Genetic Mapping of Quantitative Trait Loci Associated with Plant Height and Yield Component Traits in a Wheat (Triticum aestivum L.) Doubled Haploid Population Derived from Tugela-DN × Elands
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Field Trials, and Phenotypic Evaluations
2.2. Statistical Analysis
2.3. Genotyping and Construction of Genetic Map
2.4. QTL Analysis
3. Results
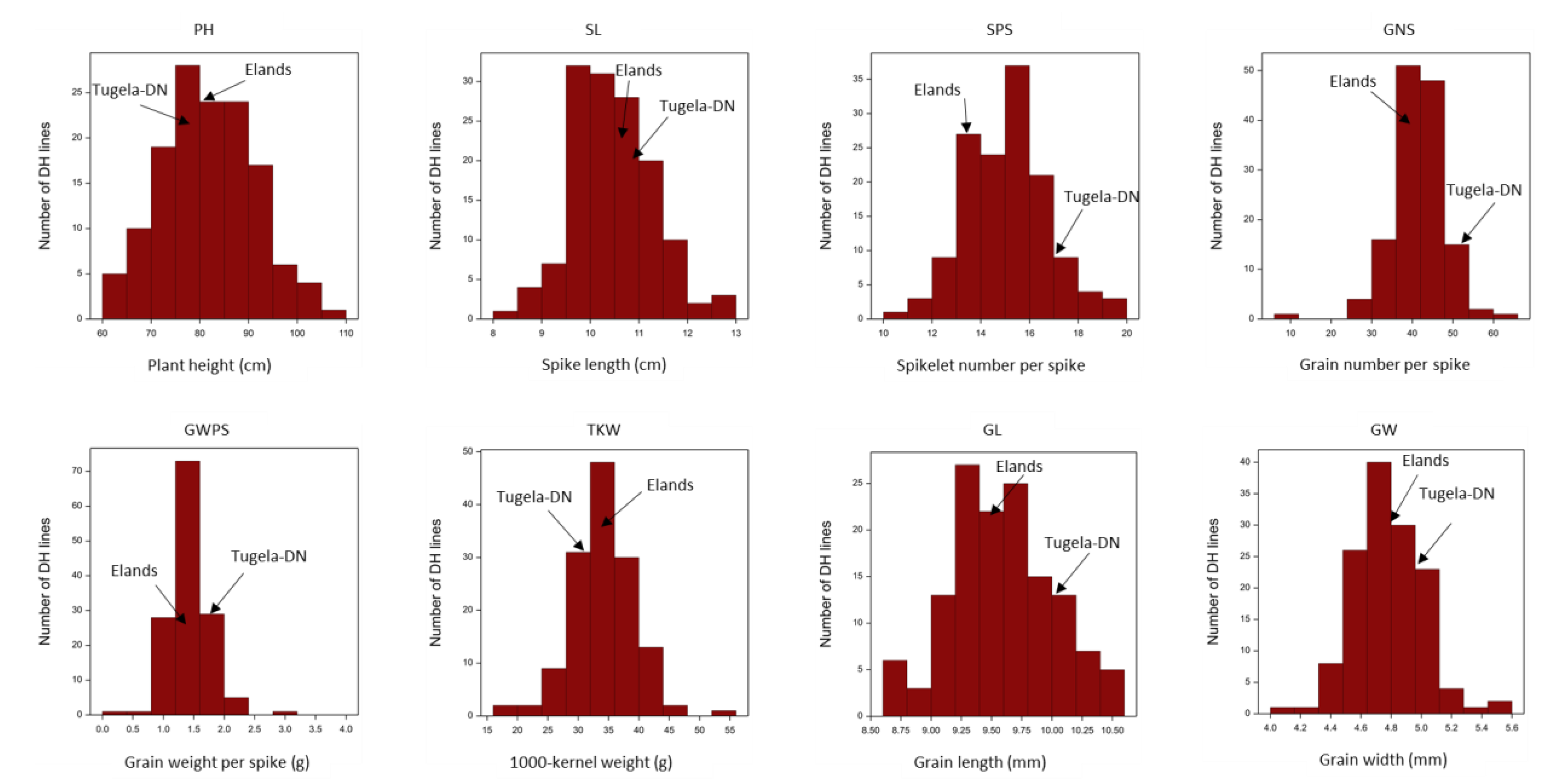
3.1. Phenotypic Performance of DH Population
3.2. Correlations between Traits
3.3. Linkage Map Construction
3.4. QTL Mapping Analysis
3.4.1. Plant Height (PH)
3.4.2. Spike Length (SL)
3.4.3. Grain Number Per Spike (GNS)
3.4.4. Grain Weight Per Spike (GWPS)
3.4.5. Grain Length (GL)
| Trait a | Chromosome (Position) b | Marker Interval/Flanking Marker | QTL c | Detected Environments d | QTL Effects e | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LOD | Add | PVE (%) | LOD | Add | PVE (%) | ||||||
| PH | 6A (18–22) | 3025617|F|0–30:C>T; 3222505|F|0–31:C>G; 4910650|F|0–18:C>T | QPh.sgi-6A.2+ | E1, E2, E3, C | 3.30 | 3.41 | 10.45 | 2.81 | 4.45 | 8.15 | [7,23,33,72] |
| 9.94 | 6.59 | 24.91 | 7.57 | 5.22 | 20.47 | ||||||
| 4D (17–32) | 3957744|F|0–35:C>T; ALMT1; 1254717 | QPh.sgi-4D+ | E2, E3, C | 2.76 | −4.67 | 7.05 | 3.94 | −4.32 | 8.61 | [10,15,30,33,73] | |
| 4.30 | −3.90 | 10.74 | |||||||||
| SL | 6A (18) | 3025617|F|0–30:C>T | QSl.sgi-6A.2+ | E1, E4, C | 4.68 | 0.51 | 14.11 | 6.10 | 0.54 | 17.50 | [7,74,75,76,77] |
| 4.57 | 0.34 | 11.56 | |||||||||
| 7A (51–84) | 4911195|F|0–8:T>C; 3952934|F|0–27:G>A; 3064815|F|0–27:A>G | QSl.sgi-7A+ | E3, E4, E5 | 2.74 | 0.49 | 15.66 | 3.04 | 0.36 | 10.47 | [34,78,79] | |
| 4.39 | 0.60 | 25.05 | |||||||||
| GNS | 3B (7–50) | 3025468|F|0–18:T>G; 3948041 | QGns.sgi-3B+ | E4, E5 | 4.19 | −2.91 | 12.87 | 2.57 | 2.13 | 7.23 | [10,80,81] |
| GWPS | 7B (54–98) | 3222513|F|0–21:T>C; 1004245|F|0–36:G>A | QGwps.sgi-7B+ | E1, E2 | 3.94 | −0.19 | 14.96 | 3.97 | 0.26 | 15.56 | |
| GL | 3B (4–55) | 3950390|F|0–5:G>C; 4539741; 5969907|F|0–9:C>A; 4910077|F|0–9:G>C | QGl.sgi-3B+ | E1, E4 | 4.58 | 0.26 | 17.23 | 3.34 | 0.25 | 12.82 | [82] |
| GW | 7A (27–62) | 4394765|F|0–8:C>G; 1164940|F|0–59:G>C | QGw.sgi-7A+ | E2, E5 | 3.02 | 0.16 | 11.56 | 2.94 | 0.16 | 11.11 | [83] |
| 2A (42–83) | 3064828|F|0–5:G>C; 4993789|F|0–5:C>A | QGw.sgi-2A+ | E4, E5 | 5.10 | 0.22 | 25.17 | 4.36 | 0.21 | 17.58 | [28,77,84] | |
3.4.6. Grain Weight (GW)
4. Discussion
4.1. Phenotypic Variations
4.2. QTL Analysis for Agronomic Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Online Statistical Database: Food Balance. Food and Agricultural Organization of the United Nations. 2017. Available online: http://www.fao.org/faostat/en/ (accessed on 28 March 2022).
- Berkhout, P.; Bergevoet, R.; van Berkum, S. A brief analysis of the impact of the war in Ukraine on food security. (Policy document/Wageningen Economic Research: No. 2022-033). Wagening. Econ. Res. 2022, 1–26. [Google Scholar] [CrossRef]
- Lang, T.; McKee, M. The reinvasion of Ukraine threatens global food supplies. BMJ 2022, 376, o676. [Google Scholar] [CrossRef] [PubMed]
- Kuzay, S.; Xu, Y.; Zhang, J.; Katz, A.; Pearce, S.; Su, Z. Identification of a candidate gene for a QTL for spikelet number per spike on wheat chromosome arm 7AL by high-resolution genetic mapping. Theor. Appl. Genet. 2019, 132, 2689–2705. [Google Scholar] [CrossRef]
- Li, T.; Deng, G.; Tang, Y.; Su, Y.; Wang, J.; Cheng, J.; Yang, Z.; Qiu, X.; Pu, X.; Zhang, H.; et al. Identification and Validation of a Novel Locus Controlling Spikelet Number in Bread Wheat (Triticum aestivum L.). Front. Plant Sci. 2021, 12, 611106. [Google Scholar] [CrossRef] [PubMed]
- Brinton, J.; Uauy, C. A reductionist approach to dissecting grain weight and yield in wheat. J. Integr. Plant Biol. 2019, 61, 337–358. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Conway, B.; Miller, D.; Marshall, D.; Cooper, A.; Murphy, P.; Chao, S.; Brown-Guedira, G.; Costa, J. Quantitative Trait Loci Mapping for Spike Characteristics in Hexaploid Wheat. Plant Genome 2017, 10, 1–15. [Google Scholar] [CrossRef]
- Li, X.; Xu, X.; Liu, W.; Li, X.; Yang, X.; Ru, Z.; Li, L. Dissection of superior alleles for yield-related traits and their distribution in important cultivars of wheat by association mapping. Front. Plant Sci. 2020, 11, 175. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Mao, X.G.; Wang, C.S.; Jing, R. Overexpression of a common wheat gene TaSnRK2.8 enhances tolerance to drought, salt and low temperature in Arabidopsis. PLoS ONE 2010, 5, e16041. [Google Scholar] [CrossRef]
- Gao, F.; Wen, W.; Liu, J.; Rasheed, A.; Yin, G.; Xia, X.; Wu, X.; He, Z. Genome-wide linkage mapping of QTL for yield components, plant height and yield-related physiological traits in the Chinese wheat cross Zhou 8425B/Chinese Spring. Front. Plant Sci. 2015, 6, 1099. [Google Scholar] [CrossRef]
- Zhai, H.; Feng, Z.; Du, X.; Song, Y.; Liu, X.; Qi, Z.; Song, L.; Li, J.; Li, L.; Peng, H.; et al. A novel allele of TaGW2-A1 is located in a finely mapped QTL that increases grain weight but decreases grain number in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2018, 131, 539–553. [Google Scholar] [CrossRef]
- Ellis, M.H.; Rebetzke, G.J.; Azanza, F.; Richards, R.A.; Spielmeyer, W. Molecular mapping of gibberellin-responsive dwarfing genes in bread wheat. Theor. Appl. Genet. 2005, 111, 423–430. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, R.A.; Dubcovsky, J.; Rogers, W.J.; Morris, C.; Xia, X.C. Catalogue of gene symbols for wheat. In Proceedings of the 12th International Wheat Genetics Symposium, Tulln, Austria, 23–28 April 2017. [Google Scholar]
- Mo, Y.; Vanzetti, L.S.; Hale, I.; Spagnolo, E.J.; Guidobaldi, F.; Al-Oboudi, J.; Odle, N.; Pearce, S.; Helguera, M.; Dubcovsky, J. Identification and characterization of Rht25, a locus on chromosome arm 6AS affecting wheat plant height, heading time, and spike development. Theor. Appl. Genet. 2018, 131, 2021–2035. [Google Scholar] [CrossRef] [PubMed]
- Würschum, T.; Langer, S.M.; Longin, C.F.H. Genetic control of plant height in European winter wheat cultivars. Theor. Appl. Genet. 2015, 128, 865–874. [Google Scholar] [CrossRef]
- Zhai, H.; Feng, Z.; Li, J.; Liu, X.; Xiao, S.; Ni, Z.; Sun, Q. QTL analysis of spike morphological traits and plant height in winter wheat (Triticum aestivum L.) using a high-density SNP and SSR-based linkage map. Front. Plant Sci. 2016, 7, 1617. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wen, W.; Xie, L.; Fu, L.; Xu, D.; Fu, C.; Wang, D.; Chen, X.; Xia, X.; Chen, Q.; et al. Molecular mapping of reduced plant height gene Rht24 in bread wheat. Front. Plant Sci. 2017, 8, 1379. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Xin, M.; Dong, C.; Chen, Z.; Zhai, H.; Zhuang, J.; Cheng, X.; Wang, N.; Geng, J.; Wang, X.; et al. A natural variation in Ribonuclease H-like gene underlies Rht8 to confer “Green Revolution” trait in wheat. Mol. Plant 2022, 15, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Zhou, C.; Fu, M.; Guo, H.; Xie, Y.; Zhao, L.; Gu, J.; Zhao, S.; Ding, Y.; Li, Y.; et al. Cloning and functional characterization of Rht8, a “Green Revolution” replacement gene in wheat. Mol. Plant 2022, 15, 373–376. [Google Scholar] [CrossRef]
- Rebetzke, G.J.; Richards, R.A.; Fischer, V.M.; Mickelson, B.J. Breeding long coleoptile, reduced height wheats. Euphytica 1999, 106, 159–168. [Google Scholar] [CrossRef]
- Zhang, J.; Dell, B.; Biddulph, B.; Drake-Brockman, F.; Walker, E.; Khan, N.; Wong, D.; Hayden, M.; Appels, R. Wild-type alleles of Rht-B1 and Rht-D1 as independent determinants of thousand-grain weight and kernel number per spike in wheat. Mol. Breed. 2013, 32, 771–783. [Google Scholar] [CrossRef]
- Gasperini, D.; Greenland, A.; Hedden, P.; Dreos, R.; Harwood, W.; Griffiths, S. Genetic and physiological analysis of Rht8 in bread wheat: An alternative source of semi-dwarfism with a reduced sensitivity to brassinosteroids. J. Exp. Bot. 2012, 63, 4419. [Google Scholar] [CrossRef]
- Würschum, T.; Langer, S.M.; Longin, C.F.H.; Tucker, M.R.; Leiser, W.L. A modern Green Revolution gene for reduced height in wheat. Plant J. 2017, 92, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xia, X.; Xiao, Y.; He, Z.; Wang, D.; Trethowan, R.; Wang, H.; Chen, X. QTL mapping for plant height and yield components in common wheat under water-limited and full irrigation environments. Crop Pasture Sci. 2015, 66, 660–670. [Google Scholar] [CrossRef]
- Tian, X.; Xia, X.; Xu, D.; Liu, Y.; Xie, L.; Hassan, M.A.; Song, J.; Li, F.; Wang, D.; Zhang, Y.; et al. Rht24b, an ancient variation of TaGA2ox-A9, reduces plant height without yield penalty in wheat. New Phytol. 2022, 233, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Gegas, V.C.; Nazari, A.; Griffiths, S.; Simmonds, J.; Fish, L.; Orford, S.; Sayers, L.; Doonan, J.H.; Snape, J.W. A genetic framework for grain size and shape variation in wheat. Plant Cell 2010, 22, 1046–1056. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ding, J.; Effgen, S.; Turck, F.; Koornneef, M. Multiple loci and genetic interactions involving flowering time genes regulate stem branching among natural variants of Arabidopsis. New Phytol. 2013, 199, 843–857. [Google Scholar] [CrossRef]
- Wu, Q.H.; Chen, Y.X.; Zhou, S.H.; Fu, L.; Chen, J.J.; Xiao, Y.; Zhang, D.; Ouyang, S.H.; Zhao, X.J.; Cui, Y.; et al. High-density genetic linkage map construction and QTL mapping of grain shape and size in the wheat population Yanda1817× Beinong6. PLoS ONE 2015, 10, e0118144. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, L.; Meng, Y.; Hao, Y.; Xu, H.; Hao, M.; Lan, S.; Zhang, Y.; Lv, L.; Zhang, K.; et al. Dissection of Genetic Basis Underpinning Kernel Weight-Related Traits in Common Wheat. Plants 2021, 10, 713. [Google Scholar] [CrossRef]
- Cui, F.; Ding, A.; Li, J.; Zhao, C.; Wang, L.; Wang, X.; Qi, X.; Li, X.; Li, G.; Gao, J.; et al. QTL detection of seven spike-related traits and their genetic correlations in wheat using two related RIL populations. Euphytica 2012, 186, 177–192. [Google Scholar] [CrossRef]
- International Wheat Genome Sequencing Consortium (IWGSC). Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191. [Google Scholar] [CrossRef]
- Shi, S.; Azam, F.I.; Li, H.; Chang, X.; Li, B.; Jing, R. Mapping QTL for stay-green and agronomic traits in wheat under diverse water regimes. Euphytica 2017, 213, 1–19. [Google Scholar] [CrossRef]
- Guan, P.; Lu, L.; Jia, L.; Kabir, M.R.; Zhang, J.; Lan, T.; Zhao, Y.; Xin, M.; Hu, Z.; Yao, Y.; et al. Global QTL analysis identifies genomic regions on chromosomes 4A and 4B harboring stable loci for yield-related traits across different environments in wheat (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 529. [Google Scholar] [CrossRef] [PubMed]
- El-Feki, W.M.; Byrne, P.F.; Reid, S.D.; Scott, D.; Haley, S.D. Mapping Quantitative Trait Loci for Agronomic Traits in Winter Wheat under Different Soil Moisture Levels. Agronomy 2018, 8, 133. [Google Scholar] [CrossRef]
- Wang, B.; Liu, H.; Liu, Z.; Dong, X.; Guo, J.; Li, W.; Chen, J.; Gao, C.; Zhu, Y.; Zheng, X.; et al. Identification of minor effect QTLs for plant architecture related traits using super high density genotyping and large recombinant inbred population in maize (Zea mays). BMC Plant Biol. 2018, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Zhang, X.; Zhang, W.; Zhang, N.; Song, L.; Liu, L.; Xue, X.; Liu, G.; Liu, J.; Meng, D.; et al. QTL detection for kernel size and weight in bread wheat (Triticum aestivum L.) using a high-density SNP and SSR-based linkage map. Front. Plant Sci. 2018, 9, 1484. [Google Scholar] [CrossRef]
- Liu, C.; Sukumaran, S.; Jarquin, D.; Crossa, J.; Dreisigacker, S.; Sansaloni, C.; Reynolds, M. Comparison of Array- and Sequencing-based Markers for Genome Wide Association Mapping and Genomic Prediction in Spring Wheat. Crop Sci. 2019, 60, 211–225. [Google Scholar] [CrossRef]
- Elshire, R.J.; Glaubitz, J.C.; Sun, Q.; Poland, J.A.; Kawamoto, K.; Buckler, E.S.; Mitchell, S.E. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 2011, 6, e19379. [Google Scholar] [CrossRef]
- Heslot, N.; Rutkoski, J.; Poland, J.; Jannink, J.L.; Sorrells, M.E. Impact of marker ascertainment bias on genomic selection accuracy and estimates of genetic diversity. PLoS ONE 2013, 8, e74612. [Google Scholar] [CrossRef]
- Alipour, H.; Bai, G.; Zhang, G.; Bihamta, M.R.; Mohammadi, V.; Peyghambari, S.A. Imputation accuracy of wheat genotyping-by-sequencing (GBS) data using barley and wheat genome references. PLoS ONE 2019, 14, e0208614. [Google Scholar] [CrossRef]
- Zou, C.; Karn, A.; Reisch, B.; Nguyen, A.; Sun, Y.; Bao, Y.; Campbell, M.S.; Church, D.; Williams, S.; Xu, X.; et al. Haplotyping the Vitis collinear core genome with rhAmpSeq improves marker transferability in a diverse genus. Nat. Commun. 2020, 11, 413. [Google Scholar] [CrossRef]
- Wenzl, P.; Carling, J.; Kudrna, D.; Jaccoud, D.; Huttner, E.; Kleinhofs, A.; Kilian, A. Diversity Arrays Technology (DArT) for whole-genome profiling of barley. Proc. Natl. Acad. Sci. USA 2004, 101, 9915–9920. [Google Scholar] [CrossRef]
- Akbari, M.; Wenzl, P.; Caig, V.; Carling, J.; Xia, L.; Yang, S.; Uszynski, G.; Mohler, V.; Lehmensiek, A.; Kuchel, H.; et al. Diversity arrays technology (DArT) for high-throughput profiling of the hexaploid wheat genome. Theor. Appl. Genet. 2006, 113, 1409–1420. [Google Scholar] [CrossRef]
- Peleg, Z.; Saranga, Y.; Suprunova, T.; Ronin, Y.; Röder, M.S.; Kilian, A.; Korol, A.B.; Fahima, T. High-density genetic map of durum wheat× wild emmer wheat based on SSR and DArT markers. Theor. Appl. Genet. 2008, 117, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Poland, J.; Endelman, J.; Dawson, J.; Rutkoski, J.; Wu, S.; Manes, Y.; Dreisigacker, S.; Crossa, J.; Sánchez-Villeda, H.; Sorrells, M.; et al. Genomic selection in wheat breeding using genotyping-by-sequencing. Plant Genome 2012, 5, 103–113. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, Z.; Peng, Z.; Yu, Y.; Liao, M.; Wei, S. Development of a high-density linkage map and mapping of the three-pistil gene (Pis1) in wheat using GBS markers. BMC Genom. 2017, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jantasuriyarat, C.; Vales, M.I.; Watson, C.J.W.; Riera-Lizarazu, O. Identification and mapping of genetic loci affecting the free-threshing habit and spike compactness in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2004, 108, 261–273. [Google Scholar] [CrossRef]
- Deng, X.F.; Zhou, Y.H.; Yang, R.W.; Ding, C.B.; Zhang, L.; Zhang, H.Q. Chromosomal location of genes for spike length in dwarfing polish wheat by monosomic analysis. Sichuan Agric. Univ. J. 2005, 23, 12–14. [Google Scholar]
- Kumar, N.; Kulwal, P.L.; Balyan, H.S.; Gupta, P.K. QTL mapping for yield and yield contributing traits in two mapping populations of bread wheat. Mol. Breed. 2007, 19, 163–177. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, D.; Zhang, C.; Zhang, Z.; Xue, S.; Lin, F.; Kong, Z.; Tian, D.; Luo, Q. Molecular genetic analysis of five spike-related traits in wheat using RIL and immortalized F2 populations. Mol. Genet. Genom. 2007, 277, 31–42. [Google Scholar] [CrossRef]
- Chu, C.G.; Xu, S.S.; Friesen, T.L.; Faris, J.D. Whole genome mapping in a wheat doubled-haploid population using SSRs and TRAPs and the identification of QTL for agronomic traits. Mol. Breed. 2008, 22, 251–266. [Google Scholar] [CrossRef]
- Yang, R.; Liu, L.; Li, H.; Zhong, H.; Yang, X.; Wang, Z.; Liu, S. QTL analysis of spike traits in an recombinant inbred lines (RILs) population derived from the cross of Triticum polonicum × T. aestivum line Zhong 13. J. Agric. Biotechnol. 2012, 20, 506–513. [Google Scholar] [CrossRef]
- Cui, F.; Zhao, C.; Li, J.; Ding, A.; Li, X.F.; Bao, Y.; Li, J.; Ji, J.; Wang, H. Kernel weight per spike: What contributes to it at the individual QTL level? Mol. Breed. 2013, 31, 256–278. [Google Scholar] [CrossRef]
- Islamovic, E.; Obert, D.E.; Oliver, R.E.; Marshall, J.M.; Miclaus, K.J.; Hang, A.; Chao, S.; Lazo, G.R.; Harrison, S.A.; Ibrahim, A.; et al. A new genetic linkage map of barley (Hordeum vulgare L.) facilitates genetic dissection of height and spike length and angle. Field Crops Res. 2013, 154, 91–99. [Google Scholar] [CrossRef][Green Version]
- Yu, M.; Mao, S.L.; Chen, G.Y.; Pu, Z.E.; Wei, Y.M.; Zheng, Y.L. QTLs for uppermost internode and spike length in two wheat RIL populations and their affect upon plant height at an individual QTL level. Euphytica 2014, 200, 95–108. [Google Scholar] [CrossRef]
- Xu, D.; Wen, W.; Fu, L.; Li, F.; Xie, L.; Xia, X.; Ni, Z.; He, Z.; Cao, S. Genetic dissection of a major QTL for kernel weight spanning the Rht-B1 locus in bread wheat. Theor. Appl. Genet. 2019, 132, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, D.; Meng, Z.; Xu, K.; Yan, J.; Xia, X.; Cao, S.; Tian, Y.; He, Z.; Zhang, Y. QTL mapping for grain yield-related traits in bread wheat via SNP-based selective genotyping. Theor. Appl. Genet. 2020, 133, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Khumalo, T.P.; Barnard, A.; Dube, E.; Tsilo, T.J. Characterization of vegetative vigor of two doubled-haploid wheat populations. J. Crop Improv. 2021, 36, 350–368. [Google Scholar] [CrossRef]
- ARC. Agricultural Research Council Guidelines for the Production of Small Grains in the Winter Rainfall Area; ARC Small Grain Institute: Bethlehem, South Africa, 1993. [Google Scholar]
- ARC. Agricultural Research Council Guidelines for the Production of Small Grains in the Summer Rainfall Area; ARC Small Grain Institute: Bethlehem, South Africa, 1999. [Google Scholar]
- Federer, W.T. Augmented designs with one-way elimination of heterogeneity. Biometrics 1961, 17, 447–473. [Google Scholar] [CrossRef]
- Lephuthing, M.C.; Tolmay, V.L.; Baloyi, T.A.; Hlongoane, T.; Oliphant, T.A.; Tsilo, T.J. Relationship of grain micronutrient concentrations and grain yield components in a doubled haploid bread wheat (Triticum aestivum) population. Crop Pasture Sci. 2021, 73, 116–126. [Google Scholar] [CrossRef]
- VSN International. Genstat for Windows 18th Edition. A Guide to QTL Analysis Genstat; VSN International: Hemel Hempstead, UK, 2015; Available online: www.genstat.co.uk (accessed on 4 October 2021).
- Tsilo, T.J.; Kolmer, J.A.; Anderson, J.A. Molecular mapping and improvement of leaf rust resistance in wheat breeding lines. Phytopathology 2014, 104, 865–870. [Google Scholar] [CrossRef]
- Khumalo, T.P.; Hlongoane, T.; Barnard, A.; Tsilo, T.J. Genomic regions influencing pre-harvest sprouting tolerance in doubled haploid wheat population (Triticum aestivum L.). Agronomy 2022, 12, 832. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R.; RStudio (version 1.1.463), Inc.: Boston, MA, USA, 2019; Available online: http://www.rstudio.com/ (accessed on 27 October 2021).
- van Ooijen, J.W. JoinMap 4, Software for the Calculation of Genetic Linkage Maps in Experimental Populations. Kyazma, B.V.: Wageningen, The Netherlands, 2006. [Google Scholar]
- Stam, P. Construction of integrated genetic linkage maps by means of a new computer package: JoinMap. Plant J. 1993, 3, 739–744. [Google Scholar] [CrossRef]
- Kosambi, D.D. The estimation of map distances from recombination values. Ann. Eugen. 1943, 12, 172–175. [Google Scholar] [CrossRef]
- Wang, S.; Basten, C.J.; Zeng, Z.B. Windows QTL Cartographer 2.5; Department of Statistics, North Carolina State University: Raleigh, NC, USA, 2012. [Google Scholar]
- Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Applied Statistics for the Behavioral Sciences; Houghton Mifflin College Division: Boston, MA, USA, 2003; Volume 663. [Google Scholar]
- Griffiths, S.; Simmonds, J.; Leverington, M.; Wang, Y.; Fish, L.; Sayers, L.; Alibert, L.; Orford, S.; Wingen, L.; Snape, J. Meta-QTL analysis of the genetic control of crop height in elite European winter wheat germplasm. Mol. Breed. 2012, 29, 159–171. [Google Scholar] [CrossRef]
- Peng, J.; Richards, D.E.; Hartley, N.M.; Murphy, G.P.; Devos, K.M.; Flintham, J.E.; Beales, J.; Fish, L.J.; Worland, A.J.; Pelica, F.; et al. ‘Green Revolution’ genes encode mutant gibberellin response modulators. Nature 1999, 400, 256–261. [Google Scholar] [CrossRef]
- Heidari, B.; Sayed-Tabatabaei, B.E.; Saeidi, G.; Kearsey, M.; Suenaga, K. Mapping QTL for grain yield, yield components, and spike features in a doubled haploid population of bread wheat. Genome 2011, 54, 517–527. [Google Scholar] [CrossRef]
- Su, Z.; Hao, C.; Wang, L.; Dong, Y.; Zhang, X. Identification of and development of a functional marker of TaGW2 associated with grain weight in bead wheat (Triticum aestivum L.). Theor. Appl. Genet. 2011, 122, 211–223. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Z.; Fan, X.; Zhou, Q.; Cao, J.; Wang, F.; Ji, G.; Yang, L.; Feng, B.; Wang, T. A genome-wide association study of wheat spike related traits in China. Front. Plant Sci. 2018, 9, 1584. [Google Scholar] [CrossRef]
- Li, T.; Li, Q.; Wang, J.; Yang, Z.; Tang, Y.; Su, Y.; Zhang, J.; Qiu, X.; Pu, X.; Pan, Z.; et al. High-resolution detection of quantitative trait loci for seven important yield-related traits in wheat (Triticum aestivum L.) using a high-density SLAF-seq genetic map. BMC Genom. Data 2022, 23, 1–16. [Google Scholar] [CrossRef]
- Pretini, N.; Vanzetti, L.S.; Terrile, I.I.; Donaire, G.; González, F.G. Mapping QTL for spike fertility and related traits in two doubled haploid wheat (Triticum aestivum L.) populations. BMC Plant Biol. 2021, 21, 353. [Google Scholar] [CrossRef]
- Fan, X.; Cui, F.; Ji, J.; Zhang, W.; Zhao, X.; Lui, J.; Meng, D.; Tong, Y.; Wang, T.; Li, J. Dissection of pleiotropic QTL regions controlling wheat spike characteristics under different nitrogen treatments using traditional and conditional QTL mapping. Front. Plant Sci. 2019, 10, 187. [Google Scholar] [CrossRef]
- Campbell, K.G.; Bergman, C.J.; Gualberto, D.G.; Anderson, J.A.; Giroux, M.J.; Hareland, G.; Fulcher, R.G.; Sorrells, M.E.; Finney, P.L. Quantitative trait loci associated with kernel traits in a soft × hard wheat cross. Crop Sci. 1999, 39, 1184–1195. [Google Scholar] [CrossRef]
- Wen, S.; Zhang, M.; Tu, K.; Fan, C.; Tian, S.; Bi, C.; Chen, Z.; Zhao, H.; Wei, C.; Shi, X.; et al. A Major Quantitative Trait Loci Cluster Controlling Three Components of Yield and Plant Height Identified on Chromosome 4B of Common Wheat. Front. Plant Sci. 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Cui, F.; Zhao, C.; Ding, A.; Li, J.; Wang, L.; Li, X.; Bao, Y.; Li, J.; Wang, H. Construction of an integrative linkage map and QTL mapping of grain yield-related traits using three related wheat RIL populations. Theor. Appl. Genet. 2014, 127, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Xu, D.; Hanif, M.; Xia, X.; He, Z. Genetic architecture underpinning yield component traits in wheat. Theor. Appl. Genet. 2020, 133, 1811–1823. [Google Scholar] [CrossRef]
- Mangini, G.; Blanco, A.; Nigro, D.; Signorile, M.A.; Simeone, R. Candidate Genes and Quantitative Trait Loci for Grain Yield and Seed Size in Durum Wheat. Plants 2021, 10, 312. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Fan, T.; Chen, S.; Ou, X.; Chen, Y.; Jiang, Q.; Diao, Y.; Sun, Z.; Peng, W.; Ren, Z.; et al. QTL Mapping and Validation for Kernel Area and Circumference in Common Wheat via High-Density SNP-Based Genotyping. Front. Plant Sci. 2021, 12, 13890. [Google Scholar] [CrossRef] [PubMed]
- Mwadzingeni, L.; Shimelis, H.; Tsilo, T.; Tesfay, S. Screening of bread wheat genotypes for drought tolerance using phenotypic and proline analyses. Front. Plant Sci. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Y.; Gao, S.; Li, Z.; Ma, J.; Deng, M.; Chen, G.; Wei, Y.; Zheng, Y. A genome-wide association study of 23 agronomic traits in Chinese wheat landraces. Plant J. 2017, 91, 861–873. [Google Scholar] [CrossRef]
- Kumar, N.; Kulwal, P.L.; Gaur, A.; Tyagi, A.K.; Khurana, J.P.; Khurana, P.; Balyan, H.S.; Gupta, P.K. QTL analysis for grain weight in common wheat. Euphytica 2006, 151, 135–144. [Google Scholar] [CrossRef]
- Cabral, A.L.; Jordan, M.C.; Larson, G.; Somers, D.J.; Humphreys, D.G.; McCartney, C.A. Relationship between QTL for grain shape, grain weight, test weight, milling yield, and plant height in the spring wheat cross RL4452/’AC Domain’. PLoS ONE 2018, 13, e0190681. [Google Scholar] [CrossRef]
- Chen, Z.; Cheng, X.; Chai, L.; Wang, Z.; Bian, R.; Li, J.; Zhao, A.; Xin, M.; Guo, W.; Hu, Z.; et al. Dissection of genetic factors underlying grain size and fine mapping of QTgw.cau-7D in common wheat (Triticum aestivum L.). Theor. Appl. Genet. 2020, 133, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Sorrells, M.E. Three-dimensional seed size and shape QTL in hexaploid wheat (Triticum aestivum L.) populations. Crop Sci. 2014, 54, 98–110. [Google Scholar] [CrossRef]
- Su, Z.; Jin, S.; Lu, Y.; Zhang, G.; Chao, S.; Bai, G. Single nucleotide polymorphism tightly linked to a major QTL on chromosome 7A for both kernel length and kernel weight in wheat. Mol. Breed. 2016, 36, 15. [Google Scholar] [CrossRef]
- Tyagi, S.; Mir, R.R.; Balyan, H.S.; Gupta, P.K. Interval mapping and meta-QTL analysis of grain traits in common wheat (Triticum aestivum L.). Euphytica 2015, 201, 367–380. [Google Scholar] [CrossRef]
- Prashant, R.; Kadoo, N.; Desale, C.; Kore, P.; Dhaliwal, H.S.; Chhuneja, P.; Gupta, V. Kernel morphometric traits in hexaploid wheat (Triticum aestivum L.) are modulated by intricate QTL × QTL and genotype× environment interactions. J. Cereal Sci. 2012, 56, 432–439. [Google Scholar] [CrossRef]
- Xie, Q.; Mayes, S.; Sparkes, D.L. Carpel size, grain filling, and morphology determine individual grain weight in wheat. J. Exp. Bot. 2015, 66, 6715–6730. [Google Scholar] [CrossRef]
- Feng, T.; Xi, Y.; Zhu, Y.-H.; Chai, N.; Zhang, X.-T.; Jin, Y.; Turner, N.C.; Li, F.-M. Reduced Vegetative Growth Increases Grain Yield in Spring Wheat Genotypes in the Dryland Farming Region of North-West China. Agronomy 2021, 11, 663. [Google Scholar] [CrossRef]
- Reynolds, M.; Foulkes, M.J.; Slafer, G.A.; Berry, P.; Parry, M.A.J.; Snape, J.W.; Angus, W.J. Raising yield potential in wheat (review paper). J. Exp. Bot. 2009, 60, 899–1918. [Google Scholar] [CrossRef]
- Würschum, T.; Leiser, W.L.; Langer, S.M.; Tucker, M.R.; Longin, C.F.H. Phenotypic and genetic analysis of spike and kernel characteristics in wheat reveals long-term genetic trends of grain yield components. Theor. Appl. Genet. 2018, 131, 2071–2084. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, N.; Wu, Y.; Sun, H.; Liu, C.; Fan, X.; Yan, X.; Xu, H.; Ji, J.; Cui, F. QTL for spike-layer uniformity and their influence on yield-related traits in wheat. BMC Genet. 2019, 20, 23. [Google Scholar] [CrossRef]
- Simons, K.J.; Fellers, J.P.; Trick, H.N.; Zhang, Z.C.; Tai, Y.S.; Gill, B.S.; Faris, J.D. Molecular characterization of the major wheat domestication gene Q. Genetics 2006, 172, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Faris, J.D.; Zhang, Z.; Garvin, D.F.; Xu, S.S. Molecular and comparative mapping of genes governing spike compactness from wild emmer wheat. Mol. Genet. Genom. 2014, 289, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Liu, W.H.; Wang, H.; Li, L.H.; Wu, J.; Yang, X.M.; Li, X.Q.; Gao, A.N. QTL mapping of yield-related traits in the wheat germplasm 3228. Euphytica 2011, 177, 277–292. [Google Scholar] [CrossRef]

| Trait a | PH | SL | GNS | SPS | GWPS | TKW | GL |
|---|---|---|---|---|---|---|---|
| SL | 0.764 ** | - | |||||
| GNS | 0.660 ** | 0.604 ** | - | ||||
| SPS | 0.811 ** | 0.756 ** | 0.823 ** | - | |||
| GWPS | 0.759 ** | 0.611 ** | 0.854 ** | 0.764 ** | - | ||
| TKW | 0.572 ** | 0.351 ** | 0.449 ** | 0.444 ** | 0.786 ** | - | |
| GL | −0.414 ** | −0.201 ** | −0.340 ** | −0.371 ** | −0.242 ** | −0.120 * | - |
| GW | 0.297 ** | 0.235 ** | 0.211 ** | 0.212 ** | 0.447 ** | 0.494 ** | 0.312 ** |
| Chromosome | No. of Markers | Map Length (cm) | Marker Density (cm/Marker) a |
|---|---|---|---|
| 1A | 19 | 49.33 | 2.60 |
| 1B | 30 | 90.05 | 3.00 |
| 1D | 25 | 76.50 | 3.06 |
| 2A | 17 | 96.05 | 5.65 |
| 2B | 40 | 75.98 | 1.90 |
| 2D | 11 | 73.56 | 6.69 |
| 3A | 8 | 49.80 | 6.22 |
| 3B | 29 | 58.84 | 2.03 |
| 3D ‡ | 15 | 89.90 | 12.33 |
| 4A | 8 | 48.35 | 6.04 |
| 4B | 23 | 82.90 | 3.60 |
| 4D | 15 | 74.35 | 4.96 |
| 5A | 14 | 53.35 | 3.81 |
| 5B | 31 | 76.91 | 2.48 |
| 5D | 15 | 34.15 | 2.28 |
| 6A | 20 | 57.27 | 2.86 |
| 6B ‡ | 58 | 93.60 | 3.38 |
| 6D | 7 | 55.74 | 7.96 |
| 7A | 34 | 126.62 | 3.72 |
| 7B | 39 | 110.63 | 2.84 |
| 7D | 25 | 42.67 | 1.71 |
| A sub-genome | 120 | 480.77 | 4.01 |
| B sub-genome | 250 | 588.92 | 2.36 |
| D sub-genome | 113 | 446.88 | 3.96 |
| Total | 483 | 1516.57 | 3.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lephuthing, M.C.; Khumalo, T.P.; Tolmay, V.L.; Dube, E.; Tsilo, T.J. Genetic Mapping of Quantitative Trait Loci Associated with Plant Height and Yield Component Traits in a Wheat (Triticum aestivum L.) Doubled Haploid Population Derived from Tugela-DN × Elands. Agronomy 2022, 12, 2283. https://doi.org/10.3390/agronomy12102283
Lephuthing MC, Khumalo TP, Tolmay VL, Dube E, Tsilo TJ. Genetic Mapping of Quantitative Trait Loci Associated with Plant Height and Yield Component Traits in a Wheat (Triticum aestivum L.) Doubled Haploid Population Derived from Tugela-DN × Elands. Agronomy. 2022; 12(10):2283. https://doi.org/10.3390/agronomy12102283
Chicago/Turabian StyleLephuthing, Mantshiuwa Christinah, Thobeka Philile Khumalo, Vicki Louise Tolmay, Ernest Dube, and Toi John Tsilo. 2022. "Genetic Mapping of Quantitative Trait Loci Associated with Plant Height and Yield Component Traits in a Wheat (Triticum aestivum L.) Doubled Haploid Population Derived from Tugela-DN × Elands" Agronomy 12, no. 10: 2283. https://doi.org/10.3390/agronomy12102283
APA StyleLephuthing, M. C., Khumalo, T. P., Tolmay, V. L., Dube, E., & Tsilo, T. J. (2022). Genetic Mapping of Quantitative Trait Loci Associated with Plant Height and Yield Component Traits in a Wheat (Triticum aestivum L.) Doubled Haploid Population Derived from Tugela-DN × Elands. Agronomy, 12(10), 2283. https://doi.org/10.3390/agronomy12102283





