Abstract
Fusarium head blight (FHB) is a major devastating wheat fungal disease. Mycotoxins act as virulent factor for FHB progression, including deoxynivalenol (DON), 15-acetyl deoxynivalenol (15-ADON), 3-acetyl deoxynivalenol (3-ADON), deoxynivalenol-3-glucoside (D3G), and zearalenone (ZEN). To identify resistant germplasm against FHB and mycotoxin accumulation, we evaluated 99 wheat cultivars for FHB severity using point inoculation by three FHB isolates under greenhouse and field conditions. FHB severity of selected varieties evaluated in the fields were correlated with that in greenhouse (p < 0.01). Inoculated spikes from 20 varieties were examined for mycotoxin accumulation, employing an LC-MS/MS method that differentiated five mycotoxins. Five cultivars exhibited resistance to both FHB and mycotoxin accumulation, with FHB severity averaging from 13.36% to 33.37%, and DON accumulation below 2400.0 µg/kg, across various conditions. Seven dominant varieties exhibited moderate resistance to FHB and mycotoxin accumulation. FHB severity was significantly positively correlated with DON accumulation, but negatively correlated to the D3G to DON ratio, across distinct groups of FHB resistance (p < 0.01) after inoculation of three distinct isolates, although no correlation was observed within-group. In the present study, Shannong20, Huaimai20, and Sunlin were identified with resistance to both FHB and mycotoxins with superior agronomic performance, providing promising materials for improving disease resistance in breeding programs.
1. Introduction
Wheat (Triticum aestivum L.) is among the world’s three most important crops, accounting for approximately 21% of caloric intake and 20% of dietary protein in developing countries [1]. Fusarium head blight (FHB), also known as scab, is a widespread and destructive fungal disease that affects wheat, maize, and other small grain cereals, particularly under humid conditions in temperate regions [2]. FHB is primarily caused by Fusarium graminearum Schwabe [teleomorph: Gibberella zeae (Schw.) Petch], although other species, such as Fusarium cerealis, F. culmorum, F. avenaceum, and F. pseudograminearum, also serve as key pathogens [3,4]. The disease typically presents as premature bleaching of wheat spikes and impaired grain development, often occurring at the soft-dough stage [5], along with the formation of shriveled kernels [2], resulting in yield losses of up to 80% [5]. Severe FHB epidemics have been reported every 4–5 years in many regions [6]. In North America alone, FHB caused economic losses exceeding USD 1 billion in wheat production and exports during the 20th century [7]. In addition to yield losses, FHB pathogens reduce grain quality by producing various mycotoxins, resulting in both direct and indirect economic damages [8].
Mycotoxins, toxic secondary metabolites produced by fungal pathogens, pose serious health risks to plants, animals, and humans. Trichothecene mycotoxins interfere with eukaryotic protein synthesis, causing symptoms such as necrosis, chlorosis, and wilting in the infected spikes [9], and also act as virulence factors that promote disease spread within the spike [10]. Even trace ingestion levels can lead to gastrointestinal and immunomodulatory disorders in both animals and humans [10,11], making mycotoxin contamination of food grains a global concern [11]. The major mycotoxins produced by Fusarium spp. include trichothecenes, zearalenone (ZEN), and fumonisins. Type B trichothecenes are frequently detected in infected wheat spikes [12] and include deoxynivalenol (DON), 3-acetyl deoxynivalenol (3-ADON), and 15-acetyl deoxynivalenol (15-ADON), which are typically produced by the F. graminearum species complex [13]. The geographic distribution of Fusarium species is shaped primarily by genetic and environmental factors [14,15,16]. F. graminearum sensu stricto (s.s.) is the predominant pathogen responsible for FHB in wheat across Canada, the United States, South America [17,18,19], and Europe [20]. In China, southern regions are predominated by F. asiaticum, followed by F. graminearum s.s. [21], whereas the main causal agent of FHB in northern China is F. graminearum s.s. [21].
In plants, UDP-glucosyltransferase (UGT) genes encode enzymes capable of catalyzing the conversion of DON into deoxynivalenol-3-glucoside (D3G), a less toxic form of DON [22,23]. However, D3G can be converted back into DON by intestinal microflora in humans and livestock, posing potential health risks [9,11,23]. Therefore, it is essential to monitor D3G levels in FHB-infected grains. Traditional methods for mycotoxin analysis include high-performance liquid chromatography (HPLC), enzyme-linked immunosorbent assay (ELISA), and mass spectrometry (MS) [24]. However, ELISA is not suitable for the simultaneous detection of DON and D3G. Thus, liquid chromatography–tandem mass spectrometry (LC–MS/MS) has been widely adopted for the simultaneous detection of multiple mycotoxins in various food and feed products [25,26,27].
Breeding wheat varieties with resistance to FHB is not only a key objective for plant breeders but also the most environmentally sustainable and effective strategy for managing the disease [28]. However, the current availability of FHB-resistant wheat germplasm remains extremely limited. Most widely used resistant cultivars trace their origins to Sumai-3. A few additional resistant sources, such as Wangshuibai, Frontana, Ernie, Nyubai, and Nobeokabouzu, have also been identified as valuable germplasm. FHB resistance in wheat is a quantitative trait governed by multiple genes. To date, more than 200 quantitative trait loci (QTLs) associated with FHB resistance have been mapped across all wheat chromosomes [28,29]. A major QTL located on chromosome 3BS, designated Fhb1, was first identified in the cultivar Sumai-3 [9]. Subsequent studies have shown that Fhb1, inherited in a Mendelian fashion, confers resistance via a rare deletion mutation in a histidine-rich calcium-binding protein [30,31]. Another gene, Fhb7, identified from Thinopyrum ponticum, encodes glutathione transferase [32]. Further research demonstrated that this enzyme transfers glutathione to the DON molecule, disrupting its epoxy structure and thereby conferring resistance to the toxin [33].
The Huang-Huai Region is a key agricultural production zone in China, contributing 35–40% of the country’s total grain yield, 60% of wheat production, and encompassing 25% of the nation’s arable land [34,35]. Shandong Province, located in the northern part of the Huang-Huai Region, is often referred to as the “Big Barn” of China. It ranks second nationwide in both total wheat output, accounting for 19.5%, and wheat planting area, which constitutes 16.99% of the national total [35]. In recent years, the spread of FHB has gradually expanded into the Huang-Huai wheat region, which was previously not considered an epidemic-prone region [34]. However, a review of national variety trials from 2005 to 2016 revealed that only 4% of registered commercial cultivars exhibited moderate resistance to FHB [36].
One of the most effective strategies to mitigate the damage caused by FHB is the breeding and cultivation of resistant wheat cultivars [37]. Type II resistance is regarded as the form with highest stability and has been most widely integrated into wheat breeding programs [38]. However, the availability of resistant cultivars remains limited. Although several FHB-resistant germplasms—such as Nobeokabouzu–Komugi, Frontana, and Sumai-3—have been identified and widely used in breeding efforts, their poor agronomic performance and limited adaptability have hindered their broader application [28,37,39]. Consequently, there is an urgent need for novel genetic resources to support the development of wheat varieties with durable FHB resistance [37].
In this study, a systematic screening of FHB-resistant genotypes was conducted on a set of 99 Triticum aestivum L. cultivars. This panel included 66 major cultivars from Shandong Province and was evaluated against different FHB isolates under diverse environmental conditions. Based on contrasting responses to FHB, 21 cultivars were selected for mycotoxin analysis using LC–MS/MS. The identification of FHB-resistant germplasm has the potential to significantly accelerate the development of wheat cultivars with improved resistance.
2. Materials and Methods
2.1. Plant Material
A total of 99 wheat varieties were selected for this study, including 66 major cultivars, each cultivated on over 6667 hectares (100,000 mu) in Shandong Province in 2022, and 33 additional varieties collected from the Huang-Huai Region of China or other countries (Supplementary Materials Table S1). Seeds were obtained from the Germplasm Bank of the Crop Germplasm Resources Institute, Shandong Academy of Agricultural Sciences, China. All cultivars were grown in a greenhouse at the Institute of Crop Research, Shandong Academy of Agricultural Sciences (Jinan, Shandong, China), under a 16/8 h day/night cycle with light intensity of 300 µmol m−2 s−1. During the 2023–2024 growing season, the average daily temperature was maintained at 26.0 °C during the day and 23.0 °C at night. Specifically, the greenhouse was set to 25.0 °C from 8:00 to 20:00 and to 23.5 °C from 20:00 to 8:00 throughout the growth period.
From the 99 wheat cultivars, 42 were selected based on their varying responses to FHB in the greenhouse and were sown in October 2023 at two experimental stations of the Shandong Academy of Agricultural Sciences—the Jinan Station (117°4′ E, 36°42′ N) and the Jiyang Station (116°58′ E, 36°58′ N)—for field-based evaluations. The cultivars Jimai22 and Sumai-3 were included as susceptible and resistant controls, respectively. A randomized block design was used at each site, with each variety planted in two rows (30 plants per row; row length = 2.0 m; row spacing = 0.3 m). Each experiment had two replications. Standard field management practices, including irrigation, fertilization, and weeding, were applied throughout the growing season.
Subsequently, spikes from 20 cultivars, comprising 5 resistant, 7 moderately resistant, and 8 susceptible genotypes, including the controls Jimai22 and Sumai-3, were selected for mycotoxin assessment (Supplementary Materials Table S2). Inoculated spikes were collected at the dough stage (DS; Feekes scale 11.2) and the physiological maturity stage (PM; Feekes scale 11.4) in four replicates per fungal isolate. Spikes were dried in an oven for 48 h prior to mycotoxin extraction.
2.2. Fungal Inoculum Material
Three isolates from the F. graminearum species complex (FGSC), including F. graminearum s.s. and F. asiaticum, namely, PH-1 (H1), 5035 (H2), and F-1 (H3), were used as inoculums to screen FHB-resistant genotypes through point inoculation in both greenhouse and field trials. These strains were provided by the Institute of Nutrition and Health, Chinese Academy of Sciences as described by Wang et al. [40]. These isolates used in this study were sequenced to be FGSC. Specifically, PH-1 belongs to F. graminearum s.s., meanwhile 5035 and F-1 both belong to F. asiaticum. The preparation of fungal inoculum suspensions followed the protocol described by Sharma et al. [41].
2.3. Standards and Chemicals for LC–MS/MS Analysis
Standards for DON, 15-ADON, 3-ADON, D3G, and ZEN were obtained from Romer Labs (Washington, MO, USA). LC–MS-grade methanol, water, acetonitrile, and formic acid were purchased from Thermo Fisher Scientific (Waltham, MA, USA).
2.4. FHB Evaluation in Greenhouse and Fields
The point inoculation protocol used to screen FHB-resistant genotypes with Type II resistance (limitation of pathogen spread within spikes) in the greenhouse was adapted from a previous study [41]. Macroconidia suspensions were injected into two central florets of each wheat spike at the anthesis stage. The inoculated spikes were then covered with plastic bags misted with sterile water to maintain high humidity and promote infection. The bags were removed four days after inoculation. Each cultivar was inoculated four times per FHB strain. Fifteen days after inoculation, the spikes were assessed for FHB severity. The number of bleached and total spikelets per spike was recorded, and FHB severity was calculated as described by Sharma et al. [41] and Bai and Shaner, using the following formula [42]: FHB severity (%) = (number of diseased spikelets per spike × 100)/total number of spikelets per spike.
For precise quantification of type II resistance, in the field trials, 8 wheat spikes per cultivar in each replicate were marked and inoculated with three different FHB isolates at anthesis. Two central florets per spike were injected between the palea and lemma with 15 µL of suspension per floret, followed by bagging to maintain humidity. Bags were removed after four days. Each variety was inoculated in eight replicates per isolate. Disease severity was assessed 21 days after inoculation using the same formula as above [16,42]. FHB severity was evaluated using two established assessment systems: the percentage of symptomatic spikelets (PSS) per spike [42,43], and a 10-point visual rating scale [44]. PSS was calculated as: (number of diseased spikelets per spike × 100)/total number of spikelets per spike. In the visual rating scale, for each sampled spike, infected spikelet proportions were categorized into predefined intervals (0%, 7%, 14%, 21%, 33%, 50%, 66%, 75%, 90%, or 100% infection). Final disease severity values represented the mean infection percentages across all evaluated spikes. For field evaluation of PSS (Type II resistance), the inoculation strategy combined two complementary methods: first, soil application of fungal-colonized corn spawn (4 g per hill) containing a mixture of three F. graminearum strains, administered 10 days before expected flowering (jointing stage); second, direct pointing inoculation at flowering, ensuring the infection and precisely knowing where the infection initiates to spread. The soil inoculation protocol involved distributing infected corn kernels across the plot surface at the jointing stage and repeating the application 14 days later to establish consistent disease pressure [43,45]. To maintain optimal infection conditions, mist irrigation was provided from flowering through early dough stages. The mist-irrigation using sprinklers was applied in four 15-minute daily sessions at 0600, 0800, 1800, and 2000 h. The final evaluations were conducted at 21 days post-inoculation, with resistance classification determined through statistical comparison to validated control varieties (resistant and susceptible checks). Wheat cultivars exhibiting FHB resistance statistically equivalent to the resistant checks were classified as resistant. Those demonstrating significantly greater resistance than susceptible controls, yet inferior to resistant standards, were designated as moderately resistant.
2.5. Mycotoxin Extraction and Purification Before Injection into LC–MS/MS
FHB-infected wheat spikes (two replicates) from each Fusarium isolate were collected at each maturation stage for analysis. Each dried spike was individually ground before threshing the seeds in a mixer. For each sample, two replicates (0.5 g each) were mixed with 5 mL of extraction buffer (acetonitrile/water/acetic acid; 80:19:1, v/v/v) in 15 mL disposable tubes. The mixture was vortexed for 1 min and then placed on an ultrasonic rotary shaker at 180 rpm for 20 min. Following extraction, the solution was centrifuged at 5000 rpm for 15 min at room temperature. A 2.5 mL aliquot of the supernatant was concentrated using a nitrogen evaporator. Then, 100 µL of acetonitrile was added to redissolve the residue. Subsequently, 5 mL of phosphate buffer (pH 3) was added, followed by vortexing for 1 min and ultrasonication for 10 s.
For purification, solid-phase extraction (SPE) cartridges (Pribolab, Qingdao, China) were first activated with 5 mL each of methanol and acetonitrile. The cartridges were equilibrated with 5 mL of PBS solution and sterile water (pH 3). Then, 5 mL of the resolved sample solution was loaded onto the SPE cartridges. Elution was performed three times by using sterile water. The cartridges were then dried using a rubber bulb. Next, 5 mL of methanol followed by 5 mL of acetonitrile were used for final elution. The eluates were combined, concentrated by nitrogen evaporation, and redissolved in 0.5 mL of a methanol/water solution (1:1, v/v) containing 0.1% formic acid. The final solution was centrifuged at 13,000 rpm for 15 min before injection into the LC–MS/MS system.
2.6. LC–MS/MS Method: Instrumentation and Analytical Conditions
Simultaneous multi-mycotoxin analysis was performed using a triple quadrupole mass spectrometer (ESI-QqQ LC8050 system; Shimadzu Corporation, Kyoto, Japan). Chromatographic separation was carried out at 40 °C on a Waters UPLC BEH C18 analytical column (2.1 × 150 mm, 1.7 µm; Milford, MA, USA), with an auto-sampler maintained at 4 °C and an injection volume of 5 µL. Compounds were separated using a gradient elution with solvent A (LC-grade water containing 2 mM ammonium bicarbonate) and solvent B (LC-grade methanol with 1 mM ammonium formate) at a flow rate of 300 µL/min over a 15-minute run. The gradient steps were as follows: 0–1.5 min, 15–25% B; 1.5–4.0 min, 25–40% B; 4.0–8.5 min, 40–100% B; 8.5–13.0 min, column flushing at 100% B; 13.0–13.1 min, 100–15% B; 13.1–15.0 min, column equilibration at 15% B (Supplementary Materials Table S3). Mass spectrometric analysis was performed in the negative electrospray ionization (ESI) mode. Instrument parameters were set as follows: capillary voltage, 3.0 kV; desolvation gas flow rate, 10 L/min; nebulizing gas flow, 1.5 L/h; and source temperature, 300 °C. Additional parameters, including collision energy, are provided in the Supplementary Materials Table S4. For quantification, external calibration standards at varying concentrations for DON, 15-ADON, 3-ADON, D3G, and ZEN were prepared in acetonitrile. Retention times and m/z transitions for these compounds are also detailed in the Supplementary Materials Tables S4 and S5.
The performance of the optimized method was evaluated based on the coefficient of determination (R2), limits of detection (LOD), limits of quantification (LOQ), recovery, and repeatability (Supplementary Materials Table S5), following the protocol by Huang et al. [46]. Matrix-matched calibration curves were constructed using 7-point serial dilutions of analytical standards in a wheat matrix to determine linearity (R2), LOD (S/N = 3), and LOQ (S/N = 10). For recovery studies, blank wheat samples were spiked in sextuplicate at three concentration levels and processed using an optimized extraction protocol. Each analytical batch included six quality control samples consisting of fortified wheat matrix, which were processed alongside the test samples to ensure accuracy and run-to-run reliability.
2.7. Plant Material and DNA Isolation
Nine wheat cultivars representing distinct FHB resistance profiles were selected for analysis, categorized as susceptible (Jimai22, SD-6, SD-66), moderately resistant (SD-18, SD-33, SD-37), and resistant (Sumai-3, Shannong20, Huaimai20). Each cultivar was challenged with three distinct F. graminearum strains under controlled conditions. For reproducibility, three biological replicates per cultivar–isolate combination were processed, with inoculated spike samples pooled prior to nucleic acid extraction.
Genomic DNA was purified using a commercial isolation kit (Tsingke, Beijing, China) following this optimized procedure: Silica membrane columns were preconditioned with 250 μL BL buffer and centrifuged (12,000× g, 1 min). Approximately 30 mg of cryogenically homogenized tissue was lysed in 400 μL gP1 buffer through vortexing (1 min) and thermal incubation (65 °C, 10–30 min) with periodic mixing. Subsequent precipitation with 150 μL gP2 buffer and ice incubation (5 min) preceded clarification centrifugation (12,000× g, 5 min). DNA binding was achieved by ethanol precipitation of supernatants, followed by column purification involving sequential washes with PW and wash buffers (500 μL each, 12,000× g, 30 s). Final elution used 50–100 μL preheated TE buffer (65 °C) after membrane drying (2 min centrifugation, 1 min air-dry).
2.8. Primer Design and Standard Preparation
The Tri6 gene (GenBank AB017495.1) was targeted using HPLC-purified primers [47] (Sangon Biotech, Shanghai, China): Tri6-10F: 5′-TCTTTGTGAGCGGACGGGACTTTA-3′; Tri6-4R: 5′-ATCTCGCATGTTATCCACCCTGCT-3′ producing a 245-bp amplicon. TA-cloned PCR products were sequence-verified to generate quantification standards. Plasmid DNA was quantified spectrophotometrically (OD260), with copy number calculated considering molecular weight (660 g/mol/bp) and Avogadro’s constant. Copy numbers/μL were calculated using the following formula: copy number = (Concentration (ng/μL) × 10−9 × 6.022 × 1023)/(Plasmid length (bp) × 660 g/mol). Plasmid concentrations were maintained at 20–100 ng/μL to ensure accuracy. Serial dilutions were prepared for 5 gradients. Each dilution was analyzed in triplicate using the same qPCR conditions as the experimental samples.
2.9. Quantitative PCR Analysis
Reactions (20 μL) contained 10 μL SYBR Green master mix (Tsingke, Beijing, China), 1 μL template DNA, and 0.8 μL each primer (10 μM). Thermal cycling parameters included initial denaturation (95 °C, 5 min); 40 cycles of 95 °C (15 s), 60 °C (20 s), 72 °C (20 s); followed by melt curve analysis (95°C, 15 s; 65°C, 1 min; 65–95 °C, 0.11 °C/s increment; 95 °C, 15 s). Fluorescence data were collected during extension and melt curve phases (5 acquisitions/°C) using a QuantStudio system (Thermo Fisher, MA, USA).
2.10. Statistical Analysis
Pearson’s correlation analysis was performed in SAS 9.4 (SAS Institute, Cary, NC, USA) to determine correlations among the variables. Multiple treatment comparisons and analyses of variance (ANOVA) for mycotoxin accumulation and FHB severity among cultivars were conducted using Tukey’s method in SAS 9.4. Box plot analyses were performed using Origin 2021 (OriginLab, Northampton, MA, USA) to illustrate variations among cultivars infected with different FHB chemotypes across distinct locations.
3. Results
3.1. Differential FHB Severity Among 99 Wheat Varieties
Significant effects of wheat genotype on FHB severity were observed (p < 0.05); however, the effect of replication was not significant, indicating the reliability of the FHB evaluation results (Table 1). The mean FHB severity values for the 99 wheat accessions inoculated with isolates H1, H2, and H3 in the greenhouse were 69.82% (range, 4.35–100%), 68.73% (range, 4.55–100%), and 70.78% (range, 5.26–100%), respectively. In field assessments, the mean FHB severity values for 43 selected accessions inoculated with H1, H2, and H3 at the Jinan site were 51.18%, 50.17%, and 51.82%, respectively, with corresponding ranges of 5.26–100%, 11.11–100%, and 5.88–100%. At the Jiyang site, the average FHB severity was 48.08%, 50.52%, and 47.14% for H1, H2, and H3 inoculations, respectively, with corresponding ranges of 4.55–100%, 1.58–100%, and 5.56–100%. Sumai-3, Wangshuibai, Shannong20, Huaimai20, and Sunlin, among the other eight varieties, exhibited FHB resistance comparable to that of Sumai-3 and were classified as resistant (R). These included only 1 of the 66 dominant cultivars (Table 2). Seventy-one varieties, including 54 dominant cultivars, exhibited susceptibility similar to Jimai22 and were designated as susceptible (S), accounting for 71.72% of all varieties and 81.82% of the dominant cultivars, respectively. Twenty varieties, such as SD-34, SD-4, SD-33, SD-17, SD-46, SD-18, and SD-37, showed intermediate severity values, significantly lower than those of the S group but higher than those of the R group, and were classified as moderately resistant (MR). These MR varieties represented 16.67% of dominant cultivars and 20.20% of the total collection (Table 2). Significant differences in FHB severity (p < 0.05) were consistently observed among the R, MR, and S groups, regardless of the isolate or environmental conditions. In the greenhouse, average FHB severities for the R group were 21.14%, 21.58%, and 29.66% for H1, H2, and H3 inoculations, respectively, significantly lower than those for the MR (64.31%, 60.77%, and 62.46%) and S (94.59%, 92.07%, and 92.04%) groups (Figure 1). Similar trends were observed in field trials, where the R group consistently exhibited significantly lower severity than the MR and S groups across all isolates. The MR group also showed significantly reduced severity compared with the S group (p < 0.05) (Figure 1). Strong correlations were observed between greenhouse and field evaluations and among different isolates, confirming that FHB severity reliably distinguishes resistance categories, regardless of the fungal strain or environmental conditions (Table 3).

Table 1.
Analysis of variance for disease severity of Fusarium head blight (FHB) among wheat varieties in greenhouse and field nurseries after inoculation with FHB isolate H1.

Table 2.
Disease severity of Fusarium head blight (FHB) in wheat spikes after inoculation of wheat cultivars with the three FHB isolates.
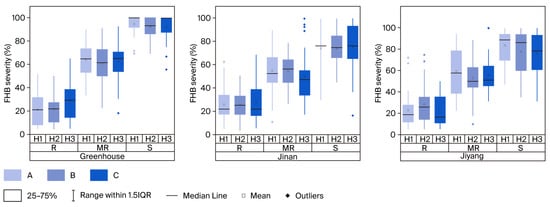
Figure 1.
Fusarium head blight (FHB) disease severity of 20 selected wheat cultivars evaluated in greenhouse, Jinan, and Jiyang field disease nurseries after inoculation with three FHB isolates. The different letters of FHB severity indicate a significant difference (p < 0.05). R = resistant; MR = moderately resistant; S = susceptible.

Table 3.
The Pearson’s correlation coefficient of FHB severity data among wheat varieties evaluated between greenhouse and field nurseries after inoculation with the three isolates.
3.2. Mycotoxin Accumulation Across Different Resistance Groups
For multi-mycotoxin assessment, five genotypes from the R group (Sumai-3, Wangshuibai, Shannong20, Huaimai20, and Sunlin), seven from the MR group (SD-34, SD-4, SD-33, SD-17, SD-46, SD-18, and SD-37), and eight cultivars from the S group were selected (Table 4). Among them, three cultivars, Shannong20, Huaimai20, and Sunlin, demonstrated dual resistance to FHB and multiple mycotoxins, comparable to Sumai-3. In addition, seven dominant cultivars from the MR group showed moderate resistance to both FHB and mycotoxin accumulation (Table 4). Mycotoxin assessments were conducted using LC–MS/MS to classify cultivars based on their FHB resistance profiles. During the PM stage, DON accumulation in the R group was statistically equivalent to that in the Sumai-3 control, whereas S group genotypes displayed vulnerability similar to Jimai22. Significant differences (p < 0.01) were observed between the R and S groups inoculated with H1 in terms of the mean levels of DON (827.01 µg/kg vs. 26,036.0 µg/kg), 15-ADON (68.78 µg/kg vs. 914.42 µg/kg), 3-ADON (36.99 µg/kg vs. 307.58 µg/kg), D3G (595.15 µg/kg vs. 7709.83 µg/kg), and the D3G/DON ratio (0.8385 vs. 0.3804), with similar trends observed for the groups inoculated with H2 and H3 (Figure 2). Furthermore, the MR group exhibited significantly lower DON (5472.15 µg/kg) and D3G (4316.37 µg/kg) levels than the S group under H1 inoculation. The D3G/DON ratio was also significantly higher in the MR group (0.8507) than in the S group (0.3461) under H2 inoculation. Under H3 inoculation, DON accumulation was significantly lower in the MR group (8242.87 µg/kg) than in the S group (18,979.0 µg/kg) (Figure 2). For mycotoxin quantification across two growth stages, no significant differences were observed between Sumai-3 and the other resistant genotypes (Table 5 and Table 6), or between the susceptible genotypes and Jimai22. However, significant differences (p < 0.01) consistently emerged between the R and S groups. These findings collectively highlight a strong association between reduced mycotoxin accumulation and FHB resistance across multiple isolates and screening environments.

Table 4.
Content of DON and D3G in wheat spikes after inoculation with the three FHB isolates.
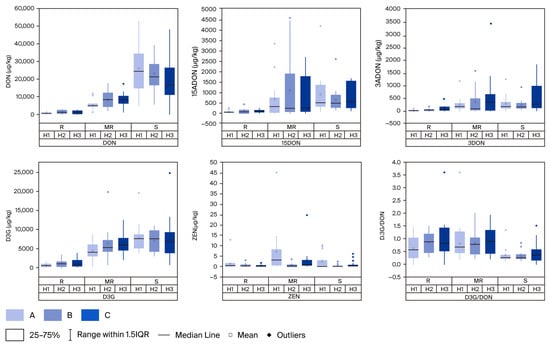
Figure 2.
Contamination with five mycotoxins in the groups of cultivars with different resistant levels after inoculation with three FHB isolates. DON = deoxynivalenol; ADON = acetyl deoxynivalenol; D3G = deoxynivalenol-3-glucoside; ZEN = zearalenone; D3G/DON = D3G-to-DON ratio; R = resistant; MR = moderately resistant; and S = susceptible.

Table 5.
Concentrations of five mycotoxins (DON, 3-ADON, 15-ADON, D3G, and ZEN) during the physiological maturity stage in 20 selected wheat cultivars determined through LC–MS/MS.

Table 6.
Concentrations of five mycotoxins (DON, 3-ADON, 15-ADON, D3G, and ZEN) during the dough stage in 20 selected wheat cultivars determined through LC–MS/MS.
3.3. Mycotoxin Accumulation Comparison Between Two Maturation Stages
Quantitative analysis revealed distinct ontogenetic variations in mycotoxin profiles across the resistance groups following H2 inoculation. Genotypes in the R and MR groups showed significantly increased 3-ADON accumulation during the PM stage compared with the DS, with levels in the R group rising from 3.45 to 43.79 µg/kg (p < 0.05), and in the MR group from 69.37 to 363.82 µg/kg (p < 0.05). Similarly, susceptible (S) genotypes exhibited significantly higher D3G concentrations at PM (6995.48 µg/kg) than at DS (5003.05 µg/kg) following H2 inoculation (p < 0.05). Notably, this stage-dependent variation appeared isolate-specific. Inoculations with H1 and H3 did not result in significant differences between DS and PM in terms of total mycotoxin concentrations or D3G/DON ratios. Across all experimental conditions, multiple comparisons consistently showed the following hierarchy in mycotoxin accumulation: DON > D3G > 15-ADON, 3-ADON, and ZEN (Figure 2). This pattern remained stable across the maturation stages, fungal isolates, and host resistance categories.
3.4. Comparison of Mycotoxin Accumulation Among Three FHB Isolates
Significant correlations among DON, 15-ADON, 3-ADON, D3G, and the D3G/DON ratio were observed in wheat spikes inoculated with the three isolates during the PM stage (p < 0.01). Specifically, DON and D3G levels showed a positive correlation (p < 0.05) in spikes inoculated with both H1 and H2 isolates during both maturation stages, as well as in H3-inoculated spikes during PM. Additionally, 3-ADON accumulation showed a strong positive correlation with D3G levels at PM in spikes infected with H1 and H2 (p < 0.01). An inverse relationship was found between DON production and the D3G/DON ratio (p < 0.05) in H1-inoculated spikes at both stages, and in H2- and H3-inoculated spikes at PM. Similarly, 3-ADON levels negatively correlated with the D3G/DON ratio (p < 0.05) in H3-inoculated spikes at PM. Among the three isolates, H3 produced significantly higher levels of 3-ADON during both maturation stages compared with H1 and H2 (p < 0.05), as determined by Tukey’s multiple comparison. While patterns of D3G conjugation varied across the isolates, no significant differences were observed in the production of other mycotoxins among the isolates.
3.5. Correlation Between FHB Severity and Different Mycotoxin Accumulation
Significant positive correlations were observed between FHB severity and mycotoxin accumulation in wheat spikes inoculated with different isolates. Specifically, FHB severity showed strong positive correlations with DON (r = 0.735, p < 0.01), D3G (r = 0.649, p < 0.01), and 15-ADON (r = 0.274, p < 0.05) in 20 selected wheat cultivars following inoculation with the three isolates in the PM stage (Table 7). In contrast, a significant negative correlation was noted between FHB severity and the D3G/DON ratio (r = −0.359, p < 0.01) across spikes inoculated with all three isolates. Additional significant correlations were also identified among mycotoxins. DON production was strongly positively correlated with D3G levels (r = 0.669, p < 0.01) and 3-ADON accumulation (r = 0.317, p < 0.05). Similarly, 15-ADON levels positively correlated with both 3-ADON (r = 0.596, p < 0.01) and D3G (r = 0.488, p < 0.01). Accumulation of 3-ADON also showed positive correlations with D3G (r = 0.367, p < 0.01) and ZEN (r = 0.356, p < 0.01). Conversely, a strong negative correlation was observed between DON accumulation and the D3G/DON ratio (r = −0.599, p < 0.01) (Table 7).

Table 7.
Pearson’s correlation coefficients for the correlation between Fusarium head blight (FHB) disease severity and multiple mycotoxin accumulation after inoculation with three FHB isolates.
3.6. Fungal Biomass Quantification
The Tri6-based qPCR assay demonstrated excellent linearity (y = −3.2826x + 40.351, R2 = 0.999) across five orders of magnitude. Negative controls showed no detectable amplification. Significant variation in fungal DNA load was observed among resistance categories (Figure 3a). Susceptible cultivars accumulated 63,345–106,167 Tri6 copies/μL, contrasting with resistant (582–647 copies/μL) and moderately resistant (1635–2498 copies/μL) lines. These differences paralleled visual disease severity ratings.
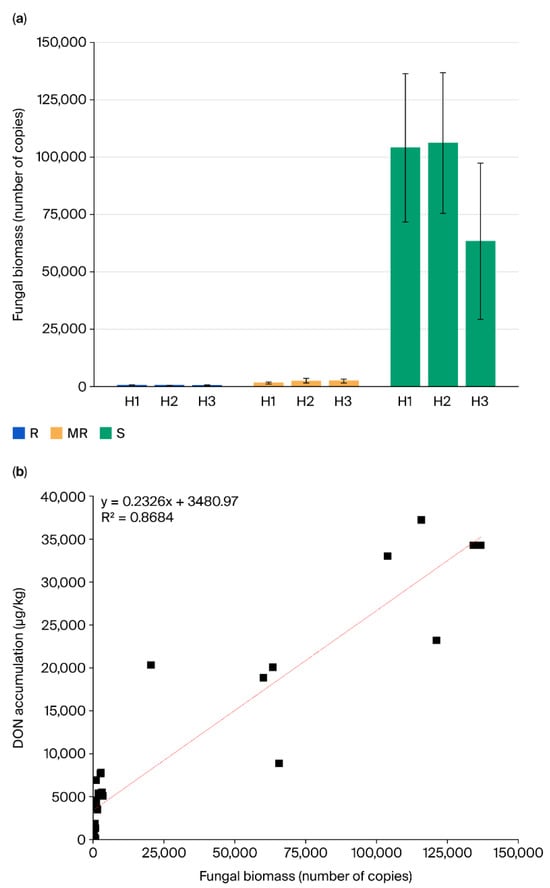
Figure 3.
(a) Tri6 gene copy numbers in nine wheat cultivars inoculated with three F. graminearum strains (three replicates). (b) Correlation between DON accumulation and fungal biomass (Tri6 copies) across cultivar–isolate combinations (three replicates).
3.7. Mycotoxin Correlation Analysis
A strong positive relationship existed between Tri6 DNA quantity and deoxynivalenol accumulation (R2 = 0.868, p < 0.01). The regression model (DON [μg/kg] = 0.2326 × [Tri6 copies] + 3480.97) indicated approximately 23 μg/kg DON increase per 100 additional fungal genome copies (Figure 3b). This linear association was consistent across all cultivar–isolate combinations.
4. Discussion
In this study, a population of 99 wheat cultivars—including 67 dominant cultivars from Shandong Province—was screened for FHB resistance through greenhouse and field trials conducted during 2023–2024. Given the region’s arid climate and low precipitation, an irrigation system was used in combination with spore suspensions to create optimal conditions for wheat growth and FHB infection [7,34]. Based on the results from both greenhouse and field assessments, as well as mycotoxin analysis, cultivars were categorized into the R, MR, and S groups. The findings indicated that both host genotype and pathogen chemotype had significant effects on FHB severity and mycotoxin accumulation, aligning with previous reports by Miedaner et al. [48]. No significant differences in FHB severity were observed across replications following inoculation with the three different isolates, confirming the reliability and reproducibility of the screening methods used.
Several cultivars demonstrated both resistance to FHB and reduced mycotoxin accumulation, making them promising candidates as new resistance sources for future breeding programs. A strong positive correlation in FHB severity between greenhouse and field evaluations across cultivars inoculated with different isolates supported the reliability of phenotypic screening for FHB resistance. This correlation aligns with the findings by Özdemir [49], Touati-Hattab et al. [50], Bjørnstad et al. [51], and Gorczyca et al. [52]. Özdemir [49] reported that the Fusarium-induced disease index showed similar trends between the greenhouse and field conditions, noting that 26 wheat lines could be categorized into the same number of resistance groups. This contrasted with the significantly higher disease index observed under growth chamber conditions.
Previous studies employing ELISA, LC–MS/MS, and GC–MS/MS have reported conflicting results regarding the correlation between mycotoxin accumulation and FHB severity—ranging from no correlation according to Yan et al. and Ji et al. [34,53], to moderate correlation as stated by He et al. [54], or strong positive correlations as reported by Góral et al. and Ochodzki et al. [55,56]. Similarly, the relationship between FHB resistance and the D3G/DON ratio remains inconclusive. In the present study, resistant and susceptible cultivars were clearly differentiated based on their accumulation of DON, D3G, and 15-ADON, as well as FHB severity. These findings are consistent with earlier reports demonstrating a positive association between DON accumulation and FHB severity in small grain cereals, including wheat [2,57]. Furthermore, the D3G/DON ratio was significantly higher in the R and MR groups than in the S group, in agreement with findings from other studies [58,59]. However, Nakagawa et al. [60] reported no significant correlation between FHB resistance and the D3G/DON ratio, highlighting the complexity of this relationship.
The D3G/DON ratio in the R and MR cultivars ranged from 0.839 to 1.113 following inoculation with three FHB isolates, significantly higher than the range of 0.346–0.471 observed in the susceptible group during the PM stage. This inverse relationship between FHB severity and the D3G/DON ratio is consistent with findings from other studies [61,62]. Moreover, transgenic wheat lines expressing the barley UGT gene HvUGT13248 exhibited significantly lower FHB severity and much higher D3G/DON ratios compared with non-transgenic controls [23,63,64], suggesting that the rapid conversion of DON into D3G may reduce the overall fungal load. This, in turn, could lead to reduced total DON and D3G accumulation and contribute to enhanced FHB resistance [9,23]. Supporting this view, Sharma et al. [41] reported distinct DON accumulation patterns in resistant versus susceptible wheat cultivars.
A gradual shift in FHB chemotypes and the expansion of FHB pathogens have been documented across multiple biogeographic regions [17,21,65]. In China, this spread has been largely driven by changing climatic conditions and the widespread adoption of conservation agriculture practices, particularly residue retention and reduced tillage systems [16,34,66]. These factors have significantly affected the northern Huang-Huai wheat region, including Shandong Province, transforming it into a high-incidence area for FHB outbreaks and mycotoxin contamination. Historically considered a non-epidemic zone, Shandong Province remains relatively understudied in terms of FHB resistance—particularly resistance to mycotoxin accumulation—in commercial cultivars. In the present study, over 80% of dominant wheat cultivars in Shandong were identified as highly susceptible to FHB, consistent with previous findings showing that 82.2% of 129 evaluated cultivars in China were susceptible [34]. This substantial deficiency in cultivars with dual resistance to both FHB and mycotoxin accumulation highlights the urgent need to incorporate mycotoxin resistance into future wheat breeding programs [34].
Furthermore, several studies have reported a phenotypic dissociation between disease resistance and mycotoxin accumulation, with some cultivars exhibiting FHB resistance while still accumulating high levels of mycotoxins [34,48,67,68]. Therefore, developing cultivars with enhanced FHB resistance and reduced mycotoxin accumulation is essential to mitigate the dual threat of yield loss and grain quality deterioration in this region.
5. Conclusions
In this study, a systematic evaluation of 99 wheat cultivars—including 66 dominant cultivars from Shandong Province—identified five cultivars (Sumai-3, Wangshuibai, Shannong20, Huaimai20, and Sunlin) exhibiting dual resistance to both FHB and mycotoxin accumulation. Additionally, seven dominant cultivars (SD-34, SD-4, SD-33, SD-17, SD-46, SD-18, and SD-37) were found to possess moderate resistance to both traits. Notably, three cultivars—Shannong20, Huaimai20, and Sunlin—demonstrated dual resistance to FHB and multiple mycotoxins for the first time in this study, along with superior agronomic performance and strong regional adaptability. These cultivars represent valuable parental resources for immediate integration into breeding programs aimed at enhancing FHB resistance while maintaining high yield potential in this strategically important wheat production region. The identification of such resistant germplasm will significantly accelerate the development of wheat cultivars with robust resistance to both FHB and mycotoxin contamination.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15071542/s1, Table S1: The genetic background of 99 wheat varieties and FHB disease ratings; Table S2: The selected 20 varieties for mycotoxin analysis; Table S3: LC–MS/MS gradient for mycotoxin analysis; Table S4: LC–MS/MS parameters for mycotoxin analysis; Table S5: Assessment of LC–MS/MS method.
Author Contributions
Conceptualization, C.H.; methodology, C.H., and X.S.; software, C.H.; validation, C.H., D.C., and Y.Z.; formal analysis, C.H.; investigation, C.H., Y.Z., Y.L., and X.S.; resources, C.H., Y.L., and D.C.; data curation, C.H.; writing—original draft preparation, C.H.; writing—review and editing, C.H., Y.L., Q.F., and Y.Z.; visualization, C.H., and X.S.; supervision, Q.F.; project administration, C.H., and Q.F.; funding acquisition, C.H., and D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32201764), and the Agricultural Scientific and Technological Innovation Project of Shandong Academy of Agricultural Sciences (CXGC2023A01).
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors wish to thank Runfang Li, from the Institute of Crop Germplasm Resources, Shandong Academy of Agricultural Sciences, for providing seeds of wheat lines for the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Peña-Bautista, R.J.; Hernandez-Espinosa, N.; Jones, J.M.; Guzmán, C.; Braun, H.J. CIMMYT series on carbohydrates, wheat, grains, and health: Wheat-based foods: Their global and regional importance in the food supply, nutrition, and health. Cereal Food World 2017, 52, 231–249. [Google Scholar] [CrossRef]
- Wegulo, S.N. Factors influencing deoxynivalenol accumulation in small grain cereals. Toxins 2012, 4, 1157–1180. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Proctor, R.H. Molecular biology of Fusarium mycotoxins. Int. J. Food Microbiol. 2007, 119, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Logrieco, A.F.; Moretti, A. Between emerging and historical problems: An overview of the main toxigenic fungi and mycotoxin concerns in Europe. In Mycotoxins: Detection Methods, Management, Public Health and Agricultural Trade; Cabi: Wallingford, UK, 2008; pp. 139–153. [Google Scholar]
- McMullen, M.; Bergstrom, G.; De Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; Van Sanford, D.A. A unified effort to fight an enemy of wheat and barley: Fusarium head blight. Plant Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef]
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat diseases—A field perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Shah, L.; Ali, A.; Yahya, M.; Zhu, Y.; Wang, S.; Si, H.; Rahman, H.; Ma, C. Integrated control of Fusarium head blight and deoxynivalenol mycotoxin in wheat. Mol. Plant-Microbe Interact. 2018, 67, 532–548. [Google Scholar] [CrossRef]
- Chen, Y.; Kistler, H.C.; Ma, Z. Fusarium graminearum Trichothecene Mycotoxins: Biosynthesis, Regulation, and Management. Annu. Rev. Phytopathol. 2019, 57, 15–39. [Google Scholar] [CrossRef]
- Lemmens, M.; Scholz, U.; Berthiller, F.; Dall’Asta, C.; Koutnik, A.; Schuhmacher, R.; Adam, G.; Buerstmayr, H.; Mesterhazy, A.; Krska, R.; et al. The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for Fusarium head blight resistance in wheat. Mol. Plant-Microbe Interact. 2005, 18, 1318–1324. [Google Scholar] [CrossRef]
- Gunupuru, L.R.; Perochon, A.; Doohan, F.M. Deoxynivalenol resistance as a component of FHB resistance. Trop. Plant Pathol. 2017, 42, 175–183. [Google Scholar] [CrossRef]
- Pierron, A.; Mimoun, S.; Murate, L.S.; Loiseau, N.; Lippi, Y.; Bracarense, A.P.; Liaubet, L.; Schatz-mayr, G.; Berthiller, F.; Moll, W.D.; et al. Intestinal toxicity of the masked mycotoxin deoxynivalenol-3-β-D-glucoside. Arch. Toxicol. 2016, 90, 2037–2046. [Google Scholar] [CrossRef]
- Mallmann, C.A.; Dilkin, P.; Mallmann, A.O.; Oliveira, M.S.; Adaniya, Z.N.C.; Tonini, C. Prevalence and levels of deoxynivalenol and zearalenone in commercial barley and wheat grain produced in Southern Brazil: An eight-year (2008 to 2015) summary. Trop. Plant Path. 2017, 42, 146–152. [Google Scholar] [CrossRef]
- Amarasinghe, C.C.; Tittlemier, S.A.; Fernando, W.G.D. Nivalenol-producing Fusarium cerealis associated with Fusarium head blight in winter wheat in Manitoba, Canada. Plant Pathol. 2015, 64, 988–995. [Google Scholar] [CrossRef]
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium ear blight scab in small grain cereals-a review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Poole, G.J.; Smiley, R.W.; Walker, C.; Huggins, D.; Rupp, R.; Abatzoglou, J.; Garland-Campbell, K.; Paulitz, T.C. Effect of climate on the distribution of Fusarium spp. causing crown rot of wheat in the Pacific Northwest of the United States. Phytopathology 2013, 103, 1130–1140. [Google Scholar] [CrossRef]
- Shi, S.; Zhao, J.; Pu, L.; Sun, D.; Han, D.; Li, C.; Feng, X.; Fan, D.; Hu, X. Identification of New Sources of Resistance to Crown Rot and Fusarium Head Blight in Wheat. Plant Dis. 2020, 104, 1979–1985. [Google Scholar] [CrossRef]
- Ward, T.J.; Clear, R.M.; Rooney, A.P.; O’Donnell, K.; Gaba, D.; Patrick, S.; Starkey, D.; Gilbert, J.; Geiser, D.; Nowicki, T. An adaptive evolutionary shift in Fusarium head blight pathogen populations is driving the rapid spread of more toxigenic Fusarium graminearum in North America. Fungal Genet. Biol. 2008, 25, 473–484. [Google Scholar] [CrossRef]
- Guo, X.W.; Fernando, W.G.D.; Seow-Brock, H.Y. Population Structure, Chemotype Diversity, and Potential Chemotype Shifting of Fusarium graminearum in Wheat Fields of Manitoba. Plant Dis. 2008, 92, 756–762. [Google Scholar] [CrossRef]
- Astolfi, P.; Reynoso, M.M.; Ramirez, M.L.; Chulze, S.N.; Alves, T.C.A.; Tessmann, D.J.; Del Ponte, E.M. Genetic population structure and trichothecene genotypes of Fusarium graminearum isolated from wheat in southern Brazil. Plant Path. 2012, 61, 289–295. [Google Scholar] [CrossRef]
- Nielsen, L.K.; Jensen, J.D.; Nielsen, G.C.; Jensen, J.E.; Spliid, N.H.; Thomsen, I.K.; Justesen, A.F.; Collinge, D.B.; Jørgensen, L.N. Fusarium head blight of cereals in Denmark: Species complex and related mycotoxins. Phytopathology 2011, 101, 960–969. [Google Scholar] [CrossRef]
- Zhang, H.; Van der Lee, T.; Waalwijk, C.; Chen, W.; Xu, J.; Xu, J.; Zhang, Y.; Feng, J. Population analysis of the Fusarium graminearum species complex from wheat in China show a shift to more aggressive strains. PLoS ONE 2012, 7, e31722. [Google Scholar]
- Poppenberger, B.; Berthiller, F.; Lucyshyn, D.; Sieberer, T.; Schuhmacher, R.; Krska, R.; Kuchler, K.; Glossl, J.; Luschnig, C.; Adam, G. Detoxification of the fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J. Biol. Chem. 2003, 278, 47905–47914. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shin, S.; Heinen, S.; Dill-Macky, R.; Berthiller, F.; Nersesian, N.; Clemente, T.; McCormick, S.; Muehlbauer, G.J. Transgenic wheat expressing a barley UDP glucosyltransferase detoxifies deoxynivalenol and provides high levels of resistance to Fusarium graminearum. Mol. Plant Microbe Interact. 2015, 28, 1237–1246. [Google Scholar] [CrossRef]
- Gilbert, J.; Pascale, M. Analytical methods for mycotoxins in the wheat chain. In Mycotoxin Reduction in Grain Chains; Leslie, J.F., Logrieco, A.F., Eds.; John Wiley and Sons Ltd.: Chichester, UK, 2014; pp. 169–188. [Google Scholar]
- Zhang, Z.; Hua, X.; Zhang, Q.; Lia, P. Determination for multiple mycotoxins in agricultural products using HPLC–MS/MS via a multiple antibody immunoaffinity column. J. Chromatogr. B 2016, 1021, 145–152. [Google Scholar] [CrossRef]
- Miró-Abella, E.; Herrero, P.; Canela, N.; Arola, L.; Borrull, F.; Ras, R.; Fontanals, N. Determination of mycotoxins in plant-based beverages using QuEChERS and liquid chromatography–tandem mass spectrometry. Food Chem. 2017, 229, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Hong, S.Y.; Kang, J.W.; Cho, S.M.; Lee, K.R.; An, T.K.; Lee, C.; Chung, S.H. Simultaneous De- termination of Multi-Mycotoxins in Cereal Grains Collected from South Korea by LC/MS/MS. Toxins 2017, 9, 106. [Google Scholar] [CrossRef]
- Jia, H.; Zhou, J.; Xue, S.; Li, G.; Yan, H.; Ran, C.; Zhang, Y.; Shi, J.; Jia, L.; Wang, X.; et al. A journey to understand wheat Fusarium head blight resistance in the Chinese wheat landrace Wangshuibai. Crop J. 2018, 6, 48–59. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Ban, T.; Anderson, J.A. QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: A review. Plant Breed. 2009, 128, 1–26. [Google Scholar] [CrossRef]
- Li, G.; Zhou, J.; Jia, H.; Gao, Z.; Fan, M.; Luo, Y.; Zhao, P.; Xue, S.; Li, N.; Yuan, Y.; et al. Mutation of a histidine-rich calcium-binding-protein gene in wheat confers resistance to Fusarium head blight. Nat. Genet. 2019, 51, 1106–1112. [Google Scholar] [CrossRef]
- Su, Z.; Bernardo, A.; Tian, B.; Chen, H.; Wang, S.; Ma, H.; Cai, S.; Liu, D.; Zhang, D.; Li, T.; et al. A deletion mutation in TaHRC confers Fhb1 resistance to Fusarium head blight in wheat. Nat. Genet. 2019, 51, 1099–1105. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, X.L.; Hou, Y.L.; Cai, J.J.; Shen, X.R.; Zhou, T.T.; Xu, H.H.; Ohm, H.W.; Wang, H.W.; Li, A.F.; et al. High-density mapping of the major FHB resistance gene Fhb7 derived from Thinopyrum ponticum and its pyramiding with Fhb1 by marker-assisted selection. Theor. Appl. Genet. 2015, 128, 2301–2316. [Google Scholar] [CrossRef]
- Wang, H.; Sun, S.; Ge, W.; Zhao, L.; Hou, B.; Wang, K.; Lyu, Z.; Chen, L.; Xu, S.; Guo, J.; et al. Horizontal gene transfer of Fhb7 from fungus underlies Fusarium head blight resistance in wheat. Science 2020, 368, 5435. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhang, H.; van der Lee, T.A.J.; Waalwijk, C.A.D.; van Diepeningen, Y.; Deng, J.; Feng, J.; Liu, T.; Chen, W. Resistance to Fusarium head blight and mycotoxin accumulation among 129 wheat cultivars from different ecological regions in China. World Mycotoxin J. 2020, 13, 189–199. [Google Scholar] [CrossRef]
- China Statistical Yearbook. 2024. Available online: https://www.stats.gov.cn/sj/ndsj/2024/indexeh.htm (accessed on 31 March 2025).
- Ma, H.X.; Zhang, X.; Yao, J.B.; Cheng, S.H. Breeding for the resistance to Fusarium head blight of wheat in China. Front. Agric. Sci. Eng. 2019, 6, 251–264. [Google Scholar] [CrossRef]
- Beres, B.L.; Brûlé-Babel, A.L.; Ye, Z.; Graf, R.J.; Turkington, T.K.; Harding, M.W.; Kutcher, H.R.; Hooker, D.C. Exploring Genotype× Environment× Management synergies to manage Fusarium head blight in wheat. Can. J. Plant Pathol. 2018, 40, 179–188. [Google Scholar] [CrossRef]
- Bai, G.H.; Su, Z.Q.; Cai, J. Wheat resistance to Fusarium head blight. Can. J. Plant Pathol. 2018, 40, 336–346. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Steiner, B.; Hartl, L.; Griesser, M.; Angerer, N.; Lengauer, D.; Miedaner, T.; Schneider, B.; Lemmens, M. Molecular mapping of QTLs for Fusarium head blight resistance in spring wheat. II. Resistance to fungal penetration and spread. Theor. Appl. Genet. 2003, 107, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, Z.; Zhou, H.; Fan, Y.; Wang, C.; Zhang, J.; Liao, Y.; Wu, A. Validation of LC-MS/MS Coupled with a Chiral Column for the Determination of 3- or 15-Acetyl Deoxynivalenol Mycotoxins from Fusarium graminearum in Wheat. Toxins 2021, 13, 659. [Google Scholar] [CrossRef]
- Sharma, P.; Gangola, M.; Huang, C.; Kutcher, H.; Ganeshan, P.; Chibbar, R. Single Nucleotide Poly-morphisms in B-Genome Specific UDP-Glucosyl Transferases Associated with Fusarium Head Blight Resistance and Reduced Deoxynivalenol Accumulation in Wheat Grain. Phytopathology 2018, 108, 124–132. [Google Scholar] [CrossRef]
- Bai, G.H.; Shaner, G. Scab of wheat: Prospects for control. Plant Dis. 1994, 78, 760–766. [Google Scholar]
- Jin, F.; Zhang, D.D.; Bockus, W.; Baenziger, P.S.; Carver, P.S.; Bai, G.H. Fusarium Head Blight Resistance in U.S. Winter Wheat Cultivars and Elite Breeding Lines. Crop Sci. 2013, 53, 2006–2013. [Google Scholar] [CrossRef]
- Stack, R.W.; McMullen, M.P. A Visual Scale to Estimate Severity of Fusarium Head Blight in Wheat; Publication PP-1095; North Dakota State University Extension Service: Fargo, ND, USA, 1995. [Google Scholar]
- Huang, C.; Gangola, M.P.; Kutcher, H.R.; Hucl, P.; Ganeshan, S.; Chibbar, R.N. In Vitro Wheat Immature Spike Culture Screening Identified Fusarium Head Blight Resistance in Wheat Spike Cultured Derived Variants and in the Progeny of Their Crosses with an Elite Cultivar. Plant Pathol. J. 2020, 36, 558–569. [Google Scholar] [CrossRef]
- Huang, C.; Gangola, M.P.; Chibbar, R.N. Utilization of wheat spike culture to assess Fusarium head blight disease progression and mycotoxin accumulation. Can. J. Plant Pathol. 2020, 42, 62–71. [Google Scholar] [CrossRef]
- Horevaj, P.; Milus, E.A.; Bluhm, B.H. A real-time qPCR assay to quantify Fusarium graminearum biomass in wheat kernels. J. Appl. Microbiol. 2011, 111, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Miedaner, T.; Reinbrecht, C.; Lauber, U.; Schollenberger, M.; Geiger, H.H. Effects of genotype and genotype—Environment interaction on deoxynivalenol accumulation and resistance to Fusarium head blight in rye, triticale, and wheat. Plant Breed. 2001, 120, 97–105. [Google Scholar] [CrossRef]
- Özdemir, F. Host Susceptibility of CIMMYT’s International Spring Wheat Lines to Crown and Root Rot Caused by Fusarium culmorum and F. pseudograminearum. Agronomy 2022, 12, 3038. [Google Scholar] [CrossRef]
- Touati-Hattab, S.; Barreau, C.; Verdal-Bonnin, M.N.; Chereau, S.; Richard-Forget, F.; Hadjout, S.; Mek-liche, L.; Bouznad, Z. Pathogenicity and trichothecenes production of Fusarium culmorum strains causing head blight on wheat and evaluation of resistance of the varieties cultivated in Algeria. Eur. J. Plant Pathol. 2016, 145, 797–814. [Google Scholar] [CrossRef]
- Bjørnstad, Å.; He, X.; Tekle, S.; Klos, K.; Huang, Y.F.; Tinker, N.A.; Dong, Y.; Skinnes, H. Genetic variation and associations involving Fusarium head blight and deoxynivalenol accumulation in cultivated oat (Avena sativa L.). Plant Breed. 2017, 136, 620–636. [Google Scholar] [CrossRef]
- Gorczyca, A.; Oleksy, A.; Gala-Czekaj, D.; Urbaniak, M.; Laskowska, M.; Waśkiewicz, A.; Stępień, Ł. Fusarium head blight incidence and mycotoxin accumulation in three durum wheat cultivars in relation to sowing date and density. Sci. Nat. 2018, 105, 2. [Google Scholar] [CrossRef]
- Ji, F.; Wu, J.; Zhao, H.; Xu, J.; Shi, J. Relationship of deoxynivalenol content in grain, chaff, and straw with Fusarium head blight severity in wheat varieties with various levels of resistance. Toxins 2015, 7, 728–742. [Google Scholar] [CrossRef]
- He, X.; Singh, P.K.; Schlang, N.; Duveiller, E.; Dreisigacker, S.; Payne, T.; He, Z. Characterization of Chinese wheat germplasm for resistance to Fusarium head blight at CIMMYT, Mexico. Euphytica 2014, 195, 383–395. [Google Scholar] [CrossRef]
- Góral, T.; Wiśniewska, H.; Ochodzki, P.; Twardawska, A.; Walentyn-Góral, D. Resistance to Fusarium Head Blight, Kernel Damage, and Concentration of Fusarium Mycotoxins in Grain of Winter Triticale (x Tritico secale Wittmack) Lines. Agronomy 2021, 11, 16. [Google Scholar] [CrossRef]
- Ochodzki, P.; Twardawska, A.; Wiśniewska, H.; Góral, T. Resistance to Fusarium Head Blight, Kernel Damage, and Concentrations of Fusarium Mycotoxins in the Grain of Winter Wheat Lines. Agronomy 2021, 11, 1690. [Google Scholar] [CrossRef]
- Chhabra, B.; Thrasu, S.; Wallace, S.; Schoen, A.; Shahoveisi, F.; Dong, Y.; Tiwari, V.; Rawat, N. Evaluation of speed breeding conditions for accelerating Fusarium head blight and deoxynivalenol screening in wheat. Crop Sci. 2024, 64, 1586–1594. [Google Scholar] [CrossRef]
- Schweiger, W.; Steiner, B.; Ametz, C.; Siegwart, G.; Wiesenberger, G.; Berthiller, F.; Lemmens, M.; Jia, H.; Adam, G.; Muehlbauer, G.J.; et al. Transcriptomic characterization of two major Fusarium resistance quantitative trait loci (QTLs), Fhb1 and Qfhs.ifa-5A, identifies novel candidate genes. Mol. Plant Pathol. 2013, 14, 772–785. [Google Scholar] [CrossRef]
- Lemmens, M.; Steiner, B.; Sulyok, M.; Nicholson, P.; Mesterhazy, A.; Buerstmayr, H. Masked mycotoxins: Does breeding for enhanced Fusarium head blight resistance result in more deoxynivalenol-3-glucoside in new wheat varieties? World Mycotoxin J. 2016, 9, 741–754. [Google Scholar] [CrossRef]
- Nakagawa, H.; He, X.; Matsuo, Y.; Singh, P.K.; Kushiro, M. Analysis of the Masked Metabolite of Deoxynivalenol and Fusarium Resistance in CIMMYT Wheat Germplasm. Toxins 2017, 9, 238. [Google Scholar] [CrossRef] [PubMed]
- Audenaert, K.; De Boevre, M.; Vanheule, A.; Callewaert, J.; Bekaert, B.; Höfte, M.; De Saeger, S.; Haesaert, G. Mycotoxin glucosylation in commercial wheat varieties: Impact on resistance to Fusarium graminearum under laboratory and field conditions. Food Control 2013, 34, 756–762. [Google Scholar] [CrossRef]
- Ovando-Martínez, M.; Ozsisli, B.; Anderson, J.; Whitney, K.; Ohm, J.B.; Simsek, S. Analysis of deoxynivalenol and deoxynivalenol-3-glucoside in hard red spring wheat inoculated with Fusarium graminearum. Toxins 2013, 5, 2522–2532. [Google Scholar] [CrossRef]
- Schweiger, W.; Boddu, J.; Shin, S.; Poppenberger, B.; Berthiller, F.; Lemmens, M.; Muehlbauer, G.J.; Adam, G. Validation of a candidate deoxynivalenol-inactivating UDP-glucosyltransferase from barley by heterologous expression in yeast. Mol. Plant-Microbe Interact. 2010, 23, 977–986. [Google Scholar] [CrossRef]
- Shin, S.; Torres-Acosta, J.A.; Heinen, S.J.; McCormick, S.; Lemmens, M.; Paris, M.P.K.; Berthiller, F.; Adam, G.; Muehlbauer, G.J. Transgenic Arabidopsis thaliana expressing a barley UDP-glucosyltransferase exhibit resistance to the mycotoxin deoxynivalenol. J. Exp. Bot. 2012, 63, 4731–4740. [Google Scholar] [CrossRef]
- Brar, G.S.; Dokken-Bouchard, F.; Peluola, C.; Sliva, T.; Stephens, D.; Singh, G.; Kutcher, H.R.; Fernandez, M.R. Fusarium head blight in common and durum wheat in Saskatchewan in 2015. Can. Plant Dis. Surv. 2016, 96, 117–119. [Google Scholar]
- Qiu, J.B.; Xu, J.H.; Shi, J.R. Molecular characterization of the Fusarium graminearum species complex in Eastern China. Eur. J. Pant Path. 2014, 139, 811–823. [Google Scholar] [CrossRef]
- Chen, L.; Song, Y.; Xu, Y. Variation in the concentrations of deoxynivalenol in the spikes of winter wheat infected by Fusarium graminearum Schw. Acta Phytopathol. Sin. 1996, 26, 25–28. [Google Scholar]
- Miller, J.D.; Young, J.C.; Sampson, D.R. Deoxynivalenol and Fusarium head blight resistance in spring cereals. J. Phytopathol. 1985, 113, 359–367. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).