Abstract
Interest in biological pest control using ants in agroforestry and agricultural systems has increased in recent decades due to the diversity and abundance of these insects in different ecosystems. Biological pest control has emerged as an alternative to reduce the impact of production on agroecosystems, and ants play a crucial role in this context. Therefore, this study aimed, based on an extensive and rigorous literature review, to describe the potential of ants as biological control agents, as well as the pests that have been targeted by this control. The search was carried out between July and November 2023, using databases such as Lilacs, Scielo, and Google Scholar. The selected descriptors were “Predatory ants”, “Natural enemy ants”, and “Chemical defense ants”, used in Portuguese, English, and Spanish. These terms were used in isolation and with the Boolean operator “AND”. A total of 47 articles published between 1976 and 2023 were reviewed. The results showed that 34 genera and 70 species of ants have potential for use in biological control. Among the most notable genera are Camponotus, Crematogaster, Oecophylla, Pheidole, Solenopsis, and Wasmannia. Their role as biological control agents can be complementary, contributing to the maintenance and balance of agroecosystems through pest predation, which can reach 100% efficiency. The predatory potential of ants has been verified, with an emphasis on biological control against invertebrate pests of cultivated plants. Among the pests potentially controlled by ants are mites, coleopterans, fruit flies, bedbugs, lepidopterans, thrips, mollusks, and other ants. The scientific literature already contains robust evidence proving the potential of ants as biological control agents, especially for invertebrate pests.
1. Introduction
The search for sustainable production in agroecosystems and the food and nutritional security of the population has been a constant, with pesticides at the center of this context over the last few decades [1]. The movement of people and international trade, combined with climate change, has resulted in the dispersal and population growth of many organisms with the potential to become agricultural pests [2]. On the one hand, the emergence of resistance to products already in use has led to a search for new molecules and new pesticides capable of meeting production demands [3] and an increase in the volume of pesticides used. On the other hand, there is increasing pressure from consumer markets for safer products and from society in general, which advocates for less environmental impact from production chains [4]. In this context, biological pest control is emerging as an alternative to reduce the effect of production on agroecosystems.
Historically, biological pest control has been consolidated as an alternative where natural enemies regulate the density of organisms with the potential to become pests while also helping to reduce damage and production costs [5]. Biological control minimizes the use of pesticides and helps maintain biodiversity [6]. Ants are among the organisms potentially contributing to biological pest control [7]. These insects occupy the most varied trophic niches in their ecosystems, emphasizing the predation of other invertebrate organisms and some species’ chemical interactions of defense and repellency [8].
Ants (Hymenoptera: Formicidae) represent a diversity of 22 subfamilies, 503 genera, and 15,876 valid species [9]. They are a dominant group of terrestrial fauna that play a variety of roles in the ecosystems they inhabit, such as the degradation of organic matter, nutrient recycling, soil aeration, seed removal, and predation [1,10]. Among the characteristics that make ants of interest in agroecosystems and biological control are their abundance, diversity, dominance, ease of sampling, sensitivity to environmental disturbances, and the numerous ecological interactions they have with other organisms such as fungi, animals, and plants [9,11].
The predatory nature of some ant taxa stands out in regulating the structure and function of the ecosystems in which they are found [12], suppressing the populations of their prey, and reducing the abundance and distribution of species with the potential to become pests [13,14]. The predatory potential of ants in controlling agricultural pests has been the subject of studies worldwide in different crops, biomes, soils, and climatic conditions, both in isolation and integrated with other control mechanisms [1].
Another mechanism developed by ants that has been studied in the context of ecology and agroecosystems is chemical defense [15]. This is a prominent feature of the adaptive behavior of some ant species that produce and release complex chemical compounds, often endowed with bioactive properties such as toxins and defensive pheromones [16,17]. The potential of ants to control agricultural pests, especially herbivores, derives from studies carried out in Africa, America, and Asia. However, this knowledge is fragmented and needs to be systematized.
Considering the need for constant progress in the search for sustainable agricultural pest management practices, the ecological role of ants as predators, and the chemical defense used by some of these insects, interest in the potential of these organisms in biological pest control is emerging [18,19]. By feeding on insects that are harmful to crops, ants help regulate pest populations naturally, reducing the need for chemical interventions [20] and, consequently, contributing to lower production costs, food safety, and human health. Therefore, this study was based on the following problem: what does the scientific literature say about the potential of ants for biological pest control in agroecosystems? Its objectives were: (a) to map scientific production on the predatory role of ants and the chemical defense of these insects against pests in agroecosystems and (b) to list the species of ants and pests that have been the target of studies on biological control.
2. Materials and Methods
2.1. Study Characterization
An integrative literature review was conducted to examine the scientific literature on ants’ potential as biological control agents. The integrative literature review is a research method that enables synthesizing and analyzing existing theoretical and empirical literature on a given phenomenon [13,21]. This method provides new questions, reflections, and criticisms, potentially identifying gaps and advancing knowledge in the field.
2.2. Search, Selection, and Inclusion of Articles
The following steps were followed in the integrative review: (1) Identifying the research question; (2) Searching the scientific literature, establishing inclusion and exclusion criteria, and selecting the studies; (3) Categorizing the results found; (4) Evaluating the selected articles; (5) Analyzing, interpreting and discussing the results; (6) Synthesizing the information and producing knowledge [21].
The following study question was formulated to guide the integrative review: What does the scientific literature say about the potential of ants in the biological control of pests in agroecosystems? A search for articles was carried out from July to November 2023, with no restriction on the time of publication, in the following databases: Lilacs, Scielo, and Google Scholar. The descriptors selected were “Predatory ants”, “Natural enemy ants”, and “Chemical defense ants”, used in Portuguese, English, and Spanish. These terms were used in isolation and with the Boolean operator “AND”.
Only articles were selected, excluding theses, dissertations, monographs, and abstracts. The inclusion criteria were articles that, after reading the title and abstract, were related to the study problem. After this stage, the selected articles were read in their entirety and once again assessed for their relevance to the topic. The consulted time series were not restricted, but only open-access studies were included. All selected articles were downloaded to a Portable Document Format (.pdf) electronic directory. A total of 531 articles were identified initially. The preliminary analysis involved reading the titles, abstracts, and keywords. Based on this pre-analysis, 40 articles that answered the study question were selected for the review (Appendix A).
The articles that met the eligibility criteria were read, and relevant information was extracted and tabulated in an Microsoft Excel database (.xlsx) v. 2010 (14.0.7268.5000). The information extracted included year of publication, scientific journal, authors, title, country where the study was carried out, objective, species or genus evaluated, focus (predators or chemical defense), summary, and conclusion. Tabulating this information allowed for systematic organization and analysis of the selected articles, facilitating the synthesis of findings and identification of key insights related to the potential of ants in biological pest control within agroecosystems.
3. Results
We included 47 articles published between 1976 and 2024. Studies have historically emphasized ants’ predatory role and, more recently, chemical defense (Figure 1).
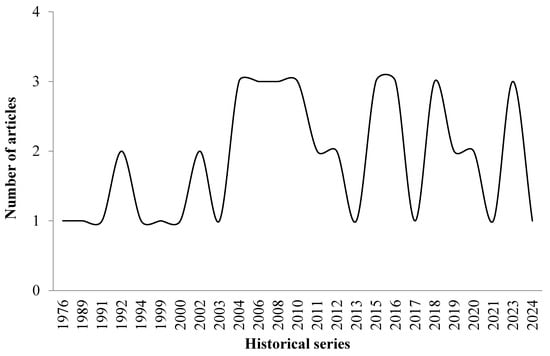
Figure 1.
Historical series and frequency of studies on potential ants for biological control in agroecosystems, 1976–2023.
The crops evaluated in the studies include cotton, peanuts, sugar beets, coffee, sugarcane, beans, corn, and soybeans. In the context of fruit growing, the studies covered banana, cashew, citrus, coconut, mango, pequi, and peach orchards. The role of ants in balancing the agroecosystem, interactions with other predators and parasitoids, and comparisons between conventional and organic crops were also covered (Table 1).

Table 1.
Objectives of studies on the potential of ants for biological control in agroecosystems, 1976–2024.
Ants from seven subfamilies with potential for biological control were listed. Myrmicinae was the most included with 12 genera and 28 species, followed by Formicinae with 8 genera and 17 species, Dolichoderinae with 7 genera and 6 species, Ponerinae with 4 genera and 8 species, Ectatomminae with 2 genus and 7 species, Pseudomyrmecinae with 1 genus and 4 species, and Dorylinae with 1 genus. The diversity reported in the articles was distributed across five continents (Africa, America, Asia, Europe, and Oceania) (Table 2).

Table 2.
Diversity of ants and pests assessed by studies on the potential of ants for biological control in agroecosystems, 1976 to 2023. Au = augmentative biological control; Co = conservation biological control; Cl = classic biological control.
The predatory potential of ants was verified, emphasizing biological control against herbivorous invertebrates of cultivated plants carried out by ants Anoplolepis sp., Dolichoderus sp., Oecophylla sp., and Wasmannia sp. Specifically, as biological control agents of the coffee berry borer Hypothenemus hampei (Ferrari, 1867) (Coleoptera: Scolytidae), the species Pseudomyrmex ejectus, Pseudomyrmex simplex, Solenopsis geminata, Tetramorium bicarinatum, Tetramorium simillimum, and Wasmannia auropunctata were identified. In the control of Diatraea saccharalis (Fabricius, 1794) (Lepidoptera, Pyralidae) in sugarcane (Saccharum officinarum L.), the ants Camponotus atriceps, Crematogaster sp., Dorymyrmex sp., Ectatomma ruidum, Pheidole sp., and Solenopsis invicta were noted. For the control of tephritid fruit flies (Diptera: Tephritidae), the ants Dorymyrmex sp., Ectatomma brunneum, Formica fusca, Leptotorax sp., Odontomachus brunneus, Oecophylla longinoda, Pheidole gertrudae, Pheidole megacephala, Pheidole oxyops, Solenopsis sp., Solenopsis invicta, and Solenopsis geminata were observed (Table 2).
4. Discussion
The potential of ants as biological control agents in agroecosystems, especially as predators, has been explored since the 1970s [22,66]. Studies have focused on different crops [23,37,38,47,52,53], types of cultivation [26,57,62,63], and the role of these insects in the ecological balance and sustainability [39,50,51] of these environments. However, since the year 2000, there has been an intensification of studies related to ant fauna. Among the studies that contributed to this increase in scientific production was the work by Hölldobler and Wilson [8], which gathered information on the biology, ecology, biodiversity, and biogeography of these insects, encouraging researchers around the world to consider them as subjects of their research. In the field of taxonomy, an important advancement was the work of Barry Bolton, which resulted in an online database that gathers and updates information from all over the world on the classification of ants [9].
Regarding distribution, the studies included in this review were conducted on five continents, with contributions from America, Europe, Africa, Asia, and Oceania. The studies conducted in the Americas addressed the role of ants in agroecosystems more comprehensively, including biological control through predation [38,41], chemical defense [31], as well as interaction with other organisms beneficial to production [31,36]. The reviewed studies from other continents emphasized biological control by ants. Similarly, the studies conducted in America covered a greater diversity of crops such as cotton, peanuts, beets, coffee, sugar cane, cabbage, beans, corn, soybeans [23,24,27,31,34], fruits such as Citrus sp. and peaches [7,43], and chemical defense against herbivores [31]. Studies conducted in Europe have emphasized the potential of the species Linepithema humile in Portugal [28], Myrmicaria opaciventris in France, and Crematogaster scutellaris in Italy [32,63], all focused on biological control. The African studies focused on the ant Oecophylla longinoda against cashew pests [49] and Pheidole megacephala in coffee crops [52]. In Asia, the potential of Monomorium floricola, Crematogaster sp., Technomyrmex albipes, and Oecophylla smaragdina in palm plantations was evaluated [23]. Studies from Oceania (both conducted in Australia) evaluated the weaver ant (Oecophylla smaragdina) for the control of the red thrips Selenothrips rubrocinctus and Amblypelta lutescens [64].
The biodiversity of ants listed in this review can be attributed to a greater concentration of studies on the American continent, where most of the subfamilies and most of the genera and species listed are native. Myrmicinae comprises a subfamily with 147 genera and 7133 valid species [9], ranging from highly specialized species to generalists, from predators to fungus cultivators [67,68]. Ant species such as Crematogaster, Pheidole, Solenopsis, and Wasmannia nest and forage in soil, leaf litter, and vegetation. They are tolerant of modified environments, including agroecosystems. Their omnivorous diet, which includes a variety of small invertebrates [69], underscores their significance in biological control and ecosystem balance.
Formicinae includes 52 genera and 3265 valid species, among which the Camponotus genus stands out, with 1087 species distributed on all continents except Antarctica [9]. Ants from this genus are notable for their variety of niches in ecosystems, their tolerance to different environments, and the generalist nature of some species [68,69]. The Formica genus comprises 179 valid species in Asia, Europe, North and Central America, and northwest Africa [9], sharing characteristics and habits with the Camponotus genus [70]. Oecophylla consists of three valid species distributed in Africa, Asia, and Oceania. Known as weaver ants, dominant in the forest canopy, their colonies can exceed 500,000 individuals and construct hundreds of nests in various trees, fiercely defending them against other colonies. They are predators of insects in both vegetation and ground habitats [8]. Conversely, Nylanderia fulva and Paratrechina longicornis are South American ants that are invasive, generalists, and opportunists in soil and vegetation [69,71], justifying their potential as biological control agents.
Dolichoderinae comprises 28 genera and 714 valid species distributed across all continents except Antarctica [9]. The genus Azteca stands out, with 84 species occurring in the Americas. These ants nest and forage in vegetation, with some species forming obligatory associations with plants of the Cecropia genus [72]. The chemical defense exhibited by Dolichoderinae ants against invertebrate pests, including other ants, has been the subject of numerous studies [69]. Ponerinae (50 genera and 1275 valid species), along with Ectatomminae (12 genera and 305 valid species), Dorylinae (27 valid genera and 756 valid species), and Pseudomyrmecinae (3 genera and 235 valid species) [9], encompass a cosmopolitan ant fauna, excluding Antarctica. These ants are predators from the soil and leaf litter to the forest canopy, specializing in various niches and preying on invertebrates and small vertebrates [66,69,73,74]. Some species tolerate environmental changes and are frequently encountered in agroecosystems, highlighting their potential as biological control agents and ecosystem regulators.
Most of the literature consulted has underscored the potential of ants as biological control agents in agroecosystems. Way et al. [23] recorded the predation of Opisina arenosella eggs by Oecophylla smaragdina. Perfecto [24] verified the predation of the corn caterpillar Spodoptera frugiperda and the corn leafhopper Dalbulus maidis by Pheidole radoszkowskii and Solenopsis geminata, as well as the reduction in damage caused by the cartridge caterpillar to corn plants. The presence of predatory ants under the plants can interfere with the presence of other arthropods [55], benefiting the crop. Knutson and Campos [38] described Solenopsis invicta ants as beneficial to production in Texas despite the pest role attributed to the species in the United States of America. Fernandes et al. [41] described the role of this ant in reducing pest populations at the start of bean cultivation, while Baldwin et al. [14] highlighted its potential in controlling Helicoverpa zea. Additionally, this ant can impact the community of epigeic arthropods, both harmful and beneficial to production in aggregate ecosystems, making its benefit dependent on the management adopted in production [45].
Population control of Diatraea saccharalis by predatory ants was verified by Rossi and Fowler [34]. Sujii et al. [35] confirmed the pasture leafhopper (Deois flavopicta) predation by Pachycondyla obscuricornis. Queiroz, Almeida, and Pereira [20] gathered evidence of the impact of predation by Formica polyctena on various insects, as well as by the ants Ectatomma ruidum and Ectatomma tuberculatum on coffee pests and Solenopsis invicta on insects attacking cotton and soybean crops. Santos et al. [37] also reported the foot-washing ant Solenopsis invicta as a predator of immature specimens of Anthonomus grandis in cotton crops. Milligan et al. [52] documented a 12% reduction in the population of Sesamia calamistis attributed to the ant Pheidole megacephala, while Larsen and Philpott [42], Morris et al. [58], and Martins et al. [65] presented evidence that ants reduce the infestation of the coffee berry borer Hypothenemus hampei.
The predation of the psyllid Cacopsylla pyricola by ants was reported by Paulson and Akre [25]. Rodrigues et al. [42] documented the role of Pseudomyrmex termitarius in predating Toxoptera citricida in mandarin orchards. Abeijon et al. [7] described the predation of Anastrepha fraterculus larvae by Pachycondyla, Pheidole, Pogonomyrmex, and Solenopsis ants, as well as their role in biological control of tephritid flies [20,60]. However, these and other reviewed studies have suggested that ants sometimes play a supportive role as predators in both orchards and agroecosystems in general [27,31]. Thus, the effectiveness of these insects in biological pest control depends on their interaction with other predators and the management practices adopted in each case.
In this regard, Way and Khoo [26] emphasized that ants serve as an alternative to chemical insecticides when such practices are not feasible or when production systems prohibit the use of synthetic compounds. Schifani et al. [63] also underscored the role of ants as supplements in integrated pest management strategies, while Lange et al. [39] noted that the no-till system can sustain a more diverse and abundant ant fauna, thereby enhancing the environmental quality of the crop.
Additionally, Way, Paiva, and Cammell [28] described the activity of the ant Linepithema humile in extensive areas due to their existence as supercolonies. Mendonça and Romanowski [30] reported the predation of Eugeniamyia dispar by ants Pseudamyrmex sp. and Pheidole sp., while Kenne et al. [32] highlighted the role of Myrmicaria opaciventris as a biological control agent against termites. The predation of thrips Selenothrips rubrocinctus by weaver ants (Oecophylla) was reported by Peng and Christian [33]. Peng, Christian, and Reilly [46] reported that the aggressive behavior of Oecophylla smaragdina repels pests from the shoots of African mahogany. Some ant species are important predators of the pasture tick (Rhipicephalus (B.) microplus). Veríssimo [47] discussed the role of Nylanderia fulva in the biological control of Atta spp. Regarding chemical defense, Vandermeer et al. [31] indicated that the ant Azteca sp. has potential as a pest control agent due to its positive effect on insect pests, highlighting its potential as a biological control agent.
Despite the outstanding potential of ants in the biological control of pests, the antagonistic role of predatory and/or generalist species of these insects on other natural enemies involved in biological control must be considered. The association between Camponotus ants and aphids (Hemiptera: Aphididae) is well documented in the scientific literature [68]. The protection from predators and parasites provided by the ants to the aphids is rewarded by the production of honeydew, thus establishing a symbiotic relationship. The negative impact of invasive ants such as Linepithema humile [62] and Solenopsis invicta [20,44] on other natural enemies and, consequently, on the ecological balance is also well known.
In this context, Milosavljević et al. [75] analyzed the effects of ants on the population regulation of Diaphorina citri Kuwayama (Hemiptera: Liviidae) by the parasitoid Tamarixia radiata (Waterston) (Hymenoptera: Eulophidae) in southern California over four years. The presence of the ant Linepithema humile increased D. citri densities threefold, indicating the need to control this ant to maximize the biological control of the psyllid. Hoddle et al. [76] found that Linepithema humile protects D. citri from natural enemies and highlighted the need to control this ant species to increase the effectiveness of natural enemies against D. citri.
On the other hand, in the context of predation, there is no consensus on the impact of ants on the ecological balance of agroecosystems. Janssen et al. [77] gathered evidence that, contrary to expectations, there is no increase in pest populations due to intraguild predation, even when the predator is the inferior natural enemy. These authors suggested that the introduction of generalist natural enemies, often intraguild predators, may not affect the biological control performed by native natural enemies.
5. Conclusions
It was possible to list 32 genera and 47 species of ants with potential for use in biological control. The most prominent genera are Camponotus, Crematogastes, Oecophylla, Pheidole, Solenopsis, and Wasmannia. Their role as biological control agents can be complementary, contributing to the maintenance and balance of agroecosystems through pest predation, with efficiency rates reaching up to 100%.
However, there are limitations to the use of ants as biological control agents. One such limitation is the need for taxonomic knowledge, as species within the same genus can sometimes become pests; for example, pests of citrus, corn, and coffee grown around the world, but with ant species’ distribution restricted to a particular region or continent. Additionally, the distribution of predator species does not always align with the dispersal area of the pests, which can be considered another significant limiting factor.
Some ants such as Camponotus are known for their relationships with sucking insects like aphids, where they provide protection for these pest species. Generalist invasive species like Linepithema humile and Solenopsis invicta can negatively impact biological control as they affect the populations of other natural enemies, thus becoming antagonists in pest control. In this context, certain ant species become the pests that need to be controlled.
Despite decades of recognizing the potential of ants as biological control agents, there are still gaps in knowledge, particularly regarding the impact of conventional pest control techniques on these insects. Consequently, their potential for pest control may have been affected. Nevertheless, the scientific literature already contains robust evidence of the potential of ants as biological control agents, especially against invertebrate pests.
Author Contributions
Conceptualization, J.A.L. and F.R.M.G.; methodology, J.A.L., F.R.M.G. and M.O.R.; software, J.A.L. and M.O.R.; formal analysis, J.A.L., C.J.L., A.O., F.S.Z., M.O.R. and F.R.M.G.; investigation, J.A.L., C.J.L., A.O., F.S.Z., M.O.R. and F.R.M.G.; resources, J.A.L.; writing—original draft preparation, J.A.L., C.J.L., A.O., F.S.Z., M.O.R. and F.R.M.G.; writing—review and editing, J.A.L. and F.R.M.G.; supervision, J.A.L.; project administration, J.A.L.; funding acquisition, J.A.L. All authors have read and agreed to the published version of the manuscript.
Funding
Coordination for the Improvement of Higher Education Personnel (CAPES); Community University of Chapecó Region (Unochapecó); CNPq for a productivity grant to FRMG.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The datasets analyzed in the present study are available from the corresponding authors on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
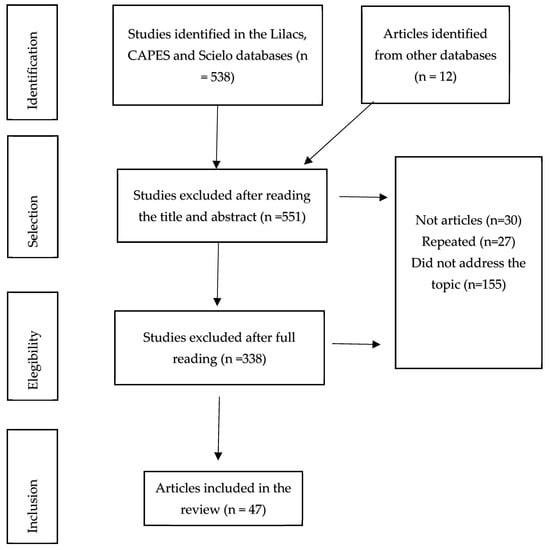
Figure A1.
Search Strategies for Articles Included in the Research on the Potential of Ants as Natural Enemies, 2023.
References
- Anjos, D.V.; Tena, A.; Viana-Junior, A.B.; Carvalho, R.L.; Torezan-Silingardi, H.; Del-Claro, K.; Perfecto, I. The effects of ants on pest control: A meta-analysis. Proc. R. Soc. 2022, 289, 20221316. [Google Scholar] [CrossRef] [PubMed]
- ONU. United Nations. Mudança Climática Influencia na Perda da Produção Agrícola Para Pragas, Conclui Estudo Apoiado Pela FAO. 2021. Available online: https://brasil.un.org/pt-br/130780-mudan%C3%A7a-clim%C3%A1tica-influencia-na-perda-da-produ%C3%A7%C3%A3o-agr%C3%ADcola-para-pragas-conclui-estudo (accessed on 30 October 2023).
- Jardim, I.C.S.F.; Andrade, J.A.; Queiroz, S.C.N. Resíduos de agrotóxicos em alimentos: Uma preocupação ambiental global–um enfoque às maçãs. Quím. Nova 2009, 32, 996–1012. [Google Scholar] [CrossRef][Green Version]
- Martine, G.; Alves, J.E.D. Economia, sociedade e meio ambiente no século 21: Tripé ou trilema da sustentabilidade? Rev. Bras. Estud. Popul. 2015, 32, 433–460. [Google Scholar] [CrossRef]
- Waage, J.K.; Greathead, D.J. Biological control: Challenges and opportunities. Philos. Trans. R. Soc. Lond. 1988, 318, 111–128. [Google Scholar] [CrossRef]
- Crowder, D.W.; Jabbour, R. Relationships between biodiversity and biological control in agroecosystems: Current status and future challenges. Biol. Control 2014, 75, 8–17. [Google Scholar] [CrossRef]
- Abeijon, L.M.; Kruger, A.P.; Lutinski, J.A.; Garcia, F.R.M. As formigas podem contribuir para o controle biológico conservador da mosca-das-frutas sul-americana? Biosci. J. 2019, 35, 941–948. [Google Scholar] [CrossRef]
- Hölldobler, B.; Wilson, E.O. The Ants; Harvard University Press: Cambridge, UK, 1990; 732p. [Google Scholar]
- AntWeb, Version 8.103.2; Academia de Ciências da Califórnia: San Francisco, CA, USA, 2024. Available online: https://www.antweb.org (accessed on 30 January 2024).
- Wilson, E.O.; Hölldobler, B. The rise of the ants: A phylogenetic and ecological explanation. Proc. Natl. Acad. Sci. USA 2005, 102, 7411. [Google Scholar] [CrossRef] [PubMed]
- Maleque, M.A.; Maeto, K.; Ishii, H.T. Arthropods as bioindicators of sustainable forest management, focusing on plantation forests. Appl. Entomol. Zool. 2009, 44, 1–11. [Google Scholar] [CrossRef]
- Lopes, D.T.; Lopes, J.; Nascimento, I.C.; Delabie, J.H. Diversidade de formigas epigeicas (Hymenoptera, Formicidae) em três ambientes no Parque Estadual Mata dos Godoy, Londrina, Paraná. Iheringia Sér. Zool. 2010, 100, 84–90. [Google Scholar] [CrossRef]
- Souza, D.R.; Stingel, E.; Almeida, L.C.; Lazarini, M.A.; Munhae, C.B.; Bueno, O.C.; Archangelo, C.R.; Morini, M.S.C. Field methods for the study of ants in sugarcane plantations in Southeastern Brazil. Sci. Agric. 2010, 67, 651–657. [Google Scholar] [CrossRef][Green Version]
- Baldwin, J.; Paula-Moraes, S.V.; Pereira, R. The Good Side of the Bad Guys: Predation of Lepidopteran Pests by Solenopsis invicta Buren (Hymenoptera: Formicidae) in the Florida Panhandle. Fla. Entomol. 2023, 103, 68–71. [Google Scholar] [CrossRef]
- Delabie, J.H.C.; Ramos, L.S.; Santos, J.R.M.; Campiolo, S.; Sanches, C.L.G. Mirmecofauna (Hymenoptera, Formicidae) da serapilheira de um cacaual inundável do agrossistema do rio mucuri, Bahia: Considerações sobre conservação da fauna e controle biológico de pragas. Agrotrópica 2007, 19, 5–12. [Google Scholar]
- Guarda, C.; Lutinski, J.A. Secreções glandulares de formigas (Hymenoptera: Formicidae): Uma revisão sobre extração, caracterização química e potencial antibiótico. Sociobiology 2020, 67, 13–25. [Google Scholar] [CrossRef]
- Parker, J.; Kronauer, D.J.C. How ants shape biodiversity. Curr. Biol. 2021, 31, R1208–R1214. [Google Scholar] [CrossRef] [PubMed]
- Philpott, S.M.; Armbrecht, I. Biodiversity in tropical agroforests and the ecological role of ants and ant diversity in predatory function. Ecol. Entomol. 2006, 31, 369–377. [Google Scholar] [CrossRef]
- Tiscoski, N.S. Controle Biológico Conservativo de Pragas no Café. Agroecol. Prod. Sustentabilidade Pesqui. 2023, 4, 49–59. [Google Scholar] [CrossRef]
- Queiroz, J.M.; Almeida, F.S.; Pereira, M.P.S. Conservação da biodiversidade e o papel das formigas (Hymenoptera: Formicidae) em agroecossistemas. Floresta Ambiente 2006, 13, 37–45. [Google Scholar]
- Mendes, K.D.S.; Silveira, R.C.C.P.; Galvão, C.M. Revisão integrativa: Método de pesquisa para a incorporação de evidências na saúde e na enfermagem. Texto Contexto-Enferm. 2008, 17, 758–764. [Google Scholar] [CrossRef]
- Coutinho, A.B. Um inimigo natural dos caramujos do gênero Biomphalaria. Rev. Soc. Bras. Med. Trop. 1976, 10, 385–387. [Google Scholar] [CrossRef][Green Version]
- Way, M.J.; Cammell, M.E.; Bolton, B.; Kanagaratnam, P. Ants (Hymenoptera: Formicidae) as egg predators of coconut pests, especially in relation to biological control of the coconut caterpillar, Opisina arenosella Walker (Lepidoptera: Xyloryctidae) in Sri Lanka. Bull. Entomol. Res. 1989, 79, 219–233. [Google Scholar] [CrossRef]
- Perfecto, I. Ants (Hymenoptera: Formicidae) as natural control agents of pests in irrigated maize in Nicaragua. J. Econ. Entomol. 1991, 84, 65–70. [Google Scholar] [CrossRef]
- Paulson, G.S.; Akre, R.D. Avaliando a eficácia das formigas como agentes de controle biológico de Pear phyla (Homoptera: Psyllidae). J. Econ. Entomol. 1992, 85, 70–73. [Google Scholar] [CrossRef]
- Way, M.J.; Khoo, K.C. Role of ants in pest management. Annu. Rev. Entomol. 1992, 37, 479–503. [Google Scholar] [CrossRef]
- Ruberson, J.; Herzog, G.; Lambert, W.; Lewis, J. Management of the beet armyworm (Lepidoptera: Noctuidae) in cotton: Role of natural enemies. Fla. Entomol. 1994, 77, 441–453. [Google Scholar] [CrossRef]
- Way, M.; Paiva, M.; Cammell, M. Natural biological control of the pine processionary moth Thaumetopoea pityocampa (Den. & Schiff.) by the Argentine ant Linepithema humile (Mayr) in Portugal. Agric. For. Entomol. 1999, 1, 27–31. [Google Scholar] [CrossRef]
- Shatters, R.G.; Vander Meer, R.K. Characterizing the interaction between fire ants (Hymenoptera: Formicidae) and developing soybean plants. J. Econ. Entomol. 2000, 93, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, J.; Romanowski, H.P. Natural enemies of the gall-maker Eugeniamyia dispar (Diptera, Cecidomyiidae): Predatory ants and parasitoids. Braz. J. Biol. 2002, 62, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Vandermeer, J.; Perfecto, I.; Ibarra, N.G. Formigas (Azteca sp.) como potenciais agentes de controle biológico na produção de café à sombra em Chiapas, México. Sist. Agroflorestais 2002, 56, 271–276. [Google Scholar] [CrossRef]
- Kenne, M.; Schatz, B.; Durand, J.L.; Dejean, A. Hunting strategy of a generalist ant species proposed as a biological control agent against termites. Entomol. Exp. Appl. 2003, 94, 31–40. [Google Scholar] [CrossRef]
- Peng, R.; Christian, K. The weaver ant, Oecophylla smaragdina (Hymenoptera: Formicidae), an effective biological control agent of the red-banded thrips, Selenothrips rubrocinctus (Thysanoptera: Thripidae) in mango crops in the Northern Territory of Australia. Int. J. Pest Manag. 2004, 50, 107–114. [Google Scholar] [CrossRef]
- Rossi, M.N.; Fowler, H.G. Predaceous ant fauna in new sugarcane fields in the state of São Paulo, Brazil. Braz. Arch. Biol. Technol. 2004, 47, 805–811. [Google Scholar] [CrossRef]
- Sujii, E.R.; Garcia, M.A.; Fontes, E.M.G.; O’Neil, R.J. Pachycondyla obscuricornis as natural enemy of the spittlebug Deois flavopicta. Pesqui. Agropecu. Bras. 2004, 39, 607–609. [Google Scholar] [CrossRef][Green Version]
- Resende, A.L.S.; Silva, E.E.; Silva, V.B.; Ribeiro, R.L.D.; Guerra, J.G.M.; Aguiar-Menezes, E.L. Primeiro registro de Lipaphis pseudobrassicae Davis (Hemiptera: Aphididae) e sua associação com insetos predadores, parasitóides e formigas em couve (Cruciferae) no Brasil. Neotrop. Entomol. 2006, 35, 551–555. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Santos, W.; Júnior, E.; Salazar, J.; Zanuncio Junior, J.; Zanuncio, C. Controle biológico de artrópodes pragas do algodoeiro com predadores e parasitóides. Rev. Bras. Ol. Fibrosas 2006, 10, 1147–1165. [Google Scholar]
- Knutson, A.E.; Campos, M. Efeito da formiga vermelha importada, Solenopsis invicta, na abundância da lagarta da espiga do milho, Helicoverpa zea, no milho no Texas. Southwest. Entomol. 2008, 33, 1–13. [Google Scholar] [CrossRef]
- Lange, D.; Fernandes, W.D.; Raizer, J.; Faccenda, O. Predacious activity of Ants (Hymenoptera: Formicidae) in conventional and in No-till agriculture systems. Braz. Arch. Biol. Technol. 2008, 51, 1199–1207. [Google Scholar] [CrossRef]
- Van Mele, P. A historical review of research on the weaver ant Oecophylla in biological control. Agric. For. Entomol. 2008, 10, 13–22. [Google Scholar] [CrossRef]
- Fernandes, F.L.; Picanco, M.C.; Fernandes, M.E.S.; Xavier, V.M.; Martins, J.C.; Silva, V.F. Controle biológico natural de pragas e ecológicos com predadores e parasitóides em feijoeiro. Biosci. J. 2010, 26, 6–14. [Google Scholar]
- Larsen, A.; Philpott, S.M. Twig-Nesting Ants: The Hidden Predators of the Coffee Berry Borer in Chiapas, Mexico. Biotropica 2010, 42, 342–347. [Google Scholar] [CrossRef]
- Rodrigues, W.C.; Spolidoro, M.V.; Zinger, K.; Cassino, P.C. R Dinâmica Populacional de pulgão preto dos Citros (Sternorrhyncha) em cultivo orgânico de tangerina (Citrus reticulata Blanco) em Seropédica-RJ. EntomoBrasilis 2010, 3, 38–44. [Google Scholar] [CrossRef]
- Drummond, F.; Choate, B. Formigas como agentes de controle biológico em sistemas de cultivo agrícola. Revisões Artrópodes Terr. 2011, 4, 157–180. [Google Scholar] [CrossRef]
- Wickings, K.G.; Ruberson, J. Impact of the red imported fire ant (Hymenoptera: Formicidae) on Epigeic Arthropods of Cotton Agroecosystems. Ann. Entomol. Soc. Am. 2011, 104, 171–179. [Google Scholar] [CrossRef]
- Peng, R.; Christian, K.; Reilly, D. Controle biológico do percevejo Amblypelta lutescens usando formigas tecelãs Oecophylla smaragdina em mognos africanos na Austrália. Agric. For. Entomol. 2012, 14, 428–433. [Google Scholar] [CrossRef]
- Veríssimo, C.J. Controle biológico do carrapato do boi, Rhipicephalus (Boophilus) microplus no Brasil. Rev. MV&Z 2013, 11, 14–23. [Google Scholar] [CrossRef]
- Chevalier, L.X.T.; Gomes, D.S.; Mayhé-Nunes, A.J.; Queiroz, J.M. Potencial de Formigas (Hymenoptera: Formicidae) como Agentes Anti-herbívoros em Cultivo de Café (Coffea canephora Pierre) e Feijão Guandu [Cajanus cajans (L.) Millsp]. EntomoBrasilis 2013, 6, 113–118. [Google Scholar] [CrossRef]
- Anato, F.; Wargui, R.; Sinzogan, A.A.C.; Offenberg, J.; Adandonon, A.; Vayssières, J.F.; Kossou, D. Reducing losses inflicted by insect pests on cashew, using weaver ants as a biological control agent. Agric. For. Entomol. 2015, 17, 285–291. [Google Scholar] [CrossRef]
- De la Mora, A.; García-Ballinas, J.A.; Philpott, S.M. Local, landscape, and diversity drivers of predation services provided by ants in a coffee landscape in Chiapas, Mexico. Agric. Ecosyst. Environ. 2015, 201, 83–91. [Google Scholar] [CrossRef][Green Version]
- Offenberg, J. Ants as tools in sustainable agriculture. J. Appl. Ecol. 2015, 52, 1197–1205. [Google Scholar] [CrossRef]
- Milligan, M.C.; Johnson, M.D.; Garfinkel, M.; Smith, C.J.; Njoroge, P. Quantifying pest control services by birds and ants in Kenyan coffee farms. Biol. Conserv. 2016, 194, 58–65. [Google Scholar] [CrossRef]
- Wang, Z.; Moshman, L.; Kraus, E.C.; Wilson, B.E.; Acharya, N.; Diaz, R. A Review of the Tawny Crazy Ant, Nylanderia fulva, an Emergent Ant Invader in the Southern United States: Is Biological Control a Feasible Management Option? Insects 2016, 7, 77. [Google Scholar] [CrossRef]
- Wickings, K.; Ruberson, J. The red imported fire ant, Solenopsis invicta, modifies predation at the soil surface and in cotton foliage: Fire ants modify predation in cotton. Ann. Appl. Biol. 2016, 169, 319–328. [Google Scholar] [CrossRef]
- Gossler, O.S.; Lange, D.F.; Wedson, D. Can the absence of ants interfere in the arthropods abundance on corn plants (Zea mays L.—Poaceae)? Comun. Scientiae 2017, 8, 9–16. [Google Scholar] [CrossRef]
- Cologna, C.T.; Rodrigues, R.S.; Santos, J. Investigação peptídica do veneno de Neoponera villosa por espectrometria de massa de alta resolução: Variações sazonais e de habitat de nidificação. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 6. [Google Scholar] [CrossRef] [PubMed]
- Diamé, L.; Rey, J.Y.; Vayssières, J.F.; Grechi, I.; Chailleux, A.; Diarra, K. Ants: Major functional elements in fruit agro-ecosystems and biological control agents. Sustainability 2018, 10, 23. [Google Scholar] [CrossRef]
- Morris, J.; Jimenez Soto, E.; Philpott, S.; Perfecto, I. Ant-mediated (Hymenoptera: Formicidae) biological control of the coffee berry borer: Diversity, ecological complexity, and conservation biocontrol. Myrmecol. News 2017, 26, 1–17. [Google Scholar] [CrossRef]
- Polania, I.Z. Invasiones de cuatro hormigas vagabundas suramericanas: Una revisión sistemática. Rev. U.D.C.A Actual. Divulg. Cient. 2019, 22, e1207. [Google Scholar] [CrossRef]
- Garcia, F.R.M.; Ovruski, S.M.; Suárez, L.; Cancino, J.; Liburd, O.E. Biological control of tephritid fruit flies in the Americas and Hawaii: A review of the use of parasitoids and predators. Insects 2020, 11, 662. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, V.B.S.; Ferro, V.G. Are ants attracted to herbivorized leaves of Caryocar brasiliense Camb. (Caryocaraceae)? Biota Neotrop. 2020, 20, e20200992. [Google Scholar] [CrossRef]
- Anjos, D.V.; Tena, A.; Torezan-Silingardi, H.M.; Pekas, A.; Janssen, A. Ants affect citrus pests and their natural enemies in contrasting ways. Biol. Control 2021, 158, 104611. [Google Scholar] [CrossRef]
- Schifani, E.; Giannetti, D.; Costi, E.; Franconi, G.; Campostrini, A.; Maistrello, L.; Grasso, D. Interactions between egg parasitoids and predatory ants for the biocontrol of the invasive brown marmorated stink bug Halyomorpha halys. J. Appl. Entomol. 2023, 147, 868–874. [Google Scholar] [CrossRef]
- Exélis, M.P.; Ramli, R.; Ibrahim, R.W.; Idris, A.H. Foraging Behaviour and Population Dynamics of Asian Weaver Ants: Assessing Its Potential as Biological Control Agent of the Invasive Bagworms Metisa plana (Lepidoptera: Psychidae) in Oil Palm Plantations. Sustentability 2023, 15, 780. [Google Scholar] [CrossRef]
- Martins, D.S.; Fornazier, M.J.; Abonizio-Santos, M.R.; Guarçoni, R.C.; Teixeira, A.F.R.; Magalhães, F.S.; Souza-Campana, D.R.; Morini, M.S. Can season and intercropping Conilon coffee favor predatory ant species? Coffee Sci. 2024, 19, e192187. [Google Scholar] [CrossRef]
- Palácio, E.E. Subfamília Ecitoninae. In Introdução às Hormigas da Região Neotropical; Fernández, F., Ed.; Instituto de Investigação de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2003; pp. 281–285. Available online: https://repository.humboldt.org.co/server/api/core/bitstreams/c39e7d79-a48e-4a47-9b56-63937f5fd55f/content (accessed on 15 April 2024).
- Adams, C.T.; Plumley, J.K.; Banks, W.A.; Lofgren, C.S. Impact of red imported fire ant, Solenopsis invicta Buren (Hymenoptera: Formicidae) on harvest of soybean in North Carolina. J. Elisha Mitchell Sci. Soc. 1977, 93, 150–152. [Google Scholar]
- Fernández, F. Introducción a Las Hormigas de la Región Neotropical; Instituto de Investigación de Recursos Biológicos Alexander Von Humboldt: Bogotá, Colombia, 2003; 404p, Available online: https://repository.humboldt.org.co/server/api/core/bitstreams/c39e7d79-a48e-4a47-9b56-63937f5fd55f/content (accessed on 10 January 2024).
- Baccaro, F.B.; Feitosa, R.M.; Fernandez, F.; Fernandes, I.O.; Izzo, T.J.; Souza, J.P.; Solar, R. Guia Para os Gêneros de Formigas do Brasil; IMPA: Manaus, Brazil, 2015; 388p. Available online: https://ppbio.inpa.gov.br/sites/default/files/Livro_Formigas_2015.pdf (accessed on 22 January 2024).
- Madsen, N.E.; Offenberg, J. Mudanças sazonais na preferência de açúcar e aminoácidos em formigas vermelhas do grupo Formica rufa. Sociobiology 2020, 67, 144–152. [Google Scholar] [CrossRef]
- Silvestre, R.; Brandão, C.R.F.; Silva, R.R. Grupos funcionales de hormigas: El caso de los gremios del cerrado. In Introducción a las Hormigas de la Región Neotropical; Fernandez, F., Ed.; Instituto de Investigación de Recursos Biológicos Alexander Von Humboldt: Bogotá, Colombia, 2003; pp. 113–148. Available online: https://repository.humboldt.org.co/server/api/core/bitstreams/c39e7d79-a48e-4a47-9b56-63937f5fd55f/content (accessed on 10 January 2024).
- Cuezzo, F. Subfamília Dolichoderinae. In Introdução às Hormigas da Região Neotropical; Fernández, F., Ed.; Instituto de Investigação de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2003; pp. 291–297. Available online: https://repository.humboldt.org.co/server/api/core/bitstreams/c39e7d79-a48e-4a47-9b56-63937f5fd55f/content (accessed on 10 January 2024).
- Latke, J.E. Subfamilia Ponerinae. In Introducción a las Hormigas de la Región Neotropical; Fernandez, F., Ed.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2003; pp. 261–276. Available online: https://repository.humboldt.org.co/server/api/core/bitstreams/c39e7d79-a48e-4a47-9b56-63937f5fd55f/content (accessed on 20 February 2024).
- Ward, P.S. Subfamília Pseudomyrmecinae. In Introducción a las Hormigas de la Región Neotropical; Fernández, F., Ed.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2003; pp. 331–333. Available online: https://repository.humboldt.org.co/server/api/core/bitstreams/c39e7d79-a48e-4a47-9b56-63937f5fd55f/content (accessed on 10 January 2024).
- Milosavljević, I.; Morgan, D.J.; Massie, R.E.; Hoddle, M.S. Density dependent mortality, climate, and Argentine ants affect population dynamics of an invasive citrus pest, Diaphorina citri, and its specialist parasitoid, Tamarixia radiata, in Southern California, USA. Biol. Control 2021, 159, 104627. [Google Scholar] [CrossRef]
- Hoddle, M.S.; Hoddle, C.D.; Morgan, D.J.; Milosavljević, I. Successful Biological Control of Asian Citrus Psyllid, Diaphorina citri, in California. In Contributions of Classical Biological Control to the U.S. Food Security, Forestry, and Biodiversity; Van Driesche, R.G., Winston, R.L., Perring, T.M., Lopez, V.M., Eds.; FHAAST-2019-05; USDA Forest Service: Morgantown, WV, USA, 2022; pp. 127–143. Available online: https://bugwoodcloud.org/resource/files/23194.pdf (accessed on 26 June 2024).
- Janssen, A.; Montserrat, M.; Hille Ris Lambers, R.; Roos, A.M.; Pallini, A.; Sabelis, M.W. Intraguild predation usually does not disrupt biological control. In Trophic and Guild in Biological Interactions Control; Brodeur, J., Boivin, G., Eds.; Progress in Biological Control; Springer: Dordrecht, The Netherlands, 2006; pp. 21–44. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).