Abstract
The root parasitic weed Phelipanche aegyptiaca (Pers.) Pomel poses a serious threat to solanaceous crops, leading to yield losses of up to 80% in tomato (Solanum lycopersicum L.). Strigolactones (SLs), derived from the carotenoid metabolic pathway, serve as key host-recognition signals for root-parasitic plants. This study investigated the molecular mechanisms of host resistance, focusing on the suppression of SL biosynthesis through altered carotenoid metabolism in the high-pigment tomato mutants hp-1 and ogc. Both pot experiment and in vitro seed germination assays demonstrated that the mutants exhibited reduced susceptibility to P. aegyptiaca and triggered lower germination rates in broomrape seeds compared to the wild-type cultivar AC. Quantitative RT-PCR analysis revealed a significant downregulation of SL biosynthesis genes (SlD27, SlCCD7, SlCCD8, SlMAX1, SlP450, SlDI4) in hp-1 at various parasitic stages post-inoculation, with a more pronounced suppression observed in hp-1 than in ogc. Notably, the extent of downregulation correlated with the enhanced resistance phenotype in hp-1. These findings highlight a synergistic resistance mechanism involving the coordinated regulation of carotenoid metabolism and SL biosynthesis, providing new insights into the molecular defense network underlying tomato-broomrape interactions.
1. Introduction
Tomato (Solanum lycopersicum L.) is one of the most important vegetable crops worldwide, valued for its high nutritional content and distinct flavor, which contribute to its broad consumer appeal. However, tomato is highly susceptible to infestation by Egyptian broomrape (Phelipanche aegyptiaca (Pers.) Pomel), a root-parasitic weed that can cause stunted growth, reduced fruit quality, and yield losses of up to 80% [1]. Therefore, elucidating the resistance mechanisms of tomato against P. aegyptiaca and breeding resistant varieties are essential for ensuring the sustainable development of tomato production, especially for the industry, mainly cultivated in open fields under dry circumstances.
Egyptian broomrape (P. aegyptiaca), an obligate holoparasitic weed of the Orobanchaceae family, lacks chlorophyll and relies entirely on host plants for water and nutrients by forming haustorial connections with their roots [2]. Notably, broomrape species (Orobanche spp. and Phelipanche spp.) germinate and initiate haustorial development only upon detecting germination stimulants released from host roots, among which strigolactones (SLs) are recognized as the primary natural inducers [3].
Current evidence indicates that SLs in tomato root exudates are synthesized through the carotenoid biosynthesis pathway [4]. This process involves a sequence of enzymatic reactions, catalyzed by D27 (β-carotene isomerase), CCD7 (carotenoid cleavage dioxygenase 7), and CCD8 (carotenoid cleavage dioxygenase 8), which convert β-carotene into carlactone (CL), the central precursor of SLs. Carlactone (CL) is then oxidized into various SL derivatives by MAX1 (More Axillary Growth 1), a cytochrome P450 monooxygenase [5,6]. At the signaling level, SL detection and signal transduction are mediated by a receptor complex composed of MAX2, an F-box leucine-rich repeat protein, and D14, an α/β-hydrolase, forming a conserved signaling module that regulates plant development and environmental responses [7].
In tomato fruits, β-carotene biosynthesis is regulated by the B gene, which encodes lycopene β-cyclase. Previous studies have shown that the old gold crimson (ogc) is a recessive allelic variant of the Crt-b gene located at the B locus [8,9]. This mutation causes a frameshift due to a single-nucleotide deletion, leading to a loss of lycopene β-cyclase function and a corresponding increase in lycopene accumulation [9]. Compared to wild-type fruits, the homozygous ogc mutant exhibits a 75% increase in lycopene content and reduced β-carotene accumulation [10]. Similarly, the tomato high pigment-1 (hp-1) mutant is an allelic variant of the DDB1 gene, which encodes a UV-damaged DNA-binding protein [11]. The hp-1 mutation involves a single-base substitution (A→T) at position 931 of the DDB1 coding sequence, leading to an elevated accumulation of both lycopene and β-carotene in tomato fruits [11,12]. Although hp-1 and ogc have been used as non-transgenic high-pigment germplasm in commercial breeding, their potential effects on carotenoid metabolism and resistance to P. aegyptiaca remain largely uncharacterized.
The current scarcity of broomrape-resistant tomato germplasm and limited understanding of resistance mechanisms continue to hinder breeding efforts. Considering the crucial role of SLs in broomrape parasitism, this study employed the high-pigment mutants hp-1 and ogc to comprehensively evaluate their susceptibility to P. aegyptiaca using pot inoculation and seed germination assays. The molecular basis of broomrape resistance in the pigment-enriched tomato mutants hp-1 and ogc was analyzed, focusing on the interaction between carotenoid metabolism and SL biosynthesis. The findings of this study provide new insights into the tomato-broomrape interaction network and offer a molecular framework for developing high-pigment cultivars with enhanced parasitic resistance through the targeted manipulation of the SL metabolic pathway.
2. Materials and Methods
2.1. Plant Materials
Three tomato genotypes were used in this study: the high-pigment mutants hp-1 (LA3538) and ogc (LA3179), along with their wild-type background AC (LA2838). All seeds were kindly provided by the Tomato Genetics Resource Center (TGRC, in Davis, California, USA). Broomrape (P. aegyptiaca) seeds were collected from infected processing tomato fields in Xinjiang, China, and stored at 4 °C until use.
2.2. Pot Experiments
The pot inoculation experiments were independently conducted at the farm of COFCO Tunhe Seed Co., Ltd. (Xinjiang, China) in Xinjiang during the 2016 and 2017 growing seasons. All tomato genotypes were initially sown in seedling trays in a greenhouse and subsequently transplanted into 20 cm diameter pots at the four-true-leaf stage for artificial inoculation. A 2:1:1 (v/v/v) mixture of peat, vermiculite, and non-infested sandy alkaline soil was used as the potting substrate. Each pot was amended with 50 mg of non-preconditioned P. aegyptiaca seeds, which were thoroughly mixed into the substrate prior to transplanting. A completely randomized block design with three replicates was employed in the open field, with each replicate consisting of five uniform plants. Water and fertilizer were applied as needed to support normal plant growth, and soil moisture was maintained at approximately 70% of field capacity.
Phenotypic data were collected 56 days after infection, when P. aegyptiaca had fully developed but had not yet flowered. Tomato roots and the attached broomrape structures were carefully excavated and rinsed. The following phenotypic parameters were recorded: (i) number of emerged broomrapes (Be; aboveground shoots); (ii) total number of broomrape attachments (Tb), defined as the sum of emerged shoots and underground tubercles, representing overall infection severity; and (iii) broomrape fresh weight (Fw), representing the total biomass of all broomrape structures per plant [13]. All phenotypic data presented in the results section represent the mean values from two independent pot experiments conducted under identical field conditions in 2016 and 2017.
2.3. Root Extract Collection
Surface-sterilized tomato seeds (1% NaClO for 3 min, followed by 75% ethanol for 3 min, and thoroughly rinsed with sterile water) were germinated on moist filter paper in 9 cm diameter Petri dishes at 25 °C in darkness for 48 h. Germinated seeds were then transferred to square Petri dishes (13 cm × 13 cm) lined with Whatman GF/A glass fiber filters, with three seedlings per dish. Plants were grown vertically in a controlled growth chamber at 25 °C under a 14 h light/10 h dark photoperiod. Hoagland nutrient solution was supplied as needed to maintain adequate nutrition.
At the six-leaf stage, 100 mg of fresh root tissue was collected from hydroponically grown tomato plants, ground into a fine powder in liquid nitrogen, and extracted with 1 mL of methanol. The extract was sonicated for 30 min and centrifuged at 6400 rpm for 2 min. The resulting supernatant was diluted 100-fold with sterile water and used as a root extract for the P. aegyptiaca seed germination assays [14].
2.4. Broomrape Seed Germination Assay
Broomrape (P. aegyptiaca) seeds were surface-sterilized as described above and placed on double-layer filter paper overlaid with Whatman GF/A glass fiber filters in 9 cm Petri dishes for stratification. Subsequently, 50 seeds were placed in each dish and treated with 5 mL of 10⁻⁴ mol/L gibberellic acid (GA₃) solution at 25 °C in darkness for 3 days to break dormancy.
For the germination assays, 20 µL of tomato root extract or control solutions (10 mg/L GR24 as the positive control and sterile distilled water as the negative control) were applied to Whatman GF/A glass fiber filters. After air drying, the pretreated seeds were placed on the filters, moistened with 40 µL of sterile water, sealed with Parafilm, and grown under at 25 °C in darkness for 7 days. The germination rate, defined as radicle emergence, was evaluated under a stereomicroscope at 10× magnification, with five biological replicates per treatment [15].
2.5. Carotenoid Content Analysis
All tomato genotypes were initially sown in seedling trays in a greenhouse free of P. aegyptiaca infestation. Fresh root tissue was collected from each plant at the six-leaf stage, with three biological replicates per genotype. Root samples were freeze-dried and stored at −80 °C until use. Prior to extraction, the dried samples were homogenized and ground into a fine powder using a mechanical mill. Then, 50 mg of dried powder was extracted with a mixed solvent of n-hexane, acetone, and ethanol, and an internal standard was added. The extract was vortexed for 20 min at room temperature and centrifuged. The resulting supernatant was evaporated to dryness under a stream of nitrogen gas and reconstituted in a methanol: MTBE solution. The final extract was filtered through a 0.22 μm membrane filter for LC–MS analysis.
Carotenoid contents were detected by MetWare (http://www.metware.cn/, accessed on 27 March 2025) based on the AB Sciex QTRAP 6500 LC-MS/MS platform.
2.6. RNA Extraction and Gene Expression Analysis by qRT-PCR
Fresh tomato root samples were harvested from a hydroponic Petri dish co-cultivation system with P. aegyptiaca at three key stages of parasitism: germination (S1, radicle emergence), attachment (S2, haustorium connection to tomato roots), and tubercle formation (S3), as described by Rubiales et al. [16].
The co-cultivation system was established by surface-sterilizing and pretreating seeds as previously described. When tomato seedlings had developed two cotyledons and roots approximately 4 cm in length, they were transferred to 13 × 13 cm Petri dishes with perforated sidewalls, with three plants per dish. Approximately 200 P. aegyptiaca seeds were uniformly applied around the tomato roots using a soft brush. The dishes were wrapped in aluminum foil, vertically placed in square plastic containers, and maintained at 25 °C under a 14 h light/10 h dark photoperiod. Sterile water was added as needed. The developmental stages of P. aegyptiaca were monitored under a stereomicroscope at 10 × magnification to guide sampling [14,16].
Total RNA was extracted using the RNA Pure Kit (Lanyi Biotechnology, Beijing, China) and subsequently reverse-transcribed into cDNA using the 5 × All-In-One RT MasterMix (Lanyi Biotechnology, Beijing, China). Quantitative real-time PCR (qRT-PCR) was then conducted on a Roche LightCycler 480 system with SYBR Green qPCR Mix (Lanyi Biotechnology, China), employing SlEF-1α as the reference gene [17]. Primer sequences are listed in Supplementary Materials Table S1. The relative expression levels of SL biosynthesis genes (SID27, SICCD7, SICCD8, SIMAX1, SIP450, SID14) and carotenoid pathway genes (PSY1, PDS1, LCY-B, CYC-B) were calculated using the 2−ΔΔCt method [18]. Each reaction was performed with three technical replicates. The primer sequence information can be found in the Supplementary Materials.
2.7. Statistical Analysis
Statistical analyses were performed using Microsoft Excel 2010, and graphical representations were generated using GraphPad Prism 10.1.2. Significant differences among treatment groups were assessed by a one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test. A significance level of α = 0.05 was used to determine statistically significant differences.
3. Results
3.1. Susceptibility of High-Lycopene Tomato Mutants to Broomrape Parasitism
To evaluate the susceptibility of the high-pigment tomato mutants hp-1 and ogc to broomrape (P. aegyptiaca), a two-year field pot experiment was conducted in Xinjiang (under consistent environmental conditions), using the wild-type AC as a control. The results demonstrate that hp-1 mutant exhibits significantly fewer emerged broomrape shoots (Be), a lower total number of broomrape attachments (Tb), and reduced broomrape fresh weight (Fw) compared to AC. Specifically, hp-1 showed reductions of 66.24% in Be, 67.68% in Tb, and 58.53% in Fw relative to AC (Figure 1). The ogc mutant also showed statistically significant differences from AC in Be (34.12% reduction) and Fw (41.07% reduction) (Figure 1a,c); no significant difference was observed in Tb (Figure 1b). Overall, both high-pigment tomato mutants hp-1 and ogc exhibited reduced susceptibility to P. aegyptiaca parasitism, with hp-1 exhibiting the lower susceptibility.

Figure 1.
Evaluation of resistance to broomrape (P. aegyptiaca) in the high-pigment tomato mutants ogc and hp-1. (a) Number of emerged broomrapes (Be; aboveground shoots). (b) Total number of broomrape attachments (Tb; sum of emerged shoots and underground tubercles, representing overall infection severity). (c) Broomrape fresh weight (Fw; total fresh weight of all broomrape structures per plant). Data represent means ± SD from two-year pot experiments (n = 15). Different lowercase letters a, b in the figure indicate significant differences at p < 0.05, as determined by Tukey’s test.
3.2. The Effect of Tomato Mutants’ Root Extract on Broomrape Germination
To determine whether the reduced P. aegyptiaca parasitism observed in pot experiments was attributable with decreased germination-stimulating activity in root extracts of high-pigment mutants, root extracts were collected from hydroponically grown ogc, hp-1, and AC plants. Pretreated P. aegyptiaca seeds were exposed to these extracts, with 10 mg/L GR24 and sterile water serving as the positive and negative controls, respectively. The results show that the germination rates of P. aegyptiaca seeds treated with ogc and hp-1 root extracts are 48.19 ± 3.63% and 39.87 ± 6.84%, respectively, both significantly lower than that induced by AC extracts (58.44 ± 4.68%) (Figure 2). These results are consistent with findings from the pot experiments, where AC exhibited the highest susceptibility to P. aegyptiaca parasitism, followed by ogc, and hp-1 showed the lowest level of infestation.
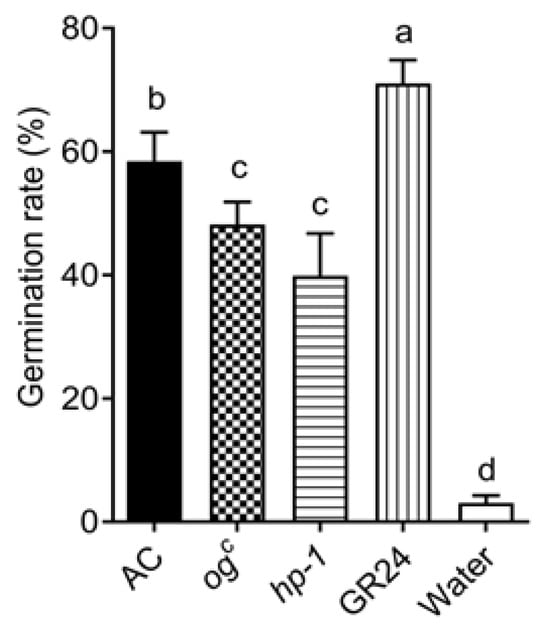
Figure 2.
Germination rate of P. aegyptiaca seeds treated with root extracts from the ogc and hp-1 mutants. Data represent the mean ± SD of five biological replicates. A 10 mg/L GR24 solution was used as the positive control, and sterile water served as the negative control. The different lowercase letters a, b, c, d in the figure indicate statistically significant differences in gene expression among treatments (p < 0.05), and the same letters indicate that the differences are not significant (p < 0.05).
3.3. Resistance Signaling Pathways in High-Lycopene Tomato Mutants
3.3.1. Expression of Lycopene Biosynthesis Pathway Genes
Field pot experiments and germination assays demonstrated that the high-pigment tomato mutants ogc and hp-1 exhibited resistance to P. aegyptiaca, with hp-1 displaying a greater degree of resistance. Previous studies have indicated that the ogc mutation inhibits β-carotene synthesis while promoting lycopene accumulation, whereas hp-1 exhibits the opposite phenotype, resulting in higher carotenoid levels in tomato fruits [9,19]. As these metabolic characteristics were originally identified in fruits, we further investigate whether similar biosynthetic processes occur in tomato roots. To this end, we performed qRT-PCR analysis to evaluate the expression of genes involved in lycopene biosynthesis (PSY1 and PDS1) and carotenoid biosynthesis (LCY-B and CYC-B) in tomato roots at different stages after P. aegyptiaca infection (Figure 3). In this experiment, S1 corresponded to germinated P. aegyptiaca seeds prior to host attachment and served as the control to capture early transcriptional responses. This design minimized the influence of direct host-parasite interactions and enabled more accurate comparisons of gene expression among tomato genotypes during parasitism. The expression levels of PSY1 and PDS1 in tomato roots increased following P. aegyptiaca infection (Figure 3a,b). Notably, both ogc and hp-1 mutants exhibited a higher expression than the wild-type AC, with hp-1 displaying the highest levels. A similar trend was observed for LCY-B and CYC-B (Figure 3c,d). Specifically, ogc showed a lower expression of LCY-B and CYC-B compared to AC, while hp-1 maintained a consistently higher expression throughout the infection stages.
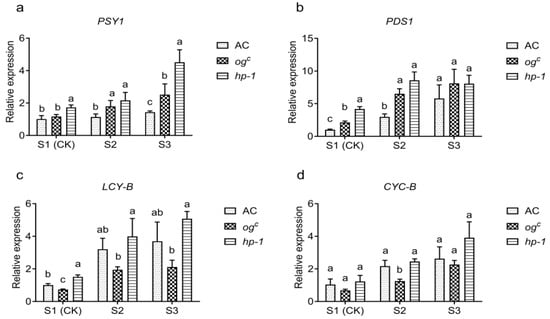
Figure 3.
Expression of β-carotenoid-related genes in the roots of AC, ogc, and hp-1 at the different stages of P. aegyptiaca infection. (a–d) Relative expression levels of genes involved in lycopene and carotenoid biosynthesis (PSY1, PDS1, LCY-B, CYC-B) in the roots of AC, ogc, and hp-1 following P. aegyptiaca infection. S1–S3 represent distinct parasitic stages: S1 (germination; radicle emergence), S2 (attachment; haustorial connection of P. aegyptiaca to tomato roots), and S3 (tubercle formation). S1 was used as the control. The different lowercase letters a, b, c in the figure indicate statistically significant differences in gene expression among treatments (p < 0.05), and the same letters indicate that the differences are not significant (p < 0.05).
3.3.2. Carotenoid Content in Roots of ogc and hp-1 Mutants
Carotenoid content in the roots of ogc and hp-1 mutants has not been previously reported [20,21]. Therefore, based on the expression patterns of LYC-B and CYC-B, which are involved in the carotenoid biosynthesis pathway, carotenoid levels were further quantified in the roots of the high-pigment mutants ogc and hp-1, as well as the wild-type AC. The results show that the β-carotene content in hp-1 roots is significantly higher than that in ogc and AC, while no significant difference was observed between ogc and AC (Table 1). These findings are consistent with the expression profiles of LYC-B and CYC-B genes in tomato roots (Figure 3c,d). In addition, the contents of lutein, violaxanthin, and neoxanthin were also significantly higher in hp-1 roots than in those of ogc and AC (Table 1).

Table 1.
Carotene content in the roots of high-pigment tomato mutants AC, ogc, and hp-1.
3.3.3. Expression of Strigolactone Biosynthesis and Signaling Genes
In tomato, SlD27, SlCCD7, SlCCD8, SlMAX1, SlP450, and SlD14 are key genes involved in SL biosynthesis and signaling [22,23]. To assess how altered carotenoid content and gene expression in ogc and hp-1 roots influence downstream SL biosynthesis and signaling, the expression level of these genes were quantified across different parasitic stages (S1–S3). In hp-1 roots, SlD27 expression was significantly lower than in AC and ogc at stages S2–S3 (Figure 4a), indicating the impaired synthesis of 9-cis-β-carotene, the initial substrate for SL biosynthesis. Similarly, SlCCD7, SlCCD8, SlMAX1, and SlP450 were markedly downregulated in hp-1 compared to AC at stages S2-S3 (Figure 4b–e). This coordinated downregulation likely led to the reduced production of carlactone (CL), a key SL precursor, thereby suppressing SL biosynthesis. In addition, SlD14 expression in hp-1 was lower than in AC during the S2–S3 stages (Figure 4f), suggesting attenuated SL signaling pathway, which may compromise host recognition by P. aegyptiaca. In contrast, ogc roots showed no significant differences in SlD27 or SlCCD7 expression compared to AC at S2–S3. However, SlCCD8 expression was downregulated in ogc at S2 (Figure 4c), suggesting a distinct mechanism of SL pathway suppression from that observed in hp-1.
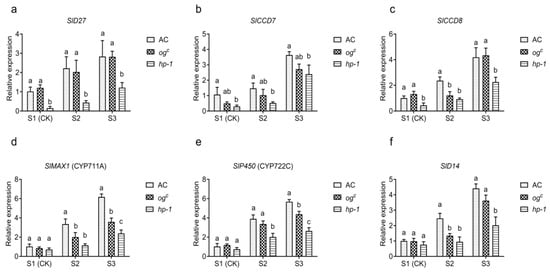
Figure 4.
Expression of SL-related genes in the roots of AC, ogc, and hp-1 at the different stages of P. aegyptiaca infection. (a–f) Relative expression levels of six genes involved in strigolactone biosynthesis and signaling (SID27, SICCD7, SICCD8, SIMAX1, SIP450, and SID14) in the roots of AC, ogc, and hp-1 following P. aegyptiaca infection. S1–S3 represent distinct parasitic stages: S1 (germination; radicle emergence), S2 (attachment; haustorial connection of P. aegyptiaca to tomato roots), and S3 (tubercle formation). S1 was used as the control. Expression levels for each gene are indicated with different lowercase letters a, b, c to denote significant differences (p < 0.05), and the same letters indicate that the differences are not significant (p < 0.05).
4. Discussion
This study demonstrates that the tomato high-pigment mutants ogc and hp-1 exhibit reduced susceptibility to P. aegyptiaca through distinct molecular mechanisms. In ogc, resistance is primarily attributed to a deficiency in β-carotene synthesis, resulting in a reduced availability of SL precursors. The β-carotene deficiency in ogc roots likely results in a decrease in SL production, contributing to the observed resistance. In contrast, resistance in hp-1 is primarily associated with the downregulation of key genes in the SL biosynthesis pathway, particularly SID27, a rate-limiting enzyme. This suppression of SL biosynthesis in hp-1 results in a significantly reduced germination-stimulating activity in root extracts and impaired parasitic plant germination.
The analysis of carotenoid biosynthesis-related genes (LYC-B and CYC-B) and carotenoid content in tomato roots confirmed that hp-1 roots accumulated substantial carotenoids, whereas ogc roots exhibited lower carotenoid contents due to a mutation in the B gene. These findings are consistent with those of previous studies on carotenoid content in tomato fruits [10,11]. Despite the higher β-carotene content in hp-1 roots, the primary factor of SL biosynthesis suppression in hp-1 appears to be the downregulation of SID27. This observation is consistent with previous findings in the hp−2dg mutant by López-Ráez et al. [24], where increased carotenoid levels correlated with reduced SL accumulation and decreased susceptibility to Orobanche ramosa. Furthermore, the low expression of SID27 significantly restricts the conversion of all-trans-β-carotene to 9-cis-β-carotene, blocking the initial step of SL biosynthesis and suppressing downstream carlactone (CL) production [25]. The reduced expression of SID27 is central to the resistance observed in hp-1.
We hypothesize that β-carotene accumulation in hp-1 roots may indirectly affect the SL biosynthesis pathway via feedback regulation. Specifically, it is possible that excess β-carotene may compete with carotenoid cleavage dioxygenases (CCDs), such as CCD7 and CCD8, inhibiting the production of carlactone, a precursor of SLs. Additionally, carotenoid-derived oxidation products, such as abscisic acid (ABA), may further modulate the expression of SL biosynthetic genes through alternative signaling pathways [26]. These findings suggest a complex interplay between carotenoid accumulation and SL biosynthesis, with further studies required to validate these hypotheses and gain deeper insights into the mechanism behind SL biosynthesis suppression in hp-1.
SLs play a crucial role in determining parasitic success through their synthesis and release, serving as germination signal molecules for parasitic plants such as P. aegyptiaca [27]. Germination assays confirmed that root extracts from ogc and hp-1 exhibited less germination-stimulating activity than AC, consistent with the resistance phenotypes observed in field trials (Figure 1 and Figure 2). The hp-1 mutant exhibits a lower P. aegyptiaca seed germination rate, associated with the downregulation of SID27 and suppression of SL biosynthetic genes (Figure 4). This disruption likely interferes with host–parasite signaling and SL-driven germination. Over the course of this study, it became evident that the reduced SL biosynthesis in hp-1 plays a central role in mediating resistance to P. aegyptiaca. Recent studies emphasize that manipulating the SL biosynthesis pathway represents an effective strategy for managing parasitic plants. For instance, sorghum LGS1 mutants with altered chemical structures of SLs exhibit reduced germination stimulant activity for Striga [28]. Similarly, tomato SL-ORT1 mutants and SlCCD8 RNAi/Cas9 lines show decreased SL release and broomrape parasitism [29,30,31]. Moreover, a recent study demonstrated that knocking out the SlABCG45 gene, which encodes a SL transporter, significantly enhanced tomato resistance to P. aegyptiaca without adversely affecting plant yield [32]. Together, these studies suggest that the targeted regulation of the SL biosynthetic and transport pathways offers a promising strategy for improving crop resistance to parasitic plants.
Although hp-1 exhibits enhanced resistance to P. aegyptiaca, SLs are multifunctional hormones that regulate numerous developmental and physiological processes, including the seed germination of parasitic plants, shoot branching, root architecture, and stress responses [27,33,34]. Therefore, future research should assess the ecological trade-offs associated with SL suppression in hp-1, particularly within host-parasite interactions. Given the extensive regulatory roles of SLs in plant development and stress responses, it is essential to explore how alterations in SL levels impact overall plant fitness, especially with regard to competition with parasitic plants [35]. Future studies should focus on the following areas: (1) Resource Allocation and Competition: SLs regulate shoot branching and root architecture, both critical for resource acquisition, particularly under nutrient stress conditions like nitrogen (N) and phosphorus (P) deficiency [36,37]. It is important to assess whether reduced SL levels in hp-1 affect branching patterns, apical dominance, and nutrient uptake, potentially influencing the plant’s ability to compete with parasitic plants. (2) Hormonal Signaling and Stress Responses: SLs interact with other hormones such as auxins, cytokinin, gibberellin and ABA to maintain hormonal balance and enhance stress tolerance [38]. Disruptions in SL signaling could negatively affect agronomic traits (e.g., plant height, branching) and stress responses, potentially influencing interactions with parasitic plants [30,31,32]. To determine whether SL-based resistance strategies compromise plant fitness and agronomic traits, further phenotypic and transcriptomic analyses are necessary. These studies will deepen our understanding of SL regulation in host-parasite interactions and inform the application of SL-based strategies in agriculture.
While this study provides initial insights into the molecular basis of broomrape resistance in hp-1, several limitations remain. First, the causal relationship between carotenoid accumulation and the downregulation of SID27 expression in hp-1 roots requires further experimental validation. Second, the absence of precise quantification of SLs and their precursors remains a constraint, primarily due to technical challenges in extracting and detecting these low-abundance compounds from hydroponically grown tomato roots. As an alternative, P. aegyptiaca seed germination was employed as a functional bioindicator to infer SL activity in root extracts. Although informative, this indirect approach does not fully capture the underlying metabolic changes associated with SL biosynthesis and signaling. Therefore, integrating multi-omics and gene-editing technologies may provide mechanistic insights into the dynamic regulation of the carotenoid-SL network and its role in host-parasitic plant interactions.
5. Conclusions
This study elucidates the molecular mechanisms by which high-pigment tomato mutants confer resistance to P. aegyptiaca through altered carotenoid metabolism and the regulation of the SL biosynthesis pathway. Contrary to the traditional view that carotenoid accumulation promotes SL production, hp-1 exhibits stronger resistance by downregulating key SL biosynthetic genes and reducing germination-stimulating activity in root extracts, despite elevated β-carotene levels. This finding challenges the conventional view that carotenoid accumulation is positively correlated with SL biosynthesis. It provides a new perspective for breeding tomato varieties resistant to Phelipanche spp., suggesting that downregulating SL biosynthetic genes or modulating the conversion efficiency from carotenoids to SLs may enhance resistance while simultaneously improving the nutritional quality of tomato fruits.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15051250/s1, Table S1: qRT-PCR Gene Primer Information.
Author Contributions
Conceptualization, J.L.; methodology, L.S. and X.L. (Xin Li); software, L.S. and J.B.; validation, formal analysis, investigation, L.S., X.L. (Xin Li), J.B., X.L. (Xiaoxiao Lu), C.P., J.H. and C.Z. (Chen Zhang); data curation, L.S. and X.L. (Xin Li); writing—original draft preparation, L.S. and J.L.; writing—review and editing, L.S., X.L. (Xin Li), L.L. and J.L.; visualization, L.S.; supervision, X.L. (Xin Li), C.Z. (Can Zhu), Y.G., X.W., Z.H., Y.D., L.L. and J.L.; project administration, L.S., X.L. (Xin Li), L.L. and J.L.; funding acquisition, X.L. (Xin Li), L.L. and J.L. All authors have read and agreed to the published version of the manuscript.
Funding
Postdoctoral Program of Chalkis Health Industry Co., Ltd.; Technology Innovation Program in Xinjiang Production and Construction Corps (Grant Number: 2024AB022); China and the Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (Grant Number CAASASTIP-IVFCAAS).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the Tomato Genetics Resource Center for providing seed stocks.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Karimmojeni, H.; Ehtemam, M.H.; Javadimoghadam, S.; Shahbazi, S.; Bazrafshan, A.H. Egyptian Broomrape (Phelipanche Aegyptiaca) Response to Silicon Nutrition in Tomato (Solanum lycopersicum L.). Arch. Agron. Soil Sci. 2017, 63, 612–618. [Google Scholar] [CrossRef]
- Xu, Y.X.; Zhang, J.X.; Ma, C.R.; Lei, Y.T.; Shen, G.J.; Jin, J.J.; Eaton, D.A.R.; Wu, J.Q. Comparative Genomics of Orobanchaceous Species with Different Parasitic Lifestyles Reveals the Origin and Stepwise Evolution of Plant Parasitism. Mol. Plant 2022, 15, 1384–1399. [Google Scholar] [CrossRef]
- Gibot-Leclerc, S.; Sallé, G.; Reboud, X.; Moreau, D. What Are the Traits of Phelipanche Ramosa (L.) Pomel That Contribute to the Success of Its Biological Cycle on Its Host Brassica napus L.? Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 512–521. [Google Scholar] [CrossRef]
- López-Ráez, J.A.; Charnikhova, T.; Gómez-Roldán, V.; Matusova, R.; Kohlen, W.; De Vos, R.; Verstappen, F.; Puech-Pages, V.; Bécard, G.; Mulder, P.; et al. Tomato Strigolactones Are Derived from Carotenoids and Their Biosynthesis Is Promoted by Phosphate Starvation. New Phytol. 2008, 178, 863–874. [Google Scholar] [CrossRef]
- Alder, A.; Jamil, M.; Marzorati, M.; Bruno, M.; Vermathen, M.; Bigler, P.; Ghisla, S.; Bouwmeester, H.; Beyer, P.; Al-Babili, S. The Path from β-Carotene to Carlactone, a Strigolactone-like Plant Hormone. Science 2012, 335, 1348–1351. [Google Scholar] [CrossRef]
- Seto, Y.; Sado, A.; Asami, K.; Hanada, A.; Umehara, M.; Akiyama, K.; Yamaguchi, S. Carlactone Is an Endogenous Biosynthetic Precursor for Strigolactones. Proc. Natl. Acad. Sci. USA 2014, 111, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-H.; Zhou, X.E.; Wu, Z.-S.; Yi, W.; Xu, Y.; Li, S.L.; Xu, T.-H.; Liu, Y.; Chen, R.-Z.; Kovach, A.; et al. Crystal Structures of Two Phytohormone Signal-Transducing α/β Hydrolases: Karrikin-Signaling KAI2 and Strigolactone-Signaling DWARF14. Cell Res. 2013, 23, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Park, Y.H.; Kang, J.S.; Choi, Y.W.; Son, B.G. A Gene-Based dCAPS Marker for Selecting Old-Gold-Crimson (Ogc) Fruit Color Mutation in Tomato. J. Life Sci. 2009, 19, 152–155. [Google Scholar] [CrossRef]
- Ronen, G.; Carmel-Goren, L.; Zamir, D.; Hirschberg, J. An Alternative Pathway to Beta -Carotene Formation in Plant Chromoplasts Discovered by Map-Based Cloning of Beta and Old-Gold Color Mutations in Tomato. Proc. Natl. Acad. Sci. USA 2000, 97, 11102–11107. [Google Scholar] [CrossRef]
- Andrade, T.M.; Maluf, W.R.; de Oliveira, C.M.; Gomes, L.A.A.; Santos, D.C.; Carvalho, R.d.C.; Gonçalves, R.J.d.S.; Gonçalves Neto, Á.C. Interaction of the Mutant Genes B, Ogc, Hp and t in the Coloring of Tomato Fruit. Euphytica 2015, 205, 773–783. [Google Scholar] [CrossRef]
- Lieberman, M.; Segev, O.; Gilboa, N.; Lalazar, A.; Levin, I. The Tomato Homolog of the Gene Encoding UV-Damaged DNA Binding Protein 1 (DDB1) Underlined as the Gene That Causes the High Pigment-1 Mutant Phenotype. Theor. Appl. Genet. 2004, 108, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, J.; Tohge, T.; Alba, R.; Osorio, S.; Caldana, C.; McQuinn, R.; Arvidsson, S.; van der Merwe, M.J.; Riaño-Pachón, D.M.; Mueller-Roeber, B.; et al. Combined Transcription Factor Profiling, Microarray Analysis and Metabolite Profiling Reveals the Transcriptional Control of Metabolic Shifts Occurring during Tomato Fruit Development. Plant J. Cell Mol. Biol. 2011, 68, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Tokasi, S.; Aval, M.B.; Mashhadi, H.R.; Ghanbari, A. Screening of Resistance to Egyptian Broomrape Infection in Tomato Varieties. Planta Dan. 2014, 32, 109–116. [Google Scholar] [CrossRef]
- Bai, J.R.; Wei, Q.; Shu, J.S.; Gan, Z.X.; Li, B.J.; Yan, D.L.; Huang, Z.J.; Guo, Y.M.; Wang, X.X.; Zhang, L.X.; et al. Exploration of Resistance to Phelipanche Aegyptiaca in Tomato. Pest Manag. Sci. 2020, 76, 3806–3821. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Yu, R.; Ma, Y.; Zhang, W.; McErlean, C.S.P. Extracts from Cotton over the Whole Growing Season Induce Orobanche Cumana (Sunflower Broomrape) Germination with Significant Cultivar Interactions. Front. Agric. Sci. Eng. 2017, 4, 228. [Google Scholar] [CrossRef]
- Yoneyama, K.; Xie, X.N.; Kusumoto, D.; Sekimoto, H.; Sugimoto, Y.; Takeuchi, Y.; Yoneyama, K. Nitrogen Deficiency as Well as Phosphorus Deficiency in Sorghum Promotes the Production and Exudation of 5-Deoxystrigol, the Host Recognition Signal for Arbuscular Mycorrhizal Fungi and Root Parasites. Planta 2007, 227, 125–132. [Google Scholar] [CrossRef]
- Rotenberg, D.; Thompson, T.S.; German, T.L.; Willis, D.K. Methods for Effective Real-Time RT-PCR Analysis of Virus-Induced Gene Silencing. J. Virol. Methods 2006, 138, 49–59. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, A.Q.; Chen, D.Y.; Ma, Q.Y.; Rose, J.K.C.; Fei, Z.J.; Liu, Y.S.; Giovannoni, J.J. The Tomato HIGH PIGMENT1/DAMAGED DNA BINDING PROTEIN 1 Gene Contributes to Regulation of Fruit Ripening. Hortic. Res. 2019, 6, 15. [Google Scholar] [CrossRef]
- Bino, R.J.; De Vos, C.H.R.; Lieberman, M.; Hall, R.D.; Bovy, A.; Jonker, H.H.; Tikunov, Y.; Lommen, A.; Moco, S.; Levin, I. The Light-Hyperresponsive High Pigment-2dg Mutation of Tomato: Alterations in the Fruit Metabolome. New Phytol. 2005, 166, 427–438. [Google Scholar] [CrossRef]
- Levin, I.; De Vos, R.; Tadmor, Y.; Bovy, A.; Lieberman-Lazarovich, M.; Oren-Shamir, M.; Segev, O.; Kolotilin, I.; Keller, M.; Ovadia, R.; et al. High pigment tomato mutants—More than just lycopene (a review). Isr. J. Plant Sci. 2006, 54, 179–190. [Google Scholar] [CrossRef]
- Abe, S.; Sado, A.; Tanaka, K.; Kisugi, T.; Asami, K.; Ota, S.; Kim, H.I.; Yoneyama, K.; Xie, X.; Ohnishi, T.; et al. Carlactone Is Converted to Carlactonoic Acid by MAX1 in Arabidopsis and Its Methyl Ester Can Directly Interact with AtD14 In Vitro. Proc. Natl. Acad. Sci. USA 2014, 111, 18084–18089. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Ueno, K.; Sugimoto, Y. Structure Elucidation and Biosynthesis of Orobanchol. Front. Plant Sci. 2022, 13, 835160. [Google Scholar] [CrossRef]
- López-Ráez, J.A.; Charnikhova, T.; Mulder, P.; Kohlen, W.; Bino, R.; Levin, I.; Bouwmeester, H. Susceptibility of the Tomato Mutant High Pigment-2dg (Hp-2dg) to Orobanche Spp. Infection. J. Agric. Food Chem. 2008, 56, 6326–6332. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Abuauf, H.; Song, S.; Wang, J.Y.; Alagoz, Y.; Moreno, J.C.; Mi, J.; Ablazov, A.; Jamil, M.; Ali, S.; et al. The Arabidopsis D27-LIKE1 Is a Cis/Cis/Trans-β-Carotene Isomerase That Contributes to Strigolactone Biosynthesis and Negatively Impacts ABA Level. Plant J. Cell Mol. Biol. 2023, 113, 986–1003. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, Q.; Yan, J.; Sun, K.; Liang, Y.; Jia, M.; Meng, X.; Fang, S.; Wang, Y.; Jing, Y.; et al. ζ-Carotene Isomerase Suppresses Tillering in Rice through the Coordinated Biosynthesis of Strigolactone and Abscisic Acid. Mol. Plant 2020, 13, 1784–1801. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Awad, A.A.; Xie, X.N.; Yoneyama, K.; Takeuchi, Y. Strigolactones as Germination Stimulants for Root Parasitic Plants. Plant Cell Physiol. 2010, 51, 1095–1103. [Google Scholar] [CrossRef]
- Gobena, D.; Shimels, M.; Rich, P.J.; Ruyter-Spira, C.; Bouwmeester, H.; Kanuganti, S.; Mengiste, T.; Ejeta, G. Mutation in Sorghum LOW GERMINATION STIMULANT 1 Alters Strigolactones and Causes Striga Resistance. Proc. Natl. Acad. Sci. USA 2017, 114, 4471–4476. [Google Scholar] [CrossRef]
- Dor, E.; Yoneyama, K.; Wininger, S.; Kapulnik, Y.; Yoneyama, K.; Koltai, H.; Xie, X.N.; Hershenhorn, J. Strigolactone Deficiency Confers Resistance in Tomato Line SL-ORT1 to the Parasitic Weeds Phelipanche and Orobanche Spp. Phytopathology 2011, 101, 213–222. [Google Scholar] [CrossRef]
- Kohlen, W.; Charnikhova, T.; Lammers, M.; Pollina, T.; Tóth, P.; Haider, I.; Pozo, M.J.; de Maagd, R.A.; Ruyter-Spira, C.; Bouwmeester, H.J.; et al. The Tomato Carotenoid Cleavage Dioxygenase8 (SlCCD8) Regulates Rhizosphere Signaling, Plant Architecture and Affects Reproductive Development through Strigolactone Biosynthesis. New Phytol. 2012, 196, 535–547. [Google Scholar] [CrossRef]
- Bari, V.K.; Nassar, J.A.; Kheredin, S.M.; Gal-On, A.; Ron, M.; Britt, A.; Steele, D.; Yoder, J.; Aly, R. CRISPR/Cas9-Mediated Mutagenesis of CAROTENOID CLEAVAGE DIOXYGENASE 8 in Tomato Provides Resistance against the Parasitic Weed Phelipanche Aegyptiaca. Sci. Rep. 2019, 9, 11438. [Google Scholar] [CrossRef] [PubMed]
- Ban, X.W.; Qin, L.; Yan, J.J.; Wu, J.X.; Li, Q.J.; Su, X.; Hao, Y.R.; Hu, Q.L.; Kou, L.Q.; Yan, Z.Y.; et al. Manipulation of a Strigolactone Transporter in Tomato Confers Resistance to the Parasitic Weed Broomrape. Innovation 2025, 6, 100815. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, B.; Yu, H.; Guo, H.Y.; Lin, T.; Kou, L.Q.; Wang, A.Q.; Shao, N.; Ma, H.Y.; Xiong, G.S.; et al. Transcriptional Regulation of Strigolactone Signalling in Arabidopsis. Nature 2020, 583, 277–281. [Google Scholar] [CrossRef]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.-P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.-C.; et al. Strigolactone Inhibition of Shoot Branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Alam, P.; Chen, Y.; Ahmad, P. Strigolactones in Plants: From Development to Abiotic Stress Management. J. Plant Growth Regul. 2024, 43, 903–919. [Google Scholar] [CrossRef]
- Yuan, K.; Zhang, H.; Yu, C.; Luo, N.; Yan, J.; Zheng, S.; Hu, Q.; Zhang, D.; Kou, L.; Meng, X.; et al. Low Phosphorus Promotes NSP1–NSP2 Heterodimerization to Enhance Strigolactone Biosynthesis and Regulate Shoot and Root Architecture in Rice. Mol. Plant 2023, 16, 1811–1831. [Google Scholar] [CrossRef]
- Kodama, K.; Rich, M.K.; Yoda, A.; Shimazaki, S.; Xie, X.; Akiyama, K.; Mizuno, Y.; Komatsu, A.; Luo, Y.; Suzuki, H.; et al. An Ancestral Function of Strigolactones as Symbiotic Rhizosphere Signals. Nat. Commun. 2022, 13, 3974. [Google Scholar] [CrossRef]
- Omoarelojie, L.O.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. Strigolactones and Their Crosstalk with Other Phytohormones. Ann. Bot. 2019, 124, 749–767. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).