Abstract
Root restriction is an agronomic technique that influences plant morphology, physiology, and productivity. This study investigates the effects of root restriction on tomato growth, fruit quality, yield, and rhizosphere microbial communities using three distinct substrates: sand, soil, and peanut shell substrate (PSS), within a Simplified Automatic Soilless Culture System (SAS). Results demonstrated that root restriction at 8 cm height significantly enhanced fruit quality indicators: soluble sugar content increased by 69.01% (sand), 53.84% (soil), and 37.67% (PSS); soluble protein increased by 77.23%, 48.14%, and 66.51%; and lycopene increased by 100.03%, 62.33%, and 74.59%, respectively, compared to the 24 cm baseline. However, single-plant yield declined by 28.30% (sand), 64.28% (soil), and 22.06% (PSS). TOPSIS analysis (Technique for Order Preference by Similarity to Ideal Solution) identified PSS at 8 cm as the optimal combination for balancing quality and yield (Cj = 0.631). Microbial amplicon sequencing revealed higher rhizosphere microbial diversity in tomatoes grown in soil and peanut shell substrate compared to sand. These three types of growing media (soil, sand, and peanut shell substrate) establish the rhizosphere of bacterial and fungal communities by selecting specific microbial taxa. Changes in container height drive the reduction–oxidation functional divergence of bacterial communities, affecting the connectivity and complexity of microbial networks.
1. Introduction
The term “soilless culture” generally refers to any method of growing plants without using soil as a rooting medium [1,2] (Lal, 2017; Savvas, 2003). Presently, the soilless culture systems (SCS) have emerged as a pivotal technological component in modern agriculture, particularly for high-value crops like tomatoes. These systems liberate crop production from dependence on natural soil—a heterogeneous medium often characterized by pathogen prevalence, degradation under monoculture, and variable fertility [3] (Savvas and Gruda, 2018). Research indicates that, compared to traditional soil-based cultivation, soilless culture systems can increase tomato yields by 30–50% and achieve an average marketable yield rate of 92.1%, whereas soil-based cultivation only reaches 77.0% [4] (Maboko et al., 2009). The global expansion of greenhouse tomato cultivation reflects its critical role in meeting rising domestic consumption and export demands, especially in regions with limited arable land. In China, tomato production under protected cultivation has seen remarkable growth, with greenhouse areas expanding significantly to support both local food security and international market supply. While soilless systems offer clear advantages over traditional soil-based cultivation, their container-based nature introduces unique challenges that require careful consideration of root-zone conditions.
Containerized plant production poses two primary challenges to healthy root development. First, unlike natural soil profiles, the shallow growing medium layer in containers rapidly saturates during irrigation. Second, the limited volume of small containers restricts water-holding capacity between irrigation events [5] (Bunt, 2012). Studies have demonstrated that a reduction in root container volume from 16 L to 6 L resulted in a significant 26.51% decrease in tomato biomass, highlighting the impact of physical root restriction on plant growth parameters [6] (Saito et al., 2008). Fundamentally, an effective growing medium must possess a physical structure capable of maintaining an optimal air–water balance during and between irrigation events, preventing root asphyxia and drought stress [7,8] (Caron and Nkongolo, 1999; Fonteno, 1993). Soil’s failure to sustain this balance within such constrained volumes has been a key catalyst for the development of soilless substrates. Indeed, these substrates represent a breakthrough innovation, enabling growers to precisely regulate water, air, and nutrient delivery to plant roots while excluding soilborne pathogens [9] (Raviv et al., 2002). In this context, research on utilizing local agricultural waste materials as sustainable alternatives is increasingly receiving attention. Green compost, as an organic substrate, is derived from plant residues after composting treatment. It is a good source of potassium and trace nutrients, suppresses diseases, has good water retention capacity, and reduces urban waste. Its composition varies, with a bulk density of 600–950 kg/m3, and may contain excessive salt levels, requiring time for composting treatment, and is prone to waterlogging [1,10] (Gianquinto et al., 2006; Lal, 2017). Among these, peanut shell substrate (PSS) represents a promising option due to its wide availability, low cost, and favorable physical properties. In major peanut-producing regions, the conversion of agricultural waste into valuable growing media not only addresses waste management challenges but also enhances economic efficiency by reducing dependence on imported substrates. In particular, the use of local agricultural waste materials as sustainable alternatives has gained increasing attention, with peanut shell substrate (PSS) emerging as a promising option due to its favorable physical properties and potential for waste valorization.
In traditional soil cultivation, plant root systems have unrestricted volume, whereas in soilless cultivation, root growth is constrained by container size, leading to varying degrees of root system limitation. Root restriction, as a form of abiotic stress, exerts both direct and indirect influences on plant morphology and physiology [11] (Gao et al., 2023). Studies demonstrate that implementing moderate root restriction (container height of 8–15 cm) enhances tomato quality parameters, with total soluble solids increasing by 35.48% and lycopene content rising by 97.05%, while potentially decreasing yield by 29.26% [11] (Gao et al., 2023). By adjusting container dimensions appropriately, this technique can enhance plant quality and container utilization efficiency. Currently, root restriction has been effectively applied to diverse crops, including wolfberry [12] (He et al., 2023), chili peppers [13] (Zakaria et al., 2020), and grapes [14] (Xu et al., 2021). Empirical studies have demonstrated that in root-restricted tomato plants, primary roots predominantly proliferate toward the container base, producing numerous shorter lateral roots (LRs) that densely fill the entire volume to form a root mat [15] (Peterson et al., 1991). This configuration enhances gas exchange efficiency by mitigating the adverse effects of oxygen deficiency while improving nutrient-uptake capacity [16] (Balliu et al., 2021). Although larger container sizes typically correlate with higher yields [16] (Balliu et al., 2021), emerging evidence highlights that root restriction elevates levels of primary metabolites, such as soluble sugars, amino acids, and lipids [17] (Leng et al., 2021), and secondary biomolecules including carotenoids, flavonoids, phenolic acids, and alkaloids in fruits [11] (Gao et al., 2023). These metabolic enhancements are conducive to improving fruit quality and bioactive compound content [18] (Leng et al., 2022). However, the effects of root restriction cannot be considered in isolation from the growing medium itself, which actively shapes the rhizosphere environment through its physical and chemical properties.
Studies have shown that soilless organic growing media have distinct ecological niches for diverse bacterial communities, which exhibit temporal functional stability [19] (Grunert et al., 2016). Newly prepared or sterilized growing media often lack a diverse and competitive microbiome [19,20,21] (Grunert et al., 2016; Postma, 2010; Raviv et al., 2019), whereas natural soil typically harbors up to 107–109 bacterial colony-forming units (CFU) and 104–106 culturable fungal propagules per gram of soil [22] (Alexander, 1978). It has been hypothesized that organic peat-based growing media used in tomato cultivation systems are primarily colonized by fungi, Actinomycetes, and Trichoderma species [23,24] (Koohakan et al., 2004; Sammar Khalil and Alsanius, 2001), while mineral-based growing media are dominated by bacterial communities [25,26] (Grunert et al., 2020; Vallance et al., 2011). In the rhizosphere, bacteria emerge as one of the most abundant and diverse microbial groups. They play pivotal roles in nutrient cycling processes, including nitrogen fixation, phosphorus solubilization, and potassium activation, while also contributing to disease suppression and plant growth promotion through the synthesis of various hormones and enzymes [27,28] (Chepsergon and Moleleki, 2023; Kong and Liu, 2022). Fungi are integral to this ecosystem: symbiotic mycorrhizal fungi form mutually beneficial associations with plant roots to enhance water and nutrient uptake, whereas free-living fungi facilitate the decomposition of organic matter, further driving nutrient cycling [29,30] (Grondin et al., 2024; Thepbandit and Athinuwat, 2024). Beneficial microorganisms such as arbuscular mycorrhizal fungi can increase plant phosphorus absorption efficiency by 7–17% [31] (Beslemes et al., 2023). This knowledge gap is particularly relevant when considering the potential of agricultural waste-derived substrates like peanut shell substrate, which may offer unique microbial habitats that influence plant performance under root restriction.
While previous studies have independently investigated soilless substrates, root restriction techniques, and rhizosphere microbiology, there is a notable lack of integrated research examining their interactive effects. Most existing research focuses on single-factor effects, overlooking the potential synergies or trade-offs that may emerge when combining these approaches. Specifically, the triple interaction among substrate type, restriction intensity, and microbial community dynamics remains poorly understood, particularly in the context of agricultural waste valorization.
This study investigates the integrated effects of root restriction and substrate type on tomato performance within a Simplified Automatic Soilless Cultivation System (SAS). By examining three distinct substrates—soil, sand, and locally sourced peanut shell substrate—across different restriction heights, we aim to identify optimal combinations that balance fruit quality, yield, and resource efficiency. Furthermore, we explore how these factors influence rhizosphere microbial communities, which play vital roles in nutrient cycling and plant health. The findings provide insights into sustainable intensification of tomato production while demonstrating the valorization of local agricultural residues (Figure 1).
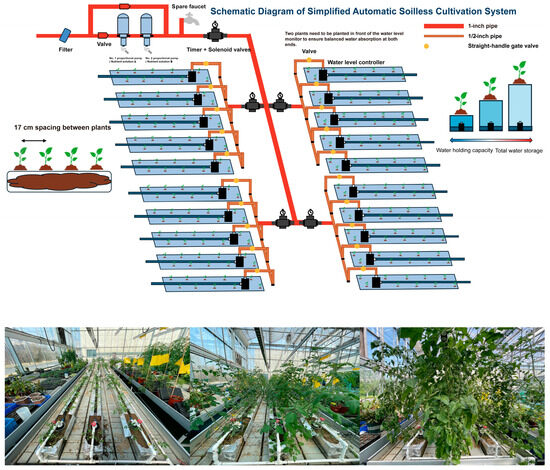
Figure 1.
Schematic diagram of a simplified automatic soilless cultivation system.
2. Materials and Methods
2.1. Plant Materials and Cultivation Facilities
The experiment used cherry tomato ‘Marinka’ as the test material. Plants were grown in a Simplified Automatic Soilless Cultivation System (SAS) located in Greenhouse A at the Agricultural Experiment Station of Zijingang Campus, Zhejiang University, Hangzhou, China. The cultivation period spanned from 14 January to 14 June 2024.
Three substrate types were evaluated:
- Fermented peanut shell substrate (PSS)
- Sand (particle size: 0.05–2.0 mm)
- Native soil (collected from the experimental station; coordinates: 120°4′54.1164″ E, 30°18′10.494″ N)
The cultivation system consisted of aluminum troughs (length × width = 280 cm × 17 cm). Each substrate was filled to three different heights: 8 cm, 16 cm, and 24 cm, corresponding to volumes of 38.08 dm3, 76.16 dm3, and 114.24 dm3, respectively. Each treatment combination was replicated three times, with 12 plants per replicate, following a factorial design.
Experimental Design Statistical Basis
The experimental design with three replicates per treatment and twelve plants per replicate was established based on the following statistical principles (Figure 2):
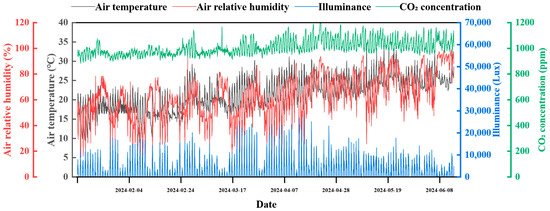
Figure 2.
The air temperature, air relative humidity, illuminance, and CO2 concentration during the experiment.
Statistical Power Considerations:
- Pre-experimental data analysis indicated that three replicates would achieve a statistical power exceeding 80% (Power > 0.8) at a significance level of α = 0.05.
- This design can detect trait differences ≥15% between treatments (including key indicators such as plant height and yield).
- Based on Cohen’s d effect size calculations, the sample size meets the detection requirements for medium effect sizes (d = 0.5).
- The number of replicates complies with conventional standards for agricultural botanical experiments.
- The factorial design (3 substrates × 3 heights) yields 9 treatment combinations, and 3 replicates provide 27 observational units, meeting the fundamental requirements for analysis of variance (ANOVA).
2.2. Environmental Control and Nutrient Management
The greenhouse environment was maintained at 25 ± 2 °C (day) and 18 ± 2 °C (night), with relative humidity at 60–70%. Natural sunlight was utilized, with shade nets adjusting light intensity to 600–800 μmol/m2·s during peak radiation. CO2 concentration was monitored daily and maintained at ambient levels (400–450 ppm).
Hoagland nutrient solution was used with the following composition: Ca(NO3)2·4H2O (945 mg/L), KNO3 (607 mg/L), NH4H2PO4 (115 mg/L), MgSO4·7H2O (493 mg/L), iron chelate (2.5 mL/L), and trace elements (5 mL/L). The electrical conductivity (EC) was maintained at 2.0–2.5 mS/cm, and pH at 6.0. Irrigation was automatically controlled via solenoid valves, with a water-level controller maintaining a 2 cm depth at the trough bottom.
2.3. Experimental Design and Sample Collection
The experiment adopted a 3 × 3 factorial design (3 substrates × 3 heights) with three replications. Irrigation frequency was adjusted based on growth stages: once every three days during seedling establishment, once daily at initial fruiting, and three times daily at peak fruiting.
At key growth stages, the following samples were collected:
- Growth parameters: Plant height, stem diameter, and chlorophyll content at peak fruiting (79–80 days after transplanting).
- Fruit quality and yield: Soluble sugars, proteins, lycopene, and yield per plant at maturity (130 days).
- Rhizosphere samples: Roots with adhering substrate were collected at harvest (150 days) and stored at –80 °C for microbial analysis.
2.4. Measurement of Plant and Soil Parameters
2.4.1. Growth Parameters Measurement
Tomato plant height was measured using a ruler, with 12 plants measured and the average value calculated. Stem diameter was measured using a vernier caliper at three points per plant (1 cm below each inflorescence), with 12 plants sampled and averaged. At the harvesting period, three uniformly growing tomato plants were selected. After excavating the entire root system, the residual substrate was rinsed off with water and blotted dry with gauze. Root activity is determined by the triphenyl tetrazolium chloride (TTC) method [32] (J. Liu et al., 2014). Chlorophyll content in leaves was determined by acetone and anhydrous ethanol extraction followed by spectrophotometric analysis [33] (Palta, 1990). Prepare an extraction solution by mixing acetone and anhydrous ethanol in equal volumes. Collect the second true leaf below the growth point and cut 0.1 g of fresh mature leaf tissue into fine filaments. Immerse the sample in a test tube containing 10 mL of the mixed extraction solution, with three replicates. Perform the extraction in the dark at 40 °C until the plant material turns completely white. Measure the absorbance of the extract at 663 nm and 645 nm using a UV-1800 spectrophotometer (Shimadzu Corporation, Kyoto, Japan), and calculate the chlorophyll content of the leaf tissue according to the formula provided below:
2.4.2. Determination of Fruit Quality and Yield
After fruit ripening, statistically record the yield data for all treatments. The lycopene content was determined by UV spectrophotometry [34] (Rao et al., 1998). Soluble protein content was measured using the Coomassie Brilliant Blue G-250 staining method [35] (Snyder and Desborough, 1978). Soluble sugar content was analyzed via the anthrone colorimetric method [36] (Y. Zhang et al., 2023). Each tomato plant was allowed to retain 5 fruit clusters, and the yield per plant was weighed using a balance. For each treatment, 12 plants were evaluated.
2.4.3. Determination of Substrate Physicochemical Properties
In order to determine the pH, organic matter (SOM), Available Nitrogen (AN), Available Phosphorus (AP), and Available Potassium (AK), it was necessary to air-dry the substrate samples and sieve them after the removal of any debris and plant tissues. The pH of the soil was determined by means of a pH meter, with a water–soil volumetric ratio of 2.5:1. SOM was determined by the external heating method using potassium dichromate. Available Nitrogen (Alkali-hydrolyzable Nitrogen) was determined by the alkaline hydrolysis diffusion method. Soil samples were hydrolyzed with 1.8 mol/L NaOH solution at 40 ± 1 °C for 24 h. The released ammonia was absorbed in a boric acid solution and then titrated with a standard hydrochloric acid solution. Available Phosphorus was extracted with 0.5 mol/L sodium bicarbonate (NaHCO3, pH 8.5) and determined by the molybdenum–antimony colorimetric method. The absorbance of the phospho-molybdenum blue complex was measured at a wavelength of 700 nm using a UV-Vis spectrophotometer. Available Potassium was extracted with 1 mol/L ammonium acetate (NH4OAc, pH 7.0) and its content was determined directly from the extract using flame photometry.
Substrate bulk density, water-holding capacity, and total water storage capacity were determined by the ring knife method [37] (Erbach, 1987). After selecting appropriate sampling points, the cutting ring was vertically driven into the soil surface until fully embedded. The surrounding soil was carefully excavated with a shovel to remove the cutting ring assembly. The upper cutting ring holder was detached, and both ends of the soil column were trimmed with a soil knife to ensure a fixed volume (five replicates for surface soil samples). The entire soil sample from the cutting ring was then transferred to a pre-weighed aluminum box (G0). The combined weight of the box and fresh soil sample was recorded (G1). Subsequently, the sample was oven-dried at 105 °C until reaching constant weight, after which the weight of the dried soil and aluminum box was measured (G2). Bulk density (g/cm3) was calculated as the dry mass of substrate divided by the volume of the cutting ring (100 cm3). The water-holding capacity was determined as the mass ratio of water retained after saturation to dry substrate. Total water storage was derived from the product of water-holding capacity and substrate volume (38.08 dm3, 76.16 dm3, and 114.24 dm3).
Result calculation:
water holding capacity (g/g) = (G1 − G2)/(G2 − G0)
bulk density (g/cm3) = (G2 − G0)/100
total water storage capacity (kg) = substrate volume × bulk density × water holding capacity.
2.4.4. Determination of Bacterial and Fungal Communities in Tomato Rhizosphere
After the tomato harvest, the root system was excavated, and rhizosphere soil was collected and stored at −80 °C for microbial community analysis. Total genomic DNA was extracted from soil samples using the conventional cetyltrimethylammonium bromide (CTAB) method. The integrity and purity of DNA were evaluated via 1% agarose gel electrophoresis, while DNA concentration and purity were assessed using NanoDrop One (Thermo Fisher Scientific, Waltham, MA, USA). The primers used for amplification were the bacterial V5V7-1 region (forward primer sequence: AACMGGATTAGATACCCKG, reverse primer sequence: ACGTCATCCCCACCTTCC) and the fungal ITS2-1 region (forward primer sequence: GCATCGATGAAGAACGCAGC, reverse primer sequence: TCCTCCGCTTATTGATATGC). For PCR amplification and product analysis, genomic DNA was used as the template. Based on the target sequencing regions, primers with barcodes and Premix Ex Taq (Probe qPCR) (TaKaRa Bio Inc., Shiga, Japan) were employed. PCR products were quantified using GeneTools Analysis Software (Version 4.03.05.0, SynGene) and pooled in equimolar ratios. The pooled products were purified using the E.Z.N.A.® Gel Extraction Kit (Omega Bio-Tek, Norcross, GA, USA) to recover target DNA fragments. Subsequent library preparation followed the NEBNext® Ultra™ DNA Library Prep Kit for Illumina® (New England Biolabs, Ipswich, MA, USA) protocol. The prepared libraries were sequenced on the Illumina HiSeq platform. Raw image data from sequencing were processed via base calling to generate raw sequencing reads (FASTQ files), which included sequence information and corresponding quality scores for each read.
2.4.5. Integrated Workflow: Fungal Isolation, Identification, and Inoculation
Fungal strains were initially isolated from peanut shell substrate under strict aseptic conditions, where approximately 1 g of sample was suspended in 9 mL of sterile dilution solution containing 0.1% Tween-80. After preparing serial dilutions, 0.1 ml aliquots were spread onto Rose Bengal Streptomycin Agar plates and incubated at 25–28 °C for 3–7 days. Distinct fungal colonies were subsequently purified by transferring hyphal tips to fresh PDA medium, followed by molecular identification through DNA sequencing and BLAST (version 2.12.0+) alignment against genomic databases. For inoculation experiments, purified fungal mycelia were first cultured in Sabouraud Dextrose Broth for 3 days before being applied to one-month-old tomato plants grown in a peat–vermiculite (2:1) substrate. The inoculation protocol consisted of three root-zone applications at 7-day intervals, with 10 mL of fungal suspension administered each time. Plant phenotypic parameters, including height, stem diameter, and biomass measurements, were systematically evaluated one month post-inoculation to assess the fungal effects on plant growth. This integrated approach ensures methodological rigor from fungal isolation to functional characterization, providing a comprehensive framework for studying plant–fungal interactions.
2.5. Composite Tomato Indicator Using TOPSIS
2.5.1. Establishment of Factors and Subfactors
- (1)
- The tomato indicators were the growth condition, fruit quality, and yield:where u1, u2, u3, and u4 are the growth condition, fruit quality, and yield, respectively.
- (2)
- Each factor was composed of subfactors. The growth condition indicators (u1) were plant height (cm), stem diameter (cm), total chlorophyll content (mg/g), and root activity [mg/(g·h)]. The fruit quality indicators (u2) were soluble sugar (mg/g), soluble protein (mg/g), and lycopene (μg/g) content. The yield indicators (u3) were the single-plant yield (kg/plant):where u11 is plant height, u12 is stem diameter, u13 is total chlorophyll content, and u14 is root activity; u21 is soluble sugar content, u22 is soluble protein content, u23 is the lycopene content, and u31 is single plant yield (Figure 3).
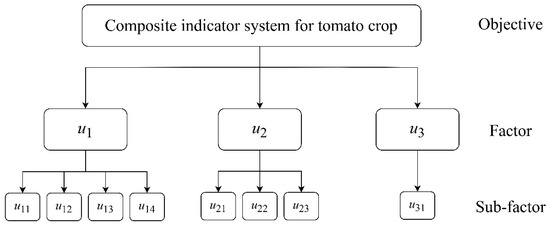 Figure 3. Composite indicator system for tomato crops in which u1, u2, and u3 are, respectively, growth condition, fruit quality, and yield. The subfactors are the following: u11 is plant height, u12 is stem diameter, u13 is total chlorophyll content, u14 is root activity, u21 is soluble sugar content, u22 is soluble protein content, u23 is lycopene content, and u31 is single plant yield.
Figure 3. Composite indicator system for tomato crops in which u1, u2, and u3 are, respectively, growth condition, fruit quality, and yield. The subfactors are the following: u11 is plant height, u12 is stem diameter, u13 is total chlorophyll content, u14 is root activity, u21 is soluble sugar content, u22 is soluble protein content, u23 is lycopene content, and u31 is single plant yield.
2.5.2. Determination of Factor Weights
To eliminate the influence of different dimensions of factors, the measurement values of all tomato indicators were normalized. The weights of the factors were determined through the Analytic Hierarchy Process (AHP).
2.5.3. Composite Indicators of Tomato Based on TOPSIS
Tomato composite indicators were evaluated by TOPSIS, and optimal substrate type and root-restriction level combination for growth, fruit quality, and yield were obtained.
Constructing the decision matrix X,
where represents the th treatment, and represents the th subfactor.
The standardized matrix is denoted as , and each element in is defined as
where is the normalized .
Determining the positive ideal solutions () and the negative ideal solutions ():
Calculating the distance () between and :
Calculating the distance () between and :
where is the weight obtained by the analytic hierarchy process.
Calculating the composite indicators of all treatments:
where . Tomato has an optimal composite indicator for growth, fruit quality, and yield when is close to 1. Ranks are obtained based on .
2.6. Data Analysis
Statistical analysis of tomato phenotype and substrate properties was performed using SPSS 26.0 (IBM Corp., Armonk, NY, USA) and Microsoft Excel 2019. Two-way ANOVA was used for testing, and multiple comparisons were conducted using Duncan’s method and Tukey’s HSD test (p < 0.05). Plotting was performed using GraphPad Prism 10. The correlation network heatmap was generated using R 4.5.0. The TOPSIS comprehensive score was calculated using MATLAB R2024a (MathWorks Inc., Natick, MA, USA). Microbial community analysis was performed using EasyAmplicon [38] (Liu et al., 2023) for batch processing of sequencing data, with bacterial functional annotation via FAPROTAX [39] (Louca et al., 2016) and fungal functional annotation using FUNGuild [40] (Nguyen et al., 2016). LEfSe analysis was conducted through the ImageGP 2 online platform [41] (Chen et al., 2024), and network analysis utilized ggClusterNet [42] (Wen et al., 2022).
3. Results
3.1. Substrate Physicochemical Properties
The results indicate that the pH of PSS is significantly lower than that of sand and soil, while the contents of SOM, AN, AP, and AK are significantly higher than those in sand and soil. There is no significant difference in TK content among the three. The SOM contents in sand are significantly lower than those in soil (Table 1).

Table 1.
Physico-chemical properties of substrate (p < 0.05).
In the peanut shell substrate (PSS), both bulk density and water-holding capacity decreased with increasing substrate height. In contrast, soil and sand exhibited no significant reduction in these properties as height increased (Figure 4a,c). Across all three substrates, total water storage capacity significantly increased with greater substrate height (Figure 4b). The total water storage of 8 cm PSS was approximately 63.37% of that of 24 cm PSS. At the 8 cm height, bulk density measurements were 1.35 g/cm3 (soil), 1.53 g/cm3 (sand), and 0.22 g/cm3 (PSS). While soil and sand showed similar bulk densities, PSS was significantly lower than both. Notably, PSS demonstrated substantially higher water holding capacity than soil and sand: at 8 cm height, values reached 0.34 g/g (soil), 0.17 g/g (sand), and 3.41 g/g (PSS). These results indicate that PSS is markedly lighter than soil or sand in containers of equivalent volume yet possesses far superior water absorption capacity. Even at shallow root-restricting heights or in limited container volumes, PSS can retain 200–300% of its own weight in water.
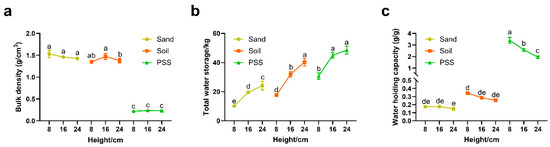
Figure 4.
Substrate physical properties under different substrates and heights: (a) bulk density; (b) total water storage; and (c) water holding capacity. The different lowercase letters (a, b, c, d, e) above the bars indicate statistically significant differences among groups based on a post-hoc Tukey’s Honestly Significant Difference (HSD) test following a significant two-way ANOVA (p < 0.05). Groups that do not share a common letter are significantly different from each other.
3.2. Tomato Phenotype
3.2.1. Growth Status
When soil or sand was used as the substrate, tomato plant height, stem diameter, and root activity increased significantly with rising substrate height. In contrast, cultivation in peanut shell substrate (PSS) resulted in no significant changes in these vegetative growth parameters across varying substrate heights (Figure 5a–c). Total chlorophyll content in leaves remained stable with increasing substrate height in soil-based cultivation (Figure 5d). At the 8 cm root-restriction height, tomatoes grown in soil and sand exhibited markedly lower plant height, stem diameter, and root activity compared to those in PSS. However, these differences progressively diminished as substrate height increased. Specifically, at 8 cm height, average stem diameters were 0.75 cm (soil), 0.88 cm (sand), and 1.05 cm (PSS). These results indicate that root restriction suppresses vegetative growth in soil and sand substrates, with more pronounced inhibition in soil than sand at minimal heights.
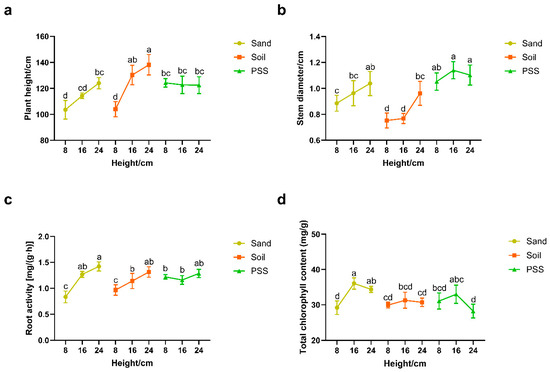
Figure 5.
Tomato growth status under different substrates and heights: (a) plant height; (b) stem diameter; (c) root activity; and (d) total chlorophyll content. The different lowercase letters (a, b, c, d) above the bars indicate statistically significant differences among groups based on a post-hoc Tukey’s Honestly Significant Difference (HSD) test following a significant two-way ANOVA (p < 0.05). Groups that do not share a common letter are significantly different from each other.
3.2.2. Fruit Quality and Yield
In tomato cultivation using soil, sand, or peanut shell substrate (PSS), the contents of soluble sugar, soluble protein, and lycopene in the fruits decreased as substrate height increased (Figure 6a–c). In contrast, single-plant yield increased with greater substrate height (Figure 6d). The root-restriction height of 8 cm increased soluble sugar (+69.01%, 53.84%, and 37.67%), protein (+77.23%, 48.14%, and 66.51%) and lycopene (+100.03%, 62.33%, and 74.59%) compared to 24 cm height in sand, soil and PSS, respectively, but reduced single-plant yield by 28.30% (sand), 64.28% (soil), 22.06% (PSS). At the 8 cm root-restriction height, soil-cultivated tomatoes exhibited significantly higher levels of soluble protein and lycopene compared to those grown in PSS or sand. Regarding yield, tomatoes in PSS at 8 cm height produced 28.30% and 61.90% higher single-plant yield than those in sand and soil. These results indicate that the choice of cultivation substrate significantly influences tomato fruit quality and yield. Root restriction imposed by reducing trough height enhanced fruit quality but reduced yield, an effect particularly pronounced in soil and sand.
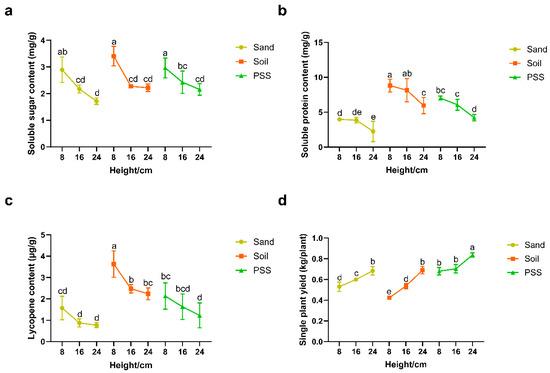
Figure 6.
Tomato quality and yield under different substrates and heights: (a) soluble sugar content; (b) soluble protein content; (c) lycopene content; and (d) single plant yield. The different lowercase letters (a, b, c, d) above the bars indicate statistically significant differences among groups based on a post-hoc Tukey’s Honestly Significant Difference (HSD) test following a significant two-way ANOVA (p < 0.05). Groups that do not share a common letter are significantly different from each other.
3.3. Multivariate Correlation Analysis of Root-Restriction Height Effects
When soil or sand served as substrates, tomato growth parameters (plant height, stem diameter), fruit quality, yield, and substrate properties were significantly affected by changes in substrate height (Figure 7a,b). In contrast, cultivation in peanut shell substrate (PSS) resulted in no significant response of tomato plant height, stem diameter, total chlorophyll content, or root activity to varying substrate heights. However, substrate height still markedly influenced PSS fruit quality, yield, and physical properties (Figure 7c). Specifically, tomato growth in soil and sand exhibited a significant positive correlation with substrate height. Across all substrates, substrate height was negatively correlated with fruit quality but positively correlated with yield. Additionally, water storage capacity showed a positive correlation with yield. Critically, variations in PSS height had no significant impact on tomato growth, indicating that PSS can sustain normal growth even at minimal root-restricting heights. Conversely, soil- and sand-imposed growth inhibition occurs under reduced substrate heights. These results demonstrate that PSS, as an effective green compost, maintains a favorable physical structure in the SAS due to its low bulk density and high water-holding capacity, thereby balancing aeration and water retention during irrigation cycles and mitigating risks of root asphyxiation and drought stress.
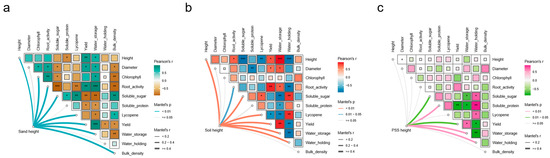
Figure 7.
Correlation analysis of root-restricted height with tomato phenotype and substrate physical properties: (a) soil; (b) sand; and (c) peanut shell substrate (PSS). Significance levels are denoted as follows: * p < 0.05, ** p < 0.01, *** p < 0.001. Statistical analysis was performed using Pearson’s correlation test.
3.4. Comprehensive Evaluation of Tomato Quality and Yield Improvement Based on TOPSIS Method
TOPSIS scores indicate that tomatoes cultivated in soil at a height of 24 cm, sand at a height of 8 cm, and peanut shell substrate (PSS) at 8 cm achieved the highest comprehensive scores within their respective substrates, with ranking orders of 2, 7, and 1, respectively. When used as SAS cultivation substrates, the comprehensive scores under different root-restricting heights followed the order: PSS > soil > sand (Table 2).

Table 2.
TOPSIS scores and rankings for different substrates and root-restriction height treatment combinations.
Based on experimental data, this study establishes an evaluation framework for determining acceptable thresholds between yield loss and quality improvement. The results demonstrate that peanut shell substrate (PSS) at the 8 cm root-restriction height exhibits the most favorable balance, where a 22.06% yield loss is exchanged for a 37.67–74.59% quality improvement. This trade-off efficiency holds significant application value for high-end quality-oriented markets. In contrast, under the same root-restriction conditions, the soil substrate requires enduring a substantial 64.28% yield loss while achieving only a 48.14–62.33% quality enhancement, indicating a considerably less favorable economic threshold.
Utilizing TOPSIS comprehensive evaluation (PSS, 8 cm score: 0.631), acceptable thresholds for different market positions were identified: the high-end organic market can tolerate a yield loss of 20–30% but requires a quality improvement of ≥40%; conversely, the conventional bulk market is better suited for an intermediate approach using PSS at 16 cm, which limits yield loss to 10–15% while achieving a 20–35% quality enhancement. This difference in thresholds is primarily regulated by the physical properties of the substrates—the high water-holding capacity of PSS (3.41 g/g) effectively buffers root-zone stress, whereas the limited aeration of soil and sand substrates narrows their tolerable threshold ranges.
The study recommends that producers adopt a quality-based graded pricing strategy; for instance, a premium of 5–8% can be set for every 10 mg/kg increase in lycopene content, thereby economically compensating for the yield loss at the production level. This threshold system provides a quantitative basis for the precise management of greenhouse tomato cultivation. Further validation of its robustness, incorporating multi-annual market fluctuation data, is necessary in the future (Table 3).

Table 3.
Optimized substrate and root restriction combinations for different market orientations.
3.5. Rhizosphere Microorganisms
3.5.1. Microbial Diversity and Relative Abundance Under Different Substrates
Changes in cultivation substrates significantly altered the α- and β-diversity of bacterial and fungal communities in the tomato rhizosphere. The Shannon index of both bacterial and fungal communities was significantly higher in soil and peanut shell substrate (PSS) than in sand (Figure 8a,b). Principal coordinate analysis (PCoA) revealed distinct compositional differences in rhizosphere microbial communities across substrates (Figure 8c,d). At the phylum level, bacterial communities in all three substrates were dominated by Proteobacteria, Actinobacteria, Bacteroidetes, Candidatus_Saccharibacteria, Chloroflexi, Acidobacteria, and Gemmatimonadetes (Figure 8e). For fungi, Ascomycota predominated in soil (86.34%) and sand (97.22%), whereas PSS comprised Ascomycota (39.69%) and Basidiomycota (51.93%). Notably, the relative abundances of Basidiomycota and Mortierellomycota in PSS significantly exceeded those in soil and sand (Figure 8f). These results demonstrate that soil and PSS supported similar α-diversity levels in rhizosphere bacterial and fungal communities, both significantly higher than sand, while fungal community composition varied substantially among substrates.
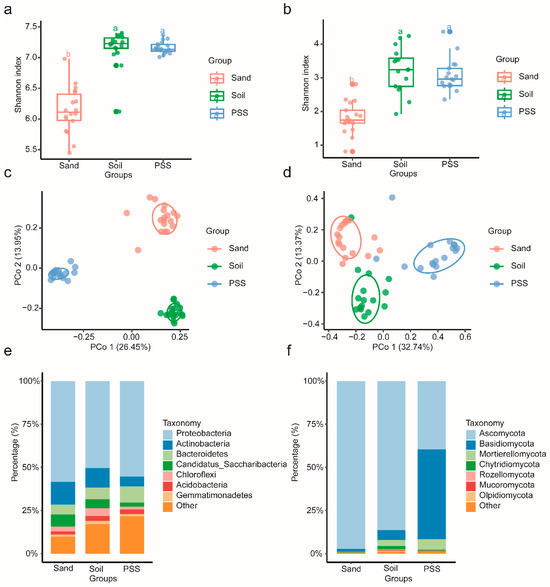
Figure 8.
(a,b) Rhizosphere bacterial and fungal α-diversity under different substrate types. (c,d) Rhizosphere bacterial and fungal β-diversity under different substrate types. (e,f) Microbial relative abundance at the phylum level for rhizosphere bacteria and fungi under different substrate types. The different lowercase letters (a, b) above the lines indicate statistically significant differences among groups based on a post-hoc Tukey’s Honestly Significant Difference (HSD) test following a significant one-way ANOVA (p < 0.05). Groups that do not share a common letter are significantly different from each other.
3.5.2. LEfSe (Linear Discriminant Analysis Effect Size) Analysis and Fungal Inoculation Experiment
Among the three substrates, compositional variations in the rhizosphere microbiome were primarily driven by significant shifts in the relative abundances of 9 specific bacterial phyla and 5 fungal phyla (Linear discriminant analysis effect size “LEfSe”, LDA score > 2, Kruskal–Wallis test, p < 0.05). At the genus level, we identified 14 specific markers in rhizosphere bacteria from soil, 8 in sand, and 17 in peanut shell substrate (PSS). For rhizosphere fungi at the genus level, 24 specific markers were identified in soil, 2 in sand, and 16 in peanut shell substrate (PSS). We noted that Sphingomonas (3.2%), Saccharibacteria_genera_incertae_sedis (2.2%), Nocardioides (1%), and Fusarium (2.4%) were indicator genera in soil; Methylotenera (1.4%), Penicillium (3.1%), and Plectosphaerella (5.2%) were indicator genera in sand; and Aspergillus (5.5%), Microascus (1.4%), and Mortierella (1.8%) were indicator genera in PSS. Notably, among the 16 marker genera detected in PSS rhizosphere fungi, they accounted for 12.5% of the relative abundance, with Aspergillus having the highest abundance at 5.5%. In sand rhizosphere fungi, the 2 detected marker genera accounted for 8.3% of the relative abundance, with Penicillium contributing 3.1% and Plectosphaerella contributing 5.2%. In summary, these results indicate that substrate properties shaped the bacterial and fungal communities inhabiting the root zone by selecting specific microbial taxa.
Correlation analysis revealed that 13 fungal species in PSS exhibited positive correlations with tomato growth, fruit quality, yield, or substrate physical properties, including the indicator genera Aspergillus (5.5%) and Mortierella (1.8%) identified by LEfSe analysis (Figure 9c). Subsequent inoculation experiments demonstrated that tomatoes inoculated with Trichoderma yunnanense (99.83% sequence identity), Aspergillus flavus, and Aspergillus tamarii—all isolated from PSS—showed significantly increased shoot or root biomass after one month, confirming the presence of growth-promoting fungi in PSS that enhance tomato development (Figure 9d–i).
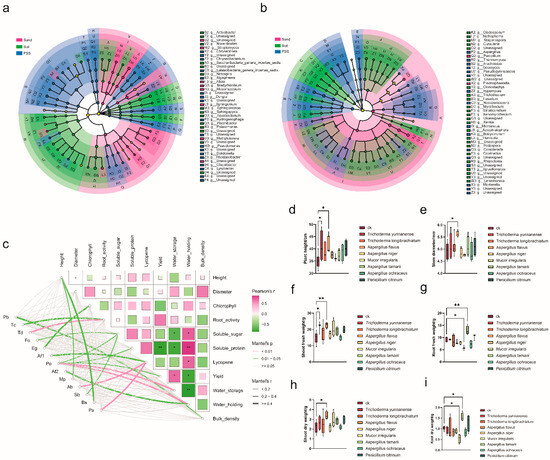
Figure 9.
(a,b) Indicator rhizosphere microorganisms of tomato under different substrates: (a) bacteria, (b) fungi. (c): Correlation analysis of fungi with tomato phenotype and substrate physical properties. Pb: Pseudallescheria boydii; Tc: Trichomonascus ciferrii; Td: Talaromyces diversiformis; Fo: Fusarium oxysporum; Eg: Emericellopsis glabra; Af1: Aspergillus fumigatus; Po: Plectosphaerella oligotrophica; Af2: Aspergillus flavus; Mp: Mortierella polygonia; Ab: Aspergillus brasiliensis; Sb: Scedosporium boydii; Bs: Botryotrichum spirotrichum; Pa: Penicillium astrolabium. (d–i) Phenotypic performance of tomato plants following fungal inoculation: (d) plant height; (e) stem diameter; (f) shoot fresh weight; (g) root fresh weight; (h) shoot dry weight; and (i) root dry weight. Significance levels are denoted as follows: * p < 0.05, ** p < 0.01. Statistical analysis was performed using Pearson’s correlation test.
3.5.3. Microbial Functional Annotation
The relative abundances of functional bacteria involved in carbon, nitrogen, and sulfur cycling were higher in soil and sand, whereas those involved in element cycling were lower in peanut shell substrate (PSS). Specifically, the tomato rhizosphere in soil tended to enrich bacteria associated with denitrification, nitrite respiration, anaerobic photoautotrophic sulfur oxidation, sulfur respiration, and hydrocarbon degradation. In contrast, sand preferentially enriched bacteria involved in nitrification, aerobic nitrite oxidation, nitrogen fixation, urea decomposition, nitrate reduction, nitrate respiration, nitrogen respiration, aerobic sulfide oxidation, and methylotrophic bacteria. By comparison, PSS showed higher relative abundances of bacteria engaged in sulfur compound respiration and sulfate respiration (Figure 10a). These results indicate that soil, with poorer aeration, harbors more anaerobic bacteria, whereas sand, with better aeration, supports bacterial communities inclined toward aerobic lifestyles. With increasing soil height, the relative abundances of functional bacteria related to dark hydrogen oxidation, denitrification, anaerobic photoautotrophic sulfur oxidation, sulfur respiration, and hydrocarbon degradation decreased. Conversely, as sand height increased, the abundances of nitrification, aerobic nitrite oxidation, aerobic sulfide oxidation, and methylotrophic bacteria rose. Additionally, higher heights in PSS correlated with increased abundances of phototrophic and aerobic ammonia-oxidizing bacteria. These findings suggest that elevating substrate height shifted the functional traits of rhizosphere bacteria toward phototrophic and aerobic metabolism.
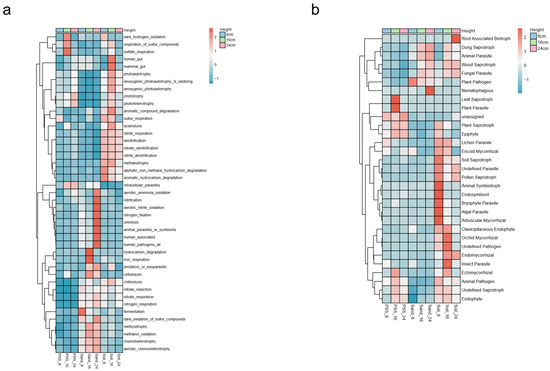
Figure 10.
Functional annotation of rhizosphere microbial communities at varying heights in PSS, sand, and soil: (a) bacteria; (b) fungi.
Saprophytic, symbiotic, parasitic, and epiphytic fungi were all widely present in soil. Fungal lifestyles in sand tended to be saprophytic and parasitic, while those in peanut shell substrate (PSS) tended to be saprophytic, symbiotic, and epiphytic (Figure 10b). These results indicate that PSS were rich in organic matter, thus favoring saprophytic and symbiotic fungal lifestyles; in contrast, sand lacked organic substances, leading fungi to adopt lifestyles such as parasitism and predation. In soil, due to its higher fungal diversity, fungal lifestyles were more diverse than those in sand and peanut shell substrate. With increasing height, the abundances of some saprophytic, symbiotic, and parasitic fungi decreased in soil. In sand, the abundances of saprophytic and parasitic fungi increased with height, while in PSS, the relative abundances of epiphytic, saprophytic, and symbiotic fungi increased with height.
3.5.4. Microbial Network Analysis Under Different Substrates
At the phylum level, we observed that the dominant bacteria involved in interactions across the three substrates were Proteobacteria (Figure 11a–c), and the dominant fungi were Ascomycota (Figure 11d–f). The rhizosphere microbial networks in sand and peanut shell substrate (PSS) exhibited significantly higher numbers of edges, density, and average node degrees compared to soil, with relatively higher modularity. Specifically, the number of edges in soil was 893 versus 8473 in sand and 4637 in PSS; the average node degree was 2.80 in soil, 18.07 in sand, and 10.78 in PSS. For rhizosphere fungi, the number of edges was 929 in soil, 2978 in sand, and 1369 in PSS; the average node degree was 3.53 in soil, 10.29 in sand, and 5.24 in PSS. These results indicate that rhizosphere microbiomes in PSS and sand—particularly those in sand—exhibit strongly clustered network topologies with high connectivity among microbial taxa. This may be attributed to the coarse-textured soil environment, which, when saturated with water, has a larger pore volume and higher connectivity [43,44,45,46] (Ebrahimi and Or, 2014; Holden, 2011; Stewart and Hartge, 1995; Vos et al., 2013). Such properties evidently promote material movement and exchange between microsites, thereby leading to elevated microbial co-occurrence patterns.
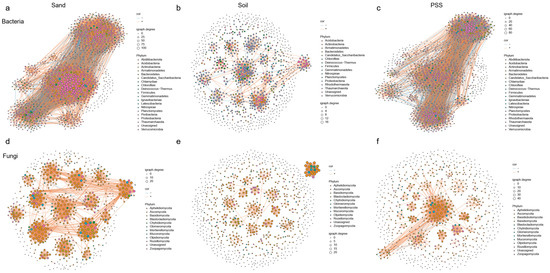
Figure 11.
Microbial networks in tomato rhizosphere under different substrates: (a) bacteria in sand; (b) bacteria in soil; (c) bacteria in peanut shell substrate (PSS); (d) fungi in sand; (e) fungi in soil; (f) fungi in peanut shell substrate (PSS). In the co-occurrence network, each node represents a microbial taxon. Edges (lines) between nodes indicate significant correlations.
3.5.5. Analysis of Microbial Networks Under Different Container Heights
In soil and peanut shell substrate (PSS), when container height increased, the number of edges (L), number of vertices (n), connectance (edge density), average degree (Average K), and relative modularity (RM) of tomato rhizosphere bacterial networks all decreased, whereas in sand, these indices increased. The mean clustering coefficient (Average.CC) increased with increasing container height in all three substrates (Figure 12a–i). At the fungal level, connectance (edge density) in sand decreased with increasing container height, while mean clustering coefficient (Average CC) in PSS increased, and both decreased in soil. Overall, the structure of rhizosphere fungal interaction networks showed no significant response to changes in substrate height (Figure 13a–i). These results indicate that in soil and PSS with greater container heights, the overall rhizosphere bacterial connectivity decreased, and bacterial interactions became less frequent. In soil and PSS at lower container heights, bacterial communities exhibited higher connectivity. The structural changes in bacterial networks of sand were opposite to those of PSS and soil; increasing container height led to more intense interactions in the rhizosphere bacterial network of sand.
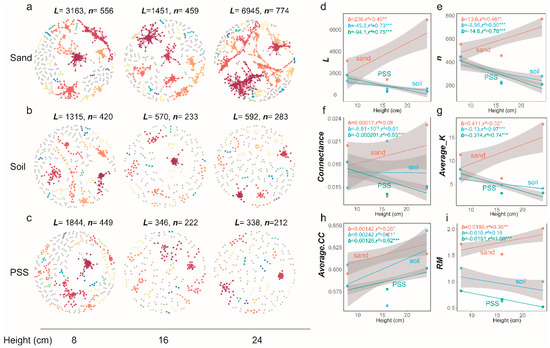
Figure 12.
(a–c) Rhizosphere bacterial network graphs under different substrate heights. Large modules with ≥5 nodes are displayed in distinct colors, while smaller modules are shown in gray. (a–c) Rhizosphere bacterial network graphs at varying heights: (a) rhizosphere bacteria in sand; (b) rhizosphere bacteria in soil; (c) rhizosphere bacteria in PSS. (d–i) Variations in topological properties of rhizosphere bacterial networks with height for sand, soil, and peanut shell substrate, including L (d), n (e), Con (f), Average K (g), Average CC (h), and RM (i). Slopes (b) and adjusted r2 and p-values from linear regressions are shown. * 0.01 < p ≤ 0.05; ** 0.001 < p ≤ 0.01; *** p ≤ 0.001.
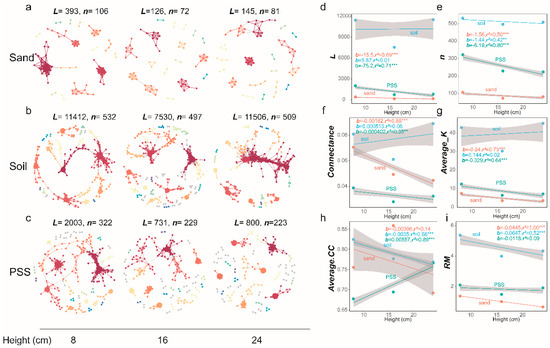
Figure 13.
(a–c) Rhizosphere fungal network graphs under different substrate heights. Large modules with ≥5 nodes are displayed in distinct colors, while smaller modules are shown in gray. (a–c) Rhizosphere fungal network graphs at varying heights: (a) rhizosphere fungi in sand; (b) rhizosphere fungi in soil; (c) rhizosphere fungi in PSS. (d–i) Variations in topological properties of rhizosphere fungal networks with height for sand, soil, and peanut shell substrate, including L (d), n (e), Con (f), Average K (g), Average CC (h), and RM (i). Slopes (b) and adjusted r2 and p-values from linear regressions are shown. ** 0.001 < p ≤ 0.01; *** p ≤ 0.001.
4. Discussion
4.1. Quality–Yield Trade-Off in Root Restriction Cultivation: The Moderating Role of Substrate Properties
In soilless cultivation systems, the height of the cultivation trough and substrate particle size were considered key factors affecting root zone water availability and aeration [3] (Savvas and Gruda, 2018). The height of the substrate layer should be maintained at a sufficiently large scale to ensure optimal drainage and aeration [47] (Heller et al., 2015), whereas substrates with a higher proportion of fine particles demonstrate better suitability for tall and narrow containers, while coarser substrates are more appropriately utilized in shallow bags or troughs [48] (Savvas, 2007). Altering the root-restriction height in the substrate can affect tomato growth, yield, and quality. Studies have shown that root-restricted cultivation of tomatoes reduces the soluble sugar content in their fruits [11,49] (Gao et al., 2023; D. Liu et al., 2023), while other studies demonstrated that root-restricted cultivation reduces tomato yield [50] (Bar-Tal and Pressman, 1996). Although reducing the root-restriction volume can improve quality, it reduces yield by approximately 20–30%. Current research lacks a comprehensive trade-off analysis between “quality gains” and “yield losses”.
Critically, the choice of substrate played a pivotal role in modulating this trade-off. Among the three substrates, PSS at 8 cm height achieved the highest TOPSIS comprehensive score (Cj = 0.631), ranking first overall, indicating an optimal balance between quality and yield (Table 1). In contrast, soil performed best at 24 cm (Cj = 0.558, rank 2), while sand had the lowest scores across heights (e.g., 8 cm sand: Cj = 0.362, rank 7). This superiority of PSS can be attributed to its unique physical properties, including low bulk density (0.22 g/cm3 at 8 cm) and high water-holding capacity (3.41 g/g at 8 cm), which maintained an optimal air–water balance in the root zone even under shallow restriction depths (Figure 4). These properties mitigated drought stress and root asphyxia, allowing sustained plant growth without compromising quality enhancements.
Our study confirms that root restriction enhanced tomato fruit quality (soluble sugars, proteins, and lycopene) but reduced single-plant yield, consistent with findings by Gao and Liu [11,49] (Gao et al., 2023; D. Liu et al., 2023) in tomato. However, the extent of this trade-off was significantly modulated by substrate type. In peanut shell substrate (PSS), the 8 cm root-restriction height caused only 22.06% yield reduction while achieving 37.67–74.59% quality improvement—a more favorable balance than in soil (64.28% yield loss) or sand (28.30% yield loss). The superiority of PSS likely stems from its unique physical structure: low bulk density (0.22 g/cm3) and high water-holding capacity (3.41 g/g) maintained a favorable root-zone water–air balance even under shallow restriction, supporting Savvas’s [48] (Savvas, 2009) proposition that “substrate physical structure determines root restriction effects.”
Notably, the TOPSIS comprehensive evaluation ranked the PSS-8 cm combination first, confirming its advantage in balancing quality and yield while providing quantitative guidance for practical production. This multi-criteria decision-making approach overcomes limitations of single-index evaluations, offering new perspectives for protected cultivation optimization.
4.2. Rhizosphere Microbial Communities: Substrate-Driven Effects from Structure to Function
LEfSe identified indicator genera in tomato rhizospheres under different substrates, revealing substrate-driven functional specialization. In soil, Sphingomonas [51,52] (Asaf et al., 2020; White et al., 1996), Saccharibacteria_genera_incertae_sedis [53] (X. Zhang et al., 2024), and Nocardioides [54] (Ma et al., 2023) have all been reported to participate in the degradation of organic pollutants such as heavy metals, petroleum, and aromatic compounds in soil, which aligns with the functional annotation results showing increased abundance of aromatic compound-degrading bacteria in soil (Figure 11a). Fusarium, on the other hand, has been reported as a genus of plant-pathogenic fungi [55,56,57] (Gordon, 2017; Han et al., 2012; Leplat et al., 2013). These results demonstrate that natural soils were contaminated with organic pollutants and harbor various plant pathogens. In sand, members of Methylotenera were typical methylotrophic bacteria [58] (Li et al., 2025). Penicillium enhances plant growth by interacting synergistically with roots, providing nutrients (e.g., soluble phosphorus) and phytohormones (e.g., IAA, GA). Some species suppress pathogens via antibiotic production, while others boost resistance through systemic resistance induction and defense signaling activation. Certain Penicillium strains are also applied in bioremediation to remediate heavy-metal-polluted soils, and they further contribute to organic matter decomposition and nutrient cycling [59] (Srinivasan et al., 2020). Most Plectosphaerella species have been reported to cause disease symptoms in tomatoes and peppers [60] (Raimondo and Carlucci, 2018). Functional studies on the microbial genera indicator in sand indicate that sand lacks plant-available carbon sources and similarly has plant pathogens. In the peanut shell substrate (PSS), the indicator genus Aspergillus played a critical role in the cycling of major nutrients such as carbon, nitrogen, phosphorus, and sulfur. It exhibited antibacterial activity against other pathogenic fungi; some common or rare species within this fungal group can also produce important plant growth hormones, including auxins, gibberellins, cytokinins, and indole-3-acetic acid (IAA) [61] (Nayak et al., 2020). Mortierella utilizes carbon from polymers (e.g., cellulose, hemicellulose, chitin), enhances bioavailable phosphorus and iron in soil, synthesizes phytohormones and 1-aminocyclopropane-1-carboxylate (ACC) deaminase, and protects crops from pathogens [62] (Ozimek and Hanaka, 2021). Some strains of this genus belonged to plant growth-promoting fungi (PGPF) [63] (Xiong et al., 2017). Previous analyses overlooked how physicochemical properties (such as the high porosity of PSS and the low water retention capacity of sand) drive microbial functional divergence. Our results indicate that substrate properties drive functional divergence of rhizosphere microbial communities through dual pathways: resource availability (e.g., cellulose-derived carbon sources in PSS) and stress selection pressure (e.g., soil pollutants). Soil legacy pollutants (e.g., heavy metals, petroleum hydrocarbons) selectively enrich pollutant-degrading bacteria (e.g., Sphingomonas, Nocardioides) but concurrently elevate risks of pathogen proliferation. In sandy substrates, carbon scarcity drives the enrichment of methylotrophic bacteria (e.g., Methylotenera), which utilize C1 compounds as energy sources. However, limited carbon availability constrains energy-intensive antagonistic traits, resulting in compromised pathogen suppression capacity. Peanut shell substrate (PSS) leverages its lignocellulosic resources and high water-holding capacity to directly recruit synergistic fungal consortia specialized in carbon transformation (e.g., Aspergillus decomposing lignin) and plant growth promotion (e.g., Mortierella synthesizing IAA/ACC deaminase). This substrate-induced microbiome engineering offers novel strategies for optimizing root-zone restriction (RZR) systems by balancing physical confinement stress with beneficial microbial functions.
Our research revealed strong substrate filtering effects on rhizosphere microbial communities. The significanty higher microbial diversity in soil and PSS compared to sand aligns with Grunert [19] (Grunert et al., 2016). More importantly, we identified clear functional differentiation: enrichment of degraders like Sphingomonas and Nocardioides in soil may relate to historical pollution; methylotrophs like Methylotenera in sand reflect carbon scarcity; prominent fungi like Aspergillus and Mortierella in PSS likely associate with its rich organic matter.
Particularly noteworthy is Mortierella as a plant growth-promoting fungi (PGPF) capable of producing auxins and ACC deaminase [62] (Ozimek and Hanaka, 2021), potentially explaining the improved growth and quality in PSS treatments. However, we emphasize that these functional inferences primarily derive from taxonomic annotations, requiring validation through isolation and inoculation experiments.
4.3. Substrate Physical Properties Influence Ecosystem Function via Microbial Networks
Rüger et al. demonstrated that fine-textured soils host small, isolated microbial subcommunities, whereas the rhizosphere microbiome in coarse-textured (high-sand) soils forms a strongly clustered network topology characterized by high microbial connectivity [64] (Rüger et al., 2023). Soil texture defines important habitat properties for soil microbiota, but it also feeds back on root elongation rate and root system architecture. Decreased axial root length in coarse-grained texture levels was compensated for by enhanced lateral root growth. Previous studies have disproportionately focused on bacterial communities, overlooking the contribution of the modular structure of fungal networks to ecosystem stability. Additionally, existing conclusions fail to distinguish the fundamental differences in microbial network formation between artificial substrates and natural soils.
Savvas et al. reported that in soilless cultivation systems, the higher the container height, the lower the water content of the growing medium inside the container and the higher the gas content [48] (Savvas, 2009). Heller et al., using lettuce as a model, investigated the water content of the growing medium in containers of varying heights and found that the water content in tall, narrow containers was lower than that in short, wide containers [47] (Heller et al., 2015). Zhang et al. revealed that diverse metabolic pathways (encompassing aerobic/anaerobic transformations of C, N, S, Fe, and Mn) in soil columns exhibited responsiveness to water table fluctuations [65] (Z. Zhang et al., 2024). Additionally, the vertical stratification of microbial communities was linked to dynamic hydrological conditions, specifically, frequent pulse recharge under infiltration regimes and recurrent groundwater variations under fluctuating regimes. Our annotation results of tomato rhizosphere bacterial communities under different heights reveal that the metabolic pathways of rhizosphere bacteria (aerobic or anaerobic pathways for C, N, and S) responded to changes in container height, and the differentiation of microbial redox functions was related to the hydrological conditions within the container.
This study referenced the methodology of Yuan et al. for analyzing microbial network complexity and examined the variations in network parameters [66] (Yuan et al., 2021)—including the number of edges (L), number of vertices (n), connectance (edge density), average degree (Average K), relative modularity (RM) and mean clustering coefficient (Average.CC)—with root restriction height. The network analysis reveals that increasing container height enhanced bacterial community complexity in sandy rhizosphere while reducing it in PSS and soil rhizospheres, with minimal impact on fungal community complexity across all three substrates (Figure 12).
Upton et al. found more complex and highly connected networks of fungal and bacterial communities in shallower soil layers of grasslands [67] (Upton et al., 2020). Zhao et al. reported that a decline in groundwater levels in grasslands reduced the complexity of microbial co-occurrence networks [68] (Zhao et al., 2023). Banerjee et al. reported that the complexity of bacterial, fungal, and archaeal networks declined with increasing soil depth, alongside reductions in key network parameters, such as node count, edge number, and maximum degree [69] (Banerjee et al., 2021). In our study, we simulated groundwater levels using a water level controller and adjusted the depth of the water level at the bottom of containers by varying container height. The results showed that even in an artificially simulated cultivation system, changes in the structure of microbial co-occurrence networks in PSS and soils were consistent with previous findings: soils and PSS in taller containers had lower water content, lower groundwater levels, reduced overall connectivity of rhizosphere bacteria, and fewer bacterial interactions. Conversely, soils and PSS in shorter containers exhibited higher connectivity in their bacterial communities.
The structural changes in the bacterial network of sand differed from those in PSS and soil. Increasing the height of the cultivation container resulted in more intense interactions in the rhizosphere bacterial network of sand. Characterized by large pores and low water and nutrient retention capacity [1,10] (Gianquinto et al., 2006; Lal, 2017), sand in taller containers formed a thicker layer. Differences in water infiltration and evaporation rates led to a vertical water gradient [65] (Zhang et al., 2024) (with the surface layer drier and the deeper layer wetter). The surface sand layer, with more direct contact with air, has higher oxygen content (an aerobic environment), whereas the deep sand layer, restricted by oxygen diffusion, may develop anaerobic or microaerophilic microenvironments. This oxygen gradient drove redox functional differentiation in the microbial community (e.g., coexistence of aerobic nitrifying bacteria and anaerobic denitrifying bacteria) [65] (Z. Zhang et al., 2024), enhancing functional interactions within the network.
Container height also influenced the intensity of the “edge effect” within the sand layer. In shorter containers, the surface sand layer experienced more frequent contact with the external environment, making it susceptible to disturbances such as temperature fluctuations, drying, or invasion by exotic microorganisms. This reduced the stability of the microbial community, but resident microbes harbored physiological tolerance mechanisms enabling adaptation to fluctuating redox potential conditions [70,71,72] (DeAngelis et al., 2010; Pett-Ridge et al., 2006; Pett-Ridge and Firestone, 2005). In contrast, taller containers provided a larger internal space within the sand layer, weakening the edge effect and stabilizing the internal microenvironment (e.g., smaller temperature and humidity fluctuations). This stability offered long-term colonization opportunities for diverse microorganisms. Such stability allowed more “rare microorganisms” (which might be outcompeted by dominant species in shorter containers) to persist and participate in interactions, thereby increasing the connectivity and complexity of the network.
Microbial co-occurrence network analysis revealed important effects of substrate physical structure on microbial interactions. The higher network complexity and connectivity in sand and PSS may relate to their superior pore structures, echoing Rüger et al. [64] (Rüger et al., 2023) on soil texture effects. The large-pore structure of sand facilitates gas diffusion and microbial migration, while PSS’s fibrous structure provides diverse microbial habitats.
Interestingly, container height differentially affected network structures: increased height reduced bacterial connectivity in soil and PSS, possibly due to moisture gradient-induced redox potential changes [70] (DeAngelis et al., 2010). Conversely, greater height enhanced network complexity in sand, likely through microenvironment differentiation promoting functional complementarity.
5. Conclusions
In this study, we employed the Simplified Automatic Soilless Culture System (SAS) to conduct root-restricted tomato cultivation at different levels. The objective was to identify the optimal coupling pattern of substrate type and container height in the SAS that promotes tomato growth, enhances fruit yield and quality, and to investigate the changes in rhizosphere microorganisms under different substrate types and container heights. The main conclusions are as follows:
- Increasing the height of the cultivation trough can enhance the total water storage capacity of the substrate and fruit yield in the SAS, whereas decreasing the height will improve the fruit quality.
- Using TOPSIS to evaluate the comprehensive indicators of tomatoes, the results indicate that the optimal root-restriction levels for different substrates are 8 cm peanut shell substrate >24 cm soil >8 cm sand.
- Sand, soil, and peanut shell substrate establish bacterial and fungal communities inhabiting roots through the selection of specific microbial taxa.
- High variation in the container can drive the functional differentiation of redox properties in microbial communities, affecting the connectivity and complexity of microbial networks.
5.1. Practical Applications and Limitations
Our findings offer clear guidance for production: PSS with moderate root restriction (8 cm) represents a viable option for premium markets where quality premiums may offset yield reduction. However, the application thresholds proposed in this study are derived from a single-growing-season experiment under controlled environments. For practical implementation, localized verification is essential, accounting for regional climatic differences, cost fluctuations, and market preference variations. Subsequent research will integrate life cycle assessment (LCA) and input–output analysis to better quantify sustainability metrics across various substrate and root-restriction cultivation regimes.
5.2. Future Research Directions
Future studies should (1) verify system stability through multi-season trials; (2) conduct targeted inoculation to confirm key microbial functions; (3) optimize irrigation-nutrition strategies to maximize root restriction benefits; and (4) perform life-cycle assessments to evaluate environmental impacts.
Author Contributions
Y.J.: writing—review and editing, writing—original draft preparation, visualization, validation, software, project administration, methodology, investigation, formal analysis, data curation, and conceptualization. S.X.: visualization, validation. H.Z.: supervision. L.W.: supervision, funding acquisition. Y.Z.: supervision. J.Z.: visualization, validation, and methodology. X.X.: visualization, validation, methodology, and funding acquisition. N.S.: writing—review and editing, conceptualization. Z.Q.: writing—review and editing, supervision, software, resources, project administration, methodology, investigation, formal analysis, data curation, and conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Science and Technology Project of Wencheng County: 2023NKY06, Innovative Development of Horticulture Discipline of Zhejiang University (B231220.0005-25), Organized Scientific Research in Horticulture Discipline of Zhejiang University (B231220.0005-26), the National Key Research and Development Program of China (2024YFD2300704) and the Fundamental Research Funds for the Zhejiang Provincial Universities (2024QZJH67).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lal, R. Encyclopedia of Soil Science; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Savvas, D. Hydroponics: A modern technology supporting the application of integrated crop management in greenhouse. J. Food Agric. Environ. 2003, 1, 80–86. [Google Scholar]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry-A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Maboko, M.; Du Plooy, C.; Bertling, I. Comparative performance of tomato cultivars in soilless vs. in-soil production systems. Acta Hortic. 2009, 843, 319–326. [Google Scholar] [CrossRef]
- Bunt, B.R. Media and Mixes for Container-Grown Plants: A Manual on the Preparation and Use of Growing Media for Pot Plants; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Saito, T.; Fukuda, N.; Iikubo, T.; Inai, S.; Fujii, T.; Konishi, C.; Ezura, H. Effects of Root-volume Restriction and Salinity on the Fruit Yield and Quality of Processing Tomato. J. Jpn. Soc. Hortic. Sci. 2008, 77, 165–172. [Google Scholar] [CrossRef]
- Caron, J.; Nkongolo, V. Aeration in growing media: Recent developments. Acta Hortic. 1999, 481, 545–551. [Google Scholar] [CrossRef]
- Fonteno, W.C. Problems & Considerations in Determining Physical Properties of Horticultural Substrates. Acta Hortic. 1993, 342, 197–204. [Google Scholar] [CrossRef]
- Raviv, M.; Wallach, R.; Silber, A.; Bar-Tal, A. Substrates and their analysis. In Hydroponic Production of Vegetables and Ornamentals; Embryo: Berlin, Germany, 2002; pp. 25–102. [Google Scholar]
- Gianquinto, G.; Orsini, F.; Michelon, N.; da Silva, D.; de Faria, F. Improving yield of vegetables by using soilless micro-garden technologies in peri-urban area of North-East Brazil. VIII Int. Symp. Prot. Cultiv. Mild Winter Clim. Adv. Soil Soil. Cultiv. Under 2006, 747, 57–65. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, X.; Liang, H.; Ji, Y.; Liu, M. Effects of Comparative Metabolism on Tomato Fruit Quality under Different Levels of Root Restriction. HortScience 2023, 58, 885–892. [Google Scholar] [CrossRef]
- He, J.; Li, X.; Tian, Y.; He, X.; Qin, K.; Zhu, L.; Cao, Y. Effect of Lycium barbarum L. Root Restriction Cultivation Method on Plant Growth and Soil Bacterial Community Abundance. Agronomy 2023, 13, 14. [Google Scholar] [CrossRef]
- Zakaria, N.I.; Ismail, M.R.; Awang, Y.; Megat Wahab, P.E.; Berahim, Z. Effect of Root Restriction on the Growth, Photosynthesis Rate, and Source and Sink Relationship of Chilli (Capsicum annuum L.) Grown in Soilless Culture. BioMed Res. Int. 2020, 2020, 2706937. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, J.; Wang, R.; Wang, L.; Zhang, C.; Xu, W.; Wang, S.; Jiu, S. The role of strigolactones in the regulation of root system architecture in grapevine (Vitis vinifera L.) in response to root-restriction cultivation. Int. J. Mol. Sci. 2021, 22, 8799. [Google Scholar] [CrossRef]
- Peterson, T.A.; Reinsel, M.D.; Krizek, D.T. Tomato (Lycopersicon esculentum Mill., cv. ‘Better Bush’) Plant Response to Root Restriction. J. Exp. Bot. 1991, 42, 1233–1240. [Google Scholar] [CrossRef]
- Balliu, A.; Zheng, Y.; Sallaku, G.; Fernández, J.A.; Gruda, N.S.; Tuzel, Y. Environmental and Cultivation Factors Affect the Morphology, Architecture and Performance of Root Systems in Soilless Grown Plants. Horticulturae 2021, 7, 243. [Google Scholar] [CrossRef]
- Leng, F.; Duan, S.; Song, S.; Zhao, L.; Xu, W.; Zhang, C.; Ma, C.; Wang, L.; Wang, S. Comparative metabolic profiling of grape pulp during the growth process reveals systematic influences under root restriction. Metabolites 2021, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Wang, Y.; Cao, J.; Wang, S.; Wu, D.; Jiang, L.; Li, X.; Bao, J.; Karim, N.; Sun, C. Transcriptomic Analysis of Root Restriction Effects on the Primary Metabolites during Grape Berry Development and Ripening. Genes 2022, 13, 281. [Google Scholar] [CrossRef] [PubMed]
- Grunert, O.; Hernandez-Sanabria, E.; Vilchez-Vargas, R.; Jauregui, R.; Pieper, D.H.; Perneel, M.; Van Labeke, M.-C.; Reheul, D.; Boon, N. Mineral and organic growing media have distinct community structure, stability and functionality in soilless culture systems. Sci. Rep. 2016, 6, 18837. [Google Scholar] [CrossRef]
- Postma, J. The Status of Biological Control of Plant Diseases in Soilless Cultivation. In Recent Developments in Management of Plant Diseases; Springer: Dordrecht, The Netherlands, 2010; pp. 133–146. [Google Scholar] [CrossRef]
- Raviv, M.; Lieth, J.H.; Bar-Tal, A. Soilless Culture: Theory and Practice: Theory and Practice; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Alexander, M. Introduction to soil microbiology. Soil Sci. 1978, 125, 331. [Google Scholar] [CrossRef]
- Koohakan, P.; Ikeda, H.; Jeanaksorn, T.; Tojo, M.; Kusakari, S.-I.; Okada, K.; Sato, S. Evaluation of the indigenous microorganisms in soilless culture: Occurrence and quantitative characteristics in the different growing systems. Sci. Hortic. 2004, 101, 179–188. [Google Scholar] [CrossRef]
- Sammar Khalil, S.K.; Alsanius, B.W. Dynamics of the indigenous microflora inhabiting the root zone and the nutrient solution of tomato in a commercial closed greenhouse system. Eur. J. Hortic. Sci. 2001, 66, 188–198. [Google Scholar] [CrossRef]
- Grunert, O.; Hernandez-Sanabria, E.; Buysens, S.; De Neve, S.; Van Labeke, M.C.; Reheul, D.; Boon, N. In-Depth Observation on the Microbial and Fungal Community Structure of Four Contrasting Tomato Cultivation Systems in Soil Based and Soilless Culture Systems. Front. Plant Sci. 2020, 11, 520834. [Google Scholar] [CrossRef]
- Vallance, J.; Déniel, F.; Le Floch, G.; Guérin-Dubrana, L.; Blancard, D.; Rey, P. Pathogenic and beneficial microorganisms in soilless cultures. Agron. Sustain. Dev. 2011, 31, 191–203. [Google Scholar] [CrossRef]
- Chepsergon, J.; Moleleki, L.N. Rhizosphere bacterial interactions and impact on plant health. Curr. Opin. Microbiol. 2023, 73, 102297. [Google Scholar] [CrossRef]
- Kong, Z.; Liu, H. Modification of Rhizosphere Microbial Communities: A Possible Mechanism of Plant Growth Promoting Rhizobacteria Enhancing Plant Growth and Fitness. Front. Plant Sci. 2022, 13, 920813. [Google Scholar] [CrossRef] [PubMed]
- Grondin, A.; Li, M.; Bhosale, R.; Sawers, R.; Schneider, H.M. Interplay between developmental cues and rhizosphere signals from mycorrhizal fungi shape root anatomy, impacting crop productivity. Plant Soil 2024, 503, 587–594. [Google Scholar] [CrossRef]
- Thepbandit, W.; Athinuwat, D. Rhizosphere Microorganisms Supply Availability of Soil Nutrients and Induce Plant Defense. Microorganisms 2024, 12, 558. [Google Scholar] [CrossRef] [PubMed]
- Beslemes, D.; Tigka, E.; Roussis, I.; Kakabouki, I.; Mavroeidis, A.; Vlachostergios, D. Effect of Arbuscular Mycorrhizal Fungi on Nitrogen and Phosphorus Uptake Efficiency and Crop Productivity of Two-Rowed Barley under Different Crop Production Systems. Plants 2023, 12, 1908. [Google Scholar] [CrossRef]
- Liu, J.J.; Wei, Z.; Li, J.H. Effects of copper on leaf membrane structure and root activity of maize seedling. Bot. Stud. 2014, 55, 47. [Google Scholar] [CrossRef]
- Palta, J.P. Leaf chlorophyll content. Remote Sens. Rev. 1990, 5, 207–213. [Google Scholar] [CrossRef]
- Rao, A.V.; Waseem, Z.; Agarwal, S. Lycopene content of tomatoes and tomato products and their contribution to dietary lycopene. Food Res. Int. 1998, 31, 737–741. [Google Scholar] [CrossRef]
- Snyder, J.C.; Desborough, S.L. Rapid estimation of potato tuber total protein content with coomassie brilliant blue G-250. Theor. Appl. Genet. 1978, 52, 135–139. [Google Scholar] [CrossRef]
- Zhang, Y.; Yun, F.; Man, X.; Huang, D.; Liao, W. Effects of Hydrogen Sulfide on Sugar, Organic Acid, Carotenoid, and Polyphenol Level in Tomato Fruit. Plants 2023, 12, 719. [Google Scholar] [CrossRef]
- Erbach, D.C. Measurement of Soil Bulk Density and Moisture. Trans. ASAE 1987, 30, 922–0931. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Ma, T.; Li, X.; Zheng, M.; Zhou, X.; Chen, L.; Qian, X.; Xi, J.; Lu, H. EasyAmplicon: An easy-to-use, open-source, reproducible, and community-based pipeline for amplicon data analysis in microbiome research. Imeta 2023, 2, e83. [Google Scholar] [CrossRef] [PubMed]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Chen, T.; Liu, Y.; Chen, T.; Yang, M.; Fan, S.; Shi, M.; Wei, B.; Lv, H.; Cao, W.; Wang, C.; et al. ImageGP 2 for enhanced data visualization and reproducible analysis in biomedical research. IMeta 2024, 3, e239. [Google Scholar] [CrossRef]
- Wen, T.; Xie, P.; Yang, S.; Niu, G.; Liu, X.; Ding, Z.; Xue, C.; Liu, Y.X.; Shen, Q.; Yuan, J. ggClusterNet: An R package for microbiome network analysis and modularity-based multiple network layouts. IMeta 2022, 1, e32. [Google Scholar] [CrossRef]
- Ebrahimi, A.N.; Or, D. Microbial dispersal in unsaturated porous media: Characteristics of motile bacterial cell motions in unsaturated angular pore networks. Water Resour. Res. 2014, 50, 7406–7429. [Google Scholar] [CrossRef]
- Holden, P.A. How do the microhabitats framed by soil structure impact soil bacteria and the processes that they regulate? Archit. Biol. Soils: Life Inn. Space 2011, 118–148. [Google Scholar] [CrossRef]
- Stewart, B.A.; Hartge, K.H. Soil Structure: Its Development and Function; CRC Press: Boca Raton, FL, USA, 1995; Volume 7. [Google Scholar]
- Vos, M.; Wolf, A.B.; Jennings, S.J.; Kowalchuk, G.A. Micro-scale determinants of bacterial diversity in soil. FEMS Microbiol. Rev. 2013, 37, 936–954. [Google Scholar] [CrossRef]
- Heller, H.; Bar-Tal, A.; Assouline, S.; Narkis, K.; Suryano, S.; de la Forge, A.; Barak, M.; Alon, H.; Bruner, M.; Cohen, S.; et al. The effects of container geometry on water and heat regimes in soilless culture: Lettuce as a case study. Irrig. Sci. 2015, 33, 53–65. [Google Scholar] [CrossRef]
- Savvas, D. Modern developments in the use of inorganic media for greenhouse vegetable and flower production. Int. Symp. Grow. Media 2007, 819, 73–86. [Google Scholar] [CrossRef]
- Liu, D.; Chen, J.; Hao, Y.; Yang, X.; Chen, R.; Zhang, Y. Effects of Extreme Root Restriction on the Nutritional and Flavor Quality, and Sucrose Metabolism of Tomato (Solanum lycopersicum L.). Horticulturae 2023, 9, 813. [Google Scholar] [CrossRef]
- Bar-Tal, A.; Pressman, E. Root restriction and potassium and calcium solution concentrations affect dry-matter production, cation uptake, and blossom-end rot in greenhouse tomato. J. Am. Soc. Hortic. Sci. 1996, 121, 649–655. [Google Scholar] [CrossRef]
- Asaf, S.; Numan, M.; Khan, A.L.; Al-Harrasi, A. Sphingomonas: From diversity and genomics to functional role in environmental remediation and plant growth. Crit. Rev. Biotechnol. 2020, 40, 138–152. [Google Scholar] [CrossRef] [PubMed]
- White, D.C.; Suttont, S.D.; Ringelberg, D.B. The genus Sphingomonas: Physiology and ecology. Curr. Opin. Biotechnol. 1996, 7, 301–306. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, M.; Zhang, T.; Gao, H.; Ou, Y.; Li, M. Effects of biochar immobilization of Serratia sp. F4 OR414381 on bioremediation of petroleum contamination and bacterial community composition in loess soil. J. Hazard. Mater. 2024, 470, 134137. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Liu, Y.; Wang, X.; Zhang, B.; Zhang, W.; Chen, T.; Liu, G.; Xue, L.; Cui, X. Nocardioides: “Specialists” for Hard-to-Degrade Pollutants in the Environment. Molecules 2023, 28, 7433. [Google Scholar] [CrossRef]
- Gordon, T.R. Fusarium oxysporum and the Fusarium Wilt Syndrome. Annu. Rev. Phytopathol. 2017, 55, 23–39. [Google Scholar] [CrossRef]
- Han, K.-S.; Lee, S.-C.; Lee, J.-S.; Soh, J.-W. First Report of Pink Mold Rot on Tomato Fruit Caused by Trichothecium roseum in Korea. Res. Plant Dis. 2012, 18, 396–398. [Google Scholar] [CrossRef]
- Leplat, J.; Friberg, H.; Abid, M.; Steinberg, C. Survival of Fusarium graminearum, the causal agent of Fusarium head blight. A review. Agron. Sustain. Dev. 2013, 33, 97–111. [Google Scholar] [CrossRef]
- Li, S.; Dong, X.; Humez, P.; Borecki, J.; Birks, J.; McClain, C.; Mayer, B.; Strous, M.; Diao, M. Proteomic evidence for aerobic methane production in groundwater by methylotrophic Methylotenera. ISME J. 2025, 19, 24. [Google Scholar] [CrossRef]
- Srinivasan, R.; Prabhu, G.; Prasad, M.; Mishra, M.; Chaudhary, M.; Srivastava, R. Penicillium. Benef. Microbes Agro-Ecol. Bact. Fungi 2020, 651–667. [Google Scholar] [CrossRef]
- Raimondo, M.L.; Carlucci, A. Characterization and pathogenicity assessment of Plectosphaerella species associated with stunting disease on tomato and pepper crops in Italy. Plant Pathol. 2018, 67, 626–641. [Google Scholar] [CrossRef]
- Nayak, S.; Samanta, S.; Mukherjee, A.K. Beneficial Role of Aspergillus sp. in Agricultural Soil and Environment. In Frontiers in Soil and Environmental Microbiology; CRC Press: Boca Raton, FL, USA, 2020; pp. 17–36. [Google Scholar] [CrossRef]
- Ozimek, E.; Hanaka, A. Mortierella species as the plant growth-promoting fungi present in the agricultural soils. Agriculture 2021, 11, 7. [Google Scholar] [CrossRef]
- Xiong, W.; Li, R.; Ren, Y.; Liu, C.; Zhao, Q.; Wu, H.; Jousset, A.; Shen, Q. Distinct roles for soil fungal and bacterial communities associated with the suppression of vanilla Fusarium wilt disease. Soil Biol. Biochem. 2017, 107, 198–207. [Google Scholar] [CrossRef]
- Rüger, L.; Feng, K.; Chen, Y.; Sun, R.; Sun, B.; Deng, Y.; Vetterlein, D.; Bonkowski, M. Responses of root architecture and the rhizosphere microbiome assembly of maize (Zea mays L.) to a soil texture gradient. Soil Biol. Biochem. 2023, 181, 109026. [Google Scholar] [CrossRef]
- Zhang, Z.; Laverman, A.; Mellage, A.; Furman, A. Microbial community structure and function in a sandy soil column under dynamic hydrologic regimes. J. Hydrol. 2024, 643, 131797. [Google Scholar] [CrossRef]
- Yuan, M.M.; Guo, X.; Wu, L.; Zhang, Y.; Xiao, N.; Ning, D.; Shi, Z.; Zhou, X.; Wu, L.; Yang, Y.; et al. Climate warming enhances microbial network complexity and stability. Nat. Clim. Change 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Upton, R.N.; Checinska Sielaff, A.; Hofmockel, K.S.; Xu, X.; Wayne Polley, H.; Wilsey, B.J.; Upton, R.N.; Sielaff, A.C.; Hofmockel, K.S.; Xu, X.; et al. Soil depth and grassland origin cooperatively shape microbial community co-occurrence and function. Ecosphere 2020, 11, e02973. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, X.; Li, Y.; Zhang, R.; Zhao, Y.; Fang, H.; Li, W. Impact of altered groundwater depth on soil microbial diversity, network complexity and multifunctionality. Front. Microbiol. 2023, 14, 1214186. [Google Scholar] [CrossRef]
- Banerjee, S.; Zhao, C.; Kirkby, C.A.; Coggins, S.; Zhao, S.; Bissett, A.; van der Heijden, M.G.; Kirkegaard, J.A.; Richardson, A.E. Microbial interkingdom associations across soil depths reveal network connectivity and keystone taxa linked to soil fine-fraction carbon content. Agric. Ecosyst. Environ. 2021, 320, 107559. [Google Scholar] [CrossRef]
- DeAngelis, K.M.; Silver, W.L.; Thompson, A.W.; Firestone, M.K. Microbial communities acclimate to recurring changes in soil redox potential status. Environ. Microbiol. 2010, 12, 3137–3149. [Google Scholar] [CrossRef]
- Pett-Ridge, J.; Silver, W.L.; Firestone, M.K. Redox fluctuations frame microbial community impacts on N-cycling rates in a humid tropical forest soil. Biogeochemistry 2006, 81, 95–110. [Google Scholar] [CrossRef]
- Pett-Ridge, J.; Firestone, M.K. Redox fluctuation structures microbial communities in a wet tropical soil. Appl. Environ. Microbiol. 2005, 71, 6998–7007. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.