Effects of Root Exudates on Ecological Function and Nitrogen Utilization Strategy of Orchard Multi-Planting System
Abstract
1. Introduction
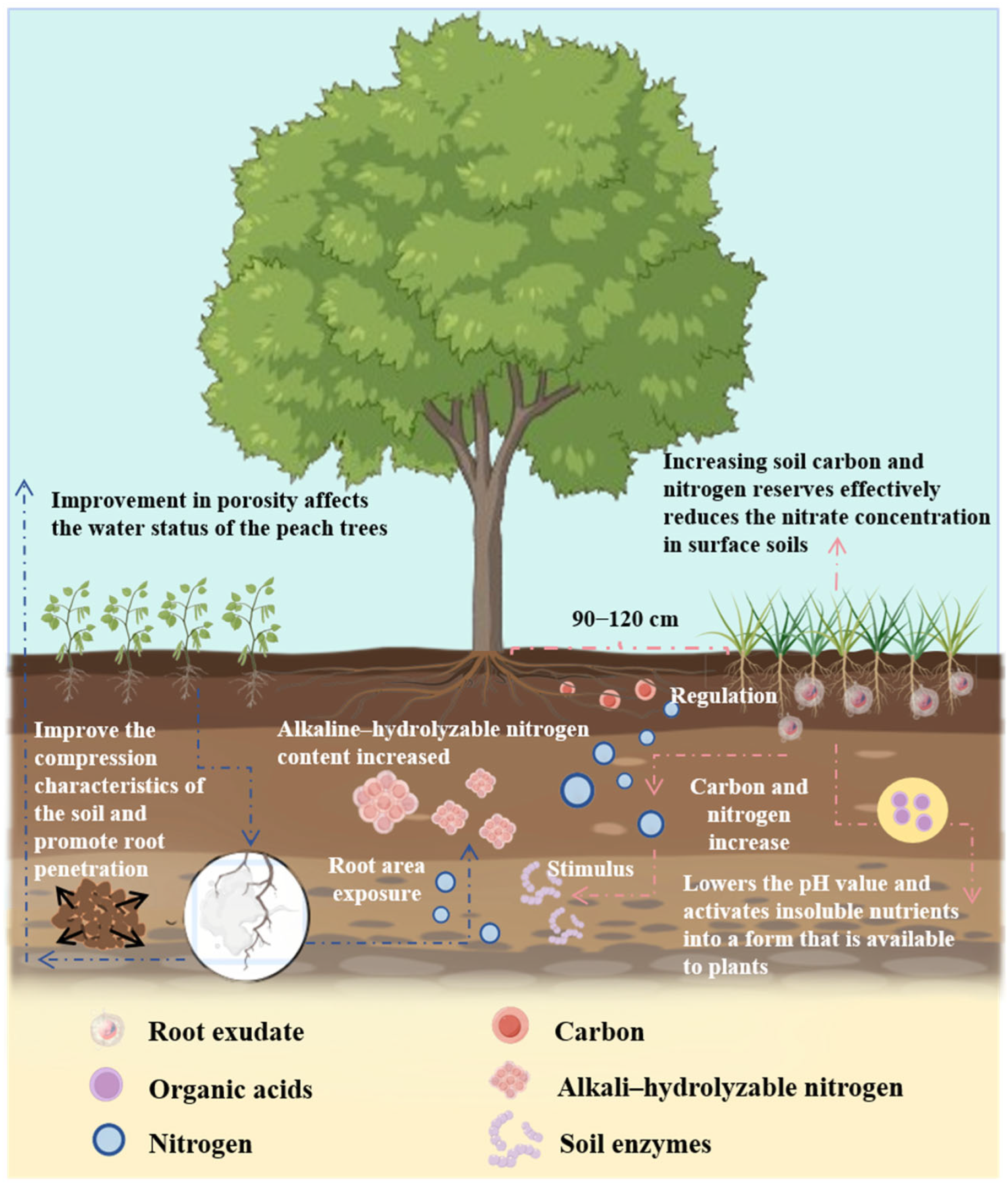
2. Root-Exudate-Mediated Improvements in Soil Properties
2.1. Soil Structure
2.2. Soil Nitrogen
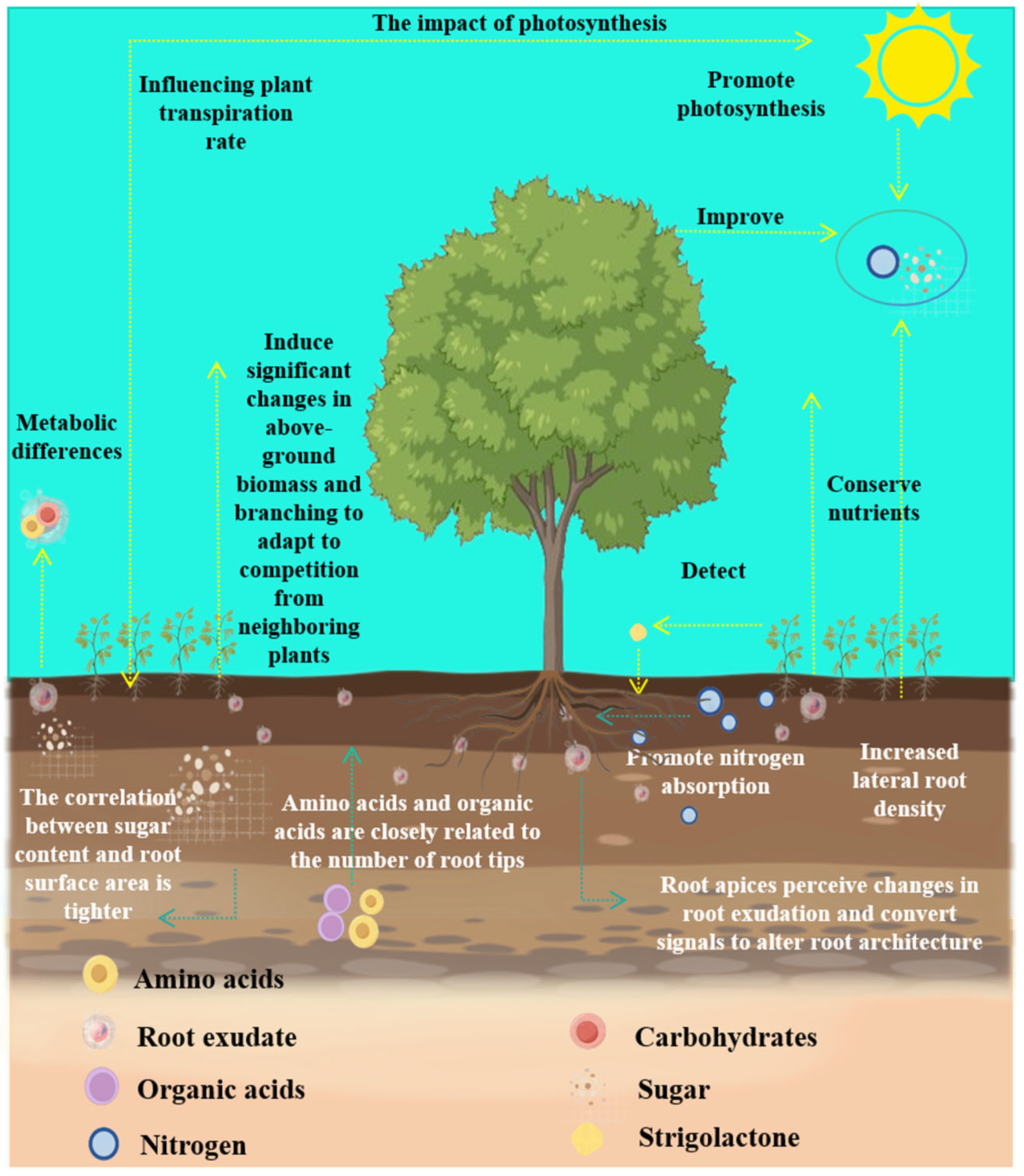
3. Root-Exudate-Mediated Improvements in Plant Traits
3.1. Root Architecture
3.2. Aboveground Trait Improvement
4. Rhizosphere Metabolite Remodeling Mediated by Root Exudates
4.1. Primary and Secondary Metabolites
4.2. Functional Metabolites—Antimicrobial Activity
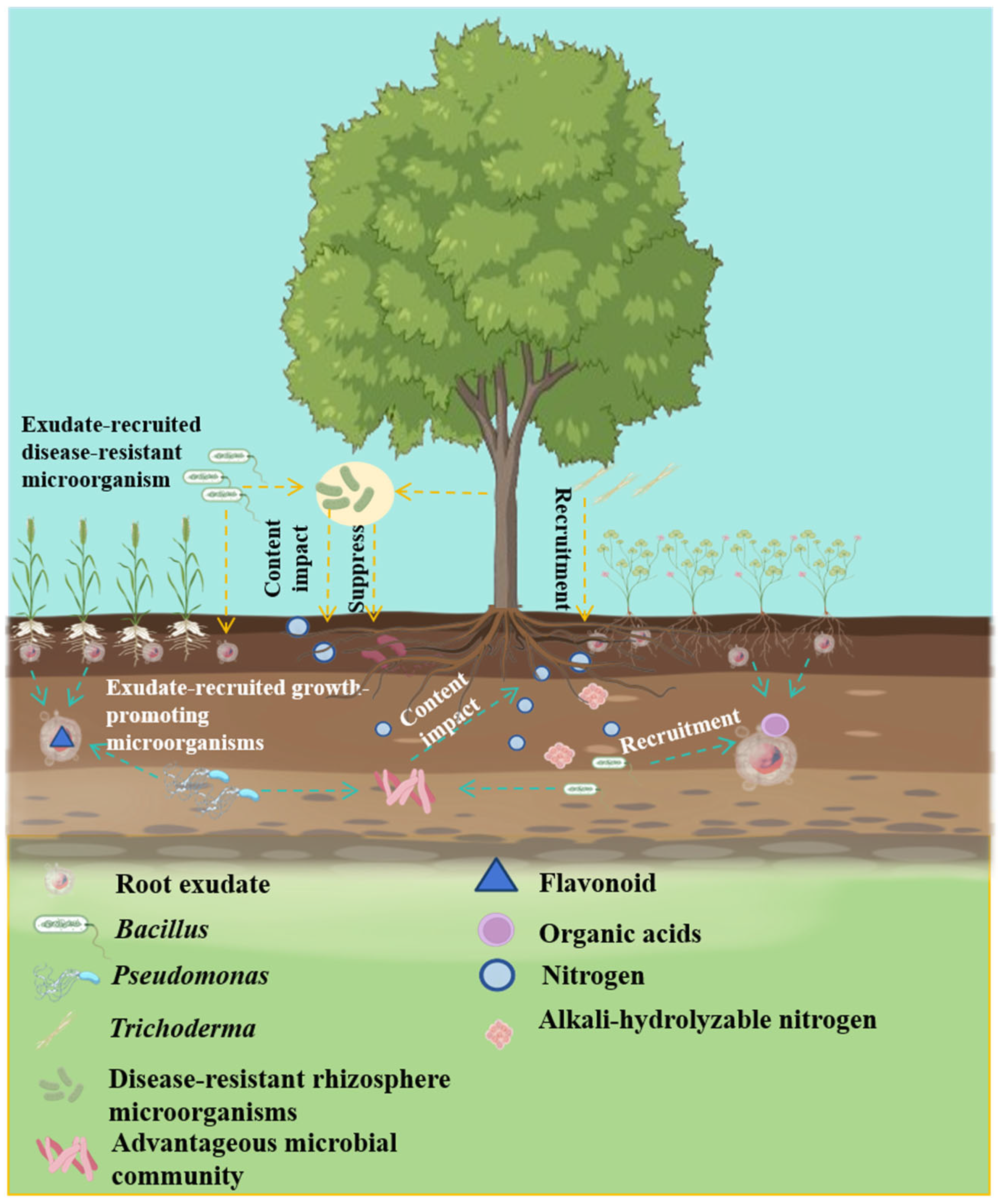
5. Root-Exudate-Mediated Recruitment of the Rhizosphere Microbiome
5.1. Recruitment of Growth-Promoting Microbiome
5.2. Recruitment of Disease-Suppressive Microbiome
6. Perspectives on Root-Exudate-Induced Nitrogen Utilization Strategies in Orchards
- (1)
- Functional genomics of associative diazotrophs: Employ metagenomic binning to screen ANF taxa (e.g., Azospirillum brasilense, Azoarcus spp.) in orchard polycultures. Integrate single-cell culturing with high-throughput screening to identify flavonoid-responsive strains, establishing genotype–nitrogenase efficiency correlations (e.g., nif cluster variants vs. exudate-induced expression).
- (2)
- Rhizosphere chemical dialogue regulation: Apply spatiotemporally resolved metabolomics–transcriptomics to quantify the dose–response relationships between companion plant exudates (e.g., flavonoids/organic acids) and diazotroph activity (acetylene reduction assays) and quorum sensing (e.g., AHL signaling). Define concentration thresholds for optimal nitrogen fixation.
- (3)
- Engineered microbial consortia: Construct synthetic communities (SynComs) integrating diazotrophs (Azotobacter vinelandii), phosphate-solubilizers (Pseudomonas putida), and biocontrol agents (Bacillus subtilis). Validate field efficacy in co-enhancing ANF and disease resistance, developing orchard-tailored biofertilizers.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Döll, S.; Koller, H.; van Dam, N.M. A simple, cost-effective and optimized protocol for collecting root exudates from soil grown plants. Rhizosphere 2024, 30, 100899. [Google Scholar] [CrossRef]
- Kengdo, S.K.; Mccormack, M.L.; Ostonen, I.; Torn, M.S. Fine-root dynamics in deeper soils: A critical but overlooked component of ecosystem responses to climate warming. New Phytol. 2025, 247, 2507–2513. [Google Scholar] [CrossRef]
- Widyati, E.; Sadino; Budiharta, S.; Akbar, A.; Susilo, A.; Kurniawan, A.; Sadili, A.; Prameswari, D.; Mirmanto, E.; Hadi, E.K.W.; et al. Changes in soil-root-organism interactions following tropical forest conversion to tree and oil palm plantations. Appl. Soil Ecol. 2025, 213, 106253. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.P. The Function of Root Exudates in the Root Colonization by Beneficial Soil Rhizobacteria. Biology 2024, 13, 95. [Google Scholar] [CrossRef]
- Yan, S.B.; Yin, L.M.; Dijkstra, F.A.; Wang, P.; Cheng, W.X. Priming effect on soil carbon decomposition by root exudate surrogates: A meta-analysis. Soil Biol. Biochem. 2023, 178, 108955. [Google Scholar] [CrossRef]
- Chen, H.H.; Zheng, Z.C.; Chen, W.S.; Rao, R.Y.; Chen, X.F.; Ye, X.; Guo, J.X.; Yang, L.T.; Chen, L.S. Regulation on copper-tolerance in Citrus sinensis seedlings by boron addition: Insights from root exudates, related metabolism, and gene expression. J. Hazard. Mater. 2023, 459, 132277. [Google Scholar] [CrossRef]
- Wu, D.Y.; He, X.M.; Jiang, L.M.; Li, W.J.; Wang, H.F.; Lv, G.H. Root exudates facilitate the regulation of soil microbial community function in the genus Haloxylon. Front. Plant Sci. 2024, 15, 1461893. [Google Scholar] [CrossRef]
- Asghar, W.; Craven, K.D.; Swenson, J.R.; Kataoka, R.; Mahmood, A.; Farias, J.G. Enhancing the Resilience of Agroecosystems Through Improved Rhizosphere Processes: A Strategic Review. Int. J. Mol. Sci. 2025, 26, 109. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, S.T.; Qi, Z.X.; Yang, C.G.; Ding, D.; Xiao, B.B.; Wang, S.H.; Yang, C.W. Regulation of Root Exudation in Wheat Plants in Response to Alkali Stress. Plants 2024, 13, 1227. [Google Scholar] [CrossRef] [PubMed]
- Santangeli, M.; Steininger-Mairinger, T.; Vetterlein, D.; Hann, S.; Oburger, E. Maize (Zea mays L.) root exudation profiles change in quality and quantity during plant development—A field study. Plants 2024, 338, 111896. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.B.; Yuan, H.M.; Liu, D.D.; Cheng, L.L. Response of soybean root exudates and related metabolic pathways to low phosphorus stress. PLoS ONE 2024, 19, e0314256. [Google Scholar] [CrossRef]
- Qu, S.; Li, H.X.; Zhang, X.K.; Gao, J.B.; Ma, R.; Ma, L.; Ma, J. Effects of Magnesium Imbalance on Root Growth and Nutrient Absorption in Different Genotypes of Vegetable Crops. Plants 2023, 12, 3518. [Google Scholar] [CrossRef]
- Cao, Y.H.; Zhao, X.W.; Nie, G.; Wang, Z.Y.; Song, X.; Zhang, M.X.; Hu, J.P.; Zhao, Q.; Jiang, Y.W.; Zhang, J.L. The salt-tolerance of perennial ryegrass is linked with root exudate profiles and microflora recruitment. Sci. Total Environ. 2024, 916, 170205. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.D.; Gou, M.C.; Yu, B.; Sun, C.; He, J.L.; Qin, S.J.; Lyu, D.G. Effects of mowing dominant grasses on root exudation and soil nitrogen cycling in a natural sod culture apple orchard. Plant Soil Environ. 2021, 67, 567–578. [Google Scholar] [CrossRef]
- Haupenthal, A.; Duddek, P.; Benard, P.; Knott, M.; Carminati, A.; Jungkunst, H.F.; Kroener, E.; Brueggemann, N. A root mucilage analogue from chia seeds reduces soil gas diffusivity. Eur. J. Soil Sci. 2024, 75, e13576. [Google Scholar] [CrossRef]
- Pantigoso, H.A.; Manter, D.K.; Fonte, S.J.; Vivanco, J.M. Root exudate-derived compounds stimulate the phosphorus solubilizing ability of bacteria. Sci. Rep. 2023, 13, 4050. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Ren, W.; Xu, J.P.; Liu, X.Y.; Liang, J.X.; Mi, G.H.; Gong, X.P.; Chen, F.J. Microbiology Combined with the Root Metabolome Reveals the Responses of Root Microorganisms to Maize Cultivars under Different Forms of Nitrogen Supply. Agronomy 2024, 14, 1828. [Google Scholar] [CrossRef]
- Joshi, S.R.; Tfaily, M.M.; Young, R.P.; Mcnear Jr, D.H. Root exudates induced coupled carbon and phosphorus cycling in a soil with low phosphorus availability. Plant Soil 2024, 498, 371–390. [Google Scholar] [CrossRef]
- Li, Z.; Guo, W.F.; Mo, C.M.; Tang, R.H.; He, L.Q.; Du, L.; Li, M.; Wu, H.N.; Tang, X.M.; Huang, Z.P.; et al. Root Metabolism and Effects of Root Exudates on the Growth of Ralstonia solanacearum and Fusarium moniliforme Were Significantly Different between the Two Genotypes of Peanuts. Genes 2023, 14, 528. [Google Scholar] [CrossRef]
- Yu, R.P.; Lambers, H.; Callaway, R.M.; Wright, A.J.; Li, L. Belowground facilitation and trait matching: Two or three to tango? Trends Plant Sci. 2021, 26, 1227–1235. [Google Scholar] [CrossRef]
- Yoneyama, K.; Bennett, T. Whispers in the dark: Signals regulating underground plant-plant interactions. Curr. Opin. Plant Biol. 2024, 77, 102456. [Google Scholar] [CrossRef]
- Lohse, M.; Santangeli, M.; Steininger-Mairinger, T.; Oburger, E.; Reemtsma, T.; Lechtenfeld, O.J.; Hann, S. The effect of root hairs on exudate composition: A comparative non-targeted metabolomics approach. Anal. Bioanal. Chem. 2023, 415, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.M.; Liao, L.L.; Wu, H.N.; Xiong, J.; Li, Z.; Huang, Z.P.; He, L.Q.; Jiang, J.; Zhong, R.C.; Han, Z.Q.; et al. Effects of Sugarcane/Peanut Intercropping on Root Exudates and Rhizosphere Soil Nutrient. Plants 2024, 13, 3257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, J.F.; Zhou, X.A.; Liu, X.X.; Wu, F.Z. Soybean triggers tomato root horizontal asymmetry by modifying capture of P rather than N under low nutrient condition. Plant Soil 2024, 499, 521–533. [Google Scholar] [CrossRef]
- Afzal, M.R.; Naz, M.; Ullah, R.; Du, D.L. Persistence of Root Exudates of Sorghum bicolor and Solidago canadensis: Impacts on Invasive and Native Species. Plants 2024, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Z.; Liu, Y.; Zeng, C.L.; Wang, J.Y.; Nyimbo, W.J.; Jiao, Y.Y.; Wu, L.K.; Chen, T.; Fang, C.X.; Lin, W.X. Intercropping with Achyranthes bidentata alleviates Rehmannia glutinosa consecutive monoculture problem by reestablishing rhizosphere microenvironment. Front. Plant Sci. 2024, 13, 1041561. [Google Scholar] [CrossRef]
- Yue, H.; Yue, W.J.; Jiao, S.; Kim, H.; Lee, Y.H.; Wei, G.H.; Song, W.N.; Shu, D.T. Plant domestication shapes rhizosphere microbiome assembly and metabolic functions. Microbiome 2023, 11, 70. [Google Scholar] [CrossRef]
- Maurer, D.; Malique, F.; Alfarraj, S.; Albasher, G.; Horn, M.A.; Butterbach-Bahl, K.; Dannenmann, M.; Rennenberg, H. Interactive regulation of root exudation and rhizosphere denitrification by plant metabolite content and soil properties. Plant Soil 2021, 467, 107–127. [Google Scholar] [CrossRef]
- Bi, B.Y.; Han, F.P. Pinus sylvestris root exudates indirectly facilitate Amorpha fruticosa growth performance by altering the nitrogen cycle. Plant Soil 2024, 504, 799–815. [Google Scholar] [CrossRef]
- Wang, H.W.; Ma, C.Y.; Xu, F.J.; Lu, F.; Zhang, W.; Dai, C.C. Root endophyte-enhanced peanut-rhizobia interaction is associated with regulation of root exudates. Microbiol. Res. 2021, 250, 126765. [Google Scholar] [CrossRef]
- Guo, L.; Liu, S.Y.; Zhang, P.Z.; Hakeem, A.; Song, H.F.; Yu, M.L.; Wang, F.L. Effects of Different Mulching Practices on Soil Environment and Fruit Quality in Peach Orchards. Plants 2024, 13, 827. [Google Scholar] [CrossRef]
- Oleghe, E.; Naveed, M.; Baggs, E.M.; Hallett, P.D. Plant exudates improve the mechanical conditions for root penetration through compacted soils. Plant Soil 2017, 421, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Haupenthal, A.; Brax, M.; Bentz, J.; Jungkunst, H.F.; Schützenmeister, K.; Kroener, E. Plants control soil gas exchanges possibly via mucilage. J. Plant Nutr. Soil Sci. 2021, 184, 320–328. [Google Scholar] [CrossRef]
- Paporisch, A.; Bavli, H.; Strickman, R.J.; Neumann, R.B.; Schwartz, N. Root Exudates Alters Nutrient Transport in Soil. Water Resour. Res. 2021, 57, e2021WR029976. [Google Scholar] [CrossRef]
- Batista, A.M.; Nunes, M.R.; Pessoa, T.N.; Libardi, P.L. Seasonal variation of the rhizosphere soil aggregation in an Oxisol. Soil Tillage Res. 2023, 231, 105741. [Google Scholar] [CrossRef]
- Sun, M.X.; Liu, X.L.; Shi, K.W.; Peng, F.T.; Xiao, Y.S. Effects of Root Zone Aeration on Soil Microbes Species in a Peach Tree Rhizosphere and Root Growth. Microorganisms 2022, 10, 1879. [Google Scholar] [CrossRef]
- Li, W.Q.; Liu, Y.J.; Duan, J.; Liu, G.P.; Nie, X.D.; Li, Z.W. Leguminous cover orchard improves soil quality, nutrient preservation capacity, and aggregate stoichiometric balance: A 22-year homogeneous experimental site. Agric. Ecosyst. Environ. 2024, 363, 108876. [Google Scholar] [CrossRef]
- Lin, Y.L.; Mei, L.L.; Wei, Q.H.; Li, B.; Zhang, P.; Sun, S.X.; Cui, G.W. Leymus chinensis resists degraded soil stress by modulating root exudate components to attract beneficial microorganisms. Front. Microbiol. 2022, 13, 951838. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, G.Z.; Tian, J.W.; Jia, Y.H.; Chu, Y.N.; Yue, H.Y.; Wang, H.X.; Li, X.L. Assessing and Quantifying the Carbon, Nitrogen, and Enzyme Dynamics Effects of Inter-row Cover Cropping on Soils and Apple Tree Development in Orchards. Hortscience 2024, 59, 1088–1096. [Google Scholar] [CrossRef]
- Li, X.; Dong, J.L.; Chu, W.Y.; Chen, Y.J.; Duan, Z.Q. The relationship between root exudation properties and root morphological traits of cucumber grown under different nitrogen supplies and atmospheric CO2 concentrations. Plant Soil 2018, 425, 415–432. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Y.Y.; Sheng, D.C.; Zhang, S.; Gu, S.C.; Yan, Y.; Zhao, F.C.; Wang, P.; Huang, S.B. Optimizing root system architecture to improve root anchorage strength and nitrogen absorption capacity under high plant density in maize. Field Crops Res. 2023, 303, 109109. [Google Scholar] [CrossRef]
- de la Peña, M.; Ruiz-Romero, R.; Castro-Arza, L.I.; Romero, H.M. Changes in the Root Architecture of Oil Palm Seedlings in Response to Nitrogen Starvation. Agronomy 2024, 14, 409. [Google Scholar] [CrossRef]
- Kang, Y.L.; Ma, Y.W.; An, X.R.; Xie, C.Y.; Mei, X.L.; Wang, Z.H.; Xu, Y.C.; Dong, C.X. Effects on the root morphology and mircostructure of young pear (Pyrus pyrifolia) tree by split-root supply of bioorganic and chemical fertilizer. Rhizosphere 2022, 22, 100504. [Google Scholar] [CrossRef]
- Wu, F.Z.; Li, M.; Cao, P.; Ma, Y.F.; Wang, L.L. Effects of wheat root exudates on cucumber growth and soil fungal community structure. Chin. J. Appl. Ecol. 2014, 25, 2861–2867. (In Chinese) [Google Scholar]
- Vengavasi, K.; Pandey, R. Root exudation potential in contrasting soybean genotypes in response to low soil phosphorus availability is determined by photo-biochemical processes. Plant Physiol. Biochem. 2018, 124, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, L.X.; Zhang, J.Q.; Lyu, D. Natural Grass Cultivation Management Improves Apple Fruit Quality by Regulating Soil Mineral Nitrogen Content and Carbon-Nitrogen Metabolism. Metabolites 2023, 13, 925. [Google Scholar] [CrossRef]
- Scandellari, F.; Ventura, M.; Gioacchini, P.; Antisari, L.V.; Tagliavini, M. Seasonal pattern of net nitrogen rhizodeposition from peach (Prunus persica (L.) Batsch) trees in soils with different textures. Agric. Ecosyst. Environ. 2010, 136, 162–168. [Google Scholar] [CrossRef]
- Ciacci, M.B.; Micheloud, N.G.; Levy, M.R.S.; Rodriguez, M.; Gariglio, N.F.; Imhoff, S. Advantages of Seeding Annual and Perennial Cover Crops Between Peach Rows. J. Soil Sci. Plant Nutr. 2023, 23, 683–692. [Google Scholar] [CrossRef]
- Xue, Z.J.; An, S.S.; Cheng, M.; Wang, W.Z. Plant functional traits and soil microbial biomass in different vegetation zones on the Loess Plateau. J. Plant Interact. 2014, 9, 889–900. [Google Scholar] [CrossRef]
- Liu, D.L.; Xu, L.Q.; Wang, H.; Xing, W.; Song, B.Q.; Wang, Q.H. Root Exudates Promoted Microbial Diversity in the Sugar Beet Rhizosphere for Organic Nitrogen Mineralization. Agriculture 2024, 14, 1094. [Google Scholar] [CrossRef]
- Liu, Y.; Evans, S.E.; Friesen, M.L.; Tiemann, L.K. Root exudates shift how N mineralization and N fixation contribute to the plant-available N supply in low fertility soils. Soil Biol. Biochem. 2022, 165, 108541. [Google Scholar] [CrossRef]
- Zhang, H.L.; Wu, R.Q.; Li, J.J.; Wang, R.Q.; Long, X.H.; Yang, C.X.; Shangguan, Z.P. Effects of root exudates C: N on soil physical and chemical characteristics and soil respiration in Robinia pseudoacacia plantation. Chin. J. Appl. Ecol. 2022, 33, 949–956. (In Chinese) [Google Scholar]
- Li, W.H.; Lin, Y.M.; Nan, X.X.; Wang, F.; Zhu, L.Z. Soil carbon and nitrogen sequestration and associated influencing factors in a sustainable cultivation system of fruit trees intercropped with cover crops. Chin. J. Appl. Ecol. 2023, 34, 471–480. (In Chinese) [Google Scholar]
- Salvador Rubio-Asensio, J.; Abbatantuono, F.; Miguel Ramirez-Cuesta, J.; Hortelano, D.; Luis Ruiz, J.; Parra, M.; Maria Martinez-Merono, R.; Intrigliolo, D.S.; Buesa, I. Effects of Cover Crops and Drip Fertigation Regime in a Young Almond Agroecosystem. Agronomy 2022, 12, 2606. [Google Scholar] [CrossRef]
- Zheng, W.; Gong, Q.L.; Lv, F.L.; Yin, Y.A.; Li, Z.Y.; Zhai, B.N. Tree-scale spatial responses of extracellular enzyme activities and stoichiometry to different types of fertilization and cover crop in an apple orchard. Eur. J. Soil Biol. 2020, 99, 103207. [Google Scholar] [CrossRef]
- Marañón-Jiménez, S.; Serrano-Ortíz, P.; Peñuelas, J.; Meijide, A.; Chamizo, S.; López-Ballesteros, A.; Vicente-Vicente, J.L.; Fernández-Ondoño, E. Effects of herbaceous covers and mineral fertilizers on the nutrient stocks and fluxes in a Mediterranean olive grove. Eur. J. Agron. 2022, 140, 126597. [Google Scholar] [CrossRef]
- Wen, Z.H.; White, P.J.; Shen, J.B.; Lambers, H. Linking root exudation to belowground economic traits for resource acquisition. New Phytol. 2022, 233, 1620–1635. [Google Scholar] [CrossRef]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root Exudation of Primary Metabolites: Mechanisms and Their Roles in Plant Responses to Environmental Stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, Ç.; Bennett, A.A.; Steininger-Mairinger, T.; Hann, S.; Puschenreiter, M.; Wirth, J.; Gfeller, A. Neighbour-induced changes in root exudation patterns of buckwheat results in altered root architecture of redroot pigweed. Sci. Rep. 2024, 14, 8679. [Google Scholar] [CrossRef]
- Yin, X.T.; Zhang, F.F.; Yu, R.P.; Liu, N.; Zhang, W.P.; Fornara, D.; Mommer, L.; Li, X.X.; Li, L. Root exudates drive root avoidance of maize in response to neighboring wheat. Plant Soil 2024, 510, 507–524. [Google Scholar] [CrossRef]
- Fujii, K. Plant strategy of root system architecture and exudates for acquiring soil nutrients. Ecol. Res. 2024, 39, 623–633. [Google Scholar] [CrossRef]
- Lecarpentier, C.; Pagès, L.; Richard-Molard, C. Genotypic diversity and plasticity of root system architecture to nitrogen availability in oilseed rape. PLoS ONE 2021, 16, e0250966. [Google Scholar] [CrossRef] [PubMed]
- Guilbeault-Mayers, X.; Lambers, H.; Laliberté, E. Coordination among leaf and fine-root traits along a strong natural soil fertility gradient. Plant Soil 2024, 1–17. [Google Scholar] [CrossRef]
- Wheeldon, C.D.; Hamon-Josse, M.; Lund, H.; Yoneyama, K.; Bennett, T. Environmental strigolactone drives early growth responses to neighboring plants and soil volume in pea. Curr. Biol. 2022, 32, 3593–3600. [Google Scholar] [CrossRef]
- Xu, Y.; Li, F.L.; Li, L.L.; Chen, X.; Meiners, S.J.; Kong, C.H. Discrimination of relatedness drives rice flowering and reproduction in cultivar mixtures. Plant Cell Environ. 2024, 47, 4572–4585. [Google Scholar] [CrossRef]
- Lu, J.H.; Liu, Y.X.; Zou, X.X.; Zhang, X.J.; Yu, X.A.; Wang, Y.F.; Si, T. Rotational strip peanut/cotton intercropping improves agricultural production through modulating plant growth, root exudates, and soil microbial communities. Agric. Ecosyst. Environ. 2024, 359, 108767. [Google Scholar] [CrossRef]
- Zhou, X.B.; Xu, W.H.; Wang, Z.Y.; Xie, D.T. Effects of Different Zn Supply Levels on Physiological Parameters, Seed Zn Concentration and Root Cell Ultrastructure in three Different Genotypes of Brassica Species. Int. J. Agric. Biol. 2017, 19, 282–290. [Google Scholar] [CrossRef]
- Asigbaase, M.; Dawoe, E.; Abugre, S.; Kyereh, B.; Nsor, C.A. Allometric relationships between stem diameter, height and crown area of associated trees of cocoa agroforests of Ghana. Sci. Rep. 2023, 13, 14897. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.B.; Chen, Y.M.; Sardans, J.; Pen, J. Ecological stoichiometric comparison of plant-litter-soil system in mixed-species and monoculture plantations of Robinia pseudoacacia, Amygdalus davidiana, and Armeniaca sibirica in the Loess Hilly Region of China. For. Ecosyst. 2023, 10, 100123. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, Z.K.; Yang, X.L.; Shen, Y.Y. The effects of cocksfoot cover crop on soil water balance, evapotranspiration partitioning, and system production in an apple orchard on the Loess Plateau of China. Soil Tillage Res. 2021, 205, 104788. [Google Scholar] [CrossRef]
- Zhao, M.N.; Sun, Y.; Dong, M.L.; Zhang, K.; Zhang, J.; Qin, X.X.; Yao, Y.C. Hexose/pentose ratio in rhizosphere exudates-mediated soil eutrophic/oligotrophic bacteria regulates the growth pattern of host plant in young apple-aromatic plant intercropping systems. Front. Microbiol. 2024, 15, 1364355. [Google Scholar] [CrossRef]
- Subrahmaniam, H.J.; Pico, F.X.; Bataillon, T.; Salomonsen, C.L.; Glasius, M.; Ehlers, B.K. Natural variation in root exudate composition in the genetically structured Arabidopsis thaliana in the Iberian Peninsula. New Phytol. 2024, 245, 1437–1449. [Google Scholar] [CrossRef]
- Wang, Y.W.; Jiang, Y.B.; Liu, X.M.; Chen, Y.D.; Zhang, Q.X.; Wang, L.; Li, W.X. Analysis of Ginkgo biloba Root Exudates and Inhibition of Soil Fungi by Flavonoids and Terpene Lactones. Plants 2024, 13, 2122. [Google Scholar] [CrossRef]
- Zhong, Y.; Xun, W.B.; Wang, X.H.; Tian, S.W.; Zhang, Y.C.; Li, D.W.; Zhou, Y.; Qin, Y.X.; Zhang, B.; Zhao, G.W.; et al. Root-secreted bitter triterpene modulates the rhizosphere microbiota to improve plant fitness. Nat. Plants 2022, 8, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Iannucci, A.; Canfora, L.; Nigro, F.; De Vita, P.; Beleggia, R. Relationships between root morphology, root exudate compounds and rhizosphere microbial community in durum wheat. Appl. Soil Ecol. 2021, 158, 103781. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.M.; Meng, L.B.; Liu, X.D.; Zhang, Y.H.; Zhao, S.C.; Zhao, H.B. Root exudation under maize/soybean intercropping system mediates the arbuscular mycorrhizal fungi diversity and improves the plant growth. Front. Plant Sci. 2024, 15, 1375194. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.; Mondal, A.; Majumder, A.; Mondal, S.K.; Banik, A. Flavonoid mediated selective cross-talk between plants and beneficial soil microbiome. Phytochem. Rev. 2022, 21, 1739–1760. [Google Scholar] [CrossRef] [PubMed]
- Ninkovic, S.; Motyka, V.; Stanisic, M.; Smailagic, D.; Zivanovic, B.; Dobrev, P.I.; Banjac, N. Phytohormone Profiling of Malus domestica and Chenopodium murale Hairy Root Exudate: Association with Allelopathic Effects. J. Plant Growth Regul. 2024, 43, 3580–3593. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, M.Z.; Song, M.N.; Tian, J.; Song, B.Z.; Hu, Y.J.; Zhang, J.; Yao, Y.C. Intercropping With Aromatic Plants Increased the Soil Organic Matter Content and Changed the Microbial Community in a Pear Orchard. Front. Microbiol. 2021, 12, 616932. [Google Scholar] [CrossRef]
- Dang, K.K.; Hou, J.F.; Liu, H.; Peng, J.W.; Sun, Y.; Li, J.A.; Dong, Y.H. Root Exudates of Ginger Induced by Ralstonia solanacearum Infection Could Inhibit Bacterial Wilt. J. Agric. Food Chem. 2023, 71, 1957–1969. [Google Scholar] [CrossRef]
- Li, S.L.; Pi, J.; Zhu, H.J.; Yang, L.; Zhang, X.G.; Ding, W. Caffeic Acid in Tobacco Root Exudate Defends Tobacco Plants From Infection by Ralstonia solanacearum. Front. Plant Sci. 2021, 12, 690586. [Google Scholar] [CrossRef]
- Li, P.; Wang, S.Y.; Liu, M.Y.; Dai, X.; Shi, H.C.; Zhou, W.H.; Sheng, S.; Wu, F. Antibacterial Activity and Mechanism of Three Root Exudates from Mulberry Seedlings against Ralstonia pseudosolanacearum. Plants 2024, 13, 482. [Google Scholar] [CrossRef]
- Kuang, M.S.; Liu, T.T.; Wu, H.B.; Lan, H.P.; Wen, Y.X.; Wu, H.B.; Li, X.M. Constituents Leached by Tomato Seeds Regulate the Behavior of Root-Knot Nematodes and Their Antifungal Effects against Seed-Borne Fungi. J. Agric. Food Chem. 2020, 68, 9061–9069. [Google Scholar] [CrossRef]
- Yang, S.Y.; Zheng, Y.R.; Cen, Z.X.; Guo, Y.T.; Yang, Z.X.; Dong, Y. Wheat root exudates suppress faba bean Fusarium wilt disease. Food Energy Secur. 2024, 13, e550. [Google Scholar] [CrossRef]
- Zhang, J.L.; Liu, Q.W.; Li, K.; Ma, L. Peanut Root Exudates Suppress Fusarium solani and Modulate the Microbial Community Structure of Rhizosphere in Grape Replant Soil. Horticulturae 2022, 8, 892. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.Z.; Liu, J.; Zhang, J.; Wang, C.L. Determination of the Effects of Pear-Morchella Intercropping Mode on M. sextelata Quality, Yield, and Soil Microbial Community. J. Fungi 2024, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.X.; Wang, G.S.; Chen, R.; Liu, X.; Chen, X.S.; Shen, X.; Yin, C.M.; Mao, Z.Q. Allium fistulosum L. Alleviates Apple Replant Disease by Suppressing Fusarium solani. J. Fungi 2022, 8, 1071. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Moreno, A.; Bollmann-Giolai, A.; Chandra, G.; Brett, P.; Davies, J.; Thornton, O.; Poole, P.; Ramachandran, V.; Brown, J.K.M.; Nicholson, P.; et al. The genotype of barley cultivars influences multiple aspects of their associated microbiota via differential root exudate secretion. PLoS Biol. 2024, 22, e3002232. [Google Scholar] [CrossRef]
- Li, J.X.; Wu, L.L.; Zhou, Y.Z.; Xie, Y.L.; Lu, F.W.; Chang, F.F.; Yang, X.; Han, X.Z.; Cheng, M.X. Kobresia humilis via root-released flavonoids recruit Bacillus for promoted growth. Microbiol. Res. 2024, 287, 127866. [Google Scholar] [CrossRef]
- Devi, T.A.P.; Sahoo, D.; Setti, A.; Sharma, C.K.; Kalita, M.C.; Devi, S.I. Bacterial rhizosphere community profile at different growth stages of Umorok (Capsicum chinense) and its response to the root exudates. Int. Microbiol. 2020, 23, 241–251. [Google Scholar]
- Yang, L.H.; Zhou, Y.; Guo, L.J.; Yang, L.Y.; Wang, J.; Liang, C.C.; Huang, J.S. The Effect of Banana Rhizosphere Chemotaxis and Chemoattractants on Bacillus velezensis LG14-3 Root Colonization and Suppression of Banana Fusarium Wilt Disease. Sustainability 2023, 15, 351. [Google Scholar] [CrossRef]
- Liu, S.S.; Tao, C.Y.; Zhang, L.Y.; Wang, Z.; Xiong, W.; Xiang, D.D.; Sheng, O.; Wang, J.B.; Li, R.; Shen, Z.Z.; et al. Plant pathogen resistance is mediated by recruitment of specific rhizosphere fungi. ISME J. 2023, 17, 931–942. [Google Scholar] [CrossRef]
- Zhou, X.G.; Zhang, J.Y.; Rahman, M.K.U.; Gao, D.M.; Wei, Z.; Wu, F.Z.; Dini-Andreote, F. Interspecific plant interaction via root exudates structures the disease suppressiveness of rhizosphere microbiomes. Mol. Plant 2023, 16, 849–864. [Google Scholar] [CrossRef]
- Xue, X.M.; Chen, R.; Xu, C.; Zhang, C.X.; Dong, L.J.; Zhao, X.Y.; Wang, X.H. Apple-marigold intercropping improves soil properties by changing soil metabolomics and bacterial community structures. Front. Microbiol. 2023, 14, 1195985. [Google Scholar] [CrossRef]
- Li, C.C.; Xie, Y.M.; Liao, Y.S.; Liu, J.T.; Li, B.; Lu, Y.S.; Yang, K.; Shan, J.W.; Wang, L.; An, K.; et al. Interplanting potato with grapes improved yield and soil nutrients by optimizing the interactions of soil microorganisms and metabolites. Front. Plant Sci. 2024, 15, 1404589. [Google Scholar] [CrossRef]
- Chang, X.L.; Wei, D.Q.; Zeng, Y.H.; Zhao, X.Y.; Hu, Y.; Wu, X.L.; Song, C.; Gong, G.S.; Chen, H.B.; Yang, C.P.; et al. Maize-soybean relay strip intercropping reshapes the rhizosphere bacterial community and recruits beneficial bacteria to suppress Fusarium root rot of soybean. Front. Microbiol. 2022, 13, 1009689. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.N.; Liu, X.Q.; He, W.X.; Xu, X.H.; Xu, Y.J.; Hashem, A.; Abd-Allah, E.F.; Wu, Q.S. Arbuscular mycorrhizal fungi and intercropping Vicia villosa mediate plant biomass, soil properties, and rhizosphere metabolite profiles of walnuts. Chem. Biol. Technol. Agric. 2024, 11, 159. [Google Scholar] [CrossRef]
- Kumar, G.A.; Kumar, S.; Bhardwaj, R.; Swapnil, P.; Meena, M.; Seth, C.S.; Yadav, A. Recent advancements in multifaceted roles of flavonoids in plant-rhizomicrobiome interactions. Front. Plant Sci. 2024, 14, 1297706. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.W.; Liang, Q.H.; Yao, Q.; Bai, Y.; Zhu, H.H. The role of the rhizobiome recruited by root exudates in plant disease resistance: Current status and future directions. Environ. Microbiome 2024, 19, 91. [Google Scholar] [CrossRef]
- Xie, S.S.; Jiang, L.; Wu, Q.; Wan, W.K.; Gan, Y.T.; Zhao, L.L.; Wen, J.J. Maize Root Exudates Recruit Bacillus amyloliquefaciens OR2-30 to Inhibit Fusarium graminearum Infection. Phytopathology 2022, 112, 1886–1893. [Google Scholar] [CrossRef]
- Duan, W.Y.; Chen, X.L.; Ding, Y.; Mao, X.Y.; Song, Z.J.; Bao, J.; Fang, L.; Guo, L.P.; Zhou, J. Intricate microbe-plant-metabolic remodeling mediated by intercropping enhances the quality of Panax quinquefolius L. Physiol. Plant. 2024, 176, e14499. [Google Scholar] [CrossRef] [PubMed]
- Sorochkina, K.; Martens-Habbena, W.; Reardon, C.L.; Inglett, P.W.; Strauss, S.L. Nitrogen-fixing bacterial communities differ between perennial agroecosystem crops. FEMS Microbiol. Ecol. 2024, 100, fiae064. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.J.; Sun, R.B.; Wang, Z.X.; Dumack, K.; Xie, X.G.; Dai, C.C.; Wang, E.R.; Zhou, J.Z.; Sun, B.; Peng, X.H.; et al. Legume rhizodeposition promotes nitrogen fixation by soil microbiota under crop diversification. Nat. Commun. 2024, 15, 2924. [Google Scholar] [CrossRef] [PubMed]

| Ecosystem | Experimental Condition | Plant | Types of Root Exudates | Key Findings | Reference |
|---|---|---|---|---|---|
| Single-planting | Laboratory-controlled | Chia | Polysaccharides | ↓ Soil penetration resistance (up to 77% in sandy loam); ↑ soil porosity under drought stress. Field validation pending. | [32] |
| Single-planting | Field | Winter crops | Organic matter inputs (indirect) | ↑ Soil aggregate stability; summer crops with high chemical fertilizers ↓ stability by suppressing microbial activity. | [35] |
| Single-planting | Field | Peach | Not specified | Root zone aeration ↑ N-fixing microbes (Bradyrhizobium) and K-solubilizers (Bacillus); ↑ soil N content and plant K:N ratio. | [36] |
| Multi-planting | Field | Citrus trees with leguminous cover crops | Not specified | ↑ Soil Quality Index (SQI) via improved aggregates; maintained nutrient balance. | [37] |
| Single-planting | Field | Grass | Citric acid and oxalic acid | ↓ Soil pH → ↑ solubilized P and chelated metals; ↑ NH4+-N/NO3−-N bioavailability by 15–70%. | [38] |
| Multi-planting | Field | Apple + cover crops | Sugars and organic acids | ↑ Soluble organic C, microbial biomass, and N fractions (>19.6%) in 0–20 cm soil. | [39] |
| Single-planting | Greenhouse | Cucumber | Sugars (fructose, glucose), organic acids (oxalic, malic), and amino acids | Sugars ↑ root surface area; organic acids/amino acids ↑ root tip number. Quantitative RSA links inform orchard management. | [40] |
| Single-planting | Field | Maize | Not characterized (N-uptake focus) | ↑ Specific root length and root angle → ↑ N absorption under high density; adaptive “foraging-avoidance” strategy. | [41] |
| Single-planting | Greenhouse | Oil palm | Not specified | N deficiency ↓ root length/surface area; ↑ angular frequency → conservative resource-use strategy. | [42] |
| Single-planting | Greenhouse | Pear | Organic acids (e.g., citrate, malate) and enzymes | Branching intensity ↑ with soil N profiles (total N, NH4+); BIO fertilizer ↑ lateral roots. | [43] |
| Multi-planting | Greenhouse | Wheat + cucumber | Not specified | Wheat REs ↑ cucumber growth; altered rhizosphere microbiome. | [44] |
| Single-planting | Greenhouse | Soybean | Organic acids, amino acids, phenolics, proteins, and sugars | P-efficient varieties ↑ photosynthetic rate, chlorophyll, and carotenoids; linked to RE metabolite profiles. | [45] |
| Multi-planting | Field | Apple + grass cover | Not specified | ↑ Photosynthesis and fruit sugar metabolism via ↑ soil N; ↓ evaporation via transpiration shift. | [46] |
| Ecosystem | Experimental Condition | Plant | Types of Root Exudates | Key Findings | Reference |
|---|---|---|---|---|---|
| Rhizosphere Metabolite Remodeling | |||||
| Single-planting | Laboratory-controlled | Ginger | Specific antimicrobial compounds (unspecified) | ↓ Bacterial wilt disease index (77.5% → 40.0%) by suppressing Ralstonia solanacearum via induced exudates. | [80] |
| Single-planting | Greenhouse | Tobacco | Caffeic acid (phenolic compound) | Disrupted R. solanacearum cell membranes (thinning, irregular cavities) at high concentrations. | [81] |
| Single-planting | Laboratory-controlled | Mulberry | Erucamide, oleamide, and bromocamphor | Inhibited R. pseudosolanacearum via ROS bursts, reduced virulence gene expression, and altered cell morphology/extracellular polysaccharides. | [82] |
| Single-planting | Laboratory-controlled | Tomato | Ferulic acid and dihydrocapsaicin | Suppressed Aspergillus flavus and Fusarium oxysporum growth via antifungal activity. | [83] |
| Multi-planting | Field | Wheat–faba bean | Salicylic acid, p-hydroxybenzoic acid, and tartaric acid | ↓ Incidence/severity of Fusarium wilt by reconstructing rhizosphere microbiota and accumulating disease-suppressive metabolites. | [84] |
| Multi-planting | Greenhouse | Peanut–grape | Unspecified antimicrobial exudates | Suppressed Fusarium solani growth in grape replant soils. | [85] |
| Multi-planting | Field | Pear–morel | Amino acids (phenylalanine, lysine), sugars (arabitol), and organic acids (quinic acid) | Inhibited Fusarium and Aspergillus pathogens via metabolite accumulation. | [86] |
| Multi-planting | Field | Chinese chive–apple | Dimethyl disulfide and diallyl disulfide | ↓ Apple replant disease by suppressing Fusarium solani mycelial growth and spore germination. | [87] |
| Microbiome Recruitment | |||||
| Multi-planting | Field | Apple–aromatic plants | Hexose-enriched exudates | Recruited Actinobacteria/Bacilli, improving soil N utilization and microbial nutrient turnover. | [79] |
| Single-planting | Field | Barley | Hexoses (sugars) | Attracted Pseudomonas spp. growth, enhancing plant growth-promoting bacteria (PGPB) colonization. | [88] |
| Single-planting | Field | Dwarf alpine sedge | Flavonoids (baicalin), sucrose, and riboflavin | Enhanced Bacillus colonization; reciprocally stimulated root growth. | [89] |
| Single-planting | Greenhouse | Capsicum | Organic acids, phenolics, and flavonoids | Shifted PGPB recruitment: Young plants → Gammaproteobacteria; Flowering/fruiting → Bacillus and Burkholderia. | [90] |
| Single-planting | Greenhouse | Banana | Citric acid, succinic acid, glycine, D-galactose, and D-maltose | Bacillus velezensis chemotaxis → ↓ Fusarium wilt severity via antagonism. | [91] |
| Single-planting | Laboratory-controlled | Banana | Shikimic acid and propylene glycol | Recruited Trichoderma and Penicillium to suppress Fusarium oxysporum f. sp. cubense (FOC). | [92] |
| Multi-planting | Field | Onion–tomato | Flavonoids (taxifolin) | Recruited Bacillus spp. to antagonize Verticillium dahliae; altered tomato root exudates to enhance colonization. | [93] |
| Multi-planting | Field | Apple–marigold | Sucrose (via starch/sucrose metabolism) | Recruited Pseudomonas and Bacillus; abundance correlated with soil N dynamics. | [94] |
| Multi-planting | Field | Maize–soybean | Lipids and organic acids | Recruited Bacillus, Kaistobacter, and Streptomyces; suppressed F. oxysporum and improved soil N concentrations. | [95,96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, Y.; He, Q.; Liu, S.; Ren, F.; Lu, A. Effects of Root Exudates on Ecological Function and Nitrogen Utilization Strategy of Orchard Multi-Planting System. Agronomy 2025, 15, 2173. https://doi.org/10.3390/agronomy15092173
Li Y, Zhang Y, He Q, Liu S, Ren F, Lu A. Effects of Root Exudates on Ecological Function and Nitrogen Utilization Strategy of Orchard Multi-Planting System. Agronomy. 2025; 15(9):2173. https://doi.org/10.3390/agronomy15092173
Chicago/Turabian StyleLi, Yufeng, Yu Zhang, Qishuang He, Shanshan Liu, Fei Ren, and Anxiang Lu. 2025. "Effects of Root Exudates on Ecological Function and Nitrogen Utilization Strategy of Orchard Multi-Planting System" Agronomy 15, no. 9: 2173. https://doi.org/10.3390/agronomy15092173
APA StyleLi, Y., Zhang, Y., He, Q., Liu, S., Ren, F., & Lu, A. (2025). Effects of Root Exudates on Ecological Function and Nitrogen Utilization Strategy of Orchard Multi-Planting System. Agronomy, 15(9), 2173. https://doi.org/10.3390/agronomy15092173





