Abstract
Boron (B) fertilizers are recognized as essential for ensuring yield and fruit quality. However, the importance of soil and foliar B fertilization in almond orchards under rainfed conditions is presently unclear. To address this literature gap, in the present study, the impact of soil and foliar application of B on leaf gas exchange, leaf photosynthetic pigments, yield, and fruit quality in almonds was investigated across three consecutive growing seasons. Boron fertilizer was applied to the soil at four rates (0, 1, 2, and 3 kg ha−1) in the presence or absence of foliar application (0.36 g L−1) of the same micronutrient. Borax pentahydrate was used as the B source. When compared to foliar B fertilization, the soil application of B positively affected the gas exchange parameters, mainly the net CO2 assimilation rate, stomatal conductance, and transpiration rate (percentage of gain between 15% and 80%), but did not influence the chlorophyll content. The almond yield and fruit characteristics were also enhanced (about 25–72%) in response to lower soil B fertilization rates. In the 3 kg ha−1 dose, B had an adverse effect on the yield and resulted in lighter fruits. On the other hand, foliar B fertilization did not benefit any of the evaluated parameters. Overall, these results suggest that, under the studied conditions, almond orchards do not respond to higher B rates in soil or foliar B fertilization.
1. Introduction
Almond (Prunus dulcis (Mill.) D.A.Webb.) constitutes an important tree crop in the Mediterranean region, as it is capable of adapting to very harsh edaphoclimatic conditions, such as drought and high temperatures [1] and poor soil nutrients [2]. Within this region, most almond orchards are grown under rainfed conditions and are located on marginal lands, generating low yields and poor fruit quality [3]. One of the best strategies for achieving high production and reducing environmental problems under these challenging conditions is based on a balanced application of fertilizers [4].
Boron (B) application is critical at the beginning of the annual growth, since it plays a pivotal role in almonds’ flowering and fruit set [5]. Boron exhibits a regulatory effect on pollen germination and pollen tube growth, helping to increase flower fertilization and, consequently, crop yield [6,7]. Boron also has an important role in cell structure and cell wall integrity [8,9], as well as in the maintenance of plasma membrane functions [10] and hull formation [6]. Moreover, this positive effect of boron could be due to its involvement in several physiological and metabolic processes [11], such as sugar transport and carbohydrate, nucleic acid, indole acetic acid (IAA), and phenol metabolism [9]. In almonds, B has high phloem mobility due to the formation of B-polyol complexes [12], which facilitate the re-translocation of sugars and other products of photosynthesis, affecting the photosynthetic capacity of plants [13]. Therefore, adequate B nutrition is essential for obtaining high yields and almond fruit quality [14]. Boron fertilizers can be applied to the soil, for uptake by plant roots, or by a foliar spray. The root uptake of B can be reduced under dry soil conditions, and in these circumstances, the leaf application of B has been seen as a complement to soil application for fulfilling boron requirements [15]. Previous studies on applying B fertilizers via the foliar route have yielded contradictory results. For example, [5] reported a positive influence of B supply on an almond yield, while [2] noted no significant effects on a kernel yield. The available evidence further indicates that the response of almond trees to B fertilization depends on the nutritional level of orchards [2], rate and time of application, and environmental conditions at the time of application [11], among other factors. According to [7], the foliar application of B improves the quality (weight, length, width, and protein content) of almond fruits. However, information on the effect of foliar B fertilization on the physiological performance of almonds under rainfed conditions is limited. Recently, [14] reported that the transpiration and assimilation rates, as well as stomatal conductance, increased seven days after the foliar application of B nanoencapsulates compared to the values obtained for nonfertilized almond trees or trees sprayed with free B, pointing to the role of B supply in the photosynthesis process [13].
Guided by the previously obtained results and the importance of an adequate B supply, the goal of the present study was to elucidate the influence of singular and combined (soil and foliar) B applications on almond trees cv. Glorieta during three consecutive years (2015–2017) under rainfed conditions. In particular, the effects of B supply were assessed through leaf gas exchange and leaf chlorophyll, as well as fruit yield and quality.
2. Materials and Methods
2.1. Site Description
The almond orchard (9.5 ha) in focus for this investigation is located near Alfândega da Fé in NE Portugal (41°21′ N, 6°57′ W, 576 m asl). This commercial orchard was planted in 2003, and individual trees (that belong to the Glorieta cultivar, grafted on GF-677 rootstock) are allocated a 6 × 4 m area. The field experiment was performed under rainfed conditions over three years (2015–2017). Although the almond trees were pruned yearly, this practice was more intensive in 2016. No phytosanitary treatments were applied during the experimental period. The soil of the plot on which the field experiment was conducted is classified as a loamy textured dystric Regosol, and its chemical properties are in Table 1.

Table 1.
Mean values of the soil chemical properties at a depth of 0–20 cm, determined at the beginning of the experiment. Adapted from [2,16].
The climate of Alfândega da Fé is of the Mediterranean type (Köppen climate classification: Csb). The mean air temperature and the annual precipitation based on the 1971–2000 measurements at the weather station of Mirandela (41°31′ N, 7°12′ W, 250 m asl) near Alfândega da Fé are, respectively, 14.3 °C and 508.6 mm. The warmest months are July and August, with an average daily temperature of 23.5 °C, and the coldest month is January, with 5.5 °C. Most of the precipitation occurs in the fall and winter, from October to January. The climatic data collected during the study from an automatic weather station located near the experimental orchard revealed that the highest daily temperature was observed in July. The precipitation exhibited marked year-to-year variations during this period, as shown in Figure 1. The highest annual rainfall (820.6 mm) was recorded in 2016, with about 75% of the annual precipitation occurring in the first five months of the year. The intense and continuous precipitation in these months affected the flowering, pollination, and, consequently, the fruit set.
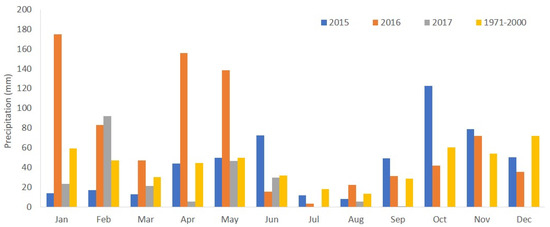
Figure 1.
Monthly precipitation during the study period of 1 January 2015–31 August 2017 and monthly precipitation for the 1971–2000 period at the Mirandela weather station (Adapted from [17]).
2.2. Boron Treatments and Experimental Design
Four rates of B fertilization (0, 1, 2, and 3 kg ha−1) were applied to the soil as borax pentahydrate (Na2B4O7.5H2O) (15% B) late in March, shortly after bloom. For foliar application, a foliar spray containing 0.36 g L−1 B of borax pentahydrate (15% B) was used before and after the harvest. Consequently, in 2015 and 2016, the foliar B application consisted of 1.4 kg B ha−1, while 0.7 kg B ha−1 was applied in 2017. All treatments received soil and foliar phosphorus (P2O5) and potassium (K2O) at 50 kg ha−1year−1. The experimental setup consisted of a randomized block design with three replications, and three similar trees were used in each replication.
2.3. Leaf Gas Exchange Measurements
The leaf gas exchange parameters [net CO2 assimilation rate (A, µmol CO2 m−2s−1), stomatal conductance (gs, mmol H2O m−2s−1), and transpiration rate (E, mmol H2O m−2s−1) were measured using an open portable Infrared Gas Analyzer System (LCpro-SD, ADC BioScentific Ltd., Hoddesdon, UK) equipped with a 6.25 cm2 leaf chamber. The measurements were performed at harvest (September) in situ at midday (14:00–15:30 h) on the uppermost fully expanded leaf from the middle of the canopy of three plants per treatment under a photosynthetic photon flux density (PPFD) that exceeded 1500 µmol m−2s−1. In addition, the intrinsic water use efficiency (iWUE) was calculated using the A/gs ratio (mmol m−2s−1) following [18].
2.4. Relative Leaf Water Content and Electrolyte Leakage Determination
After the gas exchange measurements, the leaves were immediately placed into air-tight containers to determine the relative water content (RWC). The RWC was calculated according to the expression of [19]: RWC (%) = (FW − DW)/(TW − DW) × 100, where FW is the fresh weight (g), DW is the dry weight (g) after drying at 70 °C to a constant weight, and TW is the fresh weight at full turgor (g) measured after the immersion of leaf petioles in distilled water for 24 h in the dark at 4 °C. Leaf discs 0.8 cm in diameter were cut from fully expanded leaves and were used to determine the electrolyte leakage (EL), as described by [20]. After washing with deionized water to remove all surface-adhered electrolytes, the leaf discs were placed in tubes containing 10 mL of deionized water. Then, the tubes were incubated at 25 °C on a shaker for 24 h. After this period, the initial electrical conductivity of the solution (CE1) was determined, and the tubes were then autoclaved at 120 °C for 20 min and were subsequently maintained at 25 °C. A new reading of electrical conductivity (CE2) was performed, and the EL was calculated using the following equation: EL (%) = (CE1/CE2) × 100.
2.5. Leaf Photosynthetic Pigments
The chlorophyll (chlorophyll a (chl a), chlorophyll b (chl b), and total chlorophyll (chl t)) contents in the leaf samples were determined in leaf discs of 0.8 cm in diameter, which were collected immediately after the leaf gas exchange readings, frozen in liquid nitrogen, and stored at −80 °C until required for analysis. Chlorophylls were extracted in 80% acetone (v/v), applying the method described by [21].
2.6. Yield and Fruit Quality Attributes
At harvest, all almond fruits borne by each tree were collected and weighed fresh, and the almond yield (t ha−1) was determined for each treatment. Three samples per treatment containing 50 fruits each were allowed to dry at room temperature for one month. After that, the quality attributes of the nut and kernel (mass, length, width, and thickness) were evaluated according to the almond descriptors developed by the International Plant Genetic Resources Institute or “IPGRI” [22]. The nut number (the number of fruits per kilogram) was also determined as a measure of the almond yield [23]. The nut and kernel mass were measured using a precision scale, while the nut and kernel length, width, and thickness were measured using digital calipers with 0.01-mm accuracy.
2.7. Statistical Analysis
All data were analyzed using a repeated measures ANOVA with the year of observation as the within-subject factor and the B fertilization routes (soil and foliar) as the between-group factors. A two-way ANOVA was also used to determine the effects of the B supply (soil and foliar) within each year. All data were subjected to the Shapiro–Wilk W statistic and Levene’s test to assess the distribution normality and homogeneity of variance, respectively. Mean differences were separated by applying Tukey’s HSD test at a 5% significance level. A hierarchical clustering analysis using Ward’s method and Euclidean distances was adopted to investigate the similarities among the soil B treatments.
Statistical analyses were performed using the software package IBM SPSS version 23 for Windows (Orchard Road, Armonk, NY, USA).
3. Results
3.1. Effect of Year and B Supply on Leaf Gas Exchange Parameters
The three-year data of leaf gas exchange parameters (Table S1) showed that all parameters varied greatly from one year to another (p < 0.05). Except for iWUE, the highest E, gs, and A were observed in 2016, and there was no significant difference between 2015 and 2017 (Table 2). These parameters also exhibited high variations concerning soil and foliar fertilization (Table S1).

Table 2.
Mean values (±standard error) of the leaf gas exchange parameters (E, gs, A, and iWUE); RWC; EL; photosynthetic pigments (chl a, chl b, chl t, and chl a/chl b); yield, nut number; and nut and kernel attributes (mass, length, width, and thickness) determined during the 2015–2017 period.
The photosynthetic performance of almond trees was higher in the absence of foliar B fertilization (Table 3). When the data obtained in individual years were analyzed separately, soil B fertilization (treatments B1–B3) significantly affected almost all gas exchange parameters in 2015 and 2017 (Table 2). In these years, the highest values of E, gs, and A were observed with the application of 2 kg ha−1 (B2 treatment). Conversely, no significant effect of B soil fertilization was found in iWUE (p > 0.05). The values of E, gs, A, and iWUE in the trees subjected to foliar B application (F2) were not as high as in the treatment with no foliar B application (F0) in any of the individual years (Table 3). Although significant for many of the parameters measured, the combination of soil and foliar B supply did not have a positive effect on E, gs, A, or iWUE. In fact, in all years, the highest values of the gas exchange parameters were achieved in trees fertilized by B via soil application in the absence of foliar B supplementation (Table 3).

Table 3.
Mean values (±standard error) of the gas exchange parameters (transpiration rate (E), stomatal conductance (gs), net CO2 assimilation rate (A), and water-use efficiency (iWUE)) of almond leaves measured in the 2015–2017 period.
The cluster analysis identified similarities between the B0, B1, and B3 treatments (Figure 2). However, the B0 and B1 branched more closely to each other than the B3 treatment. The B2 treatment formed a separate cluster demonstrating its superiority compared to the other soil B rates.
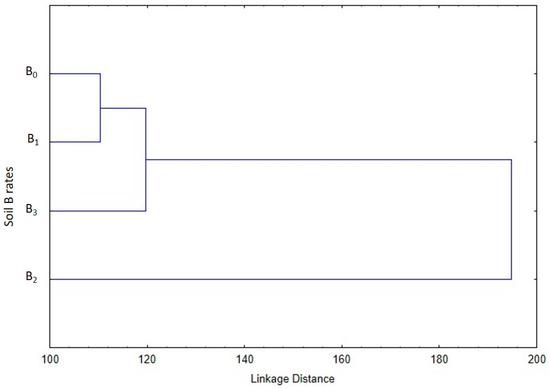
Figure 2.
Cluster analysis (Euclidean distances and Ward’s hierarchical method) based on the leaf gas exchange parameters of different soil B rates.
3.2. Effect of Year and B Supply on Leaf Relative Water Content and Electrolyte Leakage
The leaf relative water content (RWC) and electrolyte leakage (EL) were significantly influenced by year and foliar fertilization (Table S1). In general, the highest RWC values were obtained in 2016, followed by 2017 and 2015 (Table 2). In turn, the highest and the lowest EL values were obtained in 2015 and 2016, respectively (Table 2). On the other hand, throughout the study period, similar leaf RWC and EL (Table 4) were obtained for all soil treatments (p > 0.05). Foliar B application only influenced the leaf RWC in 2017 and EL in 2015 and 2017 (Table 4), but the interaction between soil and foliar fertilization did not have a significant effect on the leaf RWC or EL values (Table S1).

Table 4.
Mean values (±standard error) of the RWC and EL under different B soil rates (B0, B1, B2, and B3) associated with the absence or presence of foliar B supplementation in the 2015–2017 period. In each column, different lowercase letters indicate significant differences among the B soil rates (B0, B1, B2, and B3) within each year.
3.3. Effect of Year and B Supply on Leaf Photosynthetic Pigments
The chlorophyll content (chl a, chl b, and chl t) varied among the years (p < 0.001) (Table S2) and was the highest in 2017 (Table 2). In addition, the chl a/chl b ratio followed a decreasing trend during the three-year growing seasons (Table 2). In 2017, the chl a/chl b ratio was ca. 45% and 22% lower than the values obtained in 2015 and 2016, respectively (Table 2). In general, applying B to the soil or as a foliar spray did not influence the contents of leaf photosynthetic pigments in individual years (Table 5). In 2015 and 2016, the chl a/chl b ratio was influenced by soil B fertilization, but its effects exhibited different patterns. In 2015, this ratio was the highest in the absence of soil B application, but no differences were noted between the B1 and B3 treatments. In turn, in 2016, the highest and the lowest ratio was observed in the B1 and B0 treatments, respectively (Table 5). For most of the leaf photosynthetic pigments, the interaction between soil and foliar B fertilization did not produce significant effects (p > 0.05). The exceptions to this finding are the chl a/chl b ratio in 2015, the chl t value in 2016, and the chl a content in 2017.

Table 5.
Mean (±standard error) photosynthetic pigments content (chl a, chl b, chl t, and chl a/chl b) of almond leaves measured in the 2015–2017 period.
3.4. Effect of Year and B Supply on Almond Yield and Fruit Quality Attributes
The analyses revealed marked variations in the annual yield (Table S3), ranging from 0.09 t ha−1 in 2016 to about 4.4 t ha−1 in 2015 and 2017 (Table 2). The almond yield was also affected by soil B fertilization (Table S3), which was substantially reduced in trees fertilized with 3 kg ha−1 (B3 treatment), especially in 2017 (Table 6). Additionally, foliar B application did not improve the almond yield (p > 0.05) (Table 6).

Table 6.
Mean almond yield and nut number (± SE) under different B soil rates (B0, B1, B2, and B3) associated with the absence or presence of foliar B supplementation in the 2015–2017 period.
When the data of each year were analyzed separately, the findings indicated that foliar B application had no significant effect on the almond yield, while the soil application had a significant effect in 2017 only (Table 6). In this year, the highest almond yield was observed in the B1 treatment (5.28 ± 0.38 t ha−1), but this value was not significantly different from that obtained for the control treatment (4.08 ± 0.39 t ha−1). In all the years, the lowest nut yield was observed under the B3 treatment (Table 6).
The cumulative, three-year almond yield was significantly affected by soil B fertilization (p < 0.05). The highest yield (ca. 10.00 ± 0.83 t ha−1) was recorded with B application in the 1 kg ha−1 amount but was not statistically different from the yields obtained in the control and the B2 treatment (Figure 3). The application of B at rates exceeding 2 kg ha−1 did not positively affect the almond yield, as the lowest yield was recorded with the application of 3 kg ha−1. Moreover, foliar B application did not improve the almond yield (p > 0.05).
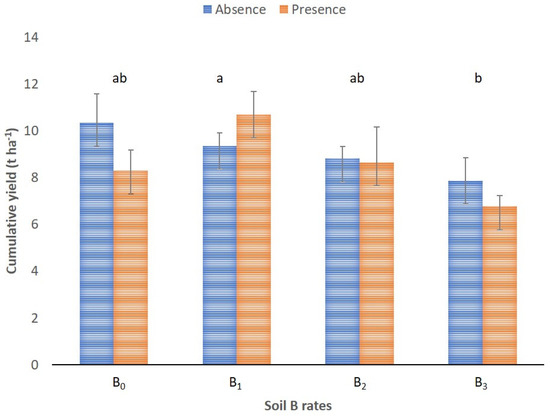
Figure 3.
Cumulative almond yield (t ha−1) after three consecutive harvests by soil B rates (B0, B1, B2, and B3) with the absence and presence of foliar B supplementation. Data are the means ± standard error. Different lowercase letters indicate significant differences among the B soil rates, according to Tukey’s HSD test (p < 0.05).
The nut number (Table S3) was affected by the study year, the soil, and foliar B fertilization (p < 0.001). As can be seen from Table 2, the nut number was the lowest in 2016 (224 ± 2) and the highest in 2017 (347 ± 2). The soil and foliar fertilization, as well as their interaction, had a significant effect on this parameter (Table S1). In all the years, the best results were observed under soil treatments B1 and B2 and in the absence of foliar fertilization (Table 6).
The year, soil, and foliar fertilization effect on the fruit characteristics were significant (Table S3). The majority of the nut and kernel attributes increased from the first to the second experimental year, decreasing substantially thereafter (Table 2). Independently of the year, both the nut and kernel dimensions were influenced by soil and foliar fertilization and their interaction (Table S3). In general, B applied via foliar sprays did not positively influence the nut and kernel dimensions, since the highest values were observed under soil fertilization (Table 7 and Table 8). Comparing all B soil treatments, the 2 kg ha−1 application rate yielded the best results for these parameters (Table 7 and Table 8).

Table 7.
Mean values (±SE) of the nut attributes (mass, length, width, and thickness) under different B soil rates (B0, B1, B2, and B3) associated with the absence or presence of foliar B supplementation in the 2015–2017 period.

Table 8.
Mean values (±SE) of the kernel attributes (mass, length, width, and thickness) under different B soil rates (B0, B1, B2, and B3) associated with the absence or presence of foliar B supplementation in the 2015–2017 period.
The cluster analysis performed on all nut and kernel data revealed high similarities between the B0 and B3 treatments, but they did not substantially differ from the B1 treatment. On the other hand, the B2 treatment formed a separate group (Figure 4).
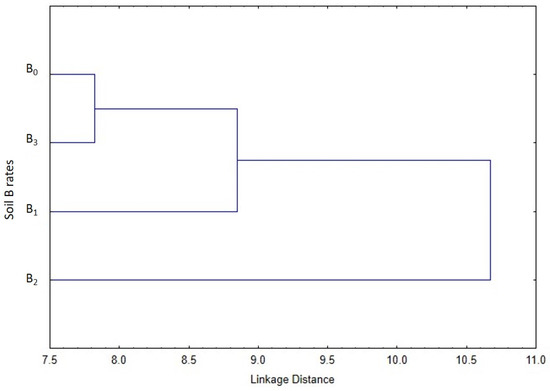
Figure 4.
Cluster analysis (Euclidean distances and Ward’s hierarchical method) based on the fruit characteristics (nut and kernel mass, length, width, and thickness) of different soil B rates.
4. Discussion
This study revealed that the boron application method had different effects on the physiological performance, yield, and fruit quality of almond trees. Under the conditions adopted for this experiment, almond trees responded favorably to boron application in the soil. The soil B rates tended to increase the leaf gas exchange parameters, including net CO2 assimilation rate, stomatal conductance, and transpiration rate. This observation is consistent with the results obtained in other studies on tree nuts and illustrates the pivotal role of B fertilization in several physiological processes affecting photosynthesis [13]. The positive effect of B on the photosynthetic performance of almond trees is especially significant when applied at a 2 kg ha−1 dose to the soil, since the highest leaf photosynthetic and transpiration rates, as well as the stomatal conductance, were observed in this treatment in all years of the study. The results of this study also indicated a tendency of increased RWC and decreased EL values in leaves subjected to this B rate, further substantiating the assertion that almond trees under this treatment exhibited better physiological performances. At a higher B rate (3 kg ha−1), the values of all the gas exchange parameters declined, suggesting that this amount of B fertilizer was unnecessary under the studied conditions. Moreover, it is widely established that excessive B application may hinder photosynthesis [24,25,26] and reduce yield [27,28]. During the study period, especially in the first and second years, B application at the highest rate (3 kg ha−1) resulted in the lowest yield, but the difference relative to other B rates was not statistically significant. This finding was unexpected, since the role of B in yield is well-documented [29]. Highly significant year-by-year yield variations were also observed, whereby similar values were obtained in 2015 and 2017, while a sharp decline was noted in 2016. This pattern is indicative of the alternate bearing that is characteristic of the species and is probably due to the harsh meteorological conditions observed in 2016. The intense precipitation in the first five months of 2016 during the bloom period caused flower drops and reduced flower pollination activity, severely impacting the yield [2]. However, it is important to point out that the observed decrease in the almond yield was less pronounced when B was applied at low rates, suggesting their beneficial influence on the yield during low production periods. This statement was also corroborated by the results obtained for the nut number and fruit quality parameters, which varied significantly among the years. The highest dimensions (mass, length, width, and thickness) of the nuts and kernels were observed in 2016 and were strongly associated with a reduction in the nut number. Comparing all soil B treatments, B application at a 2 kg ha−1 rate increased the nut and kernel size, and this effect was consistent across all three years.
Even though foliar boron application is often seen as more effective in improving plant nutrition, crop yield, and fruit quality than soil fertilization [30,31], the results did not confirm these observations. Foliar B application, either alone or in combination with soil fertilization, did not result in significant yield augmentation or the superior physiological performance of almond trees in any of the study years. Moreover, B sprays did not influence the physical attributes of almonds. The number of fruits increased, and the average yield and fruit mass (nut + kernel) decreased in response to foliar sprays. In their study focusing on hazelnut (cv. Butler), [32] noted that B sprays did not affect the fruit set and yield. Their findings contrasted with those reported by [11,33], which indicated that the foliar application of B enhanced the fruit set and yield in almond trees (cv. Butte, Mono, and Ruby). Moreover, [7] noted that foliar B significantly increased the fruit set and quality traits of almonds (cv. Azar). More recently, [14] found that the foliar application of B nanoencapsulated in almond trees (cv. Avijor) led to a significant increase in the yield. The B availability in the soil could justify the absence of the beneficial influence of foliar B fertilization in our study. According to [2], the B level in the soil of the almond orchard (1.2 ± 0.02 mg kg−1) is considered high for rainfed managed orchards, indicating that B requirements are very low. As stated by [34], foliar B application is more effective in correcting B deficiencies than soil-derived B in leaves. The discrepancy in the effectiveness of foliar B sprays in almonds can also be potentially attributed to the B requirements of cultivars and the timing of foliar B application. In the present study, the first foliar B spray occurred in the spring, and the second was performed after the harvest [2]. At this time, there was no foliage in the trees, which could have compromised the translocation and B metabolism [11].
5. Conclusions
The results of the present study indicate that foliar B application had no significant effect on the physiological performance of almond trees, yield, or almond quality, which contrasted with low B fertilization rates applied to the soil. Therefore, under the conditions at which the study was performed, foliar B supplementation and a high B fertilization rate (3 kg ha−1) are ineffective and represent an unnecessary cost. However, further studies are needed to determine the best source, doses, and time of foliar boron application in almonds grown in rainfed conditions.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy12092005/s1, Table S1: Results from repeated measures ANOVA of the effects of soil and foliar B fertilization throughout time (year) on the leaf gas exchange parameters (E (mmol m−2s−1), gs (mmol m−2s−1), A (µmol m−2s−1), and iWUE (µmol mol−1)); RWC (%); and EL (%). Table S2: Results from repeated measures ANOVA of the effects of soil and foliar B fertilization throughout time (year) on the leaf photosynthetic pigments (chl a, chl b, chl t, and chl a/chl b). Table S3: Results from repeated measures ANOVA of the effects of soil and foliar B fertilization throughout time (year) on the yield; nut number; and nut and kernel attributes (mass, length, width, and thickness).
Author Contributions
Conceptualization, A.P.S., A.C.R., M.Â.R. and B.G.; methodology, A.P.S., B.G., A.A. and M.C.M.; investigation, A.A. and M.C.M. with the help of A.P.S., B.G., A.C.R., D.B. and M.Â.R.; writing—original draft preparation, M.C.M.; and writing—review and editing, A.P.S., A.A., A.C.R. and M.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was funded by the project “Estratégias integradas para o aumento da produtividade da amendoeira em Trás-os-Montes”, no. 54611, funded by the EAFRD (European Agricultural Fund for Rural Development), and by the Portuguese State through the “Medida 4.1. Cooperação para a Inovação do programa PRODER-Programa de Desenvolvimento Rural”. The authors also acknowledge to the Foundation for Science and Technology (FCT, Portugal) and FEDER under Programme PT2020 for financial support to CIMO (UIDB/00690/2020) and CITAB (UIDB/04033/2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Ana Monteiro, Cristiana Teixeira, Iva Prgomet, Ivo Oliveira, Linton Dinis, Sara Bernardo, Sílvia Afonso, and Silvina Morais for their support in the field and in the laboratory.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Prgomet, I.; Pascual-Seva, N.; Morais, M.C.; Aires, A.; Barreales, D.; Ribeiro, A.C.; Silva, A.P.; Barros, A.I.; Gonçalves, B. Physiological and biochemical performance of almond trees under deficit irrigation. Sci. Hortic. 2020, 261, 108990. [Google Scholar] [CrossRef]
- Arrobas, M.; Ribeiro, A.; Barreales, D.; Pereira, E.L.; Rodrigues, M.A. Soil and foliar nitrogen and boron fertilization of almond trees grown under rainfed conditions. Eur. J. Agron. 2019, 106, 39–48. [Google Scholar] [CrossRef]
- Durán-Zuazo, V.H.; Rodriguez, B.C.; Gutiérrez-Gordillo, S.; Benítez, M.B.; Sacristán, P.C.; Parra, J.J.P.; García-Tejero, I.F. Rethinking irrigated almond and pistachio intensification: A shift towards a more sustainable water management paradigm. Rev. Cienc. Agrar. 2020, 43, 24–49. [Google Scholar] [CrossRef]
- Muhammad, S.; Sanden, B.L.; Saa, S.; Lampinen, B.D.; Smart, D.R.; Shackel, K.A.; DeJong, T.M.; Brown, P.H. Optimization of nitrogen and potassium nutrition to improve yield and yield parameters of irrigated almond (Prunus dulcis (Mill.) D. A. Webb). Sci. Hortic. 2018, 228, 204–212. [Google Scholar] [CrossRef]
- Nyomora, A.M.S.; Brown, P.H.; Krueger, B. Rate and time of boron application increase almond productivity and tissue boron concentration. HortScience 1999, 34, 242–245. [Google Scholar] [CrossRef]
- Nyomora, A.M.S.; Brown, P.H.; Pinney, K.; Polito, V.S. Foliar application of boron to almond trees affects pollen quality. J. Am. Soc. Hortic. Sci. 2000, 125, 265–270. [Google Scholar] [CrossRef]
- Bybordi, A.; Malakouti, M.J. Effects of foliar applications of nitrogen, boron and zinc on fruit setting and quality of almonds. Acta Hortic. 2006, 726, 2351–2358. [Google Scholar] [CrossRef]
- Lewis, D.H. Boron: The essential element for vascular plants that never was. New Phytol. 2019, 221, 1685–1690. [Google Scholar] [CrossRef]
- Shireen, F.; Nawaz, M.A.; Chen, C.; Zhang, Q.; Zheng, Z.; Sohail, H.; Sun, J.; Cao, H.; Huang, Y.; Bie, Z. Boron: Functions and approaches to enhance its availability in plants for sustainable agriculture. Int. J. Mol. Sci. 2018, 19, 1856. [Google Scholar] [CrossRef]
- Brown, P.H.; Bellaloui, N.; Wimmer, M.A.; Bassil, E.S.; Ruiz, J.; Hu, H.; Pfeffer, H.; Dannel, F.; Römheld, V. Boron in plant biology. Plant Biol. 2002, 4, 205–223. [Google Scholar] [CrossRef]
- Blevins, D.; Lukaszewski, K. Boron in plant structure and function. Annu. Rev. Plant Biol. 1998, 49, 481–500. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.H.; Hu, H. Phloem mobility of boron is species dependent: Evidence for phloem mobility in sorbitol-rich species. Ann. Bot. 1996, 77, 497–506. [Google Scholar] [CrossRef]
- Quamruzzaman, M.; Rahman, M.J.; Uddain, J.; Sarkar, M.D.; Subramaniam, S. Leaf gas exchange, reproductive development, physiological and nutritional changes of peanut as influenced by boron. J. Plant Interact. 2018, 13, 306–314. [Google Scholar] [CrossRef]
- Rios, J.J.; Lopez-Zaplana, A.; Bárzana, G.; Martinez-Alonso, A.; Carvajal, M. Foliar application of boron nanoencapsulated in almond trees allows B movement within tree and implements water uptake and transport involving aquaporins. Front. Plant Sci. 2021, 12, 752648. [Google Scholar] [CrossRef]
- Atique-Ur-Rehman; Qamar, R.; Hussain, A.; Sardar, H.; Sarwar, N.; Javeed, H.M.R.; Maqbool, A.; Hussain, M. Soil applied boron (B) improves growth, yield and fiber quality traits of cotton grown on calcareous saline soil. PLoS ONE 2020, 15, e0231805. [Google Scholar] [CrossRef]
- Morais, M.C.; Aires, A.; Barreales, D.; Rodrigues, M.Â.; Ribeiro, A.C.; Gonçalves, B.; Silva, A.P. Combined soil and foliar nitrogen fertilization effects on rainfed almond tree performance. J. Soil Sci. Plant Nutr. 2020, 20, 2552–2565. [Google Scholar] [CrossRef]
- IPMA. Ficha Climatológica Mirandela 1971–2000. Available online: https://www.ipma.pt/bin/file.data/climate-normal/cn_71-00_MIRANDELA.pdf (accessed on 2 June 2022).
- Iacono, F.; Buccella, A.; Peterlunger, E. Water stress and rootstock influence on leaf gas exchange of grafted and ungrafted grapevines. Sci. Hortic. 1998, 75, 27–39. [Google Scholar] [CrossRef]
- Weatherley, P. A convenient volumenometer for biological work. J. Exp. Bot. 1950, 1, 244–248. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Sesták, Z.; Castky, J.; Jarvis, P.G. Plant Photosynthetic Production: Manual of Methods; Dr. W. Junk Publishers: Haia, The Netherlands, 1971. [Google Scholar]
- Gülcan, R. International Board for Plant Genetic Resources. In Almonds Descriptors; IBPGR Secretariat: Rome, Italy, 1985. [Google Scholar]
- Reidel, E.J.; Brown, P.H.; Duncan, R.A.; Weinbaum, S.A. Almond productivity as related to tissue potassium. Better Crops 2001, 85, 21–23. [Google Scholar]
- Chen, L.S.; Han, S.; Qi, Y.P.; Yang, L.T. Boron stresses and tolerance in citrus. Afr. J. Biotechnol. 2012, 11, 5961–5969. [Google Scholar] [CrossRef]
- Choudhary, S.; Zehra, A.; Naeem, M.; Khan, M.M.A.; Aftab, T. Effects of boron toxicity on growth, oxidative damage, antioxidant enzymes and essential oil fingerprinting in Mentha arvensis and Cymbopogon flexuosus. Chem. Biol. Technol. Agric. 2020, 7, 8. [Google Scholar] [CrossRef]
- Landi, M.; Remorini, D.; Pardossi, A.; Guidi, L. Boron excess affects photosynthesis and antioxidant apparatus of greenhouse Cucurbita pepo and Cucumis sativus. J. Plant Res. 2013, 126, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Ben-Gal, A.; Shadi, U. Water use and yield of tomatoes under limited water and excess boron. Plant Soil 2003, 256, 179–186. [Google Scholar] [CrossRef]
- Hapuarachchi, N.S.; Kämper, W.; Wallace, H.M.; Hosseini Bai, S.; Ogbourne, S.M.; Nichols, J.; Trueman, S.J. Boron effects on fruit set, yield, quality and paternity of Hass avocado. Agronomy 2022, 12, 1479. [Google Scholar] [CrossRef]
- Brown, P.H.; Ferguson, L.; Picchioni, G. Boron boosts pistachio yields. Fluid J. 1995, 4, 11–13. [Google Scholar]
- Niu, J.; Liu, C.; Huang, M.; Liu, K.; Yan, D. Effects of foliar fertilization: A review of current status and future perspectives. J. Soil Sci. Plant Nutr. 2021, 21, 104–118. [Google Scholar] [CrossRef]
- Deliboran, A.; Cilgin, I.; Aydogdu, E.; Olmeg, H.A.; Savran, K.; Dursun, O.; Eralp, O.; Pekcan, T.; Turan, H.S.; Savran, S.; et al. Response of olive trees to different boron application in Izmir and Mugla province of Turkey. Commun. Soil Sci. Plant Anal. 2022, 53, 1294–1307. [Google Scholar] [CrossRef]
- Silva, A.P.; Rosa, E.; Haneklaus, S.H. Influence of foliar boron application on fruit set and yield of hazelnut. J. Plant Nutr. 2002, 26, 561–569. [Google Scholar] [CrossRef]
- Nyomora, A.M.S.; Brown, P.F.; Freeman, M. Fall foliar-applied boron increases tissue boron concentration and nut set of almond. J. Am. Soc. Hortic. Sci. 1997, 122, 405–410. [Google Scholar] [CrossRef]
- Shelp, B.J.; Vivekanandan, P.; Vanderpool, R.A.; Kitheka, A.M. Translocation and effectiveness of foliar-fertilizer boron in broccoli plants of varying boron status. Plant Soil 1996, 183, 309–313. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).