Abstract
As a nutrient-rich multigrain crop, buckwheat is a typical “medicinal food homology” crop that is rich in flavonoids, including rutin and various vitamins. It has positive anti-oxidant and anti-tumour properties and lowers blood pressure. However, due to strict self-crossing characteristics, slow progress has been made in Tartary buckwheat (TB) cross-breeding, resulting in the slow breeding of new varieties of new TB varieties, which has limited the improvement of yield and quality. Therefore, mutant breeding is a rapid and effective technique for broadening and innovating TB breeding. In recent years, improving qualities related to yield, lodging resistance, and stability have become key points in TB breeding. Based on the above findings, excellent, potentially valuable TB lines with rich phenotypes were obtained for the TB mutation library via ethyl methanesulfonate (EMS), laying a foundation for creating new TB germplasms. In this study, we systematically investigated more than 10 agronomic traits of JQ2 and JQ4 mutants, including plant type, leaf colour, grain type, grain colour, grain number per plant, grain length, grain width, grain weight per plant, and 1000-grain weight. The results show that the maximum number of grains per plant was 1956, the weight was 32.84 g, and the 1000-grain weight was 30.89 g. The maximum number of grains per JQ4 plant was 2308, and the weight was 44.82 g. The maximum 1000-grain weight was 24.7 g. Among the 295 JQ2 mutants and 153 JQ4 mutants, 10 flavonoids (orientin, morin, quercetin, kaempferol, luteolin, naringin, hesperetin, myricetin, hesperidin, and rutin) were detected with near infrared spectroscopy (NIR). The mutants were divided into five groups according to the flavonoid content of the JQ2 mutants, of which the first group included 31 individual lines. and the second to fifth groups included 70, 69, 72, and 53 lines, respectively. The JQ4 mutants were divided into four classes, of which 41, 50, 32, and 30 were individual lines, respectively, with the highest rutin content being 82.06 mg/g. In summary, through systematic analysis and screening of the agronomic traits and flavonoid contents of JQ2 and JQ4 mutant seeds, we obtained three lines with a high 1000-grain weight, including two JQ2 mutant lines (30.89 g) and one JQ4 mutant line, which reached 24.70 g and ten lines with high grain weight per plant. This included 8 JQ2 mutants and 2 JQ4 mutants, as well as 72 high-rutin mutants (including 31 lines from JQ2 and 41 lines from JQ4 mutants). These elite lines provide the material basis for creating TB germplasms with excellent qualities and cultivation characteristics.
1. Introduction
Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn.) is a minor crop but is a source of high-quality agricultural products that can be used in both medicine and food. Tartary buckwheat (TB) has comprehensive nutritional value because it is rich in protein, fat, high-quality starch, and rutin. It has excellent potential for development and application due to these characteristics [1]. TB plants are rich in flavonoids (such as rutin), which have specific efficacy in softening blood vessels and lowering the “three highs” (hyperlipaemia, hyperglycaemia, and hypertension). They also assist in the treatment of diabetes and cancer [2,3]. It has been reported that the flavonoid compound content in TB is 10 to 100 times greater than that in common buckwheat [4,5], a phenomenon that has important research value.
Mutants are essential basic materials in genetic research and play a critical role in genetics research and quality improvement. The establishment of a mutant library is also important for accelerating crop breeding and broadening plant genetic resources. The establishment of mutant libraries for Arabidopsis [6], rice [7], maize [8], foxtail millet [9], and wheat [10] has played an essential role in plant germplasm innovation and functional genome research. The methods of inducing mutations include physical and chemical techniques. Physical mutagenesis methods can be divided into nonionizing radiation-induced and ionizing radiation methods [11]. Common physical mutagens include various kinds of rays (X-rays, gamma rays, beta rays, alpha rays, etc.), different abnormal temperatures (high, low, variable), centrifugal forces, and mechanical damage. In wheat, mutants of various specific traits or genes, such as those conferring powdery mildew resistance and rust resistance [12,13], have been generated through various physical mutagenic methods, such as radiation and fast neutrons. Chemical mutagenesis involves treating plant materials with chemical mutagens, which can cause DNA damage and mutation. In the creation of plant mutants, ethyl methylsulfonate (EMS) is the most widely used alkylating agent, and EMS mutagenesis has been widely used for improving the quality of oil crops such as peanut, soybean, sunflower, and Brassica napus [14,15,16]. Mixed physical and chemical methods have been used, such as for rice seeds, which were first treated with gamma radiation and then treated with EMS, resulting in high-yield and high-quality varieties. This utilization was widely promoted [17].
There are four standard screening and identification methods for mutants: phenotypic identification, cytological identification, molecular biological identification, and genetic identification [18]. Currently, the available methods for buckwheat breeding include selection, mutagenesis, ploidy, cross-breeding, and marker-assisted breeding [19]. With the increasing market demand for high-quality buckwheat varieties, traditional breeding or introduced varieties are lagging. The breeders accelerated the material renewal and development of innovative varieties through mutagenesis. Li et al. [20] treated dried seeds with 100–500 Gy of Co60 γ-rays and screened 10 high-flavonoid mutants (with contents ranging from 11.37% to 14.91%). There were eight ‘Yu-621’ high-flavonoid mutants, and the flavonoid content of the mutants ranged from 10.67% to 12.46%. There were six ‘KP9920’ high-flavonoid mutants, with flavonoid content ranging from 11.32% to 12.95%. Zhao et al. [21] selected a new Tartary buckwheat variety (‘Xiqiao 1’) by combining Co60 γ-rays with colchicine and a dimethyl sulfoxide mixture. Zhang et al. [22] reported that many excellent qualities, such as drought resistance, lodging resistance, barrenness resistance, and stable yield, were obtained from ‘Jinqiao No. 3’, respectively, after treatment with Co60 γ-ray radiation. Ma et al. [23] treated buckwheat seeds with EMS and characterized the biological and agronomic traits of the obtained M2 generation materials. Four hundred eighty mutant materials were obtained, with characteristics mainly related to leaf, stem, plant type, seed, growth period, fertility, and other phenotypes. Among the grain variations, large grain, long grain, and multiedge variations were all stable and inherited by the M3 generation. Shimizu et al. [24] obtained a new TB variety, ‘Darumadattan’, by mutagenizing ‘Hokkai T8’ at a total dose of 500 Gy (25 Gy/h × 20 h). ‘Darumadattan’ was approximately 100 cm shorter than ‘Hokkai T8’, and its height decreased. However, the yield did not decrease, and the plants exhibited strong lodging resistance.
The main objectives of TB breeding are to generate varieties that exhibit high and stable yields, high quality, strong stress resistance, and adaptability. In terms of quality, a high flavonoid content is a crucial breeding index. This study investigated the excellent TB varieties Jinqiao No. 2 (JQ2) and Jinqiao No. 4 (JQ4) from Shanxi Province. The mutants obtained through EMS mutagenesis showed variation in terms of the rich phenotype. The results of this study will enrich TB gene resources and accelerate the breeding process of buckwheat. Furthermore, this study provides a good material basis for using buckwheat germplasm and for molecular biology research.
2. Materials and Methods
2.1. EMS Mutation Library Construction
Healthy and whole JQ2 and JQ4 seeds were selected, soaked in 1.2% EMS solution, shaken at 300 r/min overnight (16–18 h), neutralized with 0.2 M Na2S2O3, rinsed, and dried repeatedly before use [25].
2.2. Planting and Phenotypic Characteristic Screening of Mutagenic Materials
The JQ2 and JQ4 mutagenized seeds (0.5 kg for each variety) were sown in the experimental field with a row spacing of 30 cm in June 2020. Seeds of the M1 generation were harvested in October of the same year (plot harvest). From June 2021 to 2022, M1 generation JQ2 and JQ4 seeds were sown in line, each with a length of 2.5 m, and M2 to M3 plants were harvested to construct the mutation library (single plant harvest).
The number of seedlings, seedling emergence rate, etiolated seedlings, number of non-etiolated seedlings, and etiolated rate of the mutants were analysed in 2020.
Generation rate = Number of seedlings/seed number × 100%
Etiolation rate (%) = Number of etiolated seedlings/non etiolated seedlings × 100%
In the field investigation of agronomic traits, lines with noticeable variation in morphological traits, such as plant height, leaf colour, and plant shape, were screened, documented, and photographed.
2.3. Grain Morphology and Data Measurement
The whole grain size, longitudinal length and width, and cross-cut area were measured and compared. The measurement software used was OLYMPUS cellSens Standard (https://www.olympus-lifescience.com/en/software/cellsens/, accessed on 10 March 2023), data were statistically analyzed using Excel 2023.
The seeds from 954 lines were analysed via digital seed analyser (YTS-5D), which included the number of seeds per plant, number of grains per plant, grain length (mm), grain width (mm), grain circumference (mm), length-to-width ratio, grain weight per plant (g), and thousand-grain weight (g).
Evaluation of relations among agronomy traits was performed via Pearson correlation. The data were statistically analysed with R 4.2.3.
2.4. Flavonoid Content Determination via Near-Infrared Reflectance Spectroscopy (NIR)
NIR Model Establishment
Thirty-two TB varieties with different rutin contents were selected, and a near-infrared reflectance spectroscopy analysis model of buckwheat was established [26]. The contents of orientin, morin, quercetin, kaempferol, luteolin, naringin, hesperetin, myricetin, rutin, and hesperidin. It was performed via ultrahigh-performance liquid chromatography‒mass spectrometry (Waters, TQ-XS, Milford, CT, USA) (Supplementary Materials, Table S1). Targeted metabolomics were used to quantify 10 flavonoids in 32 TB varieties. The chromatograms of the 10 flavonoid standards are shown in Figure S1. The sample analysis was repeated 3 times. A grain analyser (Foss, DS2500, Hilleroed, Denmark) was used to perform near-infrared spectroscopy. The absorbances were recorded at 400–2500 nm. Five scans were performed for each sample, and the average spectrum was used to establish the spectrum-based TB flavonoid quantification model (Figure S2). All the data statistical analyses were performed using Excel 2023 and SPSS 21.0.0.
3. Results
3.1. Construction of the Mutant Library
After EMS mutagenesis, 12,924 JQ2 and 14,278 JQ4 seedlings were obtained in the M0 generation (Year 2020), for which the generation rates were 51.69% and 57.11%, respectively. The generation rate reached the semi-lethal level, and the mutagenesis effect was significant. In 2021, 13,464 seedlings were planted from JQ2, and 10,788 were planted from JQ4. There were 7666 JQ2-treated and 3184 JQ4-treated M2 generation seedlings. Etiolation is an essential phenotypic characteristic reflecting the occurrence of mutations (Figure S3). According to the statistics, in 2020, 257 JQ2 and 200 JQ4 etiolated seedlings were observed in the M0 generation, for which the etiolation rates were 2.03% and 1.42%, respectively (Table 1).

Table 1.
Statistics of the number of emergence and etiolation seedlings of ‘JQ2’ and ‘JQ4’ mutants.
3.2. Screening of Mutant Phenotypes
3.2.1. Plant Type and Leaf Colour Variation
When investigating the main agronomic traits of M2 generation mutants, we focused mainly on plant type, leaf colour, and grain variation. Dwarfing is an essential type of plant variation. In 2020, 13 plants of the M1 generation were found to be dwarfed. After single-plant harvest and single-row sowing in 2021, dwarf phenotypes were found in all 13 of the above lines. Within two years, a total of 94 strains of dwarfism were found (Table S2). Comparing the mutant dwarf lines with ‘JQ2, JQ4’ (Figure 1A,E) lines, the dwarf line JQ2-1510-1 was 44.5 cm tall and presented increased lateral branching and late maturation (Figure 1B). Line JQ2-319-2 had a plant height of 38.4 cm, with compact plant type, dark green leaf colour, and leaf curl (Figure 1C). Line JQ4-7-17-1 plants were 84.1 cm tall and had more lateral branches, more dispersed plant types, dark green leaves, and later maturity (Figure 1F). The height of the JQ4-8-22-4 plants was 90.4 cm, and these plants were loose (with more branches) and had light-green coloured leaves (Figure 1G). The height of the mutant plants was significantly reduced, and the height of the plants treated with JQ2 (110.4 cm) was 60.5 cm higher than that of JQ2-1510-1 (49.5 cm), and that of JQ4 (99 cm) was higher than JQ4-7-17-1 (87.2 cm) (Figure 1D,H).
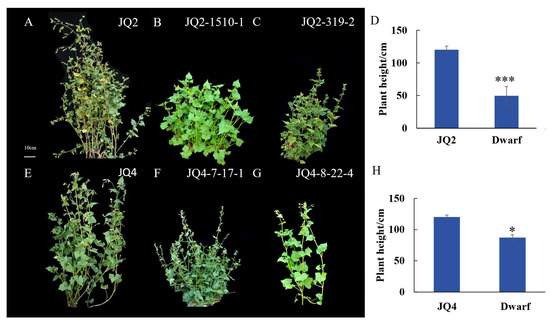
Figure 1.
Variation of plant type of ‘JQ 2’ and ‘JQ 4’ mutants. (A) JQ2. (B) JQ2-1510-1. (C) JQ2-319-2. (D) JQ2 dwarfing line statistics. (E) JQ4. (F) JQ4-7-17-1. (G) JQ4-8-22-4. (H) JQ4 dwarfing line statistics. Through Duncan’s multiple comparative analysis, ‘*’ indicated significant difference at p < 0.05 level. ‘***’ indicated significant difference at p < 0.001 level.
Two variation types, light green and dark green, were observed. According to the statistical results, 16 plants with light green leaves and 13 plants with dark green leaves were found in the M1 generation. There were 97 light green leaves and 81 dark green leaves in the M2 generation. As shown in Figure 2, which presents leaf colour for JQ2 and JQ4 (Figure 2A,E), some mutants had pale yellow leaves (Figure 2B), and green colouration was unevenly distributed (Figure 2C). However, the leaves of some of the mutants were darker than those of their parents (Figure 2D,H).
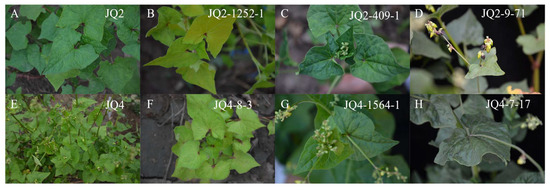
Figure 2.
Leaf colour of JQ2 green (A) and JQ2 mutants: light yellow(B), dark green (C), and dark green (D). Leaf colour of JQ4 (E) and JQ4 mutants: light green (E), light green (F), dark green (G), dark green (H).
3.2.2. Grain Variation
Grain variation included grain type variation and grain colour variation. Five grain type variations were found: barbs (i.e., spiny outgrowth at the outer wing edge of the pericarp), thin shells, four edges, and shorter or longer grains (Table S3). For example, the grains of the JQ2-281-2 mutant were longer grain (Figure 3B), while those of the JQ2-125-3 mutant were shorter than those of the JQ2 mutant (Figure S4B). Crosscutting of the grains showed that the grain edges of the JQ2-1-2 mutant did not exhibit starch filling (Figure S5B). When the grain was cut longitudinally, protruding parts on both sides could be observed, and barbs were present on the three edges of the grain (Figure S5B). The thin hull mutant JQ2-1253 had smaller grains, with an average length of 5.10 mm and a width of 2.86 μm (Figure 4C).
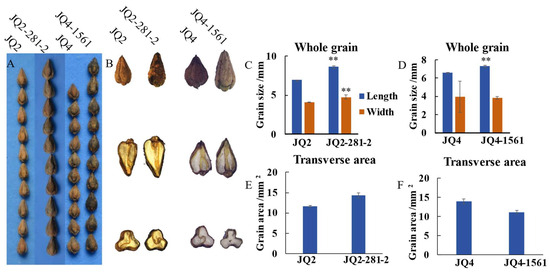
Figure 3.
Grain variation of ‘JQ 2’ and ‘JQ 4’ mutants—grain length. (A) Comparison of grain length between JQ2 and JQ2-281-2, JQ4 and JQ4-1561. (B) Comparison of grain lengths of ‘JQ2’, ‘JQ4’, and mutants under the microscope (whole grain, longitudinal cut, transverse cut from top to bottom, respectively). (C,D) Comparative analysis of grain length. (E,F) Comparative analysis of grain cross-cut area. Through Duncan’s multiple comparative analysis, the difference of ‘**’ p < 0.01 was significant.
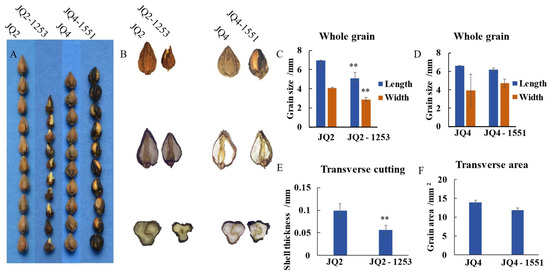
Figure 4.
Grain variation of ‘JQ2’ and ‘JQ4’ mutants—thin shell. (A) Comparison of grain types between JQ2 and JQ2-1253, JQ4 and JQ4-1551. (B) Comparison of grain morphology of JQ2, JQ4, and mutant under microscope (whole grain from top to bottom, longitudinal cut, transverse cut, respectively). (C,D) Comparative analysis of grain length. (E) Comparative analysis of grain cross-cut thickness. (F) Comparative analysis of grain cross-cut area. Through Duncan’s multiple comparative analysis, the difference of ‘**’ p < 0.01 was significant.
By screening the seeds of the JQ2 and JQ4 mutants, we found that some of the seeds were larger or smaller than those of the JQ2 and JQ4 strains, and their length and width differed. By taking pictures of the seeds, we showed that the mutant had obvious variation in grain size. The size of the JQ2 mutant seeds decreased, the size of some of the mutant seeds increased, the seeds became black, and the seeds of JQ2 were grey. As shown in Figure S6, compared with those of JQ4 and JQ4-116-12, those of the mutant seed (length: 4.08 mm, width: 2.29 mm) decreased than those of the JQ4 seed (length: 6.59 mm, width: 3.94 mm). Additionally, for JQ4-509-2, the seeds became larger and black, while those of the JQ4 seeds became grey.
In addition to the above four types of grain variation, we also found that TB was more likely to have triangular grains, and the mutant had four edges with obvious barbs (Figure S7A). The top of the grains can be seen more clearly (Figure S7B).
3.2.3. Analysis of the 1000-Grain Weights (TGWs) of the Mutants
The obtained mutants were graded according to the standard of JQ2 and JQ4, and the difference was 10% a grade; that is, 10% above the JQ2 and JQ4 was marked as +10%, and otherwise, it was marked as 10%, −10%, and so on. The classification of the JQ2 and JQ4 mutants is shown in Table S4, and the TGW of JQ2 was 18.41 g. There were 124 lines in Grade I, with TGWs ranging from 18.41 g to 20.23 g. There were 56 lines in Grade II with TGWs ranging from 20.27 g to 22.09 g, 7 lines in Grade III with TGWs ranging from 22.17 g to 23.09 g, and 2 lines in Grade IV with TGWs of 27.09 g and 30.89 g, respectively. There were 408 plants in the negative grade, and the TGWs ranged from 11.20 to 18.4 g. The highest TGWs were exhibited by two lines, and they reached 27.09 g and 30.89 g, increasing by 47.14% and 67.79%, respectively, which was greater than that of JQ2 (18.41 g).
The TGW of JQ4 was 17.31 g. There were 71 lines in Grade I, with TGWs ranging from 17.36–18.99 g; 57 lines in Grade II, with TGWs ranging from 19.09–20.72 g; and 37 lines in Grade III, with TGWs ranging from 20.79–22.4 g. Moreover, there were three lines in Grade IV, with TGW ranging from 22.67–23.19 g; 80 plants had a negative primary TGW (ranging from 15.57–17.29 g); 38 plants had a negative secondary TGW (ranging from 13.97–15.54 g); and 21 lines had a negative tertiary TGW (ranging from 11.59–13.79 g). The maximum TGW in the JQ4 mutant was 24.7 g, which was 42.69% greater than that in the JQ4 mutant.
3.2.4. Mutant Analysis of Grain Weight per Plant (GWP)
Similarly, the mutants were classified according to the grain weight per plant (GWP) of JQ2 and JQ4; a 20% difference was defined as a grade greater than that of JQ2 and JQ4 (20%), marked as +20%; otherwise, a difference lower than that of JQ2 and JQ4 (20%) was defined as −20%.
The JQ2 and JQ4 mutants were classified according to GWP (Table S5). There were 12 levels of total GWP in the JQ2 mutant. There were 75 lines in Grade I, with grain weights ranging from 10.32–12.33 g per plant. Grade II included 54 lines, with grain weights ranging from 12.35–14.38 g. There were 35 lines in Grade III, with grain weights ranging from 14.43–16.45 g. Grades IV–VI included 10, 11, and 5 lines, respectively, with grain weights ranging from 16.86–21.88 g. Eight lines at Grade VII had the highest GWPs, ranging from 24.86–32.84 g. The other 428 lines had lower GWPs than did the JQ2 line.
According to the classification method, we investigated 319 lines from JQ4 mutants, which were divided into 12 grades, of which 84 lines showed a greater GWP. The details are as follows: Grade I included 21 lines (GWP ranged from 10.42–12.16 g). Grade II included 19 lines (12.51–14.27 g). In addition, 21 Grade III lines (14.71–16.41 g), 15 Grade IV plants (16.51–18.37 g), 3 Grade V lines (18.69, 18.97 g, and 20.08 g, respectively), and 3 Grade VI lines had grain weights of 22.1 g, 23.04 g, and 25.49 g, respectively. In Grade VII, which included 2 lines, the grain weights per plant were 43.08 g and 44.82 g, respectively, which were 32.8 g and 34.54 g greater than that of JQ4 at 10.28 g. The greater the grain weight per plant was, the more grains per plant there were or the more significant the grain weight was. These results show that both yield and quality were high.
3.2.5. Correlation Analysis of Mutant Grain Traits
Correlation analysis of JQ2 mutant seeds revealed the total number of grains per plant, total number of grains per plant, number of complete seeds per plant, and the weight of grain per plant, with a significant positive correlation (Figure S8A). Grain length, grain width, and grain circumference were also strongly positively correlated. The greater the grain length and width were, the greater the grain circumference was. There was also a slight positive correlation between GWP and TGW. That might indicate that the thousand-grain weight of the plant is affected not only by the grain weight of the single plant but also by the grain size.
3.3. Analysis of Differences in Flavonoid Content in the Mutants
The flavonoid content of the JQ2 and JQ4 mutants were determined via near-infrared spectroscopy. According to the flavonoid content of the JQ2 mutants, these mutants were divided into five clusters by clustering using Euclidean distance (Figure 5). The first cluster included 31 lines in which the contents of hesperidin, kaempferol, luteolin, and quercetin were greater than those in the other lines. The highest hesperidin content was 1.65 mg/g, the kaempferol content was 23.08 mg/g, and the quercetin content reached 45.77 mg/g. The second category included 70 individual plants whose hesperidin, kaempferol, and quercetin contents were lower than those in the first category. There were 69 plants in the third group, and the hesperidin and kaempferol contents were greater than those in the second group; however, the rutin content was lower than that in the first two groups. In the fourth and fifth groups, the contents of kaempferol, morin, rutin, and quercetin were lower than those in the first three groups. The contents of these four compounds in the fifth group were the lowest, with the lowest content of kaempferol being 15.82 mg/g, that of morin being 25.52 mg/g, that of rutin being 82.90 mg/g, and that of quercetin being 41.25 mg/g. The highest rutin content was 82.60 mg/g, representing an increase of 19.30% from the JQ2 rutin content, which was 69.24 mg/g.
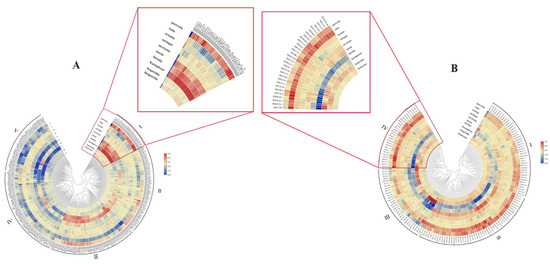
Figure 5.
Cluster heat map of flavonoid content in the mutant of ‘JQ 2’ (A) and ‘JQ 4’s (B). Note: The enlarged part showed the high rutin content lines.
The JQ4 mutants could be classified into four classes according to differences in rutin content. The first class contained 41 individual lines and 10 kinds of flavonoids, each with a moderate content. The second class contained 50 individual lines where the highest content of morin was 28.48 mg/g, the content of rutin was 82.00 mg/g, and the content of quercetin was 43.32 mg/g. All of these values were high; however, the content of kaempferol was low, and the lowest was 18.83 mg/g. There were 32 individual plants in the third class, among which the kaempferol content was the highest (17.98 mg/g), the luteolin content was 0.22 mg/g, and the morin content was 26.25 mg/g. There were 30 individual plants in the fourth class, among which the rutin content was the highest, with a maximum content of 82.06 mg/g, exhibiting an increase of 86.31% from that of JQ4, which was 44.04 mg/g.
4. Discussion
Mutants are essential materials for basic crop breeding research. Researchers have created crop mutants and bred new plant varieties. However, whether these mutant materials can aid in molecular-assisted breeding still needs to be determined, and determining whether their mutation is stable requires ongoing planting of the next generation to observe their mutation stability. In addition, analysis of the causes of mutations from a genomic perspective has become an essential direction of functional genomics research. Yield is an important trait for crop improvement. The standard components of TB yield include GWP, TGW, and field planting density, etc. [27,28,29]. In our study, we also found potential high-yield lines with a larger grain sizes or high GWPs. Through EMS mutagenesis, buckwheat germplasms with 100 grain weights and yields per plant were obtained. Qin et al. (2020) [30] treated ‘18-1’ bean seeds with EMS, conducted field phenotype screening on 2128 individual M2 plants throughout the growth period, and screened out 107 mutant lines, accounting for 5.03% of the population. Mutants with variations in leaf, stem, flower, pod, plant type, fertility, and maturity were constructed. The number and type of mutants related to middle lobe colour were the greatest, accounting for 27.0% of the total number of mutants. The numbers of mutants related to stem, flower, and fertility were equal and the lowest, accounting for 10.3% of the total number of mutants. This study provides new materials for the construction and innovation of mutant libraries. Studying the relationship between genome functional phenotypes and genetic breeding is important. On this basis, we screened the lines whose 1000-grain weight and grain weight per plant significantly increased, providing a material basis for selecting varieties with high and stable yields in the future. By investigating crop agronomic traits, we can obtain excellent mutants via selection or from hybrid materials and ultimately select excellent varieties. Ahmad et al. (2023) [31] compared the effects of different doses of gamma rays and EMS mutagenesis on the agronomic traits of TB and reported that plant height and 100-grain weight were greater in response to 0.3% EMS mutagenesis than in response to other treatments (gamma rays and 0.1% or 0.2% EMS treatment). On the basis of previous reports from our research group, when we screened the concentration of EMS, we found that the optimal concentration for TB variety HF was 1.2%; therefore, this concentration was used in this study, and better experimental results were obtained.
Flavonoids are a class of secondary metabolites that play a critical physiological role in buckwheat plants and are widely distributed in plant organs, such as seeds, buds, stalks, leaves, flowers, and roots [32,33,34]. Wang et al. [35] determined the contents of six flavonoids in 12 buckwheat varieties from different regions. The results showed that the contents of the six flavonoids and the total flavonoids in buckwheat from different regions significantly differed (p < 0.05); the content of rutin was the highest (0.1180~0.3768%), and the contents of kaempferol and hypericin were relatively low. Qin et al. [36] studied 21 TBs and 18 common buckwheat varieties and reported that the contents of rutin, quercetin, and total flavonoids in TBs were 606–1867 mg/100 g, 31–238 mg/100 g, and 665–2274 mg/100 g, respectively. The contents of rutin and total flavonoids in common buckwheat were 15–168 mg/100 g and 67–25 mg/100 g, respectively, and quercetin was detected in only two buckwheat samples, the contents of which were 5 mg/100 g and 9 mg/100 g, respectively. Researchers have evaluated the agronomic and quality traits of TB from Guizhou Province [37]. In studies of lettuce, Gurdon et al. [38] reported that mutants with high flavanol (2–4-fold increase) and phenol contents could be obtained, as could flavonoid loss mutants obtained from grass pea via EMS mutagenesis; these findings could lead to the development of good tools for gene function research and material innovation. These materials were subsequently subjected to gene function and genetic population mapping analyses, and excellent alleles for controlling flavonoid metabolism were identified.
In this study, the flavonoid content of JQ2 and JQ4 mutants was determined. Through cluster analysis, the flavonoid content of the JQ2 mutants was divided into five categories, and that of the JQ4 mutants was divided into four categories; from these categories, lines with excellent flavonoid content variation, such as those concerning the content of rutin and quercetin, were selected. Through this analysis, we screened the strains with high rutin and quercetin contents, which can provide some support for our research on rutin. Most of the literature reports that phenolic substances mainly affect crop stress tolerance and insect resistance [39,40], and these indicators could improve crop yield. However, there may be a lack of evidence, for a direct relationship between these compounds, and the relationship between high yield and high phenolic content needs further study. In addition, we found that the contents of various flavonoid compounds in the mutant grains were significantly different, indicating that some of the flavonoid compounds in the mutant lines were superior to those in the parental lines, which laid a material foundation for our later experiments and crop breeding.
5. Conclusions
The strict self-pollination traits of TBs hinder the process of cross-breeding, which limits the improvement of TB breeding. Unlike conventional breeding, mutational breeding is a rapid, efficient, and large-scale directional breeding method for new varieties. Combined with phenotype and chemical analysis, this approach can shorten the artificial breeding cycle and reduce labour costs. At the same time, combined with genetic analysis, this approach can quickly locate target genes and provide genetic resources with breeding value. In this study, two TB mutant libraries were constructed based on EMS, and high-yielding TB lines were screened by phenotype. Then, new lines with high levels of flavonoid metabolites were screened using the NIR method combined with hierarchical cluster analysis, which provided available materials and germplasm resources for further breeding of high-yielding and flavonoid-rich TB varieties.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy14030547/s1, Figure S1: Chromatogram of 10 flavonoid standards. (A) Luteolin. (B) Kaempferol. (C) Hesperidin. (D) Rutin. (E) Hesperetin. (F) Morin. (G) Quercetin. (H) Orientin. (I) Naringin. (J) Myricetin; Figure S2: A near-infrared spectroscopy model of the flavonoid content of Tartary buckwheat was constructed; Figure S3: Mutant etiolated seedlings. (A) JQ2. (B) JQ2 mutant. (C) JQ4. (D) JQ4 mutant; Figure S4. Grain variation of ‘JQ2’ and ‘JQ4’ mutants—short grain. (A) Comparison of grain length between JQ2 and JQ2-125-3 or between JQ4 and JQ4-315-4. (B) Comparison of the grain lengths of ‘JQ2’, ‘JQ4’ and the mutant under a microscope (whole grains from top to bottom, longitudinally cut, transverse cut, respectively). (C, D) Comparative analysis of grain length. (E, F) Comparative analysis of the grain cross-cut area. ‘*’ p < 0.05. ‘**’ p < 0.01; Figure S5: Grain variation of ‘Jinqiao 2’ and ‘Jinqiao 4’ mutants–barbs. (A) Comparison of grain types between JQ2 and JQ2-1-2 or between JQ4 and JQ4-1211. (B) Comparison of the morphology of JQ2, JQ4 and mutant grains under a microscope (whole grains from top to bottom, longitudinally cut, transverse cut, respectively). (C, D) Comparative analysis of grain length. (E, F) Comparative analysis of the grain cross-cut area. ‘*’ p < 0.05. ‘**’ p < 0.01; Figure S6: Comparison of mutant grain size. (A-C) Comparison of the grain size of JQ2-treated and mutant strains. (D) 1000-particle weight statistics of the JQ2 mutant. (E-G) Comparison of grain size between the JQ4-treated and mutant strains. (H) 1000-particle weight statistics of the JQ4 mutant; Figure S7: Variation in the grains of the ‘JQ2’ mutant–four edges. (A) Comparison between JQ2 and the quadrangle. (B) The grains were compared by overhead shot. (C) Grain cross cut comparison; Figure S8: Correlation analysis of ‘JQ2’ and ‘JQ4’ mutants. (A) JQ2. (B) JQ4; Table S1: Flavonoid standard quality spectrum information; Table S2: Type and leaf colour statistics of ‘JQ2’ and ‘JQ4’ mutant plants; Table S3: Grain type and grain colour statistics of ‘JQ2’ and ‘JQ4’ mutants; Table S4: Classification statistics of thousand-grain weight in ‘JQ2’ and ‘JQ4’ mutant lines; Table S5: Classification statistics of grain weight per plant in the ‘JQ2’ and ‘JQ4’ mutant lines.
Author Contributions
Conceptualization, Z.S.; methodology, H.G.; formal analysis, Z.S., Z.Q., W.R. and W.C.; investigation, H.G. and Z.Q.; writing—original draft, Z.S., H.G., H.F. and Z.Q.; writing—review and editing, Z.S., L.L. and H.G.; project administration, Z.S.; funding acquisition, Z.S. All authors have read and agreed to the published version of the manuscript.
Funding
Key R&D Project in Shanxi Province (2022ZDYF110), China Agriculture Research System of MOF and MARA (CARS-07-A-2), Graduate Students Practice Innovative Projects College of Agronomy Shanxi Agricultural University (2023YCX25), Graduate Students Practice Innovative Projects College of Agronomy Shanxi Agricultural University (2023YCX43).
Institutional Review Board Statement
Since this article is a study of plants, this study does not involve animals and humans, so this study was waived for ethical review and approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Joshi, D.C.; Chaudhari, G.V.; Sood, S.; Kant, L.; Pattanayak, A.; Zhang, K.; Fan, Y.; Janovská, D.; Meglič, V.; Zhou, M. Revisiting the versatile buckwheat: Reinvigorating genetic gains through integrated breeding and genomics approach. Planta 2019, 250, 783–801. [Google Scholar] [CrossRef]
- Joshi, D.C.; Zhang, K.; Wang, C.; Chandora, R.; Khurshid, M.; Li, J.; He, M.; Georgiev, M.I.; Zhou, M. Strategic enhancement of genetic gain for nutraceutical development in buckwheat: A genomics-driven perspective. Biotechnol. Adv. 2020, 39, 107479. [Google Scholar] [CrossRef]
- Shukla, A.; Srivastava, N.; Suneja, P.; Yadav, S.; Hussain, Z.; Rana, J.C.; Yadav, S. Genetic diversity analysis in buckwheat germplasm for nutritional traits. Indian J. Exp. Biol. 2018, 56, 827–837. [Google Scholar]
- Kwiecień, S.; Brzozowski, T.; Konturek, S.J. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J. Physiol. Pharmacol. 2002, 53, 39–50. [Google Scholar]
- Synnerstad, I.; Johansson, M.; Nylander, O.; Holm, L. Intraluminal acid and gastric mucosal integrity: The importance of blood-borne bicarbonate. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G121–G129. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, L.; Yanofsky, M.F. Establishing gene function by mutagenesis in Arabidopsis thaliana. Plant J. 2004, 39, 682–696. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, J.; Luo, Y.; Xu, T.; Zhang, Q.; Zhang, L.; Xu, M.; Wan, J.; Wang, M.B.; Zhang, C.; et al. Construction of a genomewide RNAi mutant library in rice. Plant Biotechnol. J. 2013, 11, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Wang, B.; Ding, H.; Lin, H.; Zhang, L.; Li, Q.; Wang, Y.; Zhang, B.; Liang, A.; Zheng, Q.; et al. Genome assembly of the Chinese maize elite inbred line RP125 and its EMS mutant collection provide new resources for maize genetics research and crop improvement. Plant J. 2021, 108, 40–54. [Google Scholar] [CrossRef]
- Sun, J.; Luu, N.S.; Chen, Z.; Chen, B.; Cui, X.; Wu, J.; Zhang, Z.; Lu, T. Generation and Characterization of a Foxtail Millet (Setaria italica) Mutant Library. Front. Plant Sci. 2019, 10, 369. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Wang, H.; Xu, Y.; Yang, Y.; Zhou, Y.; Chen, Z.; Zhou, Y.; Gui, L.; Guo, Y.; et al. Boosting wheat functional genomics via an indexed EMS mutant library of KN9204. Plant Commun. 2023, 4, 100593. [Google Scholar] [CrossRef]
- Kodym, A.; Afza, R. Physical and chemical mutagenesis. Methods Mol. Biol. 2003, 236, 189–204. [Google Scholar]
- Koebner, R.; Hadfield, J. Large-scale mutagenesis directed at specific chromosomes in wheat. Genome 2001, 44, 45–49. [Google Scholar] [CrossRef]
- Kinane, J.T.; Jones, P.W. Isolation of wheat mutants with increased resistance to powdery mildew from small induced variant populations. Euphytica 2001, 117, 251–260. [Google Scholar] [CrossRef]
- An, C.; Beard, W.A.; Chen, D.; Wilson, S.H.; Makridakis, N.M. Understanding the loss-of-function in a triple missense mutant of DNA polymerase β found in prostate cancer. Int. J. Oncol. 2013, 43, 1131–1140. [Google Scholar] [CrossRef][Green Version]
- Wang, F.; Travins, J.; DeLaBarre, B.; Penard-Lacronique, V.; Schalm, S.; Hansen, E.; Straley, K.; Kernytsky, A.; Liu, W.; Gliser, C.; et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science 2013, 340, 622–626. [Google Scholar] [CrossRef]
- Qu, G.; Sun, Y.; Pang, H.; Wu, Q.; Wang, F.; Hu, S. EMS mutagenesis and ALS—Inhibitor herbicide—Resistant mutants of Brassica napus L. Chin. J. Oil Crop Sci. 2014, 36, 25–31. [Google Scholar]
- Gautam, V.; Swaminathan, M.; Akilan, M.; Gurusamy, A.; Suresh, M.; Kaithamalai, B.; Joel, A.J. Early flowering, good grain quality mutants through gamma rays and EMS for enhancing per day productivity in rice (Oryza sativa L.). Int. J. Radiat. Biol. 2021, 97, 1716–1730. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Cui, Y.; Li, Y.; Wang, X.; Du, Y.; Huang, S. Inducible positive mutant screening system to unveil the signaling pathway of late blight resistance. J. Integr. Plant Biol. 2010, 52, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Liu, L.; Zhang, L.; Zhou, J.; Cui, L. Research Progress of Buckwheat Breeding. J. Shanxi Agric. Sci. 2015, 43, 240–243. [Google Scholar]
- Li, G.; Shen, H.; Fan, L.; Cao, H. Heredity analysis of flavone and agronomic characters among radiated tartary buckwheat mutants. In Proceedings of the 10th International Symposium on Buckwheat, Xianyang, China, 14–18 August 2007; pp. 178–185. [Google Scholar]
- Zhao, G.; Tang, Y.; Wang, A. Selection of a New Tartary Buckwheat Variety ‘Xiqiao No.1’. Hortic. Seed 2002, 262–264. [Google Scholar]
- Zhang, C.; Li, X.; Zhang, Y. Selection of Jin Buckwheat (Sweet) 3 and Its Cultivation Techniques. J. Shanxi Agric. Sci. 2011, 39, 316–318. [Google Scholar]
- Ma, M.; Liu, L.; Zhang, L.; Cui, L.; Zhou, J. Morphological Identification and Analysis of EMS-Induced Mutants from Ciqiao. Crops 2019, 37–41+197–198. [Google Scholar] [CrossRef]
- Shimizu, A.; Yamaguchi, H.; Degi, K.; Morishita, T. Development of ‘Darumadattan’, a semidwarf lodging-resistant Tartary buckwheat cultivar, using gamma-ray irradiation. Breed. Sci. 2020, 70, 623–630. [Google Scholar] [CrossRef]
- Sun, Z.; Hou, S.; Hao, Y.; Wang, D.; Gao, H.; Han, Y.; Li, H.; Liu, L.; Zhou, M. EMS-assisted Mutagenesis of Tartary Buckwheat and Expression Analysis of Rutin Biosynthesis Genes in Selected Mutants. J. Plant Genet. Resour. 2020, 21, 402–408. [Google Scholar]
- Yao, Y.; Cheng, X.-Z.; Ren, G.-X. Application of Near-Infrared Reflectance Spectroscopy to the Evaluation of D-chiro-lnositol, Vitexin, and Isovitexin Contents in Mung Bean. Agric. Sci. China 2011, 10, 1986–1991. [Google Scholar] [CrossRef]
- Li, R.; Chen, Z.; Zheng, R.; Chen, Q.; Deng, J.; Li, H.; Huang, J.; Liang, C.; Shi, T. QTL mapping and candidate gene analysis for yield and grain weight/size in Tartary buckwheat. BMC Plant Biol. 2023, 23, 58. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Shi, T.; Chen, Q.; Meng, Z.; Liang, C.; Chen, Q. Variation in Major Agronomic Traits and its Contribution to Grain Weight per Plant in Tartary Buckwheat Germplasm. Plant Sci. J. 2015, 33, 829–839. [Google Scholar]
- Zhou, Q.; He, P.; Tang, J.; Huang, K.; Huang, X. Increasing planting density can improve the yield of Tartary buckwheat. Front. Plant Sci. 2023, 14, 1313181. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Feng, G.; Liu, D.; Liu, C.; Yang, X.; Yan, Z. Mutagenesis of Kidney Bean Cultivar A18-1 by EMS and Mutant Library Construction. Chin. Agric. Sci. Bull. 2020, 36, 14–21. [Google Scholar]
- Ahmad, J.; Jan, S.; Javid, W.; Bhat, S.A.; Tahir, I. Assessment on induced genetic variability and divergence in the mutagenised Tartary buckwheat populations developed using gamma rays and EMS mutagenesis. Ecol. Genet. Genom. 2023, 28, 100177. [Google Scholar] [CrossRef]
- Kalinova, J.; Vrchotova, N. Level of catechin, myricetin, quercetin and isoquercitrin in buckwheat (Fagopyrum esculentum Moench), changes of their levels during vegetation and their effect on the growth of selected weeds. J. Agric. Food Chem. 2009, 57, 2719–2725. [Google Scholar] [CrossRef]
- Chen, Q.F.; Huang, X.Y.; Li, H.Y.; Yang, L.J.; Cui, Y.S. Recent Progress in Perennial Buckwheat Development. Sustainability 2018, 10, 536. [Google Scholar] [CrossRef]
- Bai, C.Z.; Feng, M.L.; Hao, X.L.; Zhong, Q.M.; Tong, L.G.; Wang, Z.H. Rutin, quercetin, and free amino acid analysis in buckwheat (Fagopyrum) seeds from different locations. Genet. Mol. Res. 2015, 14, 19040–19048. [Google Scholar] [CrossRef]
- Wang, L.; Wei, M.; Shao, J.; Du, L.; Lin, X.; Wang, L. Content determination and analysis of flavonoids in buckwheat. J. Food Saf. Food Qual. 2018, 9, 5387–5392. [Google Scholar]
- Qin, P.; Wang, Q.; Shan, F.; Hou, Z.; Ren, G. Nutritional composition and flavonoids content of flour from different buckwheat cultivars. Int. J. Food Sci. Technol. 2010, 45, 951–958. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, L.; Wen, A.; Mazhar, M.; Wang, H.; Zhu, Y. Three-solvent extracting method comprehensively evaluates phenolics profile and antioxidant activities of Tartary buckwheat. J. Food Process. Preserv. 2021, 45, e15020. [Google Scholar] [CrossRef]
- Gurdon, C.; Poulev, A.; Armas, I.; Satorov, S.; Tsai, M.; Raskin, I. Genetic and Phytochemical Characterization of Lettuce Flavonoid Biosynthesis Mutants. Sci. Rep. 2019, 9, 3305. [Google Scholar] [CrossRef]
- Attarzadeh, M.; Balouchi, H.; Rajaie, M.; Dehnavi, M.M.; Salehi, A. Improving growth and phenolic compounds of Echinacea purpurea root by integrating biological and chemical resources of phosphorus under water deficit stress. Ind. Crops Prod. 2020, 154, 112763. [Google Scholar] [CrossRef]
- Maddox, C.E.; Laur, L.M.; Tian, L. Antibacterial activity of phenolic compounds against the phytopathogen Xylella fastidiosa. Curr. Microbiol. 2010, 60, 53–58. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).