Abstract
Verticillium dahliae is one of the most devastating soilborne pathogens for horticulture production. The pathogen has a broad host range and currently there is no effective chemical disease management, therefore, novel sustainable integrated disease management strategies should be considered. In this respect, we questioned whether the soil application of common microbiological growth media can influence the plant—microbe interactions and, subsequently, offer protection against V. dahliae. Indeed, the addition of Nutrient Broth (NB) and Potato Dextrose (PD) in non-sterilized soil reduced Verticillium wilt symptoms in eggplants. The addition of NB in sterilized soil did not reduce the disease symptoms compared to controls, however the addition of PD enhanced plant protection against V. dahliae. Following the results of a split root bioassay in eggplants, the possibility that NB and PD triggered the plant defense mechanisms against V. dahliae was excluded, since NB and PD did not reduce wilt symptom. Therefore, PD may be used as an easy food source for V. dahliae, detouring the pathogen from the root system of plants, while NB may affect the soil microbiome by enhancing antagonism in rhizosphere, or antagonistic interaction between V. dahliae and rhizospheric microbiome. Subsequently, several bacterial strains were isolated from the NB-treated rhizosphere and examined for their biocontrol activity against V. dahliae. Among the examined strains, a Pseudomonas putida strain, Z13, significantly reduced Veticillium severity and incidence under greenhouse conditions.
1. Introduction
Verticillium wilt is a devastating disease, infecting plants through their root system, by colonizing the xylem vessels, causing wilting and eventually plant death. Among the most abundant species of Verticillium is V. dahliae, which causes serious economic losses for farmers in temperate zones every year. The pathogen infects more than 250 plant species, and it is inaccessible to fungicides due to its endophytic life-cycle [1,2]. Continuous research efforts have identified Verticillium pathogenicity mechanisms and R genes counteracting the pathogen [3,4]; however, resistance is a scarce phenomenon in the case of Verticillium. Due to the lack of chemical control, biological control found the grounds to development, so several biocontrol agents (BCAs) have been identified as capable of reducing the detrimental effects of V. dahliae on plants [5,6,7]. The reported BCAs are mostly root-inhabiting bacterial strains isolated mainly from Verticillium suppressive soils. Their mode of action has been mostly attributed to the production of antibiotics against the pathogen [8]. In addition, the induction of systemic resistance (ISR), competition for space and/or nutrients and, lately, epigenetic modifications have also been shown to have a central role in the activity of BCAs against Verticillium [8,9]. However, beyond the intensive research efforts, there is still no registered BCA-based formulation against V. dahliae.
The nutritional status of the soil has a central role in the outcome of the plant—pathogen interaction. Nutrient-rich soils may harbor microbial populations that outcompete pathogens from the rhizosphere, produce antibiotics and trigger ISR in plants [10,11,12]. It is known that nutrients available in the root exudates determine the ability of bacteria to colonize the rhizosphere [13]. Therefore, what will happen if common microbiological growth media are applied to a soil substrate? Will that enhance the indigenous microbial population or make the pathogens not dependent on plants for nutrients, resulting in plant protection against diseases? Indeed, the plant pathogens colonize nutrient-rich places on plant surfaces [12,14], seeking mainly carbon sources [15,16]. They are also notorious for their ability to manipulate the plant metabolism to gain nutrients from their hosts [17,18,19]. It is known that pathogenic fungi, such as V. dahliae, can acquire sugars from plant cells to satisfy their nutritional demands for growth and infection by exploiting plant SWEET sugar transporters. The overexpression of GhSWEET42 in Arabidopsis thaliana plants leads to increased glucose content and compromised their resistance to V. dahliae [20]. Therefore, it is reasonable to question whether the addition of a common microbiological growth medium in soil can reduce Verticillium wilt in plants.
In this respect, we questioned whether the addition of common microbial growth-media such as potato dextrose (PD) and nutrient broth (NB) can have a plant protective activity against V. dahliae. Our hypothesis was that the addition of PD and NB in the soil may enhance plant beneficial microbial populations and/or trigger ISR and/or become easily accessible nutrients for the pathogen, so as to divert it from plants. For this purpose, we examined initially the V. dahliae exclusion activity of PD and NB in eggplants grown in sterilized and non-sterilized soil; then, we examined the ISR triggering activity of PD and NB in a split root system. Having shown that NB protects plants against Verticillium in the non-sterilized soil but fails to do that in the sterilized soil, we isolated rhizosphere microorganisms from eggplants grown in the NB treated non-sterilized soil and examined them against V. dahliae under in vitro and in planta conditions.
2. Materials and Methods
2.1. Pathogen Preparation
A V. dahliae isolate originated from an infected eggplant was used in the experiments [21]. For the experiments, the fungus was transferred to a Petri dish containing potato dextrose agar (PDA) (Merck, Darmstadt, Germany) at 24 °C for 5 days and, subsequently, a suspension of 107 conidia mL−1 of distilled sterile water (DSW) was prepared from a culture grown for 5 days at 24 °C in a sucrose sodium nitrate liquid medium.
2.2. Evaluation of Potato Dextrose and Nutrient Broth against V. dahliae under Glasshouse Conditions
The microbial growth media, PD (38 g L−1, ingredients: 15 g L−1 Dextrose, 20 g L−1 Potato starch, Laboratorios Conda S.A, Madrid, Spain) and NB (8 g L−1, ingredients: 5 g L−1 Peptone, 3 g L−1 Beef extract, Laboratorios Conda S.A, Madrid, Spain), were applied to eggplants (third-leaf stage) of the cv. ‘Black Beauty’ (highly susceptible to V. dahliae), grown singly in plastic pots (9 cm × 9 cm × 10 cm) containing soil substrate (Potground P; Klasmann), by drenching 10 mL of NB or PD per plant. Five days later, the eggplants were infested with 10 mL of 107 V. dahliae conidia mL−1. Control plants were treated with DSW. Eggplants were maintained at 25 ± 3 °C with a 16-h light and 8-h dark cycle. The experiment was performed with a completely randomized design with ten replications (plants) per treatment and repeated three times. Symptoms were recorded every five days for 25 days. Disease severity at each observation was calculated from the number of leaves that showed Verticillium symptoms as a percentage of the total number of leaves of each plant. Subsequently, the area under the disease progress curve (AUDPC) was calculated by the trapezoidal integration method for each plant. Disease was expressed as a percentage of the maximum possible area for the whole period of the experiment, which is referred to as the relative AUDPC.
The same experimental procedure and data analysis were performed in eggplants grown in autoclaved (two successive times within a 24 h interval for 1 h at 121 °C, 1.2 atm) soil substrate (Potground P; Klasmann).
2.3. Split Root Bioassay
The ISR triggering ability of PD and NB against V. dahliae was examined in an eggplant split root system, described by Liu et al. (1995) [22]. According to Liu et al. (1995) [22], roots of cv ‘Black Beauty’ at the third leaf stage were uprooted and split into two halves. Each half of the root system was placed into separate plastic pots (9 cm × 9 cm × 10 cm) containing non-sterilized soil substrate (pH 6.0, black peat; Potground P; Klasmann). One half was drenched with 10 mL of PD or NB or DSW; then, five days later, the other half was drenched with 10 mL 107 V. dahliae conidia mL−1. The experimental plants were maintained at 25 ± 3 °C with a 16-h light and 8-h dark cycle. The experiment was performed with a completely randomized design with ten replications (plants) per treatment and repeated three times. Disease severity and progress were recorded and analyzed as already mentioned before.
2.4. Isolation and Identification of Rhizospheric Microorganisms
Eggplants, cv. ‘Black Beauty’, grown and treated with either NB or DSW (mocks), as in the previous experiments, were uprooted 15 days after treatment for determining the rhizospheric microbial population level and isolating microorganisms from the NB treatment. Soil particles attached to the roots of 5 eggplants per treatment and replication (three replications, 15 eggplants) were pooled to one sample and macerated in a mortar with 50 mM phosphate buffer, pH 7.02, containing 0.02% Tween-20 (PBT). The suspension was transferred to a McCartney bottle containing 10 mL of PBT and placed in an orbital incubator at 100 rpm and 25 °C for 45 min. Then, 10-fold dilutions were prepared and 500 μL of each dilution were plated in PDA Petri dishes and incubated for 2–4 days at 25 °C. Subsequently, single colonies of the highest dilution rate of the NB treatment were identified, based on partial sequencing of 16S rDNA. In brief, DNA extraction was performed from pure culture according to Vaneechoutte et al. (1995) [23] and amplification of the 16S rDNA was carried out using the primer pair 27F (5-AGAGTTTGATCMTGGCTCAG-3) and 1492R (5-TACGGYTACCTTGTTACGACTT-3). The PCR was run for 10 min at 95 °C before 35 cycles of 95 °C for 30 s, 51 °C for 1 min, and 72 °C for 1.5 min and, after the last cycle, 7 min at 72 °C [24]. All PCR products were purified by phenol/chloroform extraction and ethanol precipitated for sequencing analysis using the primer 1492R (CeMIA S.A., Larissa, Greece) (Supplementary Table S1).
2.5. Genome Assembly of Strain Z13 and Phylogenetic Reconstruction
For full DNA sequencing of strain Z13, the DNA was extracted from pure culture by using the NucleoSpin Microbial DNA kit (MACHEREY-NAGEL, Duren, Germany). Then, libraries were prepared using the Ion Torrent technology and Ion Chef workflows (Thermo Scientific) and sequencing was performed in the S5XLS system (CeMIA S.A., Larissa, Greece). Analysis of primary data was conducted with Ion Torrent Suite v.5.10.0 (CeMIA S.A., Larissa, Greece).
Quality analysis of the Ion Torrent sequencing files was performed with FastQC software version 0.11.9; low-quality bases and adapters were removed with Trim Galore software version 0.6.6. De novo genome assembly was performed with SPAdes software version 3.14.1 with kmer values 21, 33, 55, 77, 99, 111, 127 and a 99.4% genome completeness was obtained with CheckM software version 1.1.3. The phylogenetic reconstruction was generated with 22 Pseudomonas strains; first, clusters of orthologues groups between the 22 strains representing the core genomes were obtained with GetHomologues software v11042019, then 952 clusters representing acceptable phylogenetic markers were selected with GetPhylomarkers software v2.2.5_9 May18; finally, a maximum likelihood phylogeny representing the evolutionary relationship between the strains was obtained with RAxML-HPC software v8.2.10 with CTRCAT model and 100 bootstrap replicates [25,26,27,28,29].
2.6. In Vitro and in Planta Evaluation of Microorganisms against V. dahliae
The candidate antagonistic isolates were spotted on PDA medium and incubated for 48 h at 28 °C. After 48 h of incubation, the growing antagonists were killed by inverting Petri dishes over chloroform for 30 min. The test strain, V. dahliae, was prepared as described above, and the experimental Petri dishes were flooded with 1 mL of the spore suspension of the pathogen, dried, and incubated for 4 days at 21 °C [30]. The effectiveness of strains was evaluated by measuring the inhibition zones around antagonistic bacteria. The experiment was performed with a completely randomized design with three replications and repeated twice.
For in planta experiments, the isolated microorganisms were grown in liquid cultures of NB. All isolates were incubated in an orbital incubator at 180 rpm at 28 °C for 48 h. The bacterial cultures were centrifuged at 5000 g, 12 °C for 5 min and resuspended in 50 mM phosphate buffer, pH 7.02, before treating the plants. In the same manner as in the previous experiments, eggplants, cv ‘Black Beauty’, were singly grown in plastic pots (9 cm × 9 cm × 10 cm) containing non-sterilized soil substrate (Potground P; Klasmann) and the microbial isolates were applied at the third-leaf stage by drenching each plant, with 15 mL of 104 cfu mL−1; while, in the case of the microbial consortium, we applied a mixture of the 15 isolated microorganisms containing 1 mL of 105 cfu mL−1 per microbe, a total of 15 mL. Five days later, the eggplants were infested with 10 mL of 107 V. dahliae conidia mL−1. Control plants were treated with DSW. Eggplants were maintained at 25 ± 3 °C with a 16-h light and 8-h dark cycle. The experiment was performed with a completely randomized design with ten replications (plants) per treatment and repeated thrice. Disease severity and progress were recorded and analyzed, as previously mentioned.
2.7. Statistics
The data of disease severity of each treatment were compared with the control treatment (V. dahliae inoculated plants) for each time-point, by using the t-test analysis. While the data of the relative AUDPC were transformed with the √(x + 1) transformation before analysis of variance was applied, followed by LSD multiple range test (p < 0.05).
3. Results
3.1. Potato Dextrose and Nutrient Broth Reduce Verticillium Wilt Symptoms in Eggplants Grown in Non-Sterile Substrate but Nutrient Broth Fails in Sterile Substrate
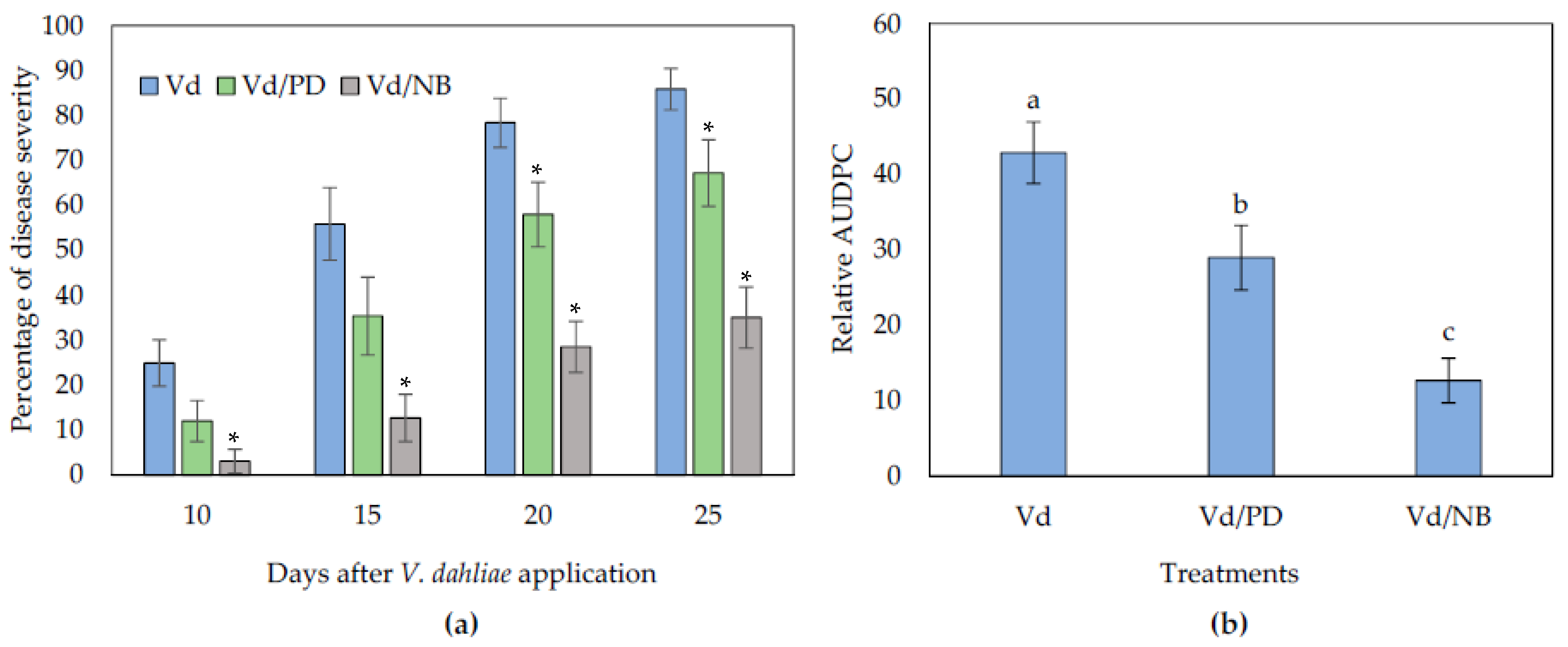
The addition of PD or NB in non-sterile soil substrate reduced Verticillium wilt symptoms in eggplants. The first symptoms appeared in the form of wilting, especially on older leaves. Symptom recording started at 10 days post-inoculation (dpi) and was recorded until 25 dpi (Figure 1). The NB-treated plants displayed significantly less symptoms than controls throughout the experimental time period (Figure 2). On the other hand, the PD-treated plants showed significantly less symptoms than controls at the later stages of the experiment (20 and 25 dpi). At the final disease scoring day (25 dpi), the disease severity was ca. 35% and 67% in the NB and PD treated plants, respectively, while in the controls it was 86%. Consequently, statistical analysis on the relative AUDPC values showed that disease severity in the NB-treated plants was the lowest among treatments, followed by PD.

Figure 1.
Verticillium wilt symptoms caused on NB (a) and PD (b) treated plants, controls (V. dahliae inoculated) (c) and mock inoculated plants (d). The photograph was taken at 20 dpi.
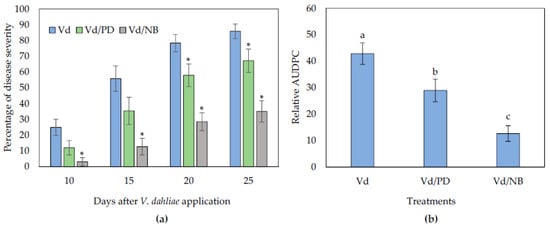
Figure 2.
Verticillium disease severity (a) and relative AUDPC (b), caused by artificial inoculation of V. dahliae (Vd), on eggplants grown in non-sterilized soil substrate amended with PD and NB. Each treatment consisted of 10 plants and the experiment was repeated three times. Columns represent means of the three replications (n = 30 plants) and the vertical bars indicate standard errors. For disease severity (a), columns with asterisks (*) are significantly different (p < 0.05) from control (Vd) according to t-test, for each time-point; for relative AUDPC (b), columns with different letters are significantly different (p < 0.05) from each other according to LSD test.
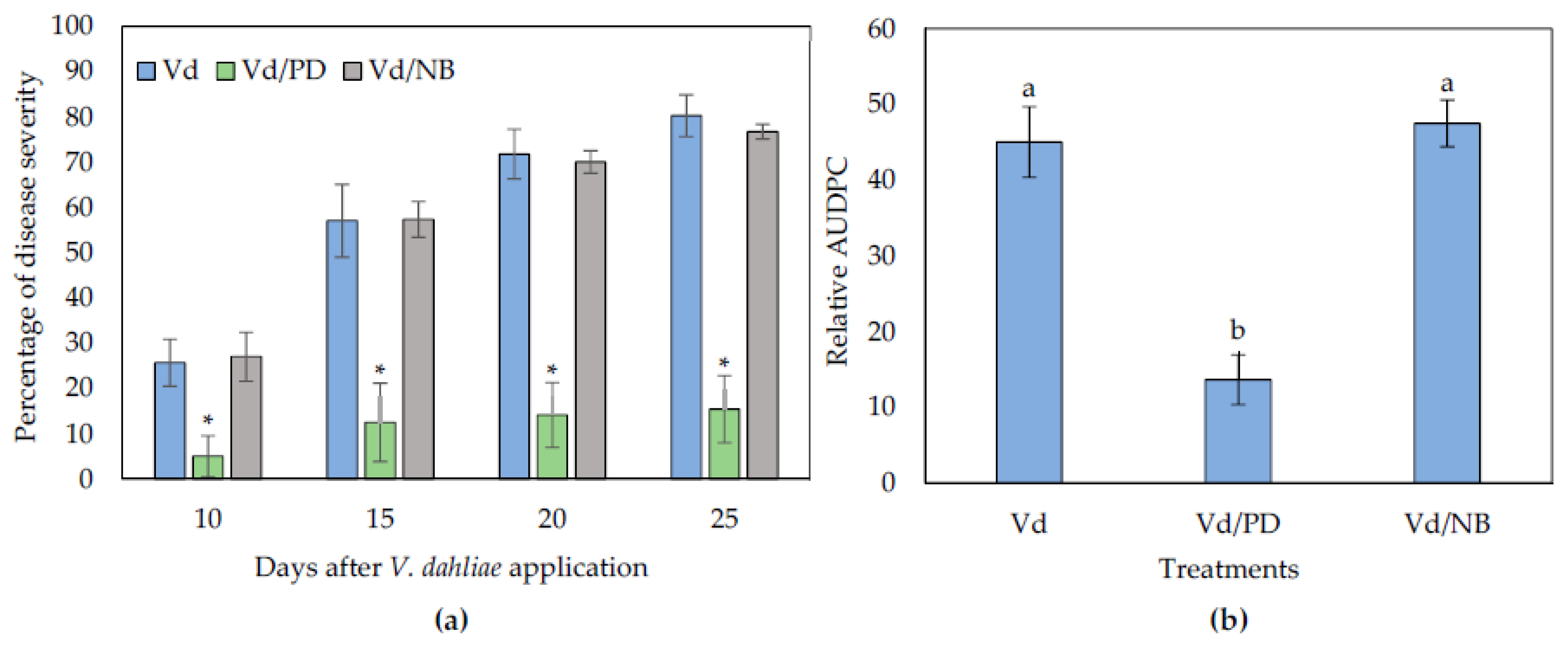
The biocontrol activity of NB and PD was also examined in eggplants grown in sterilized substrate in order to investigate if their activity was of microbial origin. It was observed that NB failed to reduce Verticillium wilt symptoms in plants compared to the controls, while PD was still protective against V. dahliae (Figure 3).
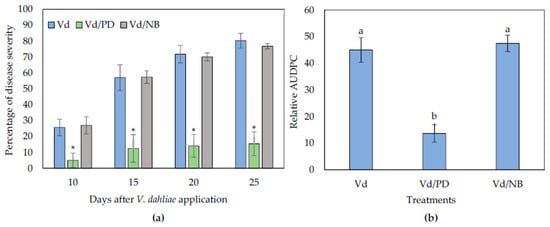
Figure 3.
Verticillium disease severity (a) and relative AUDPC (b), caused by artificial inoculation of V. dahliae (Vd), on eggplants grown in autoclaved soil substrate amended with PD and NB. Each treatment consisted of 10 plants and the experiment was repeated three times. Columns represent means of the three replications (n = 30 plants) and the vertical bars indicate standard errors. For disease severity (a), columns with asterisks (*) are significantly different (p < 0.01) from control (Vd) according to t-test, for each time point; for relative AUDPC (b), columns with different letters are significantly different (p < 0.05) from each other according to LSD test.
Interestingly, the application of PD in the autoclaved substrate was more effective than in the non-autoclaved substrate. The PD-treated plants displayed significantly less symptoms than controls throughout the experimental time period. Statistical analysis on the relative AUDPC values verified that disease severity in the PD treated plants was statistically lower than controls. Therefore, the NB biocontrol activity against V. dahliae can be of microbial origin, while the activity of PD can be disconnected from the action of microbiota.
3.2. Potato Dextrose and Nutrient Broth Fail to Reduce Verticillium Wilt Symptoms in Eggplants Grown in a Split Root System
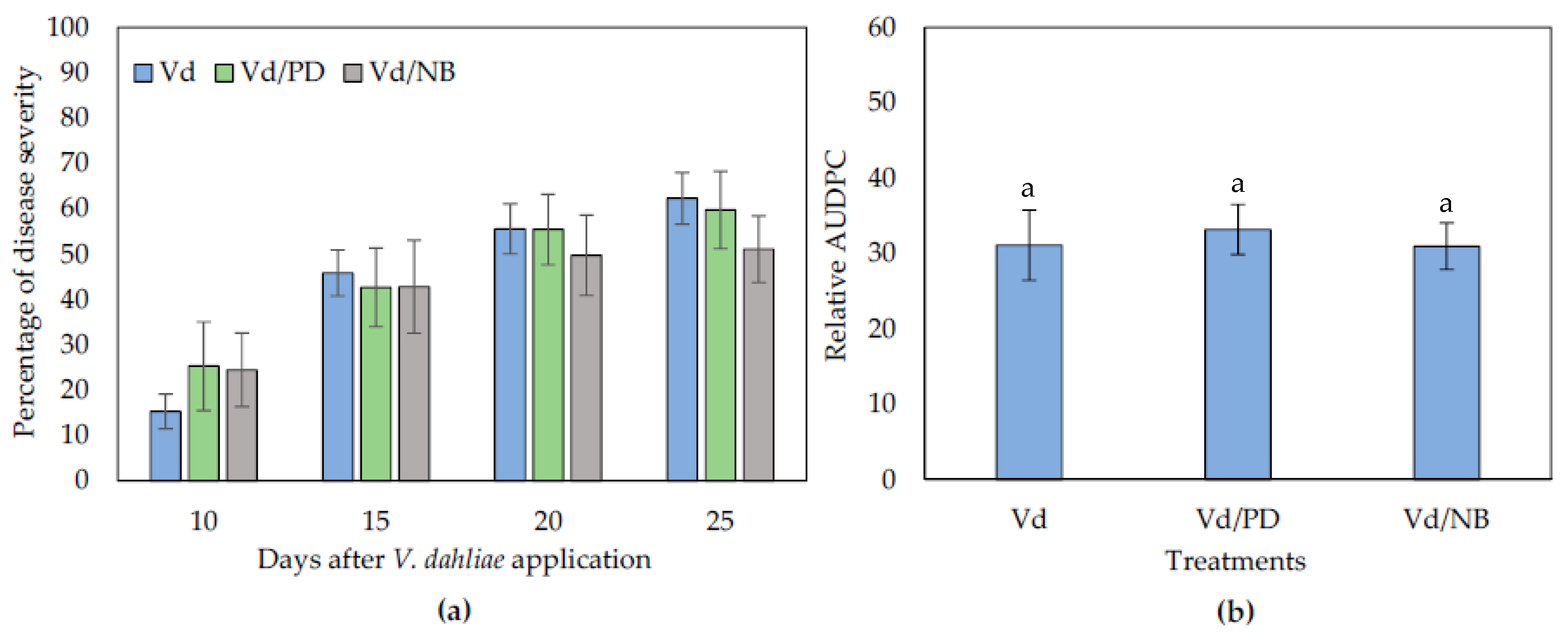
Having answered the question about the microbial origin of the PD and NB biocontrol activity, the involvement of ISR in the observed biocontrol phenomenon was examined. Similar to previous experiments, the first symptoms appeared in the form of wilting, and symptom development was recorded from 10 to 25 dpi. It was observed that NB- and PD-treated plants displayed similar disease severity levels than controls throughout the experimental time period (Figure 4).
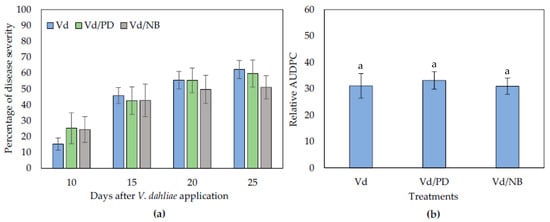
Figure 4.
Verticillium disease severity (a) and relative AUDPC (b) caused by artificial inoculation of V. dahliae (Vd), on eggplants treated with PD and NB, in a split root system. Each treatment consisted of 10 plants and the experiment was repeated three times. Columns represent means of the three replications (n = 30 plants) and the vertical bars indicate standard errors. For relative AUDPC (b), columns with different letters are significantly different (p < 0.05) from each other according to LSD test.
Therefore, the use of the split root system suggested that ISR does not have a role in the biocontrol activity of PD and NB, since both treatments did not reduce symptom development in plants compared to controls. In the case of PD, it can be suggested that it has a direct action on V. dahliae since it is neither triggering ISR nor involving the action of microorganisms.
3.3. Microorganisms Isolated from the Rhizoshere of Nutrient Broth Treated Plants, Reduce Verticillium Wilt Symptoms in Eggplants
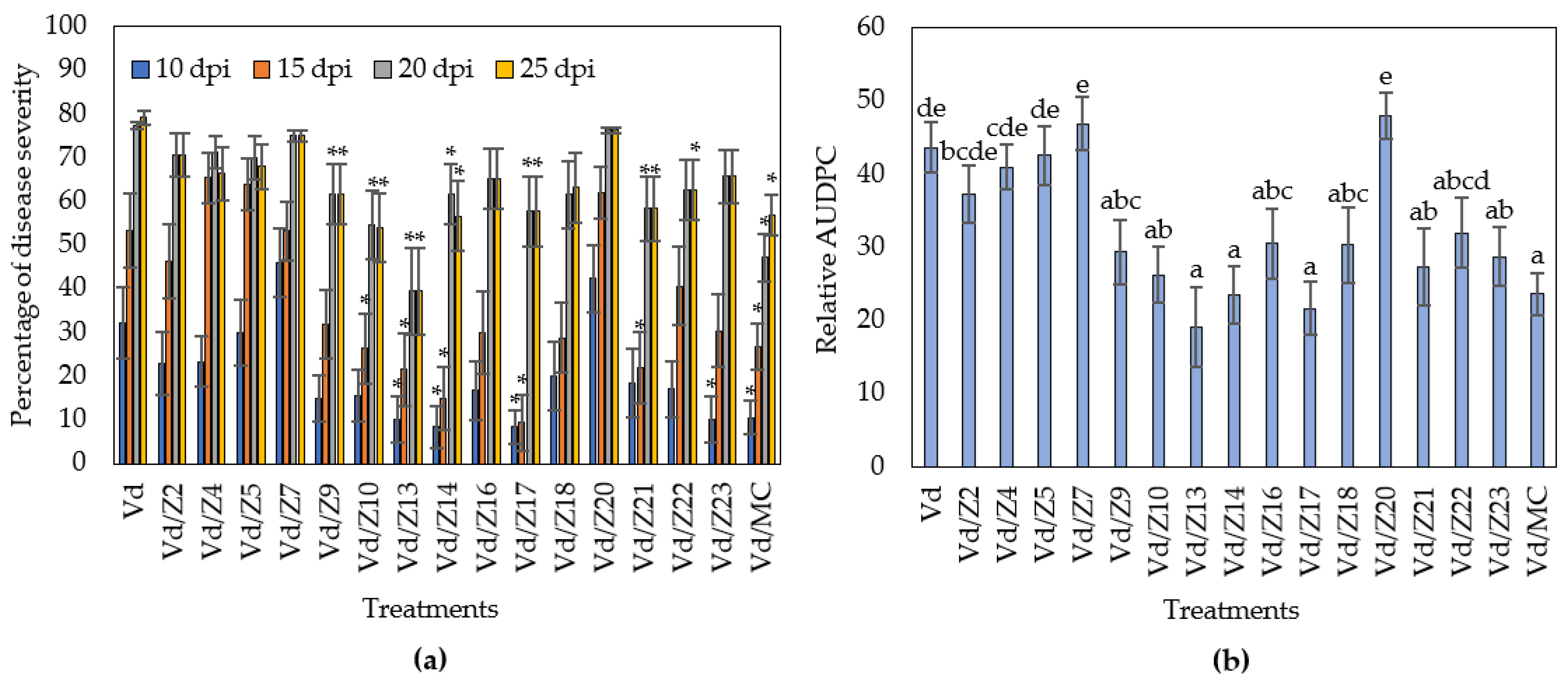
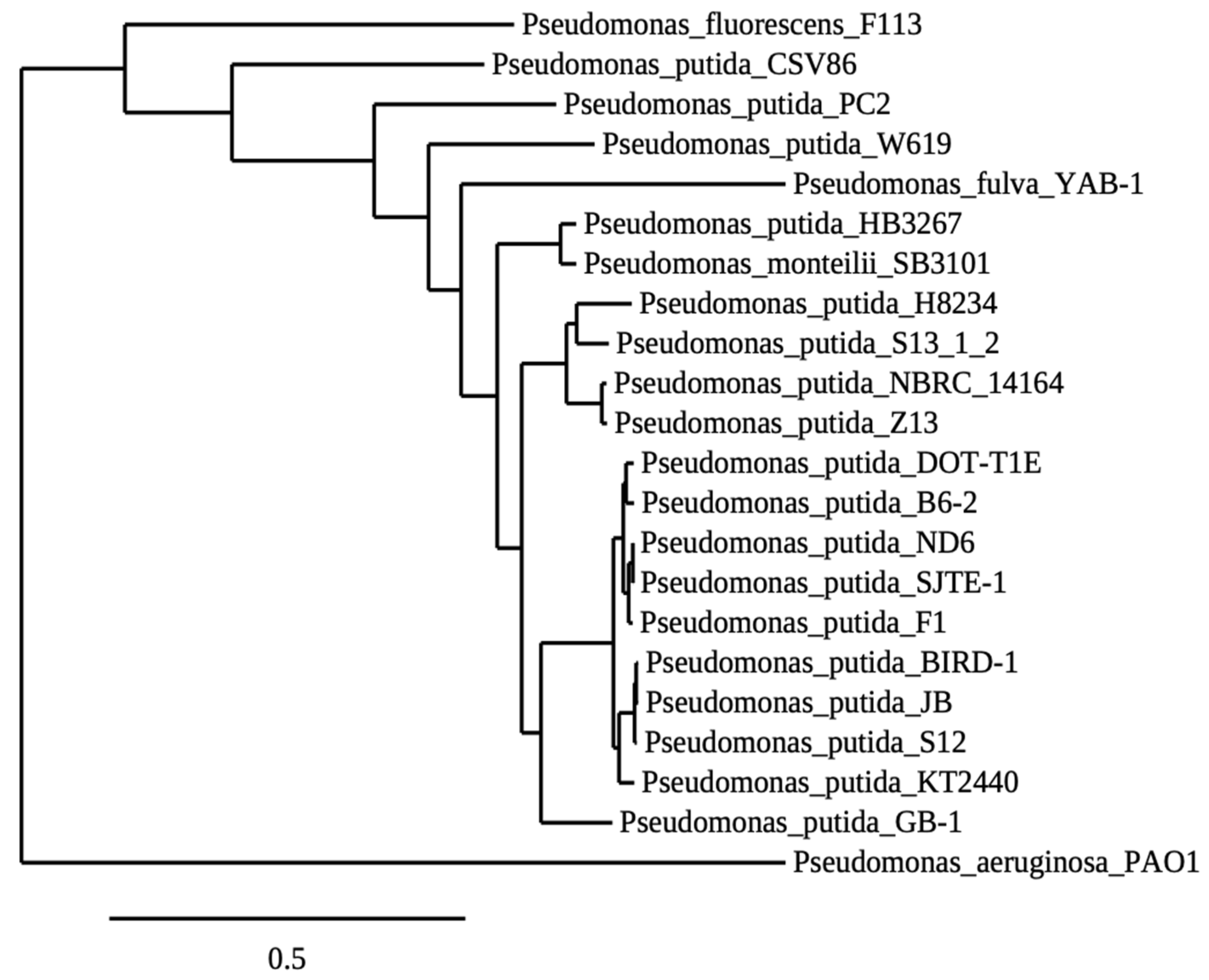
The microbial origin of the NB-observed biocontrol activity led us to investigate the rhizospheric microbial population of the NB-treated plants. It was found that the addition of NB in the plant growth substrate enhanced the rhizospheric microbiome by 14.7-fold compared to the untreated substrate (Supplementary Figure S1). Following the dilution plate technique, 15 microorganisms from the last dilution plate of the NB-treatment were isolated and identified, corresponding to a concentration of 105 cfu/g of rhizosphere. Subsequently, the biocontrol activity of the isolated microorganisms as single and consortium treatments was examined against V. dahliae. The Z13, Z14 and Z17 treatments significantly reduced Verticillium wilt symptoms in eggplants throughout the experimental time period (Figure 5 and Figure 6), while the eggplants of seven treatments (Z9, Z10, Z13, Z14, Z17, Z21, Z22) showed significantly less symptoms than controls at the final disease scoring day (25 dpi). Subsequently, statistical analysis on the relative AUDPC revealed that disease severity of nine treatments (Z10, Z9, Z13, Z14, Z17, Z18, Z21, Z22, Z23) was lower than control. Interestingly, the consortium of the microbial strains significantly reduced the Verticillium wilt symptoms compared to control; however, its relative AUDPC value was statistically similar to the most of the single bacterial treatments. Overall, the most efficacious treatment was that containing the strain Z13. The phylogeny analysis of the Z13 DNA sequence revealed that Z13 belongs to the Pseudomonas putida species. According to the analysis of 952 orthologues genes shared among 22 species of Pseudomonas, it was shown that Z13 (NCBI accession number: JAINFM000000000) is closely related to the genomes of the examined Pseudomonas putida strains and mostly to the reference genome P. putida NBRC_14164 (Figure 7).
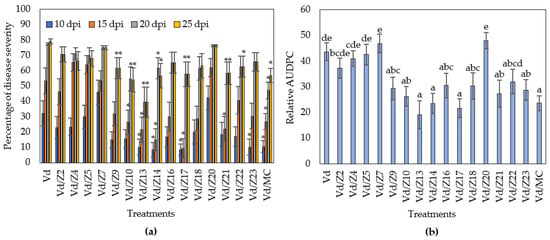
Figure 5.
Verticillium disease severity (a) and relative AUDPC (b) caused by artificial inoculation of V. dahliae (VD), on eggplants treated with microbial strains isolated from the rhizosphere of NB treated eggplants (Z2: Arthrobacter sp.; Z4: Pseudomonas sp.; Z5: Stenotrophomonas sp.; Z7: Pseudomonas sp.; Z9: Enterobacter sp.; Z10: Pseudomonas sp.; Z13: Pseudomonas putida; Z14: Burkholderia sp.; Z16; Enterobacter sp.; Z17: Burkholderia sp.; Z18: Enterobacter sp.; Z20: Enterobacter sp.; Z21: Burkholderia sp.; Z22: Pantoea sp.; Z23: Pseudomonas sp.; MC: microbial consortium, Z2–Z23). Each treatment consisted of 10 plants and the experiment was repeated three times. Columns represent means of the three replications (n = 30 plants) and the vertical bars indicate standard errors. For disease severity (a), columns with asterisks (*) are significantly different (p < 0.05) from control (Vd) according to t-test, for each time point; for relative AUDPC (b), columns with different letters are significantly different (p < 0.05) from each other according to LSD test.

Figure 6.
Verticillium wilt symptoms caused on Z13 (a) and Z7 (b) treated plants, controls (V. dahliae inoculated) (c) and mock inoculated plants (d). The photograph was taken at 20 dpi.
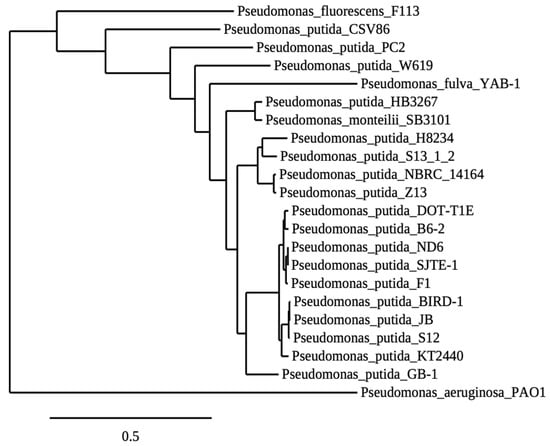
Figure 7.
Maximum Likelihood Phylogenetic Reconstruction of P. putida Z13 and other Pseudomonas strains. The phylogeny was made with 952 core genes shared by all the strains. Branch support values were obtained with 100 bootstrap replicates and every node have 100% support.
4. Discussion
Verticillium wilt, caused by V. dahliae, is a devastating disease for many crops around the world. The scarcity of disease resistance genes and the lack of chemical control amplify the impact of the disease. The fungus is soilborne, therefore, we could develop strategies to intervene in the soil-inhabiting life-stage of the pathogen, so to avoid plant infection. In the present study, we questioned whether the application of common microbiological media, PD and NB, can influence the plant—pathogen interaction offering plant protection against V. dahliae.
Our study showed that the addition of PD and NB in the soil offers protection to eggplants against Verticillium wilt. The experimental results suggest the microbial origin of the NB offered plant protection, since the addition of NB in autoclaved soil did not reduce Verticillium wilt symptoms in eggplants, compared to controls. Indeed, the rhizospheric microbial population was enhanced in the NB-treated substrate by ca 15-fold compared to the non-treated soil; this increase is not a surprise since NB is rich in carbon and nitrogen sources suitable for microbial growth. The most abundant microorganisms in the NB = treated soil were isolated and evaluated as potential BCAs against Verticillium wilt of eggplants. Interestingly, the most efficacious microbial isolate against V. dahliae was a Pseudomonas putida strain, a well-known species for its biocontrol activity against plant pathogens. The biocontrol activity of various Pseudomonas putida strains has been mainly attributed to siderophore-mediated competition for iron and ISR [31]. The most studied P. putida strain, WCS358, could trigger ISR in Arabidopsis thaliana against Pseudomonas syringae pv. tomato and Fusarium oxysporum f. sp. raphani [32,33]. In respect to Verticillium, the study of Mercado Blanco et al. [34] revealed the existence of P. putida strains with strong in vitro antibiotic activity against the defoliating and non-defoliating pathotypes of V. dahliae, but little or no ability of suppressing Verticillium wilt of olive, under field conditions. On the contrary, our results suggest that Z13 reduces Verticillium wilt symptoms in plants, even if we did not observe antibiotic production in vitro. The absence of in vitro inhibition does not exclude the possibility that Ζ13 may produce an array of inhibitory substances against V. dahliae, under favorable in vivo conditions, since factors such as oxygen, water activity and pH influence microbial production of antibiotics [35]. Nevertheless, our results suggest that Z13 neither produces deleterious compounds against V. dahliae nor triggers ISR, opening the grounds for exploring whether the observed protection against Verticillium wilt in eggplants is the outcome of competition for nutrients and/or space. Indeed, the P. putida strain PCL1760 reduces Fusarium wilt severity in tomato plants by competing for nutrients and space with Fusarium oxysporum [36]. It is known that P. putida strains form biofilms on root surface and aggregate near wound sites [37], possibly because of the presence of carbon sources, such as fructose, in root exudates [13]. The same colonization pattern has been observed for V. dahliae; it has been observed that its preference is to colonize the sites where the lateral roots emerge, and the root surface becomes discontinuous releasing exudates [38]. It is evident that further studies are needed using the “green fluorescent protein”, GFP, technology to investigate the root colonization activity of Z13 and the exclusion of V. dahliae from the same niche.
Furthermore, it was questioned whether the consortium of the isolated microorganisms could be more efficacious against V. dahliae than the single microorganisms. It is well established that plants associated microbial communities are critical for plant vigor [39,40]. For example, the disease suppressiveness of soils to the wheat pathogen Gauemannomyces graminis has been attributed to the antibiotic compound 2,4-diacetylphloroglucinol produced by a consortium of Pseudomonas species [41]. However, in our case the consortium of the isolated strains was not more efficacious than most of the single microbial treatments. Possibly, the isolated microorganisms share the same features and mode of action against V. dahliae, therefore, the microbial consortium does not offer a wider array of weapons against the pathogen than the single microbes.
In contrast to NB, the biocontrol activity of PD is not associated with the enhancement of the microbial population in the rhizosphere, since the addition of PD in autoclaved soil substrate did not result in plant protection. Furthermore, the results of the split root experiment suggest a direct effect of PD on V. dahliae, excluding the triggering of ISR. Since, PD does not have a biocontrol activity on V. dahliae, we can suggest that it is an easily accessible nutrient source for V. dahliae; therefore, V. dahliae does not have a great need to infect plants for getting access to nutrients. Verticillium species are capable of metabolizing a wide range of nutrients, mainly carbon sources, found in plants, including glucose, mannose, rhamnose, sucrose, xylose, and cellobiose [42,43,44] and also complex polysaccharides such as pectin, cellulose and starch. The study by Sarmento-Villamil et al., 2020 [45] showed that a considerable percentage of the V. dahliae transcriptome is devoted to nutrient acquisition in conjunction to pathogenicity, such as proteases, which besides having a role in the destruction of plant defenses, are also utilized to digest proteins for nutrient supply under nitrogen limited conditions [46].
5. Conclusions
In conclusion, our study showed that the addition of common microbiological growth media in a soil substrate reduced Verticillium wilt in eggplants. The results suggest that NB may enhance the antagonistic interaction between V. dahliae and the rhizospheric microbiome, while the PD medium may constitute an easily accessible food source for V. dahliae, detouring the pathogen from the root system of plants. Furthermore, we identified a P. putida strain with in planta biocontrol activity against V. dahliae, for first time in the literature. However, it is evident that further research is needed to provide more insights in the biocontrol activity of PD and NB, such as the pathogen and soil type-range of this biocontrol activity, the influence on prokaryotic and eucaryotic populations by applying Next Generation Sequence technology, plant transcriptomic changes and the influence on the soil physiochemical properties and metabolome.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agronomy11101946/s1, Figure S1: Rhizospheric microbial population in the NB treated eggplants and mocks (a). Columns represent means of three replications (soil particles attached to the roots of 5 eggplants per treatment and replication, three replications, were pooled to one sample) and the vertical bars indicate standard errors. The asterisk denotes a statistically significant difference between the NB-treated eggplants and the mock treatment, according to a t-test (p < 0.01). In (b) and (c), it is shown representative PDA amended Petri dishes (105 dilution) of the NB and mock treatments, respectively, Table S1: Identification of the isolated microorganisms according to the highest max score sequence similarity (NCBI BLAST) of the partially sequenced 16S rDNA.
Author Contributions
Conceptualization, S.E.T.; methodology, L.L., M.S. and S.E.T.; validation, L.L., M.S. and S.E.T.; formal analysis, L.L., M.S. and S.E.T.; investigation, P.Z., E.G.P., D.G. and L.L.; resources, L.L., M.S. and S.E.T.; data curation, P.Z., E.G.P. and L.L.; writing—original draft preparation, S.E.T.; writing—review and editing, L.L., M.S. and S.E.T.; supervision, S.E.T.; project administration, M.S. and S.E.T.; funding acquisition, M.S. and S.E.T. All authors have read and agreed to the published version of the manuscript.
Funding
M.S. laboratory is supported by funds from Dirección General de Asuntos del Personal Académico-UNAM (PAPIIT), grant IN203720.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank AGROFARM S.A. for kindly donating materials used for experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pegg, G.F.; Brady, B.L. Verticillium Wilts; CABI Publishing: New York, NY, USA, 2002. [Google Scholar]
- Schnathorst, W.C. Life cycle and epidemiology of Verticillium. In Fungal Wilt Diseases of Plants; Mace, M.A., Bell, A.A., Beckman, C.H., Eds.; Academic Press: New York, NY, USA, 1981; pp. 81–111. [Google Scholar]
- Poulaki, E.G.; Tsolakidou, M.-D.; Gkizi, D.; Pantelides, I.S.; Tjamos, S.E. The Ethylene Biosynthesis Genes ACS2 and ACS6 Modulate Disease Severity of Verticillium dahliae. Plants 2020, 9, 907. [Google Scholar] [CrossRef]
- Diwan, N.; Fluhr, R.; Eshed, Y.; Zamir, D.; Tanksley, S.D. Mapping of Ve in tomato: A gene conferring resistance to the broad-spectrum pathogen, Verticillium dahliae race 1. Theor. Appl. Genet. 1999, 98, 315–319. [Google Scholar] [CrossRef]
- Ruano-Rosa, D.; Prieto, P.; Rincón, A.M.; Rodríguez, R.V.; Valderrama, R.; Barroso, J.B.; Mercado-Blanco, J. Fate of Trichoderma harzianum in the olive rhizosphere: Time course of the root colonization process and interaction with the fungal pathogen Verticillium dahliae. BioControl 2016, 61, 269–282. [Google Scholar] [CrossRef]
- Cabanás, C.G.-L.; Ruano-Rosa, D.; Legarda, G.; Pizarro-Tobías, P.; Valverde-Corredor, A.; Triviño, J.C.; Roca, A.; Mercado-Blanco, J. Bacillales Members from the Olive Rhizosphere Are Effective Biological Control Agents against the Defoliating Pathotype of Verticillium dahliae. Agriculture 2018, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Tjamos, S.E.; Flemetakis, E.; Paplomatas, E.J.; Katinakis, P. Induction of Resistance to Verticillium dahliae in Arabidopsis thaliana by the Biocontrol Agent K-165 and Pathogenesis-Related Proteins Gene Expression. Mol. Plant-Microbe Interact. 2005, 18, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Deketelaere, S.; Tyvaert, L.; França, S.D.C.; Höfte, M. Desirable Traits of a Good Biocontrol Agent against Verticillium Wilt. Front. Microbiol. 2017, 8, 1186. [Google Scholar] [CrossRef] [Green Version]
- Gkizi, D.; Gil, A.G.; Pardal, A.J.; Piquerez, S.J.M.; Sergaki, C.; Ntoukakis, V.; Tjamos, S.E. The bacterial biocontrol agent Paenibacillus alvei K165 confers inherited resistance to Verticillium dahliae. J. Exp. Bot. 2021, 72, 4565–4576. [Google Scholar] [CrossRef] [PubMed]
- Somers, E.; Vanderleyden, J.; Srinivasan, M. Rhizosphere Bacterial Signalling: A Love Parade Beneath Our Feet. Crit. Rev. Microbiol. 2004, 30, 205–240. [Google Scholar] [CrossRef]
- Dennis, P.G.; Miller, T.; Hirsch, P.R. Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol. Ecol. 2010, 72, 313–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, E.; Carson, W.P. The Ecology and Natural History of Foliar Bacteria with a Focus on Tropical Forests and Agroecosystems. Bot. Rev. 2015, 81, 105–149. [Google Scholar] [CrossRef]
- Lugtenberg, B.J.J.; Kravchenko, L.V.; Simons, M. Tomato seed and root exudate sugars: Composition, utilization by Pseudomonas biocontrol strains and role in rhizosphere colonization. Environ. Microbiol. 1999, 1, 439–446. [Google Scholar] [CrossRef]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Genet. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Lindow, S.E.; Brandl, M.T. Microbiology of the Phyllosphere. Appl. Environ. Microbiol. 2003, 69, 1875–1883. [Google Scholar] [CrossRef] [Green Version]
- Monier, J.-M.; Lindow, S.E. Frequency, Size, and Localization of Bacterial Aggregates on Bean Leaf Surfaces. Appl. Environ. Microbiol. 2004, 70, 346–355. [Google Scholar] [CrossRef] [Green Version]
- Delmotte, N.; Knief, C.; Chaffron, S.; Innerebner, G.; Roschitzki, B.; Schlapbach, R.; von Mering, C.; Vorholt, J.A. Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc. Natl. Acad. Sci. USA 2009, 106, 16428–16433. [Google Scholar] [CrossRef] [Green Version]
- Chen, L. SWEET sugar transporters for phloem transport and pathogen nutrition. New Phytol. 2014, 201, 1150–1155. [Google Scholar] [CrossRef]
- Eom, J.-S.; Chen, L.-Q.; Sosso, D.; Julius, B.T.; Lin, I.; Qu, X.-Q.; Braun, D.M.; Frommer, W.B. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr. Opin. Plant Biol. 2015, 25, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Zhang, Z.; Ren, Z.; Wang, X.; Sun, W.; Feng, H.; Zhao, J.; Zhang, F.; Li, W.; Ma, X.; et al. The GhSWEET42 Glucose Transporter Participates in Verticillium dahliae Infection in Cotton. Front. Plant Sci. 2021, 12, 690754. [Google Scholar] [CrossRef] [PubMed]
- Tjamos, E.C.; Tsitsigiannis, D.I.; Tjamos, S.E.; Antoniou, P.P.; Katinakis, P. Selection and Screening of Endorhizosphere Bacteria from Solarized Soils as Biocontrol Agents Against Verticillium dahliae of Solanaceous Hosts. Eur. J. Plant Pathol. 2004, 110, 35–44. [Google Scholar] [CrossRef]
- Liu, L.; Kloepper, J.W.; Tuzun, S. Induction of Systemic Resistance in Cucumber Against Fusarium Wilt by Plant Growth-Promoting Rhizobacteria. Phytopathology 1995, 85, 695–698. [Google Scholar] [CrossRef]
- Vaneechoutte, M.; Dijkshoorn, L.; Tjernberg, I.; Elaichouni, A.; de Vos, P.; Claeys, G.; Verschraegen, G. Identification of Acinetobacter genomic species by amplified ribosomal DNA restriction analysis. J. Clin. Microbiol. 1995, 33, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Porsby, C.H.; Nielsen, K.F.; Gram, L. Phaeobacter and Ruegeria Species of the Roseobacter Clade Colonize Separate Niches in a Danish Turbot (Scophthalmus maximus)-Rearing Farm and Antagonize Vibrio anguillarum under Different Growth Conditions. Appl. Environ. Microbiol. 2008, 74, 7356–7364. [Google Scholar] [CrossRef] [Green Version]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef]
- Parks, D.H.; Imelfort, M.; Skennerton, C.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinuesa, P.; Contreras-Moreira, B. Robust Identification of Orthologues and Paralogues for Microbial Pan-Genomics Using GET_HOMOLOGUES: A Case Study of pIncA/C Plasmids. Adv. Struct. Safety Stud. 2015, 1231, 203–232. [Google Scholar] [CrossRef] [Green Version]
- Vinuesa, P.; Ochoa-Sánchez, L.E.; Contreras-Moreira, B. GET_PHYLOMARKERS, a Software Package to Select Optimal Orthologous Clusters for Phylogenomics and Inferring Pan-Genome Phylogenies, Used for a Critical Geno-Taxonomic Revision of the Genus Stenotrophomonas. Front. Microbiol. 2018, 9, 771. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Malandraki, I.; Tjamos, S.E.; Pantelides, I.; Paplomatas, E.J. Thermal inactivation of compost suppressiveness implicates possible biological factors in disease management. Biol. Control 2008, 44, 180–187. [Google Scholar] [CrossRef]
- Bakker, P.A.H.M.; Lamers, J.G.; Bakker, A.W.; Marugg, J.D.; Weisbeek, P.J.; Schippers, B. The role of siderophores in potato tuber yield increase by Pseudomonas putida in a short rotation of potato. Eur. J. Plant Pathol. 1986, 92, 249–256. [Google Scholar] [CrossRef]
- Van Wees, S.; Pieterse, C.; Trijssenaar, A.; Van’t Westende, Y.A.; Hartog, F.; Van Loon, L.C. Differential Induction of Systemic Resistance in Arabidopsis by Biocontrol Bacteria. Mol. Plant-Microbe Interact. 1997, 10, 716–724. [Google Scholar] [CrossRef] [Green Version]
- Bakker, P.A.H.M.; Raaijmakers, J.M.; Schippers, B. Role of iron in the suppression of bacterial plant pathogens by fluorescent pseudomonads. In Iron Chelation in Plants and Soil Microorganisms; Barton, L.L., Hemming, B.C., Eds.; Academic Press: San Diego, CA, USA, 1993; pp. 269–278. [Google Scholar]
- Mercado-Blanco, J.; Jurado, R.; Hervás, A.; Jiménez-Dıaz, R.M. Suppression of Verticillium wilt in olive planting stocks by root-associated fluorescent Pseudomonas spp. Biol. Control. 2004, 30, 474–486. [Google Scholar] [CrossRef]
- Raaijmakers, J.; Vlami, M.; De Souza, J.T. Antibiotic production by bacterial biocontrol agents. Antonie Leeuwenhoek 2002, 81, 537–547. [Google Scholar] [CrossRef]
- Validov, S.Z.; Kamilova, F.; Lugtenberg, B.J. Pseudomonas putida strain PCL1760 controls tomato foot and root rot in stonewool under industrial conditions in a certified greenhouse. Biol. Control. 2009, 48, 6–11. [Google Scholar] [CrossRef]
- Sun, D.; Zhuo, T.; Hu, X.; Fan, X.; Zou, H. Identification of a Pseudomonas putida as biocontrol agent for tomato bacterial wilt disease. Biol. Control. 2017, 114, 45–50. [Google Scholar] [CrossRef]
- Pantelides, I.; Tjamos, S.; Striglis, I.A.; Chatzipavlidis, I.; Paplomatas, E.J. Mode of action of a non-pathogenic Fusarium oxysporum strain against Verticillium dahliae using Real Time QPCR analysis and biomarker transformation. Biol. Control. 2009, 50, 30–36. [Google Scholar] [CrossRef]
- Hassani, M.A.; Durán, P.; Hacquard, S. Microbial interactions within the plant holobiont. Microbiome 2018, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Kemen, E. Microbe–microbe interactions determine oomycete and fungal host colonization. Curr. Opin. Plant Biol. 2014, 20, 75–81. [Google Scholar] [CrossRef]
- Kwak, Y.-S.; Weller, D.M. Take-all of Wheat and Natural Disease Suppression: A Review. Plant Pathol. J. 2013, 29, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Isaac, I. A comparative study of pathogenic isolates of Verticillium. Trans. Br. Mycol. Soc. 1949, 32, 137–157. [Google Scholar] [CrossRef]
- Malca, I.; Erwin, D.C.; Moje, W.; Jones, B. Effects of pH and carbon and nitrogen sources on the growth of Verticillium alboatrum. Phytopathology 1966, 56, 401–406. [Google Scholar]
- Cooper, R.; Wood, R. Regulation of synthesis of cell wall degrading enzymes by Veticillium alboatrum and Fusarium oxsporum f. sp. lycopersici. Physiol. Plant Pathol. 1975, 5, 135–156. [Google Scholar] [CrossRef]
- Sarmiento-Villamil, J.L.; García-Pedrajas, N.E.; Cañizares, M.C.; García-Pedrajas, M.D. Molecular Mechanisms Controlling the Disease Cycle in the Vascular Pathogen Verticillium dahliae Characterized Through Forward Genetics and Transcriptomics. Mol. Plant-Microbe Interact. 2020, 33, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Naglik, J.; Albrecht, A.; Bader, O.; Hube, B. Candida albicans proteinases and host/pathogen interactions. Cell. Microbiol. 2004, 6, 915–926. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).