Abstract
Continuous cropping is the main form of cultivation in Chinese agriculture. The bacterial community plays an important role in maintaining the healthy growth of plants. However, there are few reports on the composition and dynamics of the bacterial community structure under continuous cropping of Lonicera japonica Thunb. High-throughput sequencing was used to monitor the variation in the soil bacterial community structure of different monocropping years of Lonicera japonica Thunb., as well as the correlation between soil characteristics and bacterial community. Meanwhile, antagonistic bacteria for Fusarium oxysporum pathogens were isolated and functionally verified by culture-dependent techniques and pot experiments. Bacterial community diversity and structure changed significantly with the increase in the years of Lonicera japonica Thunb. succession. However, some beneficial bacteria, such as Bacillus and Nitrosospira, were gradually depleted. The complexity of the bacteria co-occurrence networks decreased with increasing years of cropping. FAPROTAX-based functional prediction showed that the abundance of genes related to carbon, nitrogen, sulfur metabolism and chitinlysis were reduced with the extended crop succession. Furthermore, the three Bacillus strains that were strongly antagonistic toward Fusarium oxysporum and the pot experiment demonstrated they significantly promoted Lonicera japonica Thunb. growth. Our research provides theoretical support for the development of microbial fertilizers that are beneficial to plants.
1. Introduction
Lonicera japonica Thunb., also known as honeysuckle or Jin Yin Hua in Chinese, is an extensively used traditional Chinese medicine [1,2]. Honeysuckle, with its heat-reducing, detoxification, antibacterial and anti-inflammatory functions, has been widely used in medical treatments and in other industries, with an annual market of approximately 10,000 tons and sales of over CNY 2 billion in China [1]. Furthermore, honeysuckle is a valuable antiviral agent for the treatment of SARS coronavirus [3], H1N1 influenza virus [4] and enterovirus 71 [5]. Honeysuckle is grown continuously in the same field because of the limited arable land in China and increasing market demand [6,7]. Continuous monocropping usually leads to deterioration in soil texture, increased crop disease rates and reduced yield [8]. As reported, many medicinal plants face obstacles to continuous cropping, for example, Rehmannia glutinosa, Pseudostellaria heterophylla, Panax notoginseng and Angelica sinensis [9]. However, there is little research on whether continuous cultivation of honeysuckle results in a succession of barriers. Soil microorganisms, as an important part of the soil ecosystem, are sensitive to environmental changes and can be used to measure soil health [10]. Bacteria are crucial for maintaining the health of the hosts, given that they promote host growth and defend against diseases [11,12]. For example, PGRP (plant growth-promoting rhizobacteria) reduces soil-borne diseases by increasing plant immunity to soil-borne pathogens and improving soil productivity [9]. Studies have shown that continuous cropping can lead to soil microecological imbalances [13] and increase the risk of soil root rot [14]. The number of typical pathogenic fungi, such as Fusarium, increased significantly in the soil of medicinal plants with obstacles to continuous cropping, and they occupied more ecological niches in the microbial community [15]. Root rot is a common soil-borne disease, and a study showed that Fusarium oxysporum is a cause of root rot disease that gradually weakens the ability to absorb water and nutrients [16]. The disease affects the yield and quality of the medicinal plants and results in substantial loss. Studies have shown that Fusarium oxysporum is a cause of root rot in honeysuckle [17,18]. Currently, the main method of controlling root rot is usually treatment with chemical fungicides, but the use of fungicides can have many negative effects on soil. Another method is to improve or reorganize the belowground microbial community [19]. Antagonistic bacteria control soil-borne diseases by regulating the soil microcosm, inhibiting the growth of plant pathogens or increasing the resistance of the plant [20]. The application of microbial agents and fertilizers regulates the interaction between plants and microorganisms, regulates the number of pathogenic fungi within the threshold of pathogenicity and helps to alleviate soil succession barriers [21]. To date, some antagonistic strains have been isolated and provide good control of a wide range of diseases in different plants (e.g., apple, banana, tomato and cucumber) [22,23], such as Bacillus, which has been demonstrated to play an essential role in the suppression of plant pathogens [24].
Therefore, in this study, we aim to (1) describe the composition and function of the soil bacterial community during the continuous cropping years, (2) determine the correlation between soil physicochemical properties and bacterial community structure and (3) identify and characterize the antagonistic bacteria for pathogenic fungi Fusarium oxysporum. There are few studies on the analysis of the community structure of continuously cultivated honeysuckle, and this study aims to provide theoretical support for improving management measures and establishing a scientific cultivation system for honeysuckle by analyzing the structure of the bacterial community.
2. Materials and Methods
2.1. Description of the Soil Collection
The experimental field is located in Puyang (35°71′ N, 114°98′ E), Henan Province, China. This region belongs to a temperate continental monsoon climate and has an average temperature of 13.5 °C and a mean annual precipitation of 550 mm. Soil samples were taken through a five-point sampling approach to produce the soil samples. Samples were taken from the same area and where honeysuckle had never been planted as CK, and root-associated soil samples from different consecutive cropping years were named HS1, HS2, HS3, HS4, HS5 and HS6, respectively, and used for subsequent experimental analyses. A total of 21 soil samples were collected in this study. The soil was collected from the rhizome 1–2 cm range of honeysuckle and then sieved through a 2.0 mm sieve, and the soil samples were quickly transferred to a −80 °C freezer for DNA extraction and 4 °C for soil physicochemical characterization and bacteria strain isolation, respectively.
2.2. Soil Physicochemical Property Analysis
Soil pH was measured using a pH meter (METTLER-PE28, Shanghai, China) with water:soil = 2.5:1 [25]. The contents of soil total nitrogen (TN), total phosphorus (TP) and total potassium (TK) were determined by Convinced-test Co., Ltd. (Nanjing, China). Soil TP was determined calorimetrically by wet digestion with H2SO4 and HClO4 [26], while soil TN was determined by oxidation with potassium persulfate. The TK content in soil was determined by photometry and flame photometry.
2.3. DNA Extraction, PCR Amplification and Sequencing
Total DNA was extracted from 0.5 g soil samples by the E.Z.N.A.® Soil DNA Kit (OMEGA, Norcross, GA, USA) as instructed, and the final concentration was 20 ng/μL. The 16S rRNA gene using the primers 515F/926R was amplified for the V4–V5 hypervariable region [27]; the concentration of primer was 10 μM. The PCR volum was 50 μL, and thermal cycling consisted of initial denaturation at 98 °C for 1 min, followed by 30 cycles: denaturation at 98 °C for 10 s, annealing at 50 °C for 30 s, extension at 72 °C for 30 s and finally extension at 72 °C for 5 min. The Gene JET Gel Extraction Kit (Thermo Scientific, Waltham, MA, USA) was used to purify the mixed PCR products. The purified PCR products were quantified using Qubit® 3.0 (Thermo Scientific, Waltham, MA, USA) Sequencing libraries were generated using an Illumina TruSeq DNA PCR-Free Library Preparation Kit (Illumina, San Diego, CA, USA) according to the manufacturer’s recommendations. Paired-end sequencing was performed at Novogene Technology Co., Ltd. (Beijing, China) using an Illumina MiSeq platform. After merging and quality checking, low-abundance noise was removed from the raw data [28]. The clean sequences were submitted to the NCBI Sequence Read Archive (SRA) under accession number PRJNA1012142.
2.4. Identification of Potential Isolates
Ten grams of fresh soil samples were serially diluted with sterile water, and the soil suspension was spread on LB plates. A total of 50 μL was applied to LB plates, incubated in the dark at 35 °C for 48 h and counted. Single colonies were picked for isolation and purification and then amplified by PCR. The 16S rRNA gene sequencing was conducted using bacterial universal primers 27F and 1492R. Gene sequences were aligned with those in the EzBiocloud database (https://www.ezbiocloud.net/, accessed on 19 December 2020). Multiple alignments and phylogenetic analysis were carried out using CLUSTALX (2.0) and MEGA (7.0) software.
2.5. Validation of the Antagonistic Bacteria Function
The dual culture technique was used to detect the antagonistic activities of bacterial isolates toward fungi. The pathogen Fusarium oxysporum was prepared by incubation in the center of PDA plates at 30 °C for 96 h, and then the strains were activated and inoculated at a distance of 3 cm around Fusarium oxysporum. The culture was conducted at 30 °C in an incubator for five days to observe whether the bacteria-inhibiting zone and the strains that were able to form a significant inhibition zone were considered antagonistic. The antagonistic effect was assessed by measuring the inhibition zones (mm) and the colony diameters. The percent (%) inhibition of the Fusarium oxysporum against each isolate was calculated as follows [23]:
where R1 = minimal distance between the center of the mycelial disc and the fungal colony margin toward the direction of the bacterium, and R2 = the fungal colony radius of the control plate (distance between the center of the mycelial disc and the fungal colony margin).
Percentage colony growth inhibition = [(R2 − R1)/R2] × 100
2.6. Characterization of Antagonistic Bacteria
Nitrogen fixation with Ashby medium (g/L): KH2PO4 0.2, NaCl 0.2, MgSO4·7H2O 0.2, CaCO3 5.0, CaSO4·2H2O 0.1, mannitol 10, pH = 7.0 at 28 °C for 48 h [29]. A positive reaction indicated that the strain grew very well on this plate. Phosphate solubilization assay: Inorganic phosphorus medium (g/L): glucose 10.0, KCl 1.7, (NH4)2SO4 0.5, MgSO4·7H2O 0.5, Ca3(PO4)2 5.0, FeCl3 0.005, CaCO3 0.1, pH 7.5–8.0; and organophosphorus medium (g/L): glucose 10.0, (NH4)2SO4 0.5, NaCl 0.3, KCl 0.3, MgSO4·7H2O 0.3, FeSO4·7H2O 0.03, MnSO4·H2O 0.03 and agar 20. At the same time, 10 mL of yolk solution was added (ratio of normal saline to yolk solution: 1:1) [30]. A positive reaction was indicated by a transparent ring formed around the strain on the phosphorus solution medium. Secrete indole acetic acid (IAA) test: The strain was inoculated in LB liquid medium containing L-tryptophan (100 mg/L) at 30 °C and 180 r/min for 24 h. The suspended liquid of 50 μL bacteria was dropped on a white ceramic board, and Salkowski coloration solution of equal volume was added at the same time as performed previously [31]. LB liquid medium and an equal volume of chromogenic solution were used as controls. The white ceramic plate was placed at room temperature and in the dark for 30 min for observation. The development of a pink color indicates IAA production [31,32]. Protease production assay: Protease production was inoculated on 10 g/L skim milk agar plates at 30 °C. A positive test was indicated by the appearance of a clear zone around colonies [33]. Chitinase production assay: Chitin medium (g/L), colloidal chitin 10, peptone 10, K2HPO4 0.7, KH2PO4 0.4, MgSO4·7H2O 0.5, FeSO4·7H2O 0.01, ZnSO4 0.01 and agar 15.0 g. The presence of a clear ring around the colony indicates a positive reaction [34]. β-glucanase production assay: β-glucanase production medium (g/L), β-glucan 2.0, NaNO3 1.0, K2HPO4 1.0, KCl 0.5, MgSO4 0.5, FeSO4 0.1, Congo red 0.5 and pH 7.2. The positive test was indicated by the appearance of a clear zone around colonies.
2.7. Greenhouse Pot Experiments
The pot experiments were conducted in a greenhouse in which temperature, humidity and light duration were controllable. The experiment was set up in greenhouses in pots with a diameter of 60 cm and a height of 80 cm. Two seedling honeysuckle plants were placed in each pot. The experimental treatments were carried out after two weeks of growth of honeysuckle in the pots. The pathogenic fungus was added to each bottle of PDA liquid medium at a concentration of 108 cfu/mL after 72 h. Three antagonistic strains were incubated for 48 h in LB liquid medium. The experiment was divided into different treatments as follows: (1) for CK, plants were inoculated with sterile water as a control; (2) for Bacillus tequilensis 2M311, Bacillus velezensis R38 and Bacillus paralicheniformis LB6-1 were plants inoculated with different bacteria; (3) for CK + Fusarium oxysporum, plants were inoculated with pathogenic fungus; and (4) for Bacillus tequilensis 2M311 + Fusarium oxysporum, Bacillus velezensis R38 + Fusarium oxysporum and Bacillus paralicheniformis LB6-1 + Fusarium oxysporum, plants were inoculated with both pathogenic fungus and different antibiotic bacteria. Pathogenic and antibacterial bacteria were inoculated separately by root dipping. Each treatment was repeated three times.
2.8. Data Analysis
Paired-end reads were combined using FLASH, and the sequences were analyzed using QIIME [35]. UPARSE was used to remove chimeric sequences and identify sequences with >97% similarity as the same operational classification unit (OTU) [36]. Each OTU selects a representative sequence and annotates the classification using the RDP classifier. The alpha diversity of the bacterial community in the soil was calculated using the package “vegan” in the R version 4.2.2. Alpha diversity of the bacterial community was determined by the Shannon and Chao index [37]. Principal coordinate analysis (PCoA) was used to assess the beta diversity of the changes in the bacterial community based on the Bray‒Curtis distance between samples. The PERMANOVA test was carried out using the vegan Adonis function to assess the similarity of the bacterial community structure of different continuous cropping years. Venn diagrams were used to analyze OTUs that are common and unique to continuous cropping processing. Redundancy analysis (RDA) was used to reflect the relationship between the sample and environmental factors. Co-occurrence analysis involving OTU screening was performed according to a sample discovery rate of not less than 20%, and Spearman correlation coefficients p> 0.8 and p < 0.01 between OTUs were determined in the R environment. The function of microbial communities was predicted by functional annotation of Prokaryotic Taxa (FAPROTAX) [38]. Other data analyses were performed by one-way analysis of variance (ANOVA) using SPSS version 22.0.
3. Results
3.1. Physicochemical Properties of Honeysuckle Soil
The yield of honeysuckle decreased with the increase in years of cultivation, with the highest yield in the third year of cultivation and a 50.08% decrease in yield in the sixth year (Table 1). The pH decreased with the increase in the year of cultivation, with levels 14.90% and 20.56% lower in the fifth and sixth years, respectively. The nutrient content of the soil did not vary significantly between planting years, but there was a decreasing trend in the TN, TP and TK contents of the soil in the sixth year compared to the first year.

Table 1.
The physicochemical properties of honeysuckle soil.
3.2. Alpha Diversity of Honeysuckle Root Soil Bacterial Community
All samples had coverage above 99.10%, and the rarefaction curve of each sample was close to smooth, indicating that the results can truly reflect the situation of the samples (Figure S1). The Shannon and Chao1 indices were used to characterize the alpha diversity of the bacterial community. (Figure 1a). With the increase in successive years of honeysuckle cultivation, the Chao1 indices showed a decreasing trend, and the Shannon index of the bacterial community showed an overall downward trend after the HS2 sampling period. Venn diagrams were used to reflect the number of common and unique OTUs between groups or samples (Figure 1b). A total of 2408 OTUs were found between different crop years of honeysuckle and CK, while 2492 OTUs were found in different years of honeysuckle. The highest number of unique OTUs was found in the third year of continuous cropping. However, the proportion of endemic species decreased with increasing duration of honeysuckle continuous cropping.

Figure 1.
Alpha diversity indices of the bacterial community in soil with different years of continuous cropping (a). Venn diagram reveals the number of common and unique OTUs in soil of different continuous cropping years (b). Different lowercase letters indicate a significant difference between different years of cultivation of honeysuckle.
3.3. Composition and Structure of the Bacterial Community
The PCoA analysis showed that the structure of the bacterial community was significantly separated and differed among years of succession of honeysuckle (Figure 2a). The principal component axes PCoA1 and PCoA2 explained 20.46% and 17.43% of the variation, respectively. HS1 and HS2 were closer together but separated from HS5 and HS6 by Axis 1, showing that the bacterial communities were more similar after 1 and 2 years of successive planting, but HS3 was significantly separated from all samples, indicating that the greatest differences in bacterial communities were found in the third year. This result was confirmed by the Bray–Curtis distance similarity clustering analysis (Figure 2b). The comparative analysis of distance similarity between microbial communities showed that the greatest differences in CK were in the third year.
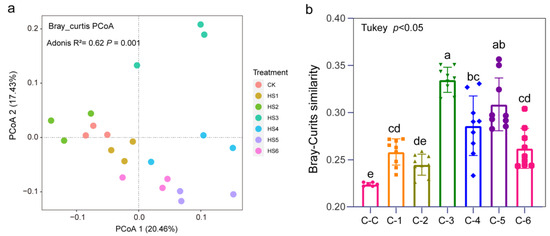
Figure 2.
Principal coordinate analysis (PCoA) of community structure based on Bray‒Curtis distance of bacteria with continuous cropping years (a). The distance similarity between microbial communities in soils of different continuous cropping years is based on Bray‒Curtis distance (b). C-C C-1, C-2, C-3, C-4, C-5 and C-6 denote CK-CK, CK-HS1, CK-HS2, CK-HS3, CK-HS4, CK-HS5 and CK-HS6, respectively. Different lowercase letters indicate a significant difference between different years of cultivation of honeysuckle.
The dominant phyla were Proteobacteria (24.08–27.58%) and Acidobacteria (18.82–24.20%), followed by Actinobacteria (8.57–14.24%), Bacteroidetes (4.34–9.28%), Chloroflexi (7.47–8.31%) and Gemmatimonadetes (4.30–6.76%) (Figure 3a). The relative abundance of Proteobacteria, Acidobacteria and Actinobacteria showed slight fluctuations in successive crop years of honeysuckle. Cyanobacteria was enriched in the third year of continuous cultivation of honeysuckle (Figure S2). Furthermore, Gammaproteobacteria (9.67–12.71%), Alphaproteobacteria (7.76–9.63%,) Deltaproteobacteria (5.52–6.54%) and Bacteroidia (5.54–9.20%) were the predominant classes (Figure 3b). Pyrinomonadaceae (3.8–5.6%), Anaerolineaceae (2.0–3.5%), unidentified_Acidobacteria (4.0–4.9%), Nitrosomonadaceae (2.58–3.945%), Microscillaceae (1.35–3.32%) and Gemmatimonadaceae (2.82–3.64%) were the domain families (Figure 3c).
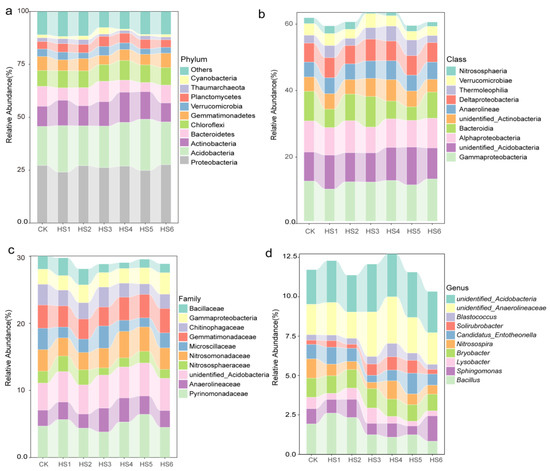
Figure 3.
The dynamics of the bacterial community composition during the continuous cropping time series at the phylum (a), class (b), family (c) and genus level (d).
At the genus level, Bacillus (0.83–2.61%), Sphingomonas (0.54–1.60%), Lysobacter (0.29–0.88%), Bryobacter (1.05–1.23%) and Nitrosospira (0.40–1.22%) were the dominant ones (Figure 3d). From the sequencing results, only 10.68–12.46% of the variation in all the samples could be identified at the genus level, and the other OTUs were annotated as unclassified.
The relative abundance of some bacteria, including Bacillus and Lysobacter, started to decrease significantly by the third year of continuous honeysuckle cultivation. However, some taxa were also enriched in the sixth year, for example, Sphingomonas (Figure 3d). The relative abundances changed in different years of crop succession for some genera, such as Bacillus, Nitrospira, Soilrubacter and Nocardioides (Figure 4a). Linear regression analysis showed that the relative abundance of Nocardia and Nitrospira, Soilrubrobacter and Nocardioides were significantly and positively correlated with honeysuckle yield (Figure 4b).
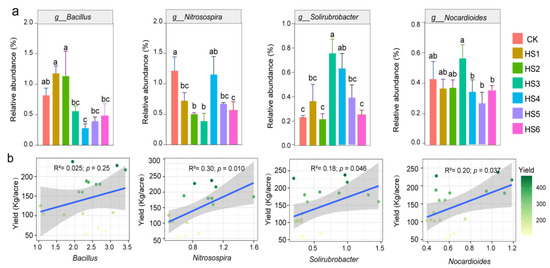
Figure 4.
Changes in the relative abundance of potential beneficial bacteria in soil during different continuous cropping years (a). Linear correlation analysis between the relative abundance of some bacteria with honeysuckle yield (b). Different lowercase letters indicate a significant difference between different years of cultivation of honeysuckle.
3.4. Correlation between Soil Physicochemical Properties and Bacterial Community
To understand the relationship between environmental factors and soil microbial communities, redundancy analysis was performed on soil microbial communities at the phylum level (Figure 5a). The first two axes of RDA explained a total of 59.84% of the community variation; the first axis explained 33.58%, and the second axis explained 26.26%. pH and TK had the greatest effects on soil microbial communities. In addition, the results of Spearman’s correlation analysis of genera with soil physicochemical properties showed that Bacillus, Candiddatus_Xiphinemmatobacter, Planctopirus, Microbacterium, Geobacter, Azoarcus and Agromyces showed a significant positive correlation (p < 0.05) with soil pH (Figure 5b). Streptomyces and Agromyces were positively correlated with TK. Other genera were significantly and negatively correlated with soil TN content, such as Rhodopirellula, Aquicella, Dongia and Steroidobacter. In addition, Geobacter and Azoarcus were significantly positively correlated with TP.
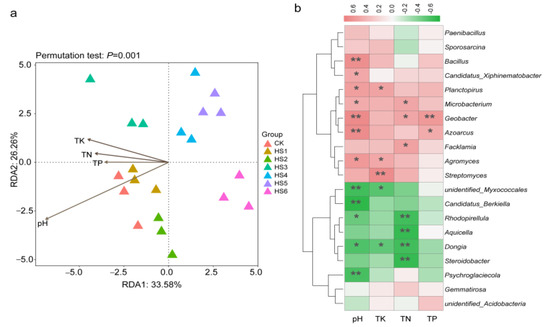
Figure 5.
Redundancy analysis (RDA) of bacterial community at phylum level and soil physicochemical properties (a). Spearman’s correlation analysis of soil properties and the abundance of bacteria at genus level (b). TN: total nitrogen; TP: total phosphorus; TK: total potassium. *, p < 0.05; **, p < 0.01.
3.5. Network Analysis of the Bacterial Community
A co-occurrence network analysis of bacterial communities was performed to further understand changes in microbial interactions across years of cropping. Two co-occurrence networks were constructed to clarify the changes in microbial interactions with increasing successive years of honeysuckle crops (Figure 6). The results showed that the network for 1–3 years had more nodes and an average degree value (Figure 6a,b). The resulting size (total nodes and links), higher average degree and higher average clustering in 1–3 years suggested that the network is more complex and the bacteria are more closely connected in 1–3 years than in 4–6 years (Figure 6c, Table S1). The modularity index of the two networks was >0.4, and the modularity index decreased with increasing years of continuous cropping. Moreover, there were more positive correlations in the 1–3 years group. Proteobacteria dominated the bacterial network at 1–3 years (28%), while Acidobacteria dominated (26.27%) at 4–6 years of continuous cropping, which may be due to the acidification of the soil environment with the increase in the number of years of continuous cropping. Moreover, the proportion of Cyanobacteria at 1–3 years was 2.86%, while that at 4–6 years was 0.67% in the network nodes. Lysobacter, Nocardioides and Burkholderiaceae, with high node degree values, were the keystone species in 1–3 years, but Solirubrobacter, Gaiella and Stenotrophobacter were keystone species in 4–6 years, which showed that the keystone species of the bacterial co-occurrence did not overlap between different years of continuous cropping (Table S2).
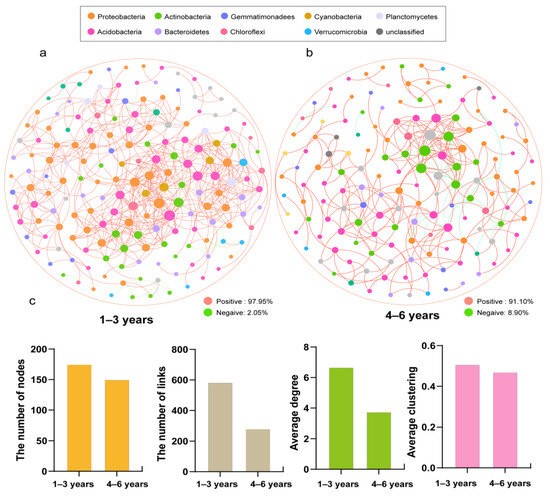
Figure 6.
Bacterial co-occurrence network at different years of continuous cropping: 1–3 years (a); 4–6 years (b). Various network topological parameters include nodes, links, average degree and average clustering (c).
3.6. Functional Prediction Analysis of the Bacteria Community
Changes in bacterial community composition resulted in changes in metabolic genes, which were predicted by FAPROTAX based on 16S rRNA gene sequences (Figure S3). Phototrophy, photoautotrophy, photosynthetic cyanobacteria, oxygenic photoautoautrophy, fermentation and chitinolysis were enriched in the third year of honeysuckle continuous cropping and decreased with increasing years of continuous cropping. Sulfate respiration, respiration of sulfur compounds and predatory or exoparasitic and aromatic compound degradation were enriched in the third to fourth years of honeysuckle continuous cropping but reduced in the sixth year. The abundance of human-associated, human pathogens and animal parasites or symbiotic parasites was significantly reduced after honeysuckle cultivation. The abundance of many functions associated with carbon and nitrogen cycling decreased after the third year of continuous monocropping of honeysuckle cultivation. In addition, the predictive function of aerobia nitrite oxidation was significantly enriched in the sixth consecutive year of the cropping.
3.7. Characteristics of the Antagonistic Bacteria
A total of 61 bacteria were screened from the rhizosphere of honeysuckle, of which the most isolated genera were Bacillus and Pseudomonas, accounting for more than 50% of the total number of isolates (Figure S4, Table S3). Based on the EzBioCloud database, the preliminary phylogenetic analysis of bacteria with antagonistic effects on the 16S rRNA gene sequences is shown in Table 2. The strain 2M311 showed the highest 16S rRNA gene similarities with Bacillus tequilensis (KCTC 13622T 99.93%), R38 showed the highest similarities with Bacillus velezensis (CR-502T 99.36%), and LB6-1 showed the highest similarities with Bacillus paralicheniformis (KJ-16T 100%).

Table 2.
Evaluation of the bacteria to antagonize Fusarium oxysporum.
To determine the inhibitory ability of the isolates, 61 strains were cultured in double culture (Table S3), of which three strains with the strongest antagonistic ability were selected for subsequent experiments (Table 2). The inhibition rates of these three strains, 2M311, R38 and LB6-1, against Fusarium oxysporum pathogens were 48.66%, 69.66 and 74.66%. The three colonies were white, opaque, and round in shape, with a wrinkled surface and slightly raised edges, and were Gram-positive (Figure 7a). The results of PGPR characterization of these strains showed that they grew well on an Ashby nitrogen-free medium; LB6-1 and 2M311 were phosphate soluble and showed clear zones of phosphate solubility around bacterial colonies based on organophosphate and inorganic phosphate plate and produced indole acetic acid (IAA) (Figure S5, Table S4). In addition, R38, LB6-1 and 2M311 all have protease and β-glucanase activity, but only strain LB6-1 exhibits chitinase activity (Figure S6).
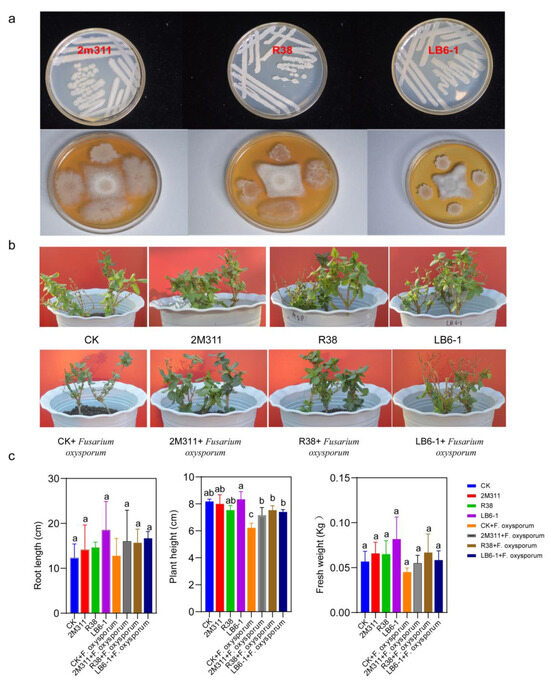
Figure 7.
Colony morphology of 2M311 (Bacillus tequilensis), R38 (Bacillus velezensis) and LB6-1 (Bacillus paralicheniformis), and 2M311, R38 and LB6-1 against Fusarium oxysporum on PDA plates (a). Pot experiment for functional validation of the strains (b). The effect of three antagonistic bacteria on the plant growth parameters of honeysuckle under greenhouse conditions over one month (c). Experimental results are presented as mean ± SD. Different lowercase letters indicate a significant difference in the treatments (p < 0.05).
3.8. Biocontrol Potential of the Antagonistic Bacteria for Pathogens Fusarium oxysporum
The biocontrol efficacy of antagonistic bacteria against Fusarium oxysporum causing root rot disease was evaluated in the greenhouse (Figure 7b,c). Compared with CK, LB6-1 had the best effect on improving the root length and plant height of honeysuckle when no pathogenic fungi were added. When pathogenic fungi were added, R38 had the best effect on improving the fresh weight and plant height of the honeysuckle. In conclusion, the results showed that the Fusarium oxysporum significantly inhibited the plant height of honeysuckle compared with the control group (p < 0.05), which may be one of the reasons why plant growth deteriorates as the years of continuous cropping increase. The three antagonistic strains had an effect on promoting the growth of honeysuckle and could inhibit the root rot of honeysuckle to some extent. High-throughput sequencing analyses of bacterial community composition showed a decrease in the abundance of Bacillus after three years of continuous cropping, which, in combination with the verified culture-dependent antagonistic pathogen characteristics of Bacillus, was hypothesized to be one of the reasons for the increase in root rot of honeysuckle with increasing years of continuous cropping.
4. Discussion
4.1. Continuous Cropping Led to Changes in Soil Physical and Chemical Properties
In our study, soil pH, TN, TP and TK contents decreased with increasing years of continuous cropping of honeysuckle (Table 1). Continuous monocropping has been reported to lead to deterioration in soil chemistry, imbalance in nutrient content and acidification, which ultimately leads to a reduction in plant yield and quality [39]. For example, continuous cropping of Panax quinquefolius L. (American ginseng) also resulted in a decrease in soil pH [40]. Soil acidification may be caused by the accumulation of root secretions, and the acidic environment will limit root development and nutrient absorption during plant growth [41]. Furthermore, it will encourage the multiplication and spread of soil pathogens [42]. Another study found that the contents of TN, TP and TK in the soil showed a downtrend with the extension of continuous cropping time [43]. Moreover, continuous cropping significantly reduced rhizosphere TN content [44]. The decrease in soil nutrient content and pH may be an obstacle to honeysuckle continuous cropping. There are many effective measures that can be taken in practical farming, such as adding organic amendments to regulate the pH of soil.
4.2. Continuous Cropping of Honeysuckle Led to Changes in Diversity of Bacterial Community
It has also been shown that bacterial diversity tends to decrease with increasing years of cultivation of American ginseng [45]. For example, continuous cropping of Rehmannia glutinosa caused a decrease in bacterial diversity [46]. This is in agreement with the results of continuous cropping of Sophora flavescens and Lycium barbarum L., which all showed that continuous cropping reduced the alpha diversity of soil bacterial communities [47]. However, some studies showed that with the increase in planting years, the Shannon index of bacteria of Andrographis paniculata and potatoes increased significantly [48,49]. This is in contrast to the results of the present study and may be related to differences in soil type, crop species and cultivation practices. In general, the greater the diversity of soil microorganisms, the more stable the microbial community and the more resistant it is to environmental disturbances [50]. Bacterial community abundance and diversity are important drivers of ecosystem sustainability and productivity [51]. Therefore, the reduced soil bacterial diversity may be one of the factors contributing to the formation of a succession barrier.
4.3. Continuous Cropping of Honeysuckle Led to Changes in Soil Bacteria Community Structure
Proteobacteria was the dominant phylum in the honeysuckle soil, and Proteobacteria was the dominant phyla of the tomato rhizosphere [52], which is consistent with our study. The main function of Proteobacteria is to break down organic matter and promote plant growth [41]. Notably, the relative abundance of Cyanobacteria reached a maximum abundance in the third year of honeysuckle cultivation (Figure S2). It has been reported that Cyanobacteria can fix N2 in the atmosphere and are increasingly being used as biological agents to inoculate and improve soil fertility and environmental quality [53]. The relative abundance of Lysobacter decreased with successive years of cultivation; in fact, it is an important potentially beneficial bacteria that is also gradually depleted during the continuous cropping of tomatoes [54]. Several bacterial genera, such as Nocardioides and Bacillus, are known to not only antagonize soil-borne fungal pathogens but also trigger plant-induced systemic resistance (ISR) and promote plant growth [55]. Nocardioides is also a plant growth-promoting rhizobacteria (PGPR) that can secrete antibiotics [56]. Nitrospirae is associated with soil nitrite oxidation, which affects the uptake of nitrogen by crops [57]. Bacillus is also a disease-inhibiting group in potato continuous cropping soil [41]. In addition, Flavisolibacter and Bryobacter decrease in abundance with increasing years of continuous cropping. Continuous cropping of Rehmannia glutinosa also caused a decrease in the abundance of Pseudomonas and Bacillus [46]. A study has shown that the number of aerobic bacteria and nitrogen-fixing bacteria decreases significantly with increasing years of continuous cropping, which may disrupt the balance of the original bacterial community structure and affect plant growth [58]. The change in the bacterial community is a complex process, but the majority of research has demonstrated that continuous cropping decreases the relative abundance of beneficial bacteria [54,59,60]. There were also bacteria that increased in relative abundance in the sixth year of continuous cropping, such as Sphingomonas. A study has shown that Sphingomonas can tolerate barren and harsh environments and that their special metabolic regulatory mechanisms can withstand unfavorable external environmental changes, as well as degrade toxic substances in the soil [61]. It is also possible that the prolonged use of chemical pesticides in the continuous cropping process results in high levels of organic contamination in the soil. This may be the reason why the genus was enriched in the sixth consecutive year of honeysuckle cultivation. These changes across different years demonstrated that continuing agricultural practices influence the structure of soil bacterial communities.
4.4. Soil Physical and Chemical Properties Are Closely Related to Bacterial Community Structure
Continuous cropping may affect soil microbial communities by influencing the soil physicochemical environment [62]. Our study showed that the bacterial community structure characteristics of honeysuckle in different years of continuous cropping were strongly correlated with soil pH, TP, TN and TK contents (Figure 5b), which was consistent with the results of watermelon and potato continuous cropping [63,64]. Nutrients released by microbial activity in soil are an important source of nutrients for plants. Continuous cropping can alter soil nutrient effectiveness and thus reduce beneficial microbial abundance [65]. A study has demonstrated that pH is an essential factor influencing the diversity and composition of bacterial communities [66]. Disturbances in the microbial community structure due to declining soil nutrient status as the number of years of continuous cropping increases may be the main reason for the decline in honeysuckle yield. Consistent with our results, continuous cropping of ramie leads to poor growth and yield reduction [67]. Therefore, continuous cropping of honeysuckle may alter the physicochemical properties of the soil and, thus, the structure of the bacterial community.
4.5. Continuous Cropping Reduced the Complexity of Bacterial Community Networks
Microbes do not exist independently but form complex social networks. In this study, the complexity of the bacterial co-occurrence network was higher at 1–3 years of continuous cropping than at 4–6 years, including the number of nodes, average degree and other network attributes. Previous studies have shown that bacterial communities with complex network structures are more resistant to environmental disturbances than simple networks [68]. A related study also found that when agricultural activity increases, the complexity of the microbial network decreases [69]. Burkholderiaceae was the keystone in the 1–3 years bacterial network, but it was altered in 4–6 years. The keystone on the network is closely associated with the functional potential of the soil [70]. Burkholderiaceae has been reported to be effective in inhibiting the growth of pathogenic fungi in the soil [71]. Positive correlation dominates in both bacterial co-occurrence networks (Figure 6a,b), indicating that the synergistic effect was the main effect among bacteria in honeysuckle soils [72]. Compared to 1–3 years of continuous cropping, the proportion of positive correlations between bacteria was also significantly reduced in the 4–6 year bacterial co-occurrence network, which was probably due to increased competition between soil bacterial communities as a result of decreased soil nutrients due to increased years of continuous cropping of plants. Therefore, prolonged cropping may possibly lead to reduced resistance of soil bacteria to environmental changes.
4.6. Continuous Cropping Changed the Function of Bacteria Community
The FAPROTAX functional predictions showed that the abundance of genes related to carbon, nitrogen and sulfur metabolism and chitinolysis in the honeysuckle soil decreased with increasing years of continuous cropping (Figure S3). Photorophy was enriched in the third year but decreased with increasing years of continuous cropping, which may be related to changes in the bacterial community, such as reduced populations of photosynthetic bacteria (Cyanobacteria), which may inhibit phototrophic, anoxic phototrophic and photoautotrophic processes and inhibit carbon fixation. Chitinolysis has been observed to create nitrate/ammonium, which could help in the degradation of invertebrate chitin [73]. Moreover, nitrate reduction would result in nutrient loss in honeysuckle continuous cropping soil due to nitrogen content loss [56], which may also be a contributing factor to the decrease in soil nutrient content of honeysuckle with increasing years of continuous cropping. Sulfuate respiration was enriched in the third year. Sulfur is essential for plant growth, and reduction in sulfur metabolism may lead to smaller plants, yellowing of leaves and ultimately to reduced crop yield and quality [74], which may also be one of the reasons for the best honeysuckle yields in the third year. Chemoheterotrophy, aerobic chemoheterotrophy and fermentation are significant biological processes related to the carbon cycle [75], and the decrease in their abundance in the fifth and sixth years indicates that the proportion of bacteria participating in the carbon cycle is reduced under a continuous cropping system, which will not be conducive to soil organic carbon mineralization. The increase in aerobic ammonia oxidation implies that under the continuous monocropping system, a greater proportion of bacteria were involved in carbon cycling.
4.7. Antagonistic Bacteria Improve Honeysuckle Growth
In our experiments, the growth of honeysuckle was improved by the addition of Bacillus sp. The possible reason why Bacillus can play a role in honeysuckle rhizosphere is that Bacillus belongs to that is easy to colonize, and the three Bacillus strains were isolated from the honeysuckle soil and can quickly adapt to the environment. At the same time, these Bacillus are capable of fixing nitrogen, solubilizing organic and inorganic phosphorus, and secreting plant growth hormones (IAA), all of which are beneficial for the improvement of plant growth environment. As reported, the addition of beneficial bacteria improves soil nutrition and quality and prevents plant disease without excessively altering soil conditions [76]. In addition, those Bacillus could inhibit the growth of pathogenic fungi Fusarium oxysporum by secreting chitinases and proteases. Fusarium oxysporum is the most common phytopathogenic fungi, infecting almost 150 plant species, which can cause vascular wilt disease in many food crops, resulting in considerable yearly economic losses throughout the world [77,78]. Chitinase and β-glucanase are major components of the fungal cell wall, and antagonistic bacteria may inhibit the growth of pathogenic fungi by secreting chitinase and β-glucanase, thereby inhibiting the growth of pathogenic fungi. The β-glucanase enzyme directly attacks glucan on fungal hyphae, inhibiting fungal growth, and the oligosaccharides produced during hydrolysis act as exciters in the plant hypersensitive response, inducing a plant defense response [79]. Bacillus has been shown to strengthen the cell wall and immunity against pathogen infections and plays an important role in tomato and rice disease resistance systems [80]. Similar to our research, a study found that Bacillus subtilis B28 has a control efficacy of approximately 44% against Fusarium wilt in chickpeas by enhancing plant growth characteristics [78]. Bacillus velezensis has been reported to form trophic interactions with indigenous species and mobilize indigenous microorganisms to achieve plant growth regulation [81]. According to a recent study, the biocontrol bacterium Beijerinckia fluminensis BFC-33 isolated from the rhizosphere of potatoes might greatly suppress the growth of Fusarium oxysporum [82]. Studies of bacterial-fungal interactions in agroecosystems are primarily concerned with the control of diseases [83,84]. Consistent with our results, some beneficial native root-associated bacteria were isolated from the rhizosphere of tobacco in a continuous cropping system, which could reduce the incidence of disease [85]. Many of the Bacillus species recognized as environmentally friendly soil regulators produce effective substances for controlling soil-borne pathogens [86]. Moreover, numerous studies have attempted to use Bacillus as biocontrol agents and biofertilizers [23,87,88]. Furthermore, the inhibitory effect on root rot was further demonstrated by the higher species diversity of cultured Bacillus, suggesting an important role for classical nonculture techniques in developing better antagonistic strategies [89]. Therefore, soil microbial community management is a viable approach to the biological control of continuous cropping barriers and will be an important aspect of future crop research. However, considering the insufficient sample size of our experiments and the fact that the strain function validation experiments were conducted in a greenhouse, where many of the environmental conditions could be controlled, subsequent field application of these strains may require several more experiments for optimization and adjustment.
5. Conclusions
The bacterial community structure and physicochemical properties of the honeysuckle soil were affected by continuous cropping years, especially when the soil pH decreased significantly. There was a reduction in the potentially beneficial bacteria Cyanobacteria and Bacillus during continuous cropping of honeysuckle. Three strains of Bacillus with antagonistic abilities against pathogenic fungi Fusarium oxysporum were isolated, identified and characterized from honeysuckle soil. Meanwhile, the disease suppression activities of Bacillus against honeysuckle root rot pathogens were further validated by pot experiments. The third year may be the accumulation enrichment phase of beneficial bacteria, and with the highest yield of honeysuckle, soil nutrient and pH condition improvement should be appropriate after the third year, which may be more favorable for the continued cultivation of honeysuckle. Our findings provide evidence for identifying and monitoring changes in plant continuous cropping bacterial communities as well as the cultivation of beneficial antagonistic bacteria to optimize medicinal plant continuous cropping scenarios to support the control of soil-borne pathogens.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy14020260/s1. Figure S1: Rarefaction curves of all samples; Figure S2: Relative abundance of Cyanobacterial in different samples; Figure S3: Heatmap of predicted soil function impacted by continuous cropping years; Figure S4: Statistics of strains obtained from pure culture; Figure S5: Verification of nitrogen fixation (a), dissolved organic phosphorus (b), dissolved inorganic phosphorus (c) and secrete indole-3-acetic acid (IAA) by three strains of Bacillus (d); Figure S6: Verification of protease, chitinase and β-glucanase production by three Bacillus. From top to bottom, the following are protease, chitinase and β-glucanase; Table S1: Topological characteristics of the bacterial network; Table S2: Nodes identified as keystones in the different cropping year of honeysuckle bacteria networks; Table S3: Detailed information of strains isolated from pure culture; Table S4: Plant-growth promoting traits of selected antagonistic bacterial isolates.
Author Contributions
Q.M. performed all experiments and composed the main text. X.H. prepared experimental materials. Y.Z. (Yiqing Zhou) and X.J. carried out the strain isolation. T.W. analyzed the data of the bacterial community. L.L. and Y.Z. (Yan Zhuang) provided suggestions for the manuscript. Z.R. conceived, organized and supervised the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (NSFC 3211101206). This research was also supported by the National Key Research and Development Program of China (2023YFE0104900) and the Agricultural Science and Technology Innovation Program (CAAS-ZDRW202308 and No. CAAS-ZDRW202202).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Author Xiaoyan Han was employed by the company Autobio Diagnostics Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Shang, X.; Pan, H.; Li, M.; Miao, X.; Ding, H. Lonicera Japonica Thunb.: Ethnopharmacology, Phytochemistry and Pharmacology of an Important Traditional Chinese Medicine. J. Ethnopharmacol. 2011, 138, 1–21. [Google Scholar] [CrossRef]
- Zeng, H.; Li, Y.; Chen, J.; Wang, X.; Qiao, Z.; Li, Y.; Cai, N.; Liu, S. Lonicera Japonica ‘Fenglei’. HortScience Horts 2017, 52, 789–791. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; He, L.; Li, Y. Chinese Herbs Combined with Western Medicine for Severe Acute Respiratory Syndrome (SARS). Cochrane Database Syst. Rev. 2012, 10, CD004882. [Google Scholar] [CrossRef]
- Ko, H.-C.; Wei, B.-L.; Chiou, W.-F. The Effect of Medicinal Plants Used in Chinese Folk Medicine on RANTES Secretion by Virus-Infected Human Epithelial Cells. J. Ethnopharmacol. 2006, 107, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, Y.; Sun, M.; Ji, H.; Dou, H.; Hu, J.; Yan, Y.; Wang, X.; Chen, L. Honeysuckle-Encoded MicroRNA2911 Inhibits Enterovirus 71 Replication via Targeting VP1 Gene. Antivir. Res. 2018, 152, 117–123. [Google Scholar] [CrossRef]
- Chen, Y.; Du, J.; Li, Y.; Tang, H.; Yin, Z.; Yang, L.; Ding, X. Evolutions and Managements of Soil Microbial Community Structure Drove by Continuous Cropping. Front. Microbiol. 2022, 13, 839494. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Y.; Liu, Q.; Zhang, Y.; Li, X.; Li, H.; Li, W. Phase Changes of Continuous Cropping Obstacles in Strawberry (Fragaria × Ananassa Duch.) Production. Appl. Soil. Ecol. 2020, 155, 103626. [Google Scholar] [CrossRef]
- Li, P.; Liu, J.; Saleem, M.; Li, G.; Luan, L.; Wu, M.; Li, Z. Reduced Chemodiversity Suppresses Rhizosphere Microbiome Functioning in the Mono-Cropped Agroecosystems. Microbiome 2022, 10, 108. [Google Scholar] [CrossRef]
- Zeeshan Ul Haq, M.; Yu, J.; Yao, G.; Yang, H.; Iqbal, H.A.; Tahir, H.; Cui, H.; Liu, Y.; Wu, Y. A Systematic Review on the Continuous Cropping Obstacles and Control Strategies in Medicinal Plants. Int. J. Mol. Sci. 2023, 24, 12470. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil Structure and Microbiome Functions in Agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Carrión, V.J.; Perez-Jaramillo, J.; Cordovez, V.; Tracanna, V.; de Hollander, M.; Ruiz-Buck, D.; Mendes, L.W.; van Ijcken, W.F.J.; Gomez-Exposito, R.; Elsayed, S.S.; et al. Pathogen-Induced Activation of Disease-Suppressive Functions in the Endophytic Root Microbiome. Science 2019, 366, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.; Wu, G.; Zhang, N.; Shen, Q.; Zhang, R. Beneficial Rhizobacterium Bacillus Amyloliquefaciens SQR9 Induces Plant Salt Tolerance through Spermidine Production. Mol. Plant. Microbe. Interact. 2017, 30, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, Z.H.; Iqbal, J.; Zhang, Q.; Chen, D.; Wei, H.; Saleem, M. Continuous Cropping Alters Multiple Biotic and Abiotic Indicators of Soil Health. Soil. Syst. 2020, 4, 59. [Google Scholar] [CrossRef]
- Wang, G.; Ren, Y.; Bai, X.; Su, Y.; Han, J. Contributions of Beneficial Microorganisms in Soil Remediation and Quality Improvement of Medicinal Plants. Plants 2022, 11, 3200. [Google Scholar] [CrossRef] [PubMed]
- Todorović, I.; Moënne-Loccoz, Y.; Raičević, V.; Jovičić-Petrović, J.; Muller, D. Microbial Diversity in Soils Suppressive to Fusarium Diseases. Front. Plant Sci. 2023, 14, 1228749. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, D.; Liu, L.; Wang, Y.; Zhang, Y. Screening and Identification of Antagonistic Bacteria from Vermicompost against Fusarium Oxysporum f. Sp. Cucumerinum. Acta Agric. Scand. Sect. B Soil. Plant Sci. 2021, 71, 266–272. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Z.; Wang, X.S. Occurrence pattern and pathogen identification of root rot of Lonicera japonica Thunb. J. Anhui Agric. Sci. 2019, 47, 129–131. (In Chinese) [Google Scholar]
- Liu, M.T.; Sun, H.T.; Zhang, D.F. A distinguish on a new root rot of Lonicera japonica and study on biological characteristic of the pathogen. Acta Agric. Boreali-Sin. 2004, 19, 109–111. (In Chinese) [Google Scholar]
- Zhang, S.; Jiang, Q.; Liu, X.; Liu, L.; Ding, W. Plant Growth Promoting Rhizobacteria Alleviate Aluminum Toxicity and Ginger Bacterial Wilt in Acidic Continuous Cropping Soil. Front. Microbiol. 2020, 11, 569512. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; Yang, X.; Li, C.; Wang, L.; Feng, J.; Chen, S.; Li, X.; Yang, Y. Effects of Integrated Biocontrol on Bacterial Wilt and Rhizosphere Bacterial Community of Tobacco. Sci. Rep. 2021, 11, 2653. [Google Scholar] [CrossRef]
- Niu, D.; Liu, H.; Jiang, C.; Wang, Y.; Wang, Q.; Jin, H.; Guo, J. The Plant Growth-Promoting Rhizobacterium Bacillus Cereus AR156 Induces Systemic Resistance in Arabidopsis Thaliana by Simultaneously Activating Salicylate- and Jasmonate/Ethylene-Dependent Signaling Pathways. Mol. Plant. Microbe. Interact. 2011, 24, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Guo, W.; Chen, X. Exogenous Addition of Alkanoic Acids Enhanced Production of Antifungal Lipopeptides in Bacillus Amyloliquefaciens Pc3. Appl. Microbiol. Biotechnol. 2019, 103, 5367–5377. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.; Fu, X.; Li, Y.; Wang, Q. Isolation and Characterization of Bacillus Amyloliquefaciens PG12 for the Biological Control of Apple Ring Rot. Postharvest Biol. Technol. 2016, 115, 113–121. [Google Scholar] [CrossRef]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological Control of Plant Pathogens by Bacillus Species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, S.; Diao, M.; Fu, J.; Borthwick, A.G.L. Microbial Community Responses to Vanadium Distributions in Mining Geological Environments and Bioremediation Assessment. J. Geophys. Res. Biogeosci. 2019, 124, 601–615. [Google Scholar] [CrossRef]
- Parkinson, J.A.; Allen, S.E. A Wet Oxidation Procedure Suitable for the Determination of Nitrogen and Mineral Nutrients in Biological Material. Commun. Soil. Sci. Plant Anal. 1975, 6, 1–11. [Google Scholar] [CrossRef]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; et al. Improved Bacterial 16S rRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. mSystems 2016, 1, e00009-15. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, J.; Liu, Y.; Chen, S. Isolation and Identification of Nitrogen-Fixing Bacilli from Plant Rhizospheres in Beijing Region. J. Appl. Microbiol. 2005, 99, 1271–1281. [Google Scholar] [CrossRef]
- Gothwal, R.; Nigam, V.; Medicherla, K.; Sasmal, D.; Ghosh, P. Phosphate Solubilization by Rhizospheric Bacterial Isolates from Economically Important Desert Plants. Indian J. Microbiol. 2006, 46, 355–361. [Google Scholar]
- MAYER, A.M. Determination of Indole Acetic Acid by the Salkowsky Reaction. Nature 1958, 182, 1670–1671. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Devi, S.; Patil, S.; Payal, C.; Negi, S. Isolation, Screening and Characterization of Bacteria from Rhizospheric Soils for Different Plant Growth Promotion (PGP) Activities: An in Vitro Study. Recent Res. Sci. Technol. 2012, 4, 1–5. [Google Scholar]
- Chang, W.T.; Hsieh, C.H.; Hsieh, H.S.; Chen, C. Conversion of Crude Chitosan to an Anti-Fungal Protease by Bacillus Cereus. World J. Microbiol. Biotechnol. 2009, 25, 375–382. [Google Scholar] [CrossRef]
- Azizoglu, Z.B.; Yilmaz, S.; Azizoglu, U.; Karabörklü, S.; Temizgul, R.; Ayvaz, A. Molecular Characterization of the Chitinase Genes of Native Bacillus Thuringiensis Isolates and Their Antagonistic Activity against Three Important Phytopathogenic Fungi. Biologia 2021, 76, 2745–2755. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and Clustering Orders of Magnitude Faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Kemp, P.F.; Aller, J.Y. Bacterial Diversity in Aquatic and Other Environments: What 16S rDNA Libraries Can Tell Us. FEMS Microbiol. Ecol. 2004, 47, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Stilianos, L.; Wegener, P.L.; Michael, D. Decoupling Function and Taxonomy in the Global Ocean Microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, G.; Cheng, Y.; Shi, P.; Yang, C.; Yang, H.; Xu, Z. Soil Acidification in Continuously Cropped Tobacco Alters Bacterial Community Structure and Diversity via the Accumulation of Phenolic Acids. Sci. Rep. 2019, 9, 12499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fan, S.; Qin, J.; Dai, J.; Zhao, F.; Gao, L.; Lian, X.; Shang, W.; Xu, X.; Hu, X. Changes in the Microbiome in the Soil of an American Ginseng Continuous Plantation. Front. Plant Sci. 2020, 11, 572199. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Hu, Y.; Han, M.; Xu, J.; Wang, X.; Liu, L.; Tang, Z.; Jiao, W.; Jin, R.; Liu, M.; et al. Effects of Continuous Cropping of Sweet Potatoes on the Bacterial Community Structure in Rhizospheric Soil. BMC Microbiol. 2021, 21, 102. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Q.; Zhou, J.; Wei, Q. Illumina Amplicon Sequencing of 16S rRNA Tag Reveals Bacterial Community Development in the Rhizosphere of Apple Nurseries at a Replant Disease Site and a New Planting Site. PLoS ONE 2014, 9, e111744. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wu, J.; Ji, Q.; Wu, W.; Dong, S.; Yu, J.; Zhang, Q.; Qin, L. Diversity of Rhizosphere and Endophytic Fungi in Atractylodes Macrocephala during Continuous Cropping. PeerJ 2020, 8, e8905. [Google Scholar] [CrossRef]
- Yao, Y.; Yao, X.; An, L.; Bai, Y.; Xie, D.; Wu, K. Rhizosphere Bacterial Community Response to Continuous Cropping of Tibetan Barley. Front. Microbiol. 2020, 11, 551444. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Z.; Niu, J.; Dang, K.; Zhang, S.; Wang, S.; Wang, Z. Changes in Physicochemical Properties, Enzymatic Activities, and the Microbial Community of Soil Significantly Influence the Continuous Cropping of Panax Quinquefolius L. (American Ginseng). Plant Soil 2021, 463, 427–446. [Google Scholar] [CrossRef]
- Wu, L.; Chen, J.; Xiao, Z.; Zhu, X.; Wang, J.; Wu, H.; Wu, Y.; Zhang, Z.; Lin, W. Barcoded Pyrosequencing Reveals a Shift in the Bacterial Community in the Rhizosphere and Rhizoplane of Rehmannia Glutinosa under Consecutive Monoculture. Int. J. Mol. Sci. 2018, 19, 850. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Liu, A.; Hou, Q.; Zhao, Q.; Guo, J.; Wang, Z. Diversity Patterns of Soil Microbial Communities in the Sophora Flavescens Rhizosphere in Response to Continuous Monocropping. BMC Microbiol. 2020, 20, 272. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, D.; Yang, Y.; Pan, Y.; Zhao, D.; Zhu, J.; Zhang, L.; Yang, Z. Dissecting the Effect of Continuous Cropping of Potato on Soil Bacterial Communities as Revealed by High-Throughput Sequencing. PLoS ONE 2020, 15, e0233356. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Li, S.; Zuo, Z.; Zhan, R.; He, R. Variations of Rhizospheric Soil Microbial Communities in Response to Continuous Andrographis Paniculata Cropping Practices. Bot. Stud. 2020, 61, 18. [Google Scholar] [CrossRef]
- Saleem, M.; Hu, J.; Jousset, A. More Than the Sum of Its Parts: Microbiome Biodiversity as a Driver of Plant Growth and Soil Health. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 145–168. [Google Scholar] [CrossRef]
- Chaer, G.; Fernandes, M.; Myrold, D.; Bottomley, P. Comparative Resistance and Resilience of Soil Microbial Communities and Enzyme Activities in Adjacent Native Forest and Agricultural Soils. Microb. Ecol. 2009, 58, 414–424. [Google Scholar] [CrossRef]
- Cheng, Z.; Lei, S.; Li, Y.; Huang, W.; Ma, R.; Xiong, J.; Zhang, T.; Jin, L.; Haq, H.U.; Xu, X.; et al. Revealing the Variation and Stability of Bacterial Communities in Tomato Rhizosphere Microbiota. Microorganisms 2020, 8, 170. [Google Scholar] [CrossRef]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A Precious Bio-Resource in Agriculture, Ecosystem, and Environmental Sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Li, H.; Wang, J.; Gao, W.; Shu, X.; Sun, X.; Wang, K.; Duan, Y.; Liu, Y.; Kuramae, E.E.; et al. Composition, Function and Succession of Bacterial Communities in the Tomato Rhizosphere during Continuous Cropping. Biol. Fertil. Soils 2023, 59, 723–732. [Google Scholar] [CrossRef]
- Lazcano, C.; Boyd, E.; Holmes, G.; Hewavitharana, S.; Pasulka, A.; Ivors, K. The Rhizosphere Microbiome Plays a Role in the Resistance to Soil-Borne Pathogens and Nutrient Uptake of Strawberry Cultivars under Field Conditions. Sci. Rep. 2021, 11, 3188. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Bai, J.L.; Yang, H.T.; Zhang, W.D.; Xiong, Y.W.; Ding, P.; Qin, S. Phylogenetic Diversity and Investigation of Plant Growth-Promoting Traits of Actinobacteria in Coastal Salt Marsh Plant Rhizospheres from Jiangsu, China. Syst. Appl. Microbiol. 2018, 41, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Daims, H.; Lücker, S.; Wagner, M. A New Perspective on Microbes Formerly Known as Nitrite-Oxidizing Bacteria. Trends Microbiol. 2016, 24, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xiao, Y.; Lv, F.; Hu, L.; Wei, L.; Yuan, Z.; Lin, H. Bacterial Community Structure and Functional Potential of Rhizosphere Soils as Influenced by Nitrogen Addition and Bacterial Wilt Disease under Continuous Sesame Cropping. Appl. Soil Ecol. 2018, 125, 117–127. [Google Scholar] [CrossRef]
- Wang, T.; Yang, K.; Ma, Q.; Jiang, X.; Zhou, Y.; Kong, D.; Wang, Z.; Parales, R.E.; Li, L.; Zhao, X.; et al. Rhizosphere Microbial Community Diversity and Function Analysis of Cut Chrysanthemum During Continuous Monocropping. Front. Microbiol. 2022, 13, 801546. [Google Scholar] [CrossRef]
- Huang, W.; Sun, D.; Fu, J.; Zhao, H.; Wang, R.; An, Y. Effects of Continuous Sugar Beet Cropping on Rhizospheric Microbial Communities. Genes 2019, 11, 13. [Google Scholar] [CrossRef]
- Matsumoto, H.; Fan, X.; Wang, Y.; Kusstatscher, P.; Duan, J.; Wu, S.; Chen, S.; Qiao, K.; Wang, Y.; Ma, B.; et al. Bacterial Seed Endophyte Shapes Disease Resistance in Rice. Nat. Plants 2021, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Hu, Y.; Zhang, S.; Noll, L.; Böckle, T.; Dietrich, M.; Herbold, C.W.; Eichorst, S.A.; Woebken, D.; Richter, A.; et al. Soil Multifunctionality Is Affected by the Soil Environment and by Microbial Community Composition and Diversity. Soil Biol. Biochem. 2019, 136, 107521. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Zhou, D.; Wei, H.; Wu, S.; Xie, B. Alleviating Soil Degradation Caused by Watermelon Continuous Cropping Obstacle: Application of Urban Waste Compost. Chemosphere 2021, 262, 128387. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Hu, Y.; Razavi, B.S.; Zhou, J.; Shen, J.; Nannipieri, P.; Wu, J.; Ge, T. Rare Taxa of Alkaline Phosphomonoesterase-Harboring Microorganisms Mediate Soil Phosphorus Mineralization. Soil Biol. Biochem. 2019, 131, 62–70. [Google Scholar] [CrossRef]
- Hontoria, C.; García-González, I.; Quemada, M.; Roldán, A.; Alguacil, M.M. The Cover Crop Determines the AMF Community Composition in Soil and in Roots of Maize after a Ten-Year Continuous Crop Rotation. Sci. Total Environ. 2019, 660, 913–922. [Google Scholar] [CrossRef]
- Wan, W.; Tan, J.; Wang, Y.; Qin, Y.; He, H.; Wu, H.; Zuo, W.; He, D. Responses of the Rhizosphere Bacterial Community in Acidic Crop Soil to pH: Changes in Diversity, Composition, Interaction, and Function. Sci. Total Environ. 2020, 700, 134418. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Y.; Xu, X.; Liu, T.; Wu, D.; Zheng, X.; Tang, S.; Dai, Q. Potential Use of High-Throughput Sequencing of Soil Microbial Communities for Estimating the Adverse Effects of Continuous Cropping on Ramie (Boehmeria Nivea L. Gaud). PLoS ONE 2018, 13, e0197095. [Google Scholar] [CrossRef]
- Santolini, M.; Barabási, A.L. Predicting Perturbation Patterns from the Topology of Biological Networks. Proc. Natl. Acad. Sci. USA 2018, 115, E6375–E6383. [Google Scholar] [CrossRef]
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; van der Heijden, M.G.A. Agricultural Intensification Reduces Microbial Network Complexity and the Abundance of Keystone Taxa in Roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.A. Keystone Taxa as Drivers of Microbiome Structure and Functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Pizzirani-Kleiner, A.A.; Araujo, W.L.; Raaijmakers, J.M. Diversity of Cultivated Endophytic Bacteria from Sugarcane: Genetic and Biochemical Characterization of Burkholderia Cepacia Complex Isolates. Appl. Environ. Microbiol. 2007, 73, 7259–7267. [Google Scholar] [CrossRef]
- Meng, L.; Xu, C.; Wu, F. Huhe Microbial Co-Occurrence Networks Driven by Low-Abundance Microbial Taxa during Composting Dominate Lignocellulose Degradation. Sci. Total Environ. 2022, 845, 157197. [Google Scholar] [CrossRef]
- Chen, X.; Wong, J.T.F.; Chen, Z.; Tang, T.W.L.; Guo, H.; Leung, A.O.W.; Ng, C.W.W.; Wong, M.H. Effects of Biochar on the Ecological Performance of a Subtropical Landfill. Sci. Total Environ. 2018, 644, 963–975. [Google Scholar] [CrossRef]
- Li, Q.; Gao, Y.; Yang, A. Sulfur Homeostasis in Plants. Int. J. Mol. Sci. 2020, 21, 8926. [Google Scholar] [CrossRef]
- Liang, Z.; Yu, Y.; Ye, Z.; Li, G.; Wang, W.; An, T. Pollution Profiles of Antibiotic Resistance Genes Associated with Airborne Opportunistic Pathogens from Typical Area, Pearl River Estuary and Their Exposure Risk to Human. Environ. Int. 2020, 143, 105934. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.M.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M.; et al. Deciphering the Rhizosphere Microbiome for Disease-Suppressive Bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- Rana, A.; Sahgal, M.; Johri, B.N. Fusarium oxysporum: Genomics, Diversity and Plant–Host Interaction BT. In Developments in Fungal Biology and Applied Mycology; Satyanarayana, T., Deshmukh, S.K., Johri, B.N., Eds.; Springer: Singapore, 2017; pp. 159–199. ISBN 978-981-10-4768-8. [Google Scholar]
- Kashiwa, T.; Kozaki, T.; Ishii, K.; Turgeon, B.G.; Teraoka, T.; Komatsu, K.; Arie, T. Sequencing of Individual Chromosomes of Plant Pathogenic Fusarium oxysporum. Fungal Genet. Biol. 2017, 98, 46–51. [Google Scholar] [CrossRef]
- Rajninec, M.; Fratrikova, M.; Boszoradova, E.; Jopcik, M.; Bauer, M.; Libantova, J. Basic β-1,3-Glucanase from Drosera Binata Exhibits Antifungal Potential in Transgenic Tobacco Plants. Plants 2021, 10, 1747. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.; Torres, M.; Blanco, L.; Béjar, V.; Sampedro, I.; Llamas, I. Plant Growth-Promoting Activity and Quorum Quenching-Mediated Biocontrol of Bacterial Phytopathogens by Pseudomonas Segetis Strain P6. Sci. Rep. 2020, 10, 4121. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xu, Z.; Xie, J.; Hesselberg-Thomsen, V.; Tan, T.; Zheng, D.; Strube, M.L.; Dragoš, A.; Shen, Q.; Zhang, R.; et al. Bacillus Velezensis Stimulates Resident Rhizosphere Pseudomonas Stutzeri for Plant Health through Metabolic Interactions. ISME J. 2022, 16, 774–787. [Google Scholar] [CrossRef]
- Shwaiman, H.A.; Shahid, M.; Elgorban, A.M.; Siddique, K.H.M.; Syed, A. Beijerinckia Fluminensis BFC-33, a Novel Multi-Stress-Tolerant Soil Bacterium: Deciphering the Stress Amelioration, Phytopathogenic Inhibition and Growth Promotion in Triticum Aestivum (L.). Chemosphere 2022, 295, 133843. [Google Scholar] [CrossRef]
- Frey-Klett, P.; Burlinson, P.; Deveau, A.; Barret, M.; Tarkka, M.; Sarniguet, A. Bacterial-Fungal Interactions: Hyphens between Agricultural, Clinical, Environmental, and Food Microbiologists. Microbiol. Mol. Biol. Rev. 2011, 75, 583–609. [Google Scholar] [CrossRef]
- Berg, G. Plant–Microbe Interactions Promoting Plant Growth and Health: Perspectives for Controlled Use of Microorganisms in Agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, R.; Luu, V.T.; Weinhold, A.; Goldberg, J.; Oh, Y.; Baldwin, I.T. Native Root-Associated Bacteria Rescue a Plant from a Sudden-Wilt Disease That Emerged during Continuous Cropping. Proc. Natl. Acad. Sci. USA 2015, 112, E5013–E5020. [Google Scholar] [CrossRef]
- Shen, Z.; Xue, C.; Taylor, P.W.J.; Ou, Y.; Wang, B.; Zhao, Y.; Ruan, Y.; Li, R.; Shen, Q. Soil Pre-Fumigation Could Effectively Improve the Disease Suppressiveness of Biofertilizer to Banana Fusarium Wilt Disease by Reshaping the Soil Microbiome. Biol. Fertil. Soils 2018, 54, 793–806. [Google Scholar] [CrossRef]
- Xun, W.; Shao, J.; Shen, Q.; Zhang, R. Rhizosphere Microbiome: Functional Compensatory Assembly for Plant Fitness. Comput. Struct. Biotechnol. J. 2021, 19, 5487–5493. [Google Scholar] [CrossRef] [PubMed]
- Xun, W.; Ren, Y.; Yan, H.; Ma, A.; Liu, Z.; Wang, L.; Zhang, N.; Xu, Z.; Miao, Y.; Feng, H.; et al. Sustained Inhibition of Maize Seed-Borne Fusarium Using a Bacillus-Dominated Rhizospheric Stable Core Microbiota with Unique Cooperative Patterns. Adv. Sci. 2023, 10, 2205215. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Yuan, X.; Yang, J.; Yang, Y.; Jv, H.; Li, R.; Jia, Z.; Ruan, Y. Selection of Rhizosphere Communities of Diverse Rotation Crops Reveals Unique Core Microbiome Associated with Reduced Banana Fusarium Wilt Disease. New Phytol. 2023, 238, 2194–2209. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).