Abstract
Foliar fertilization, an effective method to regulate plant nutrition has attracted much attention. In this study, the effects of different foliar fertilizers (F1: NPK formulation, F2: NPK+Fe-Mn-Zn formulation, F3: Fe-Mn-Zn formulation, and CK: deionized water) on plant growth, chemical composition, rhizosphere soil properties, and fungal community of Dioscorea opposita Thunb. were assessed by a field plot experiment. Rhizosphere fungal community was examined via Illumina MiSeq sequencing of ITS2 rRNA genes. Spraying foliar fertilizer directly affected the growth and chemical content of the plant, which depends on different foliar fertilizers. Ascomycota and Moraxella were the dominant fungal phyla. Nutrient elements were the main factors affecting fungal composition, and soil microelements positively influenced soil fungi, while soil macroelements negatively influenced soil fungi. Macroelement foliar fertilizer affected soil fungi by directly affecting the growth parameters and macroelement content of the plant or by directly affecting soil properties, while microelement foliar fertilizer affected soil fungi by directly affecting the microelement content of the plant or by directly affecting the soil pH and organic matter content. These factors were involved in the formation of a “leaf-root-soil microbe” conduction system similar to the “brain-gut-microbe” axis. Our results provide a basis for the application of foliar fertilizer in the cultivation of medicinal plants.
1. Introduction
Chinese yam (Dioscorea opposita Thunb., Dioscoreaceae), a twining perennial vine, has been included in the list of medicinal and food homology in China [1]. In traditional Chinese medicine, yam was mainly used to treat anorexia, chronic diarrhea, and diabetes [2,3]. In addition, the antioxidant, anti-inflammatory, neuroprotective, and anticancer properties of the polysaccharides in yam tubers have also attracted much attention [4,5,6]. In recent years, the use of a large number of chemical fertilizers has promoted the high yield of yams but also led to related problems such as low utilization rate of chemical fertilizers and soil acidification and salinization, which seriously affected the yield and quality of yams and restricted the sustainable development of the yam industry [7].
Foliar spraying is a technique used to supplement the nutrients needed for plant growth by spraying liquid fertilizer directly onto the leaves, which results in better uptake by the aerial parts of the plant [8,9]. Compared with soil fertilization, foliar fertilization has the characteristics of fast nutrient absorption, less dosage required, high nutrient utilization rate, and less soil pollution [10,11]. Anees et al. [12] reported that foliar spraying of K and Zn is an efficient fertilizer to improve maize yield and net income under rain-fed conditions. JanuškaItIenė and kacIenė [13] found that under drought stress and UV-B radiation stress, foliar spraying can reduce the oxidative stress damage of leaves by reducing the content of malondialdehyde. Ali et al. [14] found that the application of mineral K fertilizer with foliar spraying of monopotassium phosphate or potassium citrate resulted in increases in most of the potato growth and tuber chemical-composition-related parameters. Hence, foliar fertilization has become one of the important high-yield cultivation and management measures in agricultural production.
Foliar sprays allow plants to quickly absorb the nutrients they need directly from the leaves rather than through the roots, which reduces the adverse effects of fertilizers on the soil and improves the soil environment. Sun et al. [15] found that reduced soil fertilization and increased foliar spraying of tomato had no significant effect on their yield, but could result in significant reductions in nitrate and available phosphorus levels in the 0–20 cm soil layer. Compared with the control, foliar applications with monopotassium phosphate on potato leaves increased the soil Actinomycetes community and reduced soil fungal community [16]. Amerany et al. [17] reported that chitosan application to leaves enhance plant growth and flowering and reduced interaction with arbuscular mycorrhizal (AM) fungi, whereas root treatment did not affect these parameters. Khan et al. [18] reported that Vermiwash foliar sprays change the response of crops to different AM fungi in growth and nutrient utilization, which is important for selecting effective combinations of nutrient sources to improve crop growth. Aghaei et al. [19] found that foliar spraying of L-phenylalanine can improve the symbiosis of AM fungi and the effect of fertilizer and earthworm composting, which may be conducive to the organic production of medicinal plants such as Hyssopus officinalis. Therefore, it is necessary to comprehensively analyze the effects of different foliar fertilizers on plant growth and soil environment to provide a basis for reducing the total amount of fertilization and improving fertilizer efficiency.
Rhizosphere microorganisms are an important part of the soil, and they participate in almost all biochemical reactions in the soil, including carbon and nitrogen cycling, and play an important role in plant growth and development [20,21]. Rhizosphere microorganisms can directly participate in plant growth and development as growth-promoting or -inhibiting factors or indirectly act on host plants by affecting other microorganisms in the soil [22,23]. At present, there are many studies on the effects of soil fertilization on the composition of the rhizosphere’s microbial community. For example, Zheng et al. [24] reported that long-term application of N and K fertilizer and straw returning have an important impact on the diversity of methane-oxidizing bacteria in paddy fields. Hu et al. [25] considered that long-term balanced fertilization can improve soil microbial biomass carbon and nitrogen, and microbial functional activity. However, there are few documents on the effects of foliar fertilization on rhizosphere soil microorganisms of medicinal plants.
We hypothesized that there is a synergistic mode of “leaf-root-soil microorganisms” in the process of plant growth, which can be regulated by nutrient elements. In the present study, foliar fertilizer containing different nutrient elements was designed for spraying Chinese yams, and the diversity of rhizosphere soil fungi was detected using Illumina MiSeq sequencing platform, with the aim to (1) determine the changes in plant growth, chemical composition, and rhizosphere soil properties of Chinese yam after spraying foliar fertilizers; (2) determine the fungal community composition of the Chinese yam rhizosphere after spraying foliar fertilizers, and (3) assess the effects of different foliar fertilizers on rhizosphere fungal communities. These data provide a basis for evaluating the effects of foliar fertilizers for the growth of medicinal plants and the soil environment.
2. Materials and Methods
2.1. Study Sites
A field experiment of Chinese yam was conducted in Anguo Medicinal Planting site (38°42′48″ N, 115°33′30″ E), Anguo, Hebei Province, China, in April 2021. The annual mean temperature and annual mean precipitation in the study area were 12.6 °C and 567.5 mm, respectively. Soils were Alfisols and Durixeralf [26]. The soil physicochemical properties in the experimental field were as follows: soil organic matter (SOM) content 13.18 mg/g, soil pH 7.61, and the soil nutrient concentrations were 43.6 mg/kg for available nitrogen (N) and 8.39 mg/kg for available phosphorus (P).
2.2. Field Experiment Design and Sampling
A completely randomized block design was used in the field experiment, including four treatment plots: macroelement foliar fertilizer (MAE): NPK foliar fertilizer (F1: the content of urea was 2.77 g/L, potassium phosphate was 1.74 g/L, and potassium sulfate was 1.59 g/L); compound element foliar fertilizer (CE): NPK+Fe-Mn-Zn foliar fertilizer (F2: the content of urea was 2.77 g/L, potassium phosphate was 1.74 g/L, potassium sulfate was 1.59 g/L, chelated iron was 0.78 g/L, chelated manganese was 0.93 g/L, and chelated zinc was 1.16 g/L); microelement foliar fertilizer (MIE): Fe-Mn-Zn foliar fertilizer (F3: the content of chelated iron was 0.78 g/L, chelated manganese was 0.93 g/L, and chelated zinc was 1.16 g/L); and the same amount of deionized water (CK). Each foliar fertilizer treatment was repeated five times. Thus, a total of 20 experimental plots were set up. Each experimental plot had an area of 4 m2, and each plot was separated by 50 cm (the row spacing of yam planting in each plot is 30 cm × 30 cm). Before planting Chinese yams, organic fertilizer and NPK fertilizer were used as a base fertilizer in all treatment plots (the amount of organic fertilizer used is 0.875 kg/m2, and the amount of NPK compound fertilizer is 0.027 kg/m2). Chinese yams were sown in early April 2021, and in the beginning of August, foliar fertilizer was sprayed on the plant every 15 d for three consecutive times. (The equipment used for foliar fertilizer spraying is an electric sprayer with a volume of 5 L. The specific spraying methods for different foliar fertilizer treatments are: use 2.5 L of water to dilute and dissolve 10 g of NPK foliar fertilizer; use 5 L of water to dilute and dissolve 15 g of NPK+ Fe–Mn–Zn foliar fertilizer; use 1.5 L of water to dilute and dissolve 4 g of Fe–Mn–Zn foliar fertilizer.) The amount of spraying liquid was based on uniform spraying on the leaf surface without dripping water and conventional field management.
In mid-October, within each experimental plot, 10 healthy yam plants were randomly selected. Ten replicates of shoots and root samples of yam were collected, and then soil and root samples (0–30 cm soil layer) were taken from each plant in each plot. All plant and soil samples were stored in sealed self-sealing bags marked with each treatment, and taken back to the laboratory. All plant samples were used to analyze the growth and nutrient parameters of Chinese yam. Rhizosphere soil sample per repeated plant in the different treatments was sieved (<2 mm mesh) and then were divided into two parts. Subsamples were air-dried at 25 °Cto measure soil physicochemical properties, while the other was placed in a freezer at −80 °C for the analysis of fungal community structure.
2.3. Plant Biomass and Morphological Parameters
Before harvest, plant height and number of leaves in each treatment group were recorded. After the whole plant was harvested, the aboveground and underground parts were separated. The root diameters of yam plants of different treatments were determined using a straight edge of uniform size. The roots were dried at 65 °C for 72 h to calculate the root biomass. In additions, the yam roots were peeled, cut into 2.0~2.5 cm thick slices, and dried in a strong wind at 50~60 °C. Then, the dried yam slices were crushed, screened (40~60 mesh), and used for subsequent chemical analysis.
2.4. Root Protein, Amino Acid, and Polysaccharide Contents
About 0.4 g of dried root sample, 5.0 g of catalyst (K2SO4:CuSO4:5H2O = 10:1), and 10 mL of concentrated sulfuric acid were put into the digestion tube, shaken well, and digested in the digestion oven (420 °C) for about 1 h. After cooling, about 20 mL of water was slowly added. the digestion tube was placed on an automatic Kjeldahl nitrogen analyzer (SKD-1000, Shanghai Peio), alkali was added for distillation for 4 min and then titrated with boric acid absorption solution and standard hydrochloric acid solution, and the volume of hydrochloric acid titrated was recorded and the protein content of the sample was calculated.
The protein of the root samples was hydrolyzed by 6 mol/L hydrochloric acid solution for 22 h to become free amino acid. After filtration, constant volume, and concentration, it was injected into the amino acid analyzer for ion exchange separation and ninhydrin staining. The amino acid content was determined via an amino acid analyzer (GB 5009.124-2016 Determination of Amino Acid in Food).
Then, 50 g of root powder was mixed with 500 mL of double-distilled water and heated at 100 °C for 3 h. After filtering the extract, the solution was concentrated by rotary evaporation and centrifuged at 4500 rpm for 15 min. The supernatant was precipitated by adding 5 volumes of 95% ethanol overnight at 4 °C. After centrifugation at 4500 rpm for 15 min, the precipitate was dissolved with double-distilled water. The original reagent (CHCI3:CH3(CH2)3OH = 5:1) was added to the solution and stirred at 25 °C for 2 h to remove the relevant protein. The absorbance value was measured via a spectrophotometer (Mettler Toledo, UV5, Shanghai, China) at 490 nm with glucose as the reference, and the polysaccharide content was determined by the phenol sulfuric acid method [27,28].
2.5. Root Chemical Element Contents
Dried powdered samples of yam roots were digested by placing approximately 0.2 g into a mixture of 12.7 mol/L perchloric acid, 18 mol/L sulfuric acid, and water (10:1:2) using a Mars 6 microwave reaction system (CEM Inc., Matthews, NC, USA) until a clarified solution was obtained. Total N, total P, and total K were determined by Kjeldahl method, molybdenum vanadium blue colorimetry, and flame photometry [29]. Dried samples (0.4 g) were digested with HNO3/HClO4 (3:1) (v/v) mixed digestion solution at 200~250 °C. The clear solution was diluted with 0.2% HNO3 in a 50 mL volumetric flask. A flame atomic absorption spectrometer (TAS-990, Beijing Puxi Instrument Factory, Beijing, China) was used to measure the Fe, Zn, and Mn content in yam roots.
2.6. Soil Parameters
About 0.5 g of dry soil sample was digested by a Mars 6 microwave reaction system, and then a clear solution was obtained. The contents of soil organic matter, available N, available P, and available K were determined in the presence of sulfuric acid using the dichromate oxidation method [30], the alkaline hydrolysis-diffusion method, the stannous chloride reduction molybdenum phosphorus blue method [31], and flame photometry [32] method. The detection method of soil Fe, Zn, and Mn content is the same as the method for detecting microelements in yam root, and the flame atomic absorption spectrometer can be used for this determination. Soil urease activity was determined according to the method of Hoffmann and Teicher [33]. Soil acid phosphatase and alkaline phosphatase were determined according to the method of Tarafdar and Marschner [34].
2.7. Molecular Analysis
Total genomic DNA was extracted from rhizosphere soil samples (0.3 g) using the Powersoil®® DNA extraction kit (Mo Bio, Carlsbad, CA, USA). DNA quality was then examined using agarose gel electrophoresis (0.1%), while DNA concentration and purity were determined using a NanoDrop™ 1000 spectrophotometer (Thermo Fisher Scientific Inc., Oakwood, OH, USA). The two universal fungal primers used were ITS1F (5′-CTTGGTCATTTAGGAAGTAA-3′)-ITS2R and (5′-GCTGCGTTCTT CATCATGATGC-3′) [35], respectively, by targeting the ITS1 and ITS2 (internal transcribed spacer) regions of the fungus to characterize the fungal community. A 20 μL reaction system containing PCR products was performed in triplicate, and the reaction system included 4 μL of 5× FastPfu Buffer (for 16 s v3 − v4)/2v μL of 10× buffer (for ITS), 2μL of dNTPs, 0.8 μL of forward and reverse primers in total, 0.4 μL of FastPfu polymerase (16S v3 − v4)/0.2 μL rTaq polymerase (for ITS), 0.2 μL bovine serum albumin, and 10 ng template DNA. The PCR amplification procedure was as follows: 3 min initial denaturation temperature (95 °C), followed by 28 cycles of 30 s denaturation phase each (95 °C), 30 s annealing (55 °C), 45 s extension (72 °C), and finally 10 min extension (72 °C). PCR products were detected by 2% (w/v) agarose gel electrophoresis and purified using the AxyPrep™DNA Gel Extraction Kit (Axygen BioSciences Inc.,Glendale, CA, USA), and finally quantified in a QuantiFluor™-ST fluorometer (Promega, Madison, WI, USA) equipped with a PCR tube adapter in a QuantiFluor™ dsDNA system for quantification. Samples were sequenced using the paired-end option (2 × 300 bp) of the Illumina MiSeq PE 300 platform on the environmental genomic platform of NovoGen Technologies, Inc. (Beijing, China)
2.8. Bioinformatics Analysis
The relevant raw sequence reads obtained from the experiment were initially trimmed using mothur software (University of Michigan, Ann Arbor, MI, USA), and sequences that met the following three criteria were retained for use in subsequent in-depth analyses: (1) sequences with exact primers and barcodes; (2) mass fraction >30; and (3) sequences >200 bp. The above retained sequences were then further filtered using the Usearch software package to eliminate erroneous, chimeric sequences from them. Qualified sequences were then isolated using sample-specific barcode sequences and trimmed using the Illumina analysis pipeline (version 2.6). The remaining high-quality sequences were queried using local BLASTn and NCBI’s GenBank non-redundant nucleotide database (NT). The MEGAN program [36] was used to assign BLAST hits to taxa in the NCBI taxonomy.
Filtered non-chimeric sequences were clustered into operational taxonomic units (OTUs) at 97% sequence level using USEARCH V.8.0 software (Beijing Huanzhong Ruichi Technology Co., Ltd., Beijing, China). The OTU representative sequences were classified by the fungal (ITS) NITE database v.18.11.2018 and Silva (SSU123) 16S rRNA reference database using the RDP Bayesian classifier algorithm with a confidence threshold of 0.7. The RDP was then used to collate sequences from GeneBank (Release 7.3; http://fungene.cme.msu.edu/; accessed date 5 June 2022) to obtain species annotation data. To eliminate potential bias due to differences in sequence depths determined from different samples, the sequencing depths of all samples were minimized. Dilution curves, Venn diagrams, and community composition were analyzed according to OTU counts and associated taxonomic tables using R (R version 4.0.2). Fungal alpha diversity indices were calculated using mothur (v. 1.30.2). Other statistical analyses were performed in SPSS V. 22.0 (IBM, Armonk, NY, USA) and the remaining graphs were plotted using Origin V. 9.0.
2.9. Abundance and Diversity Analysis
The analysis of fungal Shannon and Chao1 indices responded to trends in the composition and variation of rhizosphere soil fungal communities, with Chao1 reflecting community abundance and Shannon representing community diversity [37]. The hierarchical abundance and sparsely curves obtained by QIIME analysis estimated species evenness, species richness and sequence depth, respectively [38]. Each representative OTU sequence in this study was used for taxonomic identification at the phylum and genus level.
2.10. Statistical Methods
The data on plant growth, chemical composition and soil parameters were found not to be normally distributed using the Shapiro–Wilk test and were therefore subjected to a nonparametric test. All boxplots show individual data points, median, interquartile range, minimum, and maximum values. The data shown in the figures are the means of ≥5 replicates. The composition of yam rhizosphere fungal communities was analyzed using non-metric multidimensional scales (NMDS) to visualize the differences in distribution between treatments. This analysis was performed mainly by running the metaMDS command in the vegan package in R 4.0.2 [39]. Rarefaction curves for the fungal OTUs were calculated using the specaccum function in the vegan package [39]. The degree of influence of plant nutrients, growth, chemical composition, and soil parameters on fungal communities was assessed using the variation partitioning method in the vegan, permute, and lattice packages. Mantel test and the structural equation model (SEM) were used to test the effects of macroelement (MAE) fertilizer, microelement (MIE) fertilizer, yam growth, and chemical composition, and soil parameters on the rhizosphere fungi using R-4.0.2 packages ecodist [40] and AMOS 21.0 (maximum likelihood).
3. Results
3.1. Growth Parameters and Chemical Composition of Chinese Yam
There were significant differences in the effects of different foliar fertilizers on the growth parameters of yam. Compared with the control treatment, F1 increased plant height, root biomass, and root diameter of yam, and F2 increased the number of leaves and plant height, while F3 had no significant effect on these parameters of yam (Figure 1). The interactions between MAE and MIE on plant height and leaf number were significant (Table 1).
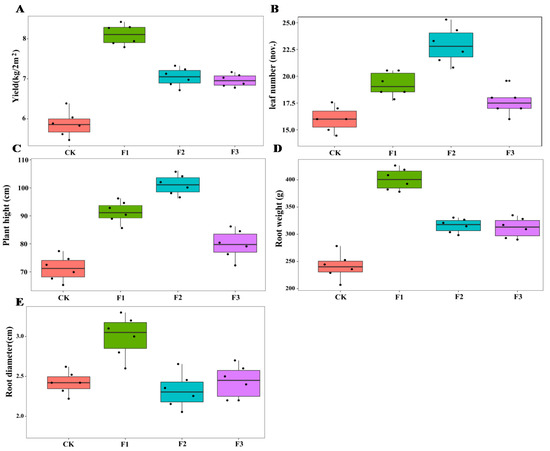
Figure 1.
Effect of different foliar fertilizer on the growth parameters of Chinese yam. Yield of yam under different foliar fertilizer treatments (A); Leaves number of yam under different foliar fertilizer treatments (B); Plant height of yam under different foliar fertilizer treatments (C); Root weight of yam under different foliar fertilizer treatments (D); Root diameter of yam under different foliar fertilizer treatments (E).CK, spray with water; F1, NPK foliar fertilizer; F2, NPK+Fe-Mn-Zn foliar fertilizer; F3, Fe-Mn-Zn foliar fertilizer.

Table 1.
Two-way ANOVA of the effect of MAE and MIE on plant growth and chemical composition parameters of Chinese yam.
The chemical composition of yam was significantly different among different foliar fertilizer treatments. F1 reduced the content of yam polysaccharides, and F3 increased the content of yam polysaccharides, while F2 had no obvious effect on yam polysaccharide, compared with the control treatment. Only F1 had a significant effect on the protein and amino acid content of yam, while F3 had a facilitative effect on amino acid content but not as effective as that of F1 (Figure 2). There was no significant interaction between MAE and MIE on these parameters (Table 1).
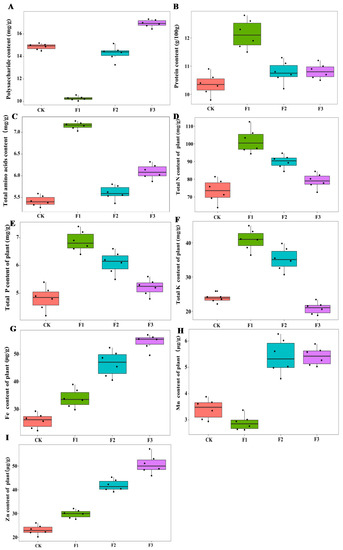
Figure 2.
Effect of different foliar fertilizer on the chemical composition content of Chinese yam. Polysaccharide content of yam under different foliar fertilizer treatments (A); Protein content of yam treated with different foliar fertilizers (B); Total amino acid content of yam treated with different foliar fertilizers (C); Total N content of yam treated with different foliar fertilizers (D); Total P content of yam treated with different foliar fertilizers (E); Total K content of yam treated with different foliar fertilizers (F); Fe content of yam treated with different foliar fertilizers (G); Mn content of yam treated with different foliar fertilizers (H); Zn content of yam treated with different foliar fertilizers (I). CK, spray with water; F1, NPK foliar fertilizer; F2, NPK+Fe-Mn-Zn foliar fertilizer; F3, Fe-Mn-Zn foliar fertilizer.
In terms of chemical elements of yam, the contents of total N, total P, and total K in the F1 treatment were significantly higher than those in other treatments. The total N, P, and K contents of the F2 treatment were lower than those of the F1 treatment but higher than the control treatment, while there was no significant difference between F3 treatment and control treatment. The contents of Fe and Zn in the F3 treatment were higher than those in other treatments, and the contents of Fe and Zn were lower in the F1 and F2 than in F3 but higher than in the control treatment. The highest Mn content was found in F2 and F3 treatments, while there was no significant difference between F1 and control treatment (Figure 2). The interaction of MAE and MIE significantly affected the total N, P, K, Fe, Zn, and Mn content in yam roots (Table 1).
3.2. Soil Parameters
Different foliar fertilizers had significant effects on the physicochemical properties and enzyme activities of rhizosphere soil (Figure 3 and Figure 4). In terms of soil physicochemical properties, the content of soil organic matter increased after spraying with micronutrients, while soil organic matter and soil pH decreased after spraying with macronutrients, compared with the control treatment. After spraying F1, soil available K content was the highest among all treatments, while the content of available soil N and P in different treatments was not significantly different, but the content of Fe, Mn, and Zn in soil decreased even lower than that of the control treatment. The Mn levels in the soil after spraying F3 were the highest among all treatments, while after spraying F2 and F3, the contents of soil Fe and Zn were higher than that of other treatments (Figure 3). The interaction between MAE and MIE significantly affected the Mn and Zn content of soil (Table 2).
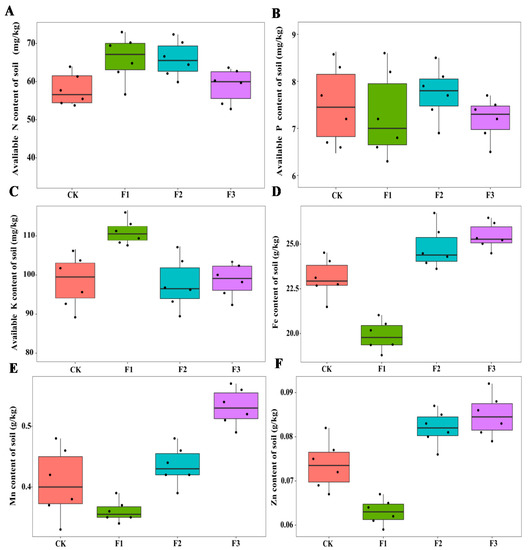
Figure 3.
Effects of different foliar fertilizer on soil physicochemical properties of Chinese yam. Avaliable N content in rhizosphere soil of yam under different foliar fertilizer treatments (A); Avaliable P content in rhizosphere soil of yam under different foliar fertilizer treatments (B); Avaliable K content in rhizosphere soil of yam under different foliar fertilizer treatments (C); Fe content in rhizosphere soil of yam under different foliar fertilizer treatments (D); Mn content in rhizosphere soil of yam under different foliar fertilizer treatments (E); Zn content in rhizosphere soil of yam under different foliar fertilizer treatments (F). CK, spray with water; F1, NPK foliar fertilizer; F2, NPK+Fe-Mn-Zn foliar fertilizer; F3, Fe-Mn-Zn foliar fertilizer.
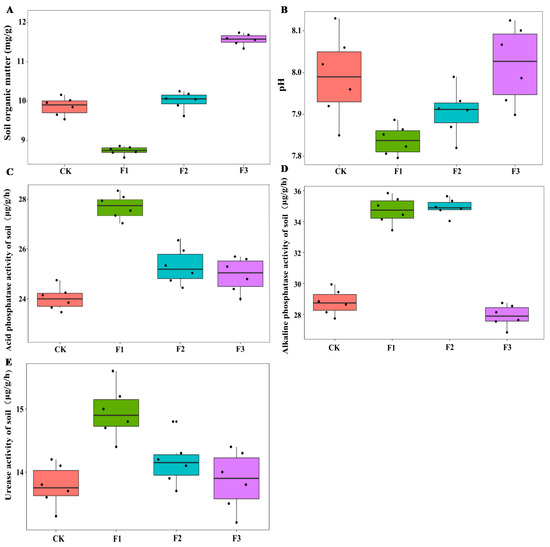
Figure 4.
Effects of different foliar fertilizer on soil enzyme activities of Chinese yam. Organic matter content in rhizosphere soil of yam under different foliar fertilizer treatments (A); pH in rhizosphere soil of yam under different foliar fertilizer treatments (B); Acid phosphatase activity in rhizosphere soil of yam under different foliar fertilizer treatments (C); Alkaline phosphatase activity in rhizosphere soil of yam under different foliar fertilizer treatments (D); Urease activity in rhizosphere soil of yam under different foliar fertilizer treatments(E). CK, spray with water; F1, NPK foliar fertilizer; F2, NPK+Fe-Mn-Zn foliar fertilizer; F3, Fe-Mn-Zn foliar fertilizer.

Table 2.
Two-way ANOVA of the effect of MAE and MIE on soil physicochemical parameters of Chinese yam.
In terms of soil enzyme activities, the ALP activity increased significantly after spraying both F1 and F2, while the ACP activity and U activity increased significantly after spraying F1. There was no significant change in soil enzyme activity after spraying F3 compared to the control treatment (Figure 4). The interaction between MAE and MIE significantly affected the soil ACP and ALP activity (Table 2).
3.3. Soil Fungal Community Composition
We obtained 2,304,715 fungal sequences. Rarity curve analysis showed that the sequencing depth was high, and it was possible to observe the fungal diversity in yam rhizosphere soils (Figure S1A). The hierarchical abundance profiles showed high species uniformity and homogeneity for all four treatments (Figure S1B). The sequencing results included biological information on most fungi in soil samples. After filtering 1,178,114 low-quality sequences, 1,489,142 valid fungal sequences were clustered into 1875 fungal OTUs with a sequence similarity of 97%. Of these 1875 fungal OTUs, 361 occurred in all four treatments, while 174, 303, 233, and 230 OTUs were found only in the CK, F1, F2, and F3 treatments, respectively (Figure 5A).
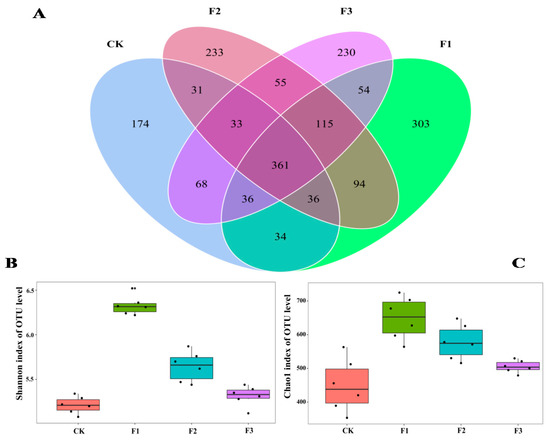
Figure 5.
Differences in OTU composition (A) and diversity index (B,C) of Chinese yam rhizosphere fungi under different foliar fertilizer treatments. Each part in the Venn diagram represents one treatment, and the number of circles and circles overlapped represents the number of OTUs shared between the samples, while the number without overlap represents the number of OTUs unique to the samples. CK, spray with water; F1, NPK foliar fertilizer; F2, NPK+Fe-Mn-Zn foliar fertilizer; F3, Fe-Mn-Zn foliar fertilizer.
The Shannon and Chao1 indices of yam rhizosphere fungi treated with F1 were the highest, while the indices of yam rhizosphere fungi treated with control and F3 were the lowest (Figure 5B,C). A total of 1875 fungal OTUs were found in yam rhizosphere soil. They were classified as Ascomycota, Moraxella, Basobacteria, Coccota, Erosobacter, Zoomycota, Myxomycetes, Apollophyla, Carbella, and some unknown fungi. Among them, Ascomycota and Moraxella were the most dominant fungal phyla, and the relative abundance of different treatments ranged from 92.7% to 99.3% (Figure 6A).
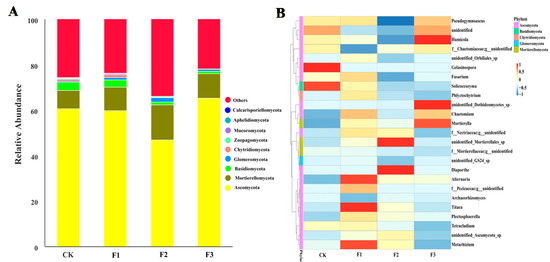
Figure 6.
The relative abundance (A) at the phylum level and the preference distribution at the genus level (B) of Chinese yam rhizosphere fungi under different foliar fertilizer. CK, spray with water; F1, NPK foliar fertilizer; F2, NPK+Fe-Mn-Zn foliar fertilizer; F3, Fe-Mn-Zn foliar fertilizer.
There were obvious preferences between fungal genera and treatments. For example, Metarhizium, Alternaria, and Titaea were the most abundant in F1; unidentified_Mortierellales_sp. and Diaporthe were the most abundant in F2; unidentified_Dothideomycetes_sp., Mortierella, and Humicola were the most abundant in F3; and Gelasinospora and Solicoccozyma were the most abundant in the control treatment (Figure 6B).
NMDS analysis showed that there were significant differences in fungal community composition in rhizosphere soil after spraying different foliar fertilizers. After spraying F1 and F2, the fungal community composition was similar (Figure 7A). LDA analysis showed that the change in soil fungal communities was explained by 32 indicators of fungal species. S_Glomus, s_Didymella_glomerata, f_Orbiliace, and p_Basidiomycota showed the highest enrichment in the CK, F1, F2, and F3 treatments, respectively (Figure 7B).
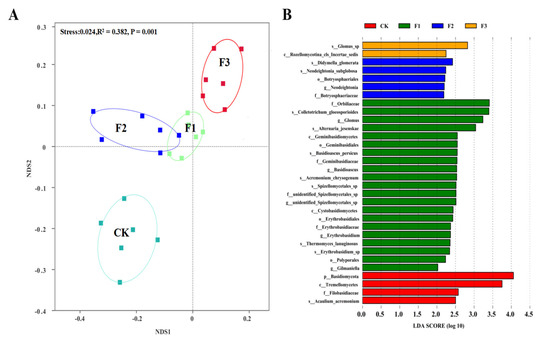
Figure 7.
Non-metric multidimensional scale (NMDS) ranking of the rhizosphere fungal community composition of Chinese yam under different foliar fertilizer (A) and indicator fungi with an LDA score of 2 or higher for fungal species under different foliar fertilizer (B). LDA Effect Size (LEfSe) algorithm was used on OTUs level to determine taxa that differentially represented between different treatments. CK, spray with water; F1, NPK foliar fertilizer; F2, NPK+Fe-Mn-Zn foliar fertilizer; F3, Fe-Mn-Zn foliar fertilizer.
3.4. Variation Partitioning of Fungal Community
Variance partitioning was used to quantify the contribution of the growth parameters, nutrient element, active components (polysaccharide, protein, and amino acid) of yam, and soil properties to fungal communities in yam rhizosphere soil (Figure 8). The combination of the growth parameters, nutrient elements, active components of yam, and soil properties explained 83.0% of the changes in rhizosphere fungal community. Nutrient elements were the most important factors affecting the fungal community, and the single explanation amount was 14.2%. The individual explanation amounts of the growth parameters, active components, and soil properties were 8.3%, 6.2%, and 3.2%, respectively. These four factors together explained 35.8% of the variation in fungal communities, while the amount of nutrient elements, growth parameters, and active components explained 10.4% (Figure 8).
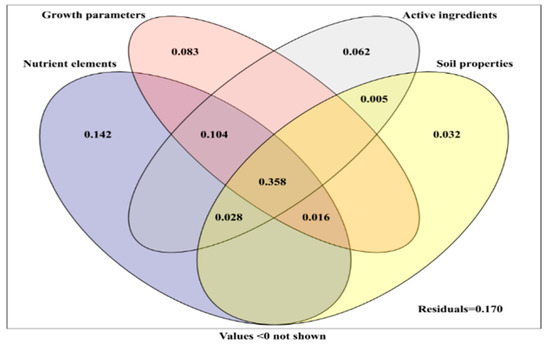
Figure 8.
Variation partitioning of plant growth parameters, plant chemical composition (active ingredient, nutrient elements), and soil parameters on relative abundance of rhizosphere fungi.
3.5. Correlation Analysis
The Mantel test and SEM were used to clarify the effects of MAE fertilizer, MIE fertilizer, yam growth parameters, yam chemical composition, and soil parameters on fungal communities (Figure 9). According to the Mantel test, there were significant relationships between MAE, MIE, growth parameters, active components, macroelements and microelements of yam, soil enzyme activity, soil macroelements, microelements, physicochemical properties (organic matter, pH), and soil fungi (Table S1). Combined with the correlation coefficient (R value), an SEM model was constructed to quantify the relative effects of different factors on fungal abundance (X2 = 173.655, df = 22, P = 0.001, GFI = 0.812, AIC = 175.061, RMSEA = 0.412). Our results revealed that MAE had a significant positive effect on the growth parameters, active ingredients, and macroelements of yam and on soil macroelements. MIE had positive effects on active components and microelements of yam and microelements and physicochemical properties of soil. Yam growth parameters had a significant positive effect on soil enzyme activity and soil fungi and had a significant negative correlation with soil microelements and physicochemical properties. There were negative effects among yam macroelements and soil microelements and soil fungi. The microelements of yam have obvious promoting effect on soil microelements. Soil enzyme activity, microelements, and physicochemical properties had a significant positive effect on soil fungi, while soil macroelements had a significant negative effect on soil fungi.
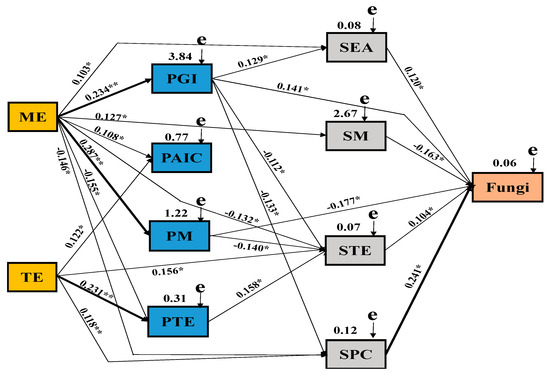
Figure 9.
Structural equation model(SEM) showing the causal relationships among macroelement foliar fertilizer, microelement foliar fertilizer, plant growth indicators, chemical composition parameters, and soil parameters and soil fungi. The final model fitted the data well: maximum likelihood, X2 = 58.227, df = 11, p = 0.008, goodness of fit index = 0.599, Akaike information criteria = 158.127, root mean square error of approximation = 0.259. Solid lines and dashed lines indicate significant and non-significant pathways, respectively. The width of the solid lines indicates the strength of the causal effect, and the numbers near the arrows indicate the standardized path coefficients (* p < 0.05 and ** p < 0.01). MAE, macroelement foliar fertilizer; MIE, microelement foliar fertilizer; PGI: plant growth parameters; PAIC, plant active ingredients; PMA, plant macroelements; PMI, plant microelements; SEA, soil enzyme activity; SMA, soil macroelements; SMI, soil microelements; SPC, soil physicochemical properties; Fungi, soil fungi.
4. Discussion
4.1. Effects of Foliar Fertilizer on the Growth and Chemical Composition of Chinese Yam
The effective utilization of nutrients is very important for the healthy growth of plants, but because of human and environmental factors, some nutrients are fixed by the soil and are not easily absorbed and transformed by plants. Therefore, it is important give necessary nutritional supplement to plants through the leaf surface to make up for the deficiency of traditional fertilization technology [7,41,42,43]. In the present study, we carried out an experiment of spraying different foliar fertilizers on yam and found that there were significant differences in the response of different growth indices and chemical composition contents of yam plants to different types of nutrients. Among them, the growth indices such as root weight and root diameter were the best with NPK fertilizer, while plant height and leaf number were better with compound fertilizer containing microelements. The application of Fe, Mn, and Zn resulted in the highest polysaccharide content in yam plants among all treatments, while the application of NPK increased the content of soluble protein and amino acids in yam plants. After spraying macroelements, the contents of NPK in yam plants were higher than those in other treatments, while the contents of Fe, Zn, and Mn were the lowest. However, the result after spraying microelements was just the opposite. The SEM analysis also showed that macroelement foliar fertilizer positively influenced plant growth, active ingredients, and macroelement contents and negatively influenced the microelement contents in yam plants, while microelement foliar fertilizer positively influenced only the contents of active ingredients and microelements. A lot of evidence has shown that foliar fertilizer plays an active role in improving crop quality, yield, and metabolism [44]. Jabborova et al. [45] reported that the application of NPK compound fertilizer can increase the contents of K, P, and Na in ginger rhizomes, and NPK + BZnFe treatment increased the contents of K, Ca, P, Mg, and Na in ginger rhizomes compared with the control without fertilization. Combined foliar spraying of macro- and micronutrients significantly improved plant growth and yield of a cotton crop [46]. Some studies showed that foliar fertilization can affect the growth of medicinal plants and the metabolic accumulation of medicinal components to some extent [47,48,49]. Our research results also contribute to the directional cultivation of high-quality yam. For example, in order to improve the yield, protein and amino acid content of yam, macroelement foliar fertilizer can be sprayed. When the polysaccharide content of yam is increased, it is better to spray microelement element foliar fertilizer.
4.2. Effect of Foliar Fertilizer on Soil Parameters of Chinese Yam Rhizosphere
Foliar spraying enables plants to absorb nutrients directly from the leaves, thus transporting them to other organs, reducing the number and amount of soil fertilizers applied, alleviating the adverse effects of chemical fertilizers on the soil, and ultimately improving the soil environment [50]. Rhizosphere soil is the main environmental place for plants to absorb nutrients. A change in the nutrient content in the rhizosphere soil will directly affect the selection and transfer of nutrients by the plants and in turn affect the growth of plants [51]. In the present study, spraying macroelement fertilizer increased the content of available K in the soil and reduced the content of soil Fe and Zn, but there was no significant difference in the content of soil-available N, available P, and Mn among different treatments. After spraying microelement fertilizer, only soil Mn content was increased. In addition, the application of macroelement fertilizer positively influenced soil NPK content and negatively influenced soil microelements, organic matter, and pH, while the application of microelement fertilizer positively influenced soil microelements, organic matter, and pH in our study. Some studies have shown the positive influence of foliar fertilization on the soil environment. For example, Zhou et al. [52] reported that spraying the leaves of Atractylodes rhizoma with 10.0 mg/m2 of Se can significantly increase the content of available N, available P, and available K in the rhizosphere soil. Srinivasan et al. [53] also found that improper application of high mineral fertilizer may reduce the contents of N, P, K, Fe, Zn, and Ca in the soil. Moreover, compared with the control treatment, spraying F1 and F2 increased the activity of soil acid phosphatase and the alkaline phosphatase, while the activity of soil urease was higher than that of other treatments after spraying F1 in our study. Niewiadomska et al. [54] reported that during the entire white lupine growing season, the foliar fertilizer decreased the activity of soil acid phosphatase and promoted nitrogenase activity. Bana et al. [55] found that the dehydrogenase activity was significantly higher with 1.0 kg/ha multi-micronutrient fertilizers as foliar spray, and when the foliar fertilizer was sprayed at 0.75 kg/ha, the activities of soil alkaline and acid phosphatase and urease were higher. Soil enzymes are biological/biochemical sensors of soil fertility and health, and their activities play a key role in the carbon, nitrogen, and phosphorus biogeochemical cycles and nutrient mineralization processes [56,57]. Therefore, we could evaluate the soil properties suitable for the growth of yam and provide the basis for the selection of high-quality yam directional cultivation bases by analyzing the plant growth and main soil physicochemical parameters under different foliar fertilizer treatments (Table S2).
4.3. Effect of Foliar Fertilizer on Fungal Community in Rhizosphere Soil of Chinese Yam
Soil microorganisms play an important role as decision-makers or conductors in the plant–soil interaction system, and their changes may have beneficial or adverse effects on plants and also impact the stability and health of the soil ecosystem [58,59]. In the present study, there were significant differences in the composition of fungal communities in the yam rhizosphere after spraying different foliar fertilizers. Among them, Ascomycota and Moraxella were the dominant phyla in different treatments, and many studies have shown that Ascomycota was dominant in the fungal community [60,61]. Interesting, Alternaria, Titaea, and Metarhizium were significantly correlated with macroelement fertilizers, and unidentified_Mortierellales_sp and Diaporthe had the highest enrichment in total element fertilizers, while Humicola, unidentified_Dothideomycetes_sp, and Mortierella had a preference for microelement fertilizers. Many studies have reported that the application of different fertilizers could affect not only the structure and distribution of the soil microbial community but also change the proportion of each flora [19,62,63].
The growth and distribution of microorganisms settled in the rhizosphere soil are closely related to the soil’s mineral nutrition. At present, research mainly focuses on the absorption of nutrients by plant roots, while there are few studies on the regulation of nutrients by plant leaves and the changes in rhizosphere soil microbial communities. In the current study, variance partitioning analysis showed that the factors affecting the changes in soil fungi are not individual but the result of the comprehensive actions of multiple factors. Of these factors, the role of soil nutrients was the most prominent. The establishment of an SEM model enables us to further understand that there are some direct or indirect conduction relationships between these factors. For example, on the one hand, macroelement fertilizer indirectly affected soil fungal diversity by directly affecting yam growth and chemical composition and soil parameters; on the other hand, macroelement fertilizer indirectly affected soil nutrition by directly affecting yam growth and chemical composition and then affected the diversity of soil fungi through the changes in soil nutrition. Our results further confirmed that there is a synergistic path of “leaf-root-soil microorganisms” in the plant and its living environment. This is similar to the “brain-gut-microorganism” relationship discussed in human biology in recent years [64,65].
5. Conclusions
In the current study, we found that spraying foliar fertilizer can directly affect the growth and chemical content of Chinese yam, transport nutrients to root tissue through leaves, and then regulate soil environments. Furthermore, the effects of foliar fertilizer on Chinese yam growth and soil environment depend on the combination of different nutrient elements. In particular, there were obvious preferences between fungal genera and nutrient elements, and soil microelements positively influenced soil fungi, while soil macroelements negatively influenced soil fungi. These data are helpful to deeply understand the synergistic relationship between “leaf-root-soil environments” and also provide guidance for the application of eco-friendly foliar fertilizer in the cultivation of medicinal plants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12092017/s1, Figure S1: The rarefaction curve (A) and the abundance ranking of operational taxonomic units (OTUs) (B) of Chinese yam rhizosphere fungi under different foliar fertilizer. CK, spray with water; F1, NPK foliar fertilizer; F2, NPK+Fe-Mn-Zn foliar fertilizer; F3, Fe-Mn-Zn foliar fertilizer. Table S1. Mantel tests showing correlationships (R values) between MAE, MIE, plant growth parameters, active ingredients parameters, soil physicochemical parameters and fungi community composition. * p < 0.05, ** p < 0.01, and *** p < 0.001. MAE, macroelements; MIE, microelements; PGI: plant growth parameters; PAIC, plant active ingredients; PMA, plant macroelements; PMI, plant microelements; SEA, soil enzyme activity; SMA, soil macroelements; SMI, soil microelements; SPC, soil physicochemical properties; Fungi, soil fungi. Table S2. Optimal range of soil parameters for yam growth under foliar fertilizer treatment.
Author Contributions
Conceptualization, C.H. and X.L.; methodology, C.H. and X.L.; formal analysis, C.H. and L.Z.; investigation, C.H. and L.Z.; resources, C.H. and X.L.; writing—original draft preparation, C.H.; writing—review and editing, C.H. and X.L.; supervision, X.L.; funding acquisition, X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Collaborative Innovation Project of Science and Technology Innovation Project of Chinese Academy of Agricultural Sciences (CAA-XTCX20190025-6).
Data Availability Statement
Complete culture-independent sequence data sets were submitted to the NCBI Short Read Archive (SRA) data-base under the accession number PRJNA789711. All data generated or analyzed during this study are included in this article and its supporting information files.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ma, F.Y.; Zhang, Y.; Wen, Y.R.; Yao, Y.N.; Zhu, J.H.; Liu, X.H.; Bell, A.; Tikkanen-Kaukanen, C. Emulsification properties of polysaccharides from Dioscorea opposita Thunb. Food Chem. 2017, 221, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bai, B.; Liu, X.; Wang, Y.; Li, M.; Zhao, D. A-Glucosidase inhibitors from Chinese yam (Dioscorea opposita Thunb.). Food Chem. 2011, 126, 203–206. [Google Scholar] [CrossRef]
- Zheng, K.Y.; Zhang, Z.; Zhou, W.; Cao, H.; Xiang, F. New phenanthrene glycosides from Dioscorea opposita. J. Asian Nat. Prod. Res. 2014, 16, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Ng, T.B. A lectin with highly potent inhibitory activity toward breast cancer cells from edible tubers of Dioscorea opposita cv. nagaimo. PLoS ONE 2013, 8, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.; Deng, J.; Chang, H.; Chen, Y.; Lee, M.; Hou, W.; Lee, C.; Huang, S.; Huang, G. Antioxidant and anti-inflammatory properties of Taiwanese yam (Dioscorea japonica Thunb.var. pseudojaponica (Hayata) Yamam.) and its reference compounds. Food Chem. 2013, 141, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Khanac, M.Z.H.; Yuan, T.T.; Zhang, Y.W.; Liu, X.H.; Du, Z.L.; Zhao, Y.Y. Preparation and characterization of Dioscorea opposita Thunb polysaccharide-zinc inclusion complex and evaluation of anti-diabetic activities. Int. J. Biol. Macromol. 2019, 121, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.H.; Liu, C.; Huang, M.L.; Liu, K.Z.; Yan, D.Y. Effects of foliar fertilization: A review of current status and future perspectives. J. Soil Sci. Plant Nut. 2021, 21, 104–118. [Google Scholar] [CrossRef]
- Nasiri, Y.; Zehtab-Salmasi, S.; Nasrullahzadeh, S.; Najafi, N.; Ghassemi-Golezani, K. Effects of foliar application of micronutrients (Fe and Zn) on flower yield and essential oil of chamomile (Matricaria chamomilla L.). J. Med. Plants Res. 2010, 4, 1733–1737. [Google Scholar] [CrossRef]
- Marzouk, N.M.; Abd-Alrahman, H.A.; Tanahy, E.L.; Mohmouud, S.H. Impact of foliar spraying of nano micronutrient fertilizers on the growth, yield, physical quality, and nutritional value of two snap bean cultivars in sandy soils. Bull. Natl. Res. Cent. 2019, 43, 84. [Google Scholar] [CrossRef]
- Reuvevi, R.; Reuveni, M. Foliar-fertilizer therapy—A concept in integrated pest management. Crop Prot. 1998, 17, 111–118. [Google Scholar] [CrossRef]
- Dong, S.F.; Cheng, L.L.; Carolyn, F.S.; Fuchigami, L.H. Nitrogen absorption, translocation and distribution from urea applied in autumn to leaves of young potted apple (Malus domestica) trees. Tree Physiol. 2002, 22, 1305–1310. [Google Scholar] [CrossRef]
- Anees, M.A.; Ali, A.; Shakoor, U.; Ahmed, F.; Hasnain, Z.; Hussain, A. Foliar applied potassium and zinc enhances growth and yield performance of maize under rainfed conditions. Int. J. Agric. Biol. 2016, 18, 1025–1032. [Google Scholar] [CrossRef]
- JanuškaItIenė, I.; kacIenė, G. The effects of foliar spray fertilizers on the tolerance of Hordeum vulgare to UV-B radiation and drought stress. Cereal Res. Commun. 2017, 45, 390–400. [Google Scholar] [CrossRef]
- Ali, M.M.E.; Petropoulos, S.A.; Selim, D.A.H.; Elbagory, M.; Othman, M.M.; Omara, A.E.D.; Mohamed, M.H. Plant growth, yield and quality of potato crop in relation to potassium fertilization. Agronomy 2021, 11, 675. [Google Scholar] [CrossRef]
- Sun, L.; He, C.L. The effects of reducing fertilizer application on tomato production, quality and soil nitrate in Chaohu lake basin. Chin. Agric. Sci. Bull. 2011, 27, 250–255. [Google Scholar]
- Moon, J.Y.; Min, B.K.; Shin, J.H.; Choi, Y.C.; Cho, H.J.; Lee, Y.H.; Min, S.R.; Heo, J.Y. Influence of foliar fertilization with monopotassium phosphate on growth and yield of sweet potato (Ipomoea batatas L.). Korean J. Soil Sci. Fert. 2019, 52, 217–225. [Google Scholar] [CrossRef]
- Amerany, F.F.; Meddich, A.; Wahbi, S.; Porzel, A.; Taourirte, M.; Rhazi, M.; Hause, B. Foliar Application of chitosan increases tomato growth and influences mycorrhization and expression of endochitinase-encoding genes. Int. J. Mol. Sci. 2020, 21, 535. [Google Scholar] [CrossRef]
- Khan, M.H.; Meghvansi, M.K.; Gupta, R.; Veer, V.; Singh, L.; Kalita, M.C. Foliar spray with vermiwash modifies the arbuscular mycorrhizal dependency and nutrient stoichiometry of Bhut Jolokia. PLoS ONE 2014, 9, e92318. [Google Scholar] [CrossRef]
- Aghaei, K.; Pirbalouti, A.G.; Mousavi, A.; Badi, H.N.; Mehnatkesh, A. Effects of foliar spraying of L-phenylalanine and application of biofertilizers on growth, yield, and essential oil of hyssop [Hyssopus officinalis L. subsp. angustifolius (Bieb.)]. Biocatal. Agri. Biotechnol. 2019, 21, 101318. [Google Scholar] [CrossRef]
- Butler, J.L.; Williams, M.A.; Bottomley, P.J.; Bottomley, P.J.; Myrold, D.D. Microbial community dynamics associated with rhizosphere carbon flow. Appl. Environ. Microbiol. 2003, 69, 6793–6800. [Google Scholar] [CrossRef]
- Cosme, M.; Wurst, S. Interactions between arbuscular mycorrhizal fungi, rhizobacteria, soil phosphorus and plant cytokinin deficiency change the root morphology, yield and quality of tobacco. Soil Biol. Biochem. 2013, 57, 436–443. [Google Scholar] [CrossRef]
- Garbeva, P.; van Elsas, J.D.; van Veen, J.A. Rhizosphere microbial community and its response to plant species and soil history. Plant Soil 2008, 302, 19–32. [Google Scholar] [CrossRef]
- Wu, Z.X.; Hao, Z.P.; Sun, Y.Q.; Guo, L.P.; Huang, L.Q.; Zeng, Y.; Wang, Y.; Yang, L.; Chen, B.D. Comparison on the structure and function of the rhizosphere microbial community between healthy and root-rot Panax notoginseng. Appl. Soil Ecol. 2016, 107, 99–107. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, L.M.; Zheng, Y.M.; Di, H.J.; He, J.Z. Abundance and community composition of methanotrophs in a Chinese paddy soil under long-term fertilization practices. J. Soils Sediments 2008, 8, 406–414. [Google Scholar] [CrossRef]
- Hu, J.L.; Lin, X.G.; Wang, J.H.; Dai, J.; Chen, R.R.; Zhang, J.B.; Wong, M.H. Microbial functional diversity, metabolic quotient, and invertase activity of a sandy loam soil as affected by long-term application of organic amendment and mineral fertilizer. J. Soils Sediments 2011, 11, 271–280. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Chen, L.; Huang, G.; Hu, J. Preparation, deproteinization, characterisation, and antioxidant activity of polysaccharide from cucumber (Cucumis saticus L.). Int. J. Biol. Macromol. 2018, 108, 408–411. [Google Scholar] [CrossRef]
- Liu, Q.; Ge, X.; Chen, L.; Cheng, D.; Yun, Z.; Xu, W.; Shao, R. Purification and analysis of the composition and antioxidant activity of polysaccharides from Helicteres angustifolia L. Int. J. Biol. Macromol. 2018, 107, 2262–2268. [Google Scholar] [CrossRef]
- Bao, S.D. Agrochemical Analysis of Soil; Chinese Agricultural Press: Beijing, China, 2000; pp. 44–49. [Google Scholar]
- Rowell, D.L. Soil Science: Methods and Applications; Longman Group: London, UK, 1994. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA Circular; US Department of Agriculture: Washington, DC, USA, 1954; Volume 939, pp. 1–18.
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India Pvt. Ltd.: New Delhi, India, 1973; pp. 38–56. [Google Scholar]
- Hoffmann, G.G.; Teicher, K. A colorimetric technique for determining urease activity in soil. Dung Boden 1961, 95, 55–63. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Marschner, H. Phosphatase activity in the rhizosphere and hyphosphere of VA mycorrhizal wheat supplied with inorganic and organic phosphorus. Soil Biol. Biochem. 1994, 26, 387–395. [Google Scholar] [CrossRef]
- Adams, R.I.; Miletto, M.; Taylor, J.W.; Bruns, T.D. Dispersal in microbes: Fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J. 2013, 7, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Huson, D.H.; Auch, A.F.; Qi, J.; Schuster, S.C. MEGAN analysis of metagenomic data. Genome Res. 2007, 17, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Maughan, H.; Wang, P.W.; Diaz Caballero, J.; Fung, P.; Gong, Y.; Donaldson, S.L.; Yuan, L.J.; Keshavjee, S.; Zhang, Y.; Yau, Y.C.W. Analysis of the cystic fibrosis lung microbiota via serial Illumina sequencing of bacterial 16S rRNA hypervariable regions. PLoS ONE. 2012, 7, e45791. [Google Scholar] [CrossRef]
- Chen, B.; Teh, B.S.; Sun, C.; Hu, S.; Lu, X.; Boland, W.; Shao, Y.Q. Biodiversity and activity of the gut microbiota across the life history of the insect herbivore Spodoptera Litt. Sci. Rep. 2016, 6, 29505. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D. Vegan: Community Ecology Package, R package version.2016.2.4–1; Available online: http://cran.r-project.org/package=vegan.
- Goslee, S.C.; Urban, D.L. The ecodist package for dissimilarity-based analysis of ecological data. J. Stat. Softw. 2007, 22, 1–19. [Google Scholar] [CrossRef]
- Fageria, N.K.; Barbosa Filho, M.P.; Moreira, A.; Guimarães, C.M. Foliar fertilization of crop plants. J. Plant Nutr. 2009, 32, 1044–1064. [Google Scholar] [CrossRef]
- Jarecki, W. The reaction of winter oilseed rape to different foliar fertilization with macro- and micronutrients. Agriculture 2021, 11, 515. [Google Scholar] [CrossRef]
- Kentelky, E.; Szekely-Varga, Z. Impact of foliar fertilization on growth, flowering, and corms production of five Gladiolus varieties. Plants 2021, 10, 1963. [Google Scholar] [CrossRef]
- Fernández, V.; Brown, P.H. From plant surface to plant metabolism: The uncertain fate of foliar-applied nutrients. Front. Plant Sci. 2013, 4, 289. [Google Scholar] [CrossRef]
- Jabborova, D.; Sayyed, R.Z.; Azimov, A.; Jabbarov, Z.; Matchanov, A.; Enakiev, Y.; Baazeem, A.; Sabagh, A.E.L.; Danish, S.; Datta, R. Impact of mineral fertilizers on mineral nutrients in the ginger rhizome and on soil enzymes activities and soil properties. Saudi J. Biol. Sci. 2021, 28, 5268–5274. [Google Scholar] [CrossRef]
- Pettigrew, W.T.; Meredith, W.R.J.; Young, L.D. Potassium fertilization effects on cotton lint yield, yield components and reniform nematode populations. Argon. J. 2005, 97, 1245–1251. [Google Scholar] [CrossRef]
- Khalid, K.A. Effect of NP and foliar spray on growth and chemical compositions of some medicinal Apiaceae plants grow in arid regions in Egypt. J. Soil Sci. Plant Nutr. 2012, 12, 617–632. [Google Scholar] [CrossRef]
- Gaj, R.; Borowski-Beszta, J. Effects of foliar fertilization with potassium and micronutrients on potato yield and quality. Eur. J. Hortic. Sci. 2020, 85, 394–400. [Google Scholar] [CrossRef]
- Singh, P.; Dwivedi, P. Micronutrients zinc and boron enhance stevioside content in Stevia rebaudiana plants while maintaining genetic fidelity. Ind. Crop Prod. 2019, 140, 111646. [Google Scholar] [CrossRef]
- Raliya, R.; Franke, C.; Chavalmane, S.; Nair, R.; Reed, N.; Biswas, P. Quantitative understanding of nanoparticle uptake in watermelon. Front. Plant Sci. 2016, 7, 1288. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Carrillo, Y.; Pendall, E.; Morgan, J.A. Rhizosphere priming: A nutrientperspective. Front. Microbiol. 2013, 4, 216. [Google Scholar] [CrossRef]
- Zhou, W.X.; Duan, Y.Y.; Zhang, Y.J.; Wang, H.; Huang, D.H.; Zhang, M.D. Effects of foliar selenium application on growth and rhizospheric soil micro-ecological environment of Atractylodes macrocephala Koidz. S. Afr. J. Bot. 2021, 137, 98–109. [Google Scholar] [CrossRef]
- Srinivasan, V.; Thankamani, C.K.; Dinesh, R.; Kandiannan, K.; Hamza, S.; Leela, N.K.; Zachariah, T.J. Variations in soil properties, rhizome yield, and quality as influenced by different nutrient management schedules in rainfed ginger. Agric. Res. 2019, 8, 218–230. [Google Scholar] [CrossRef]
- Niewiadomska, A.; Sulewska, H.; Wolna-Maruwka, A.; Ratajczak, K.; Waraczewska, Z.; Budka, A. The influence of bio-stimulants and foliar fertilizers on yield, plant features, and the level of soil biochemical activity in white lupine (Lupinus albus L.) cultivation. Agronomy 2020, 10, 150. [Google Scholar] [CrossRef]
- Bana, R.S.; Grover, M.; Kumar, V.; Jat, G.S.; Kuri, B.R.; Singh, D.; Kumar, H.; Bamboriya, S.D. Multi-micronutrient foliar fertilization in eggplant under diverse fertility scenarios: Effects on productivity, nutrient biofortification and soil microbial activity. Sci. Hort. 2022, 294, 110781. [Google Scholar] [CrossRef]
- Saha, A.; Pipariya, A.; Bhaduri, D. Enzymatic activities and microbial biomass in peanut field soil as affected by the foliar application of tebuconazole. Environ. Earth Sci. 2016, 75, 558. [Google Scholar] [CrossRef]
- Latkovic, D.; Maksimovic, J.; Dinic, Z.; Pivic, R.; Stanojkovic, A.; Stanojkovic-Sebic, A. Case study upon foliar application of biofertilizers affecting microbial biomass and enzyme activity in soil and yield related properties of maize and wheat grains. Biology 2020, 9, 452. [Google Scholar] [CrossRef] [PubMed]
- Zuppinger-Dingley, D.; Schmid, B.; Petermann, J.S.; Yadav, V.; De Deyn, G.B.; Flynn, D.F. Selection for niche differentiation in plant communities increases biodiversity effects. Nature 2014, 515, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Xu, J.; Riera, N.; Jin, T.; Li, J.Y.; Wang, N.A. Huanglongbing impairs the rhizosphere-to-rhizoplane enrichment process of the citrus root-associated microbiome. Microbiome 2017, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, M.K.; Dixon, K.; Commander, L.; Nevill, P.; Quoreshi, A.M.; Bhat, N.R.; Manuvel, A.J.; Sivadasan, M.T. Assessment of the diversity of fungal community composition associated with Vachellia pachyceras and its rhizosphere soil from Kuwait Desert. Front. Microbiol. 2019, 10, 63. [Google Scholar] [CrossRef]
- He, C.; Zeng, Q.; Chen, Y.L.; Chen, C.X.; Wang, W.Q.; Hou, J.L.; Li, X.E. Colonization by dark septate endophytes improves the growth and rhizosphere soil microbiome of licorice plants under different water treatments. Appl. Soil Ecol. 2021, 166, 103993. [Google Scholar] [CrossRef]
- Bakker, M.G.; Chaparro, J.M.; Manter, D.K.; Vivanco, J.M. Impacts of bulk soil microbial community structure on rhizosphere microbiomes of Zea mays. Plant Soil 2015, 392, 115–126. [Google Scholar] [CrossRef]
- Guo, Q.X.; Yan, L.J.; Korpelainen, H.; Niinemets, U.; Li, C.Y. Plant-plant interactions and N fertilization shape soil bacterial and fungal communities. Soil Biol. Biochem. 2019, 128, 127–138. [Google Scholar] [CrossRef]
- Esteves, A.R.; MunozPinto, M.F.; Nunes-Costa, D.; Candeias, E.; Silva, D.F.; Magalhães, J.D.; Pereira-Santos, A.R.; Ferreira, I.L.; Alarico, S.; Tiago, I.; et al. Footprints of a microbial toxin from the gut microbiome to mesencephalic mitochondria. Gut 2021, 1–17. [Google Scholar] [CrossRef]
- Kohn, N.; Szopinska-Tokov, J.; Llera Arenas, A.; Beckmann, C.F.; Arias-Vasquez, A.; Aarts, E. Multivariate associative patterns between the gut microbiota and large-scale brain network connectivity. Gut Microbes 2021, 13, e2006586. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).