Abstract
A magnetic deep eutectic solvent-based single-drop microextraction technique coupled with high-performance liquid chromatography (MDES-SDME-HPLC) was established for the determination of five triazine herbicides in environmental water samples. MDES, used as the extraction solvent, was composed of heptanoic acid, methyltrioctylammonium chloride, and iron chloride. This pretreatment method requires only 50 μL of MDES, thereby avoiding the use of large volumes of toxic organic solvents. The MDES containing the target triazine herbicides was rapidly separated from the aqueous matrix by applying an external magnetic force, thus eliminating the need for centrifugation or additional reagents to achieve phase separation. The method demonstrated a linear range of 0.2–20 μg L−1, with a limit of detection of 0.06 μg L−1. Recoveries obtained from different environmental water matrices ranged from 75.5% to 102.4%. The greenness of the method was confirmed using five independent green analytical assessment tools. This approach represents a green and efficient analytical technique for detecting triazine herbicides in environmental water samples.
1. Introduction
Triazine herbicides such as cyanazine, simazine, atrazine, propazine, and terbuthylazine are widely used to control weeds by blocking the electron transport chain in photosynthesis [1], which disrupts energy production and causes weed death. Due to their strong effectiveness and low cost, these herbicides hold a large share of the global pesticide market [2]. However, studies have shown that crops absorb less than 30% of the applied herbicides, while the rest are lost through surface runoff and eventually enter water bodies [3]. These compounds have been detected in rivers, ponds, groundwater, and seawater [4], with concentrations ranging from 0.1 to 50 μg L−1. Their presence in aquatic environments raises concerns about ecological damage [5] and potential harm to human health [6]. Long-term exposure to low concentrations may cause endocrine disruption, reproductive problems, and possibly cancer [7]. Given the typically low concentrations of these herbicides in environmental waters, there is a pressing need to develop sensitive and reliable analytical methods for their detection.
Solid-phase extraction (SPE) and liquid–liquid extraction (LLE) are widely used sample pretreatment techniques designed to isolate target analytes from complex matrices and thereby enhance detection sensitivity. SPE selectively retains target compounds on adsorbents, enabling effective purification and high analyte recovery; however, it often requires expensive synthetic adsorbents and consumes large volumes of organic solvents [8]. In contrast, LLE separates analytes based on their distribution between two immiscible liquid phases. Although the method is easy to perform, it also involves the use of large quantities of organic solvents [9]. In recent years, liquid-phase microextraction (LPME) has attracted increasing interest due to its low solvent usage and operational simplicity. Among LPME techniques, single-drop microextraction (SDME) is distinguished by its minimal use of extractant, typically requiring only a few microliters [10]. However, the extractants employed in SDME are generally not environmentally friendly, and the method depends on frequent use of syringes for extractant handling, making the process relatively cumbersome [11]. Accordingly, the advancement of SDME has centered on identifying environmentally sustainable extractants and a convenient phase separation technique.
As a novel class of environmentally friendly extractants, deep eutectic solvents (DESs) are synthesized through intermolecular interactions between hydrogen bond donors and acceptors [12]. Compared with traditional organic extractants such as chloroform and carbon tetrachloride, DESs possess several advantages, including environmental compatibility, low cost, and biodegradability [13]. When composed of naturally derived or low-toxicity components, DESs substantially reduce the environmental burden associated with the use of conventional organic solvents [14]. In addition to their green chemistry profile, DESs can selectively extract target analytes through specific interactions, including hydrogen bonding and π-π stacking [15]. Furthermore, the polarity of DESs can be adjusted by modifying their components, allowing for the extraction of analytes with different polarities [16].
The small droplet of extractant hanging at the tip of a syringe needle in SDME was used to extract target substances [17]. However, when handling complex samples or during vigorous stirring, the droplet was prone to detachment. In addition, due to its suspension at the syringe tip, the droplet size and volume were difficult to control and reproduce precisely. Therefore, it is desirable to eliminate the use of syringes in SDME. Although centrifugation was commonly used to achieve phase separation, it was both energy- and time-consuming [18]. In 2017, magnetic deep eutectic solvents (MDES) were first reported and subsequently applied to facilitate rapid extraction and phase separation [19]. Under an external magnetic force, MDES can be quickly separated from the sample without using a centrifuge, solving the problem of unstable droplets in syringe-based SDME. Transition metals (e.g., Fe, Mn, Co) and rare earth elements (e.g., Gd) can be used to make new types of DES with magnetic properties, allowing fast separation under magnetic force [20].
Green analytical chemistry assessment methods evaluate the environmental impact of analytical procedures through semi-quantitative or quantitative approaches from multiple perspectives, each with its own focus and weighting system. The Analytical Eco-Scale (AES) is valued for its simplicity and speed, whereas the Green Analytical Procedure Index (GAPI) offers a visual profile of environmental performance across the entire analytical workflow. The Analytical GREEnness (AGREE) metric provides a systematic evaluation based on the principles of green chemistry, and the Analytical GREEnness metric for sample preparation (AGREEprep) focuses specifically on sample preparation steps. In contrast, the Sample Preparation Metric of Sustainability (SPMS) adopts a broader perspective by integrating environmental, economic, and social dimensions. Employing a combination of these tools can help overcome the limitations inherent in any single metric, thereby enabling a more balanced and comprehensive assessment of an analytical method’s greenness.
In this study, heptanoic acid was selected as a hydrogen bond donor and methyltrioctylammonium chloride as a hydrogen bond acceptor, and together with iron chloride, they were combined to prepare a green MDES serving as the extractant. This MDES was applied in a single-drop microextraction process that required only a small volume to effectively extract triazine herbicides from various samples, and its magnetic properties enabled easy separation of the extractant from water matrices using an external magnetic force. To ensure reliable performance, the parameters influencing extraction efficiency were carefully optimized. The developed method, magnetic deep eutectic solvent-based single-drop microextraction coupled with high-performance liquid chromatography (MDES-SDME-HPLC), was subsequently employed for the detection of triazine herbicides in tap water, river water, lake water, and sea water.
2. Materials and Methods
2.1. Reagents and Materials
Cyanazine (95%), simazine (98%), atrazine (97%), propazine (98%), terbuthylazine (98%), pentanoic acid (98%), hexanoic acid (99%), heptanoic acid (98%), octanoic acid (99%), nonanoic acid (98%), triethylmethylammonium chloride (MTEAC, 98%), tributylmethylammonium chloride (MTBAC, 99%), and methyltrioctylammonium chloride (MTOAC, 97%) were purchased from Macklin Inc. (Shanghai, China), while iron chloride (99.9%), manganese chloride (98%), cobalt chloride (97%), and gadolinium chloride (99.9%) were obtained from Sigma Inc. (Shanghai, China).
Water samples were collected from several natural sources, including lake and river water obtained from Jinyang Lake and the Fenhe River (Taiyuan, China), and sea water collected from the East China Sea (Xiamen, China). Before analysis, all water samples were filtered through a 0.22-μm filter membrane to remove suspended particles. Details of preparation of four environmental water samples are summarized in Table S1.
2.2. Instrumentation
Quantitative analysis of the five triazine herbicides was carried out using an ACQUITY Arc HPLC system equipped with a Waters 2489 UV/Vis detector (Waters Corporation, Milford, MA, USA). Chromatographic separation was performed on a ValueLab 5 LC C18-HD column (250 × 4.6 mm, 5 μm; Agilent, Santa Clara, CA, USA), with a mobile phase composed of acetonitrile and water at a volume ratio of 70:30 and a flow rate of 0.4 mL min−1. The injection volume was set at 10 μL. The retention times for cyanazine, simazine, atrazine, propazine, and terbuthylazine were 11.5, 12.2, 14.2, 17.0, and 17.9 min, respectively (Figure S1). The column was maintained at a constant temperature of 20 °C, and the detection wavelength was set at 225 nm.
2.3. Preparation of the MDES
A green MDES was prepared by mixing heptanoic acid, MTOAC, and iron chloride in a molar ratio of 6:0.5:0.08 in a 10 mL centrifuge tube, followed by heating at 80 °C for 5 min to form a homogeneous solution. The physical properties of the MDES were provided in Table S2.
2.4. Microextraction
Microextraction was conducted using a 12-channel electronic pipette (Eppendorf, Hamburg, Germany) to dispense six aliquots (50 μL each) of the prepared MDES into six individual 10 mL centrifuge tubes, each containing 5 mL of the water sample. The mixtures were simultaneously stirred at 1200 rpm for 5 min using 6 × 10 mm stir bars to ensure efficient contact between the extractant and the aqueous phase. Phase separation was then performed by placing the tubes on a magnetic rack, allowing rapid and simultaneous isolation of the magnetic extractant droplets from all six samples. The separated extractant phases were collected and subsequently analyzed by HPLC (Figure 1).
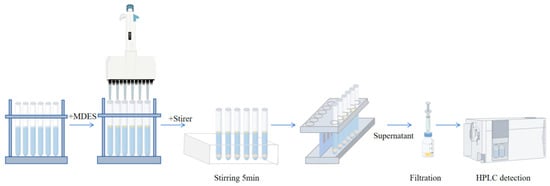
Figure 1.
Flowchart of the MDES-SDME-HPLC.
2.5. Determination of Extraction Recovery
Extraction recovery (ER) was calculated using Equation (1) as follows:
In this equation, Cm represents the measured concentration of target substances in spiked samples, Cr denotes the measured concentration in real (unspiked) samples, and Cs refers to the concentration of the substances added during spiking.
3. Results and Discussion
3.1. Optimizing the MDES-SDME-HPLC Method Conditions
After the preliminary test of the experiment, one parameter was selected for optimization, while the other parameters were kept at optimal levels. The preset conditions are as follows: the extractant MDES was synthesized using heptanoic acid as the fatty acid, MTOAC as the quaternary ammonium salt, and iron chloride as the metal chloride. The extractant volume was 50 μL. Stirring was conducted for 5 min at 1200 rpm using 6 × 10 mm stirrer bars, and the sample volume was 5 mL. Additional salt or pH buffer solution addition was not involved. Each experiment was performed under the optimized conditions to determine the optimal extraction process, and the optimization was repeated three times.
3.1.1. Effect of Fatty Acid Type
Fatty acids, serving as hydrogen bond donors, interact with quaternary ammonium salts and metal chloride to form MDES. The type of fatty acid plays a key role in influencing the viscosity, solubility, and extraction performance of the resulting MDES [21]. To identify the most suitable fatty acid, five candidates, namely pentanoic acid, hexanoic acid, heptanoic acid, octanoic acid, and nonanoic acid, were evaluated. As shown in Figure 2A, heptanoic acid yielded the highest recovery, which is likely attributable to its well-balanced intermolecular interactions. In contrast, pentanoic and hexanoic acids may interact too weakly with the quaternary ammonium salt, resulting in unstable MDES structures and reduced extraction performance. On the other hand, the stronger interactions observed with octanoic and nonanoic acids may increase the viscosity of the system, limiting mass transfer and thereby lowering extraction efficiency and recovery. Based on these results, heptanoic acid was selected for MDES preparation in the subsequent experiments.
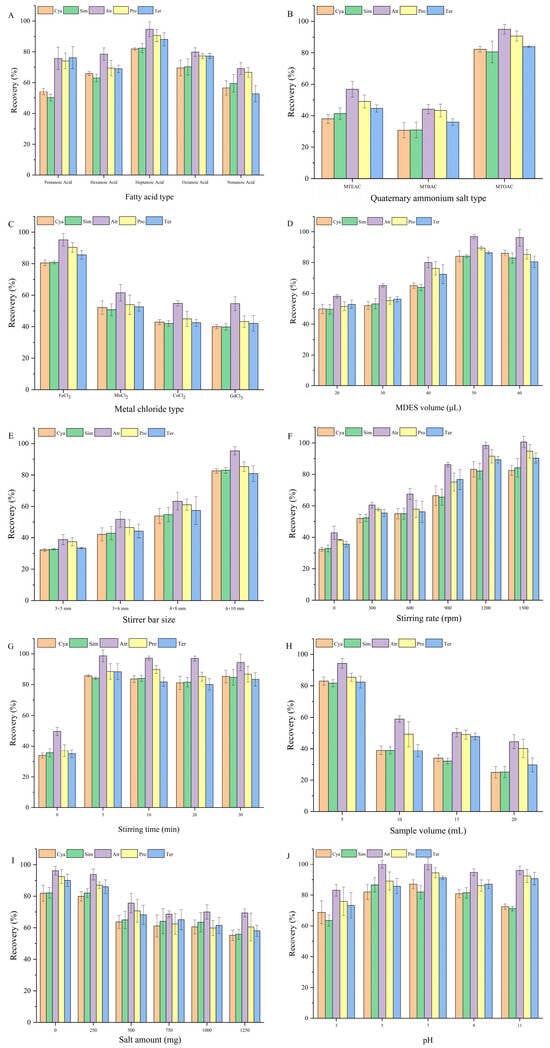
Figure 2.
Optimization of MDES-SDME-HPLC parameters: (A) type of fatty acid; (B) type of quaternary ammonium salt; (C) type of metal chloride; (D) volume of MDES; (E) size of stirrer bar; (F) stirring rate; (G) stirring time; (H) sample volume; (I) amount of salt; (J) pH value. (Cya: cyanazine; Sim: simazine; Atr: atrazine; Pro: propazine; Ter: terbuthylazine).
3.1.2. Effect of Quaternary Ammonium Salt Type
Quaternary ammonium salts, functioning as hydrogen bond acceptors, interact with fatty acids and metal chlorides to form MDES. The structure and physicochemical properties of the quaternary ammonium salt influence the polarity, surface activity, and selective extraction capability of the resulting MDES [22]. In this study, MTEAC, MTBAC, and MTOAC were evaluated to determine the most suitable type. As shown in Figure 2B, the MDES prepared using MTOAC exhibited the highest recovery. This can be attributed to the long carbon chains in MTOAC, which enhance its hydrophobicity and improve its capacity to form stable complexes with metal ions. When combined with fatty acids through hydrogen bonding, MTOAC promotes better solubility of the target analytes. Its greater hydrophobicity also contributes to more efficient extraction of target substances from the aqueous phase and facilitates effective phase separation, thereby improving both extraction efficiency and recovery. Based on these observations, MTOAC was selected for MDES preparation in subsequent experiments.
3.1.3. Effect of Metal Chloride Type
Metal chlorides, including iron chloride, manganese chloride, cobalt chloride, and gadolinium chloride, were investigated to determine the optimal metal chloride type as the magnetic source for MDES preparation [23]. As shown in Figure 2C, iron chloride resulted in the highest recovery, potentially due to its good solubility in both water and organic solvents, which allows for uniform dispersion within the MDES system and facilitates rapid extraction equilibrium, thereby improving extraction efficiency. In addition, iron ions possess multiple coordination sites, enabling the formation of stable coordination structures with quaternary ammonium salts and fatty acids. This structural stability supports efficient MDES separation and ultimately contributes to the observed increase in recovery. Based on these results, iron chloride was selected for MDES preparation in the subsequent experiment.
3.1.4. Effect of MDES Volume
The volume of MDES is closely related to extraction efficiency, as an appropriate volume ensures sufficient contact between the target substances and the extractant, thereby enhancing overall extraction performance [24]. To determine the optimal volume, different amounts of MDES, 20, 30, 40, 50, and 60 μL, were investigated. As illustrated in Figure 2D, recovery increased with extractant volume and reached its maximum at 50 μL. This trend may be explained by the insufficient capacity of smaller volumes to fully extract the target analytes, resulting in lower efficiency. At 50 μL, the distribution ratio of target substances between the extraction solvent and the sample phase reaches a stable level, and further increasing the extractant volume does not produce a noticeable improvement in extraction. Therefore, considering both environmental and economic factors, 50 μL of MDES was selected as the optimal volume for subsequent experiments.
3.1.5. Effect of Stirrer Bar Size
Stirrer bars promote the mixing of the sample with the extractant, and their size directly affects the mixing efficiency. An appropriate stirrer bar size enhances the interaction between the sample and the extractant, thereby improving extraction efficiency [25]. To determine the optimal size, commercially available stirrer bars of different dimensions (3 × 5 mm, 3 × 6 mm, 4 × 8 mm, 6 × 10 mm, and 7 × 15 mm) were tested. As illustrated in Figure 2E, the 6 × 10 mm stirrer bars yielded the highest recovery. Smaller stirrer bars may not generate sufficient force or coverage during stirring, leading to uneven mixing and reduced extraction efficiency. In contrast, an excessively large stirrer bar (7 × 15 mm) does not rotate smoothly within a 10 mL centrifuge tube and may cause splashing, which compromises the extraction process. Based on these results, the 6 × 10 mm stirrer bars were selected for use in subsequent experiments.
3.1.6. Effect of Stirring Rate
The stirring rate significantly impacts the mixing efficiency between the extractant and the target substances, thereby influencing the overall extraction performance [26]. To identify the optimal stirring rate, different rates (i.e., 0, 300, 600, 900, 1200, and 1500 rpm) were examined. As shown in Figure 2F, recovery increased with stirring rate and reached its maximum at 1200 rpm. This trend may be explained by the insufficient mixing that occurs at lower speeds, which limits the interaction between the sample and the extractant. A suitable stirring rate ensures thorough mixing, enhances the contact between phases, and improves extraction efficiency. Therefore, 1200 rpm was selected as the optimal stirring rate for the following experiments.
3.1.7. Effect of Stirring Time
An appropriate stirring time plays an important role in optimizing extraction efficiency, selectivity, and experimental stability, while also enhancing the accuracy and consistency of the analytical results [27]. To determine the optimal stirring duration, different times (i.e., 0, 5, 10, 20, and 30 min) were investigated. As shown in Figure 2G, recovery reached its highest value at 5 min. Prolonged stirring did not significantly improve extraction efficiency and, in some cases, may have led to emulsification or incomplete phase separation, which could negatively impact subsequent separation and analysis. Based on these observations, a stirring time of 5 min was selected for use in the follow-up experiments.
3.1.8. Effect of Sample Volume
The volume of the sample affects the extent of contact between the target substances and the extractant, thereby influencing extraction efficiency [28]. To determine the optimal sample volume, four different volumes, 5, 10, 15, and 20 mL, were tested. As illustrated in Figure 2H, the highest recovery was observed at 5 mL and may be attributed to the fact that, at a moderate sample volume, mass transfer between the extractant and the target substances is sufficient, promoting the effective enrichment of analytes. However, when the sample volume becomes too large, the extractant may become diluted, which weakens the extraction capacity and reduces recovery. Therefore, 5 mL was selected as the optimal sample volume for the subsequent experiments.
3.1.9. Effect of Salt Amount
The addition of an appropriate amount of sodium chloride to the sample may increase the ionic strength, reduce the water solubility of polar organic substances, and thus promote the transfer of target substances from the aqueous phase to the extractant, accelerate mass transfer, and improve extraction efficiency [29]. To determine the optimal salt amount, various additions of sodium chloride (i.e., 0, 250, 500, 750, 1000, and 1250 mg) were tested. As shown in Figure 2I, recovery remained essentially unchanged when the added salt amount did not exceed 250 mg. However, a gradual decrease in recovery was observed with further salt addition. Based on these results, no salt was added in the follow-up experiments.
3.1.10. Effect of pH
The pH value can influence the form in which target substances exist in the aqueous phase, thereby affecting their behavior during the extraction process [30]. To assess whether pH impacts extraction efficiency in this study, samples with pH values of 3, 5, 7, 9, and 11 were examined. As illustrated in Figure 2J, recovery remained stable within the pH range of 5 to 9. Therefore, the pH of real water samples was not adjusted during subsequent detection procedures.
3.2. Method Validation
Under optimal extraction conditions, several key analytical parameters of the proposed method were evaluated, including regression equations, determination coefficients (R2), limits of detection (LOD), limits of quantification (LOQ), and intra-day and inter-day relative standard deviations (RSDs), as summarized in Table 1. Calibration curves were constructed using five concentration levels (0.2, 0.4, 2, 4, and 20 μg L−1), and the corresponding regression equations were obtained by plotting peak areas against triazine herbicide concentrations. The method exhibited good linearity across the range of 0.2–20 μg L−1, with R2 values exceeding 0.998 for all target analytes. The LOD and LOQ were calculated to be 0.06 μg L−1 and 0.2 μg L−1, respectively. To assess the method’s precision, five replicate extractions were performed within a single day and across five consecutive days. Intra-day RSDs (n = 5) ranged from 4.3% to 7.2%, while inter-day RSDs (n = 5) varied between 9.7% and 13.7%, indicating acceptable repeatability and stability of the MDES-SDME-HPLC method.

Table 1.
Analytical performance of the MDES-SDME-HPLC method.
3.3. Real Samples Application
The applicability of the MDES-SDME-HPLC method for analyzing environmental water samples was evaluated, and the results are summarized in Table 2. As the concentrations of triazine herbicides in the real samples were below the respective LODs, recovery tests were performed by spiking the samples at three concentration levels (0.2, 2, and 20 μg L−1), with each level analyzed in triplicate. The recoveries of the spiked samples ranged from 75.5% to 102.4%, while the corresponding RSDs varied between 0.9% and 8.9%. These results demonstrate that the proposed method provides satisfactory accuracy and precision, confirming its reliability and applicability for the detection of triazine herbicides in various environmental water samples.

Table 2.
Detection of triazine herbicides in real samples using the MDES-SDME-HPLC method.
3.4. Comparison with Other Methods
As shown in Table 3, the MDES-SDME-HPLC method developed in this study was compared with other microextraction techniques reported over the past three years for the determination of triazine herbicides using HPLC. The comparison was based on several key parameters, including the type of triazine herbicide analyzed, pretreatment method, extractant type and volume, additional reagent and amount, auxiliary device, detection instrument, LOQ, and ER. The proposed method targets five triazine herbicides and employs only 50 μL of MDES as the extractant in the SDME procedure, highlighting its environmentally friendly nature and minimal solvent usage. Unlike other reported methods, this approach does not require adsorbents (e.g., biochar), toxic solvents (e.g., methanol and acetonitrile), or pH adjustment reagents (e.g., acids, bases, or buffers), thereby significantly reducing reagent consumption and minimizing environmental impact. Furthermore, the use of an external magnetic force for instant phase separation not only simplifies the procedure by eliminating the need for centrifugation, but also shortens the total pretreatment time by enabling the simultaneous processing of six samples. The method achieves a low LOQ of 0.2 μg L−1, which is lower than those reported in previous studies, while maintaining comparable recovery ranges. Taken together, the MDES-SDME-HPLC method established in this work offers clear advantages in terms of environmental sustainability, operational simplicity, and analytical sensitivity. Its low reagent consumption, rapid execution, and reliable performance make it a practical and green approach for the determination of triazine herbicides in environmental water samples.

Table 3.
Comparison of the MDES-SDME-HPLC method with other microextraction techniques reported in the past three years for the determination of triazine herbicides.
3.5. Green Evaluation
In this study, five green assessment tools were employed to evaluate the environmental performance of the MDES-SDME-HPLC method. The Analytical Eco-Scale (AES) evaluation [36], which considers factors such as solvent consumption, energy usage, workplace hazards, and waste generation, awarded the method a score of 86 out of 100. As scores above 75 indicate excellent green analytical practices, this result reflects the method’s minimal environmental impact. Detailed scoring results are provided in Table S3.
The Green Analytical Procedure Index (GAPI) [37], which provides a visual overview of a method’s greenness by assessing solvents, energy use, waste, and occupational hazards, yielded 8 green, 5 yellow, and 2 red fields for the MDES-SDME-HPLC method, which indicates favorable performance in most evaluated dimensions. The GAPI diagram is shown in Figure 3A, with supporting details presented in Table S4. However, it is worth noting that the outcome of the GAPI assessment may be influenced by the choice of indicators and weighting criteria, which can affect the comprehensiveness of the evaluation.
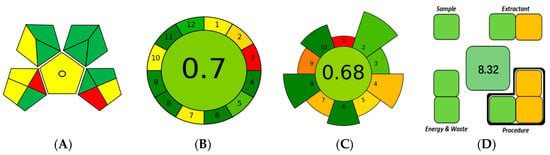
Figure 3.
Greenness evaluation of the MDES-SDME-HPLC method using: (A) GAPI; (B) AGREE; (C) AGREEprep; and (D) SPMS.
The Analytical GREEnness (AGREE) tool [38], which assesses the overall environmental impact across the entire analytical workflow, from sample collection to final measurement, takes into account multiple criteria, including solvent usage, energy requirements, waste generation, and reagent handling. The MDES-SDME-HPLC method achieved an AGREE score of 0.7, reflecting a low environmental burden. The AGREE diagram is shown in Figure 3B, and the scoring details are provided in Table S5.
The Analytical GREEnness metric for sample preparation (AGREEprep) metric [39], which focuses specifically on sample preparation, can be used to evaluate parameters such as reagent consumption, waste generation, and energy efficiency. The MDES-SDME-HPLC method received an AGREEprep score of 0.68, indicating strong environmental compatibility and alignment with green sample preparation principles. The result is illustrated in Figure 3C, and detailed metrics are available in Table S6.
Lastly, the Sample Preparation Metric of Sustainability (SPMS) [40] can be used to assess sustainability based on six dimensions: sample quantity, extractant characteristics, procedural complexity, energy consumption, total waste generation, and extractant reusability. By quantifying these aspects, SPMS provides a detailed and objective measure of sustainability. The MDES-SDME-HPLC method obtained a global score of 8.32, suggesting notable environmental advantages and good consistency with sustainable development goals. The SPMS radar chart is presented in Figure 3D, with corresponding data summarized in Table S7.
4. Conclusions
In this study, a green and efficient MDES-SDME-HPLC method was developed for the detection of five triazine herbicides in environmental water samples, including tap, river, lake, and sea water. The method employed only 50 μL of magnetic MDES as the extractant, which enabled effective extraction of target analytes without the use of toxic organic solvents. Phase separation was achieved rapidly and efficiently using an external magnetic force, eliminating the need for centrifugation. Systematic optimization of multiple parameters, including the type of fatty acid, quaternary ammonium salt, metal chloride, MDES volume, stirrer bar size, stirring rate, stirring time, sample volume, salt amount, and pH, ensured high extraction specificity and sensitivity. The method demonstrated a wide linear range, low limit of quantification, and high analytical accuracy, making it well-suited for the determination of trace levels of triazine herbicides in complex environmental water matrices. One limitation is that analyzing highly complex or turbid samples may necessitate prior filtration or dilution to reduce interference. Further optimization, including the screening of alternative MDES components and refinement of extraction kinetics, presents a clear opportunity to reduce the total processing time while maintaining high recovery. Given its simplicity, reliability, and environmental compatibility, the method holds promise for broader applications in the analysis and monitoring of other pesticide residues in environmental water samples.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy16010107/s1, Figure S1: Representative chromatogram of cyanazine, simazine, atrazine, propazine, and terbuthylazine obtained by the MDES-SDME-HPLC method.; Table S1 Preparation of four environmental water samples.; Table S2: Physical properties of the MDES.; Table S3: Detailed AES evaluation of the MDES-SDME-HPLC method.; Table S4: Detailed GAPI evaluation of the MDES-SDME-HPLC method.; Table S5: Detailed AGREE evaluation of the MDES-SDME-HPLC method.; Table S6: Detailed AGREEprep evaluation of the MDES-SDME-HPLC method.; Table S7: Detailed SPMSevaluation of the MDES-SDME-HPLC method.
Author Contributions
X.B.: Methodology, Investigation, Writing—Original Draft; W.W.: Validation, Data curation; X.X.: Formal analysis; X.J.: Conceptualization; Q.Z.: Supervision, Writing—Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
During the preparation of this manuscript, the authors used DeepSeek v3.2 for the purposes of grammar checking. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, X.-W.; Chen, H.; Zhao, H.-L.; Li, D.-W.; Ou, L.-J. Triazine Herbicide Reduced the Toxicity of the Harmful Dinoflagellate Karenia Mikimotoi by Impairing Its Photosynthetic Systems. Ecotoxicol. Environ. Saf. 2024, 269, 115740. [Google Scholar] [CrossRef]
- Zheng, X.; Guo, X.; Zhang, Y.; Han, J.; Jing, X.; Wu, J. Determination of Triazine Herbicides from Water, Tea, and Juice Samples Using Magnetic Dispersive Micro-Solid Phase Extraction and Magnetic Dispersive Liquid-Liquid Microextraction with HPLC. Food Chem. 2025, 468, 142430. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, M.; Yu, X.; Liu, L.; Gao, C.; Li, H.; Fu, S.; Wang, W.; Wang, J. Presence and Distribution of Triazine Herbicides and Their Effects on Microbial Communities in the Laizhou Bay, Northern China. Mar. Pollut. Bull. 2023, 186, 114460. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, N.; Hu, C.; Yang, L.; Wang, X.; Li, J. Reshuffling the Risk Values of Pesticides in Surface-Groundwater Systems: Evidence from Mining Intensity and Hydrogeological Vulnerabilities. Sci. Total Environ. 2025, 967, 178755. [Google Scholar] [CrossRef] [PubMed]
- Madesh, S.; Gopi, S.; Sau, A.; Rajagopal, R.; Namasivayam, S.K.R.; Arockiaraj, J. Chemical Contaminants and Environmental Stressors Induced Teratogenic Effect in Aquatic Ecosystem—A Comprehensive Review. Toxicol. Rep. 2024, 13, 101819. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, S.; Cai, M.; Zhu, J.; Xu, Z.; Zheng, H.; Xiao, K.; Wang, F. Screening Triazine Herbicides in Drinking Water in the Yangtze River Delta, China: Occurrence and Health Risk. J. Hazard. Mater. Adv. 2023, 10, 100277. [Google Scholar] [CrossRef]
- Yang, Y.; Han, W.; Zhang, H.; Qiao, H.; Zhang, Y.; Zhang, Z.; Wang, J. Insights into Interaction of Triazine Herbicides with Three Kinds of Different Alkyl Groups (Simetryne, Ametryn and Terbutryn) with Human Serum Albumin via Multi-Spectral Analysis. Pestic. Biochem. Physiol. 2024, 201, 105895. [Google Scholar] [CrossRef]
- Zhang, C.; Xing, H.; Yang, L.; Fei, P.; Liu, H. Development Trend and Prospect of Solid Phase Extraction Technology. Chin. J. Chem. Eng. 2022, 42, 245–255. [Google Scholar] [CrossRef]
- Bokhary, A.; Leitch, M.; Liao, B.Q. Liquid–Liquid Extraction Technology for Resource Recovery: Applications, Potential, and Perspectives. J. Water Process Eng. 2021, 40, 101762. [Google Scholar] [CrossRef]
- Javar, K.; Foroozandeh, A.; Souri, M.; Amoli, H.S.; Hasanzadeh, M. Critical Role of Single Drop Microextraction for Drug Isolation from Complex Matrix towards Efficient Pharmaceutical Analysis: Advances and Challenges. TrAC Trends Anal. Chem. 2025, 185, 118163. [Google Scholar] [CrossRef]
- Jeannot, M.A.; Przyjazny, A.; Kokosa, J.M. Single Drop Microextraction—Development, Applications and Future Trends. J. Chromatogr. A 2010, 1217, 2326–2336. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.; Row, K.H. Development of Deep Eutectic Solvents for Sustainable Chemistry. J. Mol. Liq. 2022, 362, 119654. [Google Scholar] [CrossRef]
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep Eutectic Solvents (DESs) as Eco-Friendly and Sustainable Solvent/Catalyst Systems in Organic Transformations. J. Mol. Liq. 2016, 215, 345–386. [Google Scholar] [CrossRef]
- Prabhune, A.; Dey, R. Green and Sustainable Solvents of the Future: Deep Eutectic Solvents. J. Mol. Liq. 2023, 379, 121676. [Google Scholar] [CrossRef]
- Shafique, S.; Belousov, A.S.; Rashid, R.; Shafiq, I.; Aziz, K.H.H.; Riaz, N.; Khan, M.S.; Shaheen, A.; Ishaq, M.; Akhter, P.; et al. Deep Eutectic Solvents (DES): Structure, Properties, and Cutting-Edge Applications in Green Catalysis. J. Mol. Liq. 2025, 419, 126769. [Google Scholar] [CrossRef]
- Tang, W.; Row, K.H. Design and Evaluation of Polarity Controlled and Recyclable Deep Eutectic Solvent Based Biphasic System for the Polarity Driven Extraction and Separation of Compounds. J. Clean. Prod. 2020, 268, 122306. [Google Scholar] [CrossRef]
- Xu, L.; Basheer, C.; Lee, H.K. Developments in Single-Drop Microextraction. J. Chromatogr. A 2007, 1152, 184–192. [Google Scholar] [CrossRef]
- Mansour, F.R.; Danielson, N.D. Solvent-Terminated Dispersive Liquid-Liquid Microextraction: A Tutorial. Anal. Chim. ACTA 2018, 1016, 1–11. [Google Scholar] [CrossRef]
- Khezeli, T.; Daneshfar, A. Synthesis and Application of Magnetic Deep Eutectic Solvents: Novel Solvents for Ultrasound Assisted Liquid-Liquid Microextraction of Thiophene. Ultrason. Sonochem. 2017, 38, 590–597. [Google Scholar] [CrossRef]
- Ortizo, R.G.G.; Sharma, V.; Tsai, M.-L.; Nargotra, P.; Wang, J.-X.; Sun, P.-P.; Chen, C.-W.; Dong, C.-D. Exploring the Potential of Magnetic Deep Eutectic Solvents and DES-Functionalized Nanomaterials for Food Analysis: Advancements and Current Trends. Food Biosci. 2024, 61, 104764. [Google Scholar] [CrossRef]
- Barani Pour, S.; Jahanbin Sardroodi, J.; Rastkar Ebrahimzadeh, A.; Pazuki, G.; Hadigheh Rezvan, V. A Comparative Study of Deep Eutectic Solvents Based on Fatty Acids and the Effect of Water on Their Intermolecular Interactions. Sci. Rep. 2024, 14, 1763. [Google Scholar] [CrossRef] [PubMed]
- Bureš, F. Quaternary Ammonium Compounds: Simple in Structure, Complex in Application. Top. Curr. Chem. 2019, 377, 14. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Cheng, X.; Zhao, W.; Wang, H.; Wang, X. Magnetic Effervescence Tablet-Assisted Switchable Hydrophilicity Solvent-Based Liquid Phase Microextraction of Triazine Herbicides in Water Samples. J. Mol. Liq. 2020, 306, 112934. [Google Scholar] [CrossRef]
- Wang, J.; Guo, H.; Liu, Y.; Yang, L.; Wang, R.; Xing, R.; Chen, X.; Hu, S. Cyclodextrin Sensitized Homogeneous Liquid-Liquid Microextraction for Six Hepatotoxic Ingredients in “Psoraleae Fructus” Based on a Switchable Deep Eutectic Supramolecular Polymer. Microchem. J. 2025, 212, 113313. [Google Scholar] [CrossRef]
- Jahangiri, S.; Hatami, M.; Farhadi, K.; Bahram, M. Hollow-Fiber-Based LPME as a Reliable Sampling Method for Gas-Chromatographic Determination of Pharmacokinetic Parameters of Valproic Acid in Rat Plasma. Chromatographia 2013, 76, 663–669. [Google Scholar] [CrossRef]
- Nojavan, S.; Tahmasebi, Z.; Davarani, S.S.H. Effect of Type of Stirring on Hollow Fiber Liquid Phase Microextraction and Electromembrane Extraction of Basic Drugs: Speed up Extraction Time and Enhancement of Extraction Efficiency. RSC Adv. 2016, 6, 110221. [Google Scholar] [CrossRef]
- Lucena, R. Extraction and Stirring Integrated Techniques: Examples and Recent Advances. Anal. Bioanal. Chem. 2012, 403, 2213–2223. [Google Scholar] [CrossRef]
- Perrucci, M.; Ricci, E.M.; Locatelli, M.; Ali, I.; Mansour, F.R.; Kabir, A.; Ulusoy, H.I. Recent Trends in Microsampling and Reduced-Volume Sample Preparation Procedures. Adv. Sample Prep. 2025, 14, 100182. [Google Scholar] [CrossRef]
- Ni, N.; El-Sayed, M.M.; Sanghvi, T.; Yalkowsky, S.H. Estimation of the Effect of NaCl on the Solubility of Organic Compounds in Aqueous Solutions. J. Pharm. Sci. 2000, 89, 1620–1625. [Google Scholar] [CrossRef]
- Yuan, B.; Braune, M.; Gröngröft, A. Liquid-Liquid Extraction of Caproic and Caprylic Acid: Solvent Properties and pH. Chem. Ing. Tech. 2023, 95, 1573–1579. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Chen, Y.; Zheng, X.; Han, J.; Jing, X.; Yang, J. Detection of Triazine Herbicides in Water, Juice, and Tea Using Deep Eutectic Solvent-Based Emulsive Liquid–Liquid Microextraction Combined with High-Performance Liquid Chromatography. Anal. Methods 2025, 17, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, F.; Zhang, Y.; Feng, J.; Wang, X.; Yang, Y.; Wang, Z.; Zhang, H. Application of Supramolecular Solvent Based on the Surface-Active Ionic Liquid in Dispersive Liquid–Liquid Microextraction of Triazine Herbicides in Tea Samples. Food Chem. 2023, 399, 133901. [Google Scholar] [CrossRef] [PubMed]
- Legesse, A.; Megersa, N.; Chandravanshi, B.S. Effervescence-Assisted Dispersive Liquid-Liquid Microextraction for the Extraction and Preconcentration of Pesticide Residues in Fruit Juice Samples. Anal. Chim. Acta 2025, 1333, 343400. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yang, S.; Meng, Y. Investigation of pH-Switchability of Hydrophobic Deep Eutectic Solvents for the Extraction and Preconcentration of Triazine Herbicides in Water Samples. Microchem. J. 2023, 194, 109198. [Google Scholar] [CrossRef]
- Díaz-Álvarez, M.; Turiel, E.; Martín-Esteban, A. Hydrophobic Natural Deep Eutectic Solvents Based on L-Menthol as Supported Liquid Membrane for Hollow Fiber Liquid-Phase Microextraction of Triazines from Water and Urine Samples. Microchem. J. 2023, 194, 109347. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.M.; Konieczka, P.; Namieśnik, J. Analytical Eco-Scale for Assessing the Greenness of Analytical Procedures. TrAC Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J. A New Tool for the Evaluation of the Analytical Procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Wojnowski, W.; Tobiszewski, M.; Pena-Pereira, F.; Psillakis, E. AGREEprep—Analytical Greenness Metric for Sample Preparation. TrAC Trends Anal. Chem. 2022, 149, 116553. [Google Scholar] [CrossRef]
- González-Martín, R.; Gutiérrez-Serpa, A.; Pino, V.; Sajid, M. A Tool to Assess Analytical Sample Preparation Procedures: Sample Preparation Metric of Sustainability. J. Chromatogr. A 2023, 1707, 464291. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.