Chemical Fractions of Soil Organic Matter and Their Interactions with Cu, Zn, and Mn in Vineyards in Southern Brazil
Abstract
1. Introduction
2. Material and Methods
2.1. Characterization of Study Sites and Soil Sampling
2.2. Preparation of Soil Samples and Chemical Analyses
2.3. Calculations and Statistical Analysis
3. Results
3.1. TOC Contents and C Associated with Humic Fractions of SOM
3.2. Carbon Distribution in the Humic Fractions of SOM
3.3. CHA/CFA and (CHA + CFA)/CHu Ratios
3.4. Molecular Composition Assessed by FTIR and RI
3.5. Distribution of Cu, Zn, and Mn in the Humic Fractions of SOM
4. Discussion
4.1. TOC Contents and C Associated with Humic Fractions of SOM
4.2. Cu, Zn, and Mn Contents in the Chemical Fractions of SOM
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Area | Layer (m) | Clay | TOC | pHH2O | SMP Index | Cu | Zn | Mn | P | K | Ca | Mg | Al | H + Al | CECef. | CECpH7.0 | m | V |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| -----g kg−1----- | ---------------------mg kg−1--------------------- | --------------------------cmolc kg−1------------------------- | -----%----- | |||||||||||||||
| Serra Gaúcha | ||||||||||||||||||
| F | 0.00–0.10 | 261 | 62.27 | 6.32 | 6.75 | 4.38 | 2.34 | 13.12 | 12.46 | 215.33 | 3.74 | 0.99 | 0.00 | 1.77 | 5.19 | 6.96 | 0.00 | 74.49 |
| 0.10–0.20 | 24.72 | 6.20 | 6.77 | 0.27 | 2.06 | 27.09 | 4.40 | 48.00 | 2.62 | 0.37 | 0.00 | 2.05 | 3.05 | 5.10 | 0.00 | 59.42 | ||
| V35 | 0.00–0.10 | 182 | 51.64 | 6.94 | 7.24 | 5.70 | 0.41 | 12.56 | 232.47 | 339.50 | 2.99 | 1.02 | 0.00 | 1.11 | 4.79 | 5.89 | 0.00 | 81.15 |
| 0.10–0.20 | 28.49 | 6.76 | 6.83 | 1.34 | 0.84 | 6.35 | 79.20 | 264.83 | 2.32 | 1.16 | 0.00 | 1.71 | 4.23 | 5.93 | 0.00 | 70.92 | ||
| V37 | 0.00–0.10 | 342 | 73.20 | 7.00 | 7.16 | 5.16 | 0.68 | 13.30 | 212.11 | 233.17 | 4.87 | 1.42 | 0.00 | 1.23 | 6.94 | 8.17 | 0.00 | 84.65 |
| 0.10–0.20 | 23.83 | 6.87 | 6.87 | 1.94 | 0.48 | 6.31 | 13.19 | 180.33 | 3.00 | 1.41 | 0.00 | 1.68 | 4.80 | 6.48 | 0.00 | 74.01 | ||
| V39 | 0.00–0.10 | 301 | 29.24 | 6.23 | 6.73 | 3.96 | 1.94 | 8.05 | 26.95 | 176.84 | 1.62 | 1.09 | 0.00 | 1.95 | 1.75 | 3.70 | 0.00 | 40.22 |
| 0.10–0.20 | 13.97 | 6.17 | 6.92 | 1.35 | 0.12 | 4.06 | 14.11 | 109.34 | 1.33 | 1.08 | 0.00 | 1.54 | 1.46 | 2.99 | 0.00 | 39.71 | ||
| Campanha Gaúcha | ||||||||||||||||||
| NG | 0.00–0.10 | 74 | 25.55 | 5.57 | 6.71 | 0.14 | 0.90 | 47.09 | 6.69 | 36.09 | 0.61 | 1.05 | 0.13 | 1.95 | 1.88 | 3.70 | 7.35 | 47.10 |
| 0.10–0.20 | 18.28 | 5.02 | 6.47 | 0.16 | 1.05 | 67.90 | 4.19 | 25.50 | 0.27 | 0.33 | 0.50 | 2.55 | 1.17 | 3.23 | 42.69 | 20.86 | ||
| V13 | 0.00–0.10 | 80 | 30.75 | 6.30 | 6.86 | 0.16 | 1.51 | 10.46 | 66.47 | 47.84 | 3.16 | 4.60 | 0.03 | 1.67 | 7.90 | 9.54 | 0.68 | 78.24 |
| 0.10–0.20 | 16.79 | 5.70 | 6.64 | 0.22 | 3.00 | 15.22 | 45.81 | 37.67 | 1.34 | 1.73 | 0.10 | 2.14 | 3.27 | 5.30 | 3.14 | 59.93 | ||
| V19 | 0.00–0.10 | 74 | 22.84 | 6.33 | 6.86 | 1.11 | 2.00 | 9.28 | 89.51 | 45.25 | 1.91 | 1.49 | 0.03 | 1.70 | 3.55 | 5.21 | 1.01 | 67.95 |
| 0.10–0.20 | 15.72 | 6.07 | 6.88 | 2.09 | 3.88 | 18.79 | 69.15 | 27.33 | 1.22 | 1.16 | 0.05 | 1.65 | 2.50 | 4.10 | 2.16 | 60.30 | ||
| V36 | 0.00–0.10 | 54 | 23.71 | 6.24 | 6.89 | 7.10 | 1.34 | 11.63 | 60.45 | 48.29 | 2.57 | 4.36 | 0.07 | 1.61 | 7.11 | 8.66 | 2.79 | 77.34 |
| 0.10–0.20 | 14.08 | 5.96 | 6.89 | 13.85 | 20.49 | 37.81 | 30.97 | 28.84 | 1.29 | 1.86 | 0.06 | 1.64 | 3.27 | 4.85 | 1.98 | 66.27 | ||
Appendix B
| Layer (m) | Serra Gaúcha | CV% | Layer (m) | Campanha Gaúcha | CV% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | V35 | V37 | V39 | V39IR | NG | V13 | V19 | V36 | V36IR | ||||
| mg kg−1 | mg kg−1 | ||||||||||||
| CuHCl | |||||||||||||
| 0.00–0.05 | 0.53 aA | 0.48 aA | 0.55 aA | 0.34 bB | 0.31 aB | 9.70 | 0.00–0.05 | 0.14 aB | 0.12 nsB | 0.14 aB | 0.13 nsB | 0.17 aA | 9.57 |
| 0.05–0.10 | 0.28 bA | 0.19 bB | 0.31 bA | 0.32 bA | 0.16 bB | 17.07 | 0.05–0.10 | 0.12 bA | 0.09 B | 0.09 bB | 0.09 B | 0.11 bA | 6.54 |
| 0.10–0.15 | 0.28 bB | 0.18 bCD | 0.20 bC | 0.43 aA | 0.16 bD | 6.40 | 0.10–0.20 | 0.12 nsb | 0.10 | 0.07 a | 0.12 | 0.10 b | 28.87 |
| 0.15–0.20 | 0.19 cB | 0.13 bCD | 0.18 bBC | 0.27 bA | 0.11 bD | 17.76 | 0.20–0.40 | 0.10 cAB | 0.11 AB | 0.08 aB | 0.10 AB | 0.11 bA | 16.95 |
| CV% | 9.19 | 10.40 | 18.42 | 7.94 | 13.57 | CV% | 3.77 | 18.61 | 14.13 | 29.29 | 7.70 | ||
| CuFA | |||||||||||||
| 0.00–0.05 | 16.5 abC | 54.4 aA | 47.3 aAB | 20.3 bBC | 53.9 aA | 41.91 | 0.00–0.05 | 1.8 nsE | 4.4 aD | 10.2 abB | 7.6 aC | 12.9 aA | 14.18 |
| 0.05–0.10 | 20.9 aC | 28.2 bB | 6.6 cD | 27.5 aB | 39.2 abA | 10.09 | 0.05–0.10 | 1.6 B | 4.2 aB | 12.1 aA | 5.3 bB | 4.9 bB | 41.47 |
| 0.10–0.15 | 10.2 bB | 27.9 bA | 26.7 bA | 10.1 cB | 11.6 abB | 14.47 | 0.10–0.20 | 1.4 B | 2.0 bB | 4.4 bcA | 4.7 bA | 4.8 bA | 12.63 |
| 0.15–0.20 | 11.2 bB | 17.4 cA | 23.5 bA | 10.5 cBC | 6.2 bC | 17.89 | 0.20–0.40 | 1.8 B | 1.8 aB | 1.5 cB | 2.8 cA | 3.5 bA | 9.89 |
| CV% | 17.87 | 10.31 | 9.18 | 14.28 | 64.37 | CV% | 20.07 | 13.98 | 38.29 | 12.81 | 10.67 | ||
| CuHA | |||||||||||||
| 0.00–0.05 | 6.2 cD | 53.6 aC | 122.9 aA | 17.0 cD | 71.4 aB | 13.63 | 0.00–0.05 | 0.9 nsC | 17.4 aB | 50.7 aA | 44.9 aA | 44.6 aA | 24.82 |
| 0.05–0.10 | 30.8 aB | 21.4 bBC | 25.0 bB | 15.2 cC | 45.0 bA | 18.99 | 0.05–0.10 | 0.8 D | 7.7 bC | 11.4 bBC | 13.3 bB | 19.5 bA | 21.56 |
| 0.10–0.15 | 14.1 bcC | 13.5 bC | 29.9 bAB | 31.8 aA | 22.2 cBC | 22.19 | 0.10–0.20 | 1.0 C | 4.0 cB | 5.1 bA | 5.5 bA | 6.0 cA | 12.11 |
| 0.15–0.20 | 18.9 bC | 11.3 bD | 27.3 bA | 24.8 bAB | 21.4 cBC | 10.15 | 0.20–0.40 | 0.7 D | 3.4 cBC | 2.4 bC | 4.3 bAB | 4.9 cA | 23.95 |
| CV% | 17.43 | 31.94 | 10.80 | 5.59 | 14.47 | CV% | 43.47 | 12.09 | 33.71 | 40.55 | 6.86 | ||
| CuHu | |||||||||||||
| 0.00–0.05 | 84.1 aE | 31.9 cD | 352.7 bC | 1127.1 aB | 1534.3 aA | 4.13 | 0.00–0.05 | 2.4 bB | 3.3 bB | 47.5 aA | 44.1 aA | 18.6 aB | 48.33 |
| 0.05–0.10 | 13.2 bE | 214.6 abD | 499.4 aC | 574.3 cB | 1110.6 bA | 5.40 | 0.05–0.10 | 2.1 bD | 1.0 bD | 20.6 abB | 37.3 bA | 6.2 bC | 15.26 |
| 0.10–0.15 | 70.1 aD | 239.2 aC | 454.5 aB | 939.7 bA | 438.0 cB | 4.21 | 0.10–0.20 | 12.4 aA | 1.3 bC | 8.2 bB | 9.4 cAB | 3.0 bC | 26.29 |
| 0.15–0.20 | 81.9 aE | 201.4 bD | 331.4 bB | 372.7 dA | 249.1 dC | 8.36 | 0.20–0.40 | 2.6 bC | 61.8 aA | 4.8 bB | 5.2 cB | 2.5 bC | 7.05 |
| CV% | 8.60 | 8.09 | 5.49 | 3.58 | 4.1 | CV% | 17.34 | 11.49 | 61.50 | 8.87 | 23.37 | ||
| CuTotal | |||||||||||||
| 0.00–0.05 | 107.3 abE | 140.4 cD | 523.4 abC | 1164.8 aB | 1660.0 aA | 1.95 | 0.00–0.05 | 5.2 bD | 25.3 bC | 108.5 aA | 96.9 aA | 76.3 aB | 14.5 |
| 0.05–0.10 | 62.0 cD | 264.4 aC | 561.5 aB | 617.4 bcA | 1195.0 bA | 4.49 | 0.05–0.10 | 4.7 bE | 13.0 bD | 36.7 bB | 55.9 bA | 30.7 bC | 7.56 |
| 0.10–0.15 | 94.5 bD | 280.7 aC | 421.6 abB | 864.0 bA | 472.1 cB | 20.77 | 0.10–0.20 | 14.9 aBC | 7.4 bD | 28.6 bAB | 19.7 cA | 13.9 cC | 11.95 |
| 0.15–0.20 | 112.2 aD | 230.2 bC | 330.0 bA | 408.3 cA | 276.9 dB | 7.16 | 0.20–0.40 | 5.3 bD | 67.1 aA | 13.9 bC | 12.3 cB | 11.0 cBC | 6.16 |
| CV% | 5.44 | 5.29 | 18.57 | 13.72 | 2.50 | CV% | 10.74 | 5.97 | 40.48 | 12.54 | 4.49 | ||
Appendix C
| Layer (m) | Serra Gaúcha | CV% | Layer (m) | Campanha Gaúcha | CV% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | V35 | V37 | V39 | V39IR | NG | V13 | V19 | V36 | V36IR | ||||
| mg kg−1 | mg kg−1 | ||||||||||||
| ZnHCl | |||||||||||||
| 0.00–0.05 | 15.16 bC | 40.3 aB | 42.5 bcB | 36.8 cB | 333.1 aA | 7.5 | 0.00–0.05 | 0.75 aB | 13.20 aA | 0.00 aB | 0.00 aB | 0.00 aB | 49.85 |
| 0.05–0.10 | 8.06 cC | 42.7 aBC | 19.5 cC | 76.6 bB | 239.5 bA | 33.46 | 0.05–0.10 | 0.27 aB | 1.41 bA | 0.00 aC | 0.00 aC | 0.00 aC | 19.82 |
| 0.10–0.15 | 5.04 cC | 3.18 bC | 123.3 aA | 106.5 aB | 127.9 cA | 9.52 | 0.10–0.20 | 0.19 aA | 0.22 bA | 0.00 aB | 0.00 aB | 0.00 aB | 64.89 |
| 0.15–0.20 | 44.6 aAB | 2.88 bB | 84.3 abA | 56.9 bcAB | 60.7 dAB | 7.58 | 0.20–0.40 | 0.19 aA | 0.16 bA | 0.00 aB | 0.00 aB | 0.00 aB | 79.36 |
| CV% | 22.07 | 12.88 | 30.93 | 11.84 | 3.99 | CV% | 65.96 | 41.12 | 0.00 | 0.00 | 0.00 | ||
| ZnHu | |||||||||||||
| 0.00–0.05 | 163.4 aBC | 174.4 nsB | 138.6 bC | 336.9 aA | 36.5 cD | 11.24 | 0.00–0.05 | 4.89 bD | 6.82 bCD | 13.08 abC | 50.70 aA | 26.18 aB | 19.93 |
| 0.05–0.10 | 102.1 bB | 227.9 A | 244.4 aA | 197.3 bA | 47.8 cC | 17.71 | 0.05–0.10 | 4.30 bC | 12.20 bB | 17.40 aA | 14.37 bAB | 16.75 bAB | 21.38 |
| 0.10–0.15 | 153.7 aB | 244.4 A | 139.2 bBC | 160.5 bB | 104.3 bC | 13.37 | 0.10–0.20 | 16.16 aA | 11.53 bB | 6.38 abC | 6.30 cC | 11.03 cC | 18.03 |
| 0.15–0.20 | 116.5 nsc | 204.9 | 144.1 ab | 184.7 b | 156.1 a | 31.52 | 0.20–0.40 | 5.76 bB | 35.68 aA | 5.63 bB | 8.70 bcB | 6.68 cB | 52.05 |
| CV% | 13.31 | 15.55 | 23.46 | 12.24 | 17.56 | CV% | 22.56 | 47.57 | 41.30 | 13.02 bc | 11.53 | ||
| ZnTotal | |||||||||||||
| 0.00–0.05 | 178.5 aB | 214.7 bB | 181.1 bB | 373.8 aA | 369.6 aA | 8.62 | 0.00–0.05 | 5.65 bD | 20.03 abB | 13.08 nsC | 50.70 aA | 26.18 aB | 16.10 |
| 0.05–0.10 | 110.2 bC | 270.7 aB | 263.9 aB | 273.9 bAB | 287.8 bA | 3.30 | 0.05–0.10 | 4.57 bB | 13.62 bA | 17.4 A | 14.37 bA | 16.75 bA | 20.82 |
| 0.10–0.15 | 158.7 aB | 247.6 aA | 243.7 aA | 266.9 bA | 232.2 cA | 9.24 | 0.10–0.20 | 16.35 aA | 11.75 bB | 7.90 C | 6.30 cC | 11.03 cC | 18.15 |
| 0.15–0.20 | 161.1 aB | 207.9 bA | 225.8 abA | 241.7 bA | 216.8 cA | 9.82 | 0.20–0.40 | 5.95 bB | 35.85 aA | 10.63 B | 8.70 bcB | 6.68 cB | 51.81 |
| CV% | 5.48 | 4.92 | 9.79 | 9.28 | 6.66 | CV% | 22.58 | 37.73 | 52.07 | 13.02 | 11.53 | ||
Appendix D
| Layer (m) | Serra Gaúcha | CV% | Layer (m) | Campanha Gaúcha | CV% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | V35 | V37 | V39 | V39IR | NG | V13 | V19 | V36 | V36IR | ||||
| mg kg−1 | mg kg−1 | ||||||||||||
| MnHCl | |||||||||||||
| 0.00–0.05 | 424.4 aA | 308.8 aC | 349.3 aB | 250.9 aD | 237.5 aD | 6.14 | 0.00–0.05 | 39.4 nsA | 60.2 aA | 41.7 aA | 0.00 aB | 0.00 aB | 63.52 |
| 0.05–0.10 | 280.7 bA | 79.8 bC | 234.6 bB | 208.6 bB | 99.4 bC | 9.26 | 0.05–0.10 | 32.7 A | 14.4 bB | 13.7 bB | 0.00 aC | 0.00 aC | 60.16 |
| 0.10–0.15 | 244.9 bA | 44.1 cD | 148.6 cC | 214.2 abB | 54.4 cD | 8.09 | 0.10–0.20 | 16.2 A | 10.4 bcB | 6.2 cC | 0.00 aD | 0.00 aD | 34.39 |
| 0.15–0.20 | 123.6 cA | 48.1 cB | 115.9 cA | 123.1 cA | 24.5 cC | 6.26 | 0.20–0.40 | 16.9 A | 4.5 cB | 2.3 cB | 0.00 aB | 0.00 aB | 80.79 |
| CV% | 7.10 | 7.74 | 6.19 | 8.01 | 11.35 | CV% | 46.83 | 13.17 | 11.45 | 0.00 | 0.00 | ||
| MnFA | |||||||||||||
| 0.00–0.05 | 122.0 aA | 37.6 aB | 29.5 aB | 6.5 bC | 45.6 aB | 24.94 | 0.00–0.05 | - | - | - | - | - | - |
| 0.05–0.10 | 80.6 bA | 8.3 bBC | 0.5 cC | 17.0 aB | 13.4 bB | 21.97 | 0.05–0.10 | - | - | - | - | - | - |
| 0.10–0.15 | 11.5 cA | 8.4 bB | 11.2 bA | 6.7 bC | 5.42 bD | 4.97 | 0.10–0.20 | - | - | - | - | - | - |
| 0.15–0.20 | 18.9 cA | 8.2 bBC | 9.27 bB | 5.9 bBC | 2.8 bC | 33.81 | 0.20–0.40 | - | - | - | - | - | - |
| CV% | 14.54 | 5.57 | 14.11 | 9.50 | 73.24 | CV% | - | - | - | - | - | ||
| MnHA | |||||||||||||
| 0.00–0.05 | 137.8 aA | 137.84 aA | 134.2 aA | 64.2 aB | 123.0 aA | 15.90 | 0.00–0.05 | 5.0 aC | 9.2 aBC | 13.5 aB | 11.6 aB | 21.4 aA | 24.00 |
| 0.05–0.10 | 83.8 bA | 41.1 bC | 55.7 bB | 57.3 bB | 17.8 bD | 14.65 | 0.05–0.10 | 3.8 aB | 5.3 bA | 4.7 bAB | 3.3 bC | 5.9 bA | 18.34 |
| 0.10–0.15 | 55.2 cA | 27.8 bB | 47.0 bA | 42.0 bA | 12.2 bC | 12.01 | 0.10–0.20 | 2.1 bB | 3.4 cA | 2.1 bB | 1.9 bA | 2.3 cB | 11.91 |
| 0.15–0.20 | 48.6 cA | 40.1 bB | 44.2 bB | 25.0 bC | 7.1 bD | 7.26 | 0.20–0.40 | 1.4 bC | 1.6 dAB | 0.8 bD | 1.6 bAB | 1.9 cA | 17.05 |
| CV% | 12.38 | 20.52 | 11.38 | 5.21 | 37.03 | CV% | 17.77 | 5.09 | 58.52 | 17.38 | 12.99 | ||
| MnHu | |||||||||||||
| 0.00–0.05 | 5157.7 aA | 1805.9 bC | 1080.2 bD | 3346.8 nsB | 3108.5 abB | 11.28 | 0.00–0.05 | 127.3 nsB | 215.4 aB | 288.6 aA | 263.7 aA | 215.0 aAB | 32.80 |
| 0.05–0.10 | 3237.7 bAB | 2426.2 abC | 2866.8 aBC | 3628.8 A | 35,394.0 aA | 7.78 | 0.05–0.10 | 113.2 C | 129.9 bB | 165.8 bA | 165.7 bA | 124.5 bC | 15.81 |
| 0.10–0.15 | 5820.3 aA | 2556.8 aD | 3326.2 aBC | 3447.8 B | 2867.2 bCD | 8.01 | 0.10–0.20 | 98.7 B | 123.8 bB | 162.0 bA | 126.5 cB | 59.6 cB | 11.85 |
| 0.15–0.20 | 5975.1 aA | 2581.0 aC | 3004.0 aC | 4100.3 B | 1909.3 cD | 9.15 | 0.20–0.40 | 93.6 B | 102.3 bA | 108.2 bB | 133.6 cB | 119.2 bB | 10.24 |
| CV% | 7.28 | 11.39 | 10.70 | 9.77 | 6.44 | CV% | 13.94 | 10.60 | 4.90 | 5.00 | 12.47 | ||
| MnTotal | |||||||||||||
| 0.00–0.05 | 5841.9 bA | 2290.1 nsC | 1593.2 bD | 3638.3 nsB | 3514.5 aB | 9.99 | 0.00–0.05 | 143.1 nsB | 193.3 aB | 343.7 aA | 275.3 aAB | 236.3 aAB | 33.26 |
| 0.05–0.10 | 3682.7 aA | 2555.5 C | 3174.5 aB | 3911.7 A | 3669.9 aA | 7.30 | 0.05–0.10 | 130.0 C | 149.5 bBC | 184.2 bA | 167.6 bAB | 65.5 cD | 13.10 |
| 0.10–0.15 | 6125.3 aA | 2637.1 C | 3094.9 aB | 3710.7 B | 2939.3 bC | 7.72 | 0.10–0.20 | 145.6 AB | 116.1 bC | 159.5 bA | 135.3 cBC | 126.8 bBC | 11.70 |
| 0.15–0.20 | 6172.7 aA | 2677.5 C | 3008.5 aC | 4254.3 B | 1943.7 cD | 8.70 | 0.20–0.40 | 131.5 B | 221.5 aA | 148.0 bB | 129.8 cB | 121.1 bB | 9.82 |
| CV% | 7.06 | 10.18 | 14.14 | 9.18 | 5.98 | CV% | 8.72 | 8.66 | 4.55 | 4.60 | 11.60 | ||
References
- Machado, P.L.O.d.A. Carbono Do Solo e a Mitigação Da Mudança Climática Global. Quím. Nova 2005, 28, 329–334. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; W. J. Riley and Sons: New York, NY, USA, 1994. [Google Scholar]
- Rossi, C.Q.; Pinto, L.A.d.S.R.; Moura, O.V.T.d.; Loss, A.; Pereira, M.G. Soil Organic Matter in Biogenic, Intermediate and Physicogenic Aggregates under Agroecological Management. Rev. Caatinga 2023, 36, 167–176. [Google Scholar] [CrossRef]
- Dick, D.P.; Novotny, E.H.; Dieckow, J.; Bayer, C. Química Da Matéria Orgânica Do Solo. In Química e Mineralogia do Solo; Melo, V.F., Alleoni, L.R.F., Eds.; SBCS: Viçosa, Brazil, 2009; pp. 1–67. [Google Scholar]
- Bahemmat, M.; Farahbakhsh, M.; Kianirad, M. Humic Substances-Enhanced Electroremediation of Heavy Metals Contaminated Soil. J. Hazard. Mater. 2016, 312, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Morton, D.W.; Johnson, B.B.; Angove, M.J. Adsorption of Humic and Fulvic Acids onto a Range of Adsorbents in Aqueous Systems, and Their Effect on the Adsorption of Other Species: A Review. Sep. Purif. Technol. 2020, 247, 116949. [Google Scholar] [CrossRef]
- Tiwari, J.; Ramanathan, A.L.; Bauddh, K.; Korstad, J. Humic Substances: Structure, Function and Benefits for Agroecosystems—A Review. Pedosphere 2023, 33, 237–249. [Google Scholar] [CrossRef]
- Brunetto, G.; Ferreira, P.A.A.; de Melo, G.W.B.; Ceretta, C.A.; Toselli, M. Heavy metals in vineyards and orchard soils. Rev. Bras. Frutic. 2017, 39, e-263. [Google Scholar] [CrossRef]
- Gattullo, C.E.; Mezzapesa, G.N.; Stellacci, A.M.; Ferrara, G.; Occhiogrosso, G.; Petrelli, G.; Castellini, M.; Spagnuolo, M. Cover Crop for a Sustainable Viticulture: Effects on Soil Properties and Table Grape Production. Agronomy 2020, 10, 1334. [Google Scholar] [CrossRef]
- Gómez-Armesto, A.; Carballeira-Díaz, J.; Pérez-Rodríguez, P.; Fernández-Calviño, D.; Arias-Estévez, M.; Novoa Muñoz, J.C.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A. Copper Content and Distribution in Vineyard Soils from Betanzos (A Coruña, Spain). Span. J. Soil. Sci. 2015, 5, 60–71. [Google Scholar] [CrossRef]
- Hummes, A.P.; Bortoluzzi, E.C.; Tonini, V.; da Silva, L.P.; Petry, C. Transfer of Copper and Zinc from Soil to Grapevine-Derived Products in Young and Centenarian Vineyards. Water Air Soil Pollut 2019, 230, 150. [Google Scholar] [CrossRef]
- Korchagin, J.; Moterle, D.F.; Escosteguy, P.A.V.; Bortoluzzi, E.C. Distribution of Copper and Zinc Fractions in a Regosol Profile under Centenary Vineyard. Environ. Earth Sci. 2020, 79, 439. [Google Scholar] [CrossRef]
- Marques, A.C.R.; Trapp, T.; de Almeida, H.S.; Morais, G.P.; Bueno, J.M.M.; Giumbelli, L.D.; Tiecher, T.L.; Silva, R.F.d.; Tiecher, T.; Dall’Orsoletta, D.J.; et al. Valores de Referência de Toxidez e Contaminação Ambiental de Cobre e Zinco Em Solos e Plantas. In Contaminação em Solos de Pomares e Vinhedos: Causas, Efeitos e Estratégias de Manejo; Brunetto, G., Trentin, E., de Melo, G.W.B., Girotto, E., Eds.; Sociedade Brasileira de Ciencia do Solo-Núcleo Regional Sul: Santa Maria, Brazil, 2022; pp. 104–129. [Google Scholar]
- Sonoda, K.; Hashimoto, Y.; Wang, S.-L.; Ban, T. Copper and Zinc in Vineyard and Orchard Soils at Millimeter Vertical Resolution. Sci. Total Environ. 2019, 689, 958–962. [Google Scholar] [CrossRef]
- Jackson, R. Viticulture. Ref. Module Food Sci. 2016, 1–14. [Google Scholar]
- OIV. State of the World Vitivinicultural Sector in 2020; International Organisation of Vine and Wine: Dijon, France, 2021. [Google Scholar]
- Monteiro, J.E.B.d.A.; Tonietto, J. Condições Meteorológicas e Sua Influência Na Vindima de 2013 Em Regiões Vitivinícolas Sul Brasileiras; Embrapa Uva e Vinho: Bento Goncalves, Brazil, 2013. [Google Scholar]
- ESALQ. Viticultura; Esalq: Piracicaba, Brazil, 2021. [Google Scholar]
- Prosdocimi, M.; Cerdà, A.; Tarolli, P. Soil Water Erosion on Mediterranean Vineyards: A Review. CATENA 2016, 141, 1–21. [Google Scholar] [CrossRef]
- Serpa, D.; Nunes, J.P.; Keizer, J.J.; Abrantes, N. Impacts of Climate and Land Use Changes on the Water Quality of a Small Mediterranean Catchment with Intensive Viticulture. Environ. Pollut. 2017, 224, 454–465. [Google Scholar] [CrossRef]
- Facco, D.B.; Trentin, E.; Drescher, G.L.; Hammerschmitt, R.K.; Ceretta, C.A.; da Silva, L.S.; Brunetto, G.; Ferreira, P.A.A. Chemical Speciation of Copper and Manganese in Solution of a Copper-Contaminated Soil and Young Grapevine Growth with Amendment Application. Pedosphere 2023, 33, 496–507. [Google Scholar] [CrossRef]
- Couto, R.d.R.; Benedet, L.; Comin, J.J.; Belli Filho, P.; Martins, S.R.; Gatiboni, L.C.; Radetski, M.; de Valois, C.M.; Ambrosini, V.G.; Brunetto, G. Accumulation of Copper and Zinc Fractions in Vineyard Soil in the Mid-Western Region of Santa Catarina, Brazil. Environ. Earth Sci. 2015, 73, 6379–6386. [Google Scholar] [CrossRef]
- Mackie, K.A.; Müller, T.; Kandeler, E. Remediation of Copper in Vineyards—A Mini Review. Environ. Pollut. 2012, 167, 16–26. [Google Scholar] [CrossRef]
- Tiecher, T.L.; Ceretta, C.A.; Tiecher, T.; Ferreira, P.A.A.; Nicoloso, F.T.; Soriani, H.H.; Rossato, L.V.; Mimmo, T.; Cesco, S.; Lourenzi, C.R.; et al. Effects of Zinc Addition to a Copper-Contaminated Vineyard Soil on Sorption of Zn by Soil and Plant Physiological Responses. Ecotoxicol. Environ. Saf. 2016, 129, 109–119. [Google Scholar] [CrossRef]
- Tiecher, T.L.; Soriani, H.H.; Tiecher, T.; Ceretta, C.A.; Nicoloso, F.T.; Tarouco, C.P.; Clasen, B.E.; De Conti, L.; Tassinari, A.; de Melo, G.W.B.; et al. The Interaction of High Copper and Zinc Doses in Acid Soil Changes the Physiological State and Development of the Root System in Young Grapevines (Vitis vinifera). Ecotoxicol. Environ. Saf. 2018, 148, 985–994. [Google Scholar] [CrossRef]
- De Conti, L.; Ceretta, C.A.; de Melo, G.W.B.; Tiecher, T.L.; Silva, L.O.S.; Garlet, L.P.; Mimmo, T.; Cesco, S.; Brunetto, G. Intercropping of Young Grapevines with Native Grasses for Phytoremediation of Cu-Contaminated Soils. Chemosphere 2019, 216, 147–156. [Google Scholar] [CrossRef]
- Andresen, E.; Peiter, E.; Küpper, H. Trace Metal Metabolism in Plants. J. Exp. Bot. 2018, 69, 909–954. [Google Scholar] [CrossRef]
- Trentin, E.; Facco, D.B.; Hammerschmitt, R.K.; Ferreira, P.A.A.; Morsch, L.; Belles, S.W.; Ricachenevsky, F.K.; Nicoloso, F.T.; Ceretta, C.A.; Tiecher, T.L.; et al. Potential of Vermicompost and Limestone in Reducing Copper Toxicity in Young Grapevines Grown in Cu-Contaminated Vineyard Soil. Chemosphere 2019, 226, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Hanke, D.; Dick, D.P. Aggregate Stability in Soil with Humic and Histic Horizons in a Toposequence under Araucaria Forest. Rev. Bras. Ciênc. Solo 2017, 41, e0160369. [Google Scholar] [CrossRef]
- Buurman, P.; Jongmans, A.G. Podzolisation and Soil Organic Matter Dynamics. Geoderma 2005, 125, 71–83. [Google Scholar] [CrossRef]
- Quéro, S.; Hatté, C.; Cornu, S.; Duvivier, A.; Cam, N.; Jamoteau, F.; Borschneck, D.; Basile-Doelsch, I. Dynamics of carbon loss from an Arenosol by a forest to vineyard land use change on a centennial scale. Soil 2022, 8, 517–539. [Google Scholar] [CrossRef]
- Ferreira, G.W.; Lourenzi, C.R.; Comin, J.J.; Loss, A.; Girotto, E.; Ludwig, M.P.; Freiberg, J.A.; Camera, D.d.O.; Marchezan, C.; Palermo, N.M.; et al. Effect of Organic and Mineral Fertilizers Applications in Pasture and No-Tillage System on Crop Yield, Fractions and Contaminant Potential of Cu and Zn. Soil. Tillage Res. 2023, 225, 105523. [Google Scholar] [CrossRef]
- Mikhailova, E.A.; Post, C.J.; Nelson, D.G. Integrating United Nations Sustainable Development Goals in Soil Science Education. Soil Syst. 2024, 8, 29. [Google Scholar] [CrossRef]
- Ferreira, G.W.; Bordallo, S.U.; Meyer, E.; Duarte, Z.V.S.; Schmitt, J.K.; Garlet, L.P.; Kokkonen Da Silva, A.A.; Moura-Bueno, J.M.; Bastos De Melo, G.W.; Brunetto, G.; et al. Heavy Metal-Based Fungicides Alter the Chemical Fractions of Cu, Zn, and Mn in Vineyards in Southern Brazil. Agronomy 2024, 14, 969. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy; Natural Resources Conservation Service, US Department of Agriculture: Washington, DC, USA, 2022.
- Brunetto, G.; Benedet, L.; Ambrosini, V.G.; Comin, J.J.; de Melo, G.W.B.; dos Santos, M.A.; Lourenzi, C.R.; Loss, A.; Belli Filho, P.; Schmitt, D.E.; et al. Copper and Zinc Fractions in the Profile of an Inceptisol Cultivated with Apple in Southern Brazil. Bragantia 2018, 77, 333–347. [Google Scholar] [CrossRef]
- EMBRAPA. Manual de Métodos de Análise de Solo; Embrapa Solos: Rio de Janeiro, Brazil, 2011. [Google Scholar]
- Tedesco, M.J.; Gianello, C.; Bissani, C.A.; Bohnen, H.; Volkweiss, S.J. Análises de Solo, Plantas e Outros Materiais; Departamento de Solos, Universidade Federal do Rio Grande do Sul: Porto Alegrem, Brazil, 1995. [Google Scholar]
- CQFS-RS/SC. Manual de Calagem e Adubação Para Os Estados Do Rio Grande Do Sul e Santa Catarina; Sociedade Brasileira de Ciência do Solo-Núcleo Regional Sul, Ed.; Comissão de Química e Fertilidade do Solo-RS/SC: Passo Fundo, Brazil, 2016. [Google Scholar]
- Almeida, H.C.; Dick, D.P.; Bertotto, F.L.; Chitarra, G.S. Distribution of Chemical Compartments of Soil Organic Matter and c Stocks of a Cambisol from South Brazil as Affected by Pinus Afforestation. Quím. Nova 2012, 35, 1329–1335. [Google Scholar] [CrossRef]
- Dick, D.P.; Gonçalves, C.N.; Dalmolin, R.S.D.; Knicker, H.; Klamt, E.; Kögel-Knabner, I.; Simões, M.L.; Martin-Neto, L. Characteristics of Soil Organic Matter of Different Brazilian Ferralsols under Native Vegetation as a Function of Soil Depth. Geoderma 2005, 124, 319–333. [Google Scholar] [CrossRef]
- Farmer, V.C. (Ed.) The Infrared Spectra of Minerals; Mineralogical Society of Great Britain and Ireland: Twickenham, UK, 1974. [Google Scholar]
- Tan, K. (Ed.) Infrared Spectroscopy. In Soil Sampling, Preparation and Analysis; Marcel Dekker: New York, NY, USA, 1996; pp. 278–298. [Google Scholar]
- Chefetz, B.; Hatcher, P.G.; Hadar, Y.; Chen, Y. Chemical and Biological Characterization of Organic Matter during Composting of Municipal Solid Waste. J. Environ. Qual. 1996, 25, 776–785. [Google Scholar] [CrossRef]
- Gerzabek, M.H.; Antil, R.S.; Koügel-Knabner, I.; Knicker, H.; Kirchmann, H.; Haberhauer, G. Howare soil use and management reflected by soil organic matter characteristics: A spectroscopic approach. Europ. J. Soil Sci. 2006, 57, 485–494. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Tinsley, J. Determination of Organic Carbon in Soils by Dichromate Mixtures. Trans. 4th Int. Congr. Soil Sci. 1950, 1, 161–169. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2024. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses; CRAN: Boston, MA, USA, 2020. [Google Scholar]
- Benedet, L. Efeitos de Aplicações de Dejetos Suínos Por 10 Anos Sobre a Matéria Orgânica Do Solo e a Biodisponibilidade de Cu e Zn; Universidade Federal de Santa Catarina: Florianópolis, Brazil, 2018. [Google Scholar]
- Seddaiu, G.; Porcu, G.; Ledda, L.; Roggero, P.P.; Agnelli, A.; Corti, G. Soil Organic Matter Content and Composition as Influenced by Soil Management in a Semi-Arid Mediterranean Agro-Silvo-Pastoral System. Agric. Ecosyst. Environ. 2013, 167, 1–11. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Beare, M.H.; McKim, U.F.; Skjemstad, J.O. Chemical and Biological Characteristics of Physically Uncomplexed Organic Matter. Soil Sci. Soc. Am. J. 2006, 70, 975–985. [Google Scholar] [CrossRef]
- Hoffland, E.; Kuyper, T.W.; Comans, R.N.J.; Creamer, R.E. Eco-Functionality of Organic Matter in Soils. Plant Soil 2020, 455, 1–22. [Google Scholar] [CrossRef]
- Bayer, C.; Mielniczuk, J.; Martin-Neto, L.; Ernani, P.R. Stocks and Humification Degree of Organic Matter Fractions as Affected by No-Tillage on a Subtropical Soil. Plant Soil 2002, 238, 133–140. [Google Scholar] [CrossRef]
- Midwood, A.J.; Hannam, K.D.; Forge, T.A.; Neilsen, D.; Emde, D.; Jones, M.D. Importance of Drive-Row Vegetation for Soil Carbon Storage in Woody Perennial Crops: A Regional Study. Geoderma 2020, 377, 114591. [Google Scholar] [CrossRef]
- Scandellari, F.; Caruso, G.; Liguori, G.; Meggio, F.; Palese Assunta, M.; Zanotelli, D.; Celano, G.; Gucci, R.; Inglese, P.; Pitacco, A.; et al. A Survey of Carbon Sequestration Potential of Orchards and Vineyards in Italy. Eur. J. Hortic. Sci. 2016, 81, 106–114. [Google Scholar] [CrossRef]
- Tezza, L.; Vendrame, N.; Pitacco, A. Disentangling the Carbon Budget of a Vineyard: The Role of Soil Management. Agric. Ecosyst. Environ. 2019, 272, 52–62. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of Soil Organic Matter as an Ecosystem Property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Ros, M.; García, C.; Hernández, T. A Full-Scale Study of Treatment of Pig Slurry by Composting: Kinetic Changes in Chemical and Microbial Properties. Waste Manag. 2006, 26, 1108–1118. [Google Scholar] [CrossRef]
- Rosa, D.M.; Nóbrega, L.H.P.; Mauli, M.M.; de Lima, G.P.; Pacheco, F.P. Substâncias Húmicas Do Solo Cultivado Com Plantas de Cobertura Em Rotação Com Milho e Soja. Rev. Cienc. Agron. 2017, 48, 221–230. [Google Scholar]
- Silva, I.R.; Mendonca, E.S. Matéria Organica Do Solo. In Fertilidade do Solo; Novais, R.F., Alvarez, V.H., Barros, N.F., Fontes, R.L.F., Cantarutti, R.B., Neves, J.C.L., Eds.; SBCS: Viçosa, Brazil, 2007; pp. 275–374. [Google Scholar]
- Olego, M.Á.; Cuesta Lasso, M.; Quiroga, M.J.; Visconti, F.; López, R.; Garzón-Jimeno, E. Effects of Leonardite Amendments on Vineyard Calcareous Soil Fertility, Vine Nutrition and Grape Quality. Plants 2022, 11, 356. [Google Scholar] [CrossRef]
- Sootahar, M.K.; Zeng, X.; Wang, Y.; Su, S.; Soothar, P.; Bai, L.; Kumar, M.; Zhang, Y.; Mustafa, A.; Ye, N. The Short-Term Effects of Mineral- and Plant-Derived Fulvic Acids on Some Selected Soil Properties: Improvement in the Growth, Yield, and Mineral Nutritional Status of Wheat (Triticum aestivum L.) under Soils of Contrasting Textures. Plants 2020, 9, 205. [Google Scholar] [CrossRef]
- da Silva, A.L.; Mafra, Á.L.; Klauberg Filho, O.; Kurtz, C.; Fayad, J.A. Carbono e Nitrogênio Microbiano Em Sistemas de Cultivo de Cebola Em Cambissolo Húmico. Rev. Ciênc. Agroveterinárias 2014, 13, 142–150. [Google Scholar]
- Perdue, E.M. Acidic Functional Groups of Humic Substances. In Humic Substances in Soil, Sediment, and Water: Geochemistry, Isolation, and Characterization; Aiken, G.R., McKnight, M.D., Wershaw, R.L., MacCarthy, P., Eds.; Wiley: Hoboken, NJ, USA, 1985; pp. 494–526. [Google Scholar]
- Yates, L.M.; von Wandruszka, R. Decontamination of Polluted Water by Treatment with a Crude Humic Acid Blend. Environ. Sci. Technol. 1999, 33, 2076–2080. [Google Scholar] [CrossRef]
- Kulikova, N.A.; Perminova, I.V. Interactions between Humic Substances and Microorganisms and Their Implications for Nature-like Bioremediation Technologies. Molecules 2021, 26, 2706. [Google Scholar] [CrossRef]
- Dortzbach, D.; Pereira, M.G.; Loss, A.; Santos, O.A.Q. Compartimentos Da Matéria Orgânica Do Solo Em Vinhedos Altomontanos de Santa Catarina. Braz. J. Dev. 2020, 6, 10677–10691. [Google Scholar] [CrossRef]
- Dortzbach, D.; Assunção, S.A.; Pereira, M.G.; Silva Neto, E.C. da Fractions of Soil Organic Matter in the Vineyards of Altitude Regions in Santa Catarina. Semin. Ciênc. Agrár. 2017, 38, 1799. [Google Scholar] [CrossRef]
- Agnelli, A.; Bol, R.; Trumbore, S.E.; Dixon, L.; Cocco, S.; Corti, G. Carbon and Nitrogen in Soil and Vine Roots in Harrowed and Grass-Covered Vineyards. Agric. Ecosyst. Environ. 2014, 193, 70–82. [Google Scholar] [CrossRef]
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; de Courcelles, V.d.R.; Singh, K.; et al. The Knowns, Known Unknowns and Unknowns of Sequestration of Soil Organic Carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, E.; Sun, O.J. Can No-Tillage Stimulate Carbon Sequestration in Agricultural Soils? A Meta-Analysis of Paired Experiments. Agric. Ecosyst. Environ. 2010, 139, 224–231. [Google Scholar] [CrossRef]
- Eldon, J.; Gershenson, A. Effects of Cultivation and Alternative Vineyard Management Practices on Soil Carbon Storage in Diverse Mediterranean Landscapes: A Review of the Literature. Agroecol. Sustain. Food Syst. 2015, 39, 516–550. [Google Scholar] [CrossRef]
- Abad, J.; Hermoso de Mendoza, I.; Marín, D.; Orcaray, L.; Santesteban, L.G. Cover Crops in Viticulture. A Systematic Review (1): Implications on Soil Characteristics and Biodiversity in Vineyard. OENO One 2021, 55, 295–312. [Google Scholar] [CrossRef]
- López-Vicente, M.; Calvo-Seas, E.; Álvarez, S.; Cerdà, A. Effectiveness of Cover Crops to Reduce Loss of Soil Organic Matter in a Rainfed Vineyard. Land 2020, 9, 230. [Google Scholar] [CrossRef]
- Ball, K.R.; Baldock, J.A.; Penfold, C.; Power, S.A.; Woodin, S.J.; Smith, P.; Pendall, E. Soil Organic Carbon and Nitrogen Pools Are Increased by Mixed Grass and Legume Cover Crops in Vineyard Agroecosystems: Detecting Short-Term Management Effects Using Infrared Spectroscopy. Geoderma 2020, 379, 114619. [Google Scholar] [CrossRef]
- Guimarães, D.V.; Gonzaga, M.I.S.; da Silva, T.O.; da Silva, T.L.; da Silva Dias, N.; Matias, M.I.S. Soil Organic Matter Pools and Carbon Fractions in Soil under Different Land Uses. Soil Tillage Res. 2013, 126, 177–182. [Google Scholar] [CrossRef]
- Canellas, L.P.; Espindola, J.A.A.; Rezende, C.E.; de Camargo, P.B.; Zandonadi, D.B.; Rumjanek, V.M.; Guerra, J.G.M.; Teixeira, M.G.; Braz-Filho, R. Organic Matter Quality in a Soil Cultivated with Perennial Herbaceous Legumes. Sci. Agric. 2004, 61, 53–61. [Google Scholar] [CrossRef]
- Canellas, L.P.; Teixeira Junior, L.R.L.; Dobbss, L.B.; Silva, C.A.; Medici, L.O.; Zandonadi, D.B.; Façanha, A.R. Humic Acids Crossinteractions with Root and Organic Acids. Ann. Appl. Biol. 2008, 153, 157–166. [Google Scholar] [CrossRef]
- Hanke, D. Matéria Orgânica de Solos Com Horizontes Húmicos e Hísticos Sob Floresta Ombrófila Mista: Mecanismos de Estabilização e Traçador de Paleo-Ambiente; Universidade Federal do: Rio Grande do Sul, Brazil, 2016. [Google Scholar]
- Kleber, M.; Sollins, P.; Sutton, R. A Conceptual Model of Organo-Mineral Interactions in Soils: Self-Assembly of Organic Molecular Fragments into Zonal Structures on Mineral Surfaces. Biogeochemistry 2007, 85, 9–24. [Google Scholar] [CrossRef]
- Kalbitz, K.; Solinger, S.; Park, J.-H.; Michalzik, B.; Matzner, E. Controls on the dynamics of dissolved organic matter in soils: A review. Soil Sci. 2000, 165, 277–304. [Google Scholar] [CrossRef]
- Viti, C.; Quaranta, D.; de Philippis, R.; Corti, G.; Agnelli, A.; Cuniglio, R.; Giovannetti, L. Characterizing Cultivable Soil Microbial Communities from Copper Fungicide-Amended Olive Orchard and Vineyard Soils. World J. Microbiol. Biotechnol. 2008, 24, 309–318. [Google Scholar] [CrossRef]
- Arias-Estévez, M.; Nóvoa-Muñoz, J.C.; Pateiro, M.; López-Periago, E. Influence of aging on copper fractionation in an acid soil. Soil Sci. 2007, 172, 225–232. [Google Scholar] [CrossRef]
- Croué, J.-P.; Benedetti, M.F.; Violleau, D.; Leenheer, J.A. Characterization and Copper Binding of Humic and Nonhumic Organic Matter Isolated from the South Platte River: Evidence for the Presence of Nitrogenous Binding Site. Environ. Sci. Technol. 2003, 37, 328–336. [Google Scholar] [CrossRef]
- Dresler, S.; Hanaka, A.; Bednarek, W.; Maksymiec, W. Accumulation of Low-Molecular-Weight Organic Acids in Roots and Leaf Segments of Zea Mays Plants Treated with Cadmium and Copper. Acta Physiol. Plant. 2014, 36, 1565–1575. [Google Scholar] [CrossRef]
- Masood, F.; Ahmad, S.; Malik, A. Role of Rhizobacterial Bacilli in Zinc Solubilization. In Microbial Biofertilizers and Micronutrient Availability; Springer International Publishing: Cham, Switzerland, 2022; pp. 361–377. [Google Scholar]
- Verma, D.; Meena, R.H.; Sukhwal, A.; Jat, G.; Meena, S.C.; Upadhyay, S.K.; Jain, D. Effect of ZSB with Graded Levels of Zinc Fertilizer on Yield and Zinc Uptake Under Maize Cultivation. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2023, 93, 379–385. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, B.; Ma, Y. Aging of Zinc Added to Soils with a Wide Range of Different Properties: Factors and Modeling. Environ. Toxicol. Chem. 2017, 36, 2925–2933. [Google Scholar] [CrossRef]
- Pérez-Novo, C.; Bermúdez-Couso, A.; López-Periago, E.; Fernández-Calviño, D.; Arias-Estévez, M. Zinc Adsorption in Acid Soils: Influence of Phosphate. Geoderma 2011, 162, 358–364. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry; Wiley: New York, NY, USA, 1981. [Google Scholar]
- Howe, P.D.; Malcolm, H.M.; Dobson, S. Manganese and Its Compounds: Environmental Aspects; World Health Organization: Geneva, Switzerland, 2004.
- Nádaská, G.; Lesný, J.; Michalík, I. Environmental Aspect of Manganese Chemistry. Hung. J. Sci. 2010, 1–16. [Google Scholar]
- Zahoransky, T.; Kaiser, K.; Mikutta, C. High Manganese Redox Variability and Manganate Predominance in Temperate Soil Profiles as Determined by X-Ray Absorption Spectroscopy. Geochim. Cosmochim. Acta 2022, 338, 229–249. [Google Scholar] [CrossRef]
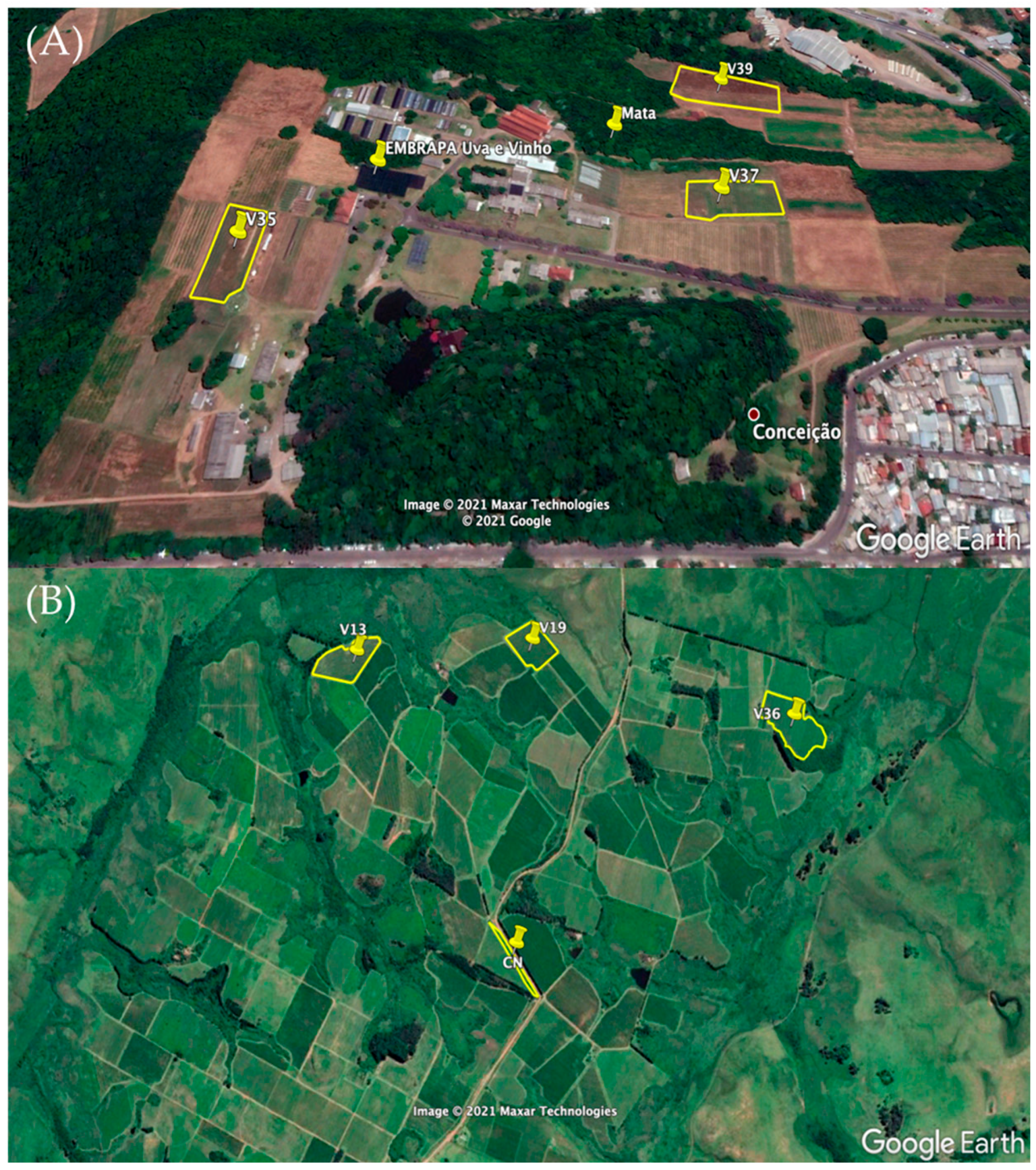

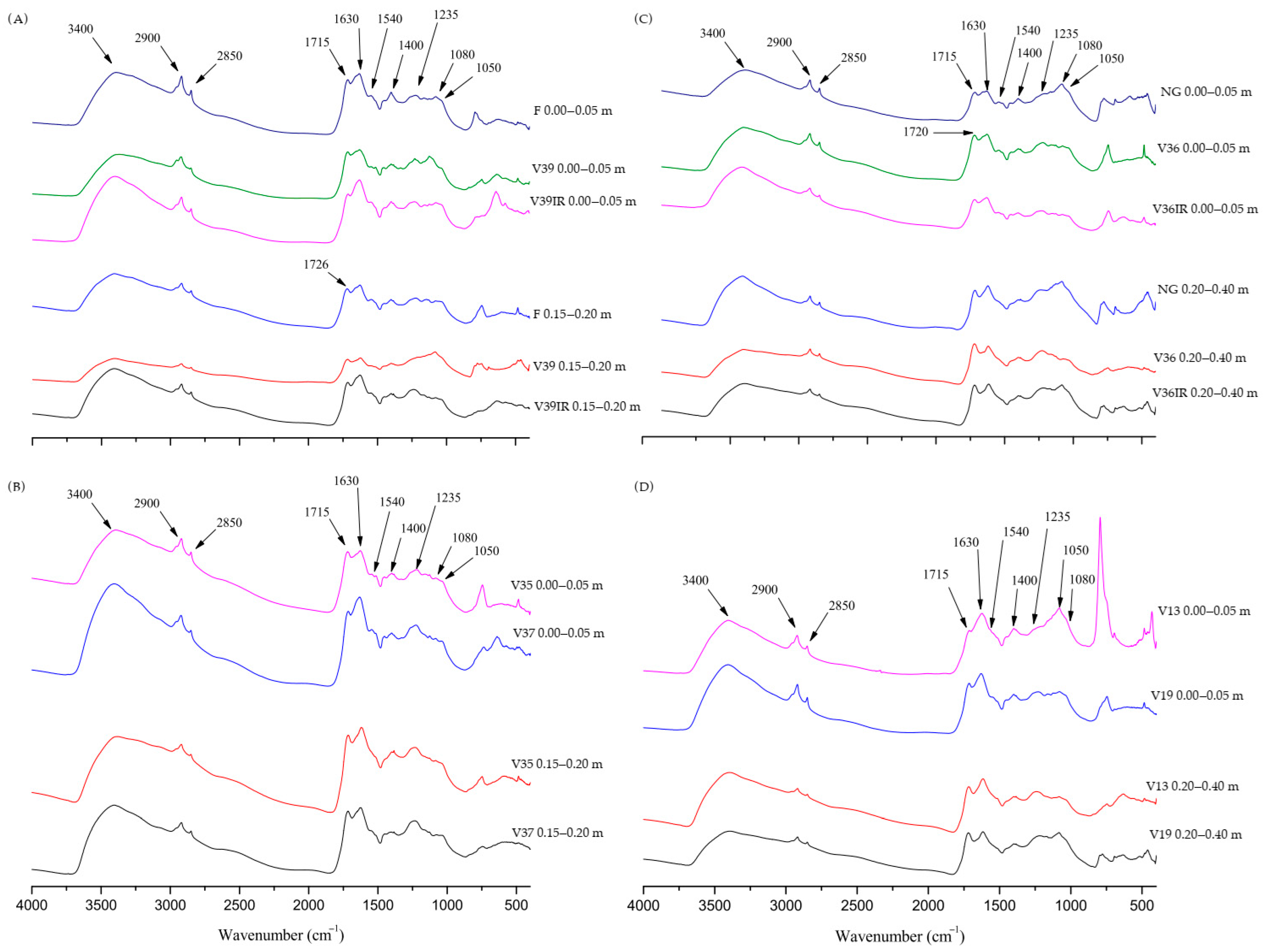
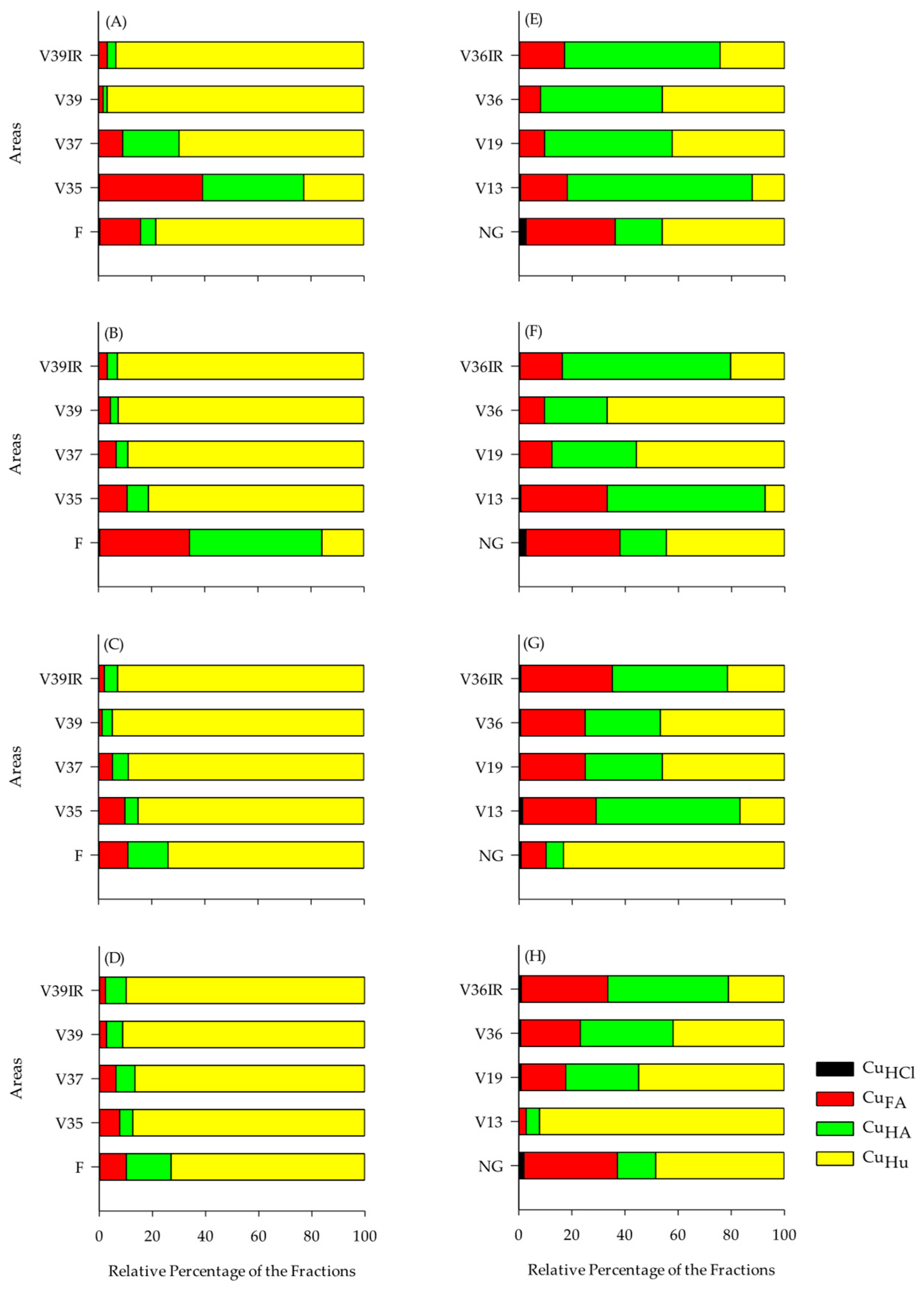

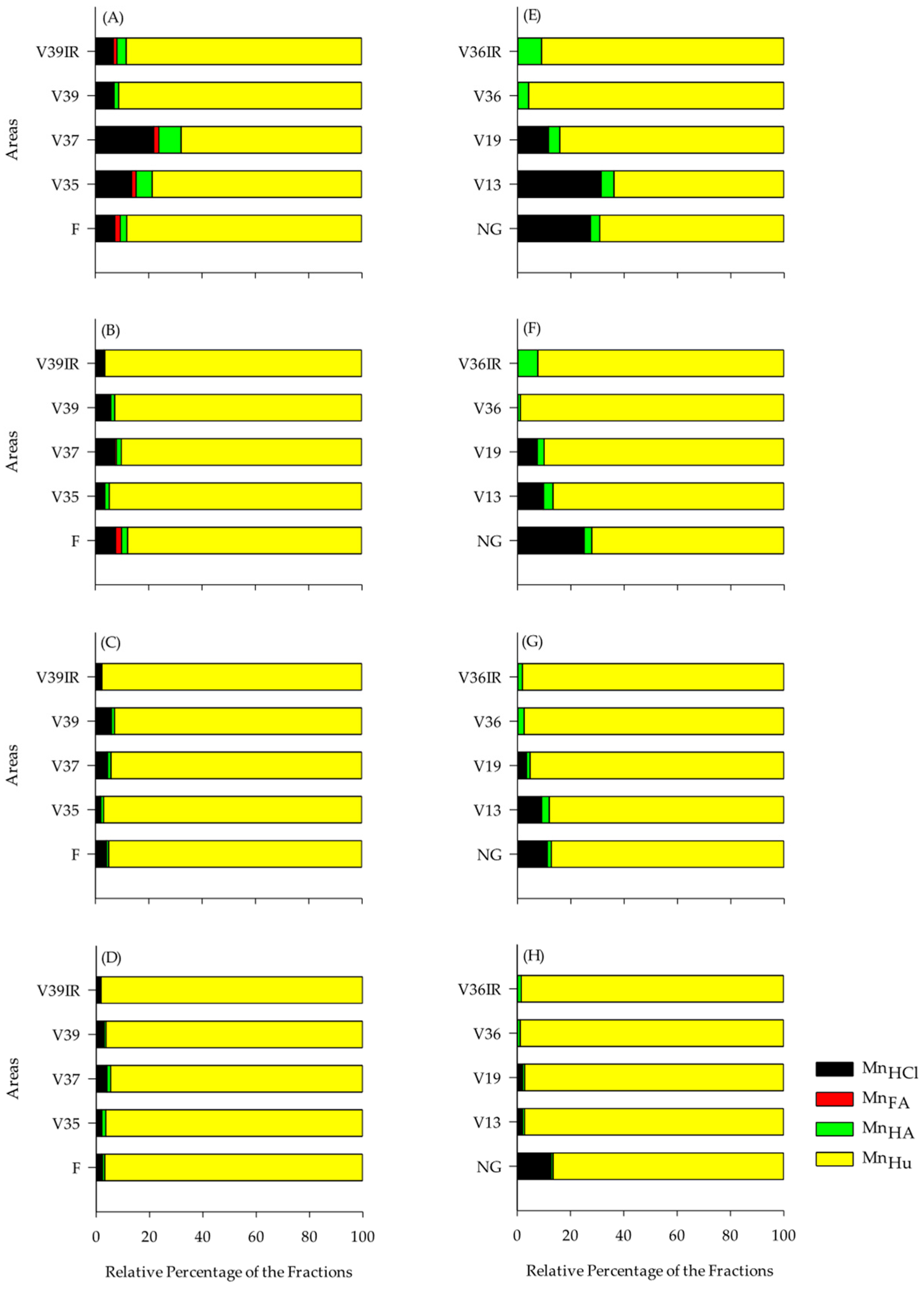
| Layer, m | Serra Gaúcha | Layer, m | Campanha Gaúcha | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | V35 | V37 | V39 | V39IR | CV% | NG | V13 | V19 | V36 | V36IR | CV% | ||
| mg kg−1 | mg kg−1 | ||||||||||||
| POM | |||||||||||||
| 0.00–0.05 | 7.93 nsC | 12.02 aBC | 17.81 aA | 13.01 aB | 15.55 aAB | 18.18 | 0.00–0.05 | 4.19 aC | 5.43 aBC | 9.01 aA | 4.48 aC | 6.35 aB | 11.63 |
| 0.05–0.10 | 7.40 C | 8.86 abB | 7.21 cC | 7.33 bC | 11.90 abA | 2.99 | 0.05–0.10 | 3.91 aA | 2.79 bC | 3.45 bAB | 3.31 bABC | 3.11 bBC | 10.32 |
| 0.10–0.15 | 7.90 B | 7.79 bB | 9.38 bA | 7.37 bB | 6.73 bB | 6.00 | 0.10–0.20 | 2.95 bB | 2.63 bB | 3.38 bA | 2.78 bB | 2.73 bB | 8.06 |
| 0.15–0.20 | 7.67 ABC | 8.53 abAB | 8.84 bA | 7.05 bC | 7.11 bBC | 10.32 | 0.20–0.40 | 2.96 bAB | 2.84 bAB | 3.17 bA | 2.84 bAB | 2.59 bB | 10.29 |
| CV% | 4.16 | 15.08 | 3.81 | 14.41 | 20.87 | 8.49 | 11.74 | 14.03 | 12.39 | 5.76 | |||
| CHCl | |||||||||||||
| 0.00–0.05 | 0.54 aA | 0.48 aA | 0.55 aA | 0.43 aB | 0.31 aB | 9.70 | 0.00–0.05 | 0.14 aB | 0.13 nsB | 0.14 aB | 0.13 nsB | 0.17 aA | 9.57 |
| 0.05–0.10 | 0.28 bA | 0.19 bB | 0.31 bA | 0.35 bA | 0.16 bB | 17.07 | 0.05–0.10 | 0.12 bA | 0.09 B | 0.09 bB | 0.09 B | 0.11 bA | 6.54 |
| 0.10–0.15 | 0.28 bB | 0.17 bC | 0.20 bE | 0.32 bA | 0.17 bCD | 6.40 | 0.10–0.20 | 0.12 nsB | 0.10 | 0.07 b | 0.12 | 0.10 b | 28.87 |
| 0.15–0.20 | 0.19 cB | 0.13 bCD | 0.18 bBC | 0.27 bA | 0.11 bD | 17.76 | 0.20–0.40 | 0.10 cAB | 0.11 AB | 0.08 bB | 0.10 AB | 0.11 bA | 16.95 |
| CV% | 9.19 | 10.40 | 18.42 | 7.94 | 13.57 | 3.77 | 18.61 | 14.13 | 29.29 | 7.70 | |||
| CFA | |||||||||||||
| 0.00–0.05 | 2.96 aA | 2.59 aA | 2.50 aA | 2.56 aA | 1.19 aB | 13.92 | 0.00–0.05 | 0.98 aB | 1.65 aA | 1.83 aA | 0.70 aB | 1.93 aA | 18.23 |
| 0.05–0.10 | 1.56 bB | 0.64 bC | 1.34 bB | 1.92 bA | 0.42 abC | 13.31 | 0.05–0.10 | 0.54 bA | 0.48 bA | 0.52 bA | 0.25 bB | 0.50 bA | 20.69 |
| 0.10–0.15 | 1.22 bA | 0.37 cC | 1.03 cB | 0.59 cCD | 0.18 bD | 12.97 | 0.10–0.20 | 0.51 bA | 0.45 bAB | 0.49 bAB | 0.26 bC | 0.36 bBC | 16.46 |
| 0.15–0.20 | 0.72 cB | 0.44 bcD | 0.84 cA | 0.26 cC | 0.08 bE | 3.80 | 0.20–0.40 | 0.26 bA | 0.28 cA | 0.20 bAB | 0.15 bB | 0.28 bA | 20.43 |
| CV% | 10.49 | 9.16 | 8.28 | 11.85 | 66.90 | 19.78 | 8.08 | 36.11 | 15.20 | 12.53 | |||
| CHA | |||||||||||||
| 0.00–0.05 | 2.57 aC | 6.08 aAB | 7.04 aA | 4.95 aB | 5.01 aB | 16.16 | 0.00–0.05 | 0.58 abC | 2.40 aB | 2.13 nsB | 3.15 aB | 5.07 aA | 27.74 |
| 0.05–0.10 | 3.00 aB | 0.72 bD | 2.65 bB | 3.65 abA | 1.24 bC | 12.58 | 0.05–0.10 | 0.89 aB | 1.08 bB | 1.06 B | 1.81 bA | 1.73 bA | 12.00 |
| 0.10–0.15 | 0.79 bC | 0.94 bC | 1.89 cB | 3.46 abA | 0.96 bC | 12.39 | 0.10–0.20 | 0.38 abC | 1.19 bAB | 1.02 B | 1.47 bA | 1.33 bA | 15.45 |
| 0.15–0.20 | 0.82 bB | 1.06 bB | 1.73 cA | 2.10 bA | 0.64 bB | 18.90 | 0.20–0.40 | 0.61 bB | 1.13 bA | 0.75 B | 1.41 bA | 1.31 bA | 17.96 |
| CV% | 18.04 | 11.53 | 8.14 | 21.89 | 24.87 | CV% | 22.93 | 7.26 | 62.86 | 15.36 | 11.40 | ||
| Layer, m | Serra Gaúcha | ||||
|---|---|---|---|---|---|
| F | V35 | V37 | V39 | V39IR | |
| mg kg−1 | |||||
| CHA/CFA | |||||
| 0.00–0.05 | 0.88 | 2.26 | 2.82 | 1.95 | 5.35 |
| 0.05–0.10 | 1.93 | 1.12 | 2.00 | 1.94 | 3.02 |
| 0.10–0.15 | 0.66 | 2.51 | 1.85 | 5.86 | 5.76 |
| 0.15–0.20 | 1.14 | 2.42 | 2.06 | 3.53 | 9.16 |
| (CFA + CHA)/CHu | |||||
| 0.00–0.05 | 0.08 | 0.21 | 0.12 | 0.74 | 0.23 |
| 0.05–0.10 | 0.15 | 0.04 | 0.16 | 2.56 | 0.27 |
| 0.10–0.15 | 0.11 | 0.06 | 0.25 | 0.59 | 0.21 |
| 0.15–0.20 | 0.13 | 0.09 | 0.24 | 0.55 | 0.32 |
| Layer, m | Campanha Gaúcha | ||||
| NG | V13 | V19 | V36 | V36IR | |
| mg kg−1 | |||||
| CHA/CFA | |||||
| 0.00–0.05 | 0.61 | 1.46 | 1.30 | 4.55 | 2.66 |
| 0.05–0.10 | 1.81 | 2.31 | 2.07 | 7.42 | 3.56 |
| 0.10–0.20 | 0.74 | 2.70 | 2.24 | 6.24 | 3.68 |
| 0.20–0.40 | 2.38 | 5.94 | 3.87 | 10.49 | 4.96 |
| (CFA + CHA)/CHu | |||||
| 0.00–0.05 | 0.07 | 0.15 | 0.84 | 0.27 | 0.45 |
| 0.05–0.10 | 0.09 | 0.09 | 0.11 | 0.18 | 0.14 |
| 0.10–0.20 | 0.06 | 0.13 | 0.14 | 0.19 | 0.19 |
| 0.20–0.40 | 0.08 | 0.17 | 0.11 | 0.18 | 0.19 |
| Area | Layer, m | Relative Intensity of the FTIR Bands | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RI2920 | RI1715 | RI1620 | RI1540 | RI1400 | RI1235 | RI1080 | AI | ||
| -------------------------%------------------------- | |||||||||
| Serra Gaúcha | |||||||||
| F | 0.00–0.05 | 11.4 | 22.9 | 22.6 | 8.8 | 9.8 | 11.4 | 13.1 | 1.9 |
| 0.15–0.20 | 7.7 | 24.2 | 23.7 | 10.1 | 8.7 | 13.0 | 12.6 | 3.1 | |
| V35 | 0.00–0.05 | 11.3 | 22.9 | 23.2 | 8.9 | 9.6 | 10.9 | 13.3 | 2.1 |
| 0.15–0.20 | 5.8 | 28.0 | 26.5 | 7.0 | 8.6 | 13.6 | 10.5 | 4.5 | |
| V37 | 0.00–0.05 | 7.7 | 25.2 | 26.9 | 10.1 | 7.3 | 12.9 | 9.8 | 3.5 |
| 0.15–0.20 | 6.7 | 26.8 | 25.5 | 9.8 | 6.7 | 15.2 | 9.4 | 3.8 | |
| V39 | 0.00–0.05 | 8.9 | 23.9 | 21.9 | 7.7 | 7.7 | 13.4 | 16.6 | 2.5 |
| 0.15–0.20 | 6.4 | 20.6 | 18.4 | 7.1 | 7.1 | 16.3 | 24.1 | 2.9 | |
| V39IR | 0.00–0.05 | 9.4 | 21.3 | 25.9 | 10.8 | 9.4 | 10.8 | 12.3 | 2.8 |
| 0.15–0.20 | 7.6 | 22.9 | 24.6 | 10.2 | 12.2 | 12.7 | 9.8 | 3.2 | |
| Campanha Gaúcha | |||||||||
| NG | 0.00–0.05 | 10.6 | 18.9 | 17.3 | 3.4 | 8.9 | 15.6 | 25.1 | 1.6 |
| 0.20–0.40 | 6.3 | 20.4 | 19.9 | 7.2 | 6.8 | 15.4 | 23.9 | 3.2 | |
| V13 | 0.00–0.05 | 9.0 | 18.9 | 23.8 | 9.0 | 9.0 | 9.8 | 20.5 | 2.6 |
| 0.20–0.40 | 5.4 | 27.0 | 27.0 | 7.6 | 7.6 | 13.5 | 11.9 | 5.0 | |
| V19 | 0.00–0.05 | 11.1 | 21.8 | 24.2 | 8.7 | 9.1 | 11.5 | 13.5 | 2.2 |
| 0.20–0.40 | 4.8 | 16.0 | 17.6 | 5.6 | 7.2 | 16.8 | 32.0 | 3.7 | |
| V36 | 0.00–0.05 | 9.7 | 24.5 | 21.8 | 8.8 | 7.4 | 13.9 | 13.9 | 2.2 |
| 0.20–0.40 | 7.6 | 28.4 | 22.5 | 7.1 | 8.3 | 15.4 | 10.7 | 2.9 | |
| V36IR | 0.00–0.05 | 9.1 | 25.2 | 25.2 | 7.7 | 7.7 | 13.3 | 11.9 | 2.8 |
| 0.20–0.40 | 5.5 | 24.1 | 20.1 | 7.0 | 7.0 | 16.1 | 20.1 | 3.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, G.W.; Bordallo, S.U.; Giumbelli, L.D.; Duarte, Z.V.S.; Brunetto, G.; Melo, G.W.B.d.; Dick, D.P.; Tiecher, T.L.; Tiecher, T.; Lourenzi, C.R. Chemical Fractions of Soil Organic Matter and Their Interactions with Cu, Zn, and Mn in Vineyards in Southern Brazil. Agronomy 2025, 15, 1937. https://doi.org/10.3390/agronomy15081937
Ferreira GW, Bordallo SU, Giumbelli LD, Duarte ZVS, Brunetto G, Melo GWBd, Dick DP, Tiecher TL, Tiecher T, Lourenzi CR. Chemical Fractions of Soil Organic Matter and Their Interactions with Cu, Zn, and Mn in Vineyards in Southern Brazil. Agronomy. 2025; 15(8):1937. https://doi.org/10.3390/agronomy15081937
Chicago/Turabian StyleFerreira, Guilherme Wilbert, Samya Uchoa Bordallo, Lucas Dupont Giumbelli, Zayne Valéria Santos Duarte, Gustavo Brunetto, George Wellington Bastos de Melo, Deborah Pinheiro Dick, Tadeu Luis Tiecher, Tales Tiecher, and Cledimar Rogério Lourenzi. 2025. "Chemical Fractions of Soil Organic Matter and Their Interactions with Cu, Zn, and Mn in Vineyards in Southern Brazil" Agronomy 15, no. 8: 1937. https://doi.org/10.3390/agronomy15081937
APA StyleFerreira, G. W., Bordallo, S. U., Giumbelli, L. D., Duarte, Z. V. S., Brunetto, G., Melo, G. W. B. d., Dick, D. P., Tiecher, T. L., Tiecher, T., & Lourenzi, C. R. (2025). Chemical Fractions of Soil Organic Matter and Their Interactions with Cu, Zn, and Mn in Vineyards in Southern Brazil. Agronomy, 15(8), 1937. https://doi.org/10.3390/agronomy15081937







