Abstract
Sugarcane, a highly productive crop, is frequently challenged by different biotic agents, such as the pokkah boeng disease that can cause drastic yield losses of up to 40%. This airborne fungal disease is caused by various Fusarium species integrated into a complex. Integrating novel compounds and biological control agents is of paramount importance to cope with these fungi in sustainable systems. In this study, we aimed to evaluate the effect and compatibility of phosphite (Phi) and Trichoderma sp. in the control of Fusarium sp., in vitro and in planta. Using in vitro tests, we evaluated the effects of Phi (0, 500, 1000, 2000, 4000, and 8000 µg mL−1) and the compatibility of Phi + Trichoderma (isolates Taz 001, 013, and 016) on the pathogen complex. Using in planta tests, we evaluated the compatibility of Phi + Trichoderma (Taz-016) in the control of Fusarium in sugarcane plants under greenhouse conditions. A synergistic effect of Phi + Trichoderma was observed in vitro on the control of the pathogen, especially when combining 1000 µg mL−1 + Trichoderma Taz-016. In the in planta trial, combining 4000 µg mL−1 Phi + Trichoderma Taz-016 showed the best control of Fusarium infection, improving plant height, culm length and leaf dry weight.
1. Introduction
Due to its high photosynthetic capacity and culm sucrose storage ability, sugarcane (Saccharum spp.) is one of the most productive crops globally [1]. Its cultivation covers more than 26.2 million hectares in more than 130 countries and territories worldwide [2], producing more than 1907 million tons of milling canes and 174 million tons of sugar for human and industrial consumption [3]. In addition to its contribution to sugar production, this crop is a main source of intermediaries and raw materials for producing alcohol, acetic acid, butanol, paper, industrial enzymes, and bioethanol as a gasoline alternative biofuel [4,5].
The growth and yield of this crop is affected by various abiotic factors, such as low soil fertility, water deficit, metal toxicity, prolonged drought, and salinity, among others [6], as well as factors of a biotic nature, such as diseases caused by viruses, bacteria, and fungi [7].
One of the biotic factors involved in the deterioration of sugarcane roots and culms is various species of phytopathogenic fungi of the Fusarium genus, which are common associates of higher plants and are among the most ubiquitous fungi in soil ecosystems [8,9]. In sugarcane, several species of Fusarium (i.e., F. sacchari, F. moniliformae, F. verticillioides, and F. moniliforme var. subglutinans) [10] form a complex that causes pokkah boeng. This disease induces deformation of the apex in sugarcane plants. This disease can decrease crop yield by 10–40% [10] and sucrose content by 40.8 to 64.5% [11].
Conventional control of this disease is through applying benzimidazole fungicides like methyl N-(1H-benzimidazol-2-yl) carbamate and methyl N-[1-(butylcarbamoyl)benzimidazol-2-yl] carbamate. However, the indiscriminate use of these products has induced microbial resistance mechanisms [12,13,14], which, together with the consequences of climate change, facilitate the proliferation of these diseases and make their control more difficult. The search for new biorational alternatives is highly prioritized in formulating sustainable management strategies for plant diseases. Alternative disease management includes using biological control through antagonistic microorganisms or disease resistance induced with inorganic salts [15,16].
The Trichoderma fungus is commonly used in the biocontrol of soil pathogens. It has been reported as an effective antagonist of phytopathogenic fungi and a stimulator of plants’ natural defense systems [17]. Phosphite (Phi) salts, which are derived from phosphorous acid (phosphonate), have been reported as an option to conventional fungicides to control plant pathogens [18,19], such as Phytophthora infestans, Fusarium circitatum, and Rhizoctonia sp., among others [15,20,21,22].
Trichoderma has different activities during the control of pathogens, including competition for the substrate, mycoparasitism, antibiosis, and deactivation of pathogenic enzymes [23]. Phosphite exerts a direct effect on phytopathogenic fungi, causing decreased conidiogenesis and inhibition of mycelial growth [22,24,25]. Additionally, Phi can act indirectly, stimulating the natural defense mechanisms of plants [16]. Phosphite salts are biocompatible chemical compounds that improve the control of various diseases in plants by stimulating induced resistance (IR) mechanisms. Their effectiveness has been proven in various horticultural crops [15]. However, using Phi may prove less effective for the control of some pathogens than chemically synthesized fungicides [26], so applying it in an integrated management strategy, combined with Trichoderma, could increase its effectiveness against plant pathogens. To integrate any beneficial fungus with an inorganic compound, knowledge of the compatibility between the two agents is required [27,28].
Although in vitro compatibility between Trichoderma species and fungicides like potassium phosphonate, fosetyl aluminum, and a mixture of cymoxanil and mancozeb has shown good results [29], this relationship has not been determined between Phi and Trichoderma in sugarcane. The evaluation of combinations of Phi and native Trichoderma isolates provides basic information to help guide field studies for the control of Fusarium sp. in sugarcane. The objective of this research was to determine the effect and compatibility of Trichoderma sp., with different Phi concentrations in the control of Fusarium sp., which causes pokkah boeng in sugarcane, through in vitro and in planta approaches.
2. Materials and Methods
The experiment was carried out in the Laboratory of Applied Microbiology of the National Institute of Technology of Mexico Campus Tierra Blanca (Tecnológico Nacional de México/ ITS Tierra Blanca) located on Av. Veracruz, at the corner with Héroes de Puebla, Pemex Section, Tierra Blanca, Veracruz, Mexico. The experimental site is located between coordinates 18°44′ North Latitude and 96°45′ West Longitude, at 53 masl, with a dry tropical climate, type Aw1 and Aw2 according to the Köppen climate classification.
2.1. Microbiological Material
The Fusarium species complex used in this study was isolated from the rhizosphere of a sugarcane plantation cultivated with the variety CP 72-2086, which exhibited pokkah boeng symptoms. The sugarcane plot was located at coordinates 18°35′31″ N and 96°29′20″ W, at approximately 50 masl. The isolate was cultivated on potato dextrose agar (PDA; Bioxon; Cuautitlán Izcalli, State of Mexico, Mexico) using the hyphal tip method until its purification and taxonomic catalog [30]. Once cataloged in the laboratory, the isolate was grown in a Binder incubator (KB 115; Tuttlingen, Germany) at 28 °C for 7 d. Finally, the isolate was stored in 10% glycerol at 4 °C until in vitro inoculation. For in planta inoculation, spores were collected using a sterile brush and kept in suspension at 4 °C in a Tween-20 solution (Thermo Fisher; Santa Clara, CA, USA) at 0.03%. For the in vitro tests, three isolates of the genus Trichoderma, Taz-001, Taz-013, and Taz-016, from the ITSTB strain bank, were selected for evaluation. The Trichoderma isolates were obtained from the rhizosphere of sugarcane varieties CP 72-2086 (18°35′30″ N; 96°29′51″ W), Mex 69–290 (18°18′09″ N; 96°25′28″ W), and CP 70-1527 (18°22′22″ N; 96°28′24″ W), respectively. The isolates were grown, cataloged, and preserved until their inoculation as described above. For the in planta tests, only the Taz-016 isolate was evaluated, which was found to be the most effective in the in vitro tests among all strains evaluated.
2.2. Plant Material
For the in planta evaluations, 5 cm long cuttings of sugarcane variety CP 72–2086 were used. This variety was derived from the La Margarita Sugar Mill experimental field located in Vicente, Oaxaca, Mexico (18°35′ N; 96°33′ W, 100 masl). Each cutting consisted of a mature culm node with a healthy, well-developed bud.
2.3. Effect of Phi on In Vitro Fusarium sp. Growth
To evaluate the effect of Phi on the development and conidiogenesis of Fusarium sp., a biological response window was established with five Phi concentrations: 500, 1000, 2000, 4000, and 8000 µg mL−1, from phosphorous acid (Sigma-Aldrich®; St. Louis, MO, USA), and control without Phi, in PDA culture medium. The pH of the growth medium was adjusted to 5.7 with NaOH 1 N. The Fusarium sp. inoculum was adjusted to a concentration of 1 × 106 spores mL−1 with 0.03% Tween-20; 5 µL of the fungus suspension was inoculated in the center of the Petri dishes. All treatments were incubated at 28 °C for 7 d. Mycelial growth was measured every 24 h with a digital caliper until the control covered the whole surface of the Petri dish. To calculate the percentage of mycelial growth inhibition (MGI (%)), the formula proposed by Pandey et al. (1982) [31] was used.
where Dc is the mean diameter of the control fungus colonies, and Dt is the mean diameter of the treatment fungus colonies.
MGI (%) = (Dc − Dt)/Dc × 100
To evaluate conidiogenesis after the incubation period, spores were collected using a sterile brush and kept in suspension 0.03% Tween-20. The concentration of each treatment was recorded with the aid of a Neubauer camera (Laboroptik; Bad-Homburg, Germany) in a 131-CLED compound microscope (National; Beijing, China) at 40×.
2.4. Compatibility of Trichoderma Isolates with Phi
To evaluate the compatibility of Trichoderma sp. with Phi, suspensions of isolates Taz-001, Taz-013 and Taz-016 isolates were prepared and inoculated on PDA medium under the same conditions and Phi concentrations as in the previous experiment. The compatibility of the Trichoderma isolates with Phi was evaluated by comparing the spore concentration obtained in the control against those obtained with each Phi concentration.
2.5. In Vitro Compatibility of Phi with Trichoderma sp. in the Control of Fusarium sp.
In vitro compatibility was studied using the dual culture technique. At the base of the standard Petri dish, a diameter line (90 mm) was drawn, and two points were marked equidistant from the center towards the edge (22.5 mm) of the Petri dish. Thus, the inoculation points for the pathogen and the antagonist were determined. For each confrontation, 5 µL of the Trichoderma sp. suspensions (Taz-001, Taz-013, or Taz-016) and Fusarium sp., adjusted with 0.03% Tween-20 to 1 × 106 mL−1 spores, were inoculated. The three Trichoderma sp. isolates combined with 1000, 4000, and 8000 µg mL−1 Phi were evaluated against Fusarium sp. in PDA medium adjusted to pH 5.7 using NaOH 1 N. The control treatment was the pathogen without the presence of Phi or Trichoderma. All the treatments were incubated at 28 °C for 10 d, and the percentage of mycelial growth inhibition (MGI) was evaluated.
2.6. Compatibility of Phi and Trichoderma sp. in the Control of Fusarium sp. in Sugarcane Plants
To evaluate the compatibility of Phi with Trichoderma sp. in the control of Fusarium sp. in sugarcane plants (in planta assay), eighty 3 L plastic pots were established under greenhouse conditions. The pots were filled to two-thirds capacity with the sterile substrate mixture (121 °C, 15 lb/in2, 15 min), consisting of ITSB agricultural soil and peat moss (Cosmopeat®) provided by Cosmocel (San Nicolás de los Garza, Nuevo León, Mexico) at a 2:3 ratio (v:v). In each container, a sugarcane cutting bud was planted, and the corresponding treatments were applied. The distribution across the experimental containers was randomized, resulting in fourteen treatments of the three factors (Trichoderma sp., Phosphite, and Fusarium sp.) listed in Table 1 for better reader understanding.

Table 1.
Treatments evaluated to test the compatibility of Trichoderma sp. and phosphite (Phi) in the control of the Fusarium species complex causing pokkah boeng in sugarcane (Saccharum spp.).
Trichoderma inoculations (T7-T13) were conducted by immersing the cuttings (each containing a healthy and well-developed bud, previously washed with 2% sodium hypochlorite) in a spore suspension adjusted with 0.03% Tween-20 to 1 × 108 cells mL−1, for 30 min. Treatments that only included Phi application from phosphorous acid (Sigma-Aldrich®) adjusted to a pH of 5.7 with NaOH 1 N were immersed in the same way for 30 min at each concentration (T2-T6). In the combination of Phi with Trichoderma (T8–12), 50 mL of each of the Phi concentrations was added directly to the cuttings previously treated with Trichoderma. The Fusarium control and absolute control treatments (T1 and T14) consisted of immersing the cuttings in 0.03% Tween-20 for the same period.
Once the sugarcane seedlings reached 30 cm in height (~30 d), treatments with Fusarium (+F) were inoculated at the base of the plant through the drenching of 50 mL Fusarium sp. suspension, adjusted to 1 × 108 spores mL−1 with 0.03% Tween-20, while the rest of the treatments without Fusarium (−F) received 50 mL 0.03% Tween-20 with no Fusarium sp. spores.
During the evaluation, Phi was applied on three occasions (at days 8, 15 and 22 after planting, like preventive treatment). Trichoderma was applied twice by drenching (100 mL), using the same inoculation concentration at days 15 and 30 after planting. Two fertilizations were applied using soluble monoammonium phosphate up to field application strength (N-P-K (12-61-0)) at a concentration of 3 g L−1. These were applied at days 0 and 30 after planting. The seedlings were watered with 250 mL tap water every morning.
After 60 days, the compatibility of the agents (Phi and Trichoderma sp.) were evaluated in the control of Fusarium sp. For this analysis, each plant was separated into culm, leaf, and root. The presence or absence of red rings at the base of the culm, caused by the infection of the Fusarium species complex, was evaluated. Plant height, culm length, and leaf dry weight were quantified in each experimental unit for each treatment tested.
At the end of the evaluation, samples were taken from the roots of the seedlings inoculated with the Trichoderma isolate (Taz-016) and Fusarium sp. for their re-isolation. Using these samples, Koch’s postulates were confirmed, and the presence of the Fusarium species complex and/or Trichoderma sp. as biocontrol agent(s) in the rhizosphere was verified.
2.7. Experimental Design
For this study, an experiment with a completely randomized block design was established to analyze the variables conidiogenesis and inhibition percentage in the in vitro tests, as well as for the analyses of the morpho-physiological variables of the sugarcane seedlings in in planta tests. Six replicates per treatment were run, and all tests were conducted twice. Two statistical models were used: (1) generalized mixed linear model (GMLM) with a negative binomial response (λij, φ) for the responses evaluated as conidiogenesis in Fusarium sp. and Trichoderma sp., and (2) mixed linear model (MLM) for the MGI (%) of Fusarium sp., to study the effect of Phi, and the compatibility of Phi with Trichoderma sp. in vitro and in planta.
2.8. Statistical Analysis
The statistical analysis of the evaluated variables was done using the glimmix procedure (PROC GLIMMIX) with Statistical Analysis System 9.4 (SAS) for Windows. Means were compared using Fisher’s LSD test with a significance of 5%.
3. Results
3.1. Effect of Phi on In Vitro Fusarium sp. Growth
In this study, the evaluated concentrations (from 500 to 8000 µg mL−1 Phi) presented a fungistatic effect on the mycelial growth of the pathogen, with an inhibition range from 26.0 to 81.0% (Figure 1).
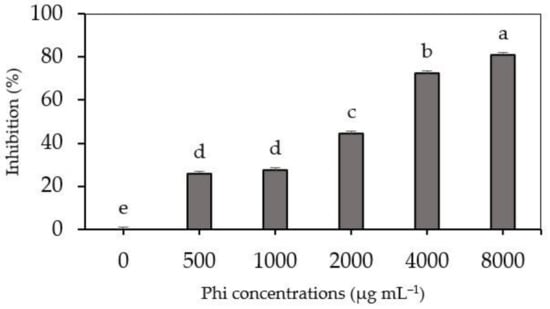
Figure 1.
Mycelial growth inhibition (MGI) of Fusarium sp. in PDA culture medium, with the addition of Phi (0, 500, 1000, 2000, 4000 and 8000 µg mL−1 Phi). Means ± SD with different letters are statistically different (Fisher’s test; α = 0.05). Phi–phosphite.
A fung-istatic effect on MGI (%) was observed when Phi was added to the medium. A fungicidal effect from 500 µg mL−1 was observed on the conidiogenesis of Fusarium sp., as it decreased by 99.7% compared to the control (Table 2).

Table 2.
Conidiogenesis of Fusarium sp. (spores mL−1) with Phi addition to the PDA culture medium.
3.2. Compatibility of Trichoderma Isolates with Phi
In the compatibility experiment between Trichoderma and Phi, there was a significant (p ≤ 0.001) decrease in the conidiogenesis of the fungus. There were significant differences in all three isolates evaluated when increasing the Phi concentration in the medium (Figure 2). Nevertheless, mycelial growth continued until the whole dish was colonized (7 d after inoculation).
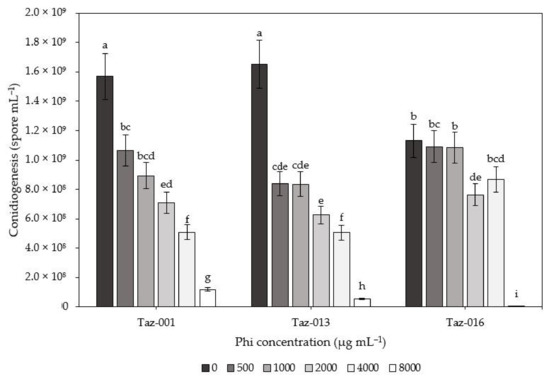
Figure 2.
Conidiogenesis of Trichoderma sp. isolations (Taz-001, Taz-013 and Taz-016) determined as the number of spores per mL established in PDA medium supplemented with different concentrations of phosphite (Phi). Means with different letters indicate significant differences among treatments (Fisher’s test; α = 0.05).
The conidiogenesis of the Taz-016 isolate showed no statistical differences between concentrations of 500 and 1000 µg mL−1 Phi concerning the control, as it only decreased by 3.5 and 4.1%, respectively. On the other hand, Taz-001 showed significant differences at the same concentrations, as its conidiogenesis decreased by 32.1 and 57.0%, respectively. Likewise, Taz-013 decreased by 49.2 and 50.7%, respectively (Figure 2). The Taz-001 strain recorded higher conidiogenesis in the treatment with 8000 µg mL−1 than the other isolates; 52.6% more than Taz-013 and 97.9% more than Taz-016.
3.3. In Vitro Compatibility of Phi with Trichoderma sp. in the Control of Fusarium sp.
In the compatibility between Phi and Trichoderma sp. for the control of Fusarium sp. experiment, total colonization of the Petri dish was observed in the control treatments at 10 d after inoculation. In addition, a higher percentage of pathogen inhibition was recorded when Phi was applied combined with Trichoderma suspensions (Figure 3), which was taken as an indicator of compatibility.
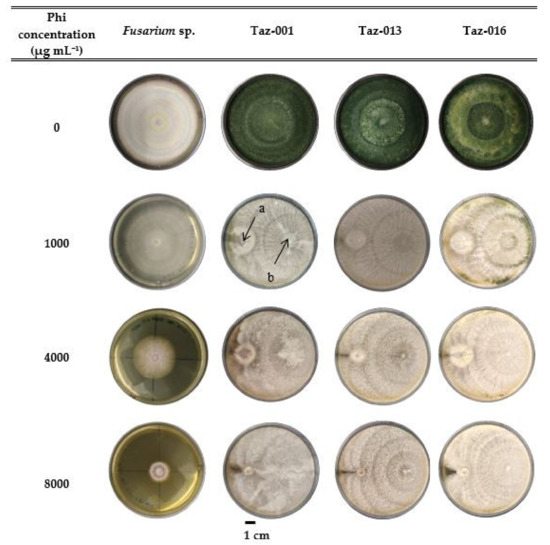
Figure 3.
Growth of Fusarium sp. and Trichoderma sp. isolates after 10 d incubation in Petri dishes with three different Phi concentrations and a control without Phi. (a) Pathogen inoculation (Fusarium sp.); (b) bioregulator inoculation (Trichoderma sp.).
Significant statistical differences were observed in the percentage of mycelial growth inhibition when increasing the Phi concentration in the medium (p ≤ 0.0001). The highest Phi concentration (8000 µg mL−1) inhibited Fusarium sp. by 77. 8%, while 4000 and 1000 µg mL−1 Phi caused 56.0 and 30.3% inhibition, respectively (Figure 4).
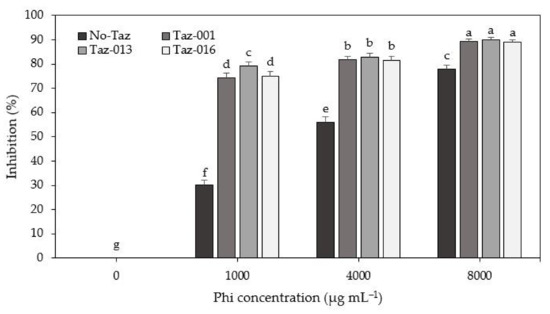
Figure 4.
Mycelial growth inhibition (MGI) of Fusarium sp. with Trichoderma sp. (Taz-001, Taz-013, and Taz-016) and adding Phi to the culture medium. Means ± SD with different letters are statistically different (Fisher’s test; α = 0.05).
The highest inhibition percentages were observed with combining Trichoderma and Phi. When Trichoderma + 1000 µg mL−1 Phi were combined, at least 74.3% inhibition was achieved. This means it increased by 44.0% more than in the Phi treatment alone, at the same concentration. When Trichoderma + 4000 µg mL−1 Phi was combined, the inhibition percentage increased by 25.2% concerning the treatment with Phi alone. Finally, when Trichoderma + 8000 µg mL−1 Phi was combined, the pathogen inhibition percentage increased by 11.2% compared to the treatment with Phi alone, at the same concentration (8000 µg mL−1) (Figure 4).
3.4. Compatibility of Phi and Trichoderma sp., in the Control of Fusarium sp. in Sugarcane Plants
In this experiment, the treatments that included only Phi application (T2-T6), only Trichoderma sp. (T7 and T13), and combining the two fungicidal agents (T8–12), showed no symptoms of the presence of Fusarium sp., nor did treatment T14 (absolute control). However, treatment T1 showed necrotic lesions at the root and a red ring at the base of the culms (Figure 5A,B).
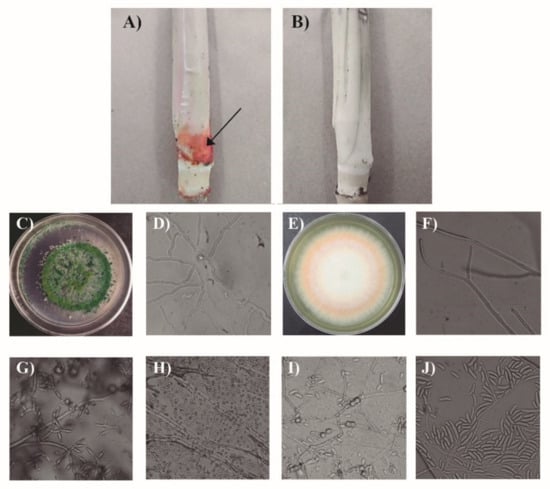
Figure 5.
Base of the sugarcane culm exposed to the tested treatments and isolates of Trichoderma sp. and a Fusarium species complex from the roots of sugarcane plants to test the Koch’s postulates. (A) Presence of a red ring at the base of the culm in plants inoculated with Fusarium sp. on the root without fungicidal agents; (B) absence of red ring at the base of the culm in plants inoculated with Fusarium sp. on the root with any of the combinations of fungicidal agents (Trichoderma + Phi); (C) Trichoderma sp. colony in PDA; (D) Trichoderma sp. septate mycelium.; (E) Fusarium sp. colony in PDA; (F) Fusarium mycelium; (G) phialides in Trichoderma isolate; (H) Trichoderma spores; (I) Fusarium resistance structures; (J) Fusarium spores.
In each of the treatments inoculated with Trichoderma sp. and Fusarium sp. the two isolates were obtained (Figure 5C–J), corroborating Koch’s postulates.
In evaluating plant height, significant differences between treatments were observed (p ≤ 0.0001). Treatment T11 (Trichoderma sp. Taz 016 + 4000 µg mL−1 Phi in the presence of Fusarium) improved plant height by 10.4% compared to the control. Moreover, the presence of Fusarium sp. without any fungicidal agent (T1) decreased plant height by 10.4%. Likewise, treatments T4 (2000 µg mL−1 Phi in the presence of Fusarium) and T5 (4000 µg mL−1 Phi in the presence of Fusarium) decreased plant height by 11.6 and 11.9%, respectively (Table 3). For culm length, treatment T11 had the best response, with 30.8% increased plant height over the control (p ≤ 0.0001). In comparison, the presence of Fusarium sp. without using control agents (T1) generated a decrease of 13.2% in the mean value of this variable compared to the control (Table 3).

Table 3.
Effect of Trichoderma sp. (Tri) and phosphite (Phi; µg mL−1) in the control of Fusarium sp. (Fus) on plant height, culm length, and leaf dry weight of sugarcane (Saccharum spp.).
4. Discussion
4.1. Phi Significantly Decreases the Growth of the Fusarium Species Complex In Vitro
The action mechanism of Phi on fungi and oomycetes is complex and is not yet known in detail [18]. However, Phi has been shown to have direct effects on mycelial growth in soil pathogens like Alternaria alternata (90.0%), Penicillium expansum (50.0%), and Phytophthora infestans (26.0%), among others [25,32]. In this work, the concentrations of Phi exhibited a fungistatic effect on the mycelial growth (Figure 1), and the inhibitory effect of the different Phi concentrations on the vegetative development of Fusarium sp. was investigated. Interestingly, our results are in full agreement with those found elsewhere [33]. Phosphite proved to be effective in inhibiting the mycelial growth of Fusarium solani under all their experimental conditions (1–6 g L−1) since none of the treatments showed mycelial growth. Phosphite-containing compounds inhibit the growth of plant pathogens through a direct fungistatic effect, which depends on the Phi concentration that accumulates in the fungus. In turn, growth is influenced by the phosphate concentration and the effectiveness of the phosphite oxidation system [33]. Potassium phosphite also partially inhibits developing Alternaria alternata, and concentrations of 229 and 531 µg mL−1 Phi cause 50.0 and 90.0% inhibition in conidial germination, respectively [32]. Furthermore, when applying 22.5–50.0 mM Phi, the sporulation of Hyaloperonospora arabidopsidis was decreased up to 97.0% [18]; hence, the amount of spores provides a valid estimate of the susceptibility of pathogens to Phi.
4.2. Compatibility between Trichoderma and Phi
A combined integrated disease control strategy that includes biological control agents and inorganic compounds requires a compatibility study of both components, especially sporulation capacity. In Trichoderma sp. and Phi, conidiogenesis provides a valid compatibility estimation, as these reproductive structures give this genus the capability to exert antagonism on plant pathogens [34]. In the present study, we observed differences in the compatibility percentages between Trichoderma sp. isolates and the different concentrations of Phi (p ≤ 0.001), demonstrating that Trichoderma has a natural capacity to tolerate agents with fungicidal activity (i.e., “natural resistance” or “inherent resistance”) [35]. The Taz-016 isolate showed greater compatibility (96.5 and 95.9%) with concentrations of 500 and 1000 µg mL−1 Phi, while Taz-001 (67.9 and 43.0%) and Taz-013 (50.8 and 49.4%) were less compatible with the same concentrations of Phi tested. The capacity of Trichoderma to resist relatively high concentrations of toxic compounds (both synthetic and natural) depends on efficient cellular detoxification mechanisms carried out by a complex system of membrane pumps, which includes ABC transporters (ATP-binding cassette) that can provide a protection mechanism against toxic compounds and xenobiotic agents [36]. ABC transporters could explain the natural tolerance of Trichoderma to fungicidal agents and its capacity to successfully survive in environments with remnant Phi molecules.
The mycoparasitic capacity of Trichoderma varies among species and fungal isolates due to their origin and host where they develop [37]. The results observed in the differences in antagonism among the isolates evaluated herein may be related to the different agronomic management and sugarcane varieties from which they were obtained. The Taz-016 isolation was obtained from plants treated with intensive management using organosynthetic products. Due to this constant selection pressure, it could have developed the resistance inherent to the medium.
Despite the generalized decrease in conidiogenesis in the three Trichoderma isolates evaluated with Phi, mycelial growth continued until the whole Petri dish was colonized. This proves the capability of Trichoderma to survive in unfavorable environments [27]. Fungicides like orthocide, propiconazole, mancozeb, and chlorothalonil at concentrations between 0.03 and 0.2% do not inhibit the growth of T. harzianum (Th09) and T. viride (Tv11) by over 10.0% [38]. Compatibilities of 34.9% in 300 ppm and 97.9% in 50 ppm of fungicides copper oxychloride and 2-(trichloromethylsulfanyl)-3a,4,7,7a-tetrahydroisoindole-1,3-dione (16.6 to 25.0%) and Trichoderma have also been reported [39]. Potassium phosphonate and fosetyl-aluminum are 100.0% compatible with T. viride with only 0.5% mycelial growth inhibition [29], which suggests that Taz-016 is compatible with Phi doses ≤1000 µg mL−1.
Several Trichoderma species can tolerate fungicidal agents since they adapt to diverse soil or substrate conditions, where they can produce lithic enzymes, antibiotics, and/or secondary metabolites and colonize them quickly [34]. Although some agricultural supplies can make it difficult for Trichoderma to act [40], they do not impede its development. This is because this genus shows ecological plasticity, developed by its wide geographical distribution, which depends more on the isolate than the species itself [41,42].
4.3. Phi Is Compatible with Trichoderma sp. in In Vitro Control of the Fusarium Species Complex Causing Pokkah Boeng in Sugarcane
The obtained results in the compatibility between Phi and Trichoderma sp. for the control of Fusarium sp. provide evidence that combining Phi and Trichoderma isolates increases the efficacy to control Fusarium sp., compared with the sole application of Phi. This can be attributed to the antifungal activity of the Phi molecules and the indirect mode of action of Phi through the stimulation of the natural defense mechanisms of plants. [18,19,34,43] and to the antagonistic capacity of the Trichoderma genus against Fusarium sp. [44,45]. By adding 0.075% and 0.1% (v/v) chitosan to a suspension of T. harzianum, the vegetative development of the opportunistic phytopathogenic fungus Sphaeropsis sapinea was significantly decreased (≥40.0%) [46]. Furthermore, combining T. viride strain-CIAH240 with fungicides like triadimefon, thiophanate methyl, mancozeb, and alcidine at 50 mg g−1 improved the percentage of disease control efficiency to more than 70.0% during postharvest of Indian plum (Ziziphus mauritiana) [47]. The results of our work show that the Trichoderma isolates (Taz-001, Taz-013, and Taz-016) and the different Phi concentrations (1000, 4000, and 8000 µg mL−1) are compatible since in all treatments, the Trichoderma isolates could colonize the entire Petri dish 10 d after inoculation. When Phi and Trichoderma are combined, they act synergistically on the inhibition of the vegetative development of Fusarium sp. In this relationship, Phi acts as a fungistatic or fungicidal agent, and the Trichoderma isolates act as antagonists by competing for space and nutrients, as well as mycoparasitism.
Under our experimental conditions, Trichoderma isolates grew considerably faster than Fusarium ones. This ability gives the antagonist an important advantage against the pathogen in competition for space and nutrients [48,49]. This mode of action is a mechanism of antagonism employed by Trichoderma sp. [50]. In our study, we considered the initial phase of the interaction between Trichoderma and Fusarium, which is characterized by a noncontact competition of mycelia. The diffusible metabolites of both organisms decide the fate of the interaction [51]. Another key biological control mechanism for most Trichoderma strains is mycoparasitism mediated by producing chitinases and other enzymes that degrade the cell wall [37,52,53,54]. Mycoparasitism is a complex process displayed by Trichoderma strains [55], which involves recognizing the pathogen, producing endochitinases, and the physical binding of Trichoderma to the pathogen. Subsequently, Trichoderma strains secrete enzymes that hydrolyze the main structural compounds of fungal cell walls, chitin and β-glucan [48,56,57,58].
Although the concentration of 8000 µg mL−1 of Phi was the most effective to inhibit the in vitro development of Fusarium sp., in this work, it was observed that 8000 µg mL−1 of Phi inhibited the development and sporulation of native microorganisms as Trichoderma sp. In addition, it has been reported that Phi at high concentrations can cause phytotoxicity [16,22]. In the integrated disease and pest management approach, efficient control of pathogens is sought, with minimal impact on the environment. Accordingly, combining 1000 µg mL−1 Phi and Trichoderma sp. was chosen, where the compatibility of both factors was recorded. Compared to applying 1000 µg mL−1 Phi, combining both Trichoderma and 1000 µg mL−1 Phi resulted in a 44.0% increase in inhibition of Fusarium.
4.4. The Combination of Phi and Trichoderma sp. Is Efficient in the Control of the Fusarium Species Complex Causing Pokkah Boeng in Sugarcane in the Planta Assay
The treatments that included only Phi and Trichoderma sp. application and combining the two fungicidal agents showed no symptoms of the presence of Fusarium sp. (Figure 5A,B). This can be attributed to the fact that combinations of Trichoderma with fungicides significantly induce defense enzymes, such as peroxidases that catalyze lignification and suberization reactions in cell walls and stimulate the synthesis of phenolic compounds in plants while limiting fungal activity [40]. Treatment T11 improved plant height compared to the control, and the presence of Fusarium sp. (T1, T4 and T5) decreased plant height (Table 3). The use of T. harzianum isolates (root immersion) combined with a fungicidal agent like tebuconazole 250 EC (direct to the soil) has been reported to increase plant height in gerbera (Gerbera jamesonii) var. Donavan yellow, where management efficiency is maximized if both agents are compatible [59]. For culm length, treatment T11 (Trichoderma sp. Taz-016 + 4000 µg mL−1 Phi in the presence of Fusarium) increased plant height compared to the control (Table 3). Interestingly, combinations of Trichoderma spp. with comothiophanate-methyl increase vegetative growth in the common bean (Phaseolus vulgaris) [40]. Trichoderma sp. Taz-016 + 4000 µg mL−1 Phi in the presence of Fusarium increased dry leaf weight (Table 3). In tomato (Solanum lycopersicum), the combined use of T. harzianum, neem (Azadirachta indica) spray, and foliar spray of benzimidazole applied to control Fusarium increased fresh and dry weight of plants compared to the control [60]. This response is attributed to a greater pressure exerted on the pathogen by combining biocontrollers with fungicidal agents. Furthermore, a host that is resistant combined with biological control agents (i.e., Purpureocillium lilacinum and Trichoderma harzianum) and organic amendments (i.e., neem) can be used in managing Fusarium in diverse agricultural systems [61].
5. Conclusions
In the present study, we demonstrated that Phi has a direct action on mycelial growth and conidiogenesis of Fusarium sp. isolated from the rhizosphere of sugarcane. We also demonstrated the capacity of Trichoderma sp. to survive in environments in the presence of Phi. The Trichoderma isolates (Taz-001, Taz-013, and Taz-016) are potential biological control agents against Fusarium sp. Furthermore, it was evident that Trichoderma sp. and Phi act synergistically, inhibiting vegetative development and conidiogenesis of Fusarium sp. in vitro and the infection caused by the pathogen in sugarcane plants. This control strategy represents an effective alternative to the conventional management of pokkah boeng in the field, under a sustainability scheme that can control the disease while stimulating plant growth.
Author Contributions
Conceptualization, F.C.G.-M. and G.H.-R.; methodology, R.S.-P., G.H.-R., and F.C.G.-M.; software, J.S.-R. and J.V.H.-C.; validation, G.H.-R. and J.S.-R.; formal analysis, R.S.-P., G.H.-R. and J.S.-R.; investigation, R.S.-P. and G.H.-R.; resources, F.C.G.-M. and G.H.-R.; writing—original draft preparation, R.S.-P. and G.H.-R.; writing—review and editing, F.C.G.-M. and G.H.-R.; supervision, F.C.G.-M. and G.H.-R.; project administration, F.C.G.-M. and G.H.-R. All authors have read and agreed to the published version of the manuscript.
Funding
Mexico’s National Science and Technology Council (CONACYT-Mexico) funded this research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The Laboratory of Plant Nutrition of the College of Postgraduates in Agricultural Sciences Campus Montecillo provided space and infrastructure for some experiments carried out during this study. The Sugar Mill La Margarita S. A de C. V. (Vicente, Oaxaca, Mexico) provided the sugarcane samples for the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moore, P.H.; Paterson, A.H.; Tew, T. Sugarcane: The Crop, the Plant, and Domestication. In Sugarcane: Physiology, Biochemistry, and Functional Biology; John Wiley & Sons Ltd.: Chichester, UK, 2013; pp. 1–17. [Google Scholar]
- Sentíes-Herrera, H.E.; Trejo-Téllez, L.I.; Gómez-Merino, F.C. The Mexican Sugarcane Production System: History, Current Status, and New Trends. In Sugarcane: Production Systems, Uses and Economic Importance; Murphy, R., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2017; pp. 39–71. [Google Scholar]
- FAO. Food and Agriculture Organization of the United Nations. FAO Stats. Crops. Sugarcane. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 9 April 2021).
- Gómez-Merino, F.C.; Trejo-Téllez, L.; Salazar-Ortiz, J.; Pérez-Sato, J.A.; Sentíes-Herrera, H.E.; Bello-Bello, J.J.; Aguilar-Rivera, N. Diversification of the Sugar Agroindustry as a Strategy for Mexico. Agroproductividad 2017, 10, 7–12. [Google Scholar]
- Aguilar-Rivera, N.; Michel-Cuello, C.; Serna-Lagunes, R.; de Jesús Debernardi-Vázquez, T.; Trujillo-Mata, A. Ethanol Production from the Mexican Sugar Industry: Perspectives and Challenges. In Sugarcane Biofuels; Springer: Cham, Switzerland, 2019; pp. 203–235. [Google Scholar]
- Azevedo, R.A.; Carvalho, R.F.; Cia, M.C.; Gratão, P.L. Sugarcane Under Pressure: An Overview of Biochemical and Physiological Studies of Abiotic Stress. Trop. Plant Biol. 2011, 4, 42–51. [Google Scholar] [CrossRef]
- Savario, C.F.; Hoy, J.W. Microbial Communities in Sugarcane Field Soils with and without a Sugarcane Cropping History. Plant Soil 2011, 341, 63–73. [Google Scholar] [CrossRef]
- Dinolfo, M.I.; Castañares, E.; Stenglein, S.A. Fusarium–Plant Interaction: State of the Art–A Review. Plant Sci. 2017, 53, 61–70. [Google Scholar] [CrossRef]
- Rampersad, S. Pathogenomics and Management of Fusarium Diseases in Plants. Pathogens 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, J.; Wang, H.; Bao, Y.; Li, Y.; Govindaraju, M.; Yao, W.; Chen, B.; Zhang, M. Molecular Characterization of Carbendazim Resistance of Fusarium species Complex that Causes Sugarcane Pokkah Boeng Disease. BMC Genomics 2019, 20, 115. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Handique, P.J.; Deka, S. Rhamnolipid Biosurfactant against Fusarium sacchari—The Causal Organism of Pokkah Boeng Disease of Sugarcane. J. Basic Microbiol. 2014, 54, 548–557. [Google Scholar] [CrossRef] [PubMed]
- McGovern, R.J. Management of Tomato Diseases Caused by Fusarium oxysporum. Crop Prot. 2015, 73, 78–92. [Google Scholar] [CrossRef]
- Sevastos, A.; Kalampokis, I.F.; Panagiotopoulou, A.; Pelecanou, M.; Aliferis, K.A. Implication of Fusarium graminearum Primary Metabolism in its Resistance to Benzimidazole Fungicides as Revealed by 1H NMR Metabolomics. Pestic. Biochem. Physiol. 2018, 148, 50–61. [Google Scholar] [CrossRef]
- Patel, P.; Shah, R.; Joshi, B.; Ramar, K.; Natarajan, A. Molecular Identification and Biocontrol Activity of Sugarcane Rhizosphere Bacteria against Red Rot Pathogen Colletotrichum falcatum. Biotechnol. Rep. 2019, 21, e00317. [Google Scholar] [CrossRef]
- Oyarburo, N.S.; Machinandiarena, M.F.; Feldman, M.L.; Daleo, G.R.; Andreu, A.B.; Olivieri, F.P. Potassium Phosphite Increases Tolerance to UV-B in Potato. Plant Physiol. Biochem. 2015, 88, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Merino, F.C.; Trejo-Tellez, L.I. Conventional and Novel Uses of Phosphite in Horticulture: Potentialities and Challenges. Italus Hortus 2016, 23, 1–13. [Google Scholar]
- Li, Y.; Sun, R.; Yu, J.; Saravanakumar, K.; Chen, J. Antagonistic and Biocontrol Potential of Trichoderma asperellum ZJSX5003 against the Maize Stalk Rot Pathogen Fusarium graminearum. Indian J. Microbiol. 2016, 56, 318–327. [Google Scholar] [CrossRef]
- Massoud, K.; Barchietto, T.; le Rudulier, T.; Pallandre, L.; Didierlaurent, L.; Garmier, M.; Ambard-Bretteville, F.; Seng, J.M.; Saindrenan, P. Dissecting Phosphite-Induced Priming in Arabidopsis Infected with Hyaloperonospora arabidopsidis. Plant Physiol. 2012, 159, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Liljeroth, E.; Lankinen, Å.; Wiik, L.; Burra, D.D.; Alexandersson, E.; Andreasson, E. Potassium Phosphite Combined with Reduced Doses of Fungicides Provides Efficient Protection against Potato Late Blight in Large-Scale Field Trials. Crop Prot. 2016, 86, 42–55. [Google Scholar] [CrossRef]
- Martínez, S. Effects of Combined Application of Potassium Phosphite and Fungicide on Stem and Sheath Disease Control, Yield, and Quality of Rice. Crop Prot. 2016, 89, 259–264. [Google Scholar] [CrossRef]
- Cerqueira, A.; Alves, A.; Berenguer, H.; Correia, B.; Gómez-Cadenas, A.; Diez, J.J.; Monteiro, P.; Pinto, G. Phosphite Shifts Physiological and Hormonal Profile of Monterey Pine and Delays Fusarium circinatum Progression. Plant Physiol. Biochem. 2017, 114, 88–99. [Google Scholar] [CrossRef]
- Trejo-Téllez, L.I.; Gómez-Merino, F.C. Phosphite as an Inductor of Adaptive Responses to Stress and Stimulator of Better Plant Performance. In Biotic and Abiotic Stress Tolerance in Plants; Springer: Singapore, 2018; pp. 203–238. [Google Scholar]
- Sharma, V.; Salwan, R.; Sharma, P.N. The Comparative Mechanistic Aspects of Trichoderma and Probiotics: Scope for Future Research. Physiol. Mol. Plant Pathol. 2017, 100, 84–96. [Google Scholar] [CrossRef]
- Amiri, A.; Bompeix, G. Control of Penicillium Expansum with Potassium Phosphite and Heat Treatment. Crop Prot. 2011, 30, 222–227. [Google Scholar] [CrossRef]
- Lopes, L.F.; Cruz, A.F.; de Barreto, M.L.A.; de Vasconcelos, T.M.M.; Blum, L.E.B. Post-Harvest Treatment with Ca-Phosphite Reduces Anthracnose without Altering Papaya Fruit Quality. J. Hortic. Sci. Biotechnol. 2018, 93, 272–278. [Google Scholar] [CrossRef]
- Deliopoulos, T.; Kettlewell, P.S.; Hare, M.C. Fungal Disease Suppression by Inorganic Salts: A Review; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1059–1075. [Google Scholar]
- Kiran, G.V.M.; Thara, S.S.; Jyothi, K.R. Studies on Compatibility of Biocontrol Agents with Chemical Fungicides for Integrated Management of Alternaria Leaf Spot of cabbage. J. Pharmacogn. Phytochem. 2018, 7, 2974–2977. [Google Scholar]
- Widmer, T.L. Compatibility of Trichoderma asperellum Isolates to Selected Soil Fungicides. Crop Prot. 2019, 120, 91–96. [Google Scholar] [CrossRef]
- Dhanya, M.K.; Anjumol, K.B.; Murugan, M.; Deepthy, K.B. Compatibility of Trichoderma viride and Pseudomonas fluorescens with Plant Protection Chemicals and Fertilizers in Cardamom. J. Trop. Agric. 2016, 54, 129–135.h. [Google Scholar]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 4th ed.; APS Press: St. Paul, MN, USA, 1998. [Google Scholar]
- Pandey, D.K.; Tripathi, N.N.; Tripathi, R.D.; Dixit, S.N. Fungitoxic and Phytotoxic Properties of the Essential Oil of Hyptis suaveolens. J. Plant Dis. Prot. 1982, 89, 344–349. [Google Scholar]
- Reuveni, M.; Sheglov, D.; Cohen, Y. Control of Moldy-Core Decay in Apple Fruits by β-Aminobutyric Acids and Potassium Phosphites. Plant Dis. 2003, 87, 933–936. [Google Scholar] [CrossRef]
- D’Addazio, V.; Ahnert dos Santos, R.A.; Bastos Leitão, A.S.; Barreto da Silva, M.; Alves Fernandes, A.; Falqueto, A.R. Evaluation of In Vitro Inhibition of Mycelial Growth of Fusarium solani f. sp. piperis by Different Products in Brazil. Afr. J. Microbiol. Res. 2016, 10, 1992–1998. [Google Scholar] [CrossRef][Green Version]
- Da Silva, M.A.F.; de Moura, K.E.; de Moura, K.E.; Salomão, D.; Patricio, F.R.A. Compatibility of Trichoderma Isolates with Pesticides Used in Lettuce Crop. Summa Phytopathol. 2018, 44, 137–142. [Google Scholar] [CrossRef]
- Chaparro, A.P.; Carvajal, L.H.; Orduz, S. Fungicide Tolerance of Trichoderma asperelloides and T. harzianum Strains. Agric. Sci. 2011, 2, 301–307. [Google Scholar] [CrossRef]
- Ruocco, M.; Lanzuise, S.; Vinale, F.; Marra, R.; Turrà, D.; Woo, S.L.; Lorito, M. Identification of a New Biocontrol Gene in Trichoderma atroviride: The Role of an ABC Transporter Membrane Pump in the Interaction with Different Plant-Pathogenic Fungi. Mol. Plant Microbe Interact. 2009, 22, 291–301. [Google Scholar] [CrossRef]
- Perveen, K.; Bokhari, N.A. Antagonistic Activity of Trichoderma harzianum and Trichoderma viride Isolated from Soil of Date Palm Field against Fusarium oxysporum. Afr. J. Microbiol. Res. 2012, 6, 3348–3353. [Google Scholar] [CrossRef]
- Bagwan, N.B. Evaluation of Trichoderma Compatibility with Fungicides, Pesticides, Organic Cakes and Botanicals for Integrated Management of Soil Borne Disease of Soybean [Glycine max (L.) Merril]. Int. J. Plant Prot. 2010, 3, 206–209. [Google Scholar] [CrossRef]
- Tapwal, A.; Kumar, R.; Gautam, N.; Pandey, S. Compatibility of Trichoderma viride for Selected Fungicides and Botanicals. Int. J. Plant Pathol. 2012, 3, 89–94. [Google Scholar] [CrossRef]
- Živković, S.; Stojanović, S.; Ivanović, Ž.; Gavrilović, V.; Popović, T.; Balaž, J. Screening of Antagonistic Activity of Microorganisms against Colletotrichum acutatum and Colletotrichum gloeosporioides. Arch. Biol. Sci. 2010, 62, 611–623. [Google Scholar] [CrossRef]
- Svistova, I.D.; Senchakova, T.Y. Ecological Plasticity of Trichoderma Fungi in Leached Chernozem. Eurasian Soil Sci. 2010, 43, 314–315. [Google Scholar] [CrossRef]
- Infante, D.; Martínez, B.; González, N.; Reyes, Y. Mecanismos de Acción de Trichoderma Frente a Hongos Fitopatógenos. Rev. Protección Veg. 2009, 24, 14–21. [Google Scholar]
- Groves, E.; Howard, K.; Hardy, G.; Burgess, T. Role of Salicylic Acid in Phosphite-Induced Protection against Oomycetes—A Phytophthora Cinnamomic-Lupinus augustifolius Model System. Eur. J. Plant Pathol. 2015, 141, 559–569. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Li, Y.; Yu, C.; Wang, Q.Q.; Wang, M.; Sun, J.; Gao, J.X.; Chen, J. Effect of Trichoderma harzianum on Maize Rhizosphere Microbiome and Biocontrol of Fusarium stalk Rot. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Chittenden, C.; Singh, T. In Vitro Evaluation of Combination of Trichoderma harzianum and Chitosan for the Control of Sapstain Fungi. Biol. Control. 2009, 50, 262–266. [Google Scholar] [CrossRef]
- Nallathambi, P.; Umamaheswari, C.; Thakore, B.B.L.; More, T.A. Post-Harvest Management of Ber (Ziziphus mauritiana Lamk) Fruit Rot (Alternaria alternata Fr. Keissler) Using Trichoderma Species, Fungicides and Their Combinations. Crop Prot. 2009, 28, 525–532. [Google Scholar] [CrossRef]
- Abd-El-Khair, H.; Elshahawy, I.E.; Haggag, H.E.K. Field Application of Trichoderma spp. Combined with Thiophanate-Methyl for Controlling Fusarium solani and Fusarium oxysporum in Dry Bean. Bull. Natl. Res. Cent. 2019, 43, 19. [Google Scholar] [CrossRef]
- El-Komy, M.H.; Saleh, A.A.; Eranthodi, A.; Molan, Y.Y. Characterization of Novel Trichoderma asperellum Isolates to Select Effective Biocontrol Agents against Tomato Fusarium Wilt. Plant Pathol. J. 2015, 31, 50–60. [Google Scholar] [CrossRef]
- Abhiram, P.; Masih, H. In Vitro Antagonism of Trichoderma viride against Fusarium oxysporum Strains. J. Pharmacogn. Phytochem. 2018, 7, 2816–2819. [Google Scholar]
- Akrami, M.; Golzary, H.; Ahmadzadeh, M. Evaluation of Different Combinations of Trichoderma Species for Controlling Fusarium Rot of Lentil. Afr. J. Biotechnol. 2011, 10, 2653–2658. [Google Scholar] [CrossRef]
- Sharma, P. Complexity of Trichoderma-Fusarium Interaction and Manifestation of Biological Control. Aust. J. Crop Sci. 2011, 5, 1027–1038. [Google Scholar]
- Tapwal, A.; Singh, U.; da Silva, J.A.T.; Singh, G.; Garg, S.; Kumar, R. In Vitro Antagonism of Trichoderma viride against Five Phytopathogens. Pest Technol. 2011, 5, 59–62. [Google Scholar]
- Cherkupally, R.; Amballa, H.; Reddy, B.N. In Vitro Antagonistic Activity of Trichoderma Species against Fusarium oxysporum f. sp. melongenae. Int. J. Appl. Agric. Res. 2017, 12, 87–95. [Google Scholar]
- Suleiman, A.S.; Gambo, M.S.; Sunusi, M. An In Vitro Antagonistic Effect of Trichoderma spp. against Fusarium oxysporum f. sp. lycopersici. FUDMA J. Sci. 2019, 3, 369–374. [Google Scholar]
- Veenstra, A.; Rafudeen, M.S.; Murray, S.L. Trichoderma asperellum Isolated from African Maize Seed Directly Inhibits Fusarium verticillioides Growth in vitro. Eur. J. Plant Pathol. 2019, 153, 279–283. [Google Scholar] [CrossRef]
- Verma, M.; Brar, S.K.; Tyagi, R.D.; Surampalli, R.Y.; Valéro, J.R. Antagonistic Fungi, Trichoderma spp.: Panoply of Biological Control. Biochem. Eng. J. 2007, 37, 1–20. [Google Scholar] [CrossRef]
- Mishra, V.K. In Vitro Antagonism of Trichoderma Species against Pythium aphanidermatum. J. Phytol. 2010, 2, 28–35. [Google Scholar]
- Rajeswari, P.; Kannabiran, B. In Vitro Effects of Antagonistic Microorganisms on Fusarium oxysporum [Schlecht. Emend. Synd & Hans] Infecting Arachis hypogaea L. J. Phytol. 2011, 3, 83–85. [Google Scholar]
- Suneeta, P.; Kumar, S.V.; Eraivan, K.; Aiyanathan, A.; Nakkeeran, S. Promissory Action of Trichoderma spp. and Fungicides in the Management of Fusarium Wilt of Gerbera. J. Pure Appl. Microbiol. 2017, 11, 241–247. [Google Scholar] [CrossRef]
- Singh, R.; Biswas, S.K.; Nagar, D.; Singh, J.; Singh, M.; Kumar Mishra, Y. Sustainable Integrated Approach for Management of Fusarium Wilt of Tomato Caused by Fusarium oxysporum f. sp. lycopersici (Sacc.) Synder & Hansen. Sustain. Agric. Res. 2015, 4. [Google Scholar] [CrossRef][Green Version]
- Mwangi, M.W.; Muiru, W.M.; Narla, R.D.; Kimenju, J.W.; Kariuki, G.M. Management of Fusarium oxysporum f. sp. lycopersici and Root-Knot Nematode Disease Complex in Tomato by Use of Antagonistic Fungi, Plant Resistance and Neem. Biocontrol Sci. Technol. 2019, 29, 229–238. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).