Assessment of Polluted Soil Remediation Using Bacterial Community Tolerance to Heavy Metals as an Indicator
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil, Bio-Sorbents and Chemicals
2.2. Experimental Design
2.2.1. Microbial Reactivation and Soil Spiking with Metals
2.2.2. Soil Remediation
- (i).
- (ii).
- (iii).
- Another sub-sample (5 g) was subjected to soil washing with 0.1 M EDTA through a laboratory column experiment. Sub-samples were placed in vertically oriented glass columns (100 mm long x 10 mm inner diameter) up to 3.9 cm in height. To set up the experiment, a peristaltic pump (Gilson Minipuls 3, Middelton, WI, USA) was connected to the lower side of the column (input). A two-way valve was used to connect the pump to two bottles containing distilled water or 0.1 M EDTA. The outgoing liquid was collected at the upper side of the column (output). Once the experiment has been set up, a 0.1 M EDTA solution was circulated through the column for 5 h (2.5 mL·h−1 flow rate), followed by 5 h of distilled water (2.5 mL·h−1 flow rate) [58]. After soil remediation, water-saturated soil was retrieved from the column, air-dried, and rewetted to restore the initial moisture of the experiment. Later, microcosms were incubated for 60 days at 22 °C in the dark.
- (iv).
- The last sub-sample (5 g) was established as control since non-remediation treatment was performed.
2.2.3. Determination of Bacterial Community Tolerance to Cu, Ni and Zn
2.3. Data Analysis
3. Results and Discussion
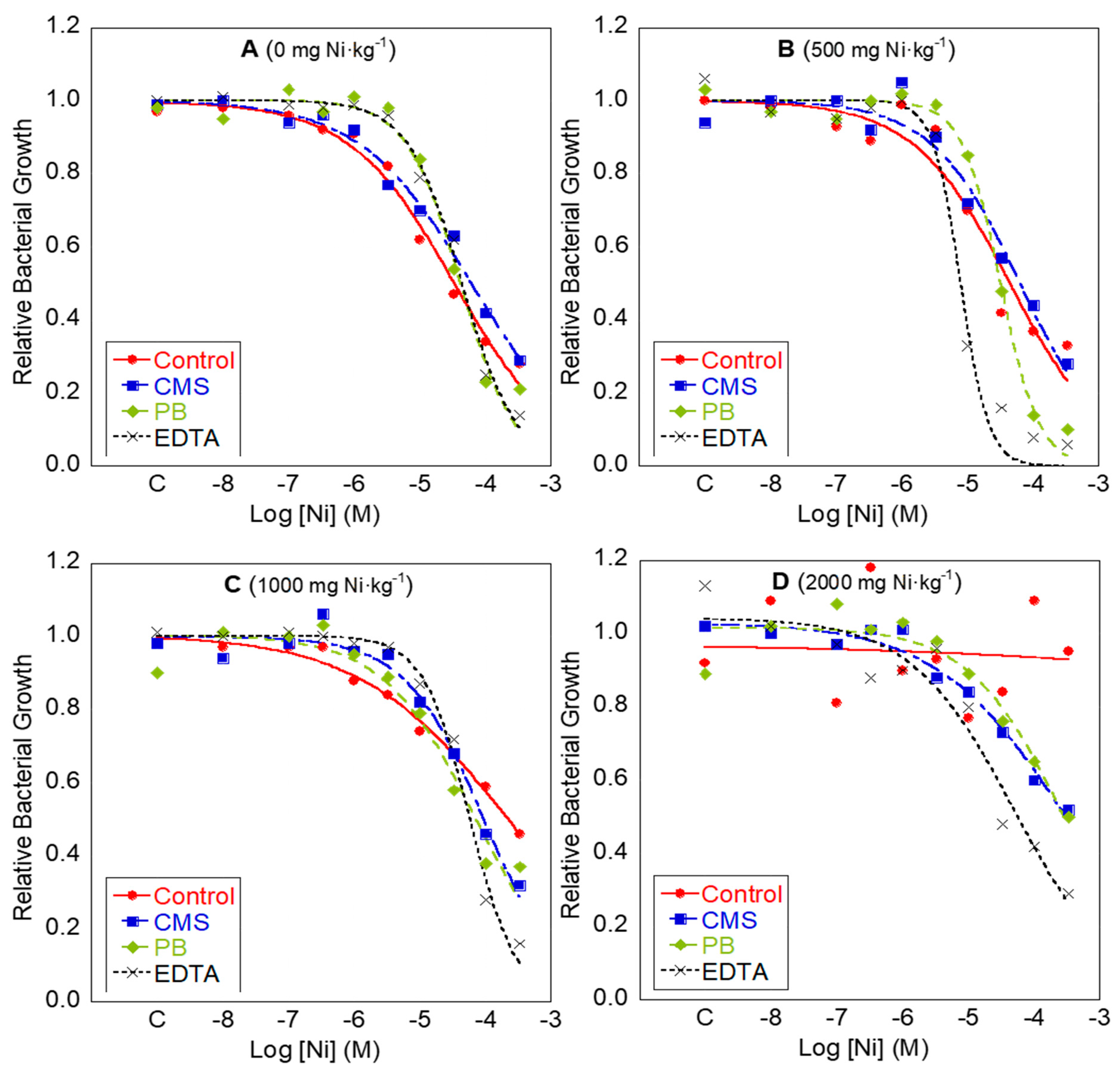
3.1. Effect of Heavy Metal Addition to Soil on the Development of Bacterial Community Tolerance
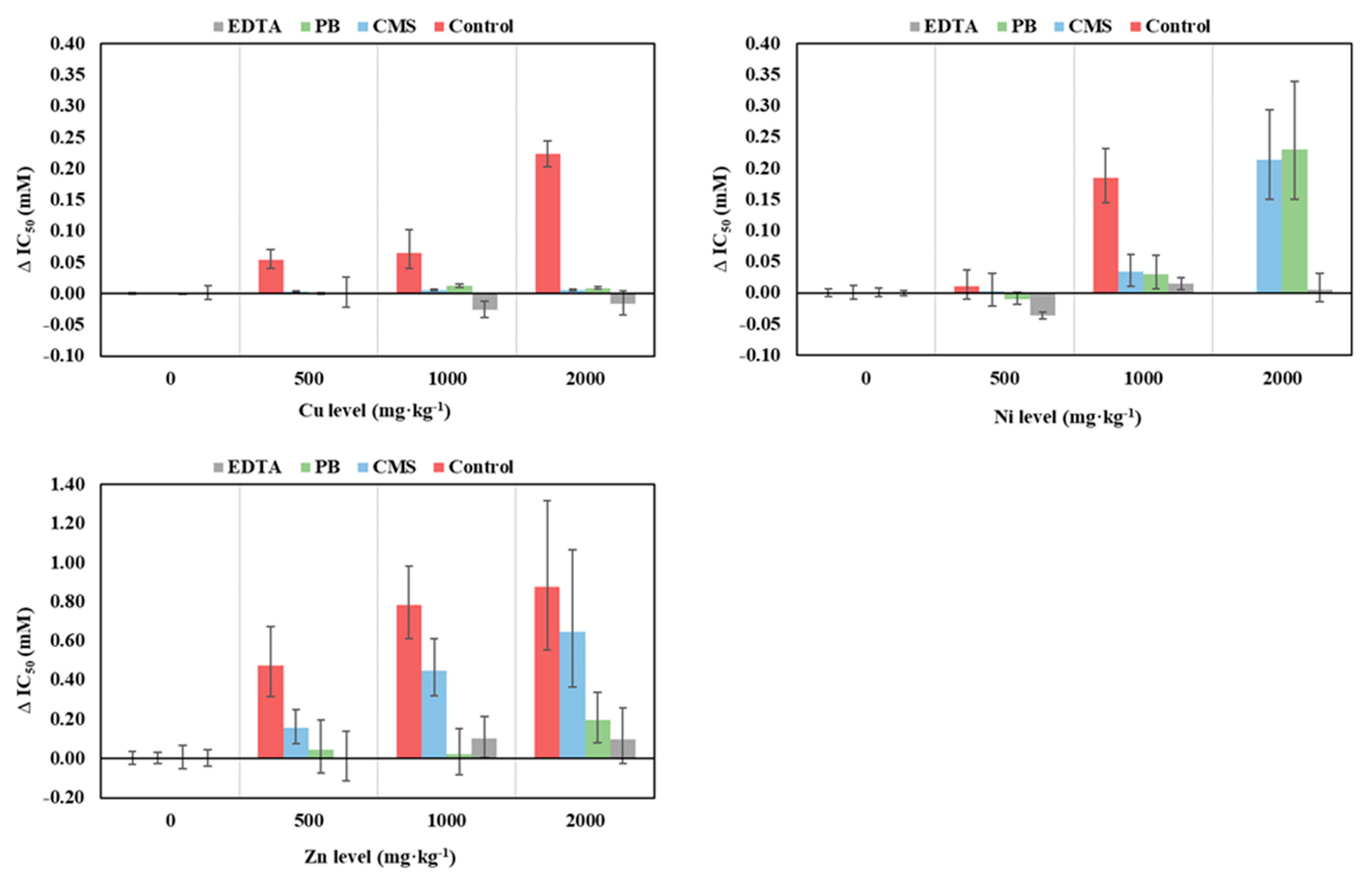
3.2. Changes in Bacterial Community Tolerance to Cu, Ni and Zn after Soil Remediation
3.2.1. Mussel Shell
3.2.2. Pine Bark
3.2.3. Soil Washing with EDTA
3.3. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy Metals, Occurrence and Toxicity for Plants: A Review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Liu, H.; Qu, M.; Chen, J.; Guang, X.; Zhang, J.; Liu, M.; Kang, J.; Zhao, Y.; Huang, B. Heavy Metal Accumulation in the Surrounding Areas Affected by Mining in China: Spatial Distribution Patterns, Risk Assessment, and Influencing Factors. Sci. Total Environ. 2022, 825, 154004. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Cheng, J.; Li, Y.; Li, F.; Li, Y.; Shi, Z. Pollution Assessment and Source Apportionment of Soil Heavy Metals in a Coastal Industrial City, Zhejiang, Southeastern China. Int. J. Environ. Res. Public Health 2022, 19, 3335. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.; Pinedo-Hernández, J.; Díez, S. Assessment of Heavy Metal Pollution, Spatial Distribution and Origin in Agricultural Soils along the Sinú River Basin, Colombia. Environ. Res. 2017, 154, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Z.; Yang, W.; Pan, L.; Gu, M.; Lee, D. Assessment of Metals Pollution on Agricultural Soil Surrounding a Lead–Zinc Mining Area in the Karst Region of Guangxi, China. Bull. Environ. Contam. Toxicol. 2013, 90, 736–741. [Google Scholar] [CrossRef]
- Enya, O.; Heaney, N.; Iniama, G.; Lin, C. Effects of Heavy Metals on Organic Matter Decomposition in Inundated Soils: Microcosm Experiment and Field Examination. Sci. Total Environ. 2020, 724, 138223. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Kuchar, L. Carbon Sequestration Rates in Organic Layers of Soils under the Grey Poplar (Populus x Canescens) Stands Impacted by Heavy Metal Pollution. In Functions of Natural Organic Matter in Changing Environment; Springer: Dordrecht, The Netherlands, 2012; pp. 365–369. [Google Scholar] [CrossRef]
- Tauqeer, H.M.; Basharat, Z.; Adnan Ramzani, P.M.; Farhad, M.; Lewińska, K.; Turan, V.; Karczewska, A.; Khan, S.A.; Faran, G.; Iqbal, M. Aspergillus Niger-Mediated Release of Phosphates from Fish Bone Char Reduces Pb Phytoavailability in Pb-Acid Batteries Polluted Soil, and Accumulation in Fenugreek. Environ. Pollut. 2022, 313, 120064. [Google Scholar] [CrossRef] [PubMed]
- Rasool, B.; Mahmood-ur-Rahman; Zubair, M.; Khan, M.A.; Ramzani, P.M.A.; Dradrach, A.; Turan, V.; Iqbal, M.; Khan, S.A.; Tauqeer, H.M.; et al. Synergetic Efficacy of Amending Pb-Polluted Soil with P-Loaded Jujube (Ziziphus Mauritiana) Twigs Biochar and Foliar Chitosan Application for Reducing Pb Distribution in Moringa Leaf Extract and Improving Its Anti-Cancer Potential. Water. Air. Soil Pollut. 2022, 233, 344. [Google Scholar] [CrossRef]
- Santás-Miguel, V.; Cutillas-Barreiro, L.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Díaz-Raviña, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A.; Fernández-Calviño, D. By-Products as an Amendment of a Mine Soil: Effects on Microbial Biomass Determined Using Phospholipid Fatty Acids. Span. J. Soil Sci. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Turan, V.; Khan, S.A.; Mahmood-ur-Rahman; Iqbal, M.; Ramzani, P.M.A.; Fatima, M. Promoting the Productivity and Quality of Brinjal Aligned with Heavy Metals Immobilization in a Wastewater Irrigated Heavy Metal Polluted Soil with Biochar and Chitosan. Ecotoxicol. Environ. Saf. 2018, 161, 409–419. [Google Scholar] [CrossRef]
- Turan, V. Confident Performance of Chitosan and Pistachio Shell Biochar on Reducing Ni Bioavailability in Soil and Plant plus Improved the Soil Enzymatic Activities, Antioxidant Defense System and Nutritional Quality of Lettuce. Ecotoxicol. Environ. Saf. 2019, 183, 109594. [Google Scholar] [CrossRef] [PubMed]
- Turan, V.; Ramzani, P.M.A.; Ali, Q.; Abbas, F.; Iqbal, M.; Irum, A.; Khan, W.-u.-D. Alleviation of Nickel Toxicity and an Improvement in Zinc Bioavailability in Sunflower Seed with Chitosan and Biochar Application in PH Adjusted Nickel Contaminated Soil. Arch. Agron. Soil Sci. 2018, 64, 1053–1067. [Google Scholar] [CrossRef]
- Turan, V. Potential of Pistachio Shell Biochar and Dicalcium Phosphate Combination to Reduce Pb Speciation in Spinach, Improved Soil Enzymatic Activities, Plant Nutritional Quality, and Antioxidant Defense System. Chemosphere 2020, 245, 125611. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Moon, D.H.; Lim, K.J.; Shope, C.L.; Lee, S.S.; Usman, A.R.A.; Kim, K.-R.; Park, J.-H.; Hur, S.-O.; Yang, J.E.; et al. An Assessment of the Utilization of Waste Resources for the Immobilization of Pb and Cu in the Soil from a Korean Military Shooting Range. Environ. Earth Sci. 2012, 67, 1023–1031. [Google Scholar] [CrossRef]
- Garrido-Rodríguez, B.; Fernández-Calviño, D.; Nóvoa Muñoz, J.C.; Arias-Estévez, M.; Díaz-Raviña, M.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A. PH-Dependent Copper Release in Acid Soils Treated with Crushed Mussel Shell. Int. J. Environ. Sci. Technol. 2013, 10, 983–994. [Google Scholar] [CrossRef]
- Ramírez-Pérez, A.M.; Paradelo, M.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Heavy Metal Retention in Copper Mine Soil Treated with Mussel Shells: Batch and Column Experiments. J. Hazard. Mater. 2013, 248–249, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Cutillas-Barreiro, L.; Ansias-Manso, L.; Fernández-Calviño, D.; Arias-Estévez, M.; Nóvoa-Muñoz, J.C.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Pine Bark as Bio-Adsorbent for Cd, Cu, Ni, Pb and Zn: Batch-Type and Stirred Flow Chamber Experiments. J. Environ. Manag. 2014, 144, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Calviño, D.; Cutillas-Barreiro, L.; Paradelo-Núñez, R.; Nóvoa-Muñoz, J.C.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A.; Arias-Estévez, M. Heavy Metals Fractionation and Desorption in Pine Bark Amended Mine Soils. J. Environ. Manag. 2017, 192, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Paradelo, R.; Cutillas-Barreiro, L.; Soto-Gómez, D.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Study of Metal Transport through Pine Bark for Reutilization as a Biosorbent. Chemosphere 2016, 149, 146–153. [Google Scholar] [CrossRef]
- Leštan, D.; Luo, C.-L.; Li, X.-D. The Use of Chelating Agents in the Remediation of Metal-Contaminated Soils: A Review. Environ. Pollut. 2008, 153, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Kaurin, A.; Lestan, D. Multi-Substrate Induced Microbial Respiration, Nitrification Potential and Enzyme Activities in Metal-Polluted, EDTA-Washed Soils. Environ. Pollut. 2018, 243, 238–245. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial Diversity and Soil Functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Pascual, J.A.; Garcia, C.; Hernandez, T.; Moreno, J.L.; Ros, M. Soil Microbial Activity as a Biomarker of Degradation and Remediation Processes. Soil Biol. Biochem. 2000, 32, 1877–1883. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, J.; Ren, L.; Zhou, Y.; Gao, J.; Luo, L.; Yang, Y.; Peng, Q.; Huang, H.; Chen, A. Diagnosis of Soil Contamination Using Microbiological Indices: A Review on Heavy Metal Pollution. J. Environ. Manag. 2019, 242, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Udovic, M.; Lestan, D. EDTA and HCl Leaching of Calcareous and Acidic Soils Polluted with Potentially Toxic Metals: Remediation Efficiency and Soil Impact. Chemosphere 2012, 88, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Clemente, R.; de la Fuente, C.; Moral, R.; Bernal, M.P. Changes in Microbial Biomass Parameters of a Heavy Metal-Contaminated Calcareous Soil during a Field Remediation Experiment. J. Environ. Qual. 2007, 36, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.J.; Häggblom, M.M.; Tate, R.L. Effects of Heavy Metal Contamination and Remediation on Soil Microbial Communities in the Vicinity of a Zinc Smelter as Indicated by Analysis of Microbial Community Phospholipid Fatty Acid Profiles. Biol. Fertil. Soils 2003, 38, 65–71. [Google Scholar] [CrossRef]
- Kelly, J.J.; Tate, R.L. Effects of Heavy Metal Contamination and Remediation on Soil Microbial Communities in the Vicinity of a Zinc Smelter. J. Environ. Qual. 1998, 27, 609–617. [Google Scholar] [CrossRef]
- Liu, S.J.; Liu, Y.G.; Tan, X.F.; Zeng, G.M.; Zhou, Y.H.; Liu, S.B.; Yin, Z.H.; Jiang, L.H.; Li, M.F.; Wen, J. The Effect of Several Activated Biochars on Cd Immobilization and Microbial Community Composition during In-Situ Remediation of Heavy Metal Contaminated Sediment. Chemosphere 2018, 208, 655–664. [Google Scholar] [CrossRef] [PubMed]
- De Mora, A.P.; Ortega-Calvo, J.J.; Cabrera, F.; Madejón, E. Changes in Enzyme Activities and Microbial Biomass after “in Situ” Remediation of a Heavy Metal-Contaminated Soil. Appl. Soil Ecol. 2005, 28, 125–137. [Google Scholar] [CrossRef]
- Kaurin, A.; Cernilogar, Z.; Lestan, D. Revitalisation of Metal-Contaminated, EDTA-Washed Soil by Addition of Unpolluted Soil, Compost and Biochar: Effects on Soil Enzyme Activity, Microbial Community Composition and Abundance. Chemosphere 2018, 193, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Blanck, H. A Critical Review of Procedures and Approaches Used for Assessing Pollution-Induced Community Tolerance (PICT) in Biotic Communities. Hum. Ecol. Risk Assess. 2002, 8, 1003–1034. [Google Scholar] [CrossRef]
- Tlili, A.; Berard, A.; Blanck, H.; Bouchez, A.; Cássio, F.; Eriksson, K.M.; Morin, S.; Montuelle, B.; Navarro, E.; Pascoal, C.; et al. Pollution-Induced Community Tolerance (PICT): Towards an Ecologically Relevant Risk Assessment of Chemicals in Aquatic Systems. Freshw. Biol. 2016, 61, 2141–2151. [Google Scholar] [CrossRef]
- Aliasgharzad, N.; Molaei, A.; Oustan, S. Pollution Induced Community Tolerance (PICT) of Microorganisms in Soil Incubated with Different Levels of Pb. Int. J. Environ. Ecol. Eng. 2011, 5, 838–842. [Google Scholar] [CrossRef]
- Bérard, A.; Capowiez, L.; Mombo, S.; Schreck, E.; Dumat, C.; Deola, F.; Capowiez, Y. Soil Microbial Respiration and PICT Responses to an Industrial and Historic Lead Pollution: A Field Study. Environ. Sci. Pollut. Res. 2016, 23, 4271–4281. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Raviña, M.; Calvo De Anta, R.; Bååth, E. Tolerance (PICT) of the Bacterial Communities to Copper in Vineyards Soils from Spain. J. Environ. Qual. 2007, 36, 1760–1764. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Arias-Estévez, M.; Díaz-Raviña, M.; Bååth, E. Bacterial Pollution Induced Community Tolerance (PICT) to Cu and Interactions with PH in Long-Term Polluted Vineyard Soils. Soil Biol. Biochem. 2011, 43, 2324–2331. [Google Scholar] [CrossRef]
- Kamitani, T.; Oba, H.; Kaneko, N. Microbial Biomass and Tolerance of Microbial Community on an Aged Heavy Metal Polluted Floodplain in Japan. Water. Air. Soil Pollut. 2006, 172, 185–200. [Google Scholar] [CrossRef]
- Brandt, K.K.; Frandsen, R.J.N.; Holm, P.E.; Nybroe, O. Development of Pollution-Induced Community Tolerance Is Linked to Structural and Functional Resilience of a Soil Bacterial Community Following a Five-Year Field Exposure to Copper. Soil Biol. Biochem. 2010, 42, 748–757. [Google Scholar] [CrossRef]
- Niklińska, M.; Chodak, M.; Laskowski, R. Pollution-Induced Community Tolerance of Microorganisms from Forest Soil Organic Layers Polluted with Zn or Cu. Appl. Soil Ecol. 2006, 32, 265–272. [Google Scholar] [CrossRef]
- Viti, C.; Quaranta, D.; De Philippis, R.; Corti, G.; Agnelli, A.; Cuniglio, R.; Giovannetti, L. Characterizing Cultivable Soil Microbial Communities from Copper Fungicide-Amended Olive Orchard and Vineyard Soils. World J. Microbiol. Biotechnol. 2008, 24, 309–318. [Google Scholar] [CrossRef]
- Demoling, L.A.; Bååth, E. No Long-Term Persistence of Bacterial Pollution-Induced Community Tolerance in Tylosin-Polluted Soil. Environ. Sci. Technol. 2008, 42, 6917–6921. [Google Scholar] [CrossRef] [PubMed]
- Santás-Miguel, V.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Díaz-Raviña, M.; Arias-Estévez, M.; Fernández-Calviño, D. Use of Waste Materials to Prevent Tetracycline Antibiotics Toxicity on the Growth of Soil Bacterial Communities. Environ. Res. 2021, 193, 110404. [Google Scholar] [CrossRef]
- Guitián, F.; Carballas, T. Técnicas de Análisis de Suelos; Pico Sacro: Santiago de Compostela, Spain, 1976; ISBN 8485170091/97884485170098. [Google Scholar]
- Day, P.R. Particle Fractionation and Particle-Size Analysis. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling; American Society of Agronomy: Madison, WI, USA, 1965; pp. 545–567. [Google Scholar] [CrossRef]
- Hoogsteen, M.J.J.; Lantinga, E.A.; Bakker, E.J.; Groot, J.C.J.; Tittonell, P.A. Estimating Soil Organic Carbon through Loss on Ignition: Effects of Ignition Conditions and Structural Water Loss. Eur. J. Soil Sci. 2015, 66, 320–328. [Google Scholar] [CrossRef]
- Reed, S.T.; Martens, D.C. Copper and Zinc. In Methods of Soil Analysis Part 3; Bigham, J., Ed.; Chemical Methods; Soil Science Society of America: Madison, WI, USA, 1996; pp. 703–722. [Google Scholar]
- Romar-Gasalla, A.; Santás-Miguel, V.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Álvarez-Rodríguez, E.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J. Chromium and Fluoride Sorption/Desorption on Un-Amended and Waste-Amended Forest and Vineyard Soils and Pyritic Material. J. Environ. Manag. 2018, 222, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Lakanen, E.; Erviö, R. A Comparison of Eight Extractans for the Determination of Plant Available Micronutrients in Soils. Acta Agric. Scand. 1971, 17, 131–139. [Google Scholar] [CrossRef]
- Naghipour, D.; Jaafari, J.; Ashrafi, S.D.; Mahvi, A.H. Remediation of Heavy Metals Contaminated Silty Clay Loam Soil by Column Extraction with Ethylenediaminetetraacetic Acid and Nitrilo Triacetic Acid. J. Environ. Eng. 2017, 143, 04017026. [Google Scholar] [CrossRef]
- Wasay, S.A.; Barrington, S.; Tokunaga, S. Organic Acids for the In Situ Remediation of Soils Polluted by Heavy Metals: Soil Flushing in Columns. Water Air Soil Pollut. 2001, 127, 301–314. [Google Scholar] [CrossRef]
- Bååth, E. Thymidine and Leucine Incorporation in Soil Bacteria with Different Cell Size. Microb. Ecol. 1994, 27, 267–278. [Google Scholar] [CrossRef]
- Bååth, E.; Pettersson, M.; Söderberg, K.H. Adaptation of a Rapid and Economical Microcentrifugation Method to Measure Thymidine and Leucine Incorporation by Soil Bacteria. Soil Biol. Biochem. 2001, 33, 1571–1574. [Google Scholar] [CrossRef]
- Meisner, A.; Bååth, E.; Rousk, J. Microbial Growth Responses upon Rewetting Soil Dried for Four Days or One Year. Soil Biol. Biochem. 2013, 66, 188–192. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Cutillas-Barreiro, L.; Nóvoa-Muñoz, J.C.; Díaz-Raviña, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A.; Arias-Estévez, M.; Rousk, J. Using Pine Bark and Mussel Shell Amendments to Reclaim Microbial Functions in a Cu Polluted Acid Mine Soil. Appl. Soil Ecol. 2018, 127, 102–111. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Cutillas-Barreiro, L.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodriguez, E.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M. Cu Immobilization and Lolium Perenne Development in an Acid Vineyard Soil Amended with Crushed Mussel Shell. Land Degrad. Dev. 2017, 28, 762–772. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Fernández-Calviño, D.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Arias-Estévez, M. Adsorption/Desorption and Transport of Sulfadiazine, Sulfachloropyridazine, and Sulfamethazine, in Acid Agricultural Soils. Chemosphere 2019, 234, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Lekfeldt, J.D.S.; Magid, J.; Holm, P.E.; Nybroe, O.; Brandt, K.K. Evaluation of the Leucine Incorporation Technique for Detection of Pollution-Induced Community Tolerance to Copper in a Long-Term Agricultural Field Trial with Urban Waste Fertilizers. Environ. Pollut. 2014, 194, 78–85. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E. Growth of Saprotrophic Fungi and Bacteria in Soil. FEMS Microbiol. Ecol. 2011, 78, 17–30. [Google Scholar] [CrossRef]
- Berg, J.; Brandt, K.K.; Al-Soud, W.A.; Holm, P.E.; Hansen, L.H.; Sørensen, S.J.; Nybroe, O. Selection for Cu-Tolerant Bacterial Communities with Altered Composition, but Unaltered Richness, via Long-Term Cu Exposure. Appl. Environ. Microbiol. 2012, 78, 7438–7446. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Bååth, E. Interaction between PH and Cu Toxicity on Fungal and Bacterial Performance in Soil. Soil Biol. Biochem. 2016, 96, 20–29. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Zhang, X.; Gao, M.; Wang, J. Effects of Cu Exposure on Enzyme Activities and Selection for Microbial Tolerances during Swine-Manure Composting. J. Hazard. Mater. 2015, 283, 512–518. [Google Scholar] [CrossRef]
- Fait, G.; Broos, K.; Zrna, S.; Lombi, E.; Hamon, R. Tolerance of Nitrifying Bacteria to Copper and Nickel. Environ. Toxicol. Chem. 2006, 25, 2000–2005. [Google Scholar] [CrossRef] [PubMed]
- Santás-Miguel, V.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Díaz-Raviña, M.; Arias-Estévez, M.; Fernández-Calviño, D. Tolerance of Soil Bacterial Community to Tetracycline Antibiotics Induced by As, Cd, Zn, Cu, Ni, Cr and Pb Pollution. Soil 2022, 8, 437–449. [Google Scholar] [CrossRef]
- Lock, K.; Janssen, C.R. Influence of Soil Zinc Concentrations on Zinc Sensitivity and Functional Diversity of Microbial Communities. Environ. Pollut. 2005, 136, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Rutgers, M.; Van’t Verlaat, I.M.; Wind, B.; Posthuma, L.; Breure, A.M. Rapid Method for Assessing Pollution-Induced Community Tolerance in Contaminated Soil. Environ. Toxicol. Chem. 1998, 17, 2210–2213. [Google Scholar] [CrossRef]
- Van Beelen, P.; Fleuren-Kemila, A.K.; Aldenberg, T. The Relation between Extrapolated Risk, Expressed as Potentially Affected Fraction, and Community Effects, Expressed as Pollution-Induced Community Tolerance. Environ. Toxicol. Chem. 2001, 20, 1133–1140. [Google Scholar] [CrossRef]
- Díaz-Raviña, M.; Bååth, E. Development of Metal Tolerance in Soil Bacterial Communities Exposed to Experimentally Increased Metal Levels. Appl. Environ. Microbiol. 1996, 62, 2970–2977. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Raviña, M.; Bååth, E.; Frostegard, A. Multiple Heavy Metal Tolerance of Soil Bacterial Communities and Its Measurement by a Thymidine Incorporation Technique. Appl. Environ. Microbiol. 1994, 60, 2238–2247. [Google Scholar] [CrossRef]
- Bradl, H.B. Adsorption of Heavy Metal Ions on Soils and Soils Constituents. J. Colloid Interface Sci. 2004, 277, 1–18. [Google Scholar] [CrossRef]
- Uchimiya, M.; Bannon, D.; Nakanishi, H.; McBride, M.B.; Williams, M.A.; Yoshihara, T. Chemical Speciation, Plant Uptake, and Toxicity of Heavy Metals in Agricultural Soils. J. Agric. Food Chem. 2020, 68, 12856–12869. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, C.A.; Yang, X.; Clark, I.M.; Zhao, F.-J.; Hirsch, P.R.; McGrath, S.P. Relative Impact of Soil, Metal Source and Metal Concentration on Bacterial Community Structure and Community Tolerance. Soil Biol. Biochem. 2010, 42, 1408–1417. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Niklińska, M.; Laskowski, R. Pollution-Induced Tolerance of Soil Bacterial Communities in Meadow and Forest Ecosystems Polluted with Heavy Metals. Eur. J. Soil Biol. 2009, 45, 363–369. [Google Scholar] [CrossRef]
- Abd El-Azeem, S.A.M.; Ahmad, M.; Usman, A.R.A.; Kim, K.-R.; Oh, S.-E.; Lee, S.S.; Ok, Y.S. Changes of Biochemical Properties and Heavy Metal Bioavailability in Soil Treated with Natural Liming Materials. Environ. Earth Sci. 2013, 70, 3411–3420. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Garrido-Rodríguez, B.; Arias-Estévez, M.; Díaz-Raviña, M.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J.; Nuñez-Delgado, A. Effect of Crushed Mussel Shell Addition on Bacterial Growth in Acid Polluted Soils. Appl. Soil Ecol. 2015, 85, 65–68. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Pérez-Armada, L.; Cutillas-Barreiro, L.; Paradelo-Núñez, R.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodriguez, E.; Arias-Estévez, M. Changes in Cd, Cu, Ni, Pb and Zn Fractionation and Liberation Due to Mussel Shell Amendment on a Mine Soil. Land Degrad. Dev. 2016, 27, 1276–1285. [Google Scholar] [CrossRef]
- Cutillas-Barreiro, L.; Paradelo, R.; Igrexas-Soto, A.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodriguez, E.; Garrote, G.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M. Valorization of Biosorbent Obtained from a Forestry Waste: Competitive Adsorption, Desorption and Transport of Cd, Cu, Ni, Pb and Zn. Ecotoxicol. Environ. Saf. 2016, 131, 118–126. [Google Scholar] [CrossRef]
- Barona, A.; Aranguiz, I.; Elías, A. Metal Associations in Soils before and after EDTA Extractive Decontamination: Implications for the Effectiveness of Further Clean-up Procedures. Environ. Pollut. 2001, 113, 79–85. [Google Scholar] [CrossRef]
- Sun, B.; Zhao, F.J.; Lombi, E.; McGrath, S.P. Leaching of Heavy Metals from Contaminated Soils Using EDTA. Environ. Pollut. 2001, 113, 111–120. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, H.; Tan, F.; Wang, H.; Qiu, R. Influence of EDTA Washing on the Species and Mobility of Heavy Metals Residual in Soils. J. Hazard. Mater. 2010, 173, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Kokalis-Burelle, N.; Rodríguez-Kábana, R. Changes in Populations of Soil Microorganisms, Nematodes, and Enzyme Activity Associated with Application of Powdered Pine Bark. Plant Soil 1994, 162, 169–175. [Google Scholar] [CrossRef]
- Wei, H.; Liu, Y.; Chao, Y.; Tsang, D.C.W.; Zhao, N.; Liu, K.; Zhang, W.; Qiu, R. Recovery of the Biological Function of Ethylenediaminetetraacetic Acid-Washed Soils: Roles of Environmental Variations and Microbes. Sci. Total Environ. 2020, 715, 137032. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Deng, S.; Zhang, J.; Hou, J.; Wang, C.; Fu, Z. Effect of Different Washing Solutions on Soil Enzyme Activity and Microbial Community in Agricultural Soil Severely Contaminated with Cadmium. Environ. Sci. Pollut. Res. 2022, 29, 54641–54651. [Google Scholar] [CrossRef] [PubMed]
- Mirlean, N.; Roisenberg, A.; Chies, J.O. Metal Contamination of Vineyard Soils in Wet Subtropics (Southern Brazil). Environ. Pollut. 2007, 149, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Seijo, A.; Alfaya, M.C.; Andrade, M.L.; Vega, F.A. Copper, Chromium, Nickel, Lead and Zinc Levels and Pollution Degree in Firing Range Soils. Land Degrad. Dev. 2016, 27, 1721–1730. [Google Scholar] [CrossRef]


| Remediation Treatment | Metal Level | Copper | Nickel | Zinc | |||
|---|---|---|---|---|---|---|---|
| mg·kg−1 | Log IC50 ± SD | R2 | Log IC50 ± SD | R2 | Log IC50 ± SD | R2 | |
| Control | 2000 | −3.64 ± 0.04 | 0.99 | - | - | −2.99 ± 0.15 | 0.97 |
| 1000 | −4.13 ± 0.17 | 0.95 | −3.66 ± 0.07 | 0.99 | −3.03 ± 0.07 | 0.99 | |
| 500 | −4.21 ± 0.10 | 0.97 | −4.35 ± 0.16 | 0.96 | −3.21 ± 0.10 | 0.97 | |
| 0 | −5.11 ± 0.08 | 0.99 | −4.47 ± 0.08 | 0.99 | −3.83 ± 0.13 | 0.96 | |
| CMS | 2000 | −5.04 ± 0.07 | 0.99 | −3.56 ± 0.10 | 0.99 | −3.11 ± 0.18 | 0.96 |
| 1000 | −5.03 ± 0.02 | 0.99 | −4.02 ± 0.07 | 0.99 | −3.24 ± 0.09 | 0.97 | |
| 500 | −5.20 ± 0.03 | 0.99 | −4.20 ± 0.10 | 0.98 | −3.55 ± 0.09 | 0.97 | |
| 0 | −5.44 ± 0.06 | 0.99 | −4.21 ± 0.07 | 0.99 | −3.91 ± 0.10 | 0.98 | |
| PB | 2000 | −4.65 ± 0.03 | 0.99 | −3.57 ± 0.14 | 0.96 | −3.34 ± 0.07 | 0.96 |
| 1000 | −4.60 ± 0.04 | 0.99 | −4.14 ± 0.12 | 0.97 | −3.55 ± 0.09 | 0.97 | |
| 500 | −4.89 ± 0.04 | 0.99 | −4.50 ± 0.04 | 0.99 | −3.52 ± 0.11 | 0.97 | |
| 0 | −4.88 ± 0.02 | 0.99 | −4.38 ± 0.07 | 0.99 | −3.59 ± 0.10 | 0.98 | |
| EDTA | 2000 | −4.47 ± 0.11 | 0.97 | −4.31 ± 0.16 | 0.97 | −3.45 ± 0.12 | 0.93 |
| 1000 | −4.62 ± 0.05 | 0.99 | −4.23 ± 0.04 | 0.99 | −3.45 ± 0.07 | 0.90 | |
| 500 | −4.30 ± 0.11 | 0.96 | −5.11 ± 0.06 | 0.99 | −3.60 ± 0.14 | 0.96 | |
| 0 | −4.30 ± 0.09 | 0.98 | −4.35 ± 0.04 | 0.99 | −3.59 ± 0.07 | 0.98 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campillo-Cora, C.; Soto-Gómez, D.; Arias-Estévez, M.; Fernández-Calviño, D. Assessment of Polluted Soil Remediation Using Bacterial Community Tolerance to Heavy Metals as an Indicator. Agronomy 2022, 12, 2280. https://doi.org/10.3390/agronomy12102280
Campillo-Cora C, Soto-Gómez D, Arias-Estévez M, Fernández-Calviño D. Assessment of Polluted Soil Remediation Using Bacterial Community Tolerance to Heavy Metals as an Indicator. Agronomy. 2022; 12(10):2280. https://doi.org/10.3390/agronomy12102280
Chicago/Turabian StyleCampillo-Cora, Claudia, Diego Soto-Gómez, Manuel Arias-Estévez, and David Fernández-Calviño. 2022. "Assessment of Polluted Soil Remediation Using Bacterial Community Tolerance to Heavy Metals as an Indicator" Agronomy 12, no. 10: 2280. https://doi.org/10.3390/agronomy12102280
APA StyleCampillo-Cora, C., Soto-Gómez, D., Arias-Estévez, M., & Fernández-Calviño, D. (2022). Assessment of Polluted Soil Remediation Using Bacterial Community Tolerance to Heavy Metals as an Indicator. Agronomy, 12(10), 2280. https://doi.org/10.3390/agronomy12102280











