant, but cultivar WL712 could be heat sensitive, as evidenced by higher water loss.
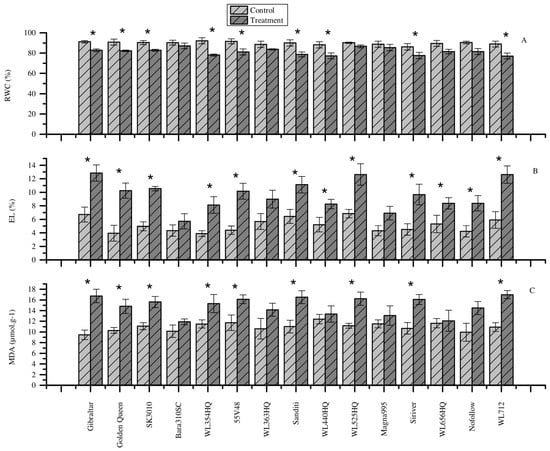
Figure 1.
Effects of heat stress on physiological parameters. (A) Relative water content. (B) Electrolyte leakage. (C) MDA content. Each bar represents the mean (n = 5) and the error bar indicates the standard deviation. Asterisks indicate statistically significant differences between control and treatment group for each cultivar (p < 0.05), independent sample t-test. EL is electrolyte leakage, MDA is malondialdehyde.
3.3. Heat Stress Increased the Electrolyte Leakage (EL) of Alfalfa
Heat stress affected the membrane integrity and stability and increased the EL of all alfalfa cultivars, as shown in Figure 1B, and was significant for the majority of the cultivars. Heat stress significantly increased the EL of Golden Queen, 55V48, Siriver, WL712, SK3010, WL354HQ, Nofollow, Gibraltar, WL525HQ, Sanditi, WL440HQ, and WL656HQ by 61.36%, 56.72%, 53.45%, 53.20%, 52.86%, 52.08%, 49.56%, 45.78%, 42.12%, 36.84%, and 36.45%, respectively (p < 0.05) (Figure 1B). However, Bara310SC cultivar had a lower increase in EL of 24.07%. The result showed that heat stress had a huge impact on the membrane stability of the majority of alfalfa cultivars, as shown by significantly higher increment in EL.
3.4. Effect of Heat Stress on Lipid Peroxidation
As indicated in EL, heat stress damaged the membrane of alfalfa and caused lipid peroxidation, which was manifested by higher MDA content in all cultivars (Figure 1C). However, heat stress had no significant effect on some alfalfa cultivars such as Bara310SC, WL363HQ, WL440HQ, Magna995, WL656HQ, and Nofollow (Figure 1C). Meanwhile, under heat stress, Gibraltar, WL712, Siriver, Sanditi, WL525HQ, and Golden Queen cultivars showed significantly higher MDA content compared to the control (p < 0.05). The results showed that different alfalfa cultivars had a different level of sensitivity to heat stress. The higher the MDA content, the higher the lipid peroxidation and the greater the membrane damage.
3.5. Heat Stress Decreased the Chlorophyll Content of Alfalfa
It is obvious that heat stress affects the chlorophyll content of plant leaves, and the same was true for all alfalfa cultivars (Figure 2). The Chl content of some cultivars was more significantly and highly affected by heat stress than others compared to the control. In particular, heat stress significantly reduced the chlorophyll content of Gibraltar, Golden Queen, SK310, WL354HQ, WL363HQ, Sanditi, WL440HQ, WL525HQ, Siriver, and WL712 compared to control. Meanwhile, higher reduction in Chl a was observed in Gibraltar, WL354HQ, Golden Queen, Siriver, WL712, and Sanditi cultivars by 40.30%, 36.06%, 35.06%, 33.41%, 31.28%, and 31.11%, respectively (Figure 2A). Similar reduction in Chl b content was observed in WL712 (44.14%), WL354HQ (34.44%), WL440HQ, (31.53%), Golden Queen (24.69%), and Gibraltar (22.59%) (Figure 2B). In addition, higher reduction in total Chl content was noted in WL712, Gibraltar, 13, and Golden Queen (36.57%, 33.11%, 31.29%, and 31.05%, respectively) (Figure 2C). The results suggested that some cultivars might be more sensitive to heat stress, as indicated by higher Chl content reduction under heat stress.
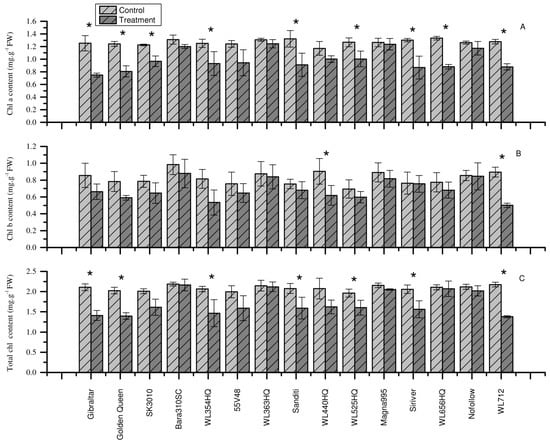
Figure 2.
Effect of heat stress on the chlorophyll content of alfalfa cultivars. (A) Chlorophyll a content. (B) Chlorophyll b content. (C) Total chlorophyll content. Each bar represents the mean (n = 5) and the error bar indicates the standard deviation. Asterisks indicate statistical significant differences between control and treatment group for each cultivar (p < 0.05), independent sample t-test.
3.6. Effect of Heat Stress on Soluble Sugar Content
The soluble sugar content of all alfalfa cultivars showed an increment under heat stress relative to the control (Figure 3A). Heat stress significantly increased the soluble sugar content of Bara310SC, Magna995, WL363HQ, Nofollow, WL525HQ, and WL354HQ by 20.20%, 15.57%, 11.53%, 7.13%, 4.68%, and 2.22%, respectively (Figure 3A). The results showed that these cultivars produce higher soluble sugar to maintain their osmotic potential and organize proteins and cellular structures under heat stress, which increases heat tolerance.
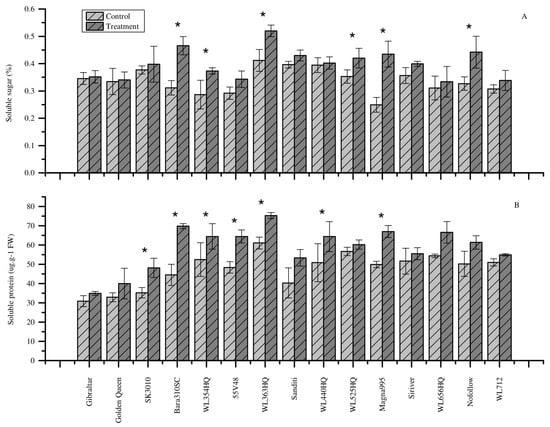
Figure 3.
Effect of heat stress on soluble sugar and soluble protein content. (A) Soluble sugar content. (B) Soluble protein content. Each bar represents the mean (n = 5) and the error bar indicates the standard deviation. Asterisks indicate statistical significant differences between control and treatment plant (p < 0.05), independent sample t-test.
3.7. Effect of Heat Stress on Soluble Protein Content
Similar to soluble sugar content, heat stress increased the soluble protein content of all alfalfa cultivars as shown in Figure 3B. Compared to the control, significantly higher soluble protein content was noted in Bara310SC, SK3010, WL363HQ, 55V48, WL440HQ, Magna995, and WL354HQ cultivars under heat stress (p < 0.05). Heat stress increased the soluble protein content of these cultivars by 36.18%, 26.73%, 25.52%, 24.91%, 24.44%, 21.01%, 18.91%, and 18.63%, respectively (Figure 3B). These results indicate that soluble protein plays an important role in alfalfa heat stress response.
3.8. A Comprehensive Evaluation of the Heat Tolerance of Alfalfa Cultivars
All biomass and physiological traits were standardized for the comprehensive heat tolerance evaluation using the heat tolerance coefficient. The heat tolerance coefficient of each biomass and physiological traits (indexes) are presented in Table 3. The heat tolerance coefficients were further used to calculate the membership function values of alfalfa cultivars.

Table 3.
Heat tolerance coefficients of biomass and physiological traits (indexes) of alfalfa cultivars.
Furthermore, correlation analysis was performed to investigate the relationship between traits. The results revealed that shoot fresh weight and shoot dry weight were strongly positively correlated with total fresh biomass and total dry biomass, respectively (r = 0.94) (p < 0.01) (Table 4). Root fresh weight was strongly positively correlated with Chl b and SS, and root dry weight was strongly positively correlated with SS, RWC, and total dry biomass (Table 4). Total fresh biomass was significantly negatively correlated with EL. Total fresh biomass was significantly positively correlated with total dry biomass, and total dry biomass was strongly positively correlated with Chl b (r = 0.80) and total Chl content (r = 0.80) (Table 4). These results indicated that electrolyte leakage and MDA content were negatively correlated with the rest of traits, which were significant for Chl b, total Chl, and SS for electrolyte leakage, and Chlb and total Chl for MDA. However, electrolyte leakage was positively correlated with MDA content (r = 0.40). Chl a (r = 0.79) and Chl b (r = 0.94) were strongly positively correlated with total Chl content. In addition, there was a positive correlation between soluble sugar and soluble protein content (Table 4). Hence, all biomass and physiological traits were used to evaluate the heat tolerance of all alfalfa cultivars.

Table 4.
Correlation analysis of biomass and physiological traits.
The membership function value calculated from heat tolerance coefficient was used to evaluate the heat tolerance of all alfalfa cultivars. As shown in Table 5, Bara310SC and Magna995 cultivars had higher mean membership function value (0.86 and 0.80), respectively. By contrast, Gibraltar (0.26) and WL712 (0.26) showed lower mean membership function value. Based on this result, Bara310SC and Magna995 were ranked “one” and “two”, respectively whereas Gibraltar and WL712 had the same rank, “14” (Table 5). Furthermore, the mean membership function value was used for Euclidean distance cluster analysis. The results showed that Bara310SC and Magna995 cultivars were clustered into one group, and Gibraltar and WL712 were clustered in another group (data are not shown). Finally, the rank and cluster results were combined to evaluate the heat tolerance of the cultivars. Thus, Bara310SC and Magna995 were found to be heat tolerant, whereas Gibraltar and WL712 were found to be heat-sensitive alfalfa cultivars. To screen the most heat-tolerant and heat-sensitive alfalfa, the four cultivars (Bara310SC, Magna995, Gibraltar, and WL712) were further evaluated by chlorophyll a fluorescence analysis.

Table 5.
Membership function value of alfalfa cultivars.
3.9. Alteration of Chlorophyll a Fluorescence under Heat Stress
Chlorophyll a fluorescence was measured to further screen the most heat-tolerant and heat-sensitive alfalfa cultivars based on the photosynthesis behavior under heat stress. OJIP curve was constructed from fluorescence transient measurement. The effect of heat stress on basic photosynthetic parameters, specific energy fluxes, quantum yield and efficiency, and performance indexes were investigated.
3.9.1. OJIP Transient Curve
OJIP transient curve was constructed based on the fluorescence measurement relative to the time (Figure 4). OJIP transient curves of control groups were higher than those of heat treatment groups for all cultivars. The JIP-test was applied to further investigate the structural alteration, functional parameters, and photosynthetic behaviors under heat stress treatment. Basic fluorescence parameters, specific energy fluxes, quantum yield efficiency, and performance index were extracted and analyzed.
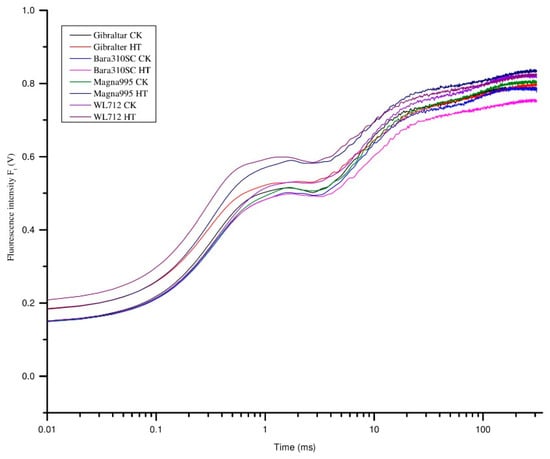
Figure 4.
Effect of heat stress on the chlorophyll a fluorescence transient (OJIP curves) of 4 alfalfa cultivars after seven days of heat treatment. CK represents control treatment and HT represents heat treatment (n = 5).
3.9.2. Basic Photosynthetic Parameters (F0, Fj, Fi, Fm, F300 μs, and Fv/Fm)
The basic fluorescence parameters were extracted from the OJIP transient curve (Table 6). Heat stress increased the F0 of Gibraltar, Magna99,5 and WL712 alfalfa cultivars, and was significant for Gibraltar and WL712 cultivars when compared with the control (Table 6). Heat stress also increased the Fm (Maximal fluorescence) of alfalfa. Relative to the control, heat stress decreased the Fv/Fm of Gibraltar, Bara310SC, Magna995, and WL712 by 6.10%, 1.25%, 2.50%, and 6.25%, respectively, and was significant in WL712 cultivar (Table 6). The results indicated that WL712 was highly affected by heat stress, which revealed high heat sensitivity.

Table 6.
Photosynthetic parameters extracted from OJIP fluorescence transients.
3.9.3. Specific Energy Fluxes (TP0/RC, ETO/RC, RE0/RC, and ABS/RC)
Heat stress affected the specific energy fluxes of alfalfa. All heat-treated plants showed higher TPO/RC and were significant for WL712 (Table 6). Cultivars showed different responses in ETO/RC under heat stress. Heat stress significantly decreased the ETO/RC of WL712 but increased in Bara310SC and Magna995 (relatively heat tolerant) (Table 6). Unlike other cultivars, heat stress reduced the RE0/RC of Bara310SC (Table 6). On the other hand, heat-treated Gibraltar, Magna995, and WL712 plants showed higher ABS/RC compared to the control and was significant for WL712 (Table 6).
3.9.4. Quantum Yield and Efficiency (φpo, φEo, δRo, and RC/ABS)
Heat stress affected all quantum yield efficiency components (Table 6). Heat stress noticeably decreased the φpo and δRo of all alfalfa cultivars except Bara310SC. Relative to control, WL712 showed significant reductions in φpo, φEo, and RC/ABS under heat stress. Meanwhile, heat stress significantly decreased the φEo of Magna995 (Table 6).
3.9.5. Performance Indexes (PIABS and PItotal)
Heat stress markedly decreased the photosynthetic performance indexes (PIABS and PItotal) of all alfalfa cultivars (Table 6). Meanwhile, heat stress significantly reduced the PIABS of Gibralter, Magna995, and WL712 by 44.44%, 35.64%, and 66.36%, respectively, compared to control (p < 0.05). In addition, WL712 showed significantly lower PItotal under heat stress, indicating its more heat sensitivity than others. However, heat stress had no significant effect on the performance indexes of Bara310SC (Table 6).
4. Discussion
High-temperature stress changes morphological, biochemical, and physiological processes of plants [,]. In this study, heat stress had an obvious negative effect on alfalfa plant biomass and most of the cultivars showed significant reductions in biomass following heat stress treatment. It has been reported that heat stress causes leaf wilting, leaf curling, leaf yellowing, reduction in shoot growth, root growth, root number, root diameter, plant height, and biomass [,]. Thus, the decrease in alfalfa plant biomass could be associated with the reduction in plant height, wilting, and falling off of leaves caused by heat stress. Our results were consistent with previous reports in maize [], sugarcane [], and wheat []. The biomass of most alfalfa cultivars was significantly affected, indicating that heat stress has a huge impact on alfalfa productivity. Like biomass, heat stress caused a significant reduction in the RWC of most alfalfa cultivars. Our results are in agreement with previous reports in rice [] and wheat []. Similar with the findings of Sita et al. [] heat-tolerant alfalfa cultivars had higher RWC than heat-sensitive ones. The decrease in leaf water content might affect plant metabolism and decrease plant growth and biomass. The reduction in leaf relative water content could be associated with the reduction in the number, mass, and growth of the roots under heat stress, which ultimately limits the supply of water and nutrients to the above-ground parts of the plant []. Taken together, significant reductions in biomass and RWC could be indicators of alfalfa heat stress sensitivity.
It is well documented that the plant membrane is sensitive to various abiotic stresses, and stress condition increased lipid peroxidation and impaired membrane selectivity []. In this study, both EL and MDA content, which are indicators of stress sensitivity, were higher in heat-treated alfalfa plants compared to the control. The membrane stability of most alfalfa cultivars was significantly affected by heat stress, which may reveal the heat sensitivity of the cultivars. Our results were in agreement with the findings of Kumar et al. [] in chickpea, Sita et al. [] in lentil, and Hu et al. [] in tall fescue, who reported higher EL under heat stress. The increase in lipid peroxidation might be as a result of the overproduction and accumulation of ROS, which then causes membrane peroxidation, protein degradation, and DNA damage to severely inhibit growth [,,]. Consequently, our results revealed that heat stress highly damaged the membrane integrity and stability of alfalfa, especially heat-sensitive cultivars. However, some cultivars like Bara310SC and Magna995 had lower EL and MDA content than others, indicating that these cultivars could maintain their membrane integrity and stability under heat stress. It has been reported that the maintenance of membrane integrity and stability under stress conditions is a major component of tolerance [] and is essential to sustained photosynthetic and respiratory performance []. Thus, Bara310SC and Magna995 with lower EL and MDA content after heat stress could be heat tolerant.
It is well established that photosynthetic pigments such as chlorophyll a and b are sensitive to high-temperature stress. Heat stress results in plant leaf pigment loss and significantly damages photosynthetic activities []. In the present study, heat treatment decreased the chlorophyll content (Chl a, Chl b, and total Chl) of all alfalfa cultivars, and a more pronounced effect was observed in heat-sensitive cultivars. The decrease in chlorophyll content might be attributed to the chlorophyll degradation or inhibition of chlorophyll biosynthesis []. In addition, the effect of high temperature on the pigments and other photosynthetic apparatus is due to the production of toxic oxygen species (oxidative damage) and reduction in antioxidative defense []. Thus, significant increases in EL and MDA content and a decrease in chlorophyll content might be interconnected, indicating the heat sensitivity of the cultivars. Our result revealed that cultivars with higher EL and MDA content had lower chlorophyll content, biomass, and RWC, suggesting that heat stress had a greater effect on some cultivars including WL712, Gibraltar, and Golden Queen.
When exposed to heat stress, plants accumulate compatible solutes, such as soluble sugar, to protect the plant from stress-induced damage by maintaining membrane stability and cell water balance, and by buffering the cellular redox potential and homeostasis []. The accumulation of compatible solute is an important adaptive mechanism, directly participating in osmotic adjustment []. Similarly, soluble proteins, which are induced by stress, play a role in stress tolerance, presumably via hydration of cellular structures []. In the current study, heat stress increased the soluble sugar and protein content of alfalfa plants. We found significant and higher soluble sugar content in Bara310SC, Magna995, WL363HQ. These cultivars could adjust their osmotic balance and cellular homeostasis, which is one of the tolerance mechanisms. Similar results were reported in lettuce [] and moth bean []. In this study, we found higher relative water content in heat-tolerant alfalfa, which was in agreement with soluble sugar and protein content, thus entailing great implications for heat tolerance []. The result revealed that soluble sugar and protein could play a considerable role for alfalfa heat tolerance by maintaining the water balance and cellular homeostasis. In this study, cultivars with higher soluble sugar and protein content had lower lipid peroxidation and membrane damage under heat stress, which implies that soluble sugar and protein ameliorates heat-induced damage in those cultivars. Our results are also supported by those of Lang-Mladek et al. [], who stated that osmolyte production under heat stress is thought to increase protein stability and stabilize the structure of the membrane bilayer. Similarly, Khan et al. [] and Kumar et al. [] found significantly higher soluble protein content in wheat under heat stress, and maximum accumulation of soluble protein content was observed in thermotolerant wheat genotypes, which was similar with our result. The overall results indicate that soluble sugar and protein play a remarkable role in alleviating heat-induced damage of alfalfa, while increasing heat tolerance.
The heat tolerance of alfalfa was evaluated comprehensively by membership function value, and the results showed that alfalfa cultivars had different sensitivities to heat stress. Cultivars with higher membership function value were considered as heat tolerant, whereas cultivars with lower membership function value were heat sensitive. As shown, Bara310SC and Magna995 had higher membership function value, 0.86 and 0.80, respectively, and were considered as relatively heat tolerant. By contrast, with lower membership function value (0.24), Gibraltar and WL712 were relatively heat-sensitive alfalfa. Furthermore, chlorophyll a fluorescence analysis was performed on the above four cultivars to identify the most heat sensitive and heat tolerant alfalfa cultivars. Chlorophyll a fluorescence analysis is a powerful and nondestructive method to study the photosynthetic behavior of plants and is widely applied to screen tolerant species [,]. In this study, heat stress affected the chlorophyll a fluorescence and altered the OJIP fluorescence transient curve of all alfalfa cultivars. The change in the OJIP fluorescence transient curve might have been caused by the oxidation of electron transport chains, which results from the reduction in the electron donor of PSII reaction centers under high temperature. Basic photosynthetic parameters were extracted from fluorescence transient and the results showed that heat stress increased the F0 and decreased Fv/Fm of all alfalfa cultivars, which were significant for sensitive cultivars (WL712). Higher F0 indicates the elevated damage of chloroplast by heat stress, resulting in blocked energy transfer to the PS II traps and a decrease of the quantum efficiency of PS II []. Fv/Fm is commonly used to analyze heat-induced damage to PSII [], and heat stress decreases Fv/Fm in a range of plant species []. For many plant species, the approximate optimal Fv/Fm value is in the range of 0.79 to 0.84, with lowered values indicating plant stress []. The heat-tolerant cultivar (Bara310SC) had 0.79 and 0.80 Fv/Fm under heat stress and control condition, respectively.
Heat stress altered the specific energy flux parameters (TP0/RC, ETO/RC, RE0/RC, and ABS/RC) of all alfalfa cultivars. ABS/RC and TR0/RC were higher under heat stress, which indicates the inactivation of absorption and trapping reaction centers. Similar results have been reported by Zushi et al. [] in tomato leaf and fruit under heat stress. In addition, heat stress markedly altered the quantum yield and efficiency parameters (φpo, φEo, and δRo). The result revealed that the behaviors of PS II on both the electron donor and acceptor side were blocked due to heat stress and PSI was less damaged than PSII []. The energy fluxes such as φpo, φEo, and RC/ABS of PS II were lower, whereas δRo was higher under heat treatment. Similarly, the decrease in RC/ABS was observed in heat-stressed Spirulina []. On the other hand, Stefanov et al. [] reported an increase of δRo in bean plants immediately after heat treatment. This result suggested a difference in energy flux between PS I and PS II in response to heat. It was reported that PS II is the most temperature-sensitive component of the photosynthetic apparatus [,]. Our results also confirmed that PS II is more sensitive to heat stress than PS I, which could be as a result of thylakoid membrane fluidity caused by heat stress.
Alteration of specific energy fluxes and quantum yield efficiency of the photosystem could affect the overall photosynthetic performance of alfalfa. Performance indexes (PIABS and PItotal) were measured to investigate the changes in leaf photosynthetic performance. The performance index (PIABS) is a parameter sensitive to various types of stress. PItotal reveals the changes in intersystem electrons and the energy conservation from exciton to the reduction of PSI end acceptors []. Heat stress noticeably decreased the performance indexes of all heat-treated alfalfa cultivars, and a significant decrease was observed in sensitive cultivar (WL712). Another heat sensitive cultivar (Gibraltar) showed a significant reduction in PIABS. A similar result was reported in tall fescue under heat stress []. In addition, Fahad et al. [] reported a significant reduction in the photosynthetic activities of two rice cultivars under high day and night temperatures. The decrease in performance indexes could indicate the lower photochemistry of PSII []. However, heat stress had no significant effect on the performance indexes of heat-tolerant cultivars (Bara310SC and Magna995), and Bara310SC showed higher performance indexes under heat stress than others. The result revealed that Bara310SC was more tolerant to heat stress compared to others under heat stress. The overall Chl a fluorescence analysis results suggested that Bara310SC and WL712 are the most heat-tolerant and heat-sensitive alfalfa cultivars, respectively. Further studies will be done to understand the molecular mechanisms of the heat tolerance of alfalfa.
5. Conclusions
Heat stress affected the biomass and physiological characteristics of alfalfa cultivars. The more pronounced effect was observed in sensitive cultivars such as WL712, Gibraltar, and Golden Queen, as evidenced by a significant decrease in biomass, RWC, chlorophyll content, and photosynthetic performance, and significant increases in EL and MDA under heat stress. Heat-tolerant cultivars showed significantly higher soluble sugar and protein content and performed better under heat stress. Among the fifteen cultivars evaluated, Bara310SC was the most heat tolerant and WL712 was the most heat-sensitive one. These cultivars can be used to explore the molecular mechanisms of heat tolerance in alfalfa plants.
Author Contributions
Conceptualization, M.W. and L.C.; methodology, M.W., W.Z. and Q.Z.; software, M.W. and K.J.; validation, M.W.; W.Z. and L.C.; formal analysis, M.W.; investigation, M.W.; resources, L.C.; data curation, M.W., Q.Z. and W.Z.; writing—original draft preparation, M.W.; writing—review and editing, M.W. and L.C.; visualization, M.W.; supervision, L.C. and K.J.; project administration, L.C.; funding acquisition, L.C.
Funding
This work was funded by the National Natural Science Foundation of China (NSFC) (Grant Nos. 31672482 and 31401915).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahuja, I.; de Vos, R.C.H.; Bones, A.M.; Hall, R.D. Plant molecular stress responses face climate change. Trends Plant Sci. 2010, 15, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Bañon, S.; Fernandez, J.A.; Franco, J.A.; Torrecillas, A.; Alarcón, J.J.; Sánchez-Blanco, M.J. Effects of water stress and night temperature preconditioning on water relations and morphological and anatomical changes of Lotus creticus plants. Sci. Hortic. 2004, 101, 333–342. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Li, S.; Li, F.; Wang, J.; Zhang, W.E.N.; Meng, Q.; Chen, T.H.H.; Lycopersicon, T. Glycinebetaine enhances the tolerance of tomato plants to high temperature during germination of seeds and growth of seedlings. Plant Cell Environ. 2011, 34, 1931–1943. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, P.; Gu, M.S. Diagnosis of abiotic and biotic stress factors using the visible symptoms in foliage. Environ. Pollut. 2005, 137. [Google Scholar] [CrossRef] [PubMed]
- Bi, A.; Fan, J.; Hu, Z.; Wang, G.; Amombo, E.; Fu, J.; Hu, T. Differential Acclimation of Enzymatic Antioxidant Metabolism and Photosystem II Photochemistry in Tall Fescue under Drought and Heat and the Combined Stresses. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Hu, T.; Liu, S.; Amombo, E.; Fu, J. Stress memory induced rearrangements of HSP transcription, photosystem II photochemistry and metabolism of tall fescue (Festuca arundinacea Schreb) in response to high-temperature stress. Front. Plant Sci. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Xu, W.; Cai, S.; Zhang, Y.; Wang, Y.; Ahammed, G.J.; Xia, X.; Campus, Z. Melatonin enhances thermotolerance by promoting cellular protein protection in tomato plants. Front. Plant Sci. 2016. [Google Scholar] [CrossRef]
- Potters, G.; Pasternak, T.P.; Guisez, Y.; Palme, K.J.; Jansen, M.A.K. Stress-induced morphogenic responses: Growing out of trouble? Trends Plant Sci. 2007, 12. [Google Scholar] [CrossRef]
- Sade, B.; Soylu, S.; Yetim, E. Drought and oxidative stress. Afr. J. Biotechnol. 2011, 10, 11102–11109. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Liang, G.; Shi, W.; Xie, J. Metabolic responses of alfalfa (Medicago Sativa L.) leaves to low and high temperature induced stresses. Afr. J. Biotechnol. 2011, 10, 1117–1124. [Google Scholar] [CrossRef]
- Song, Y.; Lv, J.; Ma, Z.; Dong, W. The mechanism of alfalfa (Medicago sativa L.) response to abiotic stress. Plant Growth Regul. 2019. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Irigoyen, J.J.; Sánchez-Díaz, M. Effect of increased temperature and drought associated to climate change on productivity of nodulated alfalfa. Quality in lucerne and medics for animal production. In Proceedings of the XIV Eucarpia Medicago spp. Group Meeting, Zaragoza and Lleida, Lleida, Spain, 12–15 January 2001. [Google Scholar]
- Aranjuelo, I.; Irigoyen, J.J.; Sanchez-Diaz, M. Effect of elevated temperature and water availability on CO2 exchange and nitrogen fixation of nodulated alfalfa plants. Environ. Exp. Bot. 2007, 59, 99–108. [Google Scholar] [CrossRef]
- Erice, G.; Irigoyen, J.J.; Sánchez-Díaz, M.; Avice, J.C.; Ourry, A. Effect of drought, elevated CO2 and temperature on accumulation of N and vegetative storage proteins (VSP) in taproot of nodulated alfalfa before and after cutting. Plant Sci. 2007, 172, 903–912. [Google Scholar] [CrossRef]
- Li, W.; Wei, Z.; Qiao, Z.; Wu, Z.; Cheng, L.; Wang, Y. Proteomics analysis of alfalfa response to heat stress. PLoS ONE 2013, 8, e82725. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Zhang, Z.; Liu, N.; Li, D.; Hu, L. Melatonin Improved Waterlogging Tolerance in Alfalfa (Medicago sativa) by Reprogramming Polyamine and Ethylene Metabolism. Front. Plant Sci. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Huang, X.; Shi, H.; Hu, Z.; Liu, A.; Amombo, E.; Chen, L.; Fu, J. ABA Is Involved in Regulation of Cold Stress Response in Bermudagrass. Front. Plant Sci. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Hu, L.; Bi, A.; Hu, Z.; Amombo, E.; Li, H.; Fu, J. Antioxidant Metabolism, Photosystem II, and Fatty Acid Composition of Two Tall Fescue Genotypes with Different Heat Tolerance Under High Temperature Stress. Front. Plant Sci. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 254, 248–254. [Google Scholar] [CrossRef]
- Tian, Z.; Yang, Y.; Wang, F. A comprehensive evaluation of heat tolerance in nine cultivars of marigold. Hortic. Environ. Biotechnol. 2015, 56, 749–755. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Al-Khaishany, M.Y.; Al-Qutami, M.A.; Al-Whaibi, M.H.; Grover, A.; Ali, H.M.; Al-Wahibi, M.S. Morphological and physiological characterization of different genotypes of faba bean under heat stress. Saudi J. Biol. Sci. 2015, 22, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Hafeez, M. Thermotolerance of pearl millet and maize at early growth stages: Growth and nutrient relations. Biol. Plant. 2004, 48, 81–86. [Google Scholar] [CrossRef]
- Wahid, A.; Close, T.J. Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol. Plant. 2007, 51, 104–109. [Google Scholar] [CrossRef]
- Hameed, A.; Goher, M.; Iqbal, N. Heat Stress-Induced Cell Death, Changes in Antioxidants, Lipid Peroxidation, and Protease Activity in Wheat Leaves. J. Plant Growth Regul. 2012, 31, 283–291. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Saud, S.; Hassan, S.; Tanveer, M. A combined application of biochar and phosphorus alleviates heat-induced adversities on physiological, agronomical and quality attributes of rice. Plant Physiol. Biochem. 2016, 103, 191–198. [Google Scholar] [CrossRef]
- Sita, K.; Sehgal, A.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.M.; Nayyar, H. Identification of High-Temperature Tolerant Lentil (Lens culinaris Medik.) Genotypes through Leaf and Pollen Traits. Front. Plant Sci. 2017, 8, 1–27. [Google Scholar] [CrossRef]
- Dias, A.S.; Barreiro, M.G.; Campos, P.S.; Ramalho, J.C.; Lidon, F.C. Wheat cellular membrane thermotolerance under heat stress. J. Agron. Crop Sci. 2010, 196, 100–108. [Google Scholar] [CrossRef]
- Kumar, S.; Thakur, P.; Kaushal, N.; Malik, J.A.; Gaur, P.; Nayyar, H. Effect of varying high temperatures during reproductive growth on reproductive function, oxidative stress and seed yield in chickpea genotypes differing in heat sensitivity. Arch. Agron. Soil Sci. 2013, 59, 823–843. [Google Scholar] [CrossRef]
- Awasthi, R.; Bhandari, K.; Nayyar, H. Temperature stress and redox homeostasis in agricultural crops. Front. Environ. Sci. 2015, 3. [Google Scholar] [CrossRef]
- Chakraborty, U.; Pradhan, B. Drought stress-induced oxidative stress and antioxidative responses in four wheat (Triticum aestivum L.) varieties. Arch. Agron. Soil Sci. 2012, 58, 617–630. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.-L.; Zhang, R.-X.; Yuan, H.-Y.; Wang, M.-M.; Yang, H.-Y.; Liang, Z.-W. Root Damage under Alkaline Stress Is Associated with Reactive Oxygen Species Accumulation in Rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bajji, M.; Kinet, J.M.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Chen, J.; Wang, P.; Mi, H.L.; Chen, G.Y.; Xu, D.Q. Reversible association of ribulose-1, 5-bisphosphate carboxylase/oxygenase activase with the thylakoid membrane depends upon the ATP level and pH in rice without heat stress. J. Exp. Bot. 2010, 61, 2939–2950. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, R.; Kaushal, N.; Vadez, V.; Turner, N.C.; Berger, J.; Siddique, K.H.M.; Nayyar, H. Individual and combined effects of transient drought and heat stress on carbon assimilation and seed filling in chickpea. Funct. Plant Biol. 2014, 41, 1148–1167. [Google Scholar] [CrossRef]
- Dutta, S.; Mohanty, S.; Tripathy, B.C. Role of Temperature Stress on Chloroplast Biogenesis and Protein Import in Pea. Plant Physiol. 2009, 150, 1050–1061. [Google Scholar] [CrossRef]
- Janska, A.; Marsik, P.; Zelenkova, S.; Ovesna, J. Cold stress and acclimation: What is important for metabolic adjustment? Plant Biol. 2010, 12, 395–405. [Google Scholar] [CrossRef]
- Gupta, N.K.; Agarwal, S.; Agarwal, V.P.; Nathawat, N.S.; Gupta, S.; Singh, G. Effect of short-term heat stress on growth, physiology and antioxidative defence system in wheat seedlings. Acta Physiol. Plant. 2013, 35, 1837–1842. [Google Scholar] [CrossRef]
- Han, Y.; Fan, S.; Zhang, Q.; Wang, Y. Effect of heat stress on the MDA, proline and soluble sugar content in leaf lettuce seedlings. Agric. Sci. 2013, 4, 112–115. [Google Scholar] [CrossRef]
- Harsh, A.; Sharma, Y.K.; Joshi, U.; Rampuria, S.; Singh, G.; Kumar, S.; Sharma, R. Effect of short-term heat stress on total sugars, proline and some antioxidant enzymes in moth bean (Vigna aconitifolia). Ann. Agric. Sci. 2016, 61, 57–64. [Google Scholar] [CrossRef]
- Lang-Mladek, C.; Popova, O.; Kiok, K.; Berlinger, M.; Rakic, B.; Aufsatz, W.; Luschnig, C. Transgenerational inheritance and resetting of stress-induced loss of epigenetic gene silencing in arabidopsis. Mol. Plant 2010, 3, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Din, J.U.; Qayyum, A.; Jan, N.E.; Jenks, M.A. Heat tolerance indicators in Pakistani wheat (Triticum aestivum L.) genotypes. Acta Bot. Croat. 2015, 74, 109–121. [Google Scholar] [CrossRef]
- Kumar, S.; Beena, A.S.; Awana, M.; Singh, A. Physiological, Biochemical, Epigenetic and Molecular Analyses of Wheat (Triticum aestivum) Genotypes with Contrasting Salt Tolerance. Front. Plant Sci. 2017, 8, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Porcar-Castell, A.; Tyystjärvi, E.; Atherton, J.; Van Der Tol, C.; Flexas, J.; Pfündel, E.E.; Berry, J.A. Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: Mechanisms and challenges. J. Exp. Bot. 2014, 65, 4065–4095. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.H.; Winter, K.; Krause, B.; Virgo, A. Light-stimulated heat tolerance in leaves of two neotropical tree species, Ficus insipida and Calophyllum longifolium. Funct. Plant Biol. 2015, 42, 42–51. [Google Scholar] [CrossRef]
- Tan, W.; wei Meng, Q.; Brestic, M.; Olsovska, K.; Yang, X. Photosynthesis is improved by exogenous calcium in heat-stressed tobacco plants. J. Plant Physiol. 2011, 168, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Zushi, K.; Kajiwara, S.; Matsuzoe, N. Chlorophyll a fluorescence OJIP transient as a tool to characterize and evaluate response to heat and chilling stress in tomato leaf and fruit. Sci. Hortic. 2012, 148, 39–46. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, J.; Gong, H.; Wen, X.; Ren, H.; Lu, C. Effects of heat stress on PSII photochemistry in a cyanobacterium Spirulina platensis. Plant Sci. 2008, 175, 556–564. [Google Scholar] [CrossRef]
- Stefanov, D.; Petkova, V.; Denev, I.D. Screening for heat tolerance in common bean (Phaseolus vulgaris L.) lines and cultivars using JIP-test. Sci. Hortic. 2011, 128, 1–6. [Google Scholar] [CrossRef]
- Apostolova, E.L.; Dobrikova, A.G. Effect of high temperature and UV-A radiation on photosystem II. In Handbook of Plant and Crop Stress; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 577–591. [Google Scholar]
- Wen, X.; Qiu, N.; Lu, Q.; Lu, C. Enhanced thermotolerance of photosystem II in salt-adapted plants of the halophyte Artemisia anethifolia. Planta 2005, 220, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Govindjee; Sarin, N.B. Overexpression of γ-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: Physiological and chlorophyll a fluorescence measurements. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1428–1438. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).