Abstract
Transgenic Bt rice (abbr. Bt rice) has provided a powerful tactics to control the striped stemborer Chilo suppressalis as one key lepidopteran pest in the paddyfields of China. Globally rising carbon dioxide (i.e., CO2) concentration has been predicted to affect the Cry protein contents in plant tissues of Bt rice and thus might affect its control efficiency to target insect pests. To reveal the resistance ability and the corresponding mechanism of C. suppressalis to Bt rice during different growth stage under elevated CO2 (eCO2), we carried out this experiment to measure the Bt toxin contents in Bt rice stems grown under ambient CO2 (aCO2) (400 ppm) and eCO2 (800 ppm) at seedling, tillering and heading stages, and to observe the larval mortality and bioassay the activity of midgut protease and the expression levels of Bt-toxin-receptor genes, aminopeptidases (APNs) in C. suppressalis larvae. Compared with aCO2, eCO2 increased the Bt-toxin content of Bt rice at seedling stage (+6.66%), and decreased that at heading stages (−13.99%), and significantly reduced the Bt-toxin content at tillering stage (−15.21%). And the larval mortality of C. suppressalis was lower as reared with Bt rice stems during tillering stage grown under eCO2 in contrast to aCO2. In addition, eCO2 significantly increased the activity of total protease, tryptase-like enzyme and aminopeptidase of C. suppressalis larvae fed on Bt rice during seedling stage, and significantly reduced the activity of tryptase-like enzyme and aminopeptidase of C. suppressails larvae fed on Bt rice during tillering and heading stages respectively. Moreover, eCO2 significantly increased the expression level of APN1 and APN5 of C. suppressails larvae fed on Bt rice during seedling stage, and significantly reduced the expression level of APN5 of C. suppressalis larvae fed on Bt rice during tillering and heading stages respectively. In summary, the control efficiency of Bt rice to target insect pests under eCO2 showed a downward trend during tillering and heading stages, and especially during tillering stage.
1. Introduction
The striped stemborer, Chilo suppressalis Walker (Lepidoptera: Pyralidae) is one of the key insect pests to rice production in Asia [1]. To control this insect pest, the genetically engineered rice plants producing Cry proteins derived from Bacillus thuringiensis (Bt) have been developed to control these target pests [2,3,4]. The transgenic Bt rice (abbr. Bt rice) expressing Cry1C, Cry1Ab, Cry1Ac, Cry2A genes and the fusion gene Cry1Ab/Ac have been proven remarkably effective in the control of target lepidopteran pests though previous laboratory and field experiments [5,6,7].
The action mode of Cry protoxins in the susceptible lepidopteran larvae is that the Bt toxins are dissolved and released in the midgut, and then the released protoxins are cleaved by midgut proteinases (e.g., trypsins, chymotrypsins) into a stable toxin core, and the toxin core is capable of binding to specific receptors on the midgut epithelium, which is recognized as an essential step for toxicity, and these events promote the toxin oligomerization and lead to the formation of toxin pores causing lysis of osmotic cells [8,9,10]. In this series of events, any physiological modification can decrease insect susceptibility to Cry toxins. For example, the significantly decreased activity of chymotrypsin-like enzyme in Bt-resistant Plodia interpunctella larvae were resulted in the decreased activation of Cry protoxins [11].
To date, several specific receptors to Bt toxins in target insect pests have been identified, including cadherin (Cad) proteins [12], alkaline phosphatases [13], glycolipids [14] and aminopeptidases (APNs) [15]. Toxin receptors mutations, such as deletion mutation and down-regulated of gene expression level, were associated with toxin resistance in target insect pests [16,17]. For instance, the lacking of APN1 expression influenced the resistance of Spodoptera exigua colony to Cry1Ca [18]. Some documents showed that the silencing of APNs expression in C. suppressalis larvae by RNA interference (RNAi) reduced the larval susceptibility to Cry1Ab, Cry1Ac and Cry1C [19,20]. In addition, the silencing of expression of CADs in C. suppressalis larvae by RNAi decreased the larval susceptibility to Cry2Aa and Cry1Ca [21].
Globally atmospheric CO2 level has risen steadily and is expected doubling (about 800 ppm) by the end of this century [22]. Generally, those plants grown under elevated CO2 (eCO2) were found to have higher photosynthetic rate, biomass and carbon: nitrogen (C: N) ratio [23,24,25]. Under eCO2, the assimilation and re-assignment of C and N resources in plant tissues of transgenic Bt crops will alter the Bt-toxin content, and further alter the control efficiency to target insect pests [26,27,28]. In previous studies, we found that the foliar Cry1Ab/1Ac content in Bt rice (cv. HH1 with Cry1Ab + Cry1Ac) during the tillering stage was significantly lower as grown under eCO2 in contrast to ambient CO2 (aCO2) [29]. However, during different growth stage (e.g., seedling, tillering or heading stage) for Bt rice grown under eCO2, how the changes of Bt-toxin content in plant tissues and how will the target insect, C. suppressalis larvae respond to the change of Bt protein content and the responsive mechanism are still unclear.
In this study, we carried out a series of experiments to measure the Bt-toxin content of Bt rice grown under aCO2 (400 ppm) and eCO2 (800 ppm) at seedling, tillering and heading stages, and observe the mortality of the target insect pest, C. suppressalis and bioassay its midgut protease activity and the expression level of Bt-toxin-receptor genes (APNs) in order to understand the physiological responses and the responsive mechanism of C. suppressalis larvae to Bt rice grown under eCO2.
2. Materials and Methods
2.1. Plants Growth Conditions
This research was performed in the artificial climate chambers (GDN-400D-4/CO2, Ningbo Southeast Instrument CO., Ningbo, China) connected with CO2 tanks for maintaining the desired CO2 level. The temperature in the chambers was maintained at 28 °C (day) and 25 °C (night) under a 16L: 8D photoperiod. Two CO2 levels were set at aCO2 (400 ppm) and eCO2 (800 ppm) with three artificial climate chambers for each CO2 treatment.
2.2. Plants and Insects
Bt rice (cv. HH1 with a fused Cry1Ab/CryAc gene driven by the actin-I promoter) and its corresponding non-Bt rice (cv. MH63) were tested in this study. The seeds of these two rice cultivars were kindly provided by Prof. Yongjun Lin and Hongxia Hua (Huazhong Agricultural University of China). The seeds were soaked in water for 1 d, and sprouted on a board covered with wet cotton gauze for 1 d, and then the rice seeds were sown into plastic foam covering (0.6 cm thick) on plastic boxes (22 cm length: 15 cm width: 12 cm height) and placed in the artificial climate chambers (GDN-400D-4/CO2, Ningbo, China) of aCO2 and eCO2 treatments. Eight plastic boxes with 8 plants per box during seedling and tillering stages were placed in each artificial climate chambers, and 4 plastic boxes with 8 plants per box were maintained in each artificial climate chambers during the heading stage. The boxes were filled with modified culture solution and the solution was replaced with fresh solution every day [29].
The larvae of C. suppressalis larvae were obtained from a laboratory colony that collected from paddy fields in Nanjing (China) in 2019. The larvae were kept at 27 ± 2 °C and 70 ± 10% RH under a 16: 8 h light/dark photoperiod in the artificial climate chambers, and maintained on an artificial diet as previously described [30].
2.3. Measurement of Bt-Toxin Content in Bt Rice Plants
At the seedling, tillering and heading stages, 5 stems of Bt rice (cv. HH1) and non-Bt rice (cv. MH63) grown under aCO2 and eCO2 were randomly selected from each artificial climate chamber to measure the Bt-toxin content by using the ELISA kit (EnviroLogix, Portland, ME, USA; catalog number AP003), respectively. The samples were respectively put into 2 mL tube with two steel balls, and homogenized in a Tissue Lyser II (Qiagen, Haan, Germany) by shaking for 2 min at 30 Hz, and then mixed with extraction buffer PBST. The Bt protein content were measured as dictated in the kit instruction.
2.4. Bioassay of Tested Insect Larvae
The 4th instar larvae of C. suppressalis fed on the stems of Bt rice (cv. HH1) and non-Bt rice (cv. MH63) grown under aCO2 and eCO2, were used for the following bioassay of C. suppressalis larvae. The stems of Bt rice and non-Bt rice were randomly selected at the seeding, tillering and heading stages, which were cut into 6 to 7-cm fragments. A thin layer of moist cotton wool was put on the bottom of a 9 cm-diameter Petri dish, and a moistened 8 cm-diameter filter paper was placed on the cotton layer. The rice stems and one 4th instar larvae of C. suppressalis were placed in a Petri dish, which was subsequently sealed with Parafilm. The Petri dishes were covered with black cloth and placed in the artificial climate chambers.
Each combination of CO2 levels (ambient vs. elevated), rice cultivars (HH1 vs. MH63) and growth stages (seedling, tillering and heading) were carried out four replicates, and each replicate contained 30 larvae of C. suppressalis. The mortality and body weight of C. suppressalis larvae were recorded every day and the rice stem fragments were changed every day. The bioassay tests were terminated until the pupation of C. suppressalis larvae.
2.5. Analysis of Midgut Enzyme Activity of C. suppressalis Larvae
Forty 4th instar larvae of C. suppressalis were individually put into each Petri dish (9 cm diameter: 2.5 cm height) and fed on the stem fragments of Bt rice (cv. HH1) and non-Bt rice (cv. MH63) grown under aCO2 and eCO2, respectively. Each combination of CO2 levels (ambient vs. elevated), rice cultivars (HH1 vs. MH63) and growth stages (seedling, tillering and heading) had 40 Petri dishes. After 1, 2, 3, 4 days of feeding exposure, the midgut of 10 larvae of C. suppressalis was daily dissected and washed with ice-cold 0.7% NaCl solution, and immediately stored at −80 °C for the following RNA extraction.
Prior to analysis, the midgut samples of C. suppressalis larvae were put in 1 mL 0.15 mol/L NaCl, and then homogenized at 4 °C and centrifuge at 15,000× g for 10 min. The supernatant was collected and the protein concentration was quantified using bovine serum albumen (BSA) as the standard method introduced by Bradford [31]. The activity of midgut protease was measured similar to that described by Zhou et al. [32] and Wang et al. [33].
The substrate of azocasein (2 mg/mL in 0.15 mol/L NaCl) was used to measure the total protease activity in the larval midgut of C. suppressalis. Ten μL enzyme sample, 100 μL azocasein solution (2 mg/mL in 0.15 mol/L NaCl) and 40 μL 0.1 mol/ L Glycine-NaOH Buffer (pH 10.0) were added into the centrifuge tube and reacted at 30 °C for 1 h. and 150 μL pre-cold 10% trichloroacetic acid was added into the tube to terminate the reaction. The solution was centrifuge at 12,000 rpm for 15 min, and the supernatant was mixed with equal volume 0.1 mol/L glycine-NaOH Buffer. Then the absorbance was measured at 415 nm.
N-α-benzoyl-DL-arginine-p-nitroanilide (BApNA) and N-succinyl-(Ala)2-Pro-Phe-p-nitroanilide (SAAPFpNA) were used as substrate to measure the activity of typsin and chymotrypsin, respectively. BApNA (50 mg/mL in DMSO) and (SAAPFpNA) (50 mg/mL in DMF) was respectively diluted to 1.0 mg/mL with universal buffer. BApNA (100 μL) and SAAPFpNA (90 μL) solution was respectively added into individual wells containing enzyme sample and Glycine-NaOH Buffer, and the reaction product absorbance was measured at 405 nm in 10 s intervals for 15 min.
Aminopeptidase (APN) activity was assayed using 10 μL enzyme sample with 100 μL 1 mM L-leucine p-nitroanilide (L-pNA) and 140 μL 50 mM Tris–HCl (pH 8.0) buffer. Then the the reaction product absorbance was measured using a UV-Vis spectrophotometer at 410 nm in 30 s intervals for 10 min.
2.6. Quantitative RT-PCR of APNs in C. suppressalis Larvae
Total RNA was extracted from the larval midgut of C. suppressalis using TRIzol reagent (Invitrogen, CA, USA) according to the kit instruction and treated with DNase I (Takara, Kyoto, Japan) to remove the DNA. Then the first-strand cDNA was immediately synthesized using the PrimeScriptTM RT Reagent Kit with gDNA Eraser (Takara, Kyoto, Japan). The sequences encoding aminopeptidase genes (i.e., APN) of C. suppressalis larvae were obtained from GenBank, including APN1 (GenBank No. DQ342305.3), APN3a (GenBank No. JF519739.1), APN3b (GenBank No. JQ088280.1), APN4 (GenBank No. HQ901596.1) and APN5 (JQ088281.1). The specific primers for the APNs in C. suppressalis larvae were designed by the Primer Premier 5.0 software (Premier Biosoft International, Palo Alto, CA, USA), and the reference gene of elongation factor (EF) and β-Actin1 were used as the internal standard to analyze the expression level of the target genes (Table 1). The expression level of APNs was quantified following the 2−ΔΔCt normalization method, respectively [34]. Three technical replicates were also performed for each cDNA sample.

Table 1.
Primers used to measure the transcript expression of APNs in the larvae of the rice stemborer Chilo suppressalis in the quantitative real time PCR.
2.7. Data Analysis
The statistical analysis was performed using SPSS 20.0 (Chicago, IL, USA). Two-way analysis of variances (ANOVAs) were used to analyze the effects of CO2 level (ambient vs. elevated), rice growth stage (seedling, tillering and heading) and their interaction on Bt content of Bt rice. And three-way repeated-measures ANOVAs were used to analyze the effects of CO2 levels, rice cultivars (Bt rice vs. non-Bt rice), growth stages and their interactions on the mortality, enzyme activity, and the transcript expression level of APNs in the midguts of C. suppressalis larvae with feeding time as the repeated measures, and the univariate-ANOVA was also used to analyze the effects of CO2 levels, rice cultivars, growth stages and their interactions on the mortality enzyme activity, and the transcript expression level of APNs in the midguts of C. suppressalis larvae with feeding time as the repeated measures. The Student’s t-test was performed to analyze significantly difference between aCO2 and eCO2, and between Bt and non-Bt rice at p < 0.05, and the Turkey test was performed to analyze significantly different among three rice growth stages at p < 0.05.
3. Results
3.1. Bt Content in Bt Rice Stems Grown under aCO2 and eCO2 during Seedling, Tillering and Heading Stages
CO2 level (F = 5.59, p = 0.027) and its interaction with rice growth stage (F = 6.26, p = 0.006) both significantly impacted the Bt content in Bt rice stems, and there were significant differences among the seedling, tillering and heading stages (F = 50.81, p < 0.001; Table 2). Compared with aCO2, eCO2 significantly reduced the Bt content in Bt rice stems during tillering stage (−15.21%, p < 0.05), and also decreased the Bt content during heading stages (−13.99%, p > 0.05), while increased the Bt content during seedling stages (+6.66%, p > 0.05; Figure 1). The Bt content in Bt rice stems continuously decreased with the plant growth from seedling stage to heading stage under aCO2 and eCO2 (Figure 1). Under aCO2, the Bt content during heading stage was significantly lower than that during seedling stage (−23.08%) and tillering stage (−17.45%), respectively (p < 0.05; Figure 1). Under eCO2, the Bt content during tillering stage (−25.93%) and heading stage (−37.97%) was significantly lower than that during seedling stage respectively (p < 0.05; Figure 1), and the Bt content during heading stage was significantly lower than that during tillering stage (−16.26%; p < 0.05, Figure 1).

Table 2.
Two-way ANOVAs of CO2 levels (ambient vs. elevated), rice growth stages (seedling, tillering and heading) and their interaction on Bt-toxin content in stems of transgenic Bt rice stems (abbr. Bt rice); and three-way repeated-measures ANOVAs of CO2 levels, rice cultivars (Bt rice vs. non-Bt rice), growth stages and their interactions with feeding time as the repeated measures on the measured indexes of enzyme activity and the transcript expression level of APNs in the midguts of rice stemborer, C. suppressalis larvae (F/p values).
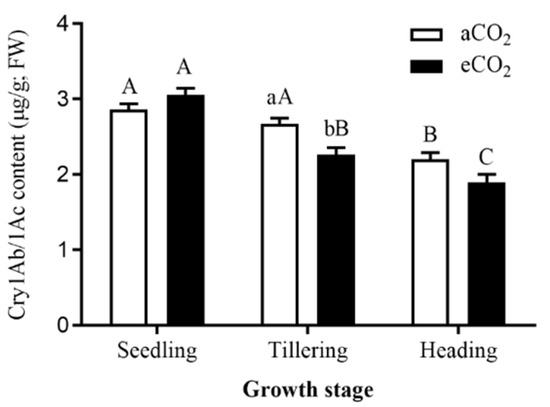
Figure 1.
Bt-toxin content in the stems of transgenic Bt rice (cv. HH1) grown under ambient CO2 (aCO2) and elevated CO2 (eCO2) during seedling, tillering and heading stages. Note: Different lowercase letters indicated significantly different between aCO2 and eCO2, at same rice growth stage by the Student’s t test at p < 0.05, and different uppercase letters indicated significantly different among three growth stages under same CO2 level by the Tukey-test at p < 0.05.
3.2. Mortality of C. suppressalis Larvae Fed on Bt and Non-Bt Rice Plants Grown under aCO2 and eCO2
The larval mortality of C. suppressalis was significantly affected by rice cultivar (F = 567.64, p < 0.001), growth stage (F = 11.81, p < 0.001) and their interactions (F = 3.25, p = 0.039) (Table 2). There was no C. suppressalis larvae survived to pupal stage as they fed on Bt rice stems grown under aCO2 and eCO2 during seedling, tillering and heading stages (Figure 2). The mortality of C. suppressalis larvae fed on Bt rice stems was significantly higher than that of C. suppressalis larvae fed on non-Bt rice stems grown under aCO2 and eCO2 during seedling, tillering and heading stages respectively (p < 0.05; Figure 2). And the mortality of C. suppressalis larvae under aCO2 was significantly higher as fed on Bt rice stems during seedling stage in contrast to tillering and heading stages, respectively (p < 0.05; Figure 2).
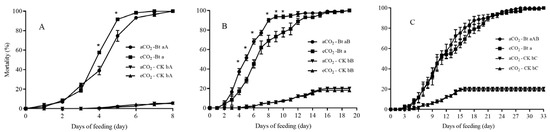
Figure 2.
Larval mortality of the rice stemborer, Chilo suppresalis fed on the stems of Bt rice (cv. HH1) and non-Bt rice (cv. MH63) grown under aCO2 and eCO2 during seedling (A), tillering (B) and heading (C) stages. Note: * and different lowercase letters indicated significantly difference between aCO2 and eCO2 for same rice cultivar and at same rice growth stage, and between Bt rice and non-Bt rice grown under same CO2 level and at same rice growth stage by the Student’s test at p < 0.05, respectively; Different uppercase letters indicated significantly difference among different rice growth stages for same rice and under same CO2 level by the Tukey test at p < 0.05.
The mortality of C. suppressalis larvae fed on Bt rice grown under eCO2 was significantly higher than that under aCO2 during seedling stage for 4 and 5 days respectively (p < 0.05; Figure 2A). After fed on Bt rice grown under eCO2 for 4, 5, 6, 8, 9 and 10 days during tillering stage, the larval mortality of C. suppressalis was significantly lower than that under aCO2 respectively (p < 0.05; Figure 2B). After fed on Bt rice grown under aCO2 for 28 days during heading stage, the mortality of C. suppressalis larvae reached 100%, and the larval mortality of C. suppressalis reached 100% after fed on Bt rice grown under eCO2 for 33 days (Figure 2C).
3.3. Enzyme Activity in C. suppressalis Larvae Fed on the Stems of Bt and Non-Bt Rice Plants Grown under aCO2 and eCO2
3.3.1. Total Protease
The total protease activity in C. suppressalis larvae was significantly affected by rice cultivar (p < 0.001), growth stage (p < 0.001) and their interaction (p < 0.001), and the interactions between CO2 level and rice cultivar (p < 0.001), and among CO2 level, rice cultivar and growth stage (p = 0.006) (Table 2). Compared with aCO2, eCO2 significantly increased the activity of total protease in C. suppressalis larvae during seedling stage (p < 0.05, Figure 3A) and significantly decreased that during tillering stage (p < 0.05, Figure 3C) when they fed on Bt rice for 4 days.
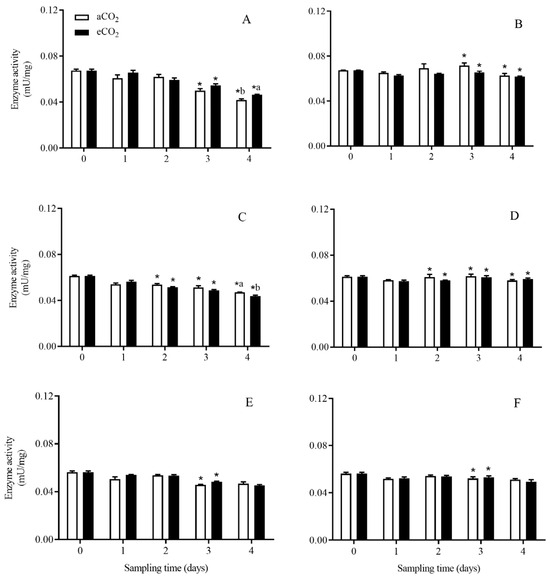
Figure 3.
The activity of total protease in C. suppressalis larvae fed on Bt rice and non-Bt rice grown under aCO2 and eCO2 for 0, 1, 2, 3 and 4 days, during seedling, tillering and heading stages. Note: (A,B)-seedling stage; (C,D)-tillering stage; (E,F)-heading stage; (A,C,E)-Bt rice, (B,D,F)-non-Bt rice. Each value represents means ± SE. *, different lowercase letters indicate significant difference between Bt and non-Bt rice grown under same CO2 level and at same rice growth stage, and between aCO2 and eCO2 for same rice cultivar and at same rice growth stage by the Student’s t-test at p < 0.05.
The activity of total protease in C. suppressalis larvae fed on Bt rice was significantly lower than that in C. suppressalis larvae fed on non-Bt rice grown under aCO2 and eCO2 during seedling stage for 3 and 4 days (p < 0.05; Figure 3A,B), and during tillering stage for 2, 3 and 4 days (p < 0.05; Figure 3C,D) and during heading stage for 3 days (p < 0.05; Figure 3E,F), respectively.
3.3.2. Trypsin-like Enzyme
The activity of trypsin-like enzyme in C. suppressalis larvae was significantly affected by rice cultivar (p < 0.001), growth stage (p < 0.001) and their interaction (p = 0.003), and the interactions between CO2 level and rice cultivar (p = 0.040), and among CO2 level, rice cultivar and growth stage (p = 0.004) (Table 2). Compared with aCO2, eCO2 significantly increased the trypsin-like enzyme activity in C. suppressalis larvae fed on Bt rice during seedling stage for 3 and 4 days (p < 0.05; Figure 4A), and significantly decreased that during tillering stage for 3 and 4 days (p < 0.05; Figure 4C) and during heading stage for 4 days, respectively (p < 0.05; Figure 4E). Moreover, the trypsin-like enzyme activity in C. suppressalis larvae fed on Bt rice was significantly lower than that in C. suppressalis larvae fed on non-Bt rice during seedling stage for 4 days under aCO2 (p< 0.05, Figure 4A,B), and during tillering stage for 3 and 4 days under eCO2 (p < 0.05, Figure 4C,D).
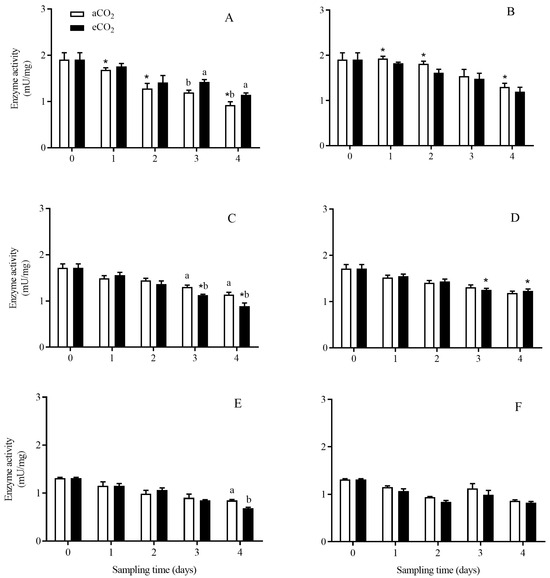
Figure 4.
The activity of trypsin-like enzyme in C. suppressalis larvae fed on Bt and non-Bt rice plants grown under aCO2 and eCO2 for 0, 1, 2, 3 and 4 days, during seedling, tillering and heading stages. Note: (A,B)—seedling stage; (C,D)—tillering stage; (E,F)—heading stage; (A,C,E)—Bt rice, (B,D,F)—non-Bt rice. Each value represents means ± SE. *, different lowercase letters indicate significant difference between Bt and non-Bt rice grown under same CO2 level and at same rice growth stage, and between aCO2 and eCO2 for same rice cultivar and at same rice growth stage by the Student’s t-test at p < 0.05.
3.3.3. Chymotrypsin-like Enzyme
The activity of chymotrypsin-like enzyme in C. suppressalis larvae was significantly affected by rice cultivar (p < 0.001) and growth stage (p < 0.001; Table 2). Compared with aCO2, eCO2 significantly decreased the chymotrypsin-like enzyme activity in C. suppressalis larvae fed on Bt rice during seedling stage for 4 days (p < 0.05, Figure 5A). The activity of chymotrypsin-like enzyme in C. suppressalis larvae fed on Bt rice was significantly lower than that in C. suppressalis larvae fed on non-Bt rice during tillering stage for 2 days (p < 0.05; Figure 5C,D).
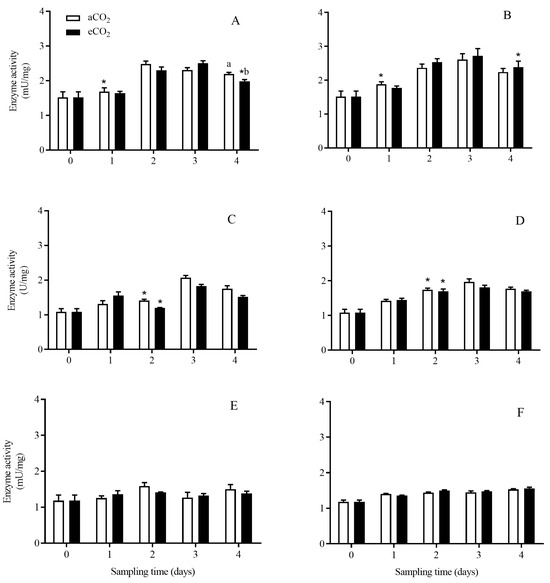
Figure 5.
The activity of chymotrypsin-like enzyme in C. suppressalis larvae fed on Bt and non-Bt rice plants grown under aCO2 and eCO2 for 0, 1, 2, 3 and 4 days, during seedling, tillering and heading stages. Note: (A,B)—seedling stage; (C,D)—tillering stage; (E,F)—heading stage; (A,C,E)—Bt rice, (B,D,F)—non-Bt rice. Each value represents means ± SE. *, different lowercase letters indicate significant difference between Bt and non-Bt rice grown under same CO2 level and at same rice growth stage, and between aCO2 and eCO2 for same rice cultivar and at same rice growth stage by the Student’s t-test at p < 0.05.
3.3.4. Aminopeptidase
The activity of aminopeptidase in C. suppressalis larvae was significantly affected by rice cultivar (p < 0.001), growth stage (p < 0.001) and their interaction (p < 0.001), and the interaction among CO2 level, rice cultivar and growth stage (p = 0.022) (Table 2). Compared with aCO2, eCO2 significantly increased the aminopeptidase activity in C. suppressalis larvae fed on Bt rice during seedling stage for 2 and 4 days (p < 0.05; Figure 6A), and significantly decreased that during tillering stage for 3 and 4 days (p < 0.05; Figure 6C) and that during heading stage for 4 days (p < 0.05; Figure 6E), respectively. The activity of aminopeptidase in C. suppressalis larvae fed on Bt rice grown under aCO2 and eCO2 during seedling and heading stage for 4 days, and that during tillering stage for 2 days was significantly higher than that in C. suppressalis larvae fed on non-Bt rice, respectively (p < 0.05; Figure 6). While the aminopeptidase activity in C. suppressalis larvae fed on Bt rice was significantly lower than that in C. suppressalis larvae fed on non-Bt rice grown under eCO2 during tillering stage for 4 days (p < 0.05; Figure 6C,D).
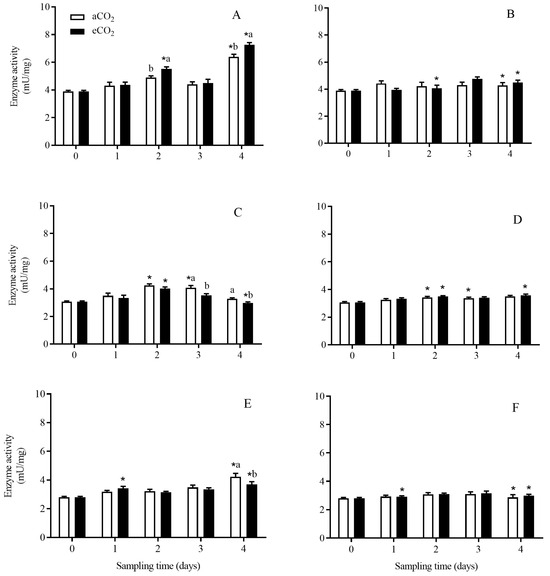
Figure 6.
The activity of aminopeptidase enzyme in C. suppressalis larvae fed on Bt and non-Bt rice plants grown under aCO2 and eCO2 for 0, 1, 2, 3 and 4 days, during seedling, tillering and heading stages. Note: (A,B)—seedling stage; (C,D)—tillering stage; (E,F)—heading stage; (A,C,E)—Bt rice, (B,D,F)—non-Bt rice. Each value represents means ± SE. *, different lowercase letters indicate significant difference between Bt and non-Bt rice grown under same CO2 level and at same rice growth stage, and between aCO2 and eCO2 for same rice cultivar and at same rice growth stage by the Student’s t-test at p < 0.05.
3.4. The Transcript Expression Level of APNs in C. suppressalis Larvae Fed on the Stems of Bt and Non-Bt Rice Plants Grown under aCO2 and eCO2
3.4.1. APN1
The expression level of APN1 in C. suppressalis larvae was significantly affected by rice cultivar (p < 0.001), growth stage (p < 0.001) and their interaction (p < 0.001), and the interaction among CO2 level, rice cultivar and growth stage (p = 0.020) (Table 2). Compared with aCO2, eCO2 significantly increased the expression level of APN1 in C. suppressalis larvae fed on Bt rice during seedling stage for 4 days (p < 0.05, Figure 7A) and significantly decreased that during tillering stage for 4 days (p < 0.05, Figure 7C). The APN1 expression level in C. suppressalis larvae fed on Bt rice was significantly higher than that in C. suppressalis larvae fed on non-Bt rice during seedling stage (aCO2: 3 d; eCO2: 1, 2, 3 and 4 d; p < 0.05, Figure 7A,B), tillering stage under aCO2 for 4 days (p < 0.05; Figure 7C,D) and heading stage under aCO2 and eCO2 for 4 days (p < 0.05; Figure 7E,F), respectively.
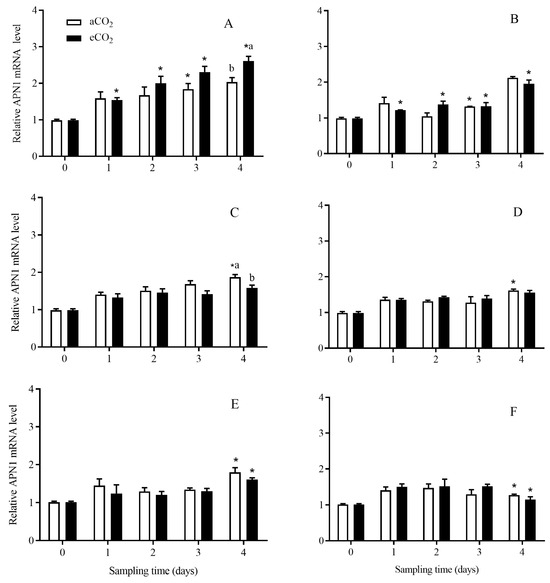
Figure 7.
The APN1 expression level in C. suppressalis larvae fed on Bt and non-Bt rice plants grown under aCO2 and eCO2 for 0, 1, 2, 3 and 4 days, during seedling, tillering and heading stages. Note: (A,B)—seedling stage; (C,D)—tillering stage; (E,F)—heading stage; (A,C,E)—Bt rice, (B,D,F)—non-Bt rice. Each value represents means ± SE. *, different lowercase letters indicate significant difference between Bt and non-Bt rice grown under same CO2 level and at same rice growth stage, and between aCO2 and eCO2 for same rice cultivar and at same rice growth stage by the Student’s t-test at p < 0.05.
3.4.2. APN3a
The expression level of APN3a in C. suppressalis larvae was significantly affected by rice cultivar (p = 0.040), growth stage (p = 0.005) and their interaction (p = 0.049) (Table 2). Compared with aCO2, eCO2 significantly reduced the expression level of APN3a in C. suppressalis larvae fed on non-Bt rice during seedling and tillering stage for 4 days, respectively (p < 0.05; Figure 8B,D). The APN3a expression level in C. suppressalis larvae fed on Bt rice was significantly higher than that in C. suppressalis larvae fed on non-Bt rice during seedling stage for 1 day under eCO2 (Figure 8A,B), tillering stage for 4 days under eCO2 (p < 0.05; Figure 8C,D) and heading stage for 4 days under aCO2 (p < 0.05; Figure 8E,F), respectively.
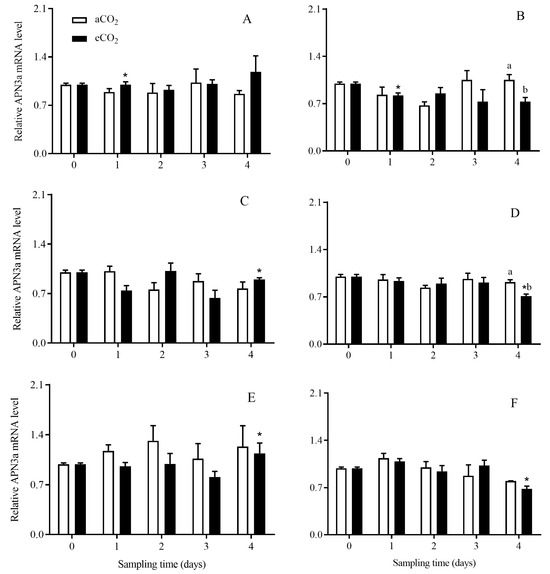
Figure 8.
The APN3a expression level in C. suppressalis larvae fed on Bt and non-Bt rice plants grown under aCO2 and eCO2 for 0, 1, 2, 3 and 4 days, during seedling, tillering and heading stages. Note: (A,B)—seedling stage; (C,D)—tillering stage; (E,F)—heading stage; (A,C,E)—Bt rice, (B,D,F)—non-Bt rice. Each value represents means ± SE. *, different lowercase letters indicate significant difference between Bt and non-Bt rice grown under same CO2 level and at same rice growth stage, and between aCO2 and eCO2 for same rice cultivar and at same rice growth stage by the Student’s t-test at p < 0.05.
3.4.3. APN3b
The expression level of APN3b in C. suppressalis larvae was significantly affected by rice cultivar (p < 0.001; Table 2). The APN3b expression level in C. suppressalis larvae fed on Bt rice was significantly lower than that in C. suppressalis larvae fed on non-Bt rice during seedling stage for 4 days under aCO2 (p < 0.05; Figure 9A), tillering stage for 3 days under eCO2 (p < 0.05, Figure 9C,D) and heading stage for 4 days under eCO2 (p < 0.05; Figure 9E), respectively.
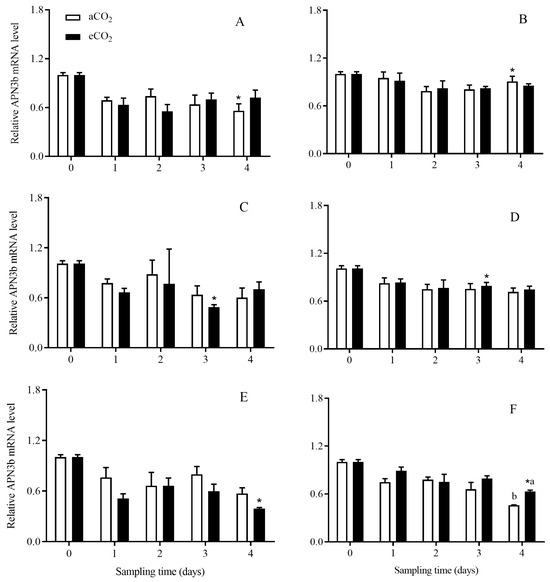
Figure 9.
The APN3b expression level in C. suppressalis larvae fed on Bt and non-Bt rice plants grown under aCO2 and eCO2 for 0, 1, 2, 3 and 4 days, during seedling, tillering and heading stages. Note: (A,B)—seedling stage; (C,D)—tillering stage; (E,F)—heading stage; (A,C,E)—Bt rice, (B,D,F)—non-Bt rice. Each value represents means ± SE. *, different lowercase letters indicate significant difference between Bt and non-Bt rice grown under same CO2 level and at same rice growth stage, and between aCO2 and eCO2 for same rice cultivar and at same rice growth stage by the Student’s t-test at p < 0.05.
3.4.4. APN4
The expression level of APN4 in C. suppressalis larvae was significantly affected by rice cultivar (p = 0.023), growth stage (p < 0.001), and the interaction among CO2 level, rice cultivar and growth stage (p = 0.037) (Table 2). Compared with aCO2, eCO2 significantly increased the APN4 expression level in C. suppressalis larvae fed on Bt rice during seedling stage for 3 days (p < 0.05; Figure 10A), and significantly decreased that during heading stage for 3 days (p < 0.05; Figure 10E). The APN4 expression level in C. suppressalis larvae fed on Bt rice was significantly lower than that in C. suppressalis larvae fed on non-Bt rice grown under aCO2 during seedling stage for 1, 3 and 4 days, respectively (p < 0.05; Figure 10A,B).
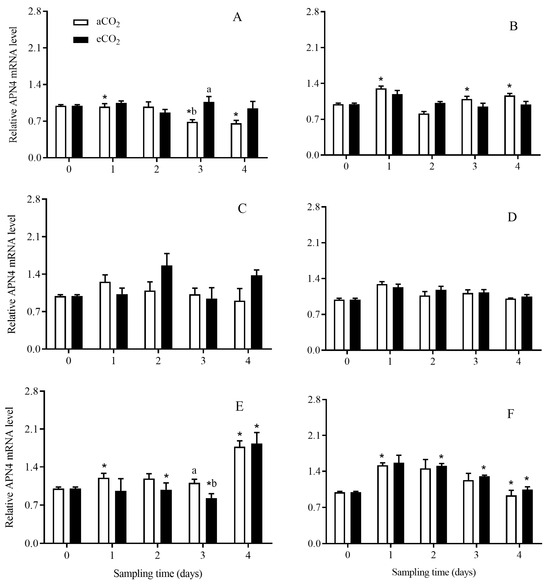
Figure 10.
The APN4 expression level in C. suppressalis larvae fed on Bt and non-Bt rice plants grown under aCO2 and eCO2 for 0, 1, 2, 3 and 4 days, during seedling, tillering and heading stages. Note: (A,B)—seedling stage; (C,D)—tillering stage; (E,F)—heading stage; (A,C,E)—Bt rice, (B,D,F)—non-Bt rice. Each value represents means ± SE. *, different lowercase letters indicate significant difference between Bt and non-Bt rice grown under same CO2 level and at same rice growth stage, and between aCO2 and eCO2 for same rice cultivar and at same rice growth stage by the Student’s t-test at p < 0.05.
3.4.5. APN5
The expression level of APN5 in C. suppressalis larvae was significantly affected by CO2 level (p = 0.003), rice cultivar (p < 0.001), growth stage (p = 0.014), and the interactions between CO2 level and rice cultivar (p = 0.004), between CO2 level and growth stage (p < 0.001), between rice cultivar and growth stage (p < 0.001), and among CO2 level, rice cultivar and growth stage (p = 0.003) (Table 2). Compared with aCO2, eCO2 significantly enhanced the APN5 expression level in C. suppressalis larvae fed on Bt rice for 3 and 4 days during seedling stage (p < 0.05; Figure 11A), and significantly decreased that during tillering stage for 3 and 4 days (p < 0.05; Figure 11C) and heading stage for 4 days (p < 0.05; Figure 11E), respectively. The expression level of APN5 in C. suppressalis larvae fed on Bt rice was significantly higher than that in C. suppressalis larvae fed on non-Bt rice during seedling stage for 1, 2, 3 and 4 days under aCO2 and eCO2 (p < 0.05; Figure 11A,D), during tillering stage (aCO2: 1, 2, 3 and 4 d; eCO2: 1, 2, 3 d; p < 0.05, Figure 11C,D) and heading stage (aCO2: 1 and 4 d; eCO2:1, 3 and 4 d; p < 0.05, Figure 11E,F), respectively.
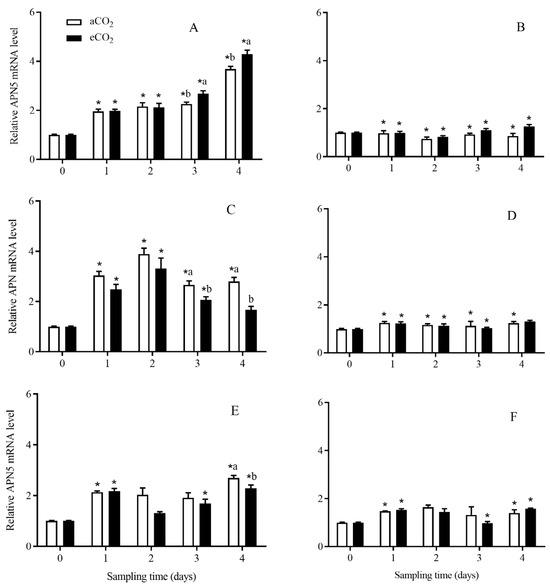
Figure 11.
The APN5 expression level in C. suppressalis larvae fed on Bt and non-Bt rice plants grown under aCO2 and eCO2 for 0, 1, 2, 3 and 4 days, during seedling, tillering and heading stages. Note: (A,B)—seedling stage; (C,D)—tillering stage; (E,F)—heading stage; (A,C,E)—Bt rice, (B,D,F)—non-Bt rice. Each value represents means ± SE. *, different lowercase letters indicate significant difference between Bt and non-Bt rice grown under same CO2 level and at same rice growth stage, and between aCO2 and eCO2 for same rice cultivar and at same rice growth stage by the Student’s t-test at p < 0.05.
4. Discussion
Previous studies showed that Bt-toxin content in plant tissues fluctuates during the growing season of transgenic Bt crops [35,36]. Our results showed that the Bt-toxin content in Bt rice stems were high at seedling and tillering stages, but relatively low at heading stage, which is consistent with the results of Chen et al. [35] and Wang et al. [4], that eCO2 significantly enhanced exogenous toxin amount per stem in seedlings of Bt rice in contrast to aCO2. The decreased Cry protein content in later growth stages may be related to that most of the energy materials in the late stage of Bt rice growth, which were mainly used to synthesize yield materials instead of just Bt protein [37]. In addition, our laboratory bioassay showed that the Bt rice (cv. HH1) had high resistance to the target stemborers, C. suppressalis. The high resistance of Bt rice to C. suppressalis was also indicated in the laboratory and field experiments [4,6]. Thus, our findings confirmed that although the Bt-toxin content in Bt rice stems was relatively lower at the heading stage, it was relatively high during all rice growth stages, which guarantees a high efficiency for consistently controlling of target insect pests throughout the whole growth season.
In this study, the results indicated that eCO2 significantly reduced Bt-toxin content in Bt rice stems during tillering stage compared with aCO2,. In our previous study, we found that the foliar Bt-toxin content of Bt rice grown under eCO2 during tillering stage was significantly lower than that under aCO2 [29]. So the response of Bt rice to eCO2 way be specific in different plant tissues under global climate change. For transgenic Bt cotton, there are many reports describing the depletion of Bt toxin in response to eCO2 levels [38,39]. However, the specific mechanism behind the lower Bt-toxin content in transgneic Bt plants exposed to eCO2 remains unclear [40]. Previous studies showed that the target insect-resistance of Bt rice was positively correlated with the Bt-toxin contents in plant tissues [35,36,41,42]. The mortality of C. suppressalis larvae fed on Bt rice stems during tillering stage grown under eCO2 was lower than that under aCO2, and simultaneously lower Bt-toxin content in Bt rice stems was also detected under eCO2.
Serine proteinases are some common luminal midgut digestive enzymes of dietary nutriens in many species of herbivorous insects including lepidopterans [43]. Protoxins are activated by midgut proteases in caterpillar midgut [44]. Among the insect serine proteinases, midgut trypsin and chymotrypsin are important in both solubilization and activation of Bt toxins [45]. In this study, the results showed that the activity of total protease and tryptase-like enzyme in C. suppressalis larvae fed on Bt rice stems were lower than that in C. suppressalis larvae fed on non-Bt rice stems regardless of CO2 level. The tryptase-like enzyme activity in Mythimna separata larvae fed on Cry1Ac diet was significantly lower than that in M. separata larvae fed on the control diet [46]. The total protease activity of Asian corn borer Ostrinia furnacalis fed on Bt corn was significantly lower than that in O. furnacalis larvae fed on non-Bt corn, and the tryptase-like enzyme activity was significantly higher than that fed on non-Bt corn [47]. The changing trend of proteinase activity may be related to the insects faced with different stress of insecticidal proteins [46]. In addition, some studies focus on the resistance of target insects to Cry toxins, which showed that the resistance can be reduced by the decreased proteinase activity, and could lead to low activation of Bt protoxins to active toxins [48,49,50,51,52]. So it can be presumed that the resistance change of target insects to Bt toxins can also be alterred by the upregulation of midgut proteinases, which causes of the increased Bt-toxin degradation [53].
A key step for the toxicity of Cry toxins is the binding of Cry-toxin core to specific receptors at midgut epithelium of target insects. One recent study shown that the Bt resistance of target insects largely focused on the reduced binding of Bt toxin to protein receptors in the midgut of insects and the reduced binding was resulting from mutation or altered expression of genes encoding aminopeptidase N, cadherin or alkaline phosphatase [44]. In this study, the aminopeptidase activity of C. suppressalis larvae fed on Bt rice increased with the fed time prolonged, and it was higher than that in C. suppressalis larvae fed on non-Bt rice. Similarly, the activity of aminopeptidase activity in Cnaphalocrocis medinalis fed on Bt rice was higher than that in C. medinalis fed on non-Bt rice [54]. Valaitis (2008) found that massive APNs of Lymantria dispar larvae rapidly shed from midgut epithelial cells into the luminal fluid after force-feeding B. thuringiensis, and the shedding APNs might have function as competitive inhibitors, preventing toxin interaction with the cell surface receptors [15]. In this study, the increased aminopeptidase activity of C. suppressalis larvae fed on Bt rice stems may protect themselves by preventing the combination of Bt toxin and aminopeptidase in midgut epithelial cells.
As a type of Bt-toxin receptor, APN is a key factor for Bt-resistance mechanism of target insects. Our result showed that eCO2 significantly increased the expression level of APN5 in C. suppressails larvae fed on Bt rice during seedling stage, and significantly reduced the expression level of APN5 of C. suppressalis larvae fed on Bt rice during tillering and heading stages. The different expression trend of APN5 in C. suppressalis larvae detected as fed on rice plants during different growth stages in this study may be a result of adaptation to the different Bt-toxin content of Bt rice during different growth stage especially under elevated CO2 condition. In addition, APN5 expression level was up-regulated at 24 h after fed on Bt rice during seedling, tillering and heading stages regardless of CO2 level. Consistent with the result of Zhang et al. (2017) that HcAPN3 in Hyphantria cunea was up-regulated at the 24 h after Cry1Ab35 infection [55]. Our results also showed that the gene expression levels varied for the five APNs in C. suppressalis larvae. Yang et al. (2010) founded that the expression level of APN1, 2 and 3 in Diatraea saccharalis was different [56]. The APN1, 2, 3 and 4 expression level of H. armigera was approximately 1000-fold higher than that of APN5 [57].
5. Conclusions
In summary, eCO2 can enhance the Bt-toxin content in Bt rice during seedling stage, and accordingly might enhance the resistance of Bt rice to the target insect pest, C. suppressails during this plant growth stage. During the tillering and heading stages, eCO2 decreased the resistance of Bt rice to C. suppressails due to decreasing of Bt-toxin content in Bt rice stems grown under eCO2 during these plant growth stages. In addition, the activity of tryptase-like enzyme and aminopeptidase, and the expression level of APNs of C. suppressails larvae were changed when they fed on the stems of Bt rice grown under eCO2. Under eCO2, the resistance of Bt rice to C. suppressails showed a decreased trend during tillering and heading stages. It’s necessary to strength monitor and control of these target insect pests especially during the later growth stages of Bt rice under global climate change.
Author Contributions
Methodology, Y.L. and F.C.; Formal analysis, Y.W. and Y.L.; Investigation, Y.L., Y.W., G.C., S.L. and B.X.; Writing-original draft, Y.L. and G.C.; Writing-review and editing, Y.L. and F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Nature Science Foundations of China (NSFC) (31871963), the National Key Research and Development Program of China (2023YFC2604900; 2023YFD1400800), the Special Program for New Transgenic Variety Breeding of the Ministry of Science and Technology, China (2016ZX08012005), the Fundamental Research Funds for the Central Universities (KYZ201818), the Qing-Lan Project of Jiangsu Province of China.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sheng, C.F.; Wang, H.T.; Sheng, S.Y.; Gao, W.D.; Xuan, W.J. Pest status and loss assessment of crop damage caused by the rice borers, Chilo suppressalis and Tryporyza incertulas in China. Entomol. Knowl. 2003, 40, 289–294. [Google Scholar]
- Gao, Y.L.; Fu, Q.; Wang, F.; Lai, F.X.; Luo, J.; Peng, Y.F.; Zhang, Z.T. Effects of transgenic rice harboring cry1Ac and CpTI genes on survival of Chilo suppressalis and Sesamia inferens and field composition of rice stem borers. Chin. J. Rice Sci. 2006, 20, 543–548. [Google Scholar]
- Qi, Y.; Chen, L.; He, X.; Jin, Q.; Zhang, X.; He, Z. Marker-free, tissue-specific expression of Cry1Ab as a safe transgenic strategy for insect resistance in rice plants. Pest Manag. Sci. 2013, 69, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Ke, K.Q.; Li, Y.H.; Han, L.Z.; Liu, Y.M.; Hua, H.X.; Peng, Y.F. Comparison of three transgenic Bt rice lines for insecticidal protein expression and resistance against a target pest, Chilo suppressalis (Lepidoptera: Crambidae). Insect Sci. 2016, 23, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Zhang, G.A.; Datta, K.; Xu, C.G.; He, Y.Q.; Zhang, Q.F.; Khush, G.S.; Datta, S.K. Field performance of transgenic elite commercial hybrid rice expressing Bacillus thuringiensis δ-endotoxin. Nat. Biotechnol. 2000, 18, 1101–1104. [Google Scholar] [CrossRef]
- Li, Z.Y.; Sui, H.; Xu, Y.B.; Han, L.Z.; Chen, F.J. Effects of insect-resistant transgenic Bt rice with a fused cry1Ab+cry1Ac gene on population dynamic of the stem borers, Chilo suppressalis and Sesamia inferens, occurring in paddy field. Acta Ecol. Sin. 2012, 32, 1783–1789. [Google Scholar]
- Liu, Q.S.; Hallerman, E.; Peng, Y.F.; Li, Y.H. Development of Bt rice and Bt maize in China and their efficacy in target pest control. Int. J. Mol. Sci. 2016, 17, 1561. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Cowles, E.A.; Pietrantonio, P.V. The mode of action of Bacillus-thuringiensis endotoxins. Annu. Rev. Entomol. 1992, 37, 615–636. [Google Scholar] [CrossRef]
- Lightwood, D.J.; Ellar, D.J.; Jarrett, P. Role of proteolysis in determining potency of Bacillus thuringiensis Cry1Ac delta-endotoxin. Appl. Environ. Microbiol. 2000, 66, 5174–5181. [Google Scholar] [CrossRef]
- Pardo-López, L.; Soberón, M.; Bravo, A. Bacillus thuringiensis insecticidal three-domain Cry toxins: Mode of action, insect resistance and consequences for crop protection. FEMS Microbiol. Rev. 2013, 37, 3–22. [Google Scholar] [CrossRef]
- Candas, M.; Loseva, O.; Oppert, B.; Kosaraju, P.; Bulla, L.A. Insect resistance to Bacillus thuringiensis. Mol. Cell. Proteom. 2003, 2, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Vadlamudi, R.K.; Weber, E.; Ji, I.; Ji, T.H.; Bulla, J.L.A. Cloning and expression of a receptor for an insecticidal toxin of Bacillus thuringiensis. J. Biol. Chem. 1995, 270, 5490–5494. [Google Scholar] [CrossRef]
- Jurat-Fuentes, J.L.; Adang, M.J. Characterization of a Cry1Ac receptor alkaline phosphatase in susceptible and resistant Heliothis virescens larvae. Eur. J. Biochem. 2004, 271, 3127–3135. [Google Scholar] [CrossRef]
- Griffitts, J.S.; Haslam, S.M.; Yang, T.; Graczynski, S.K.; Mulloy, B.; Morris, H.; Cremer, P.S.; Dell, A.; Adang, M.J.; Aroian, R.V. Glycolipids as receptors for Bacillus thuringiensis crystal toxin. Science 2005, 307, 922–925. [Google Scholar] [CrossRef]
- Valatis, A.P. Bacillus thuringiensis pore-forming to txins trigger massive shedding of GPI-anchored aminopeptidase N from gypsy moth midgut epithelial cells. Insect Biochem. Mol. Biol. 2008, 38, 611–618. [Google Scholar] [CrossRef]
- Gahan, L.J.; Gould, F.; Heckle, D.G. Identification of a gene associated with Bt resistance in Heliothis virescens. Science 2001, 293, 857–860. [Google Scholar] [CrossRef]
- Pigott, C.R.; Ellar, D.J. Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol. Mol. Biol. Rev. 2007, 71, 255–281. [Google Scholar] [CrossRef] [PubMed]
- Herrero, S.; Gechev, T.; Bakker, P.L.; Moar, W.J.; Maagd, R.A. Bacillus thuringiensis, Cry1Ca-resistant Spodoptera exigua, lacks expression of one of four Aminopeptidase N genes. BMC Genom. 2005, 6, 96–105. [Google Scholar] [CrossRef]
- Qiu, L.; Fan, J.X.; Zhang, B.Y.; Liu, L.; Wang, X.P.; Lei, C.L.; Lin, Y.J.; Ma, W.H. RNA interference knockdown of aminopeptidase N genes decrease the susceptibility of Chilo suppressalis larvae to transgenic Cry1Ab/Cry1Ac and Cry1Ca-expressing transgenic rice. J. Invertebr. Pathol. 2017, 145, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Du, L.X.; Liu, C.X.; Gong, L.; Han, L.Z.; Peng, Y.F. RNAi in the striped stem borer, Chilo suppressalis, establishes a functional role for aminopeptidase N in Cry1Ab intoxication. J. Invertebr. Pathol. 2017, 143, 1–10. [Google Scholar] [CrossRef]
- Zhang, T.T.; Coates, B.S.; Wang, Y.Q.; Wang, Y.D.; Bai, S.X.; Wang, Z.Y.; He, K.L. Down-regulation of aminopeptidase N and ABC transporter subfamily G transcripts in Cry1Ab and Cry1Ac resistant Asian corn borer, Ostrinia furnacalis, (Lepidoptera: Crambidae). Int. J. Biol. Sci. 2017, 13, 835–851. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Impacts, Adaptation and Vulnerability. In Working Group II Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change 1132; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising CO2: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Liu, Y.M.; Dang, Z.H.; Wang, Y.; Parajulee, M.N.; Chen, F.J. Interactive effects of CO2 and temperature on plant chemistry of transgenic Bt rice and population dynamics of a non-target planthopper, Nilaparvata lugens (stål) under different levels of soil nitrogen. Toxins 2019, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, M.M.; Martel, A.B.; Strugnell, C.A. Environmental factors regulate plant secondary metabolites. Plants 2023, 12, 447. [Google Scholar] [CrossRef]
- Coviella, C.E.; Stipanovic, R.D.; Trumble, J.T. Plant allocation to defensive compounds: Interactions between elevated CO2 and nitrogen in transgenic cotton plants. J. Exp. Bot. 2002, 53, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Coviella, C.E.; Trumble, J.T. Effect of elevated atmospheric carbon dioxide on the use of foliar application of Bacillus thuringiensis. Biocontrol 2000, 45, 325–336. [Google Scholar] [CrossRef]
- Jiang, S.L.; Lu, Y.Q.; Dai, Y.; Qian, L.; Muhammad, A.B.; Li, T.; Wan, G.J.; Parajulee, M.N.; Chen, F.J. Impacts of elevated CO2 on exogenous Bacillus thuringiensis toxins and transgene expression in transgenic rice under different levels of nitrogen. Sci. Rep. 2017, 7, 14716. [Google Scholar] [CrossRef]
- Liu, Y.M.; Wang, Y.H.; Chen, G.; Li, C.X.; Jiang, S.L.; Parajulee, M.N.; Chen, F.J. Elevated CO2 alters transgene methylation not only in promoterregion but also in codingregion of Bt rice under different N-fertilizer levels. Sci. Rep. 2020, 10, 18138. [Google Scholar] [CrossRef]
- Han, L.Z.; Li, S.B.; Liu, P.L.; Peng, Y.F.; Hou, M.L. New artifcial diet for continuous rearing of Chilo suppressalis (Lepidoptera: Crambidae). Ann. Entomol. Soc. Am. 2012, 105, 253–258. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zhou, Y.C.; Xu, Y.Y.; Chen, X.P.; Han, L.Z.; Jia, B.T.; Peng, Y.F. Comparison of fitness and midgut proteinase activities of Cry1Ac-tolerant and -susceptible Chilo suppressalis (Lepidoptera: Crambidae). J. Environ. Entomol. 2015, 37, 996–1002. [Google Scholar]
- Wang, C.Z.; Qin, J.D. Partial characterization of protease activity in the midgut of Helicoverpa armigera larvae. Acta Entomol. Sin. 1996, 39, 7–14. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.J.; Wu, G.; Ge, F.; Parajulee, M.N. Relationships between exogenous-toxin quantity and increased biomass of transgenic Bt crops under elevated carbon dioxide. Ecotoxicol. Environ. Saf. 2011, 74, 1074–1080. [Google Scholar] [CrossRef]
- Wan, G.J.; Dang, Z.H.; Wu, G.; Parajulee, M.N.; Ge, F.; Chen, F.J. Single and fused transgenic Bacillus thuringiensis rice alter the species-specific responses of non-target planthoppers to elevated carbon dioxide and temperature. Pest Manag. Sci. 2014, 70, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; He, S.; Han, L.Z.; Peng, Y.F. Evaluation of transgenic cry1Ab + cry1C rice lines for its resistance to Chilo suppressalis. Chin. J. Biol. Control. 2018, 34, 71–78. [Google Scholar]
- Chen, F.J.; Wu, G.; Ge, F.; Parajulee, M.N.; Shrestha, R.B. Effects of elevated CO2 and transgenic Bt cotton on plant chemistry, performance, and feeding of an insect herbivore, the cotton bollworm. Entomol. Exp. Appl. 2005, 115, 341–350. [Google Scholar] [CrossRef]
- Wu, G.; Chen, F.J.; Ge, F.; Xiao, N.W. Impacts of elevated CO2 on expression of plant defensive compounds in Bt-transgenic cotton in response to infestation by cotton bollworm. Agric. For. Entomol. 2011, 13, 77–82. [Google Scholar] [CrossRef]
- Girón-Calva, P.S.; Twyman, R.M.; Albajes, R.; Gatehouse, A.M.R.; Christou, P. The impact of environmental stress on Bt crop performance. Trends Plant Sci. 2020, 25, 264–278. [Google Scholar] [CrossRef]
- Han, L.Z.; Liu, P.L.; Wu, K.M.; Peng, Y.F.; Wang, F. Population dynamics of Sesamia inferens on transgenic rice expressing Cry1Ac and CpTI in southern China. Environ. Entomol. 2008, 37, 1361–1370. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li, Y.H.; Zhang, Y.; Chen, Y.; Wu, K.M.; Peng, Y.F.; Guo, Y.Y. Seasonal expression of Bt proteins in transgenic rice lines and the resistance against Asiatic rice borer Chilo suppressalis (Walker). Environ. Entomol. 2011, 40, 1323–1330. [Google Scholar] [CrossRef]
- Terra, W.R.; Ferreira, C. Insect digestive enzymes: Properties, compartmentalization and function. Comp. Biochem. Physiol. 1994, 109, 1–62. [Google Scholar] [CrossRef]
- Pinos, D.; Andrés-Garrido, A.; Ferré, J.; Hernández-Martínez, P. Response mechanisms of invertebrates to Bacillus thuringiensis and its pesticidal proteins. Microbiol. Mol. Biol. Rev. 2021, 85, e00007-20. [Google Scholar] [CrossRef]
- Oppert, B. Protease interactions with Bacillus thuringiensis insecticidal toxins. Arch. Insect Biochem. Physiol. 1999, 42, 1–12. [Google Scholar] [CrossRef]
- Xie, N.; Jiang, X.F.; Luo, L.Z.; Zhang, L. Effects of Cry1Ac toxin on activities of some enzymes in the larval midgut of the oriental armyworm, Mythimna separata (Walker) (Lepidoptera: Noctuidae). Acta Entomol. Sin. 2012, 55, 168–175. [Google Scholar]
- Xu, Y.L.; Wang, Z.Y.; He, K.L.; Bai, S.X. Effects of transgenic Bt corn expressing Cry1Ab toxin on activities of some enzymes in larvae of the Asian corn borer, Ostrinia furnacalis (Guenée) (Lepidoptera: Pyralidae). Acta Entomol. Sin. 2006, 49, 562–567. [Google Scholar]
- Herrero, S.; Oppert, B.; Ferre, J. Different mechanisms of resistance to Bacillus thuringiensis toxins in the Indianmeal moth. Appl. Environ. Microbiol. 2001, 67, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Oppert, B.; Higgins, R.A.; Huang, F.; Bushman, L.L.; Gao, J.R.; Zhu, K.Y. Characterization of cDNAs encoding three trypsin-like proteinases and mRNA quantitative analysis in Bt-resistant and -susceptible strains of Ostrinia nubilalis. Insect Biochem. Mol. Biol. 2005, 35, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Oppert, B.; Higgins, R.A.; Huang, F.; Zhu, K.Y.; Bushman, L.L. Comparative analysis of proteinase activities of Bacillus thuringiensis-resistant and-susceptible Ostrinia nubilalis (Lepidoptera: Crambidae). Insect Biochem. Mol. Biol. 2004, 34, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Oppert, B.; Kramer, K.J.; Beeman, R.W.; Johnson, D.; McGaughey, W.H. Proteinase-mediated insect resistance to Bacillus thuringiensis toxins. J. Biol. Chem. 1997, 272, 23473–23476. [Google Scholar] [CrossRef] [PubMed]
- Oppert, B.; Kramer, K.J.; Johnson, D.; Upton, S.J.; McGaughey, W.H. Luminal proteinases from Plodia interpunctella and hydrolysis of Bacillus thuringiensis CryIAc protoxin. Insect Biochem. Mol. Biol. 1996, 26, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Forcada, C.; Alcácer, E.; Garcerá, M.D.; Tato, A.; Martínez, R. Resistance to Bacillus thuringiensis Cry1Ac toxin in three strains of Heliothis virescens: Proteolytic and SEM study of the larval midgut. Arch. Insect Biochem. Physiol. 1999, 42, 51–63. [Google Scholar] [CrossRef]
- Wu, Z.H.; Yang, Y.J.; Xu, H.S.; Tian, J.C.; Lu, Y.H.; Lu, Z.X. Changes in growth and development and main enzyme activities in midgut of Cnaphalocrocis medinalis intermittently trend with low amount of Bt rice leaves over generations. Chin. J. Rice Sci. 2015, 29, 417–423. [Google Scholar]
- Zhang, Y.; Zhao, D.; Yan, X.; Guo, W.; Bao, Y.; Wang, W.; Wang, X. Identification and characterization of Hyphantria cunea aminopeptidase N as a binding protein of Bacillus thuringiensis Cry1Ab35 toxin. Int. J. Mol. Sci. 2017, 18, 2575. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Y.C.; Ottea, J.; Husseneder, C.; Leonard, B.R.; Abel, C.; Huang, F. Molecular characterization and RNA interference of three midgut aminopeptidase N isozymes from Bacillus thuringiensis-susceptible and -resistant strains of sugarcane borer, Diatraea saccharalis. Insect Biochem. Mol. Biol. 2010, 40, 592–603. [Google Scholar] [CrossRef]
- Angelucci, C.; Barrett-Wilt, G.A.; Hunt, D.F.; Akhurst, R.J.; East, P.D.; Gordon, K.H.; Campbell, P.M. Diversity of aminopeptidases, derived from four lepidopteran gene duplications, and polycalins expressed in the midgut of Helicoverpa armigera: Identification of proteins binding the delta-endotoxin, Cry1Ac of Bacillus thuringiensis. Insect Biochem. Mol. Biol. 2008, 38, 685–696. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).