The Impact of Fulvic Acid on the Growth Physiology, Yield, and Quality of Tomatoes Under Drought Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Material
2.2. Experimental Design
2.2.1. Seedling Treatment
2.2.2. Result Period Processing
2.3. Parameter Measurement
2.3.1. Observation and Determination of Tomato Seedling Growth and Root Morphology
2.3.2. Determination of Physiological Indexes of Tomato Seedlings
2.3.3. Determination of Photosynthetic Indices in Tomato Seedlings
2.3.4. Determination of the Growth Index of Tomato at the Flowering Stage
2.3.5. Determination of Photosynthetic Enzymes in Tomato Leaves
2.3.6. Determination of Fruit Character, Yield, and Quality of Tomato
2.4. Statistical Analysis
3. Results
3.1. Analysis of Growth and Physiological Response of Tomato Seedlings Under Drought Stress with Different Concentrations of FA
3.1.1. Effects of Tomato Seedling Growth and Root Morphology
3.1.2. Effects of Osmoregulatory Substances and MDA on Tomato Seedlings
3.1.3. Effect of Reactive Oxygen Species on Tomato Seedlings
3.2. Effects of Photosynthetic Characteristics on Tomato Seedlings
3.2.1. Effects on Chlorophyll
3.2.2. Effects on Photosynthetic Parameters and IWUE
3.2.3. Effect on Chlorophyll Fluorescence
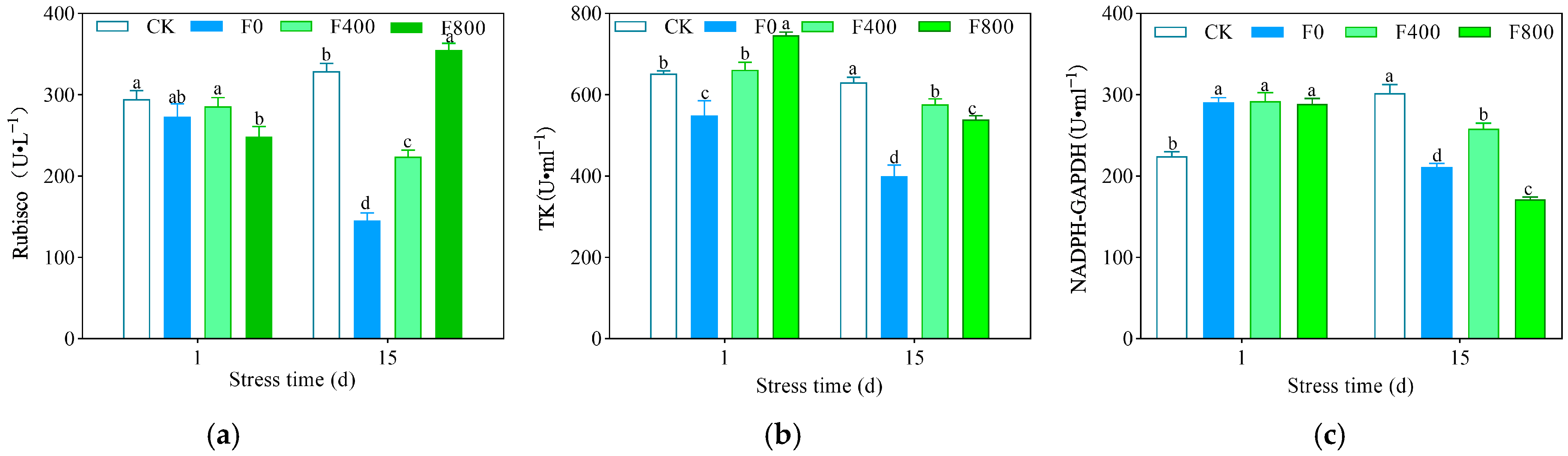
3.2.4. Effect on the Activity of Photosynthetic Enzyme
3.3. Effects of FA on the Growth, Yield, and Quality of Tomato Seedlings Under Drought Conditions
3.3.1. Effect of Tomato Growth After Colonization
3.3.2. Effects of Photosynthetic Enzymes and Water Availability in the Fruiting Period
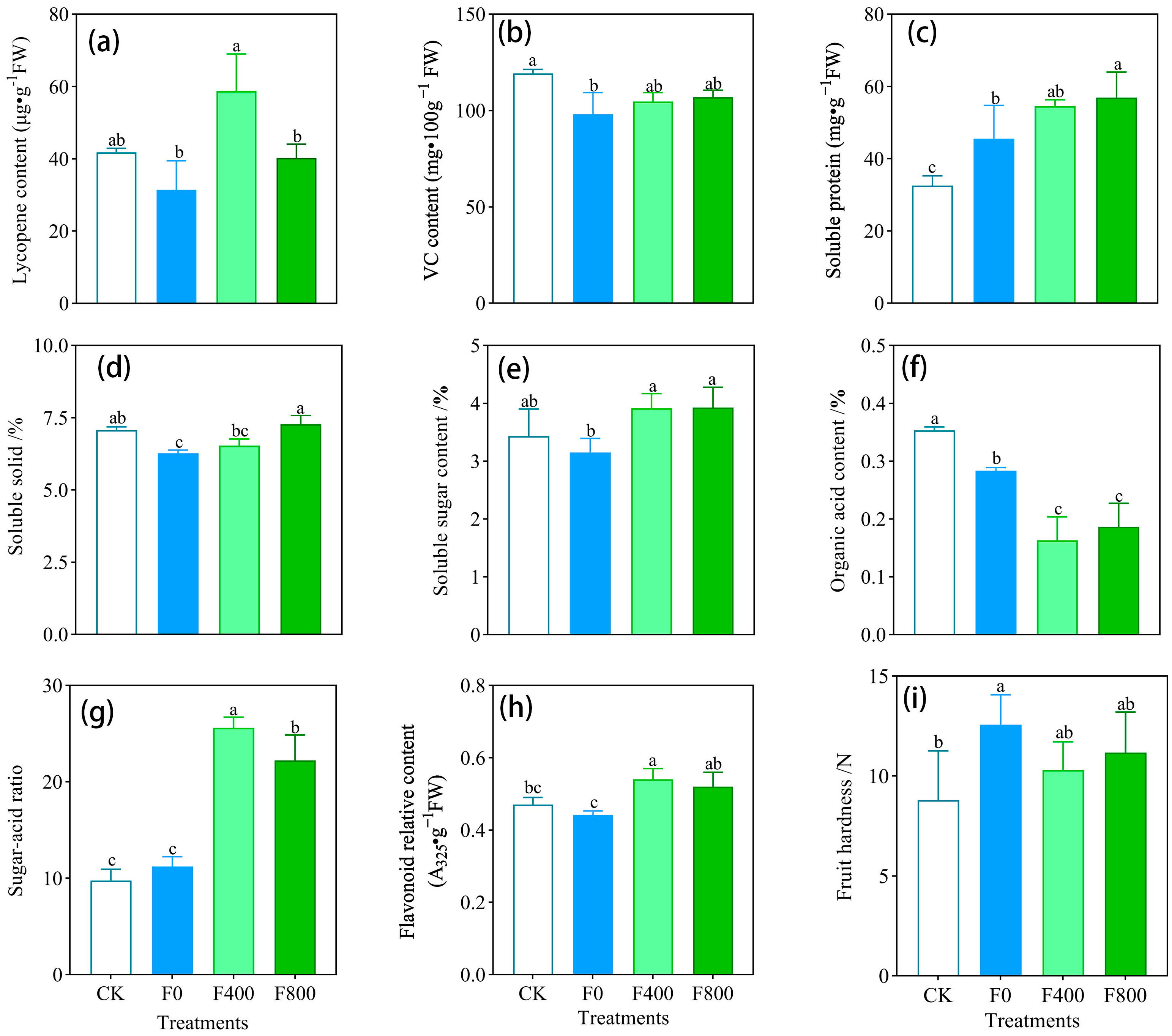
3.3.3. Effect on Yield and Quality of Tomato
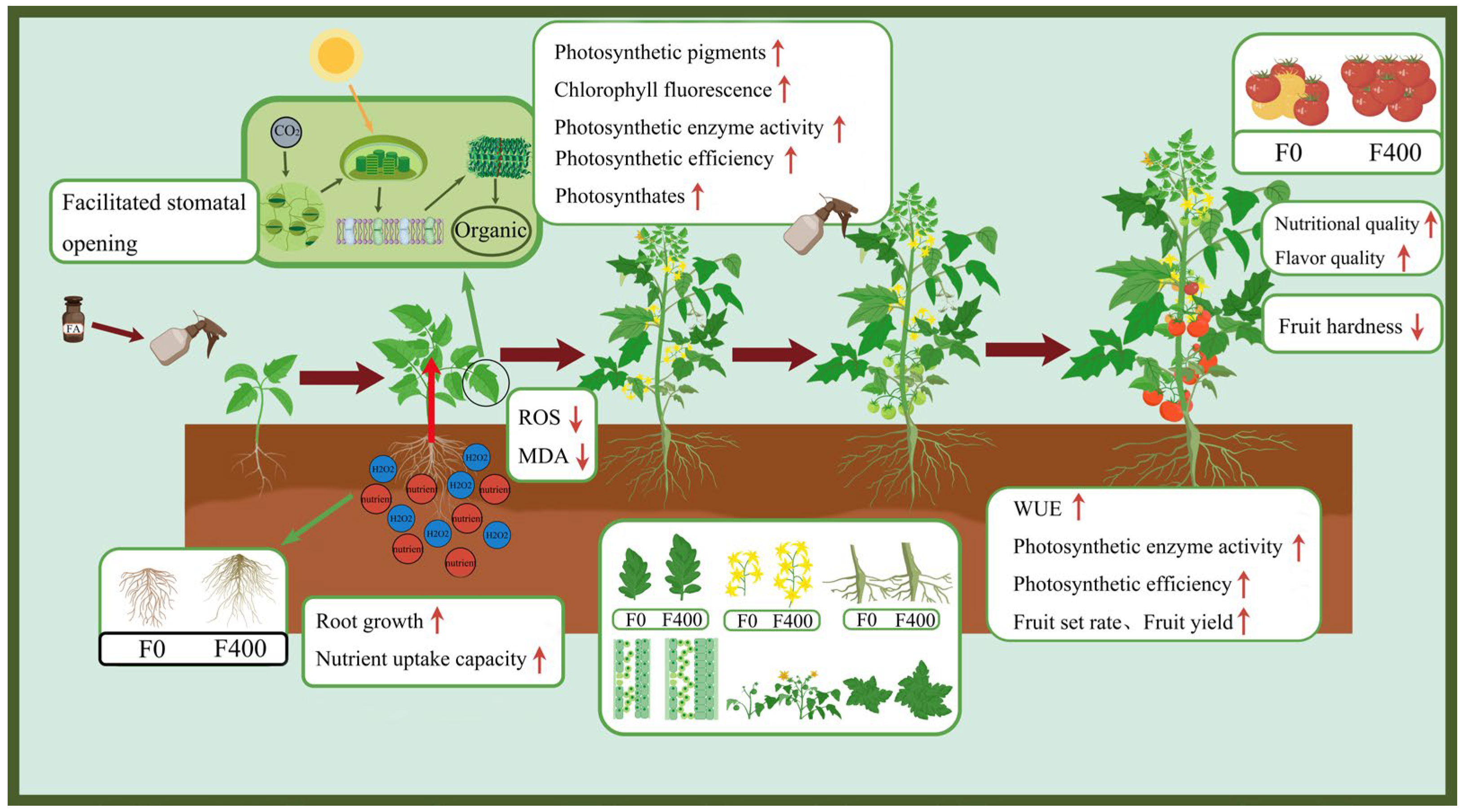
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Song, M.; Liu, J.; Wang, X.; Zhang, Z.; Yang, J. Effects of chitooligosaccharides on physiological indexes of tomato seedlings under drought stress. Soil Fertil. Sci. China 2023, 1, 163–168. [Google Scholar] [CrossRef]
- Yang, Y.; Shuanghe, S.; Yihao, M.; Runyuan, W.; Hong, Z. Advances in the Effects of Drought on Crop Growth and Research on Drought Resistance Techniques. Bull. Sci. Technol. 2020, 36, 8–15. [Google Scholar] [CrossRef]
- Li, Y.; Lin, J.; Xin, X.; Wang, K.; Tian, S.; Ding, J.; Tan, X.; Lin, G.; Zhang, Y.; Wang, G.; et al. Research Progress on Substrate-based Soilless Culture of Tomato in China. China Fruit Veg. 2022, 42, 61–65. [Google Scholar] [CrossRef]
- Chen, R.; Xue, L. Influences of selenium and copper nanoparticles on tomato growth, photosynthetic characteristics and yield under drought stress. Jiangsu Agric. Sci. 2022, 50, 127–134. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, L.; Shen, Q.; Yang, J.; Han, X.; Tian, F.; Wu, J. Effects of water stress on photosynthesis, yield, and water use efficiency in winter wheat. Water 2020, 12, 2127. [Google Scholar] [CrossRef]
- Li, W.; Li, D.; Li, X.; Gao, J.; Jin, K.; Han, W.; Su, X.; Wang, J.; Huang, X.; Liu, Y. Effects of Different Rotation Patterns on Tomato Quality and Yield and Soil Fungi Diversity in Dryland. Acta Agric. Boreali-Sin. 2022, 37, 82–89. [Google Scholar] [CrossRef]
- Li, B.; Feng, H.; Wu, P. Effects of Drought Stress and Rewater on Growth and Yield of Tomato in Seeding Stage. Plant Soil 2008, 2, 63–65. [Google Scholar] [CrossRef]
- Qiao, M.; Hong, C.; Jiao, Y.; Hou, S.; Gao, H. Impacts of drought on photosynthesis in major food crops and the related mechanisms of plant responses to drought. Plants 2024, 13, 1808. [Google Scholar] [CrossRef]
- Ali, M.M.; Yousef, A.F.; Li, B.; Chen, F. Effect of environmental factors on growth and development of fruits. Trop. Plant Biol. 2021, 14, 226–238. [Google Scholar] [CrossRef]
- Klunklin, W.; Savage, G. Effect on quality characteristics of tomatoes grown under well-watered and drought stress conditions. Foods 2017, 6, 56. [Google Scholar] [CrossRef]
- Cui, J.; Shao, G.; Lu, J.; Keabetswe, L.; Hoogenboom, G. Yield, quality and drought sensitivity of tomato to water deficit during different growth stages. Jiangsu Agric. Sci. 2019, 77, e20180390. [Google Scholar] [CrossRef]
- Lv, X.; Li, Q.; Deng, X.; Ding, S.; Sun, R.; Chen, S.; Yun, W.; Dai, C.; Luo, B. Fulvic acid application increases rice seedlings performance under low phosphorus stress. BMC Plant Biol. 2024, 24, 703. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Qiu, C.; Ding, Y.; Wang, Y.; Sun, L.; Fan, K.; Gai, Z.; Dong, G.; Wang, J.; Li, X. Fulvic acid ameliorates drought stress-induced damage in tea plants by regulating the ascorbate metabolism and flavonoids biosynthesis. BMC Genom. 2020, 21, 411. [Google Scholar] [CrossRef]
- Jannin, L.; Arkoun, M.; Ourry, A.; Laîné, P.; Goux, D.; Garnica, M.; Fuentes, M.; Francisco, S.S.; Baigorri, R.; Cruz, F.; et al. Microarray analysis of humic acid effects on Brassica napus growth: Involvement of N, C and S metabolisms. Plant Soil 2012, 359, 297–319. [Google Scholar] [CrossRef]
- Haghighi, M.; Kafi, M.; Fang, P. Photosynthetic activity and N metabolism of lettuce as affected by humic acid. Int. J. Veg. Sci. 2012, 18, 182–189. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, X.; Zhang, X.; Zhao, D.; Tao, J. Behavior. Effects of fulvic acid on the photosynthetic and physiological characteristics of Paeonia ostii under drought stress. Plant Signal. Behav. 2020, 15, 1774714. [Google Scholar] [CrossRef]
- Zhu, S.-S.; Mi, J.-Z.; Zhao, B.-P.; Wu, J.-Y.; Wang, Y.; Liu, J.-H. Effect of fulvic acid on photosynthesis and antioxidant enzyme activities of Avena sativa under drought stress. Acta Bot Boreal-Occident Sin. 2022, 42, 1902–1909. [Google Scholar] [CrossRef]
- Liu, C.-J.; Lyu, C.-Y.; Ai, X.-Z.; Bi, H.-G.J. Effects of fulvic acid on photosynthetic characteristics, yield and quality of cucumber under drought stress. J. Appl. Ecol. 2022, 33, 1300–1310. [Google Scholar]
- Yu, B.; Wang, L.; Cui, D.; Gao, W.; Xue, X.; Nie, P. Effects of fulvic acid on growth and nitrogen utilization efficiency in M9T337 seedlings. Plants 2023, 12, 3937. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, B.; Cao, J.; Song, Z. Effects of Water and Fertilizer Coupling on Growth and Sound Seedling Index of Green Cabbage Seedlings. Agric. Sci. Technol. 2017, 18, 805–838. [Google Scholar] [CrossRef]
- Ors, S.; Ekinci, M.; Yildirim, E.; Sahin, U.; Turan, M.; Dursun, A. Interactive effects of salinity and drought stress on photosynthetic characteristics and physiology of tomato (Lycopersicon esculentum L.) seedlings. South Afr. J. Bot. 2021, 137, 335–339. [Google Scholar] [CrossRef]
- Lin, S.; Meihua, L.; Lita, Y.; Peng, X. Effects of Exogenous Calcium on Growth and Physiological Characteristics of Poplars under Cadmium Stress. Asian J. Ecotoxicol. 2024, 19, 390–403. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ishiyama, K.; Cho, A.; Takegahara-Tamakawa, Y.; Wada, S.; Miyake, C.; Makino, A.; Nutrition, P. Effects of co-overproduction of Rubisco and chloroplast glyceraldehyde-3-phosphate dehydrogenase on photosynthesis in rice. Soil Sci. Plant Nutr. 2021, 67, 283–287. [Google Scholar] [CrossRef]
- Bi, H.; Li, F.; Wang, H.; Ai, X. Overexpression of transketolase gene promotes chilling tolerance by increasing the activities of photosynthetic enzymes, alleviating oxidative damage and stabilizing cell structure in Cucumis sativus L. Physiol. Plant. 2019, 167, 502–515. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Hareem, M.; Danish, S.; Obaid, S.A.; Ansari, M.J.; Datta, R. Mitigation of drought stress in chili plants (Capsicum annuum L.) using mango fruit waste biochar, fulvic acid and cobalt. Sci. Rep. 2024, 14, 14270. [Google Scholar] [CrossRef]
- Li, B.; Fan, R.; Sun, G.; Sun, T.; Fan, Y.; Bai, S.; Guo, S.; Huang, S.; Liu, J.; Zhang, H.; et al. Flavonoids improve drought tolerance of maize seedlings by regulating the homeostasis of reactive oxygen species. Plant Soil 2021, 461, 389–405. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Xia, G.; Wu, Q.; Chi, D. Continuous regulated deficit irrigation enhances peanut water use efficiency and drought resistance. Agric. Water Manag. 2021, 255, 106997. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Zheng, J.; Shen, Z.; Xu, X. Exogenous foliar application of fulvic acid alleviate cadmium toxicity in lettuce (Lactuca sativa L.). Ecotoxicol. Environ. Saf. 2019, 167, 10–19. [Google Scholar] [CrossRef]
- Li, S.; Zhang, K.; Tian, J.; Chang, K.; Yuan, S.; Zhou, Y.; Zhao, H.; Zhong, F.; Research, P. Fulvic acid mitigates cadmium toxicity-induced damage in cucumber seedlings through the coordinated interaction of antioxidant enzymes, organic acid, and amino acid. Environ. Sci. Pollut. Res. 2023, 30, 28780–28790. [Google Scholar] [CrossRef]
- Cheraghi, M.; Mousavi, S.M.; Zarebanadkouki, M. Functions of rhizosheath on facilitating the uptake of water and nutrients under drought stress: A review. Plant Soil 2023, 491, 239–263. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, T.; Cong, A.; Lian, J. Integrated application of fertilization and reduced irrigation improved maize (Zea mays L.) yield, crop water productivity and nitrogen use efficiency in a semi-arid region. Agric. Water Manag. 2023, 289, 108566. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; Elbar, O.H.A.; Farag, R.; Hikal, M.; El-Kelish, A.; El-Yazied, A.A.; Alkahtani, J.; El-Gawad, H. Melatonin counteracts drought induced oxidative damage and stimulates growth, productivity and fruit quality properties of tomato plants. Plants 2020, 9, 1276. [Google Scholar] [CrossRef] [PubMed]
- Talbi, S.; Rojas, J.A.; Sahrawy, M.; Rodríguez-Serrano, M.; Cárdenas, K.E.; Debouba, M.; Sandalio, L.; Botany, E. Effect of drought on growth, photosynthesis and total antioxidant capacity of the saharan plant Oudeneya africana. Environ. Exp. Bot. 2020, 176, 104099. [Google Scholar] [CrossRef]
- Salem, A.; Khandaker, M.M.; Mahmud, K.; Alsufyani, S.J.; Majrashi, A.A.; Rashid, Z.M.; Alenazi, M.M.; Osman, N.; Badaluddin, N. Enhancing photosynthesis and root development for better fruit quality, aroma, and lessening of radioactive materials in key lime (Citrus aurantifolia) using Trichoderma harzianum and Bacillus thuringiensis. Plant Physiol. Biochem. 2024, 206, 108295. [Google Scholar] [CrossRef]
- Naseer, M.A.; Hussain, S.; Nengyan, Z.; Ejaz, I.; Ahmad, S.; Farooq, M.; Xiaolong, R.; Science, C. Shading under drought stress during grain filling attenuates photosynthesis, grain yield and quality of winter wheat in the Loess Plateau of China. J. Agron. Crop Sci. 2022, 208, 255–263. [Google Scholar] [CrossRef]
- Fan, H.-M.; Li, T.; Sun, X.; Sun, X.-Z.; Zheng, C.-S. Effects of humic acid derived from sediments on the postharvest vase life extension in cut chrysanthemum flowers. Postharvest Biol. Technol. 2015, 101, 82–87. [Google Scholar] [CrossRef]
- Nie, P.X.; Li, M.L.; Liu, X.; Zhang, D.; Wang, L.; Xue, X. Effects of exogenous fulvic acid treatments on synthesis and distribution of photoassimilates in apple leaves during fruit expanding period. China Fruits 2024, 2, 12–17. [Google Scholar] [CrossRef]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in Portulaca oleracea L. Plants 2021, 10, 845. Plants 2021, 10, 845. [Google Scholar] [CrossRef]
- Huang, S.; Xiong, B.; Sun, G.; He, S.; Liao, L.; Wang, J.; Wang, B.; Wang, Z. Effects of fulvic acid on photosynthetic characteristics of citrus seedlings under drought stress. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Changsha, China, 18–20 September 2020; p. 032007. [Google Scholar]
- Huang, M.; Jiang, P.; Zhang, Z.; Wu, J.; Li, Y. Effects of Drought and Cultivars on Net Photosynthetic Rate, Key, Photosynthetic Enzymes Activities in Flag Leaves in the Afternoon During Grain Filling Stage and Grain Yield of Wheat. Acta Agric. Boreali-Sin. 2024, 39, 90–98. [Google Scholar] [CrossRef]
- Doron, L.; Xu, L.; Rachmilevitch, S.; Stern, D.; Botany, E. Transgenic overexpression of rubisco subunits and the assembly factor RAF1 are beneficial to recovery from drought stress in maize. Environ. Exp. Bot. 2020, 177, 104126. [Google Scholar] [CrossRef]
- Gao, F.; Liu, C.; Qu, C.; Zheng, L.; Yang, F.; Su, M.; Hong, F. Was improvement of spinach growth by nano-TiO2 treatment related to the changes of Rubisco activase? Biometals 2008, 21, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Zhou, J.; Liu, C.; Yang, F.; Wu, C.; Zheng, L.; Yang, P. Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol. Trace Elem. Res. 2005, 105, 269–279. [Google Scholar] [CrossRef] [PubMed]
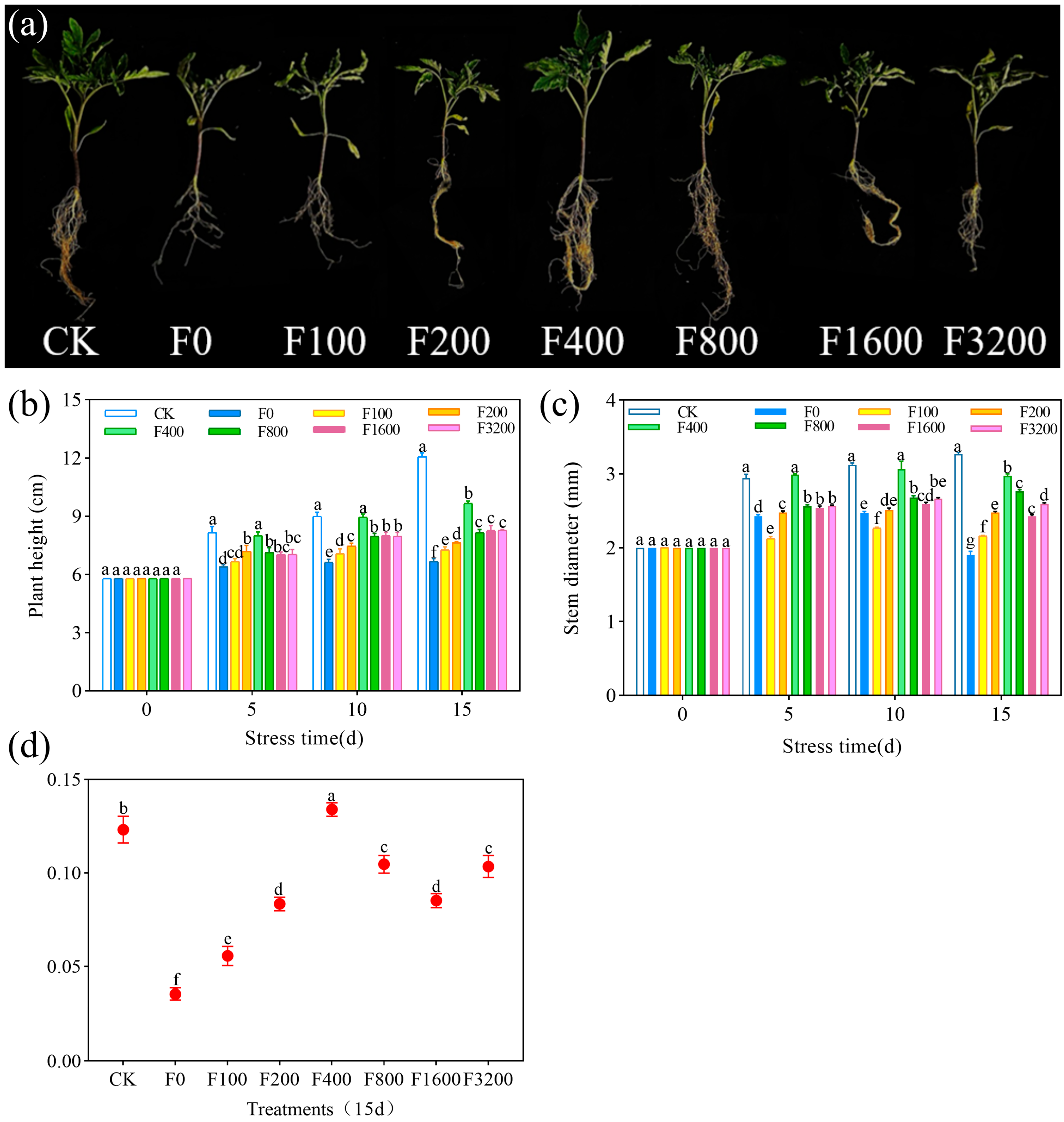
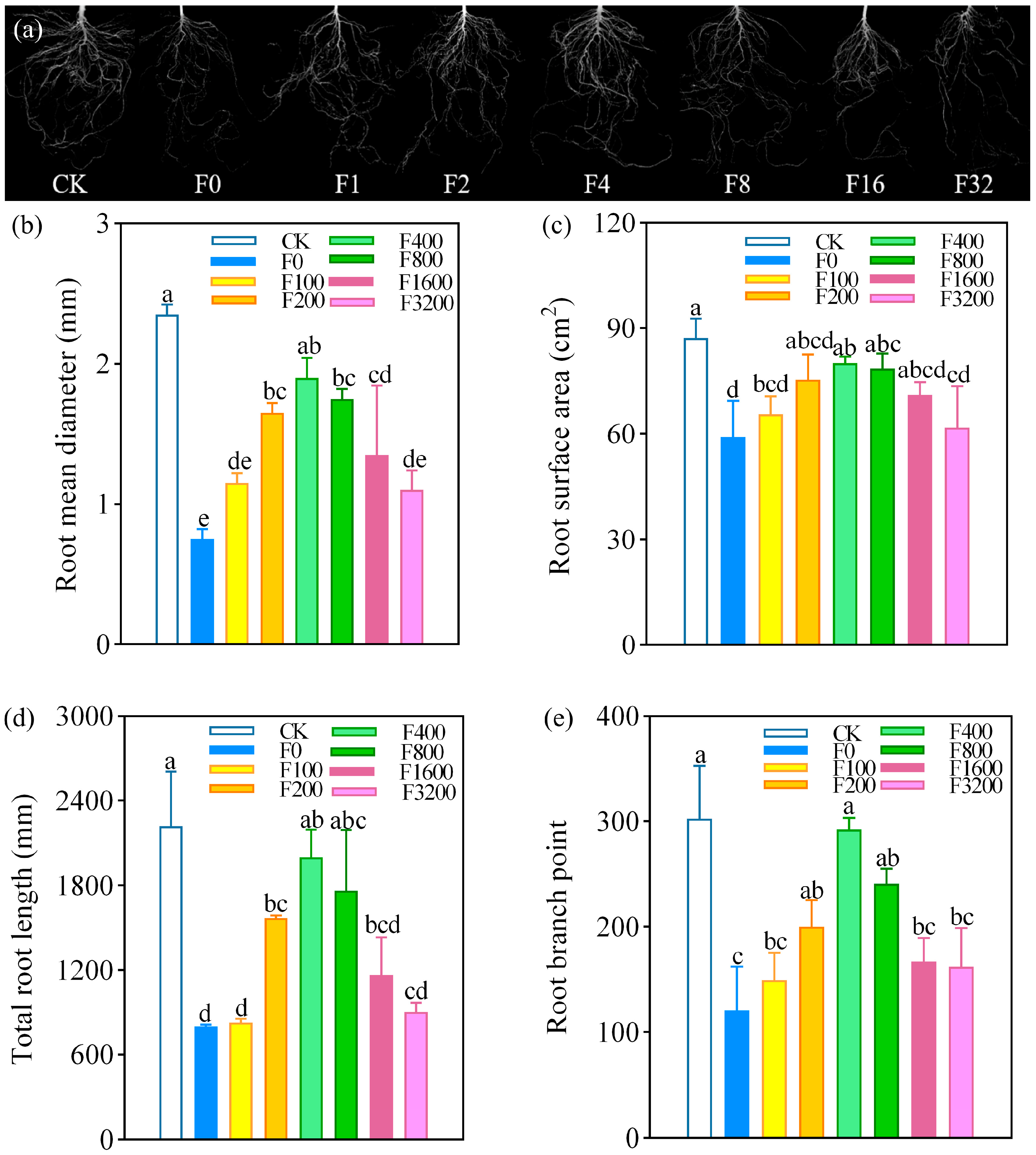

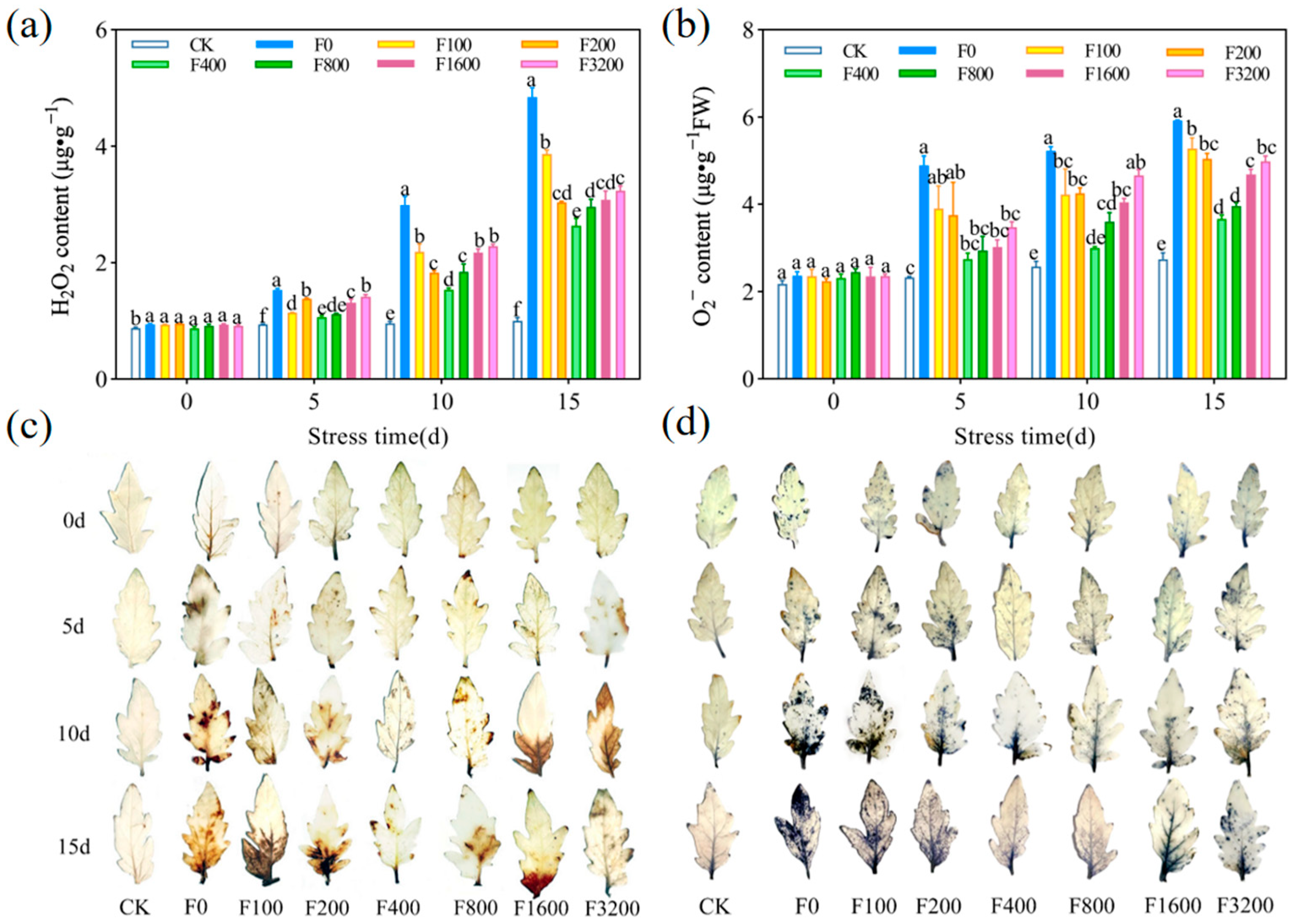


| Number | Treatments |
|---|---|
| CK | Normal irrigation |
| F0 | Drought + 0 mg·L−1 FA |
| F100 | Drought + 100 mg·L−1 FA |
| F200 | Drought + 200 mg·L−1 FA |
| F400 | Drought + 400 mg·L−1 FA |
| F800 | Drought + 800 mg·L−1 FA |
| F1600 | Drought + 1600 mg·L−1 FA |
| F3200 | Drought + 3200 mg·L−1 FA |
| Treatments | Growth Rate of Plant Height/% | Stem Growth Rate/% | Leaf Area Growth Rate/% | Number of Leaves | Specific Leaf Weight (Thickness) (mg·cm−2) |
|---|---|---|---|---|---|
| CK | 135.7 ± 5.4 a | 10.9 ± 1.6 a | 9.90 ± 0.003 a | 20.8 ± 1.4 b | 34.8 ± 1.5 a |
| F0 | 116.0 ± 3.7 b | 7.7 ± 0.4 c | 6.36 ± 0.002 b | 17.8 ± 1.1 c | 19.5 ± 2.6 b |
| F400 | 132.3 ± 1.9 a | 10.4 ± 0.8 a | 9.37 ± 0.006 a | 21.2 ± 1.3 a | 32.8 ± 1.2 a |
| F800 | 131.5 ± 1.9 a | 9.0 ± 0.4 b | 7.63 ± 0.014 ab | 19.6 ± 1.2 c | 23.3 ± 5.1 b |
| Treatments | Rubisco (U·L−1) | TK (U·mL−1) | NADPH-GAPDH (U·mL−1) | WUE/% |
|---|---|---|---|---|
| CK | 369.8 ± 4.4 a | 633.3 ± 21.3 a | 258.6 ± 9.9 a | 35 ± 0.06 a |
| F0 | 167.7 ± 9.0 d | 312.6 ± 14.0 c | 183.5 ± 6.9 b | 21 ± 0.05 b |
| F400 | 317.7 ± 5.5 b | 620.4 ± 15.9 a | 260.9 ± 8.8 a | 35 ± 0.03 a |
| F800 | 190.6 ± 2.8 c | 496.2 ± 35.6 b | 141.2 ± 5.0 c | 32 ± 0.01 a |
| Treatments | Fruit Shape Index | Weight of Single Fruit | Percentage of Fruit Setting/% | Average Yield per Plant | t·ha−1 |
|---|---|---|---|---|---|
| CK | 0.8 ± 0.06 ab | 245.2 ± 31.0 a | 88.0 ± 4.0 a | 22.3 ± 3.9 a | 124.5 ± 20.76 a |
| F0 | 0.7 ± 0.04 b | 171.5 ± 25.3 b | 68.0 ± 4.0 b | 17.0 ± 2.8 b | 67.6 ± 12.39 b |
| F400 | 0.8 ± 0.06 ab | 246.4 ± 20.8 a | 85.6 ± 4.6 a | 21.7 ± 4.3 a | 93.5 ± 13.59 a |
| F800 | 0.9 ± 0.03 a | 233.3 ± 6.8 a | 81.0 ± 6.1 a | 20.3 ± 2.1 a | 90.1 ± 4.14 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Zhu, W.; Guo, Z.; Song, T.; Wang, J.; Gao, C.; Zhang, H.; Shen, R. The Impact of Fulvic Acid on the Growth Physiology, Yield, and Quality of Tomatoes Under Drought Conditions. Agronomy 2025, 15, 1528. https://doi.org/10.3390/agronomy15071528
Song H, Zhu W, Guo Z, Song T, Wang J, Gao C, Zhang H, Shen R. The Impact of Fulvic Acid on the Growth Physiology, Yield, and Quality of Tomatoes Under Drought Conditions. Agronomy. 2025; 15(7):1528. https://doi.org/10.3390/agronomy15071528
Chicago/Turabian StyleSong, Hongxia, Weilong Zhu, Ziqing Guo, Tianyue Song, Jiayu Wang, Chongzhen Gao, Hongtao Zhang, and Ruixue Shen. 2025. "The Impact of Fulvic Acid on the Growth Physiology, Yield, and Quality of Tomatoes Under Drought Conditions" Agronomy 15, no. 7: 1528. https://doi.org/10.3390/agronomy15071528
APA StyleSong, H., Zhu, W., Guo, Z., Song, T., Wang, J., Gao, C., Zhang, H., & Shen, R. (2025). The Impact of Fulvic Acid on the Growth Physiology, Yield, and Quality of Tomatoes Under Drought Conditions. Agronomy, 15(7), 1528. https://doi.org/10.3390/agronomy15071528





