Abstract
Large nitrogen (N) losses during fertilization in agricultural production may result in energy wastage, soil and water contamination, and potentially influence crop development. Thus, with the help of a 15N-labeled tracer, we carried out a field monitoring analysis of NH3 emissions in a long-term (9-year) conservation tillage agroecosystem of Mollisols in northeast China, in order to determine whether a no-tillage regime and four levels of stover mulching (0%, 33%, 67%, and 100%), combined with urease and nitrification inhibitors, could improve fertilizer utilization efficiency in agricultural systems by reducing ammonia volatilization. Our results showed that in comparison with ridge tillage, no-tillage with stover mulching levels of 33%, 67%, and 100% significantly reduced NH3 emission rates and cumulative volatilization from 159.67 to 130.42 g N ha−1 and 15N-NH3 cumulative volatilization emission by 26% (on average). Furthermore, the application of urease and nitrification inhibitors significantly reduced 15N-NH3 volatilization levels from 1.19 to 0.98 g N ha−1. Our research results demonstrate that a long-term no-tillage regime and straw mulching can significantly reduce NH3 volatilization in fertilizers. Furthermore, when combined with the use of urease and nitrification inhibitors, these practices further enhance the reduction in NH3 volatilization. Although the volatilization of 15N-NH3 is minimally studied in Mollisols, these findings provide a solid foundation for improving fertilizer utilization efficiency, reducing crop production costs and mitigating subsequent environmental pollution.
1. Introduction
In recent years, the world economy has faced many challenges, such as climate change and environmental pollution, resulting in global food security issues [1]. Nitrogen (N) fertilizer is one of the essential fertilizers in crop production, and the growing demand for food increases the use of N fertilizers. However, the improper application of N fertilizer can easily lead to the loss of N through NH3 volatilization, N leaching, and runoff immediately following application [2], eventually resulting in an increasing loss of active N, such as through ammonia (NH3) volatilization to the environment, along with the corresponding adverse impacts [3]. Urea is the most widely traded N fertilizer in the world, and China accounts for 29% of its world-wide production [4]. It has been shown that the application of synthetic N fertilizer is one of the largest sources of NH3 emissions in the world [5], and has thus become the main N loss pathway in agricultural soils [6]. Furthermore, the low utilization rate associated with nitrogen fertilizer will further reduce agricultural productivity and seriously damage natural ecosystems [7,8]. NH3 emission can indirectly lead to the acidification and eutrophication of soil and water systems and is an indirect source of the greenhouse gas N2O [9,10,11]. NH3 can react with HNO3 and H2SO4 in the atmosphere to form secondary inorganic aerosols such as (NH4)2SO4, NH4NO3, and NH4HSO4. These aerosols account for an estimated 25–60% of China’s overall PM2.5 levels [12,13,14]. Thus, as one of the key factors governing haze formation [15], a reduction in NH3 volatilization by 50% may lead to a reduction in PM2.5 by over 10% [16]. Therefore, considering the critical importance of reducing NH3 emissions, resulting from the application of fertilizer, the development of effective conservation management strategies is urgently needed to enhance the quantity and quality of agricultural production in sustainable agricultural systems.
Agricultural systems have the potential to benefit from conservation agriculture with sustainable management practices, which includes practices like crop residue mulching, minimally or entirely avoiding the disturbance of soil, and soil fertility improvement [17,18]. Previous studies showed that conservation tillage offers advantages over conventional tillage in terms of increasing soil organic carbon content [19], microbial biomass levels and diversity [20]. Crop residue mulching can affect soil biological, physical, thermal and hydrological properties [21,22]. For example, a no-tillage regime or straw mulching can improve the availability of plant nutrients through reducing the amount of N loss via leaching and runoff, and further increase crop yields [23,24]. However, researchers have reported that NH3 emissions under conservation tillage are higher than those under conventional tillage following the application of urea [25], because straw residues prevent urea from entering the soil and subsequently impair the effectiveness of urea fertilizer [26]. Additionally, urea exposed to soil is more readily hydrolyzed, resulting in increased urease activity [27]. In contrast, other studies have suggested that conservation tillage can significantly reduce NH3 emissions [28] affected by the degree of straw mulching [21]. Therefore, it is crucial to understand the impacts of tillage practices with different levels of straw mulching on NH3 volatilization, especially those derived from ammonium and urea N fertilizers, and find effective conservation management techniques to boost crop output and increase N use efficiency.
Urease inhibitors, for example N-(n-butyl) thiophosphoric triamide (NBPT), are widely used additives that stabilize urea by delaying its hydrolysis to NH3 and slow the microbial autotrophic oxidation of ammonium to nitrate [29,30], potentially leading to a reduction in N loss [31]. It was reported that urease inhibitors can reduce NH3 emission by up to 88% [32], while nitrification inhibitors, for example 3,4-Dimethyl-1H-pyrazolium dihydrogen phosphate (DMPP), can indirectly increase crop yields [33] due to their inhibitory effects on nitrification and denitrification processes [34]. Despite their potential adverse effects during application, studies have demonstrated that the benefits of nitrification inhibitors (NIs) outweigh the risks when considering all the relevant environmental and crop factors [33]. For instance, nitrification inhibitors may prolong the time that ammonium stays in the soil and increase the risks of NH3 volatilization [35,36]. Moreover, whether the concurrent application of urease and nitrification inhibitors can balance the loss of NH3 and nitrate to reduce the total N loss remains in question [37]. Studies have reported that the combined application of urease/nitrification inhibitors can reduce NH3 loss [38] and nitrate leaching [29], compared with a single application of one inhibitor. However, other studies have found that the combination of two inhibitors may also increase NH3 emissions [39,40,41]. Therefore, the high-resolution monitoring of NH3 emissions derived from urea is needed in relation to testing the impacts of the combined use of two inhibitors and other conservation management practices.
Northeast China is a major agricultural production area with respect to Mollisols, in which intensive agricultural management is potentially precipitating severe nutrient losses, indicating the need for sustainability in conservation management practices. Here, we present a long-term (9-year) continuous conservation management study conducted on a maize cropping system in Northeast China, in which 15N-labeled tracer techniques were used to investigate the impact of no-till farming and varying levels of maize stover mulching, in combination with urease and nitrification inhibitors, on NH3 volatilization from synthetic fertilizer N. Our previous studies showed that no-tillage farming and maize stover mulching with urease/nitrification inhibitors could improve N fertilizer retention in the studied Mollisols [42,43]. Thus, in this study, we hypothesized that: (1) applying maize stover mulching in no-till farming would significantly reduce NH3 volatilization from urea; and (2) the combined use of urease/nitrification inhibitors would further minimize the loss of urea to NH3, leading to a lower N loss potential in conservation agricultural ecosystems.
2. Materials and Methods
2.1. Study Site
The study site, located in Lishu County, Siping City, Jilin Province (43°19′ N, 124°14′ E), is characterized as a temperate region with a humid to semi-humid continental monsoon climate. Its annual mean temperature is 6.9 °C with a range of −13.5 °C (January) to 23.7 °C (July). The changes in temperature and rainfall that occurred in the sampling site during the experiment are shown in Figure 1. This region receives an annual mean precipitation level of approximately 614 mm, about 75% of which occurs in the summer from June to September. The soil in the study area is classified as Mollisol, according to the U.S. Soil Taxonomy system. Table 1 presents the essential chemical properties of the top 20 cm soil layer in April of 2007, prior to the implementation of the no-till treatment. The prevailing agricultural practice in the study area is maize cultivation at a planting density of 60,000 ha−1. After the harvesting of maize in autumn, no other crops were grown. To manage weeds and pests, herbicides were used annually for one week following sowing, while pesticides were applied during the 5–6 leaf and heading stages of maize using an automated high-stem dispenser.
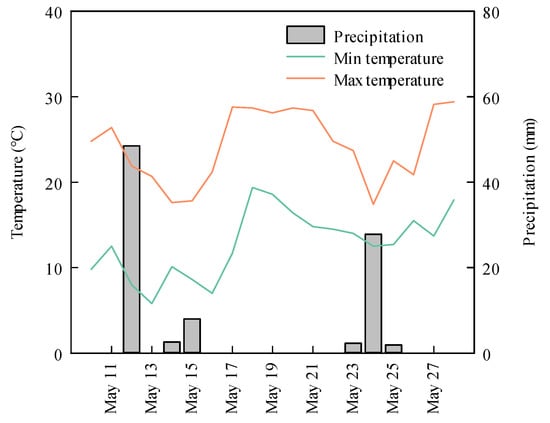
Figure 1.
Precipitation and environmental temperature variations in the analyzed area during study time from 11 May to 27 May in 2016.

Table 1.
Physical and chemical properties of top 20 cm soil layer.
2.2. Design of the Experiment
The present study reports on a long-term field experiment initiated in April of 2007, which was designed as a completely randomized block with four replicates. Each plot (replication) measured 261 m2 (8.7 m × 30 m). The experiment comprised six treatments: (1) conventional ridge tillage (RT); (2) no-tillage farming with 0% maize stover mulching (NT0); (3) NT farming with 33% mulching (NT33); (4) NT farming with 67% mulching (NT67); (5) NT farming with 100% mulching (NT100); and (6) NT100 farming with 20% NPK reduction (NT100R). The no-till plots were left undisturbed, with the exception of maize planting during the spring season. After each harvest, maize stover was uniformly spread across the field’s surface. A maize stover mulching rate of 100% was implemented within the plot, with a quantity of 7500 kg ha−1.
To accurately quantify the transformation and fate of fertilizer-derived N in the soil–crop systems, a 15N-labeled micro-plot test was conducted from 2016 to the present within the 24 plots of above-mentioned field experiment. To do so, a micro-plot with an area of 4 m2 (2 m × 2 m) was established in each plot. Meanwhile, an additional micro-plot of the same area was created in each NT100R plot for treatment with urease and nitrification inhibitors (NT100RI). The urease inhibitor employed was N-(n-Butyl) thiophosphorictriamide (NBPT), and the nitrification inhibitor used was 3,4-Dimethyl-1H-pyrazolium dihydrogen phosphate (DMPP). Subsequently, seven 15N-labeled micro-plot test treatments were examined: (1) RT, (2) NT0, (3) NT33, (4) NT67, (5) NT100, (6) NT100R, and (7) NT100RI. Each treatment included four replicates, resulting in 28 micro-plots. Twenty-four maize plants were planted in each micro-plot on 10 May 2016, according to a plant density of 60,000 ha−1.
Similar application amounts and forms of synthetic fertilizers, including urea, concentrated super-phosphate, and KCl, were employed in the micro-plot test and the long-term field experiment. On 10 May 2016, urea fertilizer, labeled with a 9.80% 15N abundance from Shanghai Research Institute of Chemical Industry, Shanghai, China, was applied in each micro-plot. Repeat measurements of NH3 volatilization were then carried out daily from 11 May to 27 May 2016, amounting to a total of 17 days. In each micro-plot, synthetic fertilizers (basal fertilizers) were applied once a year at a soil depth of 10−15 cm, utilizing traditional NPK rates of 240 kg N ha−1, 110 kg P2O5 ha−1, and 110 kg K2O ha−1. With the basal fertilizers, NBPT and DMPP were administered in every micro-plot at a rate equivalent to 1% of the urea fertilization rate.
2.3. Sampling and Analytical Methods
After applying fertilizer to agricultural soils, nitrification may convert NH4+-N into highly mobile NO3−-N within 2 weeks [41]. NH3 emission was measured at 8:00 on days 1, 4, 5, 6, 7, 8, 10, 12, 14, and 17 after fertilization. The following equipment was utilized for the measurement of NH3 volatilization: two sponges with a diameter of 16 cm and a thickness of 2 cm, which were soaked evenly in 15 mL of glycerol phosphate solution (50 mL of phosphoric acid + 40 mL of glycerin, with a volume fixed to 1000 mL) and placed inside a rigid plastic tube. The lower sponge was positioned 5 cm above the base of the tube, and the upper sponge was positioned flat against the top of the tube [44]. The samples were collected on the following day at 8:00. During sampling, the lower sponge was expeditiously transferred and segregated into a sanitized plastic pouch. In the meantime, another sponge soaked in phosphoglyceride replaced the previous one. The NH4+-N concentration in the sponge was analyzed by subjecting it to a 1 M KCl extraction using a mechanical shaker at 25 °C for 60 min, and then analyzing the filtrates using a continuous chemical analyzer (SmartChem200, Roma, Italy), while the 15N enrichment of extractable NH4+-N ascertained by the KCl extract was concentrated using a modified diffusion method described in [45]. Subsequently, the extract was analyzed using a Finnigan Delta-plus XP stable isotope-ratio mass spectrometer manufactured by Thermo Finnigan, Silicon Valley, CA, USA.
Flux and cumulation of NH3 volatilization derived from soil and labeled urea N were calculated using the following formula [46].
where M is the average ammonia amount (NH3-N, mg) measured using a single device via the closed ventilation method; A is the cross-sectional area of the capture device (m2); and D is the duration of each successive capture.
where a is the 15N abundance of extractable NH4+-N for each sampling (%); b represents the 15N abundance of urea applied in 2016 (9.80%); and c represents the natural 15N abundance (0.366%).
2.4. Statistical Analysis
A completely randomized block design was adopted in this field study, so the treatments and blocks were treated as fixed and random factors, respectively, using the linear mixed-effects model. One-way analysis of variance (ANOVA) was used to evaluate the impacts of the treatments on cumulative NH3 and 15N-labeled NH3 emissions, as well as the ratios of 15N-labelled NH3 to NH3 emissions. The repeated-measures one-way ANOVA was used to evaluate the effects of the treatments on NH3 and 15N-labeled NH3 emission rates for 17 days of monitoring. One-way ANOVA with repeated measures was used to compare group means, where the participants were the same in each group, and means were measured multiple times to observe changes to an intervention. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Total NH3 and Fertilizer-Derived 15N-NH3 Emission Rates
Generally, the NH3 volatilization rates across all treatments were relatively high during the first five days after the application of urea, and gradually decreased from 74.22 g N ha−1 day−1 on day 1 to 10.77 g N ha−1 day−1 on day 6, and they tended to stabilize between 1.65 and 6.21 g N ha−1 day−1 with the extension of time to day 17 (Figure 2). Specifically, the repeated-measures ANOVA showed that the NH3 emission rates throughout the 17-day monitoring in conventional tillage of RT (19.22 g N ha−1 day−1) and no-till farming without mulching of NT0 (19.34 g N ha−1 day−1) were significantly greater than no-till farming with mulching of NT33 (16.18 g N ha−1 day−1) and NT67 (15.62 g N ha−1 day−1) (Figure 2A, p < 0.05), although NT100 (18.04 g N ha−1 day−1), NT100R (14.81 g N ha−1 day−1), and NT100RI (15.77 g N ha−1 day−1) showed no significant difference between each other (Figure 2B).
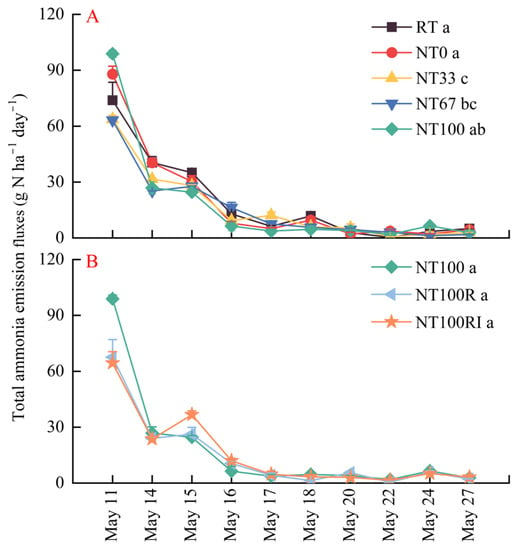
Figure 2.
Variation in total ammonia emission fluxes during the seeding stage of spring maize. The conventional and conservation tillage treatments were placed into group (A) (RT, NT0, NT33, NT67, and NT100), and conservation tillage combined with other measures were arranged into group (B) (NT100, NT100R, and NT100RI). Different lower case letters indicate significant differences at p < 0.05. Error bars indicate standard errors (n = 4). RT: conventional ridge tillage; NT0: no-tillage farming with 0% maize straw mulching; NT33: no-tillage farming with 33% (2500 kg ha−1) maize straw mulching; NT67: no-tillage farming with 67% (5000 kg ha−1) maize straw mulching; NT100: no-tillage farming with 100% (7500 kg ha−1) maize straw mulching; NT100R: NT100 plus 20% reduced fertilization; NT100RI: NT100R plus urease/nitrification inhibitors.
Similar to the NH3 volatilization rates, the fertilizer-derived 15N-NH3 emission rates across all treatments were higher for the first five days after the application of urea, although large fluctuation existed throughout the 17-day monitoring period (Figure 3). For ease of comparison, we grouped the conventional and conservation tillage treatments into one group (RT, NT0, NT33, NT67, and NT100) (Figure 3A), and the treatments where conservation tillage was combined with other measures were arranged into another group (NT100, NT100R, and NT100RI) (Figure 3B). Overall, the repeated-measures ANOVA indicated that no-tillage farming with all four levels of stover mulching, i.e., NT0, NT33, NT67, and NT100 (0.12–0.13 g N ha−1 day−1), could significantly reduce 15N-NH3 volatilization rates in comparison with the conventional tillage of RT (0.16 g N ha−1 day−1) (Figure 3A, p < 0.05). In addition, the fertilization reduction and urease/nitrification inhibitors of NT100RI further reduced the 15N-NH3 volatilization rates (from 0.13 g N ha−1 day−1 to 0.08 g N ha−1 day−1) by 38%, compared with those associated with NT100 and NT100R (Figure 3B, p < 0.05).
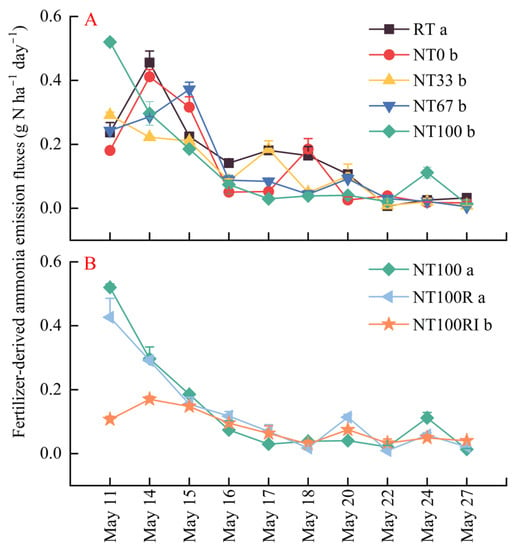
Figure 3.
Variation in fertilizer-derived ammonia emission fluxes during the seeding stage of spring maize. The conventional and conservation tillage treatments were placed into group (A) (RT, NT0, NT33, NT67, and NT100), and conservation tillage combined with other measures were arranged into group (B) (NT100, NT100R, and NT100RI). Different lower case letters indicate significant differences at p < 0.05. Error bars indicate standard errors (n = 4).
3.2. Cumulative Total NH3 and Fertilizer-Derived 15N-NH3 Emissions
To better understand the NH3 volatilization reduction, cumulative NH3 emissions were calculated for each treatment of 17 days after the application of urea (Figure 4). Soil NH3 volatilization was highest in the RT treatment (159.66 g N ha−1), followed by the NT0 treatment (151.62 g N ha−1). Compared to RT and NT0, the no-till and stover mulching treatments of NT33 (133.07 g N ha−1), NT67 (126.54 g N ha−1), and NT100 (132.66 g N ha−1) all significantly reduced cumulative NH3 volatilization by averaging 16% (p < 0.05). However, the NH3 volatilization of NT100R (119.30 g N ha−1) and NT100RI (130.80 g N ha−1) was not significantly different from NT100. There was no significant difference between RT and NT0, nor between NT33, NT67, NT100, NT100R, and NT100RI.
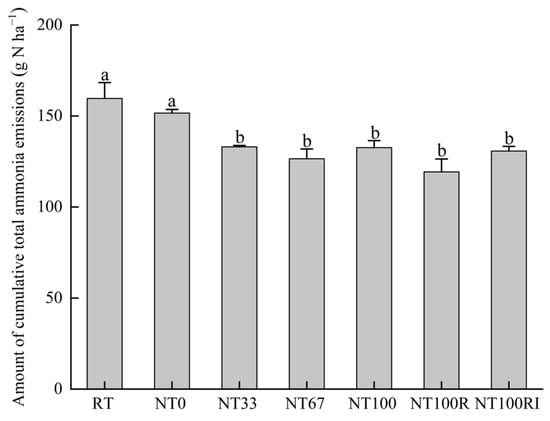
Figure 4.
Amount of cumulative total ammonia emissions during the seeding stage of spring maize. Different lower case letters indicate significant differences at p < 0.05. Error bars indicate standard errors (n = 4).
To investigate the NH3 volatilization loss directly from urea, cumulative 15N-NH3 emissions were calculated for each treatment of 17 days after the application of urea (Figure 5). The 15N-NH3 volatiles of RT were significantly higher than those of the other treatments, while there was no significant difference between NT0, NT33, NT67, NT100, and NT100R. Compared to the conventional tillage of RT (1.62 g N ha−1), no-tillage treatment of NT0 (1.29 g N ha−1) and no-till with mulching treatments of NT33 (1.13 g N ha−1), NT67 (1.26 g N ha−1), and NT100 (1.19 g N ha−1) all significantly reduced cumulative 15N-NH3 emissions by an average of 25% (p < 0.05). More importantly, urease/nitrification inhibitors of NT100RI (0.98 g N ha−1) further reduced 15N-NH3 volatilization by 20% compared with the fertilization reduction treatment of NT100R (1.22 g N ha−1) (p < 0.05).
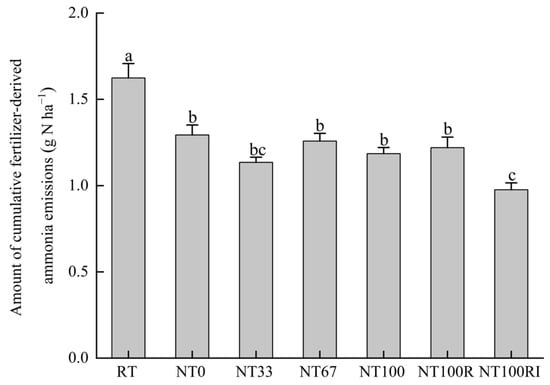
Figure 5.
Amount of cumulative fertilizer-derived ammonia emissions during the seeding stage of spring maize. Different lower case letters indicate significant differences at p < 0.05. Error bars indicate standard errors (n = 4).
To determine the extent of NH3 volatilization loss directly from urea, the ratios of 15N-NH3 to NH3 emissions were calculated for each treatment of the 17 days after the application of urea (Figure 6). Compared to the conventional tillage of RT (1.02%), no-tillage treatment of NT0 (0.85%) and no-till with mulching treatments of NT33 (0.85%) and NT100 (0.89%) all significantly reduced the ratios of 15N-NH3 to NH3 emissions by averaging 15% (p < 0.05). Interestingly, the percentage was 15% greater in the reduced fertilization treatments of NT100R (1.02%), compared to the NT100 treatment, while reduced fertilization and urease/nitrification inhibitors of NT100RI (0.75%) reduced the percentages by 20%, compared with the fertilization reduction treatment of NT100R (p < 0.05).
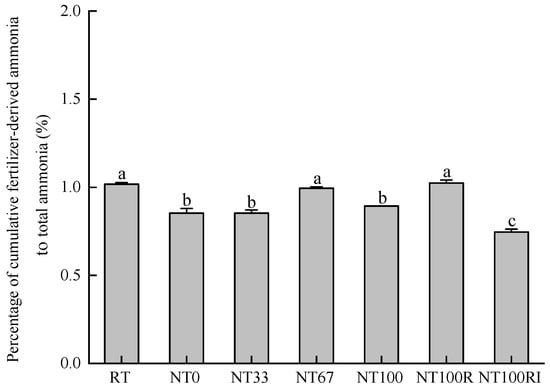
Figure 6.
Percentage of cumulative fertilizer-derived ammonia with respect to total ammonia during the seeding stage of spring maize. Different lower case letters indicate significant differences at p < 0.05. Error bars indicate standard errors (n = 4).
4. Discussion
Fertilizers are easily lost through various ways, so reducing fertilizer loss caused by NH3 volatilization can improve fertilizer utilization rates and reduce subsequent air and water pollution. This study investigated whether a series of conservation management measures, used alone or in combination, would have an impact on NH3 volatilization.
The tillage technique, fertilization timing, mulching technique, and other factors may have an impact on the NH3 emission rates. In our study, NH3 emission rates in all treatments decreased over time. This could be because urea hydrolyzes quickly in soil, releasing most NH3 emissions in the first few days following application [41]. Consistent with our research, when urea was applied to crop surface, researchers found that the loss rate of NH3 reached its peak at 23 h after application with most NH3 loss occurring within 50 h [47]. Due to biological processes, such as nitrogen infiltration, nitrification, and denitrification as well as plant and animal absorption and consumption, the substrate’s content reduces with time [48], so the rate of NH3 volatilization is highest in the days after urea application, and then gradually drops to a lower and stable level [49]. In conclusion, the volatilization of NH3 usually occurs within two weeks after the application of fertilizer in the studied Mollisols, so although our experimental period was relatively short, it was already possible to cover a clear process of NH3 volatilization and the final results were significant.
The results of earlier research on background NH3 emissions were varied. One study reported that the background NH3 emission levels of croplands in China reached 0.99–2.18 Tg N yr−1 [2]. However, other studies have suggested that NH3 volatilization is predominantly caused by the application of base fertilizer during crop planting [50], and that the background NH3 volatilization of unfertilized plots is often below the detection limit [29]. In our study, two weeks after fertilization, the total NH3 emissions ranged from 126 to 160 g N ha−1, while the 15N-NH3 emissions derived from fertilizer ranged from 0.98 to 1.62 g N ha−1. Although the degree of volatilization of NH3 in our study was very small, there was still a significant decreasing trend associated with six different experimental treatments. The low amount of NH3 volatilization observed may be related to the combined effects of conservation tillage, the reduced application of fertilizer, and the dual inhibitors employed. This low level of NH3 volatilization was also corroborated by an earlier study carried out at the same location as ours [51]. Therefore, it is possible to further limit the loss of N fertilizer through the hydrolysis of ammonia by combining other N management methods, such as urease/nitrification inhibitors, reduced fertilization, conservation tillage, and other agricultural measures [52], which would improve fertilizer utilization.
In our study, in addition to no tillage farming alone, it was determined that treatment under conservation tillage can significantly reduce total NH3 and NH3 emissions from fertilizer, and the addition of dual inhibitors can further reduce NH3 emissions from fertilizer. This may be because maize straw with high C/N ratios, combined with N inputs from fertilizers, provides a sufficient C substrate from which soil microorganisms can assimilate native soil N, reducing nitrate leaching and gaseous N emission, and thereby reducing the potential for N loss [53,54,55]. However, previous studies showed that conservation tillage management is not always effective in reducing the volatilization of NH3. For example, contrary to the results of our study, it was reported that no-till soils seeded with urea presented a loss of NH3 volatilization 1.3 times that of reduced-till soils [56]; NH3 volatilization in no-till soil even accounted for 24% of topdressing urea [57], and sometimes the cumulative volatilization of NH3 can even reach three times higher than those in conventionally tilled soils [58]. The reason behind this discrepancy may be that no-till practices typically apply urea to the soil surface, while crop residues act as a contact barrier between urea and soil, resulting in reduced nitrogen permeability and soil adsorption of NH4+ and the increased volatilization of NH3 [59]. However, in our study, fertilizer was applied at a soil depth of 10–15 cm, which might have reduced the exposure of the fertilizer to the air and thus reduced NH3 volatilization. Previous studies also showed that when urea is deeply buried in the soil, the top-cover materials of maize straw can act as a physical barrier that significantly reduces N loss [60,61]. Our previous studies showed that stover mulching can regulate the active organic N from soil mineral N pools to more stable soil-fixed NH4+ and organic N pools, in which N can be slowly released or mineralized into plant available pools, resulting in higher fertilizer N-use efficiency in soil-crop systems [42], and thereby reducing the volatilization of NH3. Furthermore, combining no tillage farming with stover mulching can regulate soil microbial growth and crop stover decomposition [20,62], affecting the urea hydrolysis process that controls NH3 volatilization from the applied urea.
To accurately quantify NH3 emissions stemming from fertilizer, 15N-labeled tracer techniques must be used to measure gaseous N loss in the form of NH3 directly derived from fertilizers [10]. Our results showed that although the variation in the volatilization rate of 15N-NH3 after fertilization was less obvious than that of the overall NH3 emission rate; the volatilization rate still showed a trend of reduction and stabilization with the extension of time (Figure 3). Compared to the other six treatments, the emission rate of 15N-NH3 was significantly reduced on the first day after fertilization for NT100RI, and the overall change in the 15N-NH3 emission rate was stable. This indicates that the dual inhibitor effectively inhibited the emission of NH3. Previous studies also demonstrated that dual inhibitors delay NH3 volatilization and reduce the NH3 volatilization peak, and one application of a urease inhibitor is generally regarded to be a more effective way of reducing NH3 volatilization [41,49]. However, because no single urease inhibitor or nitrification inhibitor was used in this experiment, the effect of a single inhibitor combined with other agricultural management strategies on NH3 volatilization must be examined further. Previous research has demonstrated that NH3 emissions increase exponentially in response to higher nitrogen inputs [63]. Therefore, the volatilization of NH3 is largely dependent on the quantity of nitrogen fertilizer applied [64]. However, compared to the conservation tillage treatments alone, the combination of conservation tillage methods with a 20% reduction in fertilization had no discernible impact on total NH3 and 15N-NH3 volatilization in our study (Figure 4 and Figure 5). This may be because a 20% reduction in nitrogen inputs may not be sufficient to significantly reduce NH3 volatilization. Moreover, the amount of nitrogen lost through NH3 volatilization of surface-applied nitrogen fertilizer can also be influenced by a range of complex soil and environmental factors in the field [65]. Interestingly, the application of dual inhibitors significantly reduced 15N-NH3 emissions and the percentage of 15N-NH3 to NH3 emissions, compared with NT100 and NT100R under conditions of reduced fertilizer application and conservation tillage practices (Figure 5 and Figure 6). It was further demonstrated that the dual inhibitor effectively reduced NH3 volatilization from the fertilizer. This result might have occurred because the dual inhibitor was buried deep underground with urea and directly affected the loss of NH3 in the fertilizer [66]. Some studies also showed that a combination of urease/nitrification inhibitors can reduce NH3 emissions [67,68]; however, it was also reported that the combination of urease and nitrification inhibitors may prolong the retention time of NH4+ [69], thereby increasing NH3 emissions [30]. Our research supports the former because the heterogeneity of the environment can also potentially lead to different inhibitors having different effects on NH3 emissions [70]. For example, in Zaman’s outdoor studies [40], rainfall occurred after fertilization, preventing the fertilizer from fully entering the soil and contributing to the rise in NH3 volatilization following the application of dual inhibitors. Moreover, previous studies showed that, when compared with the urease inhibitor alone, the simultaneous application of urease and nitrification inhibitors can promote NH3 volatilization, but also inhibit the volatilization of NH3 compared to controls without either inhibitor [41].
5. Conclusions
Our long-term conservation tillage study shows that the time of greatest NH3 volatilization was concentrated in the first five days after N fertilization. Using 15N-labeled tracer techniques, we found that conservation tillage can reduce the total NH3 and NH3 volatilization from fertilizers. On the basis of conservation tillage, the loss of NH3 volatilization in fertilizers can be further reduced through the application of urease/nitrification inhibitors. This detailed assessment of NH3 volatilization could be helpful in a range of issues regarding the loss of fertilizer in soil as NH3. Our findings can provide a scientific basis for understanding gaseous N loss under a conservation management strategy, consisting of no-tillage farming and crop residue application with implications for the development of sustainable agriculture.
Author Contributions
Conceptualization, methodology, writing—original draft preparation, J.Z., L.Y. and C.L.; investigation, visualization, formal analysis, H.X. and H.C.; writing—review and editing, H.C., X.C., H.H. and X.Z.; funding acquisition, H.H. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key R&D Program of China (grant numbers 2022YFD1500302, 2021YFD1500100, and 2022YFD1500305), the National Natural Science Foundation of China (grant numbers 42177005, 41877098, and 41671290), the Science and Technology Program of Shenyang (22-317-2-01), the Innovation team for research, development and application of conservation tillage in Liaoning Province (XLYC2008015), and the Special Research Assistant Project of the Chinese Academy of Sciences (2022000137).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dokić, D.; Gavran, M.; Gregić, M.; Gantner, V. The impact of trade balance of agri-food products on the state’s ability to withstand the crisis. HighTech Innov. J. 2020, 1, 107–111. [Google Scholar] [CrossRef]
- Ma, R.; Yu, K.; Xiao, S.; Liu, S.; Ciais, P.; Zou, J. Data-driven estimates of fertilizer-induced soil NH3, NO and N2O emissions from croplands in China and their climate change impacts. Glob. Chang. Biol. 2022, 28, 1008–1022. [Google Scholar] [CrossRef]
- Fowler, D.; Pyle, J.A.; Raven, J.A.; Sutton, M.A. The global nitrogen cycle in the twenty-first century: Introduction. Phil. Trans. R. Soc. B 2013, 368, 20130165. [Google Scholar] [CrossRef] [PubMed]
- Fixen, P.E.; West, F.B. Nitrogen fertilizers: Meeting contemporary challenges. Ambio 2002, 31, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Paulot, F.; Jacob, D.J.; Pinder, R.W.; Bash, J.O.; Travis, K.; Henze, D.K. Ammonia emissions in the United States, European Union, and China derived by high-resolution inversion of ammonium wet deposition data: Interpretation with a new agricultural emissions inventory (MASAGE_NH3). J. Geophys. Res. Atmos. 2014, 119, 4343–4364. [Google Scholar] [CrossRef]
- Martens, D.A.; Bremner, J.M. Soil properties affecting volatilization of ammonia from soils treated with urea. Commun. Soil Sci. Plant Anal. 1989, 20, 1645–1657. [Google Scholar] [CrossRef]
- Forrestal, P.J.; Harty, M.A.; Carolan, R.; Watson, C.; Lanigan, G.; Wall, D.; Hennessy, D.; Richards, K.G. Can the agronomic performance of urea equal calcium ammonium nitrate across nitrogen rates in temperate grassland? Soil Use Manag. 2017, 33, 243–251. [Google Scholar] [CrossRef]
- Engel, R.; Jones, C.; Wallander, R. Ammonia volatilization from urea and mitigation by NBPT following surface application to cold soils. Soil Sci. Soc. Am. J. 2011, 75, 2348–2357. [Google Scholar] [CrossRef]
- Sutton, M.A.; Moncrieff, J.B.; Fowler, D. Deposition of atmospheric ammonia to moorlands. Environ. Pollut. 1992, 75, 15–24. [Google Scholar] [CrossRef]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Behera, S.N.; Sharma, M.; Aneja, V.P.; Balasubramanian, R. Ammonia in the atmosphere: A review on emission sources, atmospheric chemistry and deposition on terrestrial bodies. Environ. Sci. Pollut. Res. 2013, 20, 8092–8131. [Google Scholar] [CrossRef] [PubMed]
- Steinfeld, J.I. Atmospheric chemistry and physics: From air pollution to climate change. Environ. Sci. Policy Sustain. Dev. 1998, 40, 26. [Google Scholar] [CrossRef]
- He, K.; Yang, F.; Ma, Y.; Zhang, Q.; Yao, X.; Chan, C.K.; Cadle, S.; Chan, T.; Mulawa, P. The characteristics of PM2.5 in Beijing, China. Atmos. Environ. 2001, 35, 4959–4970. [Google Scholar] [CrossRef]
- Fang, M.; Chan, C.K.; Yao, X. Managing air quality in a rapidly developing nation: China. Atmos. Environ. 2009, 43, 79–86. [Google Scholar] [CrossRef]
- Fu, H.; Luo, Z.B.; Hu, S.Y. A temporal -spatial analysis and future trends of ammonia emissions in China. Sci. Total Environ. 2020, 731, 138897. [Google Scholar] [CrossRef]
- An, Z.S.; Huang, R.J.; Zhang, R.Y.; Tie, X.X.; Li, G.H.; Cao, J.J.; Zhou, W.J.; Shi, Z.G.; Han, Y.M.; Gu, Z.L.; et al. Severe haze in northern China: A synergy of anthropogenic emissions and atmospheric processes. Proc. Natl. Acad. Sci. USA 2019, 116, 8657–8666. [Google Scholar] [CrossRef]
- Pretty, J. Agricultural sustainability: Concepts, principles and evidence. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2008, 363, 447–465. [Google Scholar] [CrossRef]
- Kassam, A.; Friedrich, T.; Shaxson, F.; Pretty, J. The spread of conservation agriculture: Justification, sustainability and uptake. Int. J. Agric. Sust. 2009, 7, 292–320. [Google Scholar] [CrossRef]
- Page, K.L.; Dang, Y.P.; Dalal, R.C. The ability of conservation agriculture to conserve soil organic carbon and the subsequent impact on soil physical, chemical, and biological properties and yield. Front. Sustain. Food Syst. 2020, 4, 31. [Google Scholar] [CrossRef]
- Jiang, Y.; Ma, N.; Chen, Z.; Xie, H. Soil macrofauna assemblage composition and functional groups in no-tillage with corn stover mulch agroecosystems in a mollisol area of northeastern China. Appl. Soil Ecol. 2018, 128, 61–70. [Google Scholar] [CrossRef]
- Soane, B.D.; Ball, B.C.; Arvidsson, J.; Basch, G.; Moreno, F.; Roger-Estrade, J. No-till in northern, western and south-western Europe: A review of problems and opportunities for crop production and the environment. Soil Till. Res. 2012, 118, 66–87. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.L.; Pu, C.; Zhang, X.Q.; Xue, J.F.; Ren, Y.X.; Zhao, X.L.; Chen, F.; Lal, R.; Zhang, H.L. Crop yields under no-till farming in China: A meta-analysis. Eur. J. Agron. 2017, 84, 67–75. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Lal, R.; Post, W.M.; Owens, L.B. Changes in long-term no-till corn growth and yield under different rates of stover mulch. Agron. J. 2006, 98, 1128. [Google Scholar] [CrossRef]
- Wilhelm, W.W.; Doran, J.W.; Power, J.F. Corn and soybean yield response to crop residue management under no-tillage production systems. Agron. J. 1986, 78, 184–189. [Google Scholar] [CrossRef]
- Griggs, B.R.; Norman, R.J.; Wilson, C.E.; Slaton, N.A. Ammonia volatilization and nitrogen uptake for conventional and conservation tilled dry-seeded, delayed-flood rice. Soil Sci. Soc. Am. J. 2007, 71, 745–751. [Google Scholar] [CrossRef]
- Mkhabela, M.; Madani, A.; Gordon, R.; Burton, D.; Cudmore, D.; Elmi, A.; Hart, W. Gaseous and leaching nitrogen losses from no-tillage and conventional tillage systems following surface application of cattle manure. Soil Till. Res. 2008, 98, 187–199. [Google Scholar] [CrossRef]
- Rochette, P.; Angers, D.A.; Chantigny, M.H.; MacDonald, J.D.; Bissonnette, N.; Bertrand, N. Ammonia volatilization following surface application of urea to tilled and no-till soils: A laboratory comparison. Soil Till. Res. 2009, 103, 310–315. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, C.; Li, N.; Han, K.; Meng, Y.; Tian, X.; Wang, L. Effects of conservation tillage practices on ammonia emissions from Loess Plateau rain-fed winter wheat fields. Atmos. Environ. 2015, 104, 59–68. [Google Scholar] [CrossRef]
- Afshar, R.K.; Lin, R.; Mohammed, Y.A.; Chen, C. Agronomic effects of urease and nitrification inhibitors on ammonia volatilization and nitrogen utilization in a dryland farming system: Field and laboratory investigation. J. Clean. Prod. 2018, 172, 4130–4139. [Google Scholar] [CrossRef]
- Klimczyk, M.; Siczek, A.; Schimmelpfennig, L. Improving the efficiency of urea-based fertilization leading to reduction in ammonia emission. Sci. Total Environ. 2021, 771, 145483. [Google Scholar] [CrossRef]
- Thilakarathna, S.K.; Hernandez-Ramirez, G. How does management legacy, nitrogen addition, and nitrification inhibition affect soil organic matter priming and nitrous oxide production? J. Environ. Qual. 2021, 50, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Cobena, A.; Misselbrook, T.; Camp, V.; Vallejo, A. Effect of water addition and the urease inhibitor NBPT on the abatement of ammonia emission from surface applied urea. Atmos. Environ. 2011, 45, 1517–1524. [Google Scholar] [CrossRef]
- Gao, J.; Luo, J.; Lindsey, S.; Shi, Y.; Sun, Z.; Wei, Z.; Wang, L. Benefits and risks for the environment and crop production with application of nitrification inhibitors in China. J. Soil Sci. Plant Nut. 2021, 21, 497–512. [Google Scholar] [CrossRef]
- Pasda, G.; Hähndel, R.; Zerulla, W. Effect of fertilizers with the new nitrification inhibitor DMPP (3,4-dimethylpyrazole phosphate) on yield and quality of agricultural and horticultural crops. Biol. Fertil. Soils 2001, 34, 85–97. [Google Scholar] [CrossRef]
- Cui, L.; Li, D.; Wu, Z.; Xue, Y.; Xiao, F.; Zhang, L.; Song, Y.; Li, Y.; Zheng, Y.; Zhang, J. Effects of nitrification inhibitors on soil nitrification and ammonia volatilization in three soils with different pH. Agron. J. 2021, 11, 1674. [Google Scholar] [CrossRef]
- Gioacchini, P.; Nastri, A.; Marzadori, C.; Giovannini, C.; Vittori Antisari, L.; Gessa, C. Influence of urease and nitrification inhibitors on N losses from soils fertilized with urea. Biol. Fertil. Soils 2002, 36, 129–135. [Google Scholar] [CrossRef]
- Thapa, R.; Chatterjee, A.; Johnson, J.M.; Awale, R. Stabilized nitrogen fertilizers and application rate influence nitrogen losses under rainfed spring wheat. Agron. J. 2015, 107, 1885–1894. [Google Scholar] [CrossRef]
- Kim, D.G.; Saggar, S.; Roudier, P. The effect of nitrification inhibitors on soil ammonia emissions in nitrogen managed soils: A meta-analysis. Nutr. Cycl. Agroecosyst. 2012, 93, 51–64. [Google Scholar] [CrossRef]
- Dell, C.J.; Han, K.; Bryant, R.B.; Schmidt, J.P. Nitrous oxide emissions with enhanced efficiency nitrogen fertilizers in a rainfed system. Agron. J. 2014, 106, 723–731. [Google Scholar] [CrossRef]
- Zaman, M.; Nguyen, M.; Blennerhassett, J.; Quin, B. Reducing NH3, N2O and NO3--N losses from a pasture soil with urease or nitrification inhibitors and elemental S-amended nitrogenous fertilizers. Biol. Fertil. Soils 2008, 44, 693–705. [Google Scholar] [CrossRef]
- Soares, J.R.; Cantarella, H.; de Campos Menegale, M.L. Ammonia volatilization losses from surface-applied urea with urease and nitrification inhibitors. Soil Biol. Biochem. 2012, 52, 82–89. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Lü, L.; Yuan, L.; Jia, J.; Chen, X.; Ma, J.; Zhao, J.; Liang, C.; Xie, H.; et al. Effects of no-tillage and stover mulching on the transformation and utilization of chemical fertilizer N in Northeast China. Soil Till. Res. 2021, 213, 105131. [Google Scholar] [CrossRef]
- Yuan, L.; Chen, X.; Jia, J.; Chen, H.; Shi, Y.; Ma, J.; Liang, C.; Liu, Y.; Xie, H.; He, H. Stover mulching and inhibitor application maintain crop yield and decrease fertilizer N input and losses in no-till cropping systems in Northeast China. Agric. Ecosyst. Environ. 2021, 312, 107360. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Ju, X.; Zhang, F. Field in situ determination of ammonia volatilization from soil: Venting. method. Plant Nutr. Fertil. Sci. 2002, 8, 205–209, (In Chinese with English Abstract). [Google Scholar]
- Stark, J.M.; Hart, S.C. Diffusion technique for preparing salt solutions, Kjeldahl digests, and persulfate digests for nitrogen-15 analysis. Soil Sci. Soc. Am. J. 1996, 60, 1846–1855. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Zhou, F.; Zhang, X.C.; Luo, J.F.; Li, Y.; Lindsey, S.; Shi, Y.L.; He, H.B.; Zhang, X.D. Effects of maize residue return rate on nitrogen transformations and gaseous losses in an arable soil. Agric. Water Manag. 2019, 211, 132–141. [Google Scholar] [CrossRef]
- Keller, G.D.; Mengel, D.B. Ammonia volatilization from nitrogen fertilizers surface applied to no-till corn. Soil Sci. Soc. Am. J. 1986, 50, 1060–1063. [Google Scholar] [CrossRef]
- Hou, H.; Zhou, S.; Hosomi, M.; Toyota, K.; Yosimura, K.; Mutou, Y.; Nisimura, T.; Takayanagi, M.; Motobayashi, T. Ammonia emissions from anaerobically-digested slurry and chemical fertilizer applied to flooded forage rice. Water Air Soil Pollut. 2007, 183, 37–48. [Google Scholar] [CrossRef]
- Rawluk, C.; Grant, C.; Racz, G. Ammonia volatilization from soils fertilized with urea and varying rates of urease inhibitor NBPT. Can. J. Soil Sci. 2001, 81, 239–246. [Google Scholar] [CrossRef]
- Kang, F.; Meng, F. Ammonia volatilization from winter wheat cropland in Northern China based on a literature analysis. Trans. Chin. Soc. Agric. Eng. 2020, 36, 228–234, (In Chinese with English Abstract). [Google Scholar]
- Li, Q.; Li, Y.; Gao, Q.; Li, S.; Chen, X.; Zhang, F.; Liu, X. Effect of conventional and optimized nitrogen fertilization on spring maize yield, ammonia volatilization and nitrogen balance in soil-maize system. J. Plant Nutr. Fert. 2015, 21, 571–579, (In Chinese with English Abstract). [Google Scholar]
- Mikkelsen, R. Ammonia emissions from agricultural operations: Fertilizer. Better Crops Plant Food 2009, 93, 9–11. [Google Scholar]
- Lu, C.; Ma, J.; Chen, X.; Zhang, X.; Shi, Y.; Huang, B. Effect of nitrogen fertilizer and maize straw incorporation on NH4+-15N and NO3−-15N accumulation in black soil of northeast China among three consecutive cropping cycles. J. Soil Sci. Plant Nutr. 2010, 10, 443–453. [Google Scholar] [CrossRef]
- Gai, X.; Liu, H.; Liu, J.; Zhai, L.; Wang, H.; Yang, B.; Ren, T.; Wu, S.; Lei, Q. Contrasting impacts of long-term application of manure and crop straw on residual nitrate-N along the soil profile in the North China Plain. Sci. Total Environ. 2019, 650, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, L.; Peng, Y.; Zhang, S.; Lv, S.; Li, J.; Abdo, A.I.; Zhou, C.; Wang, L. Film mulching, residue retention and N fertilization affect ammonia volatilization through soil labile N and C pools. Agric. Ecosyst. Environ. 2021, 308, 107272. [Google Scholar] [CrossRef]
- Palma, R.; Saubidet, M.; Rimolo, M.; Utsumi, J. Nitrogen losses by volatilization in a corn crop with two tillage systems in the Argentine Pampa. Commun. Soil Sci. Plant Anal. 1998, 29, 2865–2879. [Google Scholar] [CrossRef]
- Bacon, P.; Freney, J. Nitrogen loss from different tillage systems and the effect on cereal grain yield. Fert. Res. 1989, 20, 59–66. [Google Scholar] [CrossRef]
- Al-Kanani, T.; MacKenzie, A.F. Effect of tillage practices and hay straw on ammonia volatilization from nitrogen fertilizer solutions. Can. J. Soil Sci. 1992, 72, 145–157. [Google Scholar] [CrossRef]
- Bandel, V.A.; Dzienia, S.; Stanford, G. Comparison of N fertilizers for no-till corn. Agron. J. 1980, 72, 337–341. [Google Scholar] [CrossRef]
- Yu, Y.L.; Wang, M.F.; Yang, B.; He, S.Y.; Duan, J.J.; Yang, L.Z.; Xue, L.H. Effects of film materials on ammonia volatilization emissions from a paddy system after reducing nitrogen fertilizer application. Environ. Sci. 2021, 42, 477–484, (In Chinese with English Abstract). [Google Scholar]
- Xia, L.; Lam, S.K.; Chen, D.; Wang, J.; Tang, Q.; Yan, X. Can knowledge-based N management produce more staple grain with lower greenhouse gas emission and reactive nitrogen pollution? A meta-analysis. Glob. Chang. Biol. 2017, 23, 1917–1925. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, N.; Zhang, Z.; Xu, J.; Tao, B.; Meng, Y. Short-term responses of soil organic carbon and carbon pool management index to different annual straw return rates in a rice–wheat cropping system. Catena 2015, 135, 283–289. [Google Scholar] [CrossRef]
- Jiang, Y.; Deng, A.; Bloszies, S.; Huang, S.; Zhang, W. Nonlinear response of soil ammonia emissions to fertilizer nitrogen. Biol. Fertil. Soils 2017, 53, 269–274. [Google Scholar] [CrossRef]
- Brentrup, F.; Küsters, J.; Kuhlmann, H.; Lammel, J. Application of the Life Cycle Assessment methodology to agricultural production: An example of sugar beet production with different forms of nitrogen fertilisers. Eur. J. Agron. 2001, 14, 221–233. [Google Scholar] [CrossRef]
- Bouwman, A.; Boumans, L.; Batjes, N. Estimation of global NH3 volatilization loss from synthetic fertilizers and animal manure applied to arable lands and grasslands. Glob. Biogeochem. Cycles 2002, 16, 8-1–8-14. [Google Scholar] [CrossRef]
- Francis, D.D.; Vigil, M.F.; Mosier, A.R. Gaseous Losses of Nitrogen Other than Through Denitrification. In Nitrogen in Agricultural Systems; Agronomy Monographs; Schepers, J.S., Raun, W.R., Eds.; ASS, CSSA, and SSSA: Madison, WI, USA, 2008; Volume 49, pp. 255–279. [Google Scholar]
- Clay, D.; Malzer, G.; Anderson, J. Ammonia volatilization from urea as influenced by soil temperature, soil water content, and nitrification and hydrolysis inhibitors. Soil Sci. Soc. Am. J. 1990, 54, 263–266. [Google Scholar] [CrossRef]
- Zaman, M.; Saggar, S.; Blennerhassett, J.; Singh, J. Effect of urease and nitrification inhibitors on N transformation, gaseous emissions of ammonia and nitrous oxide, pasture yield and N uptake in grazed pasture system. Soil Biol. Biochem. 2009, 41, 1270–1280. [Google Scholar] [CrossRef]
- Zaman, M.; Blennerhassett, J.D. Effects of the different rates of urease and nitrification inhibitors on gaseous emissions of ammonia and nitrous oxide, nitrate leaching and pasture production from urine patches in an intensive grazed pasture system. Agric. Ecosyst. Environ. 2010, 136, 236–246. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Y.; Dong, G.; Du, Z.; Wu, W.; Chadwick, D.; Bol, R. The importance of ammonia volatilization in estimating the efficacy of nitrification inhibitors to reduce N2O emissions: A global meta-analysis. Environ. Pollut. 2021, 271, 116365. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).