Abstract
Banana is a globally important food crop. As a large herbaceous plant with a shallow root system, its yield is highly susceptible to drought stress. ANP family genes play crucial roles in plant drought resistance. However, the ANP gene family has not been systematically studied in bananas, and how Piriformospora indica (P. indica) induces its expression remains unclear. A comprehensive identification of the ANP family is thus a necessary foundation for functional studies. In this study, we systematically identified 13 ANP family members in banana for the first time through genome-wide analysis. Using bioinformatics, RT-qPCR and subcellular localization techniques, we characterized their structural features, phylogenetic relationships, and the expression patterns of MaNPK1 under drought stress and P. indica colonization. The results revealed that banana ANP family members are highly evolutionarily conserved. MaNPK1-1 was specifically induced and upregulated by P. indica under drought conditions and subcellular localization showed that it played a role in the nucleus. This research provides theoretical insights into the function of the banana ANP family and its regulatory role in the P. indica mediated drought stress response, offering potential applications for breeding stress resistant banana varieties.
1. Introduction
Banana (Musa spp.) is a large herbaceous plant that is recognized by the Food and Agriculture Organization of the United Nations (FAO) as the fourth most important food crop in developing countries [1]. China is the world’s second largest producer of banana. In 2022, the cultivation area of banana in China reached 505,000 hectares, with a total output of 11.77 million tons [2]. These data highlight the importance of the banana industry in China’s agricultural economy. However, as a large monocotyledonous plant with a shallow root system, banana has a high water requirement. Although major growing regions in China, such as Taiwan, Guangxi, and Fujian, receive relatively abundant rainfall, they still experience seasonal droughts in spring and autumn. Compounded by the increased frequency of abnormal drought events due to global warming, drought has become a critical constraint on the sustainable development of the banana industry. The ‘Tianbao Banana’ (AAA group) is Fujian’s main cultivar, covers over 80% of the province’s banana cultivation area and is prized for its high quality. However, it is highly susceptible to drought, with high temperatures and drought conditions causing growth stagnation, leading to a 30–50% yield loss [3]. Conventional hybrid breeding in banana has been slow due to its triploid nature. Therefore, it is of great theoretical significance to discover the stress-resistant gene resources and analyze the mechanism of banana drought resistance domestication for the subsequent cultivation of stress-resistant germplasm through genetic engineering.
The mitogen-activated protein kinase (MAPK) cascade pathway is one of the core signal transduction pathways for plants to respond to external environmental stresses and regulate growth and development. This cascade consists of three key tiers: MAP kinase kinase kinases (MAPKKKs), MAP kinase kinases (MAPKKs), and MAP kinases (MAPKs), which sequentially phosphorylate and activate downstream components to propagate the signal [4,5,6]. Members of the MAPK cascade have been extensively identified in various plant species. A total of 20 MAPKs, 10 MAPKKs, and 80 MAPKKKs were identified in Arabidopsis thaliana [7,8]; the genome of Fagopyrum tataricum encodes 8 MAPKs, 1 MAPKKs, and 56 MAPKKKs [9]; while that of Gossypium hirsutum contains 55 MAPKs, 66 MAPKKs, and 157 MAPKKKs [10,11,12]. In the genome of Populus trichocarpa includes 21 MAPKs, 11 MAPKKs, and 104 MAPKKKs [13]. The MAPKKK family, as the starting node of the cascade reaction, has a larger number of members relative to the MAPKK and MAPK families. This family is responsible for receiving and integrating upstream signals from cell membrane receptors (such as receptor kinases and reactive oxygen species ROS), and precise control of MAPKKK activation is critical for ensuring the specificity and strength of downstream signal propagation [14,15,16].
The NPK1 (nucleus- and phragmoplast-localized protein kinase 1) gene is the first identified gene encoding MAPKKK in plants [17]. It was named for its subcellular localization in the nucleus and phragmoplast and was initially demonstrated to regulate cytokinesis during mitotic anaphase, where it interacts via its C-terminal domain with NACK1 to ensure cell plate formation [18,19,20]. Subsequent studies identified its orthologs in Arabidopsis thaliana, ANP1, ANP2, and ANP3, which together constitute a distinct clade within the MAPKKK phylogenetic tree, referred to as the ANP family [21]. Studies have shown that defects in MAPK signaling in the double recessive mutants of AtANP2 and AtANP3 lead to the inhibition of phosphorylation of microtubule-associated protein MAP65-1, resulting in abnormal cell division [22], which suggests functional conservation between AtANPs and NPK1. However, the functions of ANP family members are not limited to regulating cell division [23]. They serve as key signaling nodes and play pivotal roles in plant responses to abiotic stresses such as drought, salinity, and oxidative stress [24,25,26,27]. The plant cell wall serves as the first barrier against environmental challenges. Studies on the triple mutant of Arabidopsis ANPs have revealed that the loss of ANPs leads to altered cell wall composition and causes developmental defects, indicative of a classical cell wall damage syndrome (CWDS) [28]. Abiotic stresses usually lead to the imbalance of ROS homeostasis in plants. As the key regulators of oxidative stress signaling, AtANP1 is activated by ROS and in turn initiates two downstream stress MAPKs: AtMAPK3 and AtMAPK6. This cascade promotes the removal of intracellular ROS and helps maintain redox homeostasis, thereby mitigating oxidative damage [29]. In addition, ANP family members are also involved in plant hormone signaling through the negative regulation of auxin signaling, which modulates plant developmental processes and defense responses to stress [30].
Currently, the potential of NPK1 in improving drought resistance has been demonstrated in several important crop species. For instance, overexpression of NPK1 in sugarcane (Saccharum spp.) under drought conditions has been shown to improve both yield and quality [31]. In maize (Zea mays L.), overexpression of NPK1 significantly improved yield under drought stress [32,33]. Likewise, transgenic sorghum (Sorghum bicolor L.) overexpressing NPK1 maintained better growth vigor and exhibited enhanced drought tolerance, thereby protecting the plants from drought damage [34]. However, systematic studies on the ANP family in monocots, particularly in the economically important but drought-sensitive banana, remain scarce.
The endophytic fungus Piriformospora indica (P. indica) has been shown to significantly promote plant growth and enhance stress resistance after colonization [35,36,37,38,39]. Its beneficial effects have been demonstrated in a variety of plant species, including strawberry (Fragaria spp.) [40], gerbera (Gerbera hybrida) [41], banana [42], and winter jujube (Ziziphus jujuba Mill. cv. Dongzao) [43]. The colonization by P. indica can mitigate oxidative damage caused by environmental stresses through activation of the host antioxidant system, such as enhancing the activities of antioxidant enzymes and increasing proline content, thereby reducing hydrogen peroxide (H2O2) accumulation [44,45,46]. Similarly, GO and KEGG analysis showed that P. indica could activate the expression of genes related to MAPK cascade signaling pathway to improve the drought resistance of plants [47,48,49]. As an important member of MAPKKK, ANP family genes are located in the upstream of this signaling pathway. Based on previous studies from our research group, it has been shown that colonization of ‘Tianbao Banana’ roots by P. indica effectively improves drought tolerance, and the expression of MaNPK1 was significantly upregulated only under the condition of drought and inoculation of P. indica [50]. Therefore, we speculate that P. indica is likely to improve drought resistance of banana by regulating the ANP gene family member MaNPK1 and activating the downstream MAPK signaling pathway. However, how P. indica regulates drought resistance through modulation of the ANP family members in banana remains unclear.
Therefore, this study aims to identify all members of the ANP family in banana through genome-wide analysis, characterize their gene structures, conserved motifs and cis-acting elements, and investigate the expression pattern of MaNPK1 under combined drought and P. indica treatment. The findings are expected to provide a theoretical basis and candidate genes for molecular breeding of stress-resistant banana.
2. Materials and Methods
2.1. Plant Material and Treatments
The plant material in this study was the ‘Tianbao Banana’ (AAA group, M. acuminata). The RNA samples were obtained from our laboratory’s previous work and had been stored at −80 °C [50]. Banana RNA was extracted using a polysaccharide polyphenol plant total RNA extraction kit (Tiangen, Beijing, China) according to the manufacturer’s instructions. The OD value and concentration of RNA were determined using an ultra-micro spectrophotometer (Thermo Electron Corp, Waltham, MA, USA), and its integrity was detected by using a 1% agarose gel electrophoresis. Plants were subjected to four treatments: CK: well-watered, no P. indica inoculation; H1: well-watered, P. indica inoculation; H2: drought stress, no P. indica inoculation; H3: drought stress, P. indica inoculation. The drought stress treatment was achieved when the wilting state of banana seedlings and the soil was weighed to maintain the soil moisture content at about 30%. The plant remained wilting for three consecutive days and showed no signs of recovery. Then, the soil was rehydrated until the soil moisture content was maintained at about 70% and the wilting state disappears. Three drought–rehydration treatments were repeated.
2.2. Genomic Data Sources
Genome sequences and annotation files for M. acuminata DH-Pahang V4, M. balbisiana PKW, M. itinerans, M. schizocarpa V2, M. textilis V1, and M. cavendish Baxijiao V1 were downloaded from the Banana Genome Hub (https://banana-genome-hub.southgreen.fr/, accessed on 6 June 2025). Protein sequences of ANP family members from Arabidopsis thaliana (AT1G09000, AT1G54960 and AT3G06030) were retrieved from TAIR (https://www.arabidopsis.org/, accessed on 6 June 2025). Genome sequences and annotation files for Zea mays Zm-B73-REFERENCE-NAM-5.0 (maize), Oryza sativa AGIS1.0 (rice), Solanum lycopersicum SLM_r2.1 (tomato), Glycine max V4 (soybean) and protein sequences of ANP family members from Nicotiana tabacum (tobacco, LOC107796336) were obtained from the NCBI database (https://www.ncbi.nlm.nih.gov/, accessed on 6 June 2025).
2.3. Identification of ANP Family Members and Physicochemical Analysis
Using tobacco NPK1 and Arabidopsis ANP proteins as query sequences, 10 genomes were compared using the BLAST (E-value < 1 × e−5) algorithm in TBtools-II v2.114 analysis software [51]. Download the file and deleted the duplicate value of the genome ID column, retained the unique value, and summarized it in the new table. The conserved domain of ANP protein was searched by Pfam website (http://pfam.xfam.org/, accessed on 8 June 2025). Candidate proteins were further screened using NCBI’s Conserved Domain Database (CDD, https://www.ncbi.nlm.nih.gov/cdd, accessed on 8 June 2025) to confirm the presence of the STKc_MAPKKK domain (Accession: cd06606) [52]. Physicochemical properties (number of amino acids, molecular weight, theoretical isoelectric point, instability index, aliphatic index, grand average of hydropathicity) of the identified ANP proteins were predicted using the ProtParam tool on the ExPASy server (https://web.expasy.org/protparam/, accessed on 8 June 2025) [53]. Signal peptides were predicted using SignalP 6.0 (https://services.healthtech.dtu.dk/services/SignalP-6.0/, accessed on 9 June 2025) [54]. Transmembrane helices were predicted using TMHMM 2.0 (https://services.healthtech.dtu.dk/services/TMHMM-2.0/, accessed on 9 June 2025) [55]. Subcellular localization was predicted using Plant-mPLoc (available within the Cell-PLoc 2.0 suite: http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/, accessed on 9 June 2025) [56]. Secondary structure was predicted using SOPMA (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_sopma.html, accessed on 9 June 2025) [57]. Phosphorylation sites were predicted using NetPhos 3.1 (https://services.healthtech.dtu.dk/services/NetPhos-3.1/, accessed on 9 June 2025) with a prediction threshold > 0.5 [58].
2.4. Gene Structure and Conserved Motif Analysis
Gene structure diagrams were generated using TBtools-II v2.114 based on the respective genome annotation files (Gff3 format). Conserved protein motifs were identified using the MEME suite (https://meme-suite.org/meme/tools/meme, accessed on 10 June 2025) with the following parameters: maximum number of motifs is 20, Motif Site Distribution: ZOOPS, motif width range is 6–50 aa) [59]. The resulting MEME XML output file was imported into TBtools-II v2.114 for visualization alongside the gene structure.
2.5. Phylogenetic Analysis and Protein Sequence Alignment
Full-length protein sequences of ANPs from banana, Arabidopsis, tobacco, rice, tomato, maize and soybean were aligned using the Muscle algorithm implemented in MEGA7 [60]. Phylogenetic trees were constructed using the Neighbor-Joining (NJ) method with 1000 bootstrap replicates. The Poisson model was used, and pairwise deletion was applied to handle missing data. The resulting trees were visualized with the iTOL online platform (https://itol.embl.de/, accessed on 11 June 2025). Amino acid sequence homology between banana and Arabidopsis ANPs was analyzed using the MegAlign module within the DNASTAR Lasergene 11 software suite [61]. The tertiary structure of the MaNPK1-1 protein was predicted using SWISS-MODEL (https://swissmodel.expasy.org/, accessed on 11 June 2025). The template with the highest GMQE score was selected and the corresponding PDB file was downloaded [62]. The multiple sequence alignment FASTA file and the PDB file were uploaded to ESPript3.0 (https://espript.ibcp.fr/ESPript/ESPript/, accessed on 11 June 2025) to obtain the protein sequence alignment image.
2.6. Promoter cis-Acting Element Analysis
Promoter sequences (2000 bp upstream of the ATG) of the ANP genes were extracted from the banana genome using TBtools-II v2.114. All sequences were subjected to cis-acting element prediction through the PlantCARE online analysis website (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 12 June 2025) [63]. The cis-acting elements were sorted using the downloaded prediction file, with blank entries removed. Elements involved in environmental stress responses and hormone signaling were retained. Retain elements with environmental stress response and hormone response regulation functions. The sorted files are visualized by TBtools-II v2.114.
2.7. Prediction of the Interaction Network
psRNATarget (https://www.zhaolab.org/psRNATarget/, accessed on 12 June 2025) and PlantTFDB (http://planttfdb.gao-lab.org/, accessed on 12 June 2025) were used to predict miRNAs and the transcription factors that regulate MaNPK1, respectively [64,65]. The miRNA prediction expected value was set to 5, the transcription factor binding site prediction parameter was set to p-value ≤ 1 × e−6, and the remaining parameters were defaulted. The results were visualized by Cytoscape 3.10.3 software [66]. The transcriptome data of ‘Tianbao Banana’ under drought and P. indica treatment were downloaded [47]. The STRING data in the transcriptome were used to screen proteins with known biological functions and potential interaction with MaNPK1. The screening results were imported into Cytoscape 3.10.3 software for network visualization [67].
2.8. Subcellular Localization of MaNPK1-1
The RevertAid Master Mix (Thermo Electron Corp, Waltham, MA, USA) was used to synthesize the first strand cDNA using four treated root RNA as a template according to the instructions. The full-length CDS sequence of MaNPK1-1 was cloned using 2X SanTaq PCR Master Mix (Sangon, Shanghai, China). The amplification primers are shown in Table S2. A 25 μL reaction system was used for amplification: cDNA 50 ng, F & R (10 μM) 1 μL, 2X SanTaq PCR Master Mix 12.5 μL, and ddH2O was added to 25 μL; the program was 95 °C 3 min (pre-denaturation), 95 °C 30 s (denaturation), 63 °C 30 s (annealing), 72 °C 2 min (extension), 34 cycles, and 72 °C 10 min (final extension).
The amplified product was separated via 1% agarose gel electrophoresis, and the gel was recovered using SanPrep Column DNA Gel Extraction Kit (Sangon, Shanghai China). The target fragment was ligated to pMD18-T Vector (Takara, Kusatsu, Shiga, Japan) using a 10 μL reaction system: DNA target fragment 100 ng, pMD18-T 0.5 μL, Solution I 5 μL, and ddH2O was added to 10 μL. After mixing and centrifugation, the connection product was obtained by connecting at 16 °C for 8 h. The ligation product was transformed into DH5α (Weidi, Shanghai, China) according to the instructions. Then, 50 μL of the bacterial solution was coated on an LB plate containing 100 μg/mL Amp antibiotics and cultured at 37 °C for 8–10 h. A single colony was picked and tested for positive insertion of fragment by PCR in a 15 μL reaction system: bacterial solution (OD600 = 0.8) 1 μL, F & R (10 μM) 0.6 μL, 2X SanTaq PCR Master Mix 7.5 μL, ddH2O 5.3 μL, and the program were consistent with amplification. The positive bacterial solution was labeled and sent to Sangon Bioengineering (Shanghai) Co., Ltd. (Shanghai, China) for sequencing. The correctly sequenced bacterial solution was expanded and cultured. The recombinant plasmid was extracted using the SanPrep Column Plasmid Mini-Preps Kit (Sangon, Shanghai, China). The recombinant plasmid and the empty plasmid of pRI101-AN were double digested with restriction endonuclease Sal I and Kpn I (Takara, Kusatsu, Shiga, Japan). The enzyme fragments were separated by 1% agarose gel electrophoresis and recovered. T4 DNA Ligase (Takara, Kusatsu, Shiga, Japan) was used to insert the digested target fragment into the linearized pRI101-AN vector. A 10 μL system was used for ligation: target fragment 100 ng, pRI101-AN 100 ng, T4 DNA Ligase 1 μL, 10X T4 DNA Ligase Buffer 1 μL, add ddH2O was added to 10 μL. The ligation product was transformed into DH5α (Weidi, Shanghai, China), and the plasmid was extracted and verified via double enzyme digestion to confirm the successful construction of the pRI101-MaNPK1-1-EGFP fusion expression vector. The recombinant plasmid was transformed into Agrobacterium tumefaciens GV3101 (Weidi, Shanghai, China) following the manufacturer’s instructions. Then 50 μL of bacterial suspension was coated on LB plates containing 50 μg/mL of Kan and Rif antibiotics and cultured at 28 °C for 24–36 h. Single colonies were picked and detected by PCR. The positive bacterial culture was amplified and then centrifuged at 5000 rpm for 5 min to harvest the cells and it was resuspended in a resuspension solution (MS+100 mM MgCl2+100 μM AS+5% sucrose) to an OD600 of 1.0, followed by incubation in darkness for 2 h.
The resuspended bacteria were injected into onion epidermal cells via syringe. The injected onion were wrapped with plastic film and incubated in the dark at 28 °C for 2 days. Onion epidermal cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) for 8–10 min, washed with ddH2O, and then prepared for microscopy. GFP signal distribution was observed using a laser scanning confocal microscope (Olympus FV1200, Tokyo, Shibuya, Japan) to determine the subcellular localization of the MaNPK1-1 protein.
2.9. Analysis of MaNPK1 and Drought Response Pathway Differential Gene Expression
Differentially expressed genes (DEGs) associated with the MAPK cascade pathway under the four treatments were initially screened from our previously generated ‘Tianbao Banana’ root transcriptome datasets using KEGG PATHWAY Database (https://www.kegg.jp/kegg/pathway.html#environmental, accessed on 18 June 2025). Total RNA from root samples corresponding to the CK, H1, H2 and H3 treatments was reverse transcribed into cDNA using MightyScript Plus First Strand cDNA Synthesis Master Mix (gDNA digester) (Sangon, Shanghai, China).
Using the MightyScript Plus First Strand cDNA Synthesis Master Mix (gDNA digester) kit (Sangon, Shanghai, China), cDNA templates were synthesized by reverse transcription of four treated root total RNA according to the instructions. RT-qPCR was performed using Roche LightCycler 96 (Roche, Basel, Sweden). The gene specific primers were designed by DNAMAN 7 software and synthesized by Bioengineering (Shanghai) Co., Ltd. Gene specific primers are listed in Table S2. The banana MaARP8 was used as an internal reference primer, and the RT-qPCR reaction system was as follows: 2X SGExcel FastSYBR Mixture 10 μL, F & R (10 μM) 0.4 μL, cDNA diluted 5 times and added 2 μL, ddH2O 7.2 μL. The RT-qPCR reaction conditions were 95 °C for 3 min, followed by 40 cycles of 95 °C for 5 s and 60 °C for 20 s. Each sample was set up for 3 technical repetitions. Melting curve analysis was performed to confirm amplification specificity. Relative gene expression levels were calculated using the 2−ΔΔCt method [68]. GraphPad Prism 8 software was used to analyze the significance of differences between groups (p-value < 0.05) by one-way ANOVA combined with Tukey’s multiple comparison test and plot.
3. Results
3.1. Identification and Characterization of Banana ANP Family Members
A total of 13 unredundant proteins containing the STKc _ MAPKKK conserved domain were identified in the six banana genomes (Figure S1), all containing the characteristic conserved motif of the ANP family (Figure 1A). They were systematically named according to their respective genomes: MaNPK1-1, MaNPK1-2 (Musa acuminata); MbNPK1-1, MbNPK1-2 (M. balbisiana); MiNPK1-1, MiNPK1-2 (M. itinerans); MsNPK1-1, MsNPK1-2 (M. schizocarpa); MtNPK1-1, MtNPK1-2 (M. textilis); BXNPK1-1~BXNPK1-3 (M. cavendish_Baxijiao).
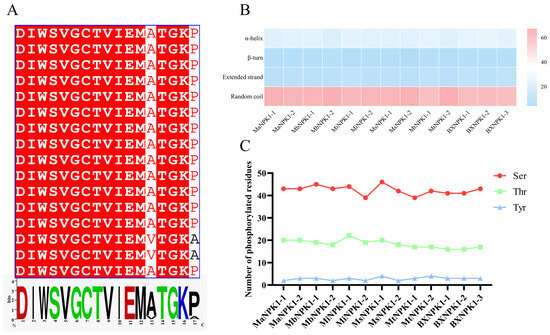
Figure 1.
Structural characteristics of banana ANP protein. (A) Multiple sequence alignment of characteristic motifs. (B) Heatmap of predicted proportions of secondary structural elements (α-helix, β-turn, extended strand and random coil). (C) Statistics of predicted number of phosphorylation sites for each member (Ser: serine; Thr: threonine; Tyr: tyrosine).
The predicted physicochemical properties of the banana ANP proteins are summarized in Table S1. Key findings include that the encoded proteins range from 592 to 669 amino acids in length. The theoretical isoelectric points of these members vary between 5.18 and 8.07. Among them, MiNPK1-2, MbNPK1-2, MsNPK1-2, and MaNPK1-1 exhibit relatively high pI values (8.07, 7.84, 7.23, and 7.22, respectively), indicating that they are basic proteins, while the remaining nine members are acidic. All 13 ANP family proteins have an instability index greater than 40 and a negative grand average of hydropathicity (GRAVY), suggesting that they are unstable hydrophilic proteins. None were predicted to contain a signal peptide. All members were found to lack transmembrane domains, except for MtNPK1-2 predicted as a transmembrane protein. Subcellular localization predictions indicate that all proteins are localized to the nucleus.
Secondary structure prediction (Figure 1B) indicated that all 13 ANP proteins comprise four elements: α-helix, β-turn, extended strands, and random coils. Random coils constituted the highest proportion (>60%), followed by α-helix (<20%), with β-turn and extended strands being the least abundant. The relatively low abundance of the structurally stabilizing elements (α-helix and β-turn) supports the prediction from physicochemical analysis that these are unstable proteins.
Prediction of protein phosphorylation sites (Figure 1C) revealed that all members of the banana ANP family contain phosphorylation sites for serine (Ser), threonine (Thr) and tyrosine (Tyr). Each protein has different number of specific kinase sites. The phosphorylation sites of each protein are mainly located on serine and threonine residues, and only a small part is located on tyrosine residues. The predominance of Ser and Thr phosphorylation sites is an important structural foundation for the activation and function of banana ANP family proteins as kinases by upstream signals.
3.2. Analysis of Conserved Motifs and Gene Structure of Banana ANP Family Proteins
A total of 20 differential conserved motifs were identified in the banana ANP family. (Figure S6). Most of the family members have similar motif number and length distribution (Figure 2A). Only half of the members have motif 20. Among them, MaNPK1-1, MbNPK1-2, MsNPK1-2 and MiNPK1-2 are highly conserved and contain all motifs except motif 20. Both MtNPK1-2 and M. cavendish members lack motif 13. Only MtNPK1-2 lacks motif 17. MtNPK1-1 lacks the largest number of motifs, with a total of five motifs missing, and only this member lacks motif 5, motif 12, motif 15 and motif 18.
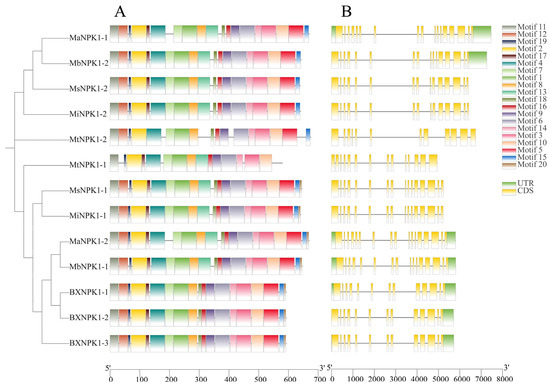
Figure 2.
Conserved motifs and gene structure of banana ANPs. (A) Conserved motifs. (B) Gene structure.
Gene structure analysis (Figure 2B) revealed that all members from the M. acuminata, M. balbisiana, and M. cavendish contain untranslated regions (UTRs). Among these, only MaNPK1-1, MaNPK1-2, MbNPK1-1 and BXNPK1-1 possess an intron in the upstream region of the gene. The exon–intron structures were largely similar across most family members. However, the number of exons varied from 13 to 17, and their positions were not uniform (Figure 2B). For instance, MaNPK1-1 not only contained longer introns but also had the highest number of exons, whereas MtNPK1-1 had only 13 exons with a compact arrangement.
3.3. Construction of ANP Phylogenetic Tree and Protein Sequence Alignment
A total of 29 ANP proteins from seven species were clustered and a phylogenetic tree was constructed (Figure 3A). The ANP family members of different plants were divided into three groups. Among them, the ANP members of monocotyledonous and dicotyledonous plants were significantly differentiated, while the banana ANP cluster was an independent branch. The genetic relationship between MaNPK1-1 and MbNPK1-2 is the closest, and the genetic relationship between MaNPK1-2 and MbNPK1-1 is close, which means that the members of M. acuminata and M. balbisiana genomes have high homology.
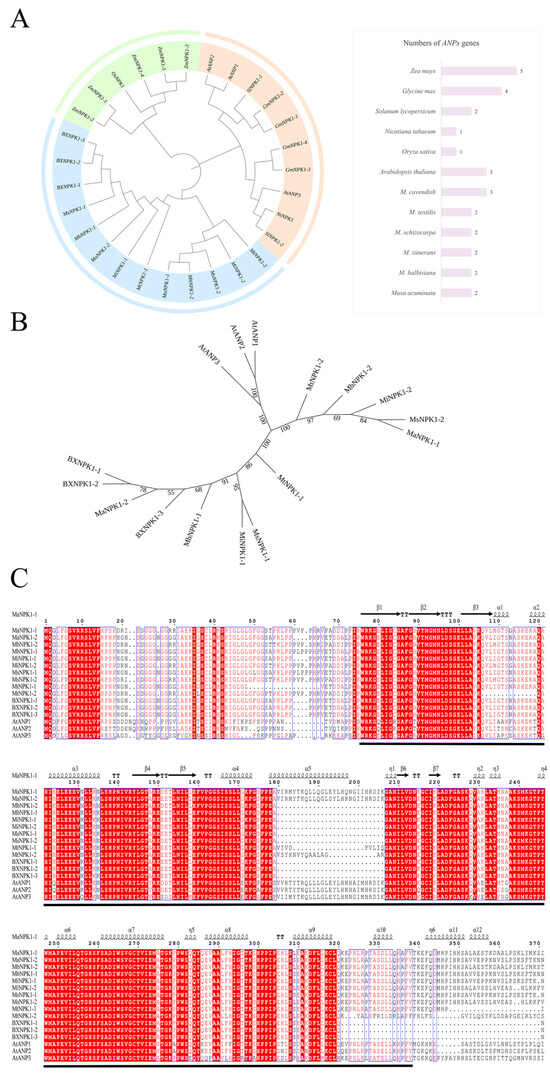
Figure 3.
Phylogenetic analysis and multiple sequence alignment of ANP family. (A) Phylogenetic analysis of the ANP family (Arabidopsis thaliana (At); Oryza sativa (Os); Nicotiana tabacum (Nt); Solanum lycopersicum (Sl); Glycine max (Gm); Zea mays (Zm); Musa acuminate (Ma); Musa balbisiana (Mb); Musa itinerans (Mi); Musa schizocarpa (Ms); Musa textilis (Mt) and Musa cavendish_Baxijiao (BX)). (B) Phylogenetic analysis of ANP proteins from banana and Arabidopsis thaliana (bootstrap values from 1000 replicates are shown at the branches). (C) Multiple amino acid sequence alignment results of banana and Arabidopsis thaliana ANP proteins (taking ANP3 as an example, the amino acid sequence of the underlined region is the sequence of STKc _ MAPKKK domain).
Given the well-characterized functions of ANPs in Arabidopsis, a comparative clustering analysis was performed between banana and Arabidopsis ANP members to infer functional relevance (Figure 3B). All banana ANP members first cluster into a single branch with AtANP3, suggesting that banana ANPs may share higher functional similarity with AtANP3. However, protein sequence homology analysis (Figure S3) revealed no significant differences in similarity between banana ANPs and various Arabidopsis ANP members, indicating that banana ANPs likely retain conserved fundamental functions similar to those of the Arabidopsis ANP family.
The ANP protein sequences from banana and Arabidopsis were aligned (Figure 3C). All members were found to contain the MAPKKK phosphorylation motif S/TxxxxxS/T, and was highly conserved in the STKc_MAPKKK catalytic domain (75–338). The average sequence similarity reached 85.7%. All proteins contained canonical kinase catalytic cores, such as the DFG motif (222–224) and GTPYWMAPE (243–251). Furthermore, banana ANP family members exhibit high sequence similarity, suggesting that there is no significant differentiation in sequence and function, which is consistent with the phylogenetic analysis. Within the α5 helical region (180–216), MaNPK1 exhibits the highest sequence similarity to AtANPs. Notably, only MaNPK1 and AtANPs contain the DIKGAN motif (203–208), while other homologs show various amino acid substitutions or deletions in this region.
3.4. Analysis of Promoter cis-Elements in Banana ANP Genes
To further investigate the potential roles of banana ANP family genes in environmental stress responses and hormonal signaling. The cis-acting element prediction analysis was performed on the promoter sequence of 2000 bp upstream of the initiation codon of banana ANPs (Figure 4). In addition to core promoter elements, a multitude of elements associated with plant growth and development were identified. Among the 17 types of responsive elements predicted, only MsNPK1-1 lacked all. With the exception of MaNPK1-2, BXNPK1-2 and BXNPK1-3, all other members lacked the zein metabolism regulation element. Notably, MtNPK1-2 was the only gene associated with seed-specific regulation, while MtNPK1-1 contained elements related to endosperm expression. All members except MsNPK1-1 and MiNPK1-2 contained methyl jasmonate (MeJA) responsive elements in varying numbers (Figure 4). The majority of members possessed anaerobic induction responsive elements, but only MsNPK1-2, MbNPK1-1, MbNPK1-2 and MaNPK1-1 could respond to drought stress. Over 70% of the members contained abscisic acid (ABA) and salicylic acid (SA) responsive elements. In contrast, auxin-responsive elements were only identified in MaNPK1-1, MbNPK1-2, MiNPK1-1 and MsNPK1-2.
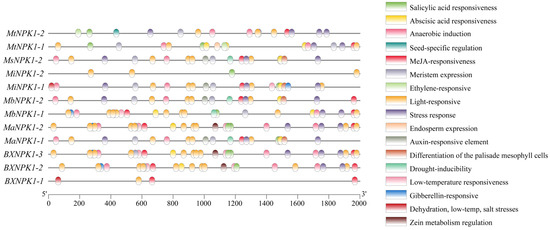
Figure 4.
Analysis of cis-acting elements of the ANP gene family in banana.
3.5. Prediction of the MaNPK1 Interaction Network
Prediction of potential transcription factor (TF) binding sites on the MaNPK1 promoter (Figure 5A) revealed 89 putative binding sites for 11 distinct TFs. Among these, only five TF families (NAC, TALE, ERF, GATA and BBR-BPC) were predicted to regulate both MaNPK1-1 and MaNPK1-2. Notably, ERF contained the highest number of binding sites, suggesting that ERF is the most plausible transcriptional regulator of MaNPK1 (Figure 5A). Furthermore, a total of 84 miRNAs targeting MaNPK1 were predicted using psRNAtarget, enabling the construction of a comprehensive ‘TF-MaNPK1-miRNA’ regulatory network (Figure 5B). The analysis revealed that both MaNPK1-1 and MaNPK1-2 are targeted by multiple distinct miRNAs. However, only 11 miRNAs are shared between them. Mechanistically, most of these miRNAs are predicted to regulate MaNPK1 expression primarily through cleavage (Figure 5B).
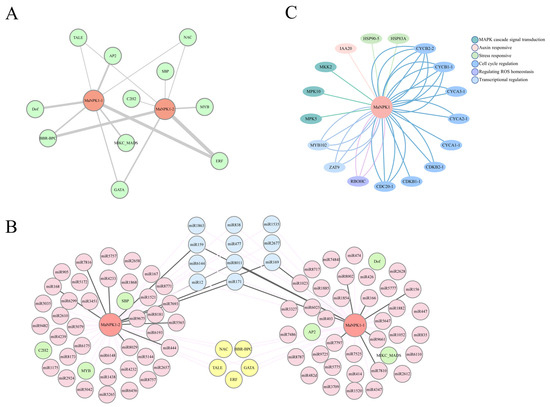
Figure 5.
Banana MaNPK1 interaction network. (A) TF-MaNPK1 interaction network (red: genes; green: transcription factors; the thicker the line, the more binding sites). (B) TF-MaNPK1-miRNA interaction network (red: genes; green: transcription factors; pink: miRNAs; yellow: transcription factors regulating two genes; blue: miRNAs regulating two genes; black lines represent translation inhibition; pink lines represent cleavage). (C) MaNPK1 protein interaction network.
Based on STRING analysis of transcriptome data, potential interactors of MaNPK1 were identified (Figure 5C). MaNPK1 was predicted to interact with several cell cycle regulators (e.g., CDC20-1, CDKB1-1, and CYCA2-1), suggesting a role in mitosis. Additionally, interactions with auxin-responsive protein IAA20 and respiratory burst oxidase homolog C (RBOHC) were predicted, implicating that MaNPK1 may be involved in auxin signaling and ROS signaling.
3.6. Subcellular Localization Analysis of MaNPK1-1 Protein
The fusion expression vector pRI101-AN-35S::MaNPK1-1 was successfully constructed using TA cloning technology (Figure S4).
Subcellular localization results are presented in Figure 6. In onion cells expressing MaNPK1-1, green fluorescence was detected only in the nucleus. It co-localized with the blue DAPI stain. In control cells with the empty vector, fluorescence was seen in the cytoplasm, membrane, and around the nucleus. This confirms the nucleus localization of MaNPK1-1, consistent with the bioinformatic prediction.

Figure 6.
Subcellular localization of banana MaNPK1-1 protein in onion. Bars = 100 μm.
3.7. P. indica Colonization Regulates MaNPK1 Expression Under Drought
Based on the transcriptome data of ‘Tianbao Banana’ colonized by P. indica under drought treatment (Figure 7A), we identified two members of the banana ANP family, MaNPK1-1 and MaNPK1-2, which were specifically induced by drought stress after P. indica colonization. In addition, a large number of differentially expressed genes related to the regulation of ROS production and auxin synthesis pathway were also found. RT-qPCR validation under four treatments showed that MaNPK1-1 expression was significantly upregulated in H3 (drought, P. indica) compared to H2 (drought, no P. indica). In contrast, MaNPK1-2 expression remained unchanged across all treatments (Figure 7B). These results indicate that MaNPK1-1 is specifically induced by P. indica colonization under drought conditions. This functional divergence aligns with the presence of drought responsive cis-elements in the MaNPK1-1 promoter and their absence in the MaNPK1-2 promoter.
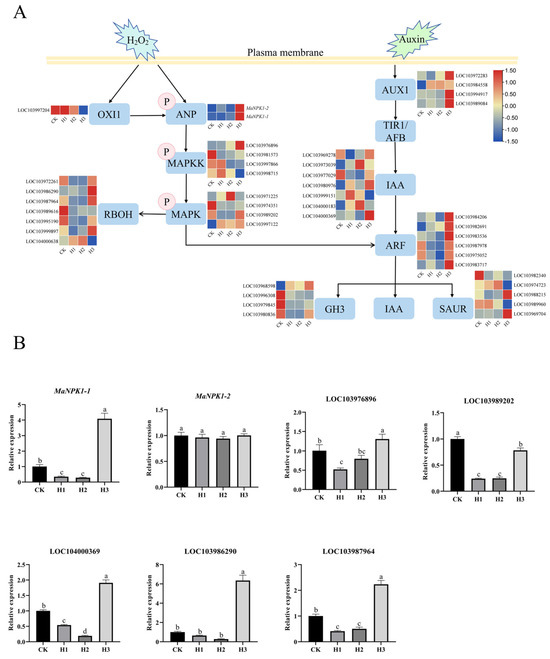
Figure 7.
P. indica affects differentially expressed genes in plant MAPK cascade pathway under drought stress. (A) Expression heatmap (CK: well-watered, no P. indica; H1: well-watered, P. indica; H2: drought, no P. indica; H3: drought, P. indica). (B) The relative expression of differentially expressed genes in the roots of ‘Tianbao Banana’. (The error bar represents the standard deviation (n = 3), different lowercase letters on the histogram indicate significant differences (p < 0.05). LOC103976896 in the MAPKK family, LOC103989202 in the MAPK family, LOC103986290 and LOC103987964 in the RBOH family and LOC104000369 in the IAA family).
Further analysis showed that multiple genes related to MAPK cascade pathway, ROS production, and auxin synthesis were also differentially expressed. Combined with protein interaction network analysis, some key differentially expressed genes were screened for expression verification (Figure 7B). It includes LOC103976896 and LOC103989202 in the MAPK cascade pathway, LOC103986290 and LOC103987964 in the RBOH family, and LOC104000369 in the IAA family. The expression patterns of these genes were consistent with MaNPK1-1, which were significantly upregulated by P. indica under drought conditions.
4. Discussion
This study identified a comparative screening using available banana genomic data and homologous NPK1 protein sequences from Arabidopsis. Finally, 2, 2, 2, 2, and 3 banana ANP family members were identified from the genomes of M. acuminata, M. balbisiana, M. itinerans, M. schizocarpa, M. textilis, and M. cavendish, respectively. The members are highly conserved and contain the characteristic conserved motif (DIWS×GCT××E××T××P) typical of the ANP gene family [69]. It was found that the evolutionary patterns of the ANP gene family were significantly different in various plants. In legume plants, multiple whole genome duplications (WGDs) occurred. This led to a clear expansion of the ANP family in these plants. However, no similar expansion was found in grass species [70]. Similarly, in this study, the number of ANP family members identified in banana is comparable to that in Arabidopsis (3) and tomato (2). This suggests that the ANP family has been relatively conserved during banana evolution. Although there is no lack of WGD events in the banana genome, which provides the possibility for the expansion of the gene family, many duplicated genes have not been retained [71]. We think that ANP is a core functional family. It may be highly conserved in evolution. Extra gene copies were unlikely to be retained. As a result, the ANP family did not expand significantly in banana.
In the prediction analysis of physical and chemical properties, we found that the members of the banana ANP family share some common traits, but also show signs of functional differences. All members are predicted to be unstable, hydrophilic nuclear proteins. It is speculated that they may function in the nucleus after being activated by upstream signals so as to respond quickly to external stimuli. Notably, there is a big difference in isoelectric points among the 13 members. Most are predicted to be acidic proteins. This difference provides a structural basis for their functional divergence. MtNPK1-2 is the only member predicted to have a transmembrane structure. Multiple sequence alignment shows that MtNPK1-2 has an extra sequence containing GxxxG in the N-terminal (640–650) region (Figure S5). Research shows this motif is important for transmembrane helix interactions [72]. Therefore, we think this hydrophobic sequence with the GxxxG motif causes MtNPK1-2 to be predicted as a transmembrane protein. This unique feature suggests it may have a different function. It might be involved in transmitting membrane-related signals.
In the comparison of characteristic conserved motifs, two amino acid differences were identified within the conserved region of AtANP1 and AtANP2. In addition to DIWSVG, the catalytic domain of NPK1 also includes two typical sequences, DIKGAN and GTPYWMAPE [17]. The DIKGAN sequence only existed in the α5 helix region of MaNPK1 and AtANPs, where MaNPK1 exhibits the highest sequence similarity to AtANPs, and other genes have amino acid substitution or deletion in this region. It is proposed that members of the banana ANP gene family, particularly MaNPK1, may exhibit functional conservation with the Arabidopsis ANP family and likely share the closest biological functions to AtANP3. Studies have indicated functional redundancy among AtANP1, AtANP2, and AtANP3, with varying degrees of contribution to plant development [73]. Only the loss of AtANP3 leads to defective phenotypes, suggesting that AtANP3 is the most critical member of the ANP family [74]. Cluster analysis showed that banana ANP members were more closely related to AtANP3. However, homology analysis did not reveal significant differences in sequence similarity between banana ANPs and Arabidopsis ANPs, unlike the pattern in Rosaceae species, where ANP1 and ANP2 are lost and only ANP3 contributes to immune function [70]. It is speculated that there is no gene loss in the banana ANP family and the redundancy of gene function is retained.
Subcellular localization prediction showed that all banana ANP members were localized in the nucleus, and subcellular localization experiments further confirmed that the GFP signal of MaNPK1-1 was specifically enriched in the nucleus. This is consistent with the established role of NPK1 in regulating mitotic processes within the nucleus [75,76,77,78]. However, protein–protein interaction network analysis revealed that MaNPK1 not only interacts with cell cycle regulators such as CDC20-1 and CYCB1-1, but also interacts with the ROS generation enzymes RBOHC and heat shock protein HSP90-5. Combined with the ‘ANP multi-organelle localization’ dynamics hypothesis proposed by Marti [79], stress signals induce conformational changes in ANP proteins, making them shuttle between the nucleus, mitochondria, and plastids to integrate oxidative stress responses. In addition, miRNA regulatory network prediction showed that miR156, miR166 and miR169 could target MaNPK1. Studies have found that these miRNAs can participate in stress response and redox homeostasis regulation [80,81]. We speculate that MaNPK1 can participate in the regulation of cellular ROS homeostasis in response to drought stress.
Analysis of promoter cis-acting elements indicated that, in addition to abundant enhancer and light responsive elements, banana ANP promoters contain multiple hormone and stress responsive elements. This provides a transcriptional regulatory basis for their involvement in abiotic stress responses. Most members carry stress response elements, supporting the view of Shen [82] that CsNPK1 is induced by stress to form the quality of Oolong tea leaves. Some members contain auxin-responsive elements, which is consistent with the results that AtANPs mediate auxin signal transduction [30]. Additionally, the promoter region of MaNPK1 contains binding sites for AP2 transcription factors, which have been reported to regulate auxin synthesis [83]. Studies have found that plants can respond to drought stress by regulating root development, and auxin plays a central role in this process [84,85]. In the protein interaction prediction map, we found the presence of auxin-responsive proteins. It is thus proposed that MaNPK1 may participate in the auxin signaling pathway and modulate cellular auxin levels, thereby contributing to drought resistance in banana.
Previous studies have shown that overexpression of NPK1 enhances drought tolerance in sorghum [34], rice [86] and maize [87,88]. P. indica enhances stress resistance in host plants by activating antioxidant systems and regulating endogenous hormone levels [89,90]. In line with this, we analyzed the root transcriptome data of ‘Tianbao Banana’ with drought and colonization of P. indica, and found that two banana ANP members MaNPK1-1 and MaNPK1-2 were significantly differentially expressed. Moreover, P. indica also induced the differential expression of a large number of ROS-generating enzymes (RBOHs) and auxin synthesis and signal transduction-related genes (IAA, ARF and GH3). In the regulatory network composed of these differential genes, MaNPK1 has potential functional links with RBOHC and IAA20. Combined with the conclusion that P. indica can enhance stress resistance by coordinating auxin levels and oxidative bursts [91,92,93,94], it is speculated that MaNPK1 is functionally conserved with Arabidopsis ANPs. It may function as an upstream regulatory node in the RBOH-mediated H2O2 signaling cascade and integrate hormones and redox signals by regulating the auxin pathway, thus playing a central role in the process of P. indica, improving the drought resistance of banana. The expression of MAPK cascade-related genes was further verified by RT-qPCR, and it was found that their expression patterns were consistent with MaNPK1-1: under drought conditions, they were significantly upregulated by P. indica, while the expression level of MaNPK1-2 remained unchanged. Promoter analysis revealed a divergence in stress responsiveness: MaNPK1-1 contains drought, auxin and oxidative stress response elements, while MaNPK1-2 lacked drought response elements, which explains their differential expression patterns. This further supports the notion that MaNPK1-1 may serve as a key regulatory node in the P. indica-mediated drought resistance pathway of banana, likely through coordinating auxin and H2O2 signaling to collectively enhance drought tolerance.
5. Conclusions
In summary, this study presents the first genome-wide systematic identification of the ANP gene family in banana. It provides a solid foundation for the functional characterization of ANP genes in the future. Through the experiment involving drought and P. indica treatment, the results demonstrated that the expression of MaNPK1-1 in the roots of ‘Tianbao Banana’ was specifically induced by P. indica colonization. This expression pattern is consistent with the presence of drought-responsive elements in the promoter region of MaNPK1-1. The interaction network prediction results showed that MaNPK1 may regulate banana drought resistance by integrating auxin and H2O2 signals. However, the specific mechanism needs further experimental verification. The nuclear localization of MaNPK1-1 was confirmed by subcellular localization assays. These results indicate that MaNPK1-1 acts as a key role in the P. indica mediated drought tolerance pathway.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15102410/s1. Figure S1: Conserved domain of banana ANP protein; Figure S2: Prediction of phosphorylation sites of banana ANPs protein; Figure S3: Homology analysis of ANP protein between banana and Arabidopsis thaliana; Figure S4: Construction of pRI-AN-35S::MaNPK1-1 fusion expression vector; Figure S5: Multiple amino acid sequence alignment results of the N-terminal of banana and Arabidopsis ANP protein; Figure S6: Amino acid sequence of differential conserved motifs. Table S1: Physicochemical properties of banana ANP family members; Table S2: RT-qPCR and PCR primer sequences; Table S3: The accessions of Arabidopsis and tobacco ANP members.
Author Contributions
Conceptualization, T.L., L.M. and Y.H.; software, T.L. and W.W.; data curation, T.L. and X.F.; formal analysis, T.L., W.W. and J.X.; writing—original draft preparation, T.L.; supervision, Y.H. and L.M.; writing—review and editing, Z.L., L.M. and Y.H.; funding acquisition, Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Fujian Province, grant number 2024J01392, 2023J01450, and the National Natural Science Foundation of China, grant number 31701900.
Data Availability Statement
The data presented in this study are available in the article and its Supplementary Materials.
Acknowledgments
During the preparation of this work the authors used DeepSeek in order to improve the language. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, X.; Wang, A.; Li, Y.; Xu, Y.; Wei, Q.; Wang, J.; Lin, F.; Gong, D.; Liu, F.; Wang, Y.; et al. A novel banana mutant “RF 1” (Musa spp. ABB, pisang awak subgroup) for improved agronomic traits and enhanced cold tolerance and disease resistance. Front. Plant Sci. 2021, 12, 730718. [Google Scholar] [CrossRef]
- Jiang, C.; Zhao, Y.; Wang, L.; Song, K.; Huang, S.; He, Y. Analysis of banana industry development and scientific research in China since the 12th Five-Year Plan. Trop. Agric. Sci. 2025, 45, 111–119. [Google Scholar]
- Lu, S.; Lin, X.; Yang, J.; Luo, J.; Lin, X.; Wu, Y. Dilemmas and countermeasures of banana industry development in Fujian Province. China Fruits 2024, 135–142+151. [Google Scholar] [CrossRef]
- Widmann, C.; Gibson, S.; Jarpe, M.; Johnson, G. Mitogen-activated protein kinase: Conservation of a three-kinase module from yeast to human. Physiol. Rev. 1999, 79, 143–180. [Google Scholar] [CrossRef]
- Ren, N.; Zhang, G.; Yang, X.; Chen, J.; Ni, L.; Jiang, M. MAPKKK28 functions upstream of the MKK1-MPK1 cascade to regulate abscisic acid responses in rice. Plant Cell Environ. 2024, 47, 5140–5157. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Chen, D.; Ahsan, N.; Jorge, G.L.; Thelen, J.J.; Stacey, G. The raf-like MAPKKK integrin-linked kinase 5 regulates purinergic receptor-mediated innate immunity in Arabidopsis. Plant Cell 2023, 35, 1572–1592. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, K.; Shinozaki, K.; Tena, G.; Sheen, J.; Henry, Y.; Champion, A.; Kreis, M.; Zhang, S.; Hirt, H.; Wilson, C. Mitogen-activated protein kinase cascades in plants: A new nomenclature. Trends Plant Sci. 2002, 7, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Colcombet, J.; Hirt, H. Arabidopsis MAPKs: A complex signalling network involved in multiple biological processes. Biochem. J. 2008, 413, 217–226. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, S.; Ren, W.; Liu, Y.; Sun, W.; Liu, M.; Lu, J.; Mi, Y.; Ma, W. Genome-wide identification of MAPK, MAPKK, and MAPKKK gene families in Fagopyrum tataricum and analysis of their expression patterns under abiotic stress. Front. Genet. 2022, 13, 894048. [Google Scholar] [CrossRef]
- Wang, F.; Liang, S.; Wang, G.; Wang, Q.; Xu, Z.; Li, B.; Fu, C.; Fan, Y.; Hu, T.; Alariqi, M.; et al. Comprehensive analysis of MAPK gene family in upland cotton (Gossypium hirsutum) and functional characterization of GhMPK31 in regulating defense response to insect infestation. Plant Cell Rep. 2024, 43, 102. [Google Scholar] [CrossRef]
- Ding, R.; Li, J.; Wang, J.; Li, Y.; Ye, W.; Yan, G.; Yin, Z. Molecular traits of MAPK kinases and the regulatory mechanism of GhMAPKK5 alleviating drought/salt stress in cotton. Plant Physiol. 2024, 196, 2030–2047. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Wang, Y.; Chen, Y.; Luo, J.; Li, D.; Li, X. Genome-wide identification and functional characterization of cotton (Gossypium hirsutum) MAPKKK gene family in response to drought stress. BMC Plant Biol. 2020, 20, 217. [Google Scholar] [CrossRef]
- Wu, J.; Liang, X.; Lin, M.; Lan, Y.; Xiang, Y.; Yan, H. Comprehensive analysis of MAPK gene family in Populus trichocarpa and physiological characterization of PtMAPK3-1 in response to MeJA induction. Physiol. Plant. 2023, 175, e13869. [Google Scholar] [CrossRef]
- Tajdel-Zielinska, M.; Janicki, M.; Marczak, M.; Ludwikow, A. Arabidopsis HECT and RING-type E3 ligases promote MAPKKK18 degradation to regulate abscisic acid signaling. Plant Cell Physiol. 2024, 65, 390–404. [Google Scholar] [CrossRef]
- Feng, Y.; Li, M.; Lu, S.; Shao, M.; Liang, G.; Mao, J. Identification of the grape MAPKKK gene family and functional analysis of the VaMAPKKK15 gene under low temperature stress. Plant Physiol. Biochem. 2025, 220, 109533. [Google Scholar] [CrossRef]
- Yu, J.; Kang, L.; Li, Y.; Wu, C.; Zheng, C.; Liu, P.; Huang, J. RING finger protein RGLG1 and RGLG2 negatively modulate MAPKKK18 mediated drought stress tolerance in Arabidopsis. J. Integr. Plant Biol. 2021, 63, 484–493. [Google Scholar] [CrossRef]
- Banno, H.; Hirano, K.; Nakamura, T.; Irie, K.; Nomoto, S.; Matsumoto, K.; Machida, Y. NPK1, a tobacco gene that encodes a protein with a domain homologous to yeast BCK1, STE11 and BYR2 protein-kinases. Mol. Cell. Biol. 1993, 13, 4745–4752. [Google Scholar] [CrossRef]
- Takahashi, Y.; Soyano, T.; Sasabe, M.; Machida, Y. A MAP kinase cascade that controls plant cytokinesis. J. Biochem. 2004, 136, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Soyano, T.; Nishihama, R.; Machida, Y. The NPK1 mitogen-activated protein kinase kinase kinase contains a functional nuclear localization signal at the binding site for the NACK1 kinesin-like protein. Plant J. 2002, 32, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Sasabe, M.; Boudolf, V.; Lieven, D.; Inze, D.; Genschik, P.; Machida, Y. Phosphorylation of a mitotic kinesin-like protein and a MAPKKK by cyclin-dependent kinases (CDKs) is involved in the transition to cytokinesis in plants. Proc. Natl. Acad. Sci. USA 2011, 108, 17844–17849. [Google Scholar] [CrossRef] [PubMed]
- Joúannic, S.; Hamal, A.; Leprince, A.; Tregear, J.; Kreis, M.; Henry, Y. Plant MAP kinase kinase kinases structure, classification and evolution. Gene 1999, 233, 1–11. [Google Scholar] [CrossRef]
- Beck, M.; Komis, G.; Mueller, J.; Menzel, D.; Samaj, J. Arabidopsis homologs of nucleus-and phragmoplast-localized kinase 2 and 3 and mitogen-activated protein kinase 4 are essential for microtubule organization. Plant Cell 2010, 22, 755–771. [Google Scholar] [CrossRef]
- Saito, T.; Fujikawa, H.; Haga, N.; Suzuki, T.; Machida, Y.; Ito, M. Genetic interaction between G2/M phase-specific transcription factor MYB3R4 and MAPKKK ANP3 for execution of cytokinesis in Arabidopsis thaliana. Plant Signal. Behav. 2015, 10, e990817. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, S.; Savic, J.; Milojevic, J.; Vinterhalter, B.; Girek, Z.; Adzic, S.; Zecevic, B.; Banjac, N. Introduction of the Nicotiana protein kinase (NPK1) gene by combining agrobacterium-mediated transformation and recurrent somatic embryogenesis to enhance salt tolerance in cauliflower. Plant Cell Tissue Organ Cult. 2020, 143, 635–651. [Google Scholar] [CrossRef]
- Ning, J.; Liu, S.; Hu, H.; Xiong, L. Systematic analysis of NPK1-like genes in rice reveals a stress-inducible gene cluster co-localized with a quantitative trait locus of drought resistance. Mol. Genet. Genom. 2008, 280, 535–546. [Google Scholar] [CrossRef]
- Takac, T.; Samajova, O.; Vadovic, P.; Pechan, T.; Kosutova, P.; Ovecka, M.; Husickova, A.; Komis, G.; Samaj, J. Proteomic and biochemical analyses show a functional network of proteins involved in antioxidant defense of the Arabidopsisanp2anp3 double mutant. J. Proteome Res. 2014, 13, 5347–5361. [Google Scholar] [CrossRef]
- Savatin, D.V.; Bisceglia, N.G.; Marti, L.; Fabbri, C.; Cervone, F.; De Lorenzo, G. The Arabidopsis nucleus-and phragmopkast-localized kinase 1 -related protein kinases are required for elicitor-induced oxidative burst and immunity. Plant Physiol. 2014, 165, 1188–1202. [Google Scholar] [CrossRef] [PubMed]
- Bisceglia, N.; Savatin, D.; Cervone, F.; Engelsdorf, T.; De Lorenzo, G. Loss of the Arabidopsis protein kinases ANPs affects root cell wall composition, and triggers the cell wall damage syndrome. Front. Plant Sci. 2018, 8, 2234. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, Y.; Chiu, W.; Tena, G.; Sheen, J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc. Natl. Acad. Sci. USA 2000, 97, 2940–2945. [Google Scholar] [CrossRef]
- Kovtun, Y.; Chiu, W.; Zeng, W.; Sheen, J. Suppression of auxin signal transduction by a MAPK cascade in higher plants. Nature 1998, 395, 716–720. [Google Scholar] [CrossRef] [PubMed]
- De Aquino, G.; Carlos, E. The NPK1 gene increases sugarcane productivity under water deficit and conventional crop management conditions. Biocatal. Agric. Biotechnol. 2022, 44, 102454. [Google Scholar] [CrossRef]
- Assem, S.; Hussein, E.; Hussein, H. Genetic transformation of the Nicotiana protein kinase (NPK1) gene confers osmotic tolerance in egyptian maize. Aust. J. Basic Appl. Sci. 2009, 3, 828–835. [Google Scholar]
- Omondi Muoma, J.; Ombori, O. Agrobacterium-mediated transformation of selected Kenyan maize (Zea mays L.) genotypes by introgression of Nicotiana protein kinase (NPK1) to enhance drought tolerance. Am. J. Plant Sci. 2014, 5, 863–883. [Google Scholar] [CrossRef]
- Assem, S.; Zamzam, M.; Saad, M.; Hussein, B.; Hussein, E. The impact of over-expression of NPK1 gene on growth and yield of sorghum under drought stress. Afr. J. Biotechnol. 2017, 16, 2267–2277. [Google Scholar] [CrossRef]
- Kundu, A.; Mishra, S.; Kundu, P.; Jogawat, A.; Vadassery, J. Piriformospora indica recruits host-derived putrescine for growth promotion in plants. Plant Physiol. 2022, 188, 2289–2307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, H.; Xie, C.; Zhu, Z.; Lin, L.; An, Q.; Zhang, X.; Wu, W.; Li, D. Piriformospora indica colonization enhances remediation of cadmium and chromium co-contaminated soils by king grass through plant growth promotion and rhizosphere microecological regulation. J. Hazard. Mater. 2024, 462, 132728. [Google Scholar] [CrossRef]
- Wang, Z.; Zong, F.; Lin, W.; Tang, X.; Xuan, S.; He, B.; Wu, B.; Guo, L. The effects of Piriformospora indica on the growth of cuttings from three species of woody ornamental plants. Ind. Crops Prod. 2025, 223, 120127. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Song, H.; Luo, C.; Cheng, C.; Mao, L. Promoting effects of Piriformospora indica on the growth and development of Asparagus (Asparagus officinalis L.) seedlings. Plants 2025, 14, 1232. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.; Huang, J.; Yang, S.; Mei, H.; Jiang, Y.; Hou, Y.; Peng, J.; Cheng, C.; Li, H.; et al. Integrated transcriptome and sRNAome analysis reveals the molecular mechanisms of Piriformospora indica-mediated resistance to fusarium wilt in banana. Int. J. Mol. Sci. 2024, 25, 12446. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Nong, C.; Lin, T.; Fang, L.; Feng, X.; Chen, Y.; Lin, Y.; Lai, Z.; Miao, L. Piriformospora indica enhances resistance to fusarium wilt in strawberry by increasing the activity of superoxide dismutase, peroxidase, and catalase, while reducing the content of malondialdehyde in the roots. Horticulturae 2024, 10, 240. [Google Scholar] [CrossRef]
- Wu, H.; Wang, B.; Hao, X.; Zhang, Y.; Wang, T.; Lu, Z.; Lai, Z.; Cheng, C. Piriformospora indica promotes the growth and enhances the root rot disease resistance of gerbera. Sci. Hortic. 2022, 297, 110946. [Google Scholar] [CrossRef]
- Zhao, B.; Li, R.; Tian, N.; Li, Q.; Cheng, C.; Wang, M. The growth-promoting effects of Piriformospora indica on banana under different concentrations of phosphorus and potassium treatmentss. Plants 2025, 14, 1878. [Google Scholar] [CrossRef]
- Shao, A.; Yang, J.; Li, H.; Li, R.; Hu, Y.; Cheng, C. Piriformospora indica culture filtrate application adds brilliance to the promoting effects of facility warming on winter jujube fruit ripening. Food Chem. X 2024, 24, 101986. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Zhang, M.; Liang, Y.; Ding, C.; Chen, Q.; Fan, X.; Meng, X.; Zhang, X.; Gao, S.; Zhai, D.; et al. Piriformospora indica enhances growth and salt tolerance in a short rotation woody crop, Paulownia elongata, under NaCl stress. Front. Plant Sci. 2025, 16, 1566470. [Google Scholar] [CrossRef]
- Zhou, G.; Wu, S.; Qiu, M.; Long, Y.; He, Q.; Zhang, J. Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress. Open Life Sci. 2025, 20, 20251111. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Feng, Y.; Qi, F.; Hao, R. Research progress of Piriformospora indica in improving plant growth and stress resistance to plant. J. Fungi 2023, 9, 965. [Google Scholar] [CrossRef]
- Chen, C. Effects of Piriformospora indica on the Growth and Drought Tolerance of ‘Tianbao Banana’. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2023. [Google Scholar]
- Lu, Y. Effects of Piriformospora indica on the Growth and Drought Tolerance of Kiwifruit. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2024. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, P.; Zhang, J.; Li, R.; Liu, R.; Cheng, C. Insights into the underlying mechanism of the Piriformospora indica-enhanced drought tolerance in blueberry. Horticulturae 2025, 11, 605. [Google Scholar] [CrossRef]
- Chen, C.; Li, J.; Luo, J.; Chen, Y.; Cheng, C.; Lai, Z.; Huang, Y. Effects of Piriformospora indica on drought resistance in banana. Chin. J. Appl. Environ. Biol. 2024, 30, 118–125. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.S.; Thanki, N.; Yamashita, R.A.; et al. The conserved domain database in 2023. Nucleic Acids Res. 2023, 51, D384–D388. [Google Scholar] [CrossRef]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the swiss bioinformatics resource portal, as designed by its users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar] [CrossRef] [PubMed]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gislason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, P.; Luo, J.; Jiang, Y. Secreted protein prediction system combining CJ-SPHMM, TMHMM, and PSORT. Mamm. Genome 2003, 14, 859–865. [Google Scholar] [CrossRef]
- Chou, K.; Shen, H. Cell-PLoc: A package of Web servers for predicting subcellular localization of proteins in various organisms. Nat. Protoc. 2008, 3, 153–162. [Google Scholar] [CrossRef]
- Geourjon, C.; Deléage, G. Sopma: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Bioinformatics 1995, 11, 681–684. [Google Scholar] [CrossRef]
- Gao, T.; Gao, Y.; Liu, X.; Nie, Z.; Sun, H.; Lin, K.; Peng, H.; Wang, S. Identification and functional analysis of the SARS-COV-2 nucleocapsid protein. BMC Microbiol. 2021, 21, 58. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Burland, T.G. DNASTAR’s Lasergene sequence analysis software. Methods Mol. Biol. 2000, 132, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Kamguia, S.D.; Njabon, E.N.; Patouossa, I.; Emadak, A.; Forlemu, N. A comparative analysis of cockroach and mosquito, octopamine receptor homologues produced using Chimera, Swiss-Model, and AlphaFold molecular modeling tools. ACS Omega 2025, 10, 7907–7919. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server (2017 release). Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef]
- Jin, J.; Tian, F.; Yang, D.; Meng, Y.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017, 45, D1040–D1045. [Google Scholar] [CrossRef]
- Ono, K.; Fong, D.; Gao, C.; Churas, C.; Pillich, R.; Lenkiewicz, J.; Pratt, D.; Pico, A.R.; Hanspers, K.; Xin, Y.; et al. Cytoscape Web: Bringing network biology to the browser. Nucleic Acids Res. 2025, 53, W203–W212. [Google Scholar] [CrossRef] [PubMed]
- Majeed, A.; Mukhtar, S. Protein-Protein Interaction network exploration using Cytoscape. Methods Mol. Biol. 2023, 2690, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Tena, G.; Asai, T.; Chiu, W.; Sheen, J. Plant mitogen-activated protein kinase signaling cascades. Curr. Opin. Plant Biol. 2001, 4, 392–400. [Google Scholar] [CrossRef]
- Sun, Y.; Bian, F.; Cui, T.; Fu, T.; Zhang, L.; Gao, N.; Zhang, Q.; Irfan, M.; Zhang, X.; Chen, L. Duplication and functional diversification of ANP3-like genes (MAPKKKs) in rosaceae. J. Plant Growth Regul. 2025, 44, 4355–4367. [Google Scholar] [CrossRef]
- D’Hont, A.; Denoeud, F.; Aury, J.-M.; Baurens, F.-C.; Carreel, F.; Garsmeur, O.; Noel, B.; Bocs, S.; Droc, G.; Rouard, M.; et al. The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 2012, 488, 213–217. [Google Scholar] [CrossRef]
- Melnyk, R.; Kim, S.; Curran, A.; Engelman, D.; Bowie, J.; Deber, C. The affinity of GXXXG motifs in transmembrane helix-helix interactions is modulated by long-range communication. J. Biol. Chem. 2004, 279, 16591–16597. [Google Scholar] [CrossRef]
- Krysan, P.; Jester, P.; Gottwald, J.; Sussman, M. An Arabidopsis mitogen-activated protein kinase kinase kinase gene family encodes essential positive regulators of cytokinesis. Plant Cell 2002, 14, 1109–1120. [Google Scholar] [CrossRef]
- Lian, K.; Gao, F.; Sun, T.; van Wersch, R.; Ao, K.; Kong, Q.; Nitta, Y.; Wu, D.; Krysan, P.; Zhang, Y. MKK6 functions in two parallel MAP kinase cascades in immune signaling. Plant Physiol. 2018, 178, 1284–1295. [Google Scholar] [CrossRef]
- Nishihama, R.; Machida, Y. The MAP kinase cascade that includes MAPKKK-related protein kinase NPK1 controls a mitotic proces in plant cells. Res. Probl. Cell Differ. 2000, 27, 119–130. [Google Scholar] [CrossRef]
- Beck, M.; Komis, G.; Ziemann, A.; Menzel, D.; Samaj, J. Mitogen-activated protein kinase 4 is involved in the regulation of mitotic and cytokinetic microtubule transitions in Arabidopsis thaliana. New Phytol. 2011, 189, 1069–1083. [Google Scholar] [CrossRef]
- Takahashi, Y.; Soyano, T.; Kosetsu, K.; Sasabe, M.; Machida, Y. HINKEL kinesin, ANP MAPKKKs and MKK6/ANQ MAPKK, which phosphorylates and activates MPK4 MAPK, constitute a pathway that is required for cytokinesis in Arabidopsis thaliana. Plant Cell Physiol. 2010, 51, 1766–1776. [Google Scholar] [CrossRef]
- Nishihama, R.; Soyano, T.; Ishikawa, M.; Araki, S.; Tanaka, H.; Asada, T.; Irie, K.; Ito, M.; Terada, M.; Banno, H.; et al. Expansion of the cell plate in plant cytokinesis requires a kinesin-like protein/MAPKKK complex. Cell 2002, 109, 87–99. [Google Scholar] [CrossRef]
- Marti, L.; Savatin, D.; Nora, G.; Valeria, D.; Cervone, F.; Giulia, D. The intracellular ROS accumulation in elicitor-induced immunity requires the multiple organelle-targeted Arabidopsis NPK1-related protein kinases. Plant Cell Environ. 2020, 44, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Gudimella, R.; Singh, P.; Mazumdar, P.; Wong, G.R.; Lau, S.-E.; Harikrishna, J.A. Genome-wide regulatory network mapping of miRNA and transcription factors in banana roots. Trop. Plant Biol. 2018, 11, 141–153. [Google Scholar] [CrossRef]
- Muthusamy, M.; Uma, S.; Backiyarani, S.; Saraswathi, M.S. Computational prediction, identification, and expression profiling of microRNAs in banana (Musa spp.) during soil moisture deficit stress. J. Hortic. Sci. Biotechnol. 2014, 89, 208–214. [Google Scholar] [CrossRef]
- Shen, C.; Yang, R.; Yue, C.; Cao, H. Cloning of three MAPKKK genes and their expression during postharvest processing in tea plant. J. Nucl. Agric. Sci. 2021, 35, 1281–1290. [Google Scholar] [CrossRef]
- Lu, R.; Hu, S.; Feng, J.; Liu, Z.; Kang, C. The AP2 transcription factor BARE RECEPTACLE regulates floral organogenesis via auxin pathways in woodland strawberry. Plant Cell 2024, 36, 4970–4987. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Wang, Y.; Li, Y.; Zhu, R.; Gu, Y.; Li, J.; Guo, H.; Ye, W.; Nabi, H.G.; Yang, T.; et al. The OsNAC41-RoLe1-OsAGAP module promotes root development and drought resistance in upland rice. Molecular Plant 2024, 17, 1573–1593. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Mehra, P.; Clark, L.; Mukkawar, V.; Bellande, K.; Martin-Arevalillo, R.; Ghosh, S.; Ingole, K.D.; Bhagat, P.K.; Brown, A.; et al. Redox-regulated Aux/IAA multimerization modulates auxin responses. Science 2025, 389, eadu1470. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Chen, X.; Xiang, C.; Tang, N.; Zhang, Q.; Xiong, L. Evaluation of seven function-known candidate genes for their effects on improving drought resistance of transgenic rice under field conditions. Mol. Plant 2009, 2, 73–83. [Google Scholar] [CrossRef]
- Shou, H.; Bordallo, P.; Wang, K. Expression of the Nicotiana protein kinase (NPK1) enhanced drought tolerance in transgenic maize. J. Exp. Bot. 2004, 55, 1013–1019. [Google Scholar] [CrossRef]
- Omer, R.; Matheka, J.; Ali, A. Transformation of tropical maize with the NPK1 gene for drought tolerance. Int. J. Genet. Eng. 2013, 3, 7–14. [Google Scholar]
- Yin, L.; Qu, P.; Wang, D.; Yan, S.; Gong, Q.; Yang, R.; Hu, Y.; Liu, N.; Cheng, C.; Wang, P.; et al. The influence of Piriformospora indica colonization on the root development and growth of cerasus humilis cuttings. Plants 2024, 13, 1482. [Google Scholar] [CrossRef]
- Jyothymol, C.; Kutty, M.; Pradeepkumar, T.; Parvathi, M.; Rashmi, C. Piriformospora indica improves water stress tolerance in watermelon (Citrullus lanatus (Thunb.) Matsum & Nakai). Plant Physiol. Rep. 2024, 29, 638–650. [Google Scholar] [CrossRef]
- Ortega-Villaizan, A.G.; King, E.; Patel, M.K.; Perez-Alonso, M.-M.; Scholz, S.S.; Sakakibara, H.; Kiba, T.; Kojima, M.; Takebayashi, Y.; Ramos, P.; et al. The endophytic fungus Serendipita indica affects auxin distribution in Arabidopsis thaliana roots through alteration of auxin transport and conjugation to promote plant growth. Plant Cell Environ. 2024, 47, 3899–3919. [Google Scholar] [CrossRef]
- Cao, J.; He, W.; Zou, Y.; Wu, Q. An endophytic fungus, Piriformospora indica, enhances drought tolerance of trifoliate orange by modulating the antioxidant defense system and composition of fatty acids. Tree Physiol. 2023, 43, 452–466. [Google Scholar] [CrossRef]
- Xu, F.; Liao, H.; Zhang, Y.; Yao, M.; Liu, J.; Sun, L.; Zhang, X.; Yang, J.; Wang, K.; Wang, X.; et al. Coordination of root auxin with the fungus Piriformospora indica and bacterium Bacillus cereus enhances rice rhizosheath formation under soil drying. ISME J. 2022, 16, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, J.; Hashem, A.; Abd Allah, E.F.; Wu, Q. Serendipita indica mitigates drought-triggered oxidative burst in trifoliate orange by stimulating antioxidant defense systems. Front. Plant Sci. 2023, 14, 1247342. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).