Improving Endogenous Nitric Oxide Enhances Cadmium Tolerance in Rice through Modulation of Cadmium Accumulation and Antioxidant Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Stress Treatments and Plant Sampling
2.3. Measurement of NO Content
2.4. Measurement of NOS Activity
2.5. Measurement of H2O2 Content, CAT Activity and POX Activity
2.6. Quantitative Real-Time PCR
2.7. Measurement of Cd Content
2.8. Measurement of Chlorophyll Content
2.9. Measurement of Melatonin Content
2.10. Statistical Analysis
3. Results
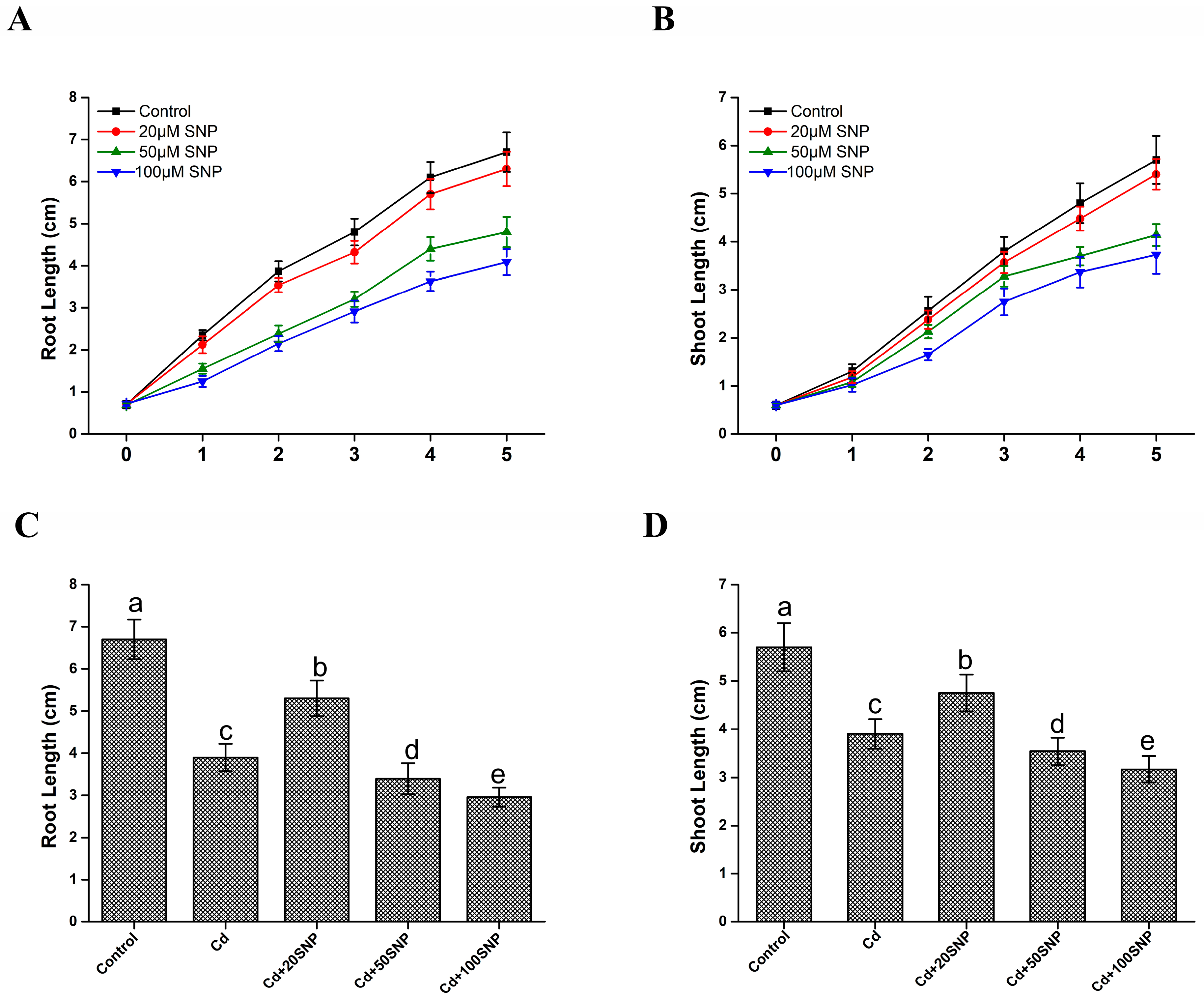
3.1. Exogenous NO Donor Alleviated Cd Toxicity in Rice Seedlings
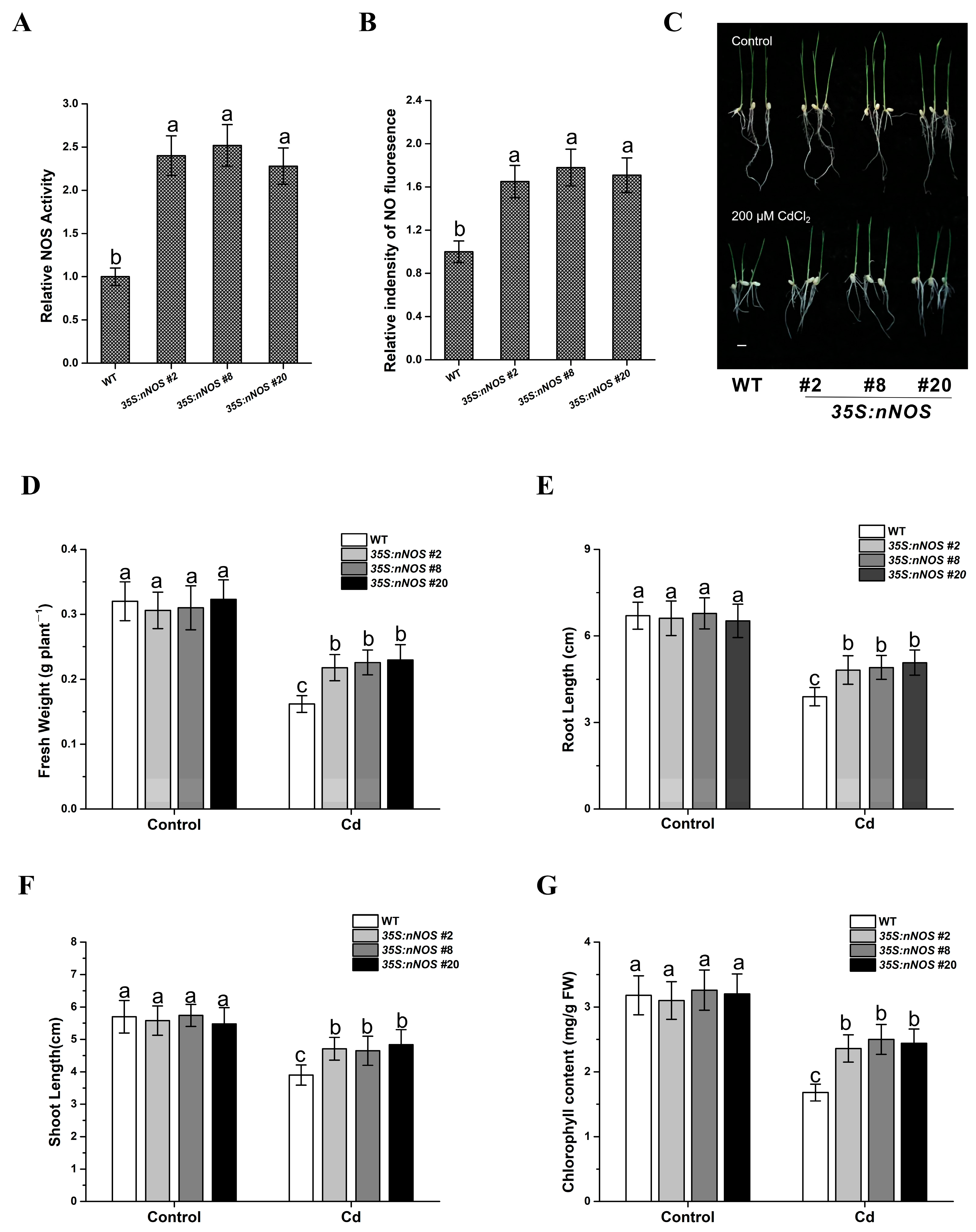
3.2. nNOS-Overexpressing Rice Plants Demonstrated Improved Tolerance to Cadmium Stress
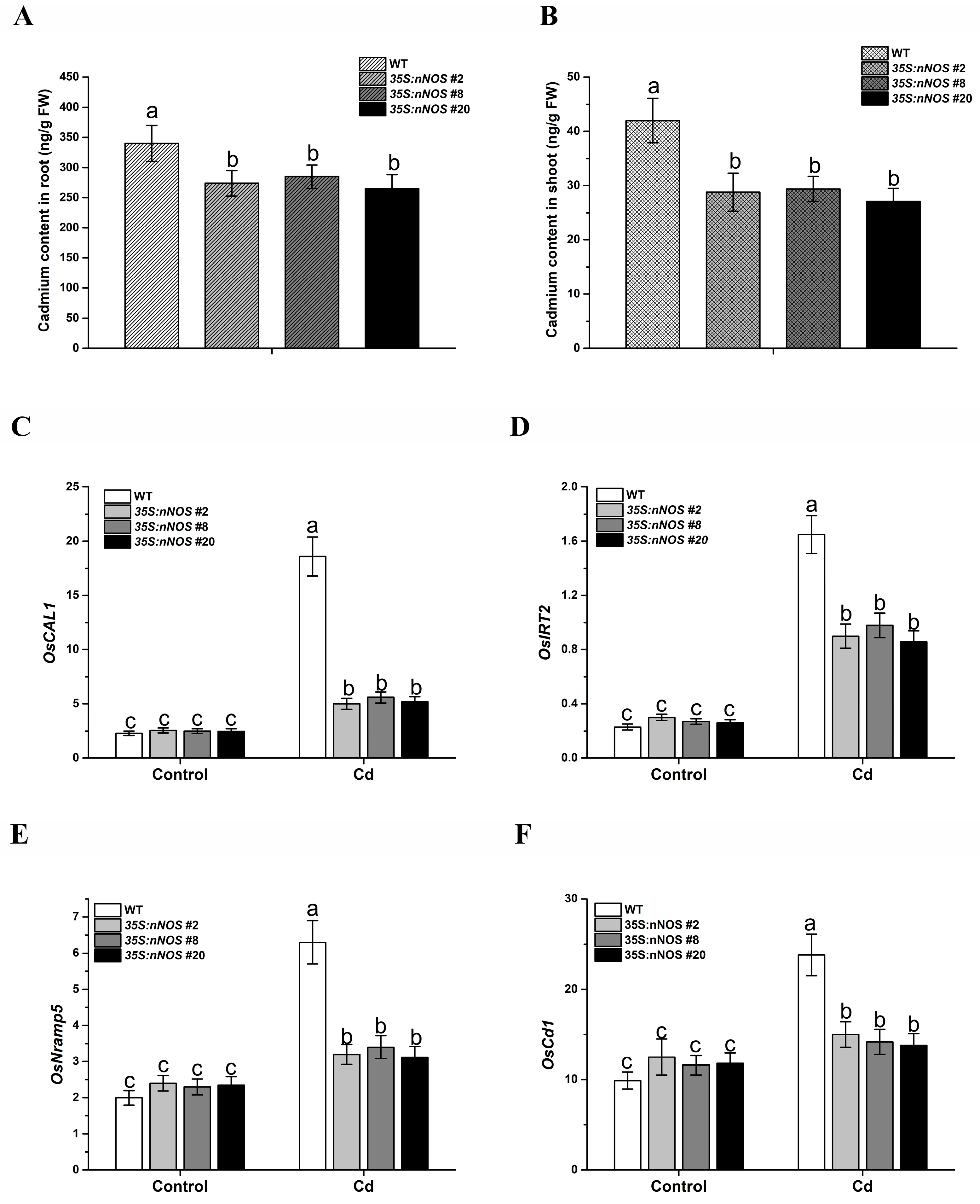
3.3. nNOS-Overexpressing Rice Plants Accumulate Less Cadmium under Cadmium Stress
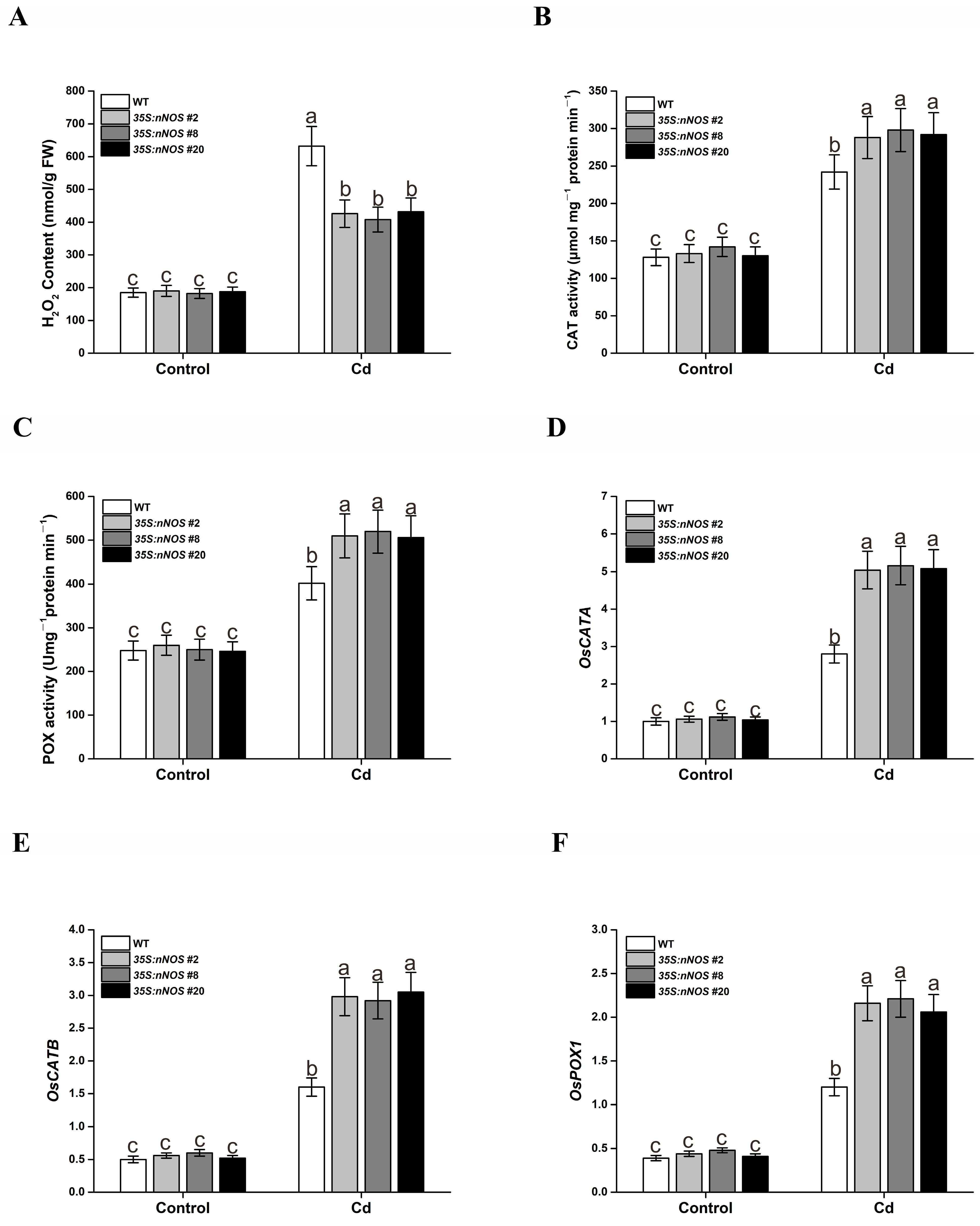
3.4. ROS-Scavenging Capacity of nNOS-Overexpressing Rice Plants Was Enhanced
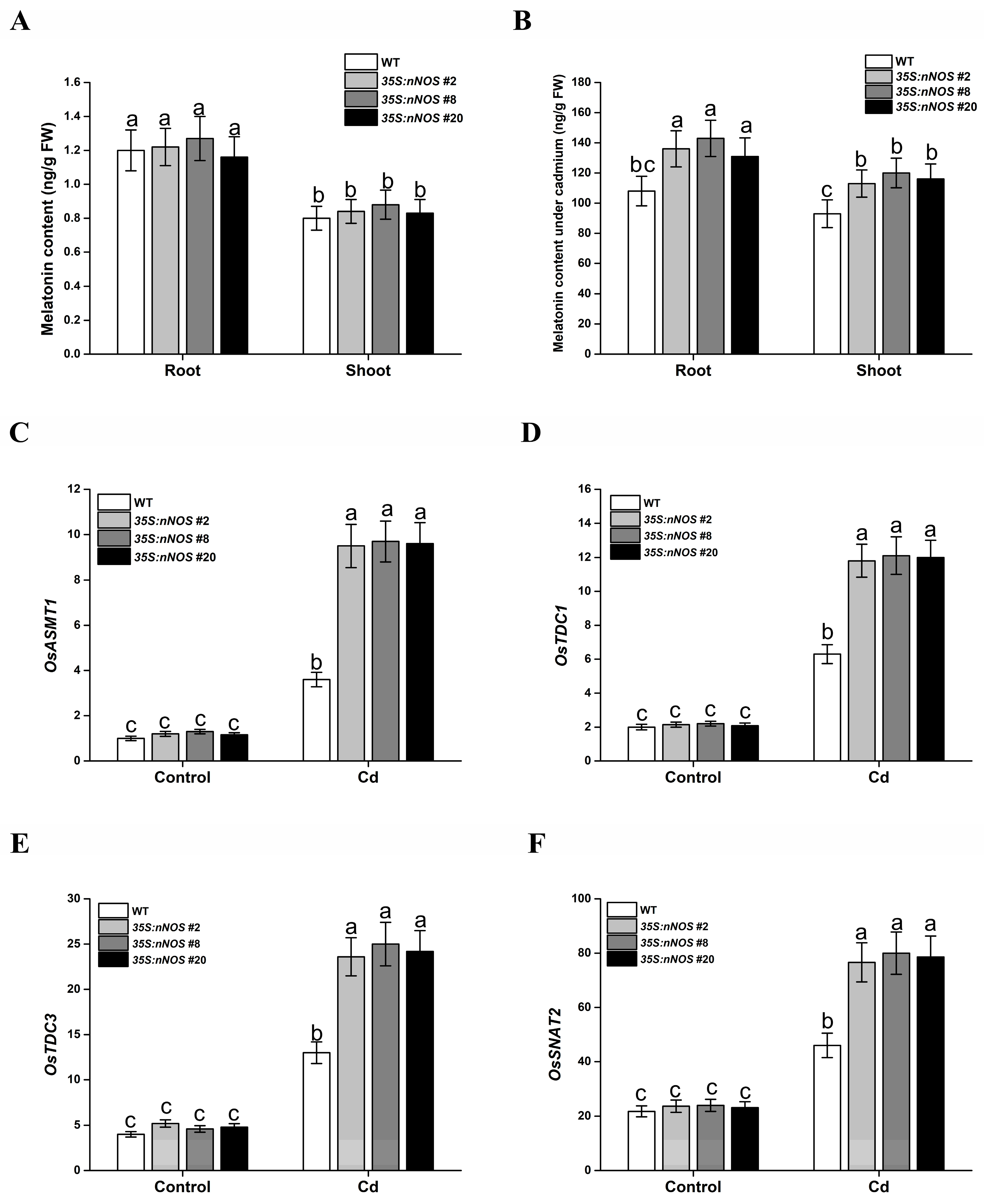
3.5. nNOS Transgenic Plants Showed Increased Melatonin Levels under Cadmium Stress
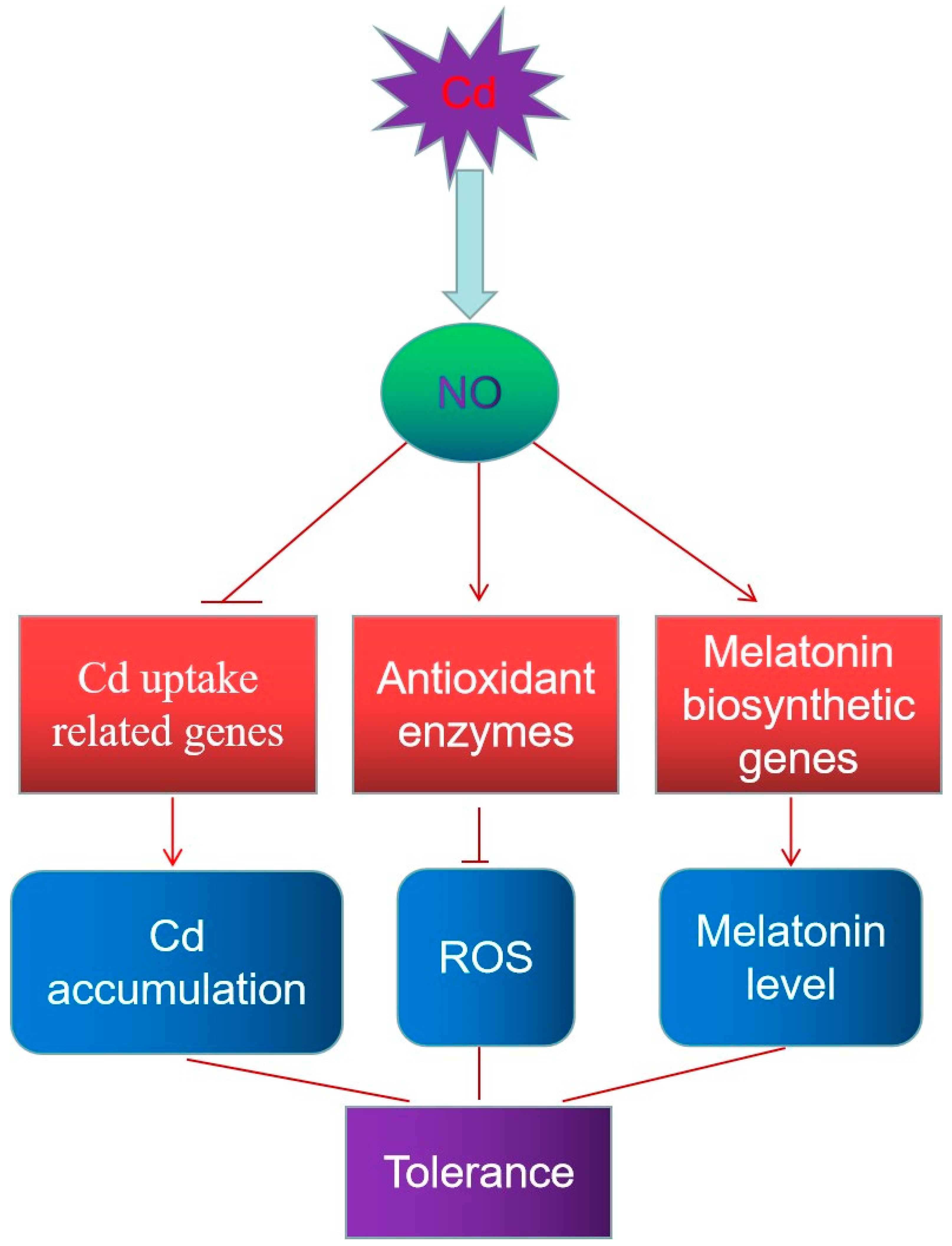
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Yu, L.; Yang, M.; Zou, X.; Yin, C.; Lin, Y. Research advances in cadmium uptake, transport and resistance in rice (Oryza sativa L.). Cells 2022, 11, 569. [Google Scholar] [CrossRef]
- Kumar, D.; Ohri, P. Say “no” to plant stresses: Unravelling the role of nitric oxide under abiotic and biotic stress. Nitric Oxide 2022, 130, 36–57. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ali, S.; Azzawi, T.N.I.A.; Yun, B. Nitric oxide acts as a key signaling molecule in plant development under stressful conditions. Int. J. Mol. Sci. 2023, 24, 4782. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.J.; Kaladhar, V.C.; Fitzpatrick, T.B.; Fernie, A.R.; Møller, I.M.; Loake, G.J. Nitric oxide regulation of plant metabolism. Mol. Plant 2021, 15, 228–242. [Google Scholar] [CrossRef]
- Gill, S.S.; Hasanuzzaman, M.; Nahar, K.; Macovei, A.; Tuteja, N. Importance of nitric oxide in cadmium stress tolerance in crop plants. Plant Physiol. Biochem. 2012, 63, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Jing, H.; Huang, J.; Shen, R.; Zhu, X. The role of nitric oxide signaling in plant responses to cadmium stress. Int. J. Mol. Sci. 2022, 23, 6901. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Shrivastava, A.K.; Singh, V.K. Arsenic and cadmium are inhibitors of cyanobacterial dinitrogenase reductase (nifh1) gene. Funct. Integr. Genom. 2014, 14, 571–580. [Google Scholar] [CrossRef]
- Xiong, J.; Lu, H.; Lu, K.; Duan, Y.; An, L.; Zhu, C. Cadmium decreases crown root number by decreasing endogenous nitric oxide, which is indispensable for crown root primordia initiation in rice seedlings. Planta 2009, 230, 599–610. [Google Scholar] [CrossRef]
- Piacentini, D.; Ronzan, M.; Fattorini, L.; Rovere, F.D.; Massimi, L.; Altamura, M.M.; Falasca, G. Nitric oxide alleviates cadmium- but not arsenic-induced damages in rice roots. Plant Physiol. Biochem. 2020, 151, 729–742. [Google Scholar] [CrossRef]
- Wang, T.; Shi, Z.Q.; Hu, L.; Xu, X.; Han, F.X.; Zhou, L.; Chen, J. Thymol ameliorates cadmium-induced phytotoxicity in the root of rice (Oryza sativa) seedling by decreasing endogenous nitric oxide generation. J. Agric. Food Chem. 2017, 65, 7396–7405. [Google Scholar] [CrossRef] [PubMed]
- Valentovicová, K.; Halusková, L.; Huttová, J.; Mistrík, I.; Tamás, L. Effect of cadmium on diaphorase activity and nitric oxide production in barley root tips. J. Plant Physiol. 2009, 167, 10–14. [Google Scholar] [CrossRef]
- Yang, L.; Ji, J.; Harris, S.; Karen, R.; Wang, H.; Wang, H.; Abd, A.; Elsayed, F.; Luo, Y.; Hu, X. The dynamic changes of the plasma membrane proteins and the protective roles of nitric oxide in rice subjected to heavy metal cadmium stress. Front. Plant Sci. 2016, 7, 190. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-serrano, M.; Romero-puertas, M.C.; Pazmiño, D.M.; Testillano, P.S.; Risueño, M.C.; Río, L.A.D.; Sandalio, L.M. Cellular response of pea plants to cadmium toxicity: Cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol. 2009, 150, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; An, L.; Lu, H.; Zhu, C. Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta 2009, 230, 755–765. [Google Scholar] [CrossRef]
- Arasimowicz-jelonek, M.; Floryszak-wieczorek, J.; Deckert, J.; Rucińska-sobkowiak, R.; Gzyl, J.; Pawlak-sprada, S.; Abramowski, D.; Jelonek, T.; Gwóźdź, E.A. Nitric oxide implication in cadmium-induced programmed cell death in roots and signaling response of yellow lupine plants. Plant Physiol. Biochem. 2012, 58, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Michele, R.D.; Vurro, E.; Rigo, C.; Costa, A.; Elviri, L.; Valentin, M.D.; Careri, M.; Zottini, M.; Toppi, L.S.D.; Schiavo, F.L. Nitric oxide is involved in cadmium-induced programmed cell death in arabidopsis suspension cultures. Plant Physiol. 2009, 150, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.; Mehta, S.K.; Shekhawat, G.S. Nitric oxide (NO) counteracts cadmium induced cytotoxic processes mediated by reactive oxygen species (ROS) in brassica juncea: Cross-talk between ROS, NO and antioxidant responses. Biometals 2013, 26, 255–269. [Google Scholar] [CrossRef]
- Singh, P.; Shah, K. Evidences for reduced metal-uptake and membrane injury upon application of nitric oxide donor in cadmium stressed rice seedlings. Plant Physiol. Biochem. 2014, 83, 180–184. [Google Scholar] [CrossRef]
- Leitner, M.; Vandelle, E.; Gaupels, F.; Bellin, D.; Delledonne, M. NO signals in the haze: Nitric oxide signalling in plant defence. Curr. Opin. Plant Biol. 2009, 12, 451–458. [Google Scholar] [CrossRef]
- Grün, S.; Lindermayr, C.; Sell, S.; Durner, J. Nitric oxide and gene regulation in plants. J. Exp. Bot. 2006, 57, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, W.; Yin, H.; Liu, X.; Sun, H.; Mi, Q. Exogenous nitric oxide improves antioxidative capacity and reduces auxin degradation in roots of Medicago truncatula seedlings under cadmium stress. Plant Soil 2009, 326, 321. [Google Scholar] [CrossRef]
- Lee, K.; Choi, G.H.; Back, K. Cadmium-induced melatonin synthesis in rice requires light, hydrogen peroxide, and nitric oxide: Key regulatory roles for tryptophan decarboxylase and caffeic acid O-methyltransferase. J. Pineal Res. 2017, 63, e12441. [Google Scholar] [CrossRef]
- Gu, Q.; Wang, C.; Xiao, Q.; Chen, Z.; Han, Y. Melatonin confers plant cadmium tolerance: An update. Int. J. Mol. Sci. 2021, 22, 11704. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Chen, Z.; Yu, X.; Cui, W.; Pan, J.; Zhao, G.; Xu, S.; Wang, R.; Shen, W. Melatonin confers plant tolerance against cadmium stress via the decrease of cadmium accumulation and reestablishment of microrna-mediated redox homeostasis. Plant Sci. 2017, 261, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Okant, M.; Ugurlar, F.; Alyemeni, M.N.; Ashraf, M.; Ahmad, P. Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 2019, 225, 627–638. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhuang, X.; Zhou, J.; Sun, L.; Wan, H.; Li, H.; Lyu, D. Exogenous melatonin alleviates cadmium uptake and toxicity in apple rootstocks. Tree Physiol. 2020, 40, 746–761. [Google Scholar] [CrossRef] [PubMed]
- Terrón-Camero, L.C.; Peláez-Vico, M.Á.; Del-val, C.; Sandalio, L.M.; Romero-Puertas, M.C. Role of nitric oxide in plant responses to heavy metal stress: Exogenous application versus endogenous production. J. Exp. Bot. 2019, 70, 4477–4488. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.J.; Fernie, A.R.; Kaiser, W.M.; Dongen, J.T.V. On the origins of nitric oxide. Trends Plant Sci. 2010, 16, 160–168. [Google Scholar] [CrossRef]
- Lin, A.; Wang, Y.; Tang, J.; Xue, P.; Li, C.; Liu, L.; Hu, B.; Yang, F.; Loake, G.J.; Chu, C. Nitric oxide and protein S-nitrosylation are integral to hydrogen peroxide-induced leaf cell death in rice. Plant Physiol. 2012, 158, 451–464. [Google Scholar] [CrossRef]
- Chun, H.J.; Park, H.C.; Koo, S.C.; Lee, J.H.; Park, C.Y.; Choi, M.S.; Kang, C.H.; Baek, D.; Cheong, Y.H.; Yun, D.; et al. Constitutive expression of mammalian nitric oxide synthase in tobacco plants triggers disease resistance to pathogens. Mol. Cells 2012, 34, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Li, R.; Cai, W.; Liu, W.; Wang, C.; Lu, Y. Increasing nitric oxide content in arabidopsis thaliana by expressing rat neuronal nitric oxide synthase resulted in enhanced stress tolerance. Plant Cell Physiol. 2011, 53, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Liu, W.; Wang, W.; Fu, Z.; Han, T.; Lu, Y. Overexpression of rat neurons nitric oxide synthase in rice enhances drought and salt tolerance. PLoS ONE 2015, 10, e0131599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cai, W.; Ji, T.; Ye, L.; Lu, Y.; Yuan, T. WRKY13 enhances cadmium tolerance by promoting D-cysteine desulfhydrase and hydrogen sulfide production. Plant Physiol. 2020, 183, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Song, F.; Xia, J.; Wang, R. A Glucuronic acid-producing endophyte pseudomonas sp. MCS15 reduces cadmium uptake in rice by inhibition of ethylene biosynthesis. Front. Plant Sci. 2022, 13, 876545. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, G.; Chang, Y.; Lin, D.; Reiter, R.J.; He, C.; Shi, H. Melatonin biosynthesis enzymes recruit wrky transcription factors to regulate melatonin accumulation and transcriptional activity on w-box in cassava. J. Pineal Res. 2018, 65, e12487. [Google Scholar] [CrossRef]
- He, H.; He, L. Crosstalk between melatonin and nitric oxide in plant development and stress responses. Physiol. Plantarum 2020, 170, 218–226. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Z.; Zhu, C. Endogenous nitric oxide mediates alleviation of cadmium toxicity induced by calcium in rice seedlings. J. Environ. Sci. 2012, 24, 940–948. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, M.M.; Ansary, M.M.U.; Fujita, M.; Tran, L.P. Interactive effects of salicylic acid and nitric oxide in enhancing rice tolerance to cadmium stress. Int. J. Mol. Sci. 2019, 20, 5798. [Google Scholar] [CrossRef]
- Delledonne, M.; Zeier, J.; Marocco, A.; Lamb, C. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc. Natl. Acad. Sci. USA 2001, 98, 13454–13459. [Google Scholar] [CrossRef]
- Wang, T.; Song, J.; Liu, Z.; Liu, Z.; Cui, J. Melatonin alleviates cadmium toxicity by reducing nitric oxide accumulation and IRT1 expression in chinese cabbage seedlings. Environ. Sci. Pollut. Res. 2020, 28, 15394–15405. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, W.; Wang, W.; Deng, H.; Chen, B.; Zhang, G.; Wang, P.; Yuan, T.; Zhu, Y. Improving Endogenous Nitric Oxide Enhances Cadmium Tolerance in Rice through Modulation of Cadmium Accumulation and Antioxidant Capacity. Agronomy 2023, 13, 1978. https://doi.org/10.3390/agronomy13081978
Cai W, Wang W, Deng H, Chen B, Zhang G, Wang P, Yuan T, Zhu Y. Improving Endogenous Nitric Oxide Enhances Cadmium Tolerance in Rice through Modulation of Cadmium Accumulation and Antioxidant Capacity. Agronomy. 2023; 13(8):1978. https://doi.org/10.3390/agronomy13081978
Chicago/Turabian StyleCai, Wei, Wenshu Wang, Hui Deng, Bin Chen, Guo Zhang, Ping Wang, Tingting Yuan, and Yongsheng Zhu. 2023. "Improving Endogenous Nitric Oxide Enhances Cadmium Tolerance in Rice through Modulation of Cadmium Accumulation and Antioxidant Capacity" Agronomy 13, no. 8: 1978. https://doi.org/10.3390/agronomy13081978
APA StyleCai, W., Wang, W., Deng, H., Chen, B., Zhang, G., Wang, P., Yuan, T., & Zhu, Y. (2023). Improving Endogenous Nitric Oxide Enhances Cadmium Tolerance in Rice through Modulation of Cadmium Accumulation and Antioxidant Capacity. Agronomy, 13(8), 1978. https://doi.org/10.3390/agronomy13081978





