Abstract
In arid regions, yields from safflower plants are appreciably lower than normal. Foliar application of zinc or glycine betaine has been reported to increase yields in other grown crops. A field experiment was conducted to compare the specific effects and mechanisms of foliar-applied zinc or glycine betaine on safflower yield in this study. Seven foliar spraying treatments were implemented, including a control (spraying water), three concentrations of zinc sulfate (Zn1: 0.6 g L−1, Zn2: 0.8 g L−1, Zn3: 1.0 g L−1), and three concentrations of glycine betaine (GB1: 0.23 g L−1, GB2: 0.47 g L−1, GB3: 0.70 g L−1). Results showed that Zn1 treatment had the highest grain yield at 2197 kg ha−1, which was 45.4% higher than the control. GB3 treatment resulted in a grain yield at 2127 kg ha−1, which was 40.8% higher than the control. The yield increase mechanism for the zinc treatment was primarily due to optimized plant morphology and improved photosynthetic performance, while glycine betaine improved yield mainly through antioxidant regulation. This study has important implications for water-saving and sustainable agriculture development in arid regions.
1. Introduction
Environmental problems such as water shortages and soil salinization are seriously affecting the production of safflowers (Carthamus tinctorius L.), an economic crop in arid areas [1,2,3]. Foliar spraying of trace elements or exogenous substances is expected to enhance the stress resistance of safflowers, and therefore increase the yield [4,5].
Zinc is an essential micronutrient and contributes to plant tolerance to drought stress through improving various physiological and molecular processes, such as plant water relations, osmolyte accumulation, stomatal regulation, water-use efficiency and photosynthesis [6]. In recent years, researchers have made significant progress in foliar spraying zinc fertilizer to improve drought resistance and safflower yield. According to a two-year field experiment on five safflower varieties, foliar spraying zinc at 1.2 kg ha−1 effectively alleviated drought stress by leading to an enhancement in proline, carbohydrate accumulation, and chlorophyll content (SPAD), which resulted in improved grain yield of safflowers [7]. Another study found that when comparing with the control, spraying zinc at 1 kg ha−1 twice at the rosette stage and the full-bloom stage increased grain yield with 430 kg ha−1, or approximately by 16.8% [8]. Drought stress increased antioxidant enzyme activity, while the foliar application of zinc enhanced the drought-stress tolerance of safflowers by altering the physiological and biochemical traits, such as the SPAD index and catalase enzyme [8]. Recent research has demonstrated that spraying zinc fertilizer at the rosette stage when the safflower has five to seven leaves has a better yield-increasing impact than spraying at the full-bloom stage [4]. Foliar fertilization provides a more efficient solution to nutrient deficiencies, especially in suboptimal soil conditions [9]. However, recent study indicates that soil-applied mycorrhizae and zinc [10] is also an effective method for increasing safflower yield.
The foliar application of exogenous osmotic regulators, such as glycine betaine, has been shown to enhance drought resistance and the yield of crops [11]. Glycine betaine plays an adaptive role in mediating osmotic adjustment and protecting the sub-cellular structures in stressed plants, protection of the transcriptional and translational machineries, and intervention as a molecular chaperone in the refolding of enzymes [12]. Previous research has examined the impact of foliar spraying glycine betaine on enhancing plant biomass, chlorophyll contents, stomatal conductance, and sub-stomatal CO2 concentration of safflower under well-watered or water-deficit conditions [5]. Seed priming with glycine betaine increased catalase and superoxide dismutase enzyme activities, which facilitated germination and the primary growth of safflower seedling under salinity-stress conditions [13]. Results of a controlled pot experiment showed that foliar application of glycine betaine was more effective than zinc to enhance maize yield under well-watered or drought-stress conditions [11]. However, both the effect of spraying glycine betaine on safflower yield and the main mechanisms by which glycine betaine improves safflower plant physiology are still less known.
The purposes of this study are (1) to explore the potential yield-increasing effect of foliar spraying glycine betaine on safflowers, and (2) to compare the effects between foliar spraying of zinc or glycine betaine on increasing safflower yield. This study is expected to provide a technical reference for achieving high- and stable-state yields of safflowers in arid areas.
2. Materials and Methods
2.1. Study Area
The experiment was conducted on Sanping farmland, located at 87°22′48″ E, 43°56′24″ N near Urumqi city, Xinjiang Uygur Autonomous Region, China. The study area is situated at the northern base of the Tianshan Mountains, on the southern edge of the Junggar Basin, and on the alluvial fan of the Toutun River. This region has a typical arid, continental climate with ample sunlight and heat resources. The annual sunshine hours can reach up to 2800 h, and the potential evapotranspiration rate is high, ranging from 1780 to 2131.2 mm annually. Precipitation is unevenly distributed, with an average of 64.3 to 181.6 mm year−1. The average annual temperature is 6 °C, and the frost-free period lasts for approximately 163 days. The general groundwater depth is 2.0 m. The soil type is Calci-Orthic Aridosols, as classified by the Chinese Soil Taxonomy [14]. Soil organic matter is 13.7 g kg−1 in the top layer. Most soils in Xinjiang are zinc deficient, and soil-available zinc contents of about 84% farmlands are less than 1 μg g−1 [15].
2.2. Field Experiment
The safflower variety used in this experiment was Yumin, which is a thornless variety. The experiment was conducted on 30 April 2024, with a seeding rate at 30 kg ha−1. The rows of safflowers were spaced 30 cm apart, with 15 cm between every two plants in a row.
Seven treatments were set in this study, including a control (CK, spraying with water), ZnSO4·7H2O treatments (Zn1: 0.6 g L−1, Zn2: 0.8 g L−1, Zn3: 1.0 g L−1), and glycine betaine treatments (GB1: 0.23 g L−1, GB2: 0.47 g L−1, GB3: 0.70 g L−1). Each treatment was repeated in three plots, with each plot covering an area of 4 m × 3 m, with a 1.2 m protection row. During the rosette stage (23 May), elongation stage (10 June), branching stage (19 June), and bud stage (24 June) of safflowers, each treatment was sprayed once for a total of four times. Sunny afternoons were chosen for foliar spraying, using a fine and uniform spray to ensure complete coverage of safflower leaves. The same volume of water/solution was sprayed for all the seven treatments. The spraying amounts for the entire growth period were Zn1: 13.33 kg ha−1, Zn2: 17.78 kg ha−1, Zn3: 22.22 kg ha−1, GB1: 5.11 kg ha−1, GB2: 10.44 kg ha−1, and GB3: 15.56 kg ha−1. Drip irrigation was used under normal irrigation conditions, with a total irrigation quota at 2850 m3 ha−1 for the entire growth period. Safflower filaments and seeds were harvested in early July 2024.
2.3. Determination of Monitoring Indicators
2.3.1. Agronomic Characteristics
Three representative safflower plants were selected in each plot. The height (cm) from the ground to the top of the plant was measured by tape during the flowering period. The stem diameter (mm) was measured by a vernier caliper at 10 cm above the base. The number of primary branches were counted as the number of direct branches on the main stem, while the number of secondary branches were counted as the number of smaller lateral branches from the leaf axils of the primary branches. The diameter of the fruit ball was measured using the vernier caliper to measure the maximum transverse diameter (mm); the number of fruit balls per plant were recorded at full flowering stage.
2.3.2. Leaf Photosynthetic Indices and SPAD Value
The measurement time was from 9:00 to 11:30 in the morning on a sunny day. The air temperature was between 23 °C and 30 °C during measurement. Photosynthetic parameters of safflower leaves were measured using a portable photosynthesis system (Li-6400, LI-COR Biosciences, Lincoln, NE, USA) at the full-bloom stage. Before the measurements, light intensity, CO2 concentration, and gas flow rate was set to 1600 μmol m−2 s−1, 400 μmol mol−1, and 500 μmol s−1 in the sample chamber, respectively. A CO2 buffer bottle was used to stabilize the gas environment. Before each measurement, the instrument was zero-calibrated to eliminate any baseline errors. The leaf was clamped in the chamber and allowed to stabilize for 1 min. Once the parameters, such as net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr), and intercellular CO2 concentration (Ci) reached a stable state (with fluctuations less than 5%), the data were recorded. The fifth fully expanded functional leaf was selected. Each leaf was measured five times continuously, and the mean value was recorded. Five representative leaves were selected in each of the three selected safflower plants in one plot. Therefore, a total of fifteen representative leaves were measured in each plot.
The chlorophyll relative content (SPAD value) of safflower leaves was measured using a portable SPAD-502 chlorophyll meter (KonicaMinolta, Tokyo, Japan) at the full-bloom stage. For the measurement, fully expanded functional leaves were selected, and the instrument’s sensing window was positioned perpendicular to the direction of the main vein of leaves. This was done to avoid the obvious vein area and to make sure the measurement was taken in the middle of leaves. Each leaf was measured five times continuously, and the mean value was recorded.
2.3.3. Antioxidant Enzymes and MDA
At the full-bloom stage, fresh leaf samples were sampled. Catalase (CAT) activity (U g−1) and peroxidase (POD) activity (U g−1) were determined using the guaiacol coloration method and ultraviolet absorption method, respectively [16]. The activity was measured to reflect the efficiency of hydrogen peroxide decomposition. Superoxide dismutase (SOD) activity (U g−1) was assessed using the nitrogen blue tetrazolium photoreduction inhibition method [13], and was used to indicate the plant’s ability to scavenge superoxide radicals. The level of malondialdehyde (MDA) (nmol g−1) was measured using the thiobarbituric acid colorimetric method [13], and was used to assess the degree of lipid peroxidation in the cell membrane.
2.3.4. Filament and Grain Yields of Safflowers
During the full-bloom stage, the filaments within a 12 m2 area of each plot were manually harvested three times. After drying in a cool and ventilated manner until passing a constant weight, the filament yield of each treatment was weighed. The yield per hectare was then calculated based on the unit area.
In addition, a representative sample plot of 1 m2 was chosen from each plot. The entire plant was harvested and threshed. The grains were then dried at 60 °C until passing a constant weight and converted into grain yield per unit area (kg ha−1) by multiplying 10,000.
2.4. Water-Use Efficiency
The water-use efficiency (WUE, μmol mmol−1) of safflower leaves was calculated using the ratio of net photosynthetic rate to transpiration rate [17] as the following:
where Pn represents leaf net photosynthetic rate (μmol m−2 s−1), and Tr indicates transpiration rate (mmol m−2 s−1).
2.5. Data Analysis
The experimental data was calculated and organized using Excel 2016. The data was analyzed using SPSS 26.0 through one-way ANOVA for test of mean comparison. Pearson correlation analysis was used to find out whether significant relationships exist among all the measured parameters. Additionally, Origin 2022 was utilized for data visualization.
3. Results
3.1. Effects of Zinc and Glycine Betaine on Agronomic Traits
Spraying high concentration of zinc fertilizer significantly (p < 0.05) improved plant height, stem diameter, and the number of secondary branches. However, glycine betaine treatment had an insignificant effect on the plant height of safflowers (Figure 1A). Among all these treatments, the tallest average safflower plant was measured at 74.0 cm for the Zn3 treatment, which was 14.3% higher than the control. Additionally, the average stem diameter of Zn3 was 10.88 mm, which was 49.5% higher than the control. The Zn3 treatment also had the largest average number of secondary branches at 9.0, which was 58.7% higher than the control (Figure 1D).
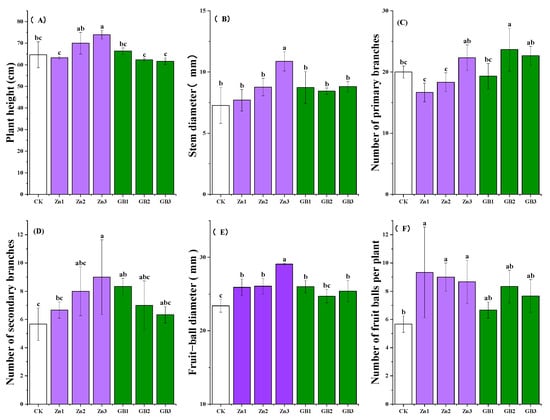
Figure 1.
(A) plant height, (B) stem diameter, (C) number of primary branches, (D) number of secondary branches, (E) fruit-ball diameter, and (F) number of fruit balls per plant of safflower at full flowering stage under application of Zn or glycine betaine. Different lowercase letters indicate significant differences according to the Tukey test at the 0.05 level for each treatment; vertical bars in each mean indicate standard errors.
No significant difference was observed in the number of primary branches of safflowers at the full flowering stage among the zinc fertilizer and glycine betaine treatments. However, the average number of primary branches in the GB2 treatment was the highest at 23.7, which was 18.4% higher than the control. The Zn3 treatment had a relatively higher number of primary branches at 22.3, which was 11.7% higher than the control (Figure 1C).
Overall, the application of zinc fertilizer or glycine betaine had significant impacts on increasing the fruit-ball diameter at the full-bloom stage. Specifically, fruit-ball diameters for all treatments were higher than that of the control. The largest average fruit-ball diameter was observed for the Zn3 treatment, with was 29.07 mm (Figure 1E). A low level of zinc fertilizer greatly increased the number of fruit balls per plant. However, spraying glycine betaine had no significant impact on the number of fruit balls per plant (Figure 1F). At the full flowering stage, plants treated with Zn1 had the highest number of fruit balls at 9.3, which was 63.2% higher than the control (Figure 1F).
3.2. Effects of Zinc or Glycine Betaine on Photosynthetic Parameters
Compared to the control, foliar spraying of zinc or glycine betaine significantly increased the net photosynthetic rate (Pn) and transpiration rate (Tr) of safflowers at the full-bloom stage (Figure 2). The average Pn value of the Zn3 treatment was the highest at 57.8 μmol m−2 s−1, which was 5.45 times higher than the control (Figure 2A). A higher Pn value indicates the greater accumulation of organic matter, which is beneficial for crop growth and yield formation [18]. Spraying a high concentration of zinc fertilizer or a low concentration of glycine betaine significantly increased the Tr value of safflowers at the full-bloom stage. The average Tr value of the Zn3 treatment was 9.20 μmol m−2 s−1, which was 2.65 times higher than the control (Figure 2B). A high Tr value promotes nutrient transport when water is sufficient, suggesting that a high Tr value is beneficial for crop-yield improvement. The average leaf water-use efficiency values were relatively high for the Zn1 and GB2 treatments, which were 3.08 and 2.28 times higher than the control, respectively (Figure 2C).
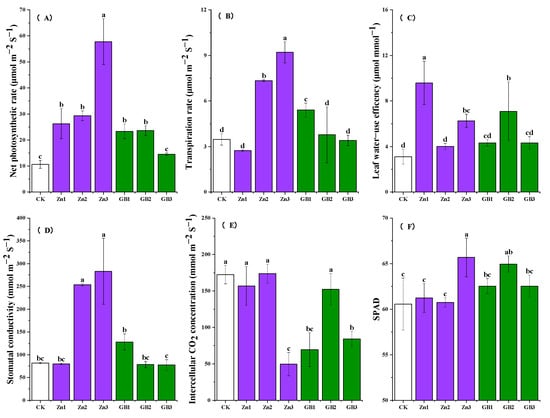
Figure 2.
(A) net photosynthetic rate, (B) transpiration rate, (C) leaf water-use efficency, (D) stomatal conductivity, (E) intercellular CO2 concentration, and (F) chlorophyll relative content (SPAD) of safflower at full-bloom stage under application of Zn or glycine betaine. Different lowercase letters indicate significant differences according to the Tukey test at the 0.05 level for each treatment; vertical bars in each mean indicate standard errors.
Stomatal conductance (Gs) is a measure of the stomatal opening degree, and a high Gs value can enhance both CO2 absorption and transpiration [5]. For the Zn3 treatment, the average stomatal conductance was 283 mmol m−2 s−1, which was 3.46 times higher than that of the control treatment. Similarly, the average stomatal conductance of the GB1 treatment was 128 mmol m−2 s−1, which was 1.57 times higher than that of the control (Figure 2D). A high intercellular CO2 concentration (Ci) indicates a limitation in leaf metabolism [19]. In this study, the Ci values of both the Zn3 and GB1 treatments were significantly lower than that of the control (Figure 2E), which may be due to an increase in photorespiration. A high SPAD value typically indicates better photosynthetic performance and growth potential, which are beneficial for yield and stress resistance [20]. In general, foliar spraying of zinc or glycine betaine increased the SPAD value of safflowers, with the effect of Zn3 treatment being more pronounced (Figure 2F).
3.3. Effects of Zinc or Glycine Betaine on Enzyme Activity
Overall, the foliar application of zinc (Zn) or glycine betaine (GB) significantly increased the activity of superoxide dismutase (SOD). Catalase (CAT) is a key enzyme responsible for scavenging hydrogen peroxide, and its activity reflects the plant’s resistance to oxidative stress. In safflower leaves, the activity of CAT was significantly increased by Zn2 and GB1 treatments in the field at the full flowering stage. The CAT activity of GB1 treatment was the highest (1562.89 U g−1), which was 130.7% higher than the control (Figure 3A). However, the decrease in CAT activity at high glycine betaine concentrations suggests that excessive activation of CAT may inhibit other antioxidant pathways. As the first line of defense in the antioxidant system, SOD is responsible for converting superoxide radicals into H2O2. Overall, spraying glycine betaine or zinc fertilizer significantly increased SOD activity at the full-bloom stage. The GB3 treatment (521.25 U g−1) and Zn3 treatment (474.80 U g−1) had higher SOD activity, which was 49.3% and 36.0% higher than the control, respectively (Figure 3B).
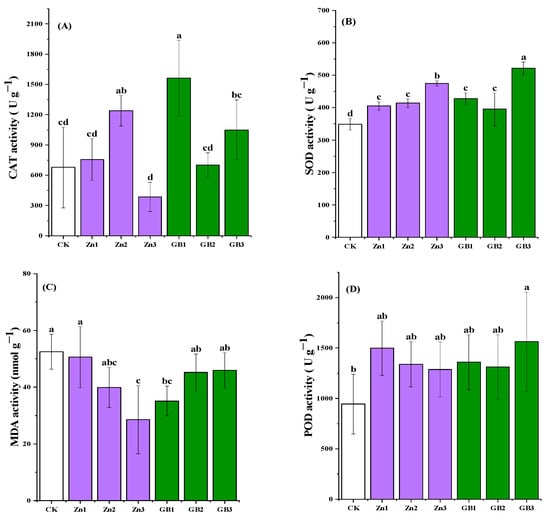
Figure 3.
(A) catalase (CAT) activity, (B) superoxide dismutase (SOD) activity, (C) malondialdehyde (MDA) activity, and (D) peroxidase (POD) activity at full-bloom stage of safflower under application of Zn or glycine betaine. Different lowercase letters indicate significant differences according to the Tukey test at 0.05 level for each treatment; vertical bars in each mean indicate standard errors.
Peroxidase (POD) plays a crucial role in scavenging reactive oxygen species and participating in lignin synthesis. However, results showed no significant difference in POD activity among these spraying treatments. This might be due to the functional diversity of POD, resulting in a weak response to different spraying treatments. The activity of GB3 treatment (1562.67 U g−1) and Zn1 treatment (1498.67 U g−1) was 21.3% and 16.4% higher than the control, respectively (Figure 3D). Malondialdehyde (MDA) is a marker of membrane lipid peroxidation, and its content reflects the degree of oxidative damage. Results showed a significant difference in MDA content among the treatments (p < 0.05). The MDA content of Zn3 treatment (28.55 nmol g−1) was the lowest, which was 32.8% lower than the control, suggesting that a high zinc concentration can effectively inhibit the oxidative damage of safflowers.
3.4. Effects of Zinc or Glycine Betaine on Safflower Yield
At the harvesting stage, there was a significant difference in grain yield among the treatments in the field (p < 0.01). According to the yield gradation standard of Meng et al. [21], the Zn1 treatment had the highest grain yield at 2196.67 kg ha−1, passing the level of a high-yield field (≥1800 kg ha−1). This was 45.5% higher than the control. The GB3 treatment (2126.67 kg ha−1) also falls in the high-yield field category, which was 40.8% higher than the control. The average yield of Zn treatments (Zn1–Zn3) (2166.67–2196.67 kg ha−1) was higher than those of GB treatments, indicating that foliar spraying of zinc fertilizer had a more significant effect on increasing safflower grain yield. In terms of dry filament weight, there was no significant difference (p > 0.05) among the treatments at the full flowering stage (Figure 4B). The value of each treatment ranged from 329.00 to 386.34 kg ha−1, with the Zn3 treatment having the highest filament yield value (386.34 kg ha−1), which was 6.9% higher than the control (361.31 kg ha−1).
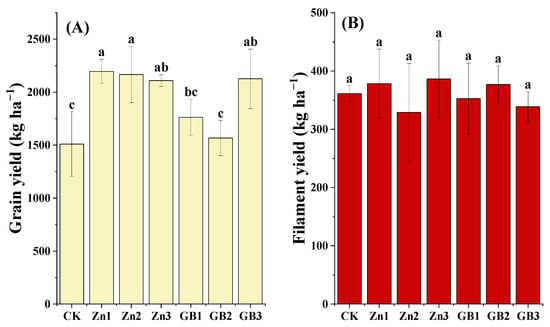
Figure 4.
(A) grain yield and (B) filament yield of safflower under application of Zn or glycine betaine. Different lowercase letters indicate significant differences according to the Tukey test at 0.05 level for each treatment; vertical bars in each mean indicate standard errors.
3.5. Correlation Among the Indices and Safflower Yield
The safflower yield is influenced by both physical structure and physiological functions. In order to achieve a high yield, it is necessary to optimize multiple traits in a collaborative manner [22]. According to Pearson correlation analysis, the number of fruit balls per plant, SOD activity, and POD activity are all significantly and positively correlated with grain yield (p < 0.05). Additionally, the number of seed heads per plant is significantly and positively correlated with transpiration rate and stomatal conductance (p < 0.01), while being significantly and negatively correlated with intercellular CO2 concentration and MDA content (p < 0.05). This suggests that transpiration rate and stomatal conductance indirectly promote yield, while high intercellular CO2 concentration and MDA content indirectly inhibit yield formation. The morphological characteristics of safflower plants, such as plant height, stem diameter, and number of primary branches, are also significantly and positively correlated with the number of fruit balls per plant, indicating that the aboveground biomass of safflowers plays a crucial role in achieving a high yield. There was no significant correlation between dry filament yield and any of the variables in this study (Figure 5).
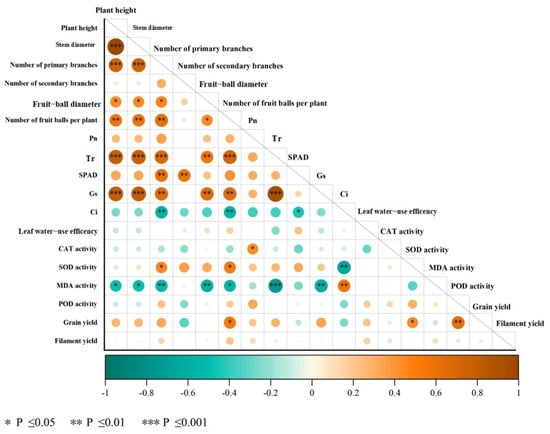
Figure 5.
Correlations among all the measured parameters. Pn: net photosynthetic rate, Tr: transpiration rate, SPAD: chlorophyll relative content, Gs: stomatal conductance, Ci: intercellular CO2 concentration, CAT: catalase, SOD: superoxide dismutase, MDA: malondialdehyde, POD: peroxidase.
4. Discussion
4.1. Technical Measures for Increasing Safflower Yield
The amount of grain yield is one of the basic factors influencing production efficiency. Previous reports showed that a higher grain yield of barley could be obtained after spraying microelement Zn [23,24]. Safflower yield is influenced by various factors, including climatic conditions, soil moisture, and management practices. The use of extensive planting techniques and limited irrigation water resources are significant contributors to the low yield (<900 kg ha−1) of safflowers. In Ili River Valley of Xinjiang, China, the survival rates of safflowers under micro-rainwater harvesting measures were higher than the control [25]. Utilizing runoff from hillslopes and erosion gullies [26] to cultivate rain-fed safflowers can serve as a model for sustainable safflower production in arid regions. The deep roots of safflowers, which can reach up to 150 cm, play a crucial role in soil conservation and contribute to an ecological positive cycle of “rainwater harvesting-soil conservation-yield improvement”.
In this study, compared with the control, the grain yield of the Zn1 treatment increased by 45.5%, which was higher than the increasing rate 16.8% of Rahmani et al. [7] in an arid area of Iran. This may be due to the fact that four times foliar spraying of zinc in this study could better provide trace elements in line with the dynamic demand of safflowers. Results of a pot experiment showed that, under well-watered conditions, foliar-applied zinc and foliar-applied glycine betaine could improve the grain yield of maize by 5.92% and 16.1%, respectively. However, under water-deficient conditions, the yield-increasing rates were 20.0% and 60.5% for zinc and glycine betaine, respectively [11]. Foliar application of zinc or glycine betaine could achieve significant yield improvements in both water-deficit conditions and under normal-irrigation conditions, indicating that foliar spraying technology is not only suitable for water-limited environments, but can also be used as a conventional measure to improve the production of high-yield fields.
4.2. Mechanism of Safflower Yield Improvement
Zinc treatment has been shown to improve the lodging resistance of safflowers by increasing stem diameter, branch number, and other parameters [27]. This treatment also optimizes safflower plant architecture. Adequate zinc levels promote cell division and subsequent cell expansion in the fruit meristem by maintaining auxin levels, directly increasing the fruit-ball diameter [28]. This improvement in plant architecture leads to an increase in the net photosynthetic rate per plant, allowing for better utilization of abundant light resources in arid areas. Results of a two-year field experiment showed that foliar application of 1 kg ha−1 Zn improved the biological yield and seed yield by 21.9% and 16.8%, respectively [8]. The effects of foliar-applied Zn on mass distribution within safflower plants varied due to genotypic differences. Averagely, foliar Zn application enhanced plant above-ground dry mass, root dry mass, and grain yield of the four studied genotypes by 17.2%, 20.6%, and 16.2%, respectively [10]. As a result, the main mechanism for foliar spraying zinc fertilizer to increase safflower grain yield is through the improvement of agronomic traits and the promotion of photosynthesis (Figure 6).
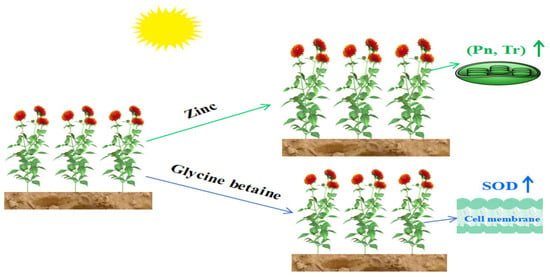
Figure 6.
Physiological mechanism by which zinc or glycine betaine increases safflower yield. Pn: net photosynthetic rate, Tr: transpiration rate, SOD: superoxide dismutase.
Glycine betaine is a natural osmotic adjustment substance [29]. Results of a pot experiment showed that under well-watered conditions, exogenous application of 100 mM glycine betaine improved the root dry weight and shoot dry weight of safflowers by 76.2% and 31.1%, respectively [5]. However, no literature has been found regarding the field effect of foliar spraying glycine betaine on the grain yield of safflowers. This study found that glycine betaine treatment significantly increased physiological indexes such as superoxide dismutase and peroxidase, suggesting that the mechanism behind the yield-increasing effect of spraying glycine betaine lies in its ability to improve the internal antioxidant system of safflowers, resulting in an increase in yield indicators such as the number of fruit balls per plant (Figure 5). Furthermore, glycine betaine has been shown to enhance leaf water-use efficiency by promoting its photosynthetic stability (Figure 6).
5. Conclusions
The application of zinc or glycine betaine through foliar spraying could increase safflower yield through different mechanisms. The highest grain yield at 2196.67 kg ha−1 was achieved for foliar application of 0.6 g L−1 zinc, which was 45.5% higher than the control. This increase can be attributed to the synergistic effects of optimizing plant morphology and enhancing photosynthetic performance. However, foliar spraying with 7.03 g L−1 glycine betaine showed significant antioxidant regulation, resulting in an increase in safflower yield by improving the activity of physiological indexes such as SOD. In comparison to control, the leaf water-use efficiency was increased by 208% for zinc treatment and 128% for glycine betaine treatment. This demonstrates the potential of foliar spraying zinc or glycine betaine to maintain high- and stable-state yields, making foliar application of micronutrients or osmoprotectants a promising technique for water-saving agriculture in arid regions.
Author Contributions
Writing—original draft preparation, software, visualization, data curation, J.L.; conceptualization, methodology, supervision, founding acquisition, G.H.; validation, investigation, writing—review and editing, W.Z.; formal analysis, resources, writing—review and editing, J.W.; project administration, material, founding acquisition, Q.G. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the Open Project of Xinjiang Key Laboratory of Soil and Plant Ecological Processess (XJKL202309), the Key Research and Development Plan of Xinjiang Uygur Autonomous Region (2024B03023-1) and the Technology Innovation Guidance Plan of Shandong Province (YDZX2023010).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
We declare that we have no financial or personal relationships with other people or organizations that can inappropriately influence our work and that there is no professional or other personal interest of any nature in any product, service, and/or company that could be construed as influencing the position presented in, or the review of, this manuscript.
References
- Kummu, M.; Guillaume, J.H.A.; de Moel, H.; Eisner, S.; Flörke, M.; Porkka, M.; Siebert, S.; Veldkamp, T.I.E.; Ward, P.J. The world’s road to water scarcity: Shortage and stress in the 20th century and pathways towards sustainability. Sci. Rep. 2016, 6, 38495. [Google Scholar] [CrossRef] [PubMed]
- Omidi, A.H.; Khazaei, H.; Monneveux, P.; Stoddard, F. Effect of cultivar and water regime on yield and yield components in safflower (Carthamus tinctorius L.). Turk. J. Field Crops 2012, 17, 10–15. [Google Scholar]
- Elliott, J.; Deryng, D.; Müller, C.; Frieler, K.; Konzmann, M.; Gerten, D.; Glotter, M.; Flörke, M.; Wada, Y.; Best, N. Constraints and potentials of future irrigation availability on agricultural production under climate change. Proc. Natl. Acad. Sci. USA 2014, 111, 3239–3244. [Google Scholar] [CrossRef]
- Ahmadi, R.; Mahmoudi, M.; Shekari, F.; Afsahi, K.; Shekari, K.; Saba, J.; Mastinu, A. Application methods of zinc sulphate increased safflower seed yield and quality under end-season drought stress. Horticulturae 2024, 10, 963. [Google Scholar] [CrossRef]
- Nazar, Z.; Akram, N.A.; Saleem, M.H.; Ashraf, M.; Ahmed, S.; Ali, S.; Alsahli, A.A.; Alyemeni, M.N. Glycinebetaine-induced alteration in gaseous exchange capacity and osmoprotective phenomena in safflower (Carthamus tinctorius L.) under water deficit conditions. Sustainability 2020, 12, 10649. [Google Scholar] [CrossRef]
- Hassan, M.U.; Aamer, M.; Chattha, M.U.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y.; et al. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 0396. [Google Scholar] [CrossRef]
- Rahmani, F.; Sayfzadeh, S.; Jabbari, H.; Valadabadi, S.A.; Masouleh, E.H. Alleviation of drought stress effects on safflower yield by foliar application of zinc. Int. J. Plant Prod. 2019, 13, 297–308. [Google Scholar] [CrossRef]
- Manvelian, J.; Weisany, W.; Tahir, N.A.; Jabbari, H.; Diyanat, M. Physiological and biochemical response of safflower (Carthamus tinctorius L.) cultivars to zinc application under drought stress. Ind. Crops Prod. 2021, 172, 114069. [Google Scholar] [CrossRef]
- Ssemugenze, B.; Ocwa, A.; Kuunya, R.; Gumisiriya, C.; Bojtor, C.; Nagy, J.; Széles, A.; Illés, Á. Enhancing maize production through timely nutrient supply: The role of foliar fertiliser application. Agronomy 2025, 15, 176. [Google Scholar] [CrossRef]
- Boostanian, M.; Ehsanzadeh, P. Mycorrhizae and zinc supply benefits safflower: Evidence from the correction of minerals nutrition, physiological and yield penalties of saline water. J. Soil Sci. Plant Nutr. 2025, 25, 2187–2205. [Google Scholar] [CrossRef]
- Shemi, R.; Wang, R.; Gheith, E.S.M.S.; Hussain, H.A.; Hussain, S.; Irfan, M.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress. Sci. Rep. 2021, 11, 3195. [Google Scholar] [CrossRef]
- Wani, S.H.; Singh, N.B.; Haribhushan, A.; Mir, J.I. Compatible solute engineering in plants for abiotic stress tolerance -Role of glycine betaine. Curr. Genom. 2013, 14, 157–165. [Google Scholar] [CrossRef]
- Alasvandyari, F.; Mahdavi, B.; Hosseini, S.M. Glycine betaine affects the antioxidant system and ion accumulation and reduces salinity-induced damage in safflower seedlings. Arch. Biol. Sci. 2017, 69, 139–147. [Google Scholar] [CrossRef]
- Gong, Z.T.; Huang, R.J.; Zhang, G.L. Soil Geography of China; Science Press: Beijing, China, 2014. [Google Scholar]
- Cui, W.C. Soil in Xinjiang; Science Press: Beijing, China, 1996. [Google Scholar]
- Al Shamsi, S.R.H.A.; Rabert, G.A.; Kurup, S.S.; Alyafei, M.A.M.; Jaleel, A. Biochemical changes and antioxidant variations in date palm (Phoenix dactylifera L.) varieties during flower induction and development. Plants 2021, 10, 2550. [Google Scholar] [CrossRef]
- De Santana, T.A.; Oliveira, P.S.; Silva, L.D.; Laviola, B.G.; De Almeida, A.F.; Gomes, F.P. Water use efficiency and consumption in different brazilian genotypes of Jatropha curcas L. subjected to soil water deficit. Biomass Bioenerg. 2015, 75, 119–125. [Google Scholar] [CrossRef]
- Shen, D.; Zhang, G.; Xie, R.; Ming, B.; Hou, P.; Xue, J.; Li, S.; Wang, K. Improvement in photosynthetic rate and grain yield in super-high-yield maize (Zea mays L.) by optimizing irrigation interval under mulch drip irrigation. Agronomy 2020, 10, 1778. [Google Scholar] [CrossRef]
- Yasin, S.; Zavala-García, F.; Niño-Medina, G.; Rodríguez-Salinas, P.A.; Gutiérrez-Diez, A.; Sinagawa-García, S.R.; Lugo-Cruz, E. Morphological and physiological response of maize (Zea mays L.) to drought stress during reproductive stage. Agronomy 2024, 14, 1718. [Google Scholar]
- Song, H.; Jiang, Y.; Xu, Z.; Zhou, G. Response of photosynthetic physiological parameters of maize to drought during the whole growth period and after the jointing stage. Acta Ecol. Sin. 2019, 39, 2405–2415. (In Chinese) [Google Scholar] [CrossRef]
- Meng, J.; Zhang, W.; Hu, G.; Wang, L.; Geng, Q.; Wang, X. Yield grading and yield increasing measures of safflower in Xinjiang. Hubei Agric. Sci. 2024, 63, 110–114. (In Chinese) [Google Scholar]
- Zhao, X.; Shi, X.; Dong, T.; Gao, W.; Deng, C.; Lu, L. Correlation and path analysis of main agronomic traits and single plant grain yield in safflower. J. Henan Norm. Univ. (Nat. Sci. Ed.) 2016, 44, 140–144. (In Chinese) [Google Scholar]
- Stadnik, B.; Tobiasz-Salach, R.; Migut, D. Influence of foliar application of microelements on yield and yield components of spring malting barley. Agriculture 2024, 14, 505. [Google Scholar] [CrossRef]
- Boorboori, M.; Asli, D.E.; Tehrani, M. The effect of dose and different methods of iron, zinc, manganese and copper application on yield components, morphological traits and grain protein percentage of barley plant (Hordeum vulgare L.) in greenhouse conditions. Adv. Environ. Biol. 2012, 6, 740–746. [Google Scholar]
- Pan, Y.; Zhang, W.; Hu, G.; Li, Y. Effects of different micro rainwater harvesting measures on safflower growth. Bull. Soil Water Conserv. 2023, 43, 104–110. (In Chinese) [Google Scholar]
- Li, Z.; Zhang, W.; Aikebaier, Y.; Dong, T.; Huang, G.; Qu, T.; Zhang, H. Sustainable development of arid rangelands and managing rainwater in gullies, Central Asia. Water 2020, 12, 2533. [Google Scholar] [CrossRef]
- Soheili-Movahhed, S.; Khomari, S.; Sheikhzadeh, P.; Alizadeh, B. Improvement in seed quantity and quality of spring safflower through foliar application of boron and zinc under end-season drought stress. J. Plant Nutr. 2019, 42, 942–953. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Adelaide, Australia, 2012. [Google Scholar]
- Felitsky, D.J.; Cannon, J.G.; Capp, M.W.; Hong, J.; Van Wynsberghe, A.W.; Anderson, C.F.; Record, M.T., Jr. The exclusion of glycine betaine from anionic biopolymer surface: Why glycine betaine is an effective osmoprotectant but also a compatible solute. Biochemistry 2004, 43, 14732–14743. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).