Antifungal Activity of Oregano Essential Oil Against Fusarium oxysporum f. sp. cubense Race 1 and Fusarium Wilt Disease on Silk Banana Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Essential Oil Extraction
2.2. Chemical Characterization
2.3. Plant Pathogenic Fungi
2.4. In Vitro Evaluation of Antifungal Activity
2.5. Determination of Antifungal Activity on Fusarium Wilt Control
2.6. Disease Index
2.7. Statistical Analysis
3. Results
3.1. Composition of Oregano Essential Oil
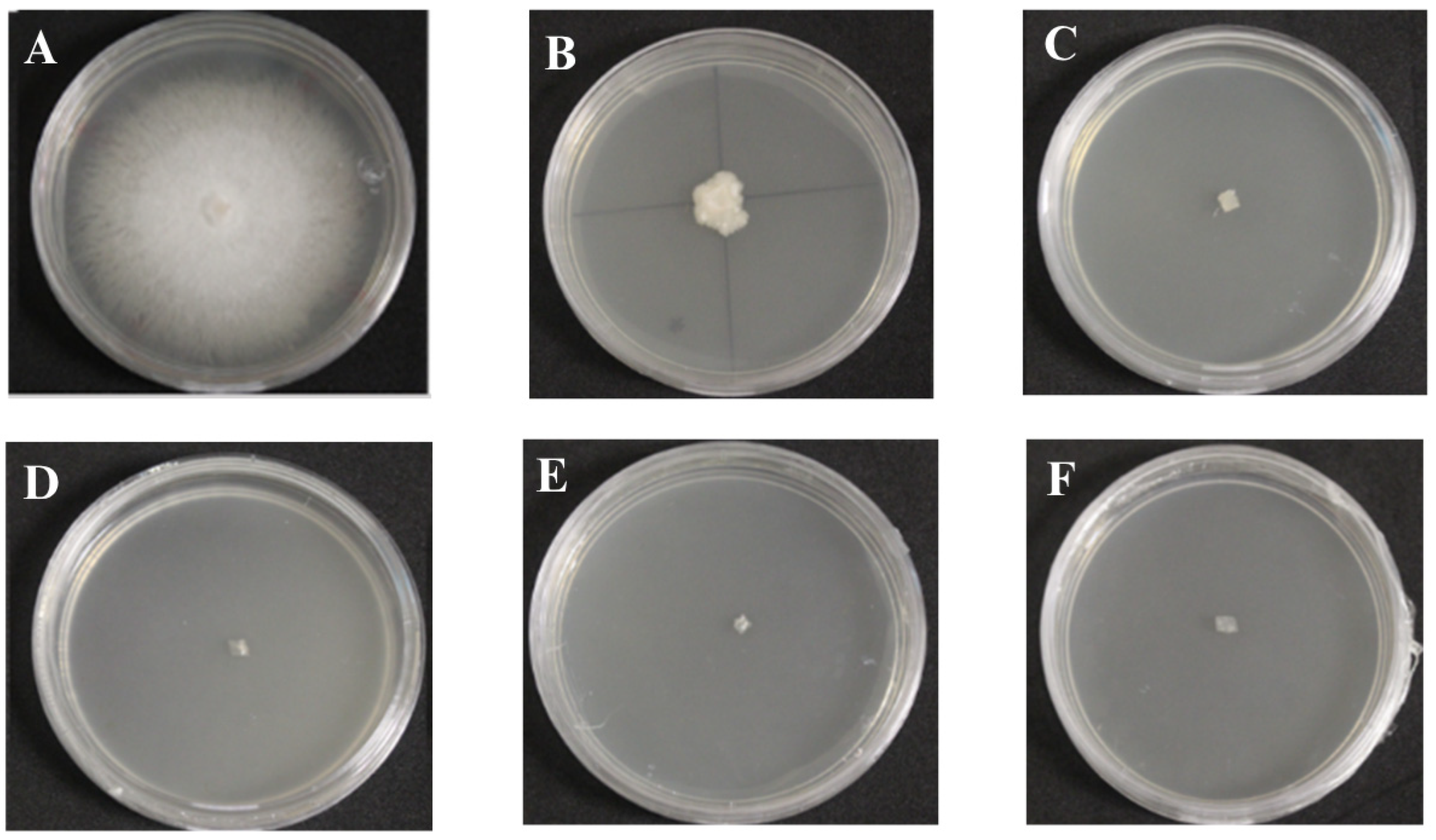
3.2. Antifungal Assays on Fusarium oxysporum f. sp. Cubense Race 1
3.3. In Vivo Antifungal Activity of Oregano Essential Oil Against FOC Race 1
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Organización de las Naciones Unidas para la Alimentación y la Agricultura (FAO). FAOSTAT, Cultivos y Productos de Ganadería. Available online: https://www.fao.org/faostat/es/#data/QCL (accessed on 5 August 2025).
- Dirección General del Servicio de Información Agroalimentaria y Pesquera (DGSIAP). SIAP. Anuario Estadístico de la Producción Agrícola. Available online: https://nube.agricultura.gob.mx/cierre_agricola/ (accessed on 10 August 2025).
- Maryani, N.; Lombard, L.; Poerba, Y.S.; Subandiyah, S.; Crous, P.W.; Kema, G.H.J. Phylogeny and genetic diversity of the banana Fusarium wilt pathogen Fusarium oxysporum f. sp. cubense in the Indonesian centre of origin. Stud. Mycol. 2019, 92, 155–194. [Google Scholar] [CrossRef]
- Ploetz, R.; Kema, G.H.J.; Ma, L.J. Impact of diseases on export and smallholder production of banana. Annu. Rev. Phytopathol. 2015, 53, 269–288. [Google Scholar] [CrossRef]
- Kaliapan, K.; Mazlin, S.N.A.; Chua, K.O.; Rejab, N.A.; Mohd-Yusuf, Y. Secreted in Xylem (SIX) genes in Fusarium oxysporum f. sp. cubense (Foc) unravels the potential biomarkers for early detection of Fusarium wilt disease. Arch. Microbiol. 2024, 206, 271. [Google Scholar] [CrossRef]
- Molina, A.B.; Fabregar, E.; Sinohin, V.G.; Yi, G.; Viljoen, A. Recent Occurrence of Fusarium oxysporum f. sp. cubense Tropical Race 4 in Asia. Acta Hortic. 2009, 828, 109–114. [Google Scholar] [CrossRef]
- Viljoen, A.; Mostert, D.; Chiconela, T.; Beukes, I.; Fraser, C.; Dwyer, J.; Murray, H.; Amisse, J.; Matabuana, E.L.; Tazan, G.; et al. Occurrence and spread of the banana fungus Fusarium oxysporum f. sp. cubense TR4 in Mozambique. S. Afr. J. Sci. 2020, 116, 88–98. [Google Scholar] [CrossRef]
- García, B.F.A.; Quintero, V.J.C.; Ayala, V.M.; Schermer, T.; Seidi, M.F.; Santos, P.M.; Noguera, A.M.; Aguilera, G.C.; Wittenberg, A.; Hofstede, R.; et al. First report of Fusarium wilt tropical race 4 in Cavendish bananas caused by Fusarium odoratissimum in Colombia. Plant Dis. 2019, 104, 994. [Google Scholar] [CrossRef]
- Acuña, R.; Rouard, M.; Leiva, A.M.; Marques, C.; Olortegui, J.A.; Ureta, C.; Cabrera-Pintado, R.M.; Rojas, J.C.; Lopez-Alvarez, D.; Cenci, A.; et al. First report of Fusarium oxysporum f. sp. cubense Tropical Race 4 causing Fusarium wilt in Cavendish bananas in Peru. Plant Dis. 2022, 106, 2268. [Google Scholar] [CrossRef]
- Mejías, H.R.; Hernández, Y.; Magdama, F.; Mostert, D.; Bothma, S.; Paredes, S.E.M.; Terán, D.; González, E.; Angulo, R.; Angel, L.; et al. First Report of Fusarium wilt of Cavendish bananas caused by Fusarium oxysporum f. sp. cubense Tropical Race 4 in Venezuela. Plant Dis. 2023, 107, 3297. [Google Scholar] [CrossRef]
- Martínez, G.; Olivares, B.O.; Rey, J.C.; Rojas, J.; Cardenas, J.; Puentes, C.; Dawson, C. The advance of Fusarium wilt Tropical Race 4 in musaceae of Latin America and the Caribbean: Current situation. Pathogens 2023, 12, 277. [Google Scholar] [CrossRef]
- Ndayihanzamaso, P.; Karangwa, P.; Mostert, D.; Mahuku, G.; Blomme, G.; Swennen, R.; Viljoen, A. The development of a multiplex PCR assay for the detection of Fusarium oxysporum f. sp. cubense lineage VI strain in East and Central Africa. Eur. J. Plant Pathol. 2020, 158, 495–509. [Google Scholar] [CrossRef]
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.S.G.; Staver, C.P. Fusarium Wilt of Banana: Current Knowledge on Epidemiology and Research Needs Toward Sustainable Disease Management. Front. Plant Sci. 2018, 9, 1468. [Google Scholar] [CrossRef]
- Fang, H.; Zhong, C.; Sun, J.; Chen, H. Revealing the different mechanisms of banana ‘Guijiao 9’to Fusarium oxysporum f. sp. cubense tropical race 4 using comparative proteomic analysis. J. Proteom. 2023, 283–284, 104937. [Google Scholar] [CrossRef]
- Mintoff, S.J.L.; Nguyen, T.V.; Kelly, C.; Cullen, S.; Hearden, M.; Williams, R.; Daniells, J.W.; Tran-Nguyen, T.T. Banana cultivar field screening for resistance to Fusarium oxysporum f. sp. cubense Tropical race 4 in the Northern territory. J. Fungi 2021, 7, 627. [Google Scholar] [CrossRef]
- Dale, J.; James, A.; Paul, J.Y.; Khanna, H.; Smith, M.; Peraza-Echeverria, S.; García-Bastidas, F.; Kema, G.; Waterhouse, P.; Mengersen, K.; et al. Transgenic Cavendish bananas with resistance to Fusarium wilt tropical race 4. Nat. Commun. 2017, 8, 1496. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-García, L.F.; Carmona-Gutierrez, S.L.; Moreno-Velandia, C.A.; Villarreal-Navarrete, A.D.P.; Burbano-David, D.M.; Quiroga-Mateus, R.Y.; Gómez-Marroquín, M.R.; Rodríguez-Yzquierdo, G.A.; Betancourt-Vásquez, M. Microbial based biofungicides mitigate the damage caused by Fusarium oxysporum f. sp. cubense race 1 and improve the physiological performance in banana. J. Fungi 2024, 10, 419. [Google Scholar] [CrossRef]
- Wang, J.; Cai, B.; Li, K.; Zhao, Y.; Li, C.; Liu, S.; Xiang, D.; Zhang, L.; Xie, J.; Wang, W. Biological control of Fusarium oxysporum f. sp. cubense Tropical Race 4 in banana plantlets using newly isolated Streptomycin sp. WHL7 from marine soft coral. Plant Dis. 2022, 106, 254–259. [Google Scholar] [CrossRef]
- Lopez-Moya, F.; Zorrilla-Fontanesi, Y.; Lozano-Soria, A.; Ganado, N.F.L.; Moreno-Gonzalez, C.M.; Hernández, A.; Torres, A.; Gonzalez-Silvera, D.; Gunsé, B.; López-Jiménez, J.A.; et al. Chitosan induces salicylic acid and methyl salicylate in banana plants and reduces colonization by Fusarium oxysporum f. sp. cubense TR4. Curr. Plant Biol. 2025, 42, 100457. [Google Scholar] [CrossRef]
- Yang, J.; Duan, Y.; Liu, X.; Sun, M.; Wang, Y.; Liu, M.; Zhu, Z.; Shen, Z.; Gao, W.; Wang, B.; et al. Reduction of banana Fusarium wilt associated with soil microbiome reconstruction through green mature intercropping. Agric. Ecosyst. Environ. 2022, 337, 108065. [Google Scholar] [CrossRef]
- Zhang, H.; Mallik, A.; Zeng, R.S. Control of Panama Disease of banana by rotating and intercropping with Chinese Chive (Allium tuberosum Rottler): Role of plant volatiles. J. Chem. Ecol. 2013, 39, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Ju, H.; Lu, M.; Wang, B.; Zhao, Y.; Ruan, Y. Significant decline in banana Fusarium wilt disease is associated with soil microbiome reconstruction under chili pepper-banana rotation. Eur. J. Soil Biol. 2020, 97, 103154. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, D.; Shan, S.; Zhang, W.; Li, R.; Zhang, A. Fluorine-containing amphiphilic quaternary ammonium salts for the suppression of Banana fusarium wilt. React. Funct. Polym. 2022, 182, 105488. [Google Scholar] [CrossRef]
- Salacinas, M.; Meijer, H.J.G.; Mamora, S.H.; Corcolon, B.; Gohari, A.M.; Ghimire, B.; Kema, G.H.J. Efficacy of disinfectants against Tropical Race 4 causing Fusarium wilt in Cavendish bananas. Plant Dis. 2022, 106, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Cannon, S.; Kay, W.; Kilaru, S.; Schuster, M.; Gurr, S.J.; Steinberg, G. Multi-site fungicides suppress banana Panama disease, caused by Fusarium oxysporum f. sp. cubense Tropical Race 4. PLoS Pathog. 2022, 18, e1010860. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the bacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Sivakumar, D.; Bautista, B.S. A review on the use of essential oils for post harvest decay control and maintenance of fruit quality during storage. Crop Prot. 2014, 64, 27–37. [Google Scholar] [CrossRef]
- Li, Z.; Wang, T.; He, C.; Cheng, K.; Zeng, R.; Song, Y. Control of Panama disease of banana by intercropping with Chinese chive (Allium tuberosum Rottler): Cultivar differences. BMC Plant Biol. 2020, 20, 432. [Google Scholar] [CrossRef] [PubMed]
- Paramalingam, P.; Baharum, N.A.; Abdullah, J.O.; Hong, J.K.; Saidi, N.B. Antifungal potential of Melaleuca alternifolia against fungal pathogen Fusarium oxysporum f. sp. cubense Tropical Race 4. Molecules 2023, 28, 4456. [Google Scholar] [CrossRef]
- Abdullahi, A.; Khairulmazmi, A.; Yasmeen, S.; Ismail, I.S.; Norhayu, A.; Sulaiman, M.R.; Ahmed, O.H.; Ismail, M.R. Phytochemical profiling and antimicrobial activity of ginger (Zingiber officinale) essential oils against important phytopathogens. Arab. J. Chem. 2020, 13, 8012–8025. [Google Scholar] [CrossRef]
- Ramírez-Mejía, J.M.; Aguilera-Galvez, C.; Kema, G.H.J.; Valencia-Riascos, L.M.; Zapata-Henao, S.; Gómez, L.A.; Villegas-Escobar, V. Combining cyclic lipopeptides and cinnamon extract enhance antifungal activity against Fusarium oxysporum strains pathogenic to banana and delay Fusarium wilt under greenhouse conditions. Trop. Plant Pathol. 2024, 49, 838–849. [Google Scholar] [CrossRef]
- Goncalves, D.C.; de Queiroz, V.T.; Costa, A.V.; Lima, W.P.; Belan, L.L.; Moraes, W.B.; Iorio, N.L.P.P.; Póvoa, H.C.C. Reduction of Fusarium wilt symptoms in tomato seedlings following seed treatment with Origanum vulgare L. Essential oil and carvacrol. Crop Prot. 2021, 141, 105487. [Google Scholar] [CrossRef]
- Sarathambal, C.; Rajagopal, S.; Viswanathan, R. Mechanism of antioxidant and antifungal properties of Pimenta dioica (L.) leaf essential oil on Aspergillus flavus. J. Food Sci. Technol. 2020, 58, 2497–2506. [Google Scholar] [CrossRef]
- Kamsu, N.P.; Tchinda, S.E.; Tchamenj, N.S.; Jazet, D.P.M.; Madjouko, M.A.; Youassi, O.Y.; Sameza, M.L.; Tchoumbougnang, F.; Menut, C. Antifungal activities of essential oils of cinnamon (Cinnamomum zeylanicum) and lemongrass (Cymbopogon citratus) on crown rot pathogens of banana. Indian Phytopathol. 2018, 72, 131–137. [Google Scholar] [CrossRef]
- Orr, R.; Nelson, P.N. Eucalyptus cloeziana mulch suppresses Fusarium wilt of banana. Crop Prot. 2021, 147, 105694. [Google Scholar] [CrossRef]
- Asbahani, A.E.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; Mousadik, A.E.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing: Oxford, UK, 2006. [Google Scholar] [CrossRef]
- Li, W.; Dita, M.; Wu, W.; Hu, G.; Xie, J.; Ge, X.J. Resistance sources to Fusarium oxysporum f. sp. cubense tropical race 4 in banana wild relatives. Plant Pathol. 2014, 64, 1061–1067. [Google Scholar] [CrossRef]
- Thomidis, T.; Filotheou, A. Evaluation of five essential oils as bio-fungicides on the control of Pilidiella granati rot in pomegranate. Crop Prot. 2017, 89, 66–71. [Google Scholar] [CrossRef]
- Pola, C.C.; Medeiros, E.A.A.; Pereira, O.L.; Souza, V.G.L.; Otoni, C.G.; Camilloto, G.P.; Soares, N.F.F. Cellulose acetate active films incorporated with oregano (Origanum vulgare) essential oil and organophilic montmorillonite clay control the growth of phytophatogenic fungi. Food Packag. Shelf Life 2016, 9, 69–78. [Google Scholar] [CrossRef]
- Lavin, P.; Gómez, S.S.; Guiamet, P. Scopulariopsis sp. and Fusarium sp, in the documentary heritage: Evaluation of their biodeterioration ability and antifungal effect of two essential oils. Microb. Ecol. 2016, 71, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Valente, J.S.; Fonseca, A.O.S.; Denardi, L.B.; Ben, V.S.D.; Filho, F.S.M.; Baptista, C.T.; Braga, C.Q.; Zambrano, C.G.; Alves, S.H.; Botton, S.A.; et al. In vitro susceptibility of Pythium insidiosum to Melaleuca alternifolia, Mentha piperita and Origanum vulgare essential oils combinations. Mycopathologia 2016, 181, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Wang, R.C.; Li, C.H.; Zuo, C.W.; Wei, Y.R.; Zhang, L.; Yi, G.J. Control of Fusarium wilt in banana with Chinese leek. Eur. J. Plant Pathol. 2012, 134, 87–95. [Google Scholar] [CrossRef]
- Dima, C.; Cotarlet, M.; Alexe, P.; Dima, S. Reprint of “Microencapsulation of essential oil of pimento (Pimenta dioica (L) Merr.) by chitosan/k-carrageenan complex coacervation method”. Inn. Food Sci. Emerg. Technol. 2014, 25, 97–105. [Google Scholar] [CrossRef]
- Monteiro, O.S.; Souza, A.G.; Soledade, L.E.B.; Queiroz, N.; Souza, A.L.; Filho, V.E.M.; Vasconcelos, A.F.F. Chemical evaluation and thermal analysis of the essential oil from the fruits of the vegetable species Pimenta dioica Lindl. J. Therm. Anal. Calorim. 2011, 106, 595–600. [Google Scholar] [CrossRef]
- Jiang, Z.T.; Feng, X.; Li, R.; Wang, Y. Composition comparison of essential oils extracted by classical hydro distillation and microwave-assisted hydrodistillation from Pimenta dioica. J. Essent. Oil-Bear. Plants 2013, 16, 45–50. [Google Scholar] [CrossRef]
- De Elguea-Culebras, G.O.; Panamá_Tapia, L.A.; Melero-Bravo, E.; Cerro-Ibáñez, N.; Calvo-Martínez, A.; Sánchez-Vioque, R. Comparison of the phenolic composition and biological capacities of wastewater from Origanum vulgare L., Rosmarinus officialIs L., Salvia lavandulifolia Vahl. and Thymus mastichina L. resulting from two hydrostillation systems: Clevenger and MAE. J. Appl. Res. Med. Aromat. Plants 2023, 34, 100480. [Google Scholar] [CrossRef]
- Marques, C.S.; Carvalho, S.G.; Bertoli, L.D.; Villanova, J.C.O.; Pinheiro, P.F.; dos Santos, D.C.M.; Yoshida, M.I.; de Freitas, J.C.C.; Cipriano, D.F.; Bernardes, P.C. B-Cyclodextrin inclusion complexes with essential oils: Obtention, characterization, antimicrobial activity and potential application for food preservative sachets. Food Res. Int. 2019, 119, 499–509. [Google Scholar] [CrossRef]
- Pezzani, R.; Vitalini, S.; Iriti, M. Bioactivities of Origanum vulgare L.: An update. Phytochem. Rev. 2017, 16, 1253–1268. [Google Scholar] [CrossRef]
- Zhang, M.; Ge, J.; Yu, X. Transcriptome analysis reveals the mechanism of fungicidal of thymol against Fusarium oxysporum f. sp. niveum. Curr. Microbiol. 2018, 75, 410–419. [Google Scholar] [CrossRef]



| Compound | Concentration (%) | TR * (min) |
| 1-Methyl-4-(1-methylethenyl)-cyclohexene (D-Limonene) | 6.19 | 7.13 |
| β-cis-Terpineol | 2.20 | 7.85 |
| 5-Isopropyl-2-methylphenol (Carvacrol) | 1.06 | 12.28 |
| 3-Allyl-6-methoxyphenol (Eugenol) | 76.31 | 14.64 |
| α-Bergamotene | 3.10 | 17.27 |
| 3-tert-butil-4-hidroxianisole | 2.35 | 18.28 |
| Eucalyptol | 0.24 | 19.18 |
| β-Bisaboleno | 0.44 | 19.65 |
| β-Tujeno | 0.80 | 6.41 |
| o-Cimeno | 0.63 | 6.95 |
| Borneol | 0.56 | 8.75 |
| 3-Tujen-2-ona | 0.46 | 10.67 |
| Metileugenol | 0.39 | 15.86 |
| P-thymol | 0.21 | 14.30 |
| Caryophilen | 0.21 | 16.82 |
| 2-isopropil-5-methylisol | 0.23 | 10.74 |
| 4-tert-Butilcatecol | 0.21 | 17.62 |
| Caryophilen oxid | 0.27 | 22.28 |
| β-Bisabolen | 0.44 | 19.65 |
| α-Cariophylen | 0.89 | 17.81 |
| β-cis-Ocimen | 0.39 | 7.27 |
| Viridifloreno | 0.79 | 19.18 |
| Essential Oil | EO Concentration (μL L−1)/FOC Race 1 Inhibition of Mycelial Growth (%) | Pr > F | ||||
|---|---|---|---|---|---|---|
| 100 | 500 | 1000 | 3000 | 5000 | ||
| O. vulgare | 34.8 b * | 100 ± 0.0 a | 100 a | 100 a | 100 a | 0.0001 |
| Essential Oil | B ± EE & | MIC (μL L−1) * | P > X2 *** | |
|---|---|---|---|---|
| MIC50 * | MIC95 ** | |||
| O. vulgare | 2.31 ± 0.11 | 111.2 (104.1–118.9) | 174.1 (155.9–208.2) | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oltehua-Vázquez, M.; Martínez-Bolaños, S.S.; López-Martínez, V.; Diaz-Trujillo, C.; Orozco-Santos, M.; Vallejo-Pérez, M.R.; Manzo-Sánchez, G.; Martínez-Bolaños, L. Antifungal Activity of Oregano Essential Oil Against Fusarium oxysporum f. sp. cubense Race 1 and Fusarium Wilt Disease on Silk Banana Plants. Agronomy 2025, 15, 2682. https://doi.org/10.3390/agronomy15122682
Oltehua-Vázquez M, Martínez-Bolaños SS, López-Martínez V, Diaz-Trujillo C, Orozco-Santos M, Vallejo-Pérez MR, Manzo-Sánchez G, Martínez-Bolaños L. Antifungal Activity of Oregano Essential Oil Against Fusarium oxysporum f. sp. cubense Race 1 and Fusarium Wilt Disease on Silk Banana Plants. Agronomy. 2025; 15(12):2682. https://doi.org/10.3390/agronomy15122682
Chicago/Turabian StyleOltehua-Vázquez, Marisol, Syl Soledad Martínez-Bolaños, Victor López-Martínez, Caucasella Diaz-Trujillo, Mario Orozco-Santos, Moisés Roberto Vallejo-Pérez, Gilberto Manzo-Sánchez, and Luciano Martínez-Bolaños. 2025. "Antifungal Activity of Oregano Essential Oil Against Fusarium oxysporum f. sp. cubense Race 1 and Fusarium Wilt Disease on Silk Banana Plants" Agronomy 15, no. 12: 2682. https://doi.org/10.3390/agronomy15122682
APA StyleOltehua-Vázquez, M., Martínez-Bolaños, S. S., López-Martínez, V., Diaz-Trujillo, C., Orozco-Santos, M., Vallejo-Pérez, M. R., Manzo-Sánchez, G., & Martínez-Bolaños, L. (2025). Antifungal Activity of Oregano Essential Oil Against Fusarium oxysporum f. sp. cubense Race 1 and Fusarium Wilt Disease on Silk Banana Plants. Agronomy, 15(12), 2682. https://doi.org/10.3390/agronomy15122682









