Hyperparasitic Fungi against Melon Powdery Mildew Pathogens: Quantitative Analysis of Conidia Released from Single Colonies of Podosphaera xanthii Parasitised by Ampelomyces
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Cultivation
2.2. Fungal Materials, Culture, Inoculation, and Incubation
2.2.1. Podosphaera xanthii KMP-6N
2.2.2. Ampelomyces Strain Xs-q
2.2.3. Inoculation Experiments for Observing Mycoparasitism in Melon PM
2.3. Morphological Observation and Infection Processes of Ampelomyces Strain Xs-q Inoculated onto Hyphae of P. xanthii KMP-6N
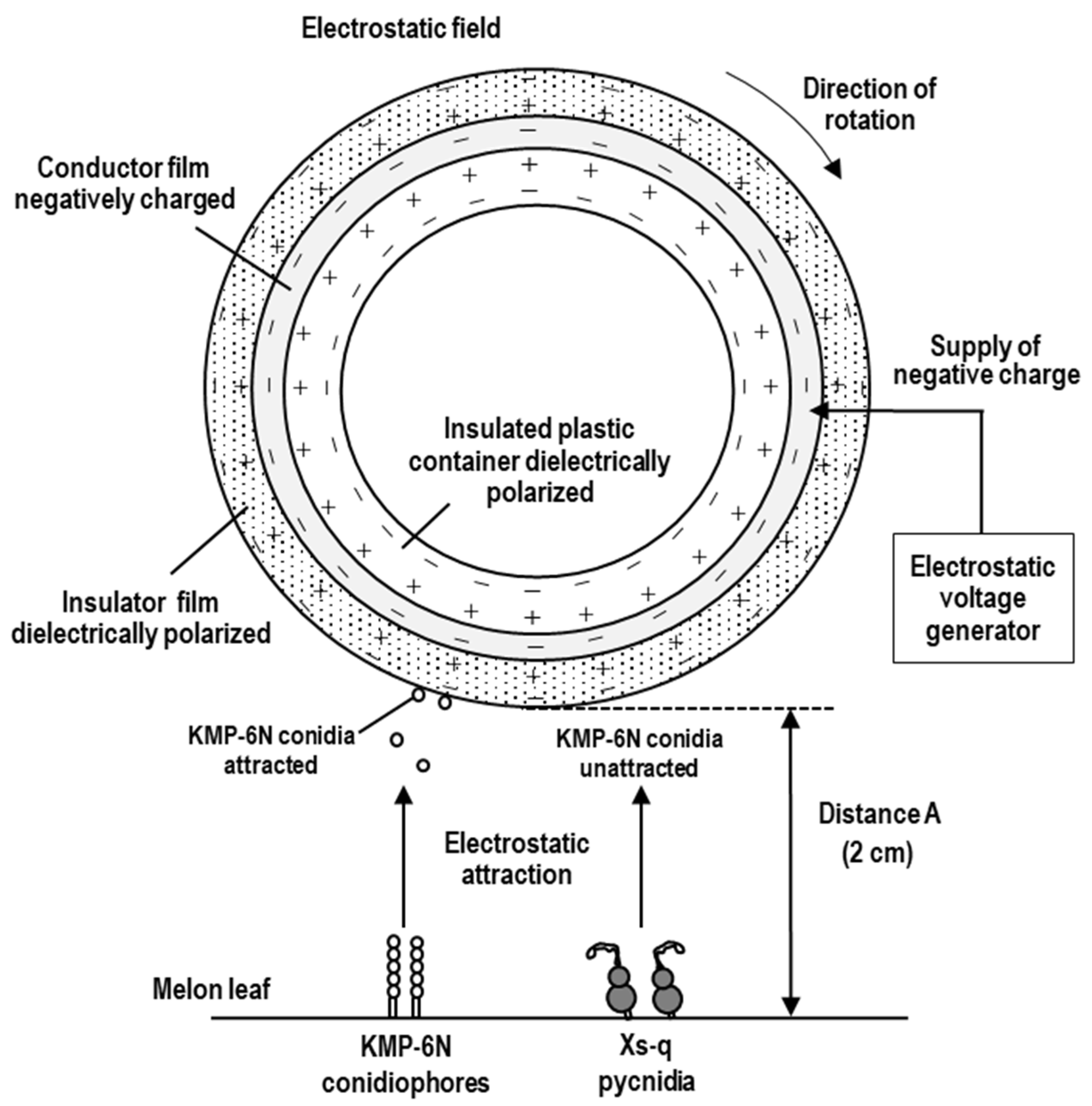
2.4. Conidial Collector and Electrostatic Spore Collection
2.5. Electrostatic Activation of the Insulator Film
2.6. Consecutive Collection of KMP-6N Conidia Released from Single Ampelomyces-Treated Colonies
2.7. Microscopic Observation of Melon PM Colonies Inoculated with Xs-q Spores
2.8. Statistical Analyses
3. Results
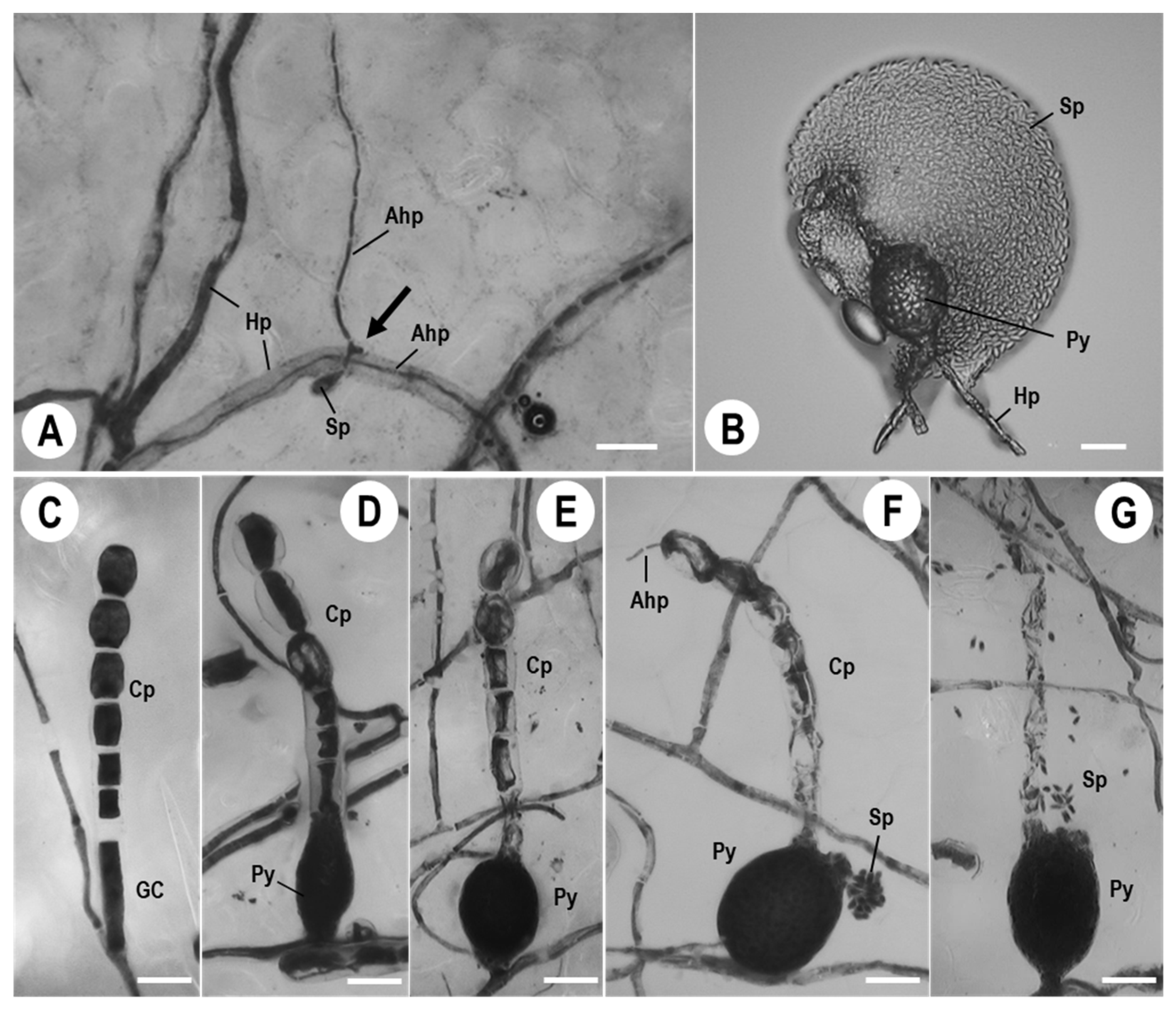
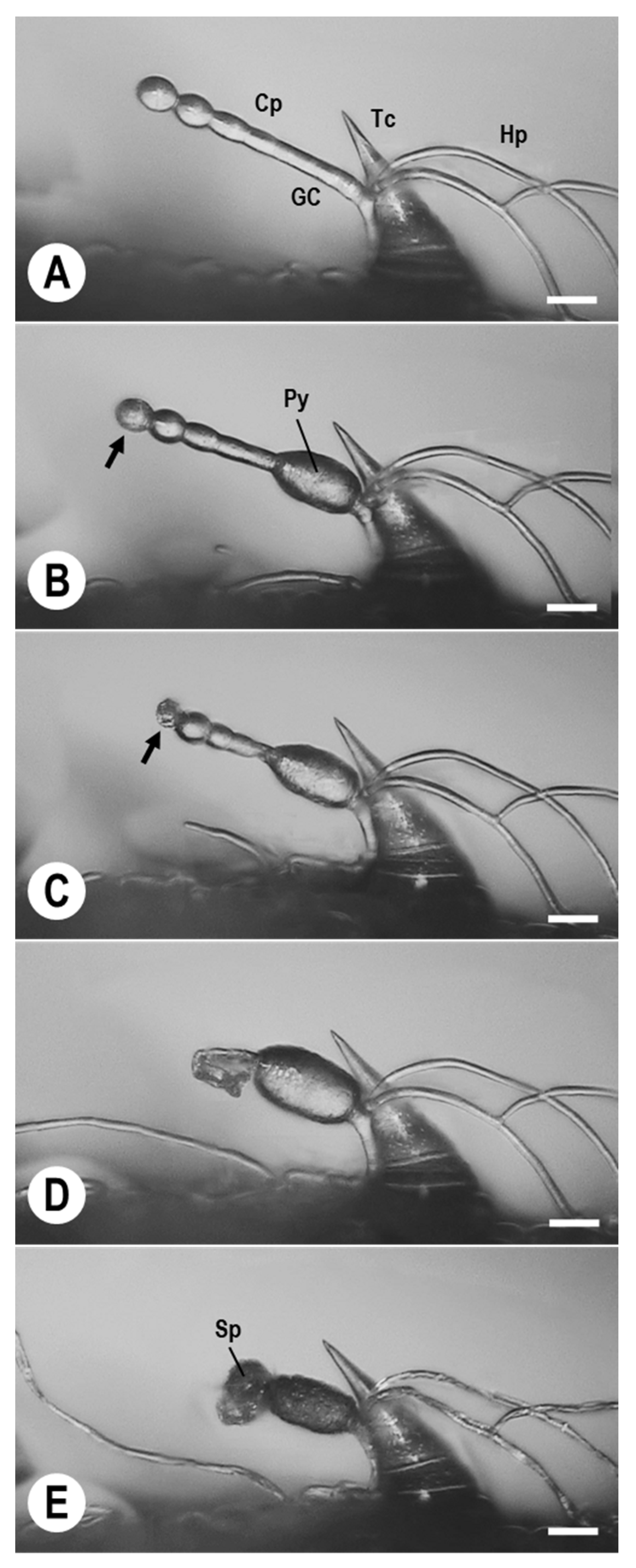
3.1. Morphological Observations of Ampelomyces Strain Xs-q in KMP-6N Colonies
3.2. KMP-6N Conidiophore Morphology following Invasion by Ampelomyces Strain Xs-q
3.3. Pycnidial Development of Ampelomyces Strain Xs-q in KMP-6N Conidiophores
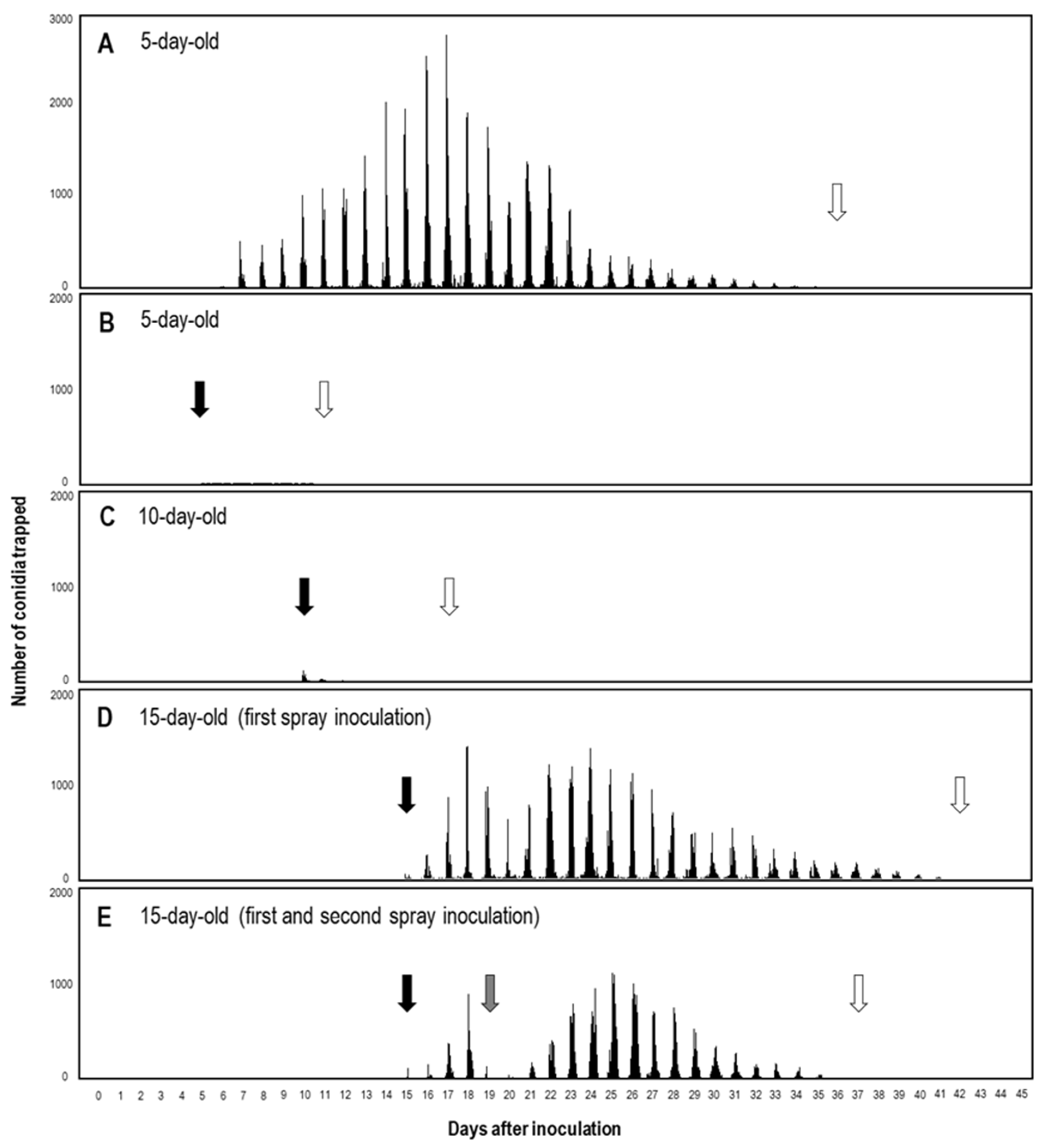
3.4. Quantitative Analysis of KMP-6N Conidia Released from Parasitised PM Colonies under Greenhouse Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sowell, G., Jr. Population shift of Sphaerotheca fuliginea on musk melon. J. Am. Soc. Hortic. Sci. 1982, 112, 156–160. [Google Scholar]
- Reifschneider, F.J.B.; Boiteux, L.S.; Occhiena, E.M. Powdery mildew on melon (Cucumis melo) caused by Sphaerotheca fuliginea in Brazil. Plant Dis. 1985, 69, 1069–1070. [Google Scholar]
- Mohamed, Y.F.; Bardin, M.; Nicot, P.C.; Pitrat, M. Causal agents of powdery mildew of cucurbits in Sudan. Plant Dis. 1995, 79, 634–636. [Google Scholar] [CrossRef]
- Hosoya, K.; Narisawa, K.; Pitrat, M.; Ezura, H. Race identification in powdery mildew (Sphaerotheca fuliginea) on melon (Cucumis melo) in Japan. Plant Breed. 1999, 118, 259–262. [Google Scholar] [CrossRef]
- Del Pino, D.; Olalla, L.; Pérez-García, A.; Rivera, M.E.; García, S.; Moreno, R.; Torés, J.A. Occurrence of races and pathotypes of cucurbit powdery mildew in southeastern Spain. Phytoparasitica 2002, 30, 459–466. [Google Scholar] [CrossRef]
- Křístková, E.; Lebeda, A.; Sedláková, B. Virulence of Czech cucurbit powdery mildew isolates on Cucumis melo genotypes MR-1 and PI 124112. Sci. Hortic. 2004, 99, 257–265. [Google Scholar] [CrossRef]
- Křístková, E.; Lebeda, A.; Sedláková, B. Species spectra, distribution and host range of cucurbit powdery mildews in the Czech Republic, and in some other European and Middle Eastern countries. Phytoparasitica 2009, 37, 337–350. [Google Scholar] [CrossRef]
- Tomason, Y.; Gibson, P.T. Fungal characteristics and varietal reactions of powdery mildew species on cucurbits in steppes of Ukraine. Agron. Res. 2006, 4, 549–562. [Google Scholar]
- Pérez-García, A.; Romero, D.; Fernández-Ortuño, D.; López-Ruiz, F.; De Vicente, A.; Torés, J.A. The powdery mildew fungus Podosphaera fusca (synonym Podosphaera xanthii), a constant threat to cucurbits. Mol. Plant Pathol. 2009, 10, 153–160. [Google Scholar] [CrossRef]
- Hong, Y.-J.; Hossain, M.R.; Kim, H.-T.; Park, J.-I.; Nou, I.-S. Identification of two new races of Podosphaera xanthii causing powdery mildew in melon in South Korea. Plant Pathol. J. 2018, 34, 182–190. [Google Scholar] [CrossRef]
- Kiss, L.; Vaghefi, N.; Bransgrove, K.; Dearnaley, J.D.W.; Takamatsu, S.; Tan, Y.P.; Marston, C.; Liu, S.Y.; Jin, D.N.; Adorada, D.L.; et al. Australia: A continent without native powdery mildews? The first comprehensive catalogue indicates recent introductions and multiple host range expansion events, and leads to the re-discovery of Salmonomyces as a new lineage of the Erysiphales. Front. Microbiol. 2020, 11, 1571. [Google Scholar] [CrossRef] [PubMed]
- Kelly, L.A.; Vaghefi, N.; Bransgrove, K.; Fechner, N.A.; Stuart, K.; Pandey, A.K.; Sharma, M.; Németh, M.Z.; Liu, S.Y.; Tang, S.R.; et al. One crop disease, how many pathogens? Podosphaera xanthii and Erysiphe vignae sp. nov. identified as the two species that cause powdery mildew of mungbean (Vigna radiata) and black gram (V. mungo) in Australia. Phytopathology 2021, 111, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- Vaghefi, N.; Kusch, S.; Németh, M.Z.; Seress, D.; Braun, U.; Takamatsu, S.; Panstruga, R.; Kiss, L. Beyond nuclear ribosomal DNA sequences: Evolution, taxonomy, and closest known saprobic relatives of powdery mildew fungi (Erysiphaceae) inferred from their first comprehensive genome-scale phylogenetic analyses. Front. Microbiol. 2022, 13, 903024. [Google Scholar] [CrossRef] [PubMed]
- Keinath, A.P.; Wintermantel, W.M.; Zitter, T.A. Compendium of Cucurbit Diseases and Pests, 2nd ed.; The American Phytopathological Society Press: St. Paul, MN, USA, 2017. [Google Scholar]
- Takikawa, Y.; Nonomura, T.; Miyamoto, S.; Okamoto, N.; Murakami, T.; Matsuda, Y.; Kakutani, K.; Kusakari, S.; Toyoda, H. Digital microscopic analysis of developmental process of conidiogenesis by powdery mildew pathogens isolated from melon leaves. Phytoparasitica 2015, 43, 517–530. [Google Scholar] [CrossRef]
- Huggenberger, F.; Collins, M.A.; Skylakakis, G. Decreased sensitivity of Sphaerotheca fuliginea to fenarimol and other ergosterol-biosynthesis inhibitors. Crop Prot. 1984, 3, 137–149. [Google Scholar] [CrossRef]
- Lebeda, A.; Sedláková, B. Fungicide resistance in population of cucurbit powdery mildew. J. Plant Pathol. 2008, 90, S2.142. [Google Scholar]
- McGrath, M.T.; Shishkoff, N. Resistance to triadimefon and benomyl: Dynamics and impact on managing cucurbit powdery mildew. Plant Dis. 2001, 85, 147–154. [Google Scholar] [CrossRef]
- McGrath, M.T.; Shishkoff, N. Frist report of the cucurbit powdery mildew fungus (Podosphaera xanthii) resistant to strobilurin fungicides in the United States. Plant Dis. 2003, 87, 1007. [Google Scholar] [CrossRef]
- Miyamoto, T.; Hayashi, K.; Ogawara, T. First report of the occurrence of multiple resistance to Flutianil and Pyriofenone in field isolates of Podosphaera xanthii, the causal fungus of cucumber powdery mildew. Eur. J. Plant Pathol. 2020, 156, 953–963. [Google Scholar] [CrossRef]
- Miyamoto, T.; Hayashi, K.; Okada, R.; Wari, D.; Ogawara, T. Resistance to succinate dehydrogenase inhibitors in field isolates of Podosphaera xanthii on cucumber: Monitoring, cross-resistance patterns and molecular characterization. Pestic. Biochem. Physiol. 2020, 169, 104646. [Google Scholar] [CrossRef]
- Vielba-Fernández, A.; Polonio, A.; Ruiz-Jiménez, L.; de Vicente, A.; Pérez-García, A.; Fernández-Ortuño, D. Fungicide resistance in powdery mildew fungi. Microorganisms 2020, 8, 1431. [Google Scholar] [CrossRef] [PubMed]
- Cesati, V. Ampelomyces quisqualis Ces. Bot. Ztg. 1852, 10, 301–302. [Google Scholar]
- Jarvis, W.R.; Slingsby, K. The control of powdery mildew of greenhouse cucumber by water sprays and Ampelomyces quisqualis. Plant Dis. Reptr. 1977, 61, 728–730. [Google Scholar]
- Sundheim, L. Control of cucumber powdery mildew by the hyperparasite Ampelomyces quisquialis and fungicides. Plant Pathol. 1982, 31, 209–214. [Google Scholar] [CrossRef]
- Kiss, L. Natural occurrence of Ampelomyces intracellular mycoparasites in mycelia of powdery mildew fungi. New Phytol. 1998, 140, 709–714. [Google Scholar] [CrossRef]
- Yarwood, C.E. An overwintering pycnidial stage of Cicinnobolus. Mycologia 1939, 31, 420–422. [Google Scholar] [CrossRef]
- Falk, S.P.; Gadoury, D.M.; Cortesi, P.; Pearson, R.C.; Seem, R.C. Parasitism of Uncinula necator cleistothecia by the mycoparasite Ampelomyces quisqualis. Phytopathology 1995, 85, 794–800. [Google Scholar] [CrossRef]
- Kiss, L. A review of fungal antagonists of powdery mildews and their potential as biocontrol agents. Pest Manag. Sci. 2003, 59, 475–483. [Google Scholar] [CrossRef]
- Huth, L.; Ash, G.J.; Idnurm, A.; Kiss, L.; Vaghefi, N. The “bipartite” structure of the first genome of Ampelomyces quisqualis, a common hyperparasite and biocontrol agent of powdery mildews, may point to its evolutionary origin from plant pathogenic fungi. Genome Biol. Evol. 2021, 13, evab182. [Google Scholar] [CrossRef]
- Sztejnberg, A. Biological control of powdery mildews by Ampelomyces quisqualis. Phytopathology 1979, 69, 1047. [Google Scholar]
- Sundheim, L. Ampelomyces quisqualis, a hyperparasitic fungus in biological control of powdery mildews on greenhouse cucumber. Acta Hortic. 1984, 156, 229–236. [Google Scholar] [CrossRef]
- Sztejnberg, A.; Mazar, S. Biocontrol of cucumber and carrot powdery mildew by Ampelomyces quisqualis. Phytopathology 1985, 75, 1301–1302. [Google Scholar]
- Sztejnberg, A.; Galper, S.; Mazar, S.; Lisker, N. Ampelomyces quisqualis for biological and integrated control of powdery mildews in Israel. J. Phytopathol. 1989, 124, 285–295. [Google Scholar] [CrossRef]
- Dik, A.J.; Verhaar, M.A.; Bélanger, R.R. Comparison of three biological control agents against cucumber powdery mildew (Sphaerotheca fuliginea) in semi-commercial-scale glasshouse trials. Eur. J. Plant Pathol. 1998, 104, 413–423. [Google Scholar] [CrossRef]
- Shishkoff, N.; McGrath, M.T. AQ10 biofungicide combined with chemical fungicides or AddQ spray adjuvant for control of cucurbit powdery mildew in detached leaf culture. Plant Dis. 2002, 86, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Angeli, D.; Maurhofer, M.; Gessler, C.; Pertot, I. Existence of different physiological forms within genetically diverse strains of Ampelomyces quisqualis. Phytoparasitica 2012, 40, 37–51. [Google Scholar] [CrossRef]
- Sztejnberg, A.; Mazar, S. Studies on the hyperparasite Ampelomyces quisqualis and preliminary trials on biological control of powdery mildew. Phytoparasitica 1983, 11, 219–220. [Google Scholar]
- Romero, D.; Rivera, M.E.; Cazorla, F.M.; De Vicente, A.; Pérez-García, A. Effect of mycoparasitic fungi on the development of Sphaerotheca fusca in melon leaves. Mycol. Res. 2003, 107, 64–71. [Google Scholar] [CrossRef]
- Park, M.-J.; Choi, Y.-J.; Hong, S.-B.; Shin, H.-D. Genetic variability and mycohost association of Ampelomyces quisqualis isolates inferred from phylogenetic analyses of ITS rDNA and actin gene sequences. Fungal Biol. 2010, 114, 235–247. [Google Scholar] [CrossRef]
- Siozios, S.; Tosi, L.; Ferrarini, A.; Ferrari, A.; Tononi, P.; Bellin, D.; Maurhofer, M.; Gessler, C.; Delledonne, M.; Pertot, I. Transcriptional reprogramming of the mycoparasitic fungus Ampelomyces quisqualis during the powdery mildew host-induced germination. Phytopathology 2015, 105, 199–209. [Google Scholar] [CrossRef]
- Legler, S.E.; Pintye, A.; Caffi, T.; Gulyás, S.; Bohár, G.; Rossi, V.; Kiss, L. Sporulation rate in culture and mycoparasitic activity, but not mycohost specificity, are the key factors for selecting Ampelomyces strains for biocontrol of grapevine powdery mildew (Erysiphe necator). Eur. J. Plant Pathol. 2016, 144, 723–736. [Google Scholar] [CrossRef]
- Falk, S.P.; Gadoury, D.M.; Pearson, R.C.; Seem, R.C. Partial control of grape powdery mildew by the mycoparasite Ampelomyces quisqualis. Plant Dis. 1995, 79, 483–490. [Google Scholar] [CrossRef]
- Hashioka, Y.; Nakai, Y. Ultrastructure of pycnidial development and mycoparasitism of Ampelomyces quisqualis parasitic on Erysiphales. Trans. Mycol. Soc. Japan 1980, 21, 329–338. [Google Scholar]
- Kiss, L.; Russell, J.C.; Szentiványi, O.; Xu, X.; Jeffries, P. Biology and biocontrol potential of Ampelomyces mycoparasites, natural antagonists of powdery mildew fungi. Biocont. Sci. Technol. 2004, 14, 635–651. [Google Scholar] [CrossRef]
- Kiss, L. Intracellular mycoparasites in action: Interactions between powdery mildew fungi and Ampelomyces. In Stress in Yeasts and Filamentous Fungi; Avery, S.V., Stratford, M., van West, P., Eds.; Academic Press: Cambridge, MA, USA, 2008; Volume 27, pp. 37–52. [Google Scholar]
- Németh, M.Z.; Pintye, A.; Horváth, Á.N.; Vági, P.; Kovács, G.M.; Gorfer, M.; Kiss, L. Green fluorescent protein transformation sheds more light on a widespread mycoparasitic interaction. Phytopathology 2019, 109, 1404–1416. [Google Scholar] [CrossRef] [PubMed]
- Németh, M.Z.; Mizuno, Y.; Kobayashi, H.; Seress, D.; Shishido, N.; Kimura, Y.; Takamatsu, S.; Suzuki, T.; Takikawa, Y.; Kakutani, K.; et al. Ampelomyces strains isolated from diverse powdery mildew hosts in Japan: Their phylogeny and mycoparasitic activity, including timing and quantifying mycoparasitism of Pseudoidium neolycopersici on tomato. PLoS ONE 2021, 16, e0251444. [Google Scholar] [CrossRef]
- Aylor, D.E. The role of intermittent wind in the dispersal of fungal pathogens. Annu. Rev. Phytopathol. 1990, 28, 73–92. [Google Scholar] [CrossRef]
- Brown, J.K.M.; Hovmøller, M.S. Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 2002, 297, 537–541. [Google Scholar] [CrossRef]
- Nonomura, T.; Matsuda, Y.; Yamashita, S.; Akahoshi, H.; Takikawa, Y.; Kakutani, K.; Toyoda, H. Natural woody plant, Mallotus japonicus, as an ecological partner to transfer different pathotypic conidia of Oidium neolycopersici to greenhouse tomatoes. Plant Protect. Sci. 2013, 49, S33–S40. [Google Scholar] [CrossRef]
- Suzuki, T.; Iwasaki, S.; Hisazumi, H.; Miyamoto, A.; Ogami, H.; Takikawa, Y.; Kakutani, K.; Matsuda, Y.; Nonomura, T. Inhibitory effects of blue light-emitting diode irradiation on Podosphaera xanthii conidial release and infection of melon seedlings. Agriculture 2022, 12, 198. [Google Scholar] [CrossRef]
- Suzuki, T.; Nakamura, R.; Takagi, N.; Takikawa, Y.; Kakutani, K.; Matsuda, Y.; Matsui, K.; Nonomura, T. Quantitative analysis of the lifelong production of conidia released from single colonies of Podosphaera xanthii on melon leaves using electrostatic techniques. Austral. Plant Pathol. 2019, 48, 297–307. [Google Scholar] [CrossRef]
- Griffith, W.T. Electrostatic phenomena. In The Physics of Everyday Phenomena: A Conceptual Introduction to Physics; Bruflodt, D., Loehr, B.S., Eds.; McGraw-Hill: New York, NY, USA, 2004; pp. 232–252. [Google Scholar]
- Halliday, D.; Resnick, R.; Walker, J. Electric charge. In Fundamentals of Physics; Johnson, S., Ford, E., Eds.; John Wiley & Sons: New York, NY, USA, 2005; pp. 561–579. [Google Scholar]
- Leach, C.M. An electrostatic theory to explain violent spore liberation by Drechslera turcica and other fungi. Mycologia 1976, 68, 63–86. [Google Scholar] [CrossRef]
- Matsuda, Y.; Ikeda, H.; Moriura, N.; Tanaka, N.; Shimizu, K.; Oichi, W.; Nonomura, T.; Kakutani, K.; Kusakari, S.; Higashi, K.; et al. A new spore precipitator with polarized dielectric insulators for physical control of tomato powdery mildew. Phytopathology 2006, 96, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Matsuda, Y.; Nonomura, T.; Ikeda, H.; Tamura, N.; Kusakari, S.; Kimbara, J.; Toyoda, H. Dual protection of hydroponic tomatoes from rhizosphere pathogens Ralstonia solanacearum and Fusarium oxysporum f. sp. radicis-lycopersici and airborne conidia of Oidium neolycopersici with an ozone-generative electrostatic spore precipitator. Plant Pathol. 2007, 56, 987–997. [Google Scholar] [CrossRef]
- Ayabe, S.; Kimura, Y.; Umei, N.; Takikawa, Y.; Kakutani, K.; Matsuda, Y.; Nonomura, T. Real-time collection of conidia released from living single colonies of Podosphaera aphanis on strawberry leaves under natural conditions with electrostatic techniques. Plants 2022, 11, 3453. [Google Scholar] [CrossRef]
- Moriura, N.; Matsuda, Y.; Oichi, W.; Nakashima, S.; Hirai, T.; Nonomura, T.; Kakutani, K.; Kusakari, S.; Higashi, K.; Toyoda, H. An apparatus for collecting total conidia of Blumeria graminis f. sp. hordei from leaf colonies using electrostatic attraction. Plant Pathol. 2006, 55, 367–374. [Google Scholar] [CrossRef]
- Suzuki, T.; Nishimura, S.; Yagi, K.; Nakamura, R.; Takikawa, Y.; Matsuda, Y.; Kakutani, K.; Nonomura, T. Effects of light quality on conidiophore formation of the melon powdery mildew pathogen Podosphaera xanthii. Phytoparasitica 2018, 94, 1105–1110. [Google Scholar] [CrossRef]
- Sameshima, T.; Kashimoto, K.; Kida, K.; Matsuda, Y.; Nonomura, T.; Kakutani, K.; Nakata, K.; Kusakari, S.; Toyoda, H. Cytological events in tomato leaves inoculated with conidia of Blumeria graminis f. sp. hordei and Oidium neolycopersici KTP-01. J. Gen. Plant Pathol. 2004, 70, 7–10. [Google Scholar] [CrossRef]
- Kiss, L. Graminicolous powdery mildew fungi as new natural hosts of Ampelomyces mycoparasites. Can. J. Bot. 1997, 75, 680–683. [Google Scholar] [CrossRef]
- Kiss, L.; Nakasone, K.K. Ribosomal DNA internal transcribed spacer sequences do not support the species status of Ampelomyces quisqualis, a hyperparasite of powdery mildew fungi. Curr. Genet. 1998, 33, 362–367. [Google Scholar] [CrossRef]
- Ranković, B. Hyperparasites of the genus Ampelomyces on powdery mildew fungi in Serbia. Mycopathologia 1997, 139, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Pintye, A.; Ropars, J.; Harvey, N.; Shin, H.D.; Leyronas, C.; Nicot, P.C.; Giraud, T.; Kiss, L. Host phenology and geography as drivers of differentiation in generalist fungal mycoparasites. PLoS ONE 2015, 10, e0120703. [Google Scholar] [CrossRef]
- Gu, Y.H.; Ko, W.H. Water agarose medium for studying factors affecting germination of conidia of Ampelomyces quisqualis. Mycol. Res. 1997, 101, 422–424. [Google Scholar] [CrossRef]
- Sundheim, L.; Krekling, T. Host-parasite relationships of the hyperparasite Ampelomyces quisqualis and its powdery mildew host Sphaerotheca fuliginea. Phytopath. Z. 1982, 104, 202–210. [Google Scholar] [CrossRef]
- Philipp, W.-D.; Crüger, G. Parasitismus von Ampelomyces quisqualis auf echten mehltaupilzen an gurken und anderen gemüsearten. Z. Pflanzenkrankh. Pflanzenschutz 1979, 86, 129–142. [Google Scholar]
- Nonomura, T.; Matsuda, Y.; Xu, L.; Kakutani, K.; Takikawa, Y.; Toyoda, H. Collection of highly germinative pseudochain conidia of Oidium neolycopersici from conidiophores by electrostatic attraction. Mycol. Res. 2009, 113, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Speer, E.O. Ampelomyces cesati (Fungi, Sphaeropsidales). Taxon 1978, 27, 549–562. [Google Scholar] [CrossRef]
- Kiss, L.; Pintye, A.; Zséli, G.; Jankovics, T.; Szentiványi, O.; Hafez, Y.M.; Cook, R.T.A. Microcyclic conidiogenesis in powdery mildews and its association with intracellular parasitism by Ampelomyces. Eur. J. Plant Pathol. 2010, 126, 445–451. [Google Scholar] [CrossRef]
- Zeng, L.F.; Jiang, Y.C. An investigation of parasitic fungi on Erysiphe spp. RoPP 1986, 65, 419. [Google Scholar]
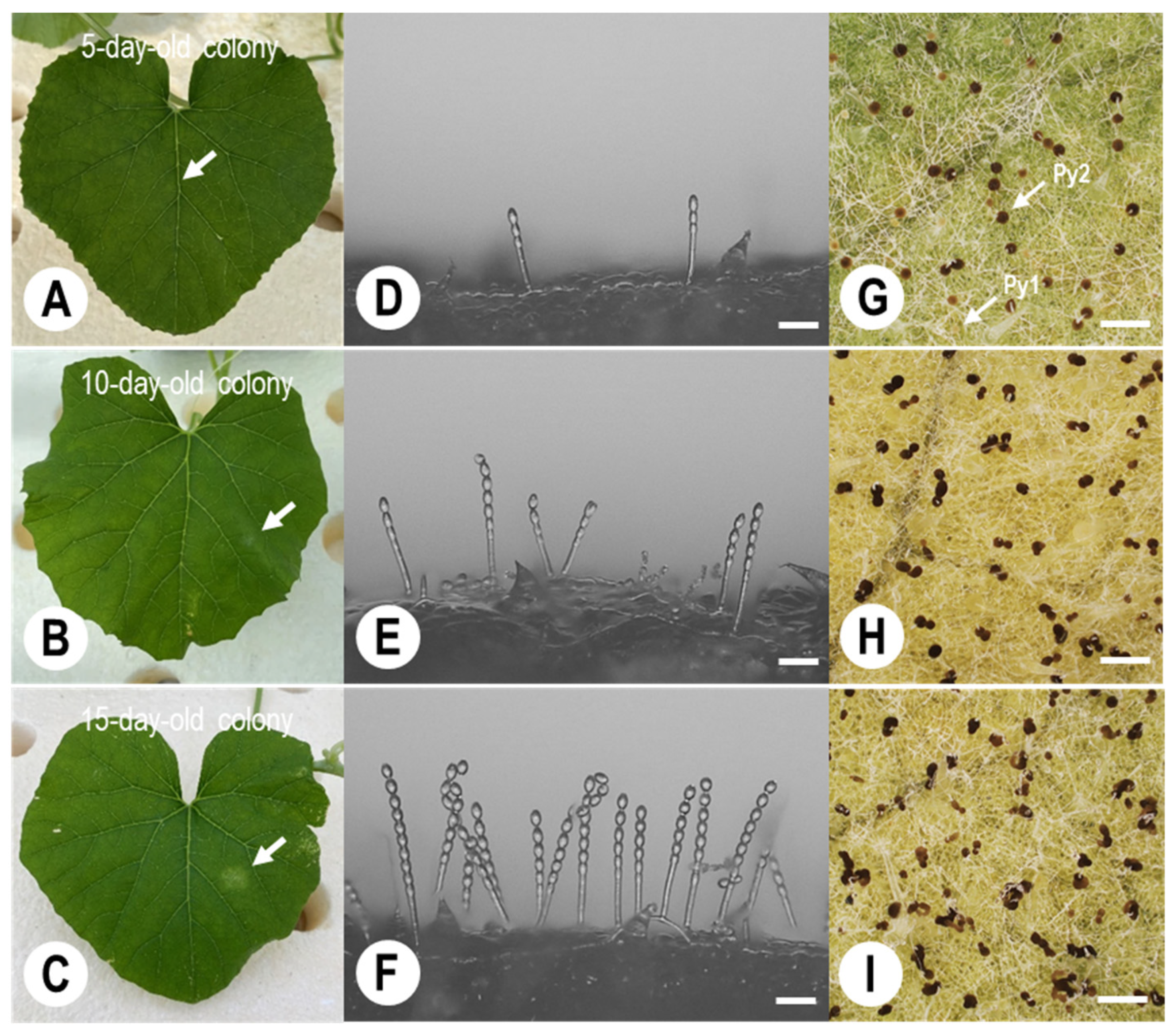

| Colony | Times of Spray Inoculation | Numbers of Pycnidia in a Single Colony x | Numbers of Spores Produced in a Mature Pycnidium y |
|---|---|---|---|
| 5 days old | Once | 313.7 ± 125.6 a | 745.3 ± 287.3 a |
| 10 days old | Once | 1602.1 ± 220.5 b | 851.0 ± 372.3 a |
| 15 days old | Once | 2321.0 ± 399.5 c | 1738.5 ± 494.2 b |
| Colony | Times of Spray Inoculation | Colony Area (cm2) | Number of Normal Conidiophores in a Single Colony | Duration of Conidial Secession (Day) | Total Conidia Collected from Xs-q-Inoculated Colonies y | ||
|---|---|---|---|---|---|---|---|
| Before Inoculation | After Inoculation w | Before Inoculation | After Inoculation x | ||||
| 5 days old | One | 0.02 ± 0.02 a | 0.04 ± 0.02 a | 12.0 ± 10.6 a | 0 a | 4.6 ± 0.9 a | 156.2 ± 108.3 a |
| 10 days old | One | 0.32 ± 0.13 b | 0.46 ± 0.14 b | 752.1 ± 170.4 b | 0 a | 8.2 ± 0.8 b | 1167.0 ± 745.9 b |
| 15 days old | One | 0.98 ± 0.14 c | 1.21 ± 0.15 c | 1130.7 ± 145.9 c | 864.8 ± 91.2 b | 24.6 ± 3.0 c | 61,530.4 ± 8785.3 c |
| 15 days old | Twice | 0.94 ± 0.18 c | 1.10 ± 0.15 c | 1255.3 ± 179.4 c | 654.4 ± 45.6 c | 20.4 ± 1.1 d | 44,866.4 ± 7654.7 d |
| Uninoculated z | One (water) | 0.05 ± 0.02 a | 2.00 ± 0.16 d | 15.4 ± 4.0 a | 1409.0 ± 100.1 d | 28.2 ± 2.2 c | 124,761.0 ± 12,157.4 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, Y.; Németh, M.Z.; Numano, K.; Mitao, A.; Shirakawa, T.; Seress, D.; Takikawa, Y.; Kakutani, K.; Matsuda, Y.; Kiss, L.; et al. Hyperparasitic Fungi against Melon Powdery Mildew Pathogens: Quantitative Analysis of Conidia Released from Single Colonies of Podosphaera xanthii Parasitised by Ampelomyces. Agronomy 2023, 13, 1204. https://doi.org/10.3390/agronomy13051204
Kimura Y, Németh MZ, Numano K, Mitao A, Shirakawa T, Seress D, Takikawa Y, Kakutani K, Matsuda Y, Kiss L, et al. Hyperparasitic Fungi against Melon Powdery Mildew Pathogens: Quantitative Analysis of Conidia Released from Single Colonies of Podosphaera xanthii Parasitised by Ampelomyces. Agronomy. 2023; 13(5):1204. https://doi.org/10.3390/agronomy13051204
Chicago/Turabian StyleKimura, Yutaka, Márk Z. Németh, Kana Numano, Asami Mitao, Tomomi Shirakawa, Diána Seress, Yoshihiro Takikawa, Koji Kakutani, Yoshinori Matsuda, Levente Kiss, and et al. 2023. "Hyperparasitic Fungi against Melon Powdery Mildew Pathogens: Quantitative Analysis of Conidia Released from Single Colonies of Podosphaera xanthii Parasitised by Ampelomyces" Agronomy 13, no. 5: 1204. https://doi.org/10.3390/agronomy13051204
APA StyleKimura, Y., Németh, M. Z., Numano, K., Mitao, A., Shirakawa, T., Seress, D., Takikawa, Y., Kakutani, K., Matsuda, Y., Kiss, L., & Nonomura, T. (2023). Hyperparasitic Fungi against Melon Powdery Mildew Pathogens: Quantitative Analysis of Conidia Released from Single Colonies of Podosphaera xanthii Parasitised by Ampelomyces. Agronomy, 13(5), 1204. https://doi.org/10.3390/agronomy13051204







