Abstract
Weed management is an important issue since weeds directly compete with crop plants for space, nutrients; serve as habitat for insect pests and diseases, and can create a significant annual reduction in crop productivity. This study focused on evaluating the contribution of the secondary metabolites of the fruit pulp of Couroupita guianensis Aubl. for its potential growth inhibitory effect. Crude extracts of C. guianensis fruit pulp were collected with different solvents and applied to test plants in petri dishes. The crude extracts of methanol and 1% dimethyl sulfoxide (DMSO) showed potential growth inhibitions with the 50% effective concentration (EC50) of 223 and 229 µg/mL in the bioassay experiment. In the greenhouse pot experiment, soil incorporated with oven-dried fruit pulp of C. guianensis was evaluated on cultivated plant species including Lactuca sativa L., Trifolium repens L., Medicago sativa L., Lolium multiflorum Lam., and Phleum pratense L. The incorporation of dried fruit pulp of C. guianensis into soil reduced shoot and root lengths and the germination percentage of test plants. It was observed that the monocot plants were more affected than the dicot plants. The fruit pulp of C. guianensis was subjected to reversed-phase high-performance liquid chromatography (HPLC) analysis to identify the active compounds. Indigo, identified as one of the candidate compounds of the C. guianensis, had high specific activity (i.e., strong inhibitory activity) in a phytotoxicity bioassay and could explain through the total activity concept the growth inhibitory effect of the C. guianensis on test plants. The results suggested that indigo has plant growth inhibitory effect, indicating the allelopathic potential of C. guianensis, which could be exploited in sustainable weed management.
1. Introduction
Weed management is one of the most fundamental concerns in crop production. The interference of weeds with field crops is responsible for multibillion dollars of annual yield loss in crop cultivation [1,2,3]. In the USA and Canada, the total loss in corn and soybean due to weed infestation was estimated to be USD 43 billion per annum [1]. In Australia, the cost was estimated to be AUD 3.3 billion [2] and USD 11 billion in India annually [3]. The excessive application of various weedicides for weed management globally has to lead to an increase in herbicide-resistant weed species [4]. Allelopathic species have been considered as valuable sources of bioactive substances for managing weeds to increase crop productivity, minimize soil degradation, and maintain biodiversity [5]. By the term, allelopathy denotes the process of releasing bioactive secondary metabolites (allelochemicals) from living organisms and the subsequent interactions with other species through beneficial or harmful natural ways [6,7]. Allelochemicals are released into the environment in different ways, including leaching, volatilization, root exudation, and decomposition of plant residues [8,9]. These allelochemicals are present in different plant parts, including leaves, barks, roots, root exudates, flowers, seeds, pollens, stems, and fruits of plants [10]. In recent years, several researchers have focused on the possibilities of exploring allelopathic species and allelochemicals in sustainable weed management [11]. Some previous studies showed the utilization of allelopathic species and their allelochemicals as herbicides in sustainable weed management [6,10,12,13]. Several bioactive compounds have been identified as allelochemicals that can potentially be utilized in a sustainable weed management aspect [14]. For example, cyanamide was isolated from hairy vetch (Vicia villosa), tricin and momilactone B from rice (Oryza sativa) [15,16], and L-3,4-dihydroxyphenylalanine (L-DOPA) from velvet bean (Mucuna pruriens) [17]. Moreover, it is reasonable for examining natural products to uncover potential novel sites of action since many allelochemicals operate by mechanisms that are not possessed by synthetic herbicides [18,19]. The cannonball tree (C. guianensis) (Lecythidaceae) is domestic to northern South America and the West Indies. This plant is also prevalent in the Indian subcontinent. All parts of this deciduous tree have been reported with medicinal value [20,21,22,23]. C. guianensis has several biological activities, including anti-inflammatory [24], antimicrobial [25,26], and antioxidant properties [27,28].
C. guianensis fruit pulp contains bioactive compounds such as indigo naturalis (indigo, indirubin, tryptanthrin), isatin, triterpenes, phenolic compounds, [29,30] alkaloids, flavonoids, and terpenoids [31]. Indigo is one of the famous natural blue dyes which has been used as a traditional Chinese medicine for centuries, where; it is also known as indigo naturalis or qing dai [32]. It has been used to reduce inflammation, fever, alleviate pain, purify the liver, and also have nematicide activity [33,34,35]. As a Chinese medicine, indigo naturalis showed anti-inflammatory, hemostatic, antibacterial, antiviral properties. Indirubin, red-colored 3,2′-isomer, a minor byproduct of indigo naturalis, has reported exhibiting anti-cancer, anti-inflammation activities, and also used to treat chronic diseases [36,37,38,39,40]. It acts as a potent inhibitor of cyclin-dependent kinases (CDKs), interacting with the kinase’s ATP-binding site [41,42,43]. Isatin acts as an antiviral agent in non-mammalian species and is also compatible with antagonistic action at the atrial natriuretic peptide (ANP) receptor. It has brain and periphery physiological functions, and a high dose even showed anticonvulsant behavior [44,45,46].
Interestingly, this fruit pulp (both fresh and dried) contains a strong unpleasant smell, and the color of the fresh pulp gradually turns into indigo (blue color) after breaking. The initial allelopathic screening study [47] identified C. guianensis fruit pulp as a potential allelopathic candidate. However, the active allelochemical of C. guianensis fruit pulp responsible for the plant growth inhibitory effect remains unknown. This study focused on the identification of the potential allelochemical from the C. guianensis fruit pulp.
Consequently, the present study aimed to (a) evaluate the inhibitory potentials of different crude extracts of C. guianensis fruit pulp; (b) identify the potential allelopathic compounds in C. guianensis fruit pulp, and (c) evaluate the phytotoxicity of the candidate allelopathic compound on other plants.
2. Materials and Methods
2.1. Collection of C. guianensis Samples and Extraction Procedure
Fruit pulps of C. guianensis were collected in February 2018 from Sher-e-Bangla Agricultural University (Dhaka, Bangladesh). The crude extracts were obtained from the oven-dried (60 °C for 48 h) fruit pulp. Finely ground 60 mg samples were extracted with five different solvents, including ethyl acetate, methanol, chloroform, 1% dimethyl sulfoxide (DMSO), and water. All the extracts were kept in the shaking condition (Recipro Shaker NR-1, speed 600 min−1) for 48 h at room temperature. The extracts were sonicated (10 min), filtered (No. 1 filter paper, Advantec Toyo Roshi Kaisha, Tokyo, Japan), centrifuged (Hitachi himac CR22N (6000 rpm, 30 min)), and the supernatants were collected. The solvents were re-extracted following the same procedure as above. The supernatants were homogenized and used as a working solution.
2.2. Chemicals and Test Plants for Bioassay
Indigo, indirubin, and isatin were purchased from Sigma-Aldrich (Suisse, Switzerland). All compounds (Figure 1) were reagent grade and were used without further purification. Seeds of five plants from three different plant families were purchased from local seed companies in Japan and used as test plants (Lactuca sativa L. cv. Legacy from Takii Seed Co., Ltd. Kyoto, Japan; Trifolium repens L. cv. Fia from Snow Brand Seed Co., Ltd. Chiba, Japan; Medicago sativa L. cv. Neotachiwakaba from Takii Seed Co., Ltd. Kyoto, Japan; Lolium multiflorum Lam. cv. Ace from Snow Brand Seed Co., Ltd. Chiba, Japan; Phleum pratense L. cv. Climax from Takii Seed Co., Ltd. Kyoto, Japan). The seeds belonged to the following plant families: Asteraceae L. sativa, Fabaceae (T. repens and M. sativa), and Poaceae (L. multiflorum and P. pratense). Among the test plants used in this study, L. sativa and M. sativa are crop species, while L. multiflorum, T. repens, and P. pratense are weed species.
Figure 1.
Chemical structure of indigo, indirubin, isatin, the compounds of Couroupita guianensis fruit pulp.
2.3. Inhibitory Effects of C. guianensis Crude Extracts and Test Compounds
The 50% effective concentration (EC50) value or specific activity of the crude extracts and the tested compounds was evaluated using lettuce as a test plant [8]. The crude extracts were obtained from C. guianensis fruit pulps, as described in the extraction procedure. The following concentrations of the crude extracts were tested on the test plants: 50, 100, 200, 400, 500, 1000, 2000, and 2500 ppm. Filter paper (Toyo Roshi Kaisha, Ltd., Tokyo, Japan) was placed in a glass Petri dish (27 mm ø). Then, 0.7 mL of the test solution was added to the filter paper and dried thoroughly in a vacuum. Lettuce seeds were pre-germinated for 18 h. Five pre-germinated seedlings were placed on the filter paper after adding 0.7 mL of 1% DMSO and incubated (CN-25C, Mitsubishi Elec., Tokyo, Japan) for 54 h at 25 °C under dark conditions. The inhibitory activity bioassays using indigo, indirubin, and isatin were performed in the same conditions as described above. The contribution of each polyphenol to the allelopathy of C. guianensis fruit pulp crude extract was estimated based on the inhibition and concentration of the compounds estimated to be present in the crude extract (i.e., total activity). The control treatments were set up without crude extract or compound but only 1% DMSO. Three replications were set for each treatment. The lengths of radicle and hypocotyl were measured after the incubation period, and the elongation percentage was calculated using Equation (1) [48]
where A = mean length of radicle/hypocotyl by treatment (pure compound/crude extract) and B = mean length of radicle/hypocotyl by (no treatment) control.
Elongation % = A/B × 100
2.4. Effects on Plant Growth Inhibition Activity of Candidate Compounds on Test Plants
Seeds of the test plant (L. sativa) were placed in 27 mm diameter Petri dishes containing 0.7 mL of 1% DMSO (control) or the synthetic compounds (indigo, indirubin, isatin) with different concentrations on filter paper. Each dish was kept in an incubator (CN-25C, Mitsubishi Elec., Tokyo, Japan) under dark conditions for three days at 25 °C. Based on the germination inhibition bioassay experiment, five test plant species from three different families were selected to investigate 50% inhibition of radicle growth. Various concentrations of pure compounds (25, 50, 75, 100, and 150 µg/mL) were tested on each of the test plant species. The lengths of radicle and hypocotyl were measured on the 3rd day and expressed as a percentage of the control.
2.5. HPLC Analysis of C. guianensis Fruit Pulps
A total of 15 mg of ground C. guianensis sample (fruit pulps) was accurately weighed into 5 mL tubes containing 100% DMSO and extracted, as shown in the extraction procedure. Then, it was diluted as an HPLC working concentration of 2000 ppm. An aliquot of the extract after centrifugation was filtered through a 0.2 µm syringe filter before injection (1 µL). HPLC analysis was performed using an LC-20AD liquid chromatograph (Shimadzu, Japan). An Inertsil ODS 3 column (250 × 4.6 mm, 5 µm particles, GL Sciences Inc, Tokyo, Japan) was used. Mobile phases A and B were water with 0.1% acetic acid and acetonitrile (ACN), respectively. The column temperature was kept at 30 °C, and the flow rate of the mobile phase was set at 0.5 mL min per minute. The following multi-step gradient with different proportions of mobile phase B was applied: 0 min, 5% B; 10 min, 40% B; 15 min, 85% B, and maintained for 10 min. The initial conditions for HPLC were maintained for 5 min. The result of the analysis was monitored by using an SPD-M20A detector at 310 nm. To examine the quantification of the compound, the comparison of the peak areas of the target compound with the availability of the compound in the corresponding standard was used in the calibration curve. All chemical analyses were replicated three times.
2.6. Effect of Incorporating C. guianensis Fruit Pulps in Soil on the Bioassay Species
The study was conducted to understand the effect of crude fruit pulp of C. guianensis on the growth of selected model test plants under greenhouse conditions. Each pot (L × W × H: 7 × 7 × 6 cm) was filled up with 150 g autoclaved soil (Kumiai Nippi, Tokyo, Japan) and mixed with 0.75, 2.25, and 3.75 g of C. guianensis fruit pulps. The treatment concentrations were expressed as 0, 0.5%, 1.5%, and 2.5% (w/w) based on the dry weight of soil. Previous studies used similar incorporation rates for plants (like as Rosmarinus officinalis, Ophiopogon japonicas, Cyperus iria L.) for allelopathic studies [49,50,51]. Five seeds from each species were sown in the pots. Five replications of each concentration were performed for each of the bioassay species. The pots were kept in the greenhouse with average day and night temperatures of 26 °C and 20 °C, respectively. Seedlings were watered (25 mL/pot) regularly to keep soil in a moist and appropriate condition for plant growth. All seeds used for this evaluation (control and the replications) were pre-germinated (L. multiflorum and P. pretense—40 h; L. sativa, T. repens, and M. sativa—18 h). For the evaluation study, for the parameters (shoot length, root length, shoot dry weight, root dry weight), pre-germinated seeds were placed in pots with five replicants. In contrast, only for the evaluation of seed germination inhibition, non-germinated seeds sown in different pots along with replications (thus, the non-germinated seeds were necessary to evaluate the germination inhibition). The seed germination inhibition test was separated from other evaluations in the same period. However, the rest of all the conditions remained the same as before. Plants were harvested after twenty-five days of planting. Each of the plants (especially roots) were carefully handled to separate then from the soil by a gentle wash to avoid the damage of the root. Seed germinations were counted after seven days. Lengths of roots and shoots, weights of dry roots and shoots were measured after harvesting. The control pot did not contain C. guianensis fruit pulp.
2.7. Statistical Analysis
Each result for the test plants was expressed as the mean percentage. Statistical differences of parameters between control and sample groups were evaluated by using a two-factor analysis of variance (ANOVA) followed by Tukey’s HSD (honestly significant difference) test for the analysis. p < 0.05 was considered as statistically significant. Statistical analysis was performed with RStudio software version 1.1.423.0.
3. Results and Discussion
3.1. Inhibitory Effect of Crude Extracts of C. guianensis Fruit Pulp on Lettuce Seedlings
To evaluate the plant extracts inhibition, lettuce seedlings (L. sativa) were tested as a model plant. Five crude extracts with different solvents were evaluated on the test plant to understand the inhibitory effect of the extraction of C. guianensis fruit pulp. Similar to this study, previous research also evaluated the allelopathic activity of other plant species on L. sativa using crude extracts from different solvents [52,53]. The growth of radicle showed more sensitivity to the extracts than hypocotyl. The higher sensitivity of lettuce radicle to allelochemicals is thought to be due to the direct interaction of the extracts and the radicle [54] along with root cell expansion, cell proliferation [55], and higher permeability of the root surface [56]. The lowest EC50 value by the crude extract of fruit pulp of C. guianensis on the test plant was found with methanol extract (223 µg/mL), followed by 1% DMSO extract (229 µg/mL), water (271 µg/mL), ethyl acetate (276 µg/mL), and chloroform extract (409 µg/mL), in that order (Figure 2). The results showed that the smallest EC50 value (highest specific activity) on radicle elongation was exhibited by methanol extract, followed by the 1% DMSO extract.
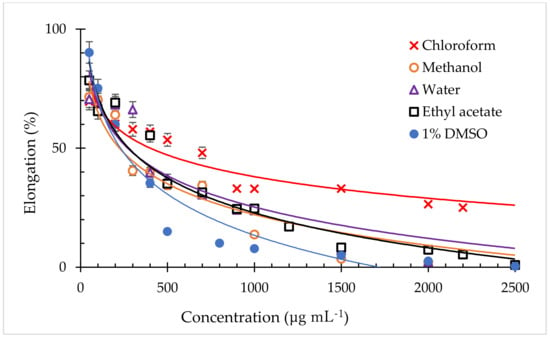
Figure 2.
Effect of different crude extracts of C. guianensis fruit pulps on the radicle growth of L. sativa seedlings. Data are the mean of 3 replications ± standard deviation (n = 3).
In a different study, the leaf extracts of C. guianensis completely inhibited the germination of L. sativa and significantly delayed the germination of barnyard grass [47]. The crude extract of C. guianensis was reported with several medicinal properties. Wound healing activity by the ethanol extract of C. guianensis whole plant (barks, leaves, flowers, and fruits) was also reported previously [25]. The chloroform extract of C. guianensis fruit showed sensible antimicrobial, antimycobacterial, and antibiofilm properties [23,57]. Shah et al. [57] showed that the ethanol extract of the fruit pulp of C. guianensis showed inhibition on Gram-positive microorganisms (Staphylococcus aureus and Bacillus subtilis) and Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa). However, there is no previous study of the plant growth inhibitory effects of the crude extract of C. guianensis fruit pulp. This study is fundamental research that investigated plant growth inhibition by the different extractions of fruit pulp of C. guianensis on the test plant.
3.2. Plant Growth Inhibitory Effects of Synthetic Compounds Present in Crude Extract of C. guianensis Fruit Pulp
The synthetic compounds of C. guianensis (Indigo, Indirubin, and Isatin) were tested for their plant growth inhibitory effect on L. sativa. The examined compounds showed varying degrees of inhibitions of radicle and hypocotyl elongation (Figure 3). The synthetic compounds inhibited the growth of the radicle more than the hypocotyl for the tested compounds. The EC50 on hypocotyl was estimated to be 120 ppm, 3600 ppm, and 3212 ppm for indigo, indirubin, and isatin, respectively. Similar to the results of this study, Fujii et al. [17] reported that the allelochemical from velvet bean (L-3,4-dihydroxyphenylalanine) had very minimal or no influence on the hypocotyl growth of lettuce seedlings. On the radicle growth, indigo showed the inhibitory effect at 70 mg/L (ppm), which was followed by 3107 mg/L (ppm) for indirubin and 597 mg/L (ppm) for isatin. Isatin showed a small inhibition effect, whereas indirubin showed nearly no impact or minimal effect on the radicle growth of the lettuce seedling. The results indicated that among the tested synthetic compounds, indigo had the highest growth inhibition on lettuce radicle elongation.
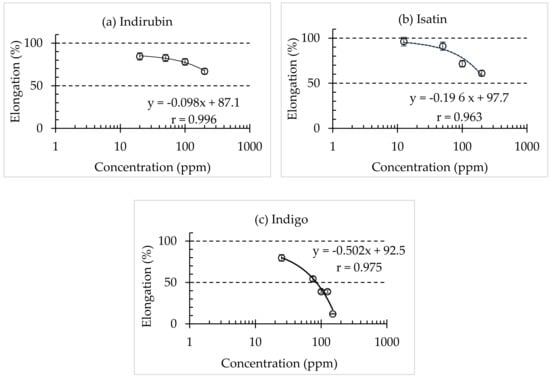
Figure 3.
The effects of synthetic compounds on the radicle growth of lettuce seedlings. (a) Indirubin, (b) isatin, and (c) indigo. The data are the means of three replications ± SD.
3.3. Estimation of the Contribution of the Candidate Compound on Growth Inhibitory Activity
The study elaborated on the prospect of inhibitory effects of the candidate compound (indigo) on tested seedlings and also accounted for the contribution to the inhibition of crude extracts of C. guianensis. Moreover, the activity of the allelochemicals and the allelopathic species can be evaluated by the specific and total activity [58]. The total activity of allelochemicals should be evaluated to determine the contribution of such compounds to the growth inhibitory effects of allelopathic species [59]. It is necessary to know the concentration of the indigo in the plant and the inhibitory effect (specific activity of EC50) to evaluate the contribution (total activity) of the candidate species.
The targeted synthetic compound (indigo) was dissolved in DMSO, and, subsequently, the inhibitory effect on lettuce by 1% DMSO extraction of C. guianensis was considered for the total activity estimation. From the HPLC analysis (Figure 4), the content of the indigo concentration was estimated at 8.08 µg/mL in the 1mg/mL crude extract of fruit pulp of C. guianensis. Among the tested compounds, indigo had the highest estimated specific activity (70 ppm) and was considered for the total activity estimation. The total activity of indigo in the fruit pulp of C. guianensis was 58 for lettuce radicle. The total activity values of around 100 of indigo for other model species, including white clover (T. repens), alfalfa (M. sativa), Italian ryegrass (L. multiflorum), and timothy grass (P. pratense), were higher than that of lettuce. The compound indigo explained the inhibitory effect of fruit pulp of C. guianensis on both the radicle and hypocotyl growth of lettuce seedlings (Figure 5) and other model test species (Figure S1). Based on this estimation, the compound indigo can be considered as one of the inhibitors/candidates for the inhibitory activity of the crude extracts of C. guianensis fruit pulp. Based on the specific activity (EC50) and the total activity of the estimated compound, several allelochemicals have been identified. Some potential allelochemicals have been identified in previous studies by using the total activity concept, including cyanamide, juglone, angelicin, L-DOPA, rutin, umbelliferone, and carnosic acid [49,60,61,62,63,64].
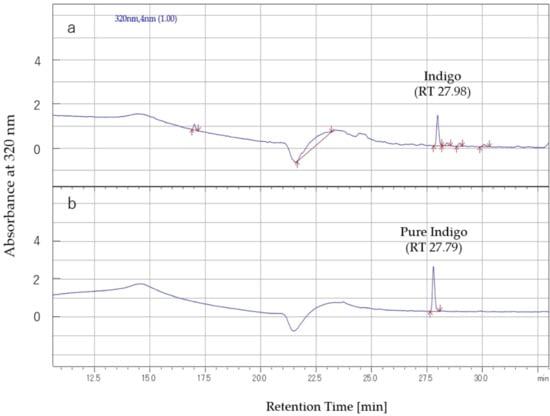
Figure 4.
HPLC chromatogram of the crude extract of the pulp of C. guianensis: (a) crude extract, (b) pure standard compound indigo.
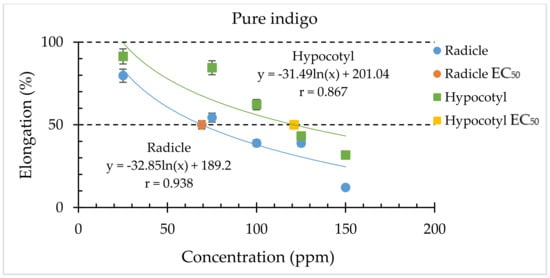
Figure 5.
The inhibitory effects of the synthetic compound (indigo) on C. guianensis fruit pulp on the radicle and hypocotyl growth of lettuce seedlings. The represented data are the ± SD; n = 3.
Usually, indigo is not synthesized directly by the plant. Indigo is derived from the secondary metabolite, indole glucoside precursors [65]. Different indigo precursors are present in other indigo producing plants. For example, indican (indoxyl-β-D-glucoside) [66] or tryptanthrin [67] are present in Polygonum tinctorium, or both indican and isatin B (indoxyl-5-ketogluconate) [66,68] are found in different Isatis spp. The precursors are colorless, and when the carbohydrate moiety is cleaved from the indoxyl group, and two of the indoxyl molecules combine oxidatively, they produce indigo. This study showed the significance of indigo as a plant growth inhibitor.
Besides indigo, the sample of dry fruit pulp of C. guianensis contains other inhibitor chemicals, including some active alkaloids, such as tryptophan. The dried fruit pulp also showed partial growth inhibition by volatile emission [69,70]. By the reduction process, indigo produced indole [71,72], which is one of the typical secondary metabolites [73]. A relatively high concentration of indole constitutes the major contributor to odor [74,75]. Plants can produce indole by tryptophanase or its analogues in the presence of indole-3-glycerol phosphate and tryptophan [76,77,78]. The fundamental findings of this study were that indigo was detected in a high concentration of the crude extract of C. guianensis fruit pulp and could be a contributor to the growth inhibitory effect of the plant.
3.4. Effect of Soil Incorporation of the Crude Extract of C. guianensis Fruit Pulp on Weeds Species
Different concentrations of C. guianensis dried fruit pulp were incorporated into the soil to evaluate the effect on the model-tested species under greenhouse conditions. A total of 150 g soil per pot was mixed with different amounts of dried fruit pulp (0.75 g, 2.25 g, and 3.75 g). Under the greenhouse conditions, incorporation of fruit pulp of C. guianensis at different rates (0.75 g to 3.75 g) with 150 g soil significantly (p < 0.05) reduced the germination, shoot length, and root length of P. pratense (Figure 6e). Other test plants, along with the model plant, L. sativa, did not respond and showed emergence after germination. All the species showed reduced shoot and root length. Neither shoot dry weight nor root dry weight of test plants was affected significantly except for T. repens. The hormesis effect can explain the significant (p < 0.05) biphasic dose-response, a low dose stimulatory effect, and a high dose inhibitory effect on the shoot and root weights of T. repens (Figure 6c). Calabrese et al. [79] and Chen and Jeffrey [80] explained this kind of dose-response relationship previously.
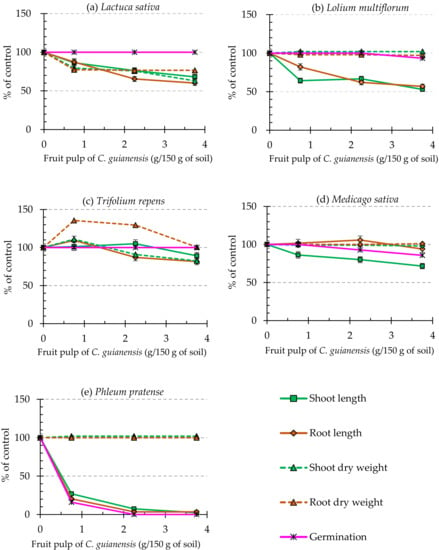
Figure 6.
Effect of soil incorporated with fruit pulp of C. guianensis on the growth of (a) L. sativa, (b) L. multiflorum, (c) T. repens, (d) M. sativa, and (e) P. pratense. The parameters here are considered as shoot length, root length, shoot dry weight, root dry weight, and effect on germination. Data are presented as the mean ± SD.
The incorporation of dried fruit pulp of C. guianensis affected the growth of L. sativa at all concentrations. At the concentration of 0.75 g/150 g of soil, the length of the shoot of L. sativa was significantly inhibited by 13.0%, and at 2.25 g/150 g of soil, the length of the root was inhibited by 34.6% (Figure 6a). In the shoot, dry weight showed significant inhibition at 0.75 g/150 g of soil. At the same concentration, the inhibition of the dry weight of L. sativa shoot was 36.4%. The pattern of root inhibition was slightly different from the shoot. The inhibition of the dry weight of the root was 22.7% at 0.75 g/150 g of soil. However, the germination of L. sativa was noticeably unaffected at any of the applied rates. Sensitivity to allelopathic substances on test plants has also been well studied [81]. Relatively different patterns of changing were reported previously in terms of allelochemical effects, such as the shape of the root, necrosis, radicle color, curling and reduction of root axis, increased number of seminal roots, and reduced dry weight accumulation [82,83].
The length of the shoot of L. multiflorum was significantly inhibited by 35.7% at the lowest concentration (0.75 g/150 g of soil). However, the root responded at the highest concentration. The length of the root showed significant inhibition by 43.2% at 3.75 g/150 g of soil (Figure 6b). The increasing concentration of fruit pulp reduced the root elongation of test plants (Figure 7a). Another study reported that the morphological variations with a variety and species affect the cellular or molecular level of the receptor plants [82]. The effect of dried fruit pulp caused brown patches or burn symptoms at the tip of the leaves of L. multiflorum (Figure 7b).
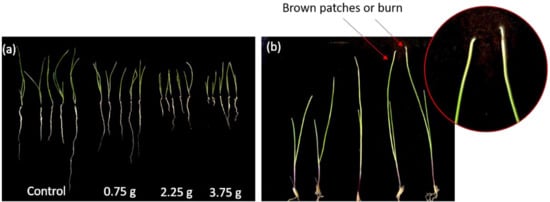
Figure 7.
Effect of C. guianensis fruit pulp on Lolium multiflorum. (a) An increasing dose of crude fruit pulp effects the length of radicle and hypocotyl compared to the control treatment; (b) 3.75 g of crude C. guianensis fruit pulp showed brown patches or burn symptoms of the leaf tip of L. multiflorum.
Similarly, in a previous study, a high amount of rape straw in the soil caused the burning of leaf-tip of barley plants, changing color to pale green, and stunted growth three weeks after incorporation [84]. The inhibition of root, shoot, and dry weight of L. multiflorum was not noticeably affected by any of the application rates of dried fruit pulp of C. guianensis. The germination percentage of L. multiflorum seed was affected by 6.67% with 3.75 g/150 g of soil fruit pulp of C. guianensis compared to the control (Figure 6b).
T. repens showed 10.9% shoot length inhibition at 3.75 g/150 g of soil and 13.0% root length inhibition at 2.25 g/150 g of soil (Figure 6c). The root and shoot lengths were not inhibited above 18%, even at the highest application rate. The weight of dry shoot showed significant inhibition at 3.75 g/150 g of soil and was inhibited by 20.9% (Figure 6c). The germination of seed remained unaffected for T. repens. Appiah et al. [49] reported that the leaf debris of rosemary affected the fresh and dry weight of the shoot more than the dry weight of the root of the test plants. In another study, the dry weight of the root of Trianthema portulacastrum was affected more than the dry weight of the shoot by Brassica campestris and sorghum residues [85]. The seed germination of T. repens was not affected at any incorporation rate (Figure 6c).
The shoot length of M. sativa was observed decreasing by 13.7%, 19.9%, and 28.4% at 0.75 g, 2.25 g, and 3.75 g/150 g of soil, respectively (Figure 6d). The length of the root, the dry weight of the shoot, and the root were unaffected and did not show any noticeable inhibition at any of the applied rates. Seed germination was decreased by 7.14% and 14.3% and at 2.25 g and 3.75 g/150 g of soil, respectively (Figure 6d). However, the decrease in seed germination was not significant. P. pratense showed the highest sensitivity to the inhibitory substances released from the fruit pulp of C. guianensis. The lengths of shoot and root of P. pratense was inhibited significantly by 74.9% and 79.5% at 0.75 g/150 g of soil, respectively (Figure 6e). This trend of inhibition increased with the increasing concentration of the fruit pulp of C. guianensis. The seed size of P. pratense was smaller than other tested weed species; hence, the germination might be affected more than other weeds.
Factors such as size, dormancy of seed, and the duration of germination could influence the effect of allelopathic compounds [86]. The results showed that the seed germination of P. pratense was inhibited significantly by 81.82% at the minimum incorporation rate of 0.75 g/150 g of soil (Figure 6e).
The other parameters did not show any significant differences. In a previous study, the germination of oat (Avena sativa), oilseed rape (Brassica napus), and sunflower (Helianthus annuus) were not significantly affected by the water extraction of the indigo plant (Amorpha fructicosa) but significantly inhibited root and shoot lengths of the test species [87]. Wu et al. [88] reported that seed germinations, as well as seedling growth indices, were affected by aqueous leaf extract of Mikania micrantha in a concentration-dependent manner. Seed germination and seedlings development are generally considered to be the most susceptible stage. Therefore, in several bioassays, seed germination and seedling development were measured after the exposure to potential allelochemicals [89,90,91]. The results stated that the allelopathic effects of C. guianensis on the shoot and root lengths and seed germination were more prominent on the tested monocot plants rather than the tested dicot plants. Both laboratory screening and field experiments indicated that rice allelochemicals are active against both monocot and dicot weeds [92]. The phenomenon of seedling growth affected by soils amended with the debris of allelopathic plants was reported in other studies [76].
4. Conclusions
Identification of new plant growth inhibitory species and compounds might provide the possibilities for new natural herbicide development that could be exploited in sustainable weed management. This study indicated that the crude extracts of C. guianensis fruit pulps contained potential inhibitory substances. This is the first report of indigo as a potential allelochemical identified from the fruit pulps of C. guianensis. The evaluation of the inhibitory effects showed by released allelochemicals may promote sustainable weed management in agriculture. Under the greenhouse conditions, the allelopathic effect by growth inhibition was more pronounced on the monocot species rather than the dicot species. Furthermore, it is necessary to understand the prospect of individual or combinations of allelopathic compounds and their dynamic mode of action. Therefore, the development of new bioherbicides using a natural product would be an excellent prospect for the development of the weed management sector in agriculture.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4395/10/9/1388/s1, Figure S1. The inhibitory effects of pure compound (indigo) of C. guianensis fruit pulp on the radicle and hypocotyl growth of (a) T. repens, (b) M. sativa, (c) L. multiflorum, (d) P. pratense seedlings. The represented data are the +/− standard deviation; n = 3.
Author Contributions
Conceptualization, K.B., N.H., and Y.F.; methodology, K.B., Y.F., T.M.; software, Microsoft Office 2016, RStudio version 1.1.423.0.; validation, N.H., K.S.A., M.S., T.M., and Y.F.; formal analysis, K.B., K.S.A., N.H., Y.F.; investigation, K.B., N.H., and T.M.; resources, N.H. and M.S.; data curation, K.B., K.S.A., Y.F.; writing—original draft preparation, K.B.; writing—review and editing, K.B., M.S., N.H., K.S.A., T.M., Y.F.; supervision, T.M., Y.F.; funding acquisition, T.M., Y.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan. This work was also partly supported by JST CREST Grant Number JPMJCR17O2 and JSPS KAKENHI Grant Number 26304024.
Acknowledgments
The authors thank the Japanese Ministry of Education, Culture, Sports, Science, and Technology (MEXT) for providing scholarships and funds to carry out this research at the Tokyo University of Agriculture and Technology.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WSSA Calculates Billions in Potential Economic Losses from Uncontrolled Weeds; National and Regional Weed Science Societies: Lawrence, KS, USA, 2016.
- Llewellyn, R.; Ronning, D.; Ouzman, J.; Walker, S.; Mayfield, A.; Clarke, M. Impact of Weeds on Australian Grain Production: The Cost of Weeds to Australian Grain Growers and the Adoption of Weed Management and Tillage Practices; Report for Grains Research & Development Corporation: Canberra, ACT, Australia, 2016. [Google Scholar]
- Gharde, Y.; Singh, P.K.; Dubey, R.P.; Gupta, P.K. Assessment of yield and economic losses in agriculture due to weeds in India. Crop Prot. 2018, 107, 12–18. [Google Scholar] [CrossRef]
- Duke, S.O.; Romagni, J.G.; Dayan, F.E. Natural products as sources for new mechanisms of herbicidal action. Crop Prot. 2000, 19, 583–589. [Google Scholar] [CrossRef]
- Wardle, D.A.; Karban, R.; Callaway, R.M. The ecosystem and evolutionary contexts of allelopathy. Trends Ecol. Evol. 2011, 26, 655–662. [Google Scholar] [CrossRef]
- Weston, L.A.; Duke, S.O. Weed and crop allelopathy. Crit. Rev. Plant Sci. 2003, 22, 367–389. [Google Scholar] [CrossRef]
- International Allelopathy Society. June 2015. Available online: http://allelopathy-society.osupytheas.fr/about/ (accessed on 9 June 2020).
- Fujii, Y. Allelopathy in the natural and agricultural ecosystems and isolation of potent allelochemicals from Velvet bean (Mucuna pruriens) and Hairy vetch (Vicia villosa). Biol. Sci. Space. 2003, 17, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Hiradate, S. Allelopathy: New Concepts & Methodology; Science Publishers, Inc.: Enfield, NH, USA, 2007; ISBN 978-1-57808-446-3. [Google Scholar]
- Soltys, D.; Krasuska, U.; Bogatek, R.; Gniazdowsk, A. Allelochemicals as Bioherbicides—Present and Perspectives. In Herbicides-Current Research and Case Studies in Use; Price, A., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-1112-2. [Google Scholar]
- Kropff, M.J.; Walter, H. EWRS and the challenges for weed research at the start of a new millennium. Weed Res. (Oxford) 2000, 40, 7–10. [Google Scholar] [CrossRef]
- Vyvyan, J.R. Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron 2002, 58, 1631–1646. [Google Scholar] [CrossRef]
- Barney, J.N.; Hay, A.G.; Weston, L.A. Isolation and characterization of allelopathic volatiles from mugwort (Artemisia vulgaris). J. Chem. Ecol. 2005, 31, 247–265. [Google Scholar] [CrossRef]
- Macías, F.A.; Marín, D.; Oliveros-Bastidas, A.; Varela, R.M.; Simonet, A.M.; Carrera, C.; Molinillo, J.M. Allelopathy as a new strategy for sustainable ecosystems development. Biol. Sci. Space 2003, 17, 18–23. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Ino, T. Rice seedlings release momilactone B into the environment. Phytochemistry 2003, 63, 551–554. [Google Scholar] [CrossRef]
- Kong, C.; Liang, W.; Xu, X.; Hu, F.; Wang, P.; Jiang, Y. Release and activity of allelochemicals from allelopathic rice seedlings. J. Agric. Food Chem. 2004, 52, 2861–2865. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Shibuya, T.; Yasuda, T. Allelopathy of velvetbean: Its discrimination and identification of L-DOPA as a candidate of allelopathic substances. Jpn. Agric. Res. Q. 1992, 25, 238–247. [Google Scholar]
- Dayan, F.E.; Owens, D.K.; Duke, S.O. Rationale for a natural products approach to herbicide discovery. Pest Manag. Sci. 2012, 68, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, B.C.; Sparks, T.C. Natural products for pest control: An analysis of their role, value and future. Pest Manag. Sci. 2014, 70, 1169–1185. [Google Scholar] [CrossRef]
- Heywood, V.H. Popular Encyclopedia of Plants; Vernon, H., Chant, S.R., Eds.; Cambridge University Press: Cambridge, UK, 1982; ISBN 978-0-521-24611-8. [Google Scholar]
- Mori, S.A.; Tsou, C.-H.; Wu, C.-C.; Cronholm, B.; Anderberg, A.A. Evolution of Lecythidaceae with an emphasis on the circumscription of neotropical genera: Information from combined ndhF and trnL-F sequence data. Am. J. Bot. 2007, 94, 289–301. [Google Scholar] [CrossRef]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands; New York, NY, USA, 2012; ISBN 978-90-481-8660-0. [Google Scholar]
- Al-Dhabi, N.A.; Balachandran, C.; Raj, M.K.; Duraipandiyan, V.; Muthukumar, C.; Ignacimuthu, S.; Khan, I.A.; Rajput, V.S. Antimicrobial, antimycobacterial and antibiofilm properties of Couroupita guianensis Aubl. fruit extract. BMC Complement. Altern. Med. 2012, 12, 1–8. [Google Scholar] [CrossRef]
- Geetha, M.; Saluja, A.K.; Shankar, M.B.; Mehta, R.S. Analgesic and anti-inflammatory activity of Couroupita guianensis Aubl. J. Nat. Remedies 2004, 4, 4. [Google Scholar]
- Umachigi, S.P.; Jayaveera, K.; Kumar, A.; Kumar, G. Antimicrobial, Wound Healing and Antioxidant potential of Couroupita guianensis in rats. Pharmacol. Online 2007, 3, 269–281. [Google Scholar]
- Patel, S.H.; Suthar, J.V.; Patel, R.K.; Zankharia, U.S.; Jani, V.R.; Gajjar, K.N. Antimicrobial activity investigation of Aegle marmelos, Couroupita guianesis, Manilkarahexandra, cow urine and dung. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1014–1022. [Google Scholar]
- Shivashankar, M.; Rajeshwari, S.; Nagananda, G.; Rajath, S.; Chandan, N. Comparative antioxidant and antimicrobial studies of cold and hot bark hydromethanolic extract of Couroupita guianensis Aubl. Res. Pharm. 2013, 3, 6–13. [Google Scholar]
- Manimegalai, S.; Sridharan, T.; Rameshpathy, M.; Devi Rajeswari, V. Antioxidant, phytochemical screening and antimicrobial activity of Couroupita guianensis flower extract. Der Pharm. Lett. 2014, 6, 251–256. [Google Scholar]
- Begum, R.; Rahman, M.S.; Chowdhury, A.M.S.; Hasan, C.M.; Rashid, M.A. Secondary metabolites (Triterpenes) from Couroupita guianensis. Orient. Pharm. Exp. Med. 2009, 9, 200–205. [Google Scholar] [CrossRef]
- Gousia, S.K.; Kumar, K.A.; Kumar, T.V.; Latha, J.N.L. Biological Activities and Medicinal Properties of Couroupita guianensis. Int. J. Pharm. Pharm. Sci. Res. 2013, 3, 140–143. [Google Scholar]
- Pandurangan, P.; Sahadeven, M.; Sunkar, S.; Dhana, S.K.N.M. Comparative analysis of biochemical compounds of leaf, flower and fruit of Couroupita guianensis and synthesis of silver nanoparticles. Pharmacogn. J. 2018, 10, 315–323. [Google Scholar] [CrossRef]
- Chanayath, N.; Lhieochaiphant, S.; Phutrakul, S. Pigment Extraction Techniques from the Leaves of Indigofera tinctoria Linn. and Baphicacanthus cusia Brem. and Chemical Structure Analysis of Their Major Components. Chemistry 2005, 1, 149–160. [Google Scholar]
- Simon, J.E.; Chadwick, A.F.; Craker, L.E. Herbs, An Indexed Bibliography, 1971–1980; Elsevier: Amsterdam, The Netherlands, 1984; ISBN 0-444-99626-5. [Google Scholar]
- Lin, Y.-K.; Wong, W.-R.; Chang, Y.-C.; Chang, C.-J.; Tsay, P.-K.; Chang, S.-C.; Pang, J.-H.S. The efficacy and safety of topically applied indigo naturalis ointment in patients with plaque-type psoriasis. Dermatology 2007, 214, 155–161. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, W.; Li, X.; Deng, B.; Xu, W.; Qu, J.; Zhang, G.; Zhang, C.; Sun, L.; Jiang, C.; et al. Evidence-based practice guideline of Chinese herbal medicine for Psoriasis vulgaris (Bai Bi). Eur. J. Integr. Med. 2014, 6, 135–146. [Google Scholar] [CrossRef]
- Qing-hua, L. The Chemical Constituents of Qing Dai. J. Integr. Plant Biol. 1987, 29, 67–72. [Google Scholar]
- Wu, G.Y.; Fang, F.D.; Liu, J.Z.; Chang, A.; Ho, Y.H. Studies on the mechanism of action of indirubin in the treatment of chronic granulocytic leukemia. I. Effects on nucleic acid and protein synthesis in human leukemic cells. Chin. Med. J. 1980, 60, 451–454. [Google Scholar]
- Zhang, S.X. Studies on the chemical constituents of Isatis indigotica root. Chin. Trad Herb Drugs 1983, 14, 247–248. [Google Scholar]
- Zheng, Q.T.; Lu, D.J.; Yang, S.L. Pharmacological studies of indirubin. I. Antitumor effect. Comm. Chin. Herb. Med. 1979, 10, 35–39. [Google Scholar]
- Zheng, Q.T.; Qi, S.B.; Cheng, Z.Y. Pharmacological studies of indirubin. II. Absorption, distribution and excretion of 3H-indirubin. Comm. Chin. Herb Med. 1979, 10, 19–21. [Google Scholar]
- Qi, T.; Li, H.; Li, S. Indirubin improves antioxidant and anti-inflammatory functions in lipopolysaccharide-challenged mice. Oncotarget 2017, 8, 36658–36663. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Woerdenbag, H.J. Traditional Chinese herbal medicine. Pharm. World Sci. 1995, 17, 103–112. [Google Scholar] [CrossRef]
- Hoessel, R.; Leclerc, S.; Endicott, J.A.; Nobel, M.E.; Lawrie, A.; Tunnah, P.; Leost, M.; Damiens, E.; Marie, D.; Marko, D. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat. Cell Biol. 1999, 1, 60–67. [Google Scholar] [CrossRef]
- Sareen, K.; Kohli, R.P.; Amma, M.K.; Gujral, M.L. Anticonvulsant drugs based on the neurochemistry of seizures. Indian J. Physiol. Pharmacol. 1962, 6, 87. [Google Scholar] [PubMed]
- Chocholova, L.; Kolinova, M. Effect of isatin (2, 3-dioxoindoline) on audiogenic seizures in rats and its relationship to electrographic and behavioural phenomena. Physiol. Bohemoslov. 1979, 28, 495. [Google Scholar]
- Glover, V.; Bhattacharya, S.K.; Chakrabarti, A.; Sandler, M. The psychopharmacology of isatin: A brief review. Stress Med. 1998, 14, 225–229. [Google Scholar] [CrossRef]
- Khan, M.S.I.; Kato-Noguchi, H. Assessment of allelopathic potential of Couroupita guianensis Aubl. Plant Omics 2016, 9, 115–120. [Google Scholar] [CrossRef]
- Chandra, S.; Chatterjee, P.; Dey, P.; Bhattacharya, S. Allelopathic effect of Ashwagandha against the germination and radicle growth of Cicer arietinum and Triticum aestivum. Pharmacogn. Res. 2012, 4, 166. [Google Scholar] [CrossRef]
- Appiah, K.S.; Mardani, H.K.; Omari, R.A.; Eziah, V.Y.; Ofosu-Anim, J.; Onwona-Agyeman, S.; Amoatey, C.A.; Kawada, K.; Katsura, K.; Oikawa, Y.; et al. Involvement of Carnosic Acid in the Phytotoxicity of Rosmarinus officinalis Leaves. Toxins 2018, 10, 498. [Google Scholar] [CrossRef]
- Iqbal, Z.; Furubayashi, A.; Fujii, Y. Allelopathic effect of leaf debris, leaf aqueous extract and rhizosphere soil of Ophiopogon japonicus Ker-Gawler on the growth of plants. Weed Biol. Manag. 2004, 4, 43–48. [Google Scholar] [CrossRef]
- Ismail, B.S.; Siddique, M.A.B. The Inhibitory Effect of Grasshopper’s Cyperus (Cyperus iria L.) on the Seedling Growth of Five Malaysian Rice Varieties. Trop. Life Sci. Res. 2011, 22, 81–89. [Google Scholar]
- Magiero, E.C.; Assmann, J.M.; Marchese, J.A.; Capelin, D.; Paladini, M.V.; Trezzi, M.M. Allelopathic effect of Artemisia annua L. on the germination and initial development of lettuce (Lactuca sativa L.) and wild poinsettia (Euphorbia heterophylla L.) seedlings. Rev. Bras. Plantas Med. 2009, 11, 317–324. [Google Scholar] [CrossRef]
- Rizzi, E.S.; Pereira, K.C.L.; de Araujo Abreu, C.A.; de Lima Silva, B.C.F.; Fernandes, R.M.; de Oliveira, A.K.M.; Matias, R. Allelopathic potential and phytochemistry of cambarazinho (Vochysia haenkeana (Spreng.) Mart.) leaves in the germination and development of lettuce and tomato. Biosci. J. 2016, 32, 98–107. [Google Scholar] [CrossRef]
- Qasem, J.R. The allelopathic effect of three Amaranthus spp. (pigweeds) on wheat (Triticum durum). Weed Res. 1995, 35, 41–49. [Google Scholar] [CrossRef]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef]
- Yoshimura, H.; Sawai, Y.; Tamotsu, S.; Sakai, A. 1, 8-cineole inhibits both proliferation and elongation of BY-2 cultured tobacco cells. J. Chem. Ecol. 2011, 37, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.J.; Mulla, S.A.; Revdiwala, S.B. Neonatal Sepsis: High Antibiotic Resistance of the Bacterial Pathogens in a Neonatal Intensive Care Unit of a Tertiary Care Hospital. J. Clin. Neonatol. 2012, 1, 72. [Google Scholar] [CrossRef]
- Rashid, M.A.; Alam, S.N.; Rouf, F.M.A.; Talekar, N.S. Socio-Economic Parameters of Eggplant Pest Control in Jessore District of Bangladesh; AVRDC-World Vegetable Center: Tainan, Taiwan, 2003; ISBN 978-92-9058-127-7. [Google Scholar]
- Dasgupta, S.; Meisner, C.; Huq, M. Health Effects And Pesticide Perception As Determinants Of Pesticide Use: Evidence From Bangladesh; Policy Research Working Papers; The World Bank: Washington, DC, USA, 2005. [Google Scholar]
- Mishyna, M.; Laman, N.; Prokhorov, V.; Maninang, J.S.; Fujii, Y. Identification of Octanal as Plant Growth Inhibitory Volatile Compound Released from Heracleum sosnowskyi Fruit. Nat. Prod. Commun. 2015, 10. [Google Scholar] [CrossRef]
- Hiradate, S.; Ohse, K.; Furubayashi, A.; Fujii, Y. Quantitative evaluation of allelopathic potentials in soils: Total activity approach. Weed Sci. 2010, 58, 258–264. [Google Scholar] [CrossRef]
- Golisz, A.; Lata, B.; Gawronski, S.W.; Fujii, Y. Specific and total activities of the allelochemicals identified in buckwheat. Weed Biol. Manag. 2007, 7, 164–171. [Google Scholar] [CrossRef]
- Morikawa, C.; Miyaura, R.; Kamo, T.; Hiradate, S.; Pérez, J.; Fujii, Y. Isolation of Umbelliferone as a Principal Allelocheical from The Peruvian Medicinal plant Diplostephium foliosissimum (Asteraceae). Rev. Soc. Quím. Perú. 2011, 77, 285–291. [Google Scholar]
- Fujii, Y.; Hiradate, S. The Regional Institute-A critical survey of allelochemicals in action-The importance of total activity and the weed suppression equation. In Proceedings of the 4th World Congress on Allelopathy, Wagga Wagga, NSW, Australia, 21–26 August 2005; pp. 73–76. [Google Scholar]
- Js, M.; White, R. The Organic Chemistry of Museum Objects; Buttersworth: London, UK, 1994. [Google Scholar]
- Gilbert, K.G.; Maule, H.G.; Rudolph, B.; Lewis, M.; Vandenburg, H.; Sales, E.; Tozzi, S.; Cooke, D.T. Quantitative Analysis of Indigo and Indigo Precursors in Leaves of Isatis spp. and Polygonum tinctorium. Biotechnol. Prog. 2004, 20, 1289–1292. [Google Scholar] [CrossRef]
- Honda, G.; Tosirisuk, V.; Tabata, M. Isolation of an antidermatophytic, tryptanthrin, from indigo plants, Polygonum tinctorium and Isatis tinctoria. Planta Med. 1980, 38, 275–276. [Google Scholar] [CrossRef]
- Epstein, E.; NABORS, M.W.; Stowe, B.B. Origin of indigo of woad. Nature 1967, 216, 547–549. [Google Scholar] [CrossRef]
- Begum, K.; Shammi, M.; Hasan, N.; Asaduzzaman, M.d.; Appiah, K.S.; Fujii, Y. Potential allelopathic candidates for land use and possible sustainable weed management in south asian ecosystem. Sustainability 2019, 11, 2649. [Google Scholar] [CrossRef]
- Begum, K.; Shammi, M.; Hasan, N.; Appiah, K.S.; Fujii, Y. Evaluation of Potential Volatile Allelopathic Plants from Bangladesh, with Sapindus mukorossi as a Candidate Species. Agronomy 2020, 10, 49. [Google Scholar] [CrossRef]
- Baeyer, A. Ueber die reduction aromatischer verbindungen mittelst zinkstaub. Justus Liebigs Ann. Der Chem. 1866, 140, 295–296. [Google Scholar] [CrossRef]
- Fischer, E.; Jourdan, F. Ueber die hydrazine der brenztraubensäure. Ber. Der Dtsch. Chem. Ges. 1883, 16, 2241–2245. [Google Scholar] [CrossRef]
- Erb, M.; Veyrat, N.; Robert, C.A.; Xu, H.; Frey, M.; Ton, J.; Turlings, T.C. Indole is an essential herbivore-induced volatile priming signal in maize. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Sato, Y.; Ebina, T.; Yokoyama, C.; Takahasi, S.; Mito, Y.; Tanabe, H.; Nishiguchi, N.; Nagaoka, K. Separation of high purity indole from coal tar by high pressure crystallization. Fuel 1991, 70, 565–566. [Google Scholar] [CrossRef]
- Mackie, R.I.; Stroot, P.G.; Varel, V.H. Biochemical identification and biological origin of key odor components in livestock waste. J. Anim. Sci. 1998, 76, 1331–1342. [Google Scholar] [CrossRef]
- Frey, M.; Stettner, C.; Paré, P.W.; Schmelz, E.A.; Tumlinson, J.H.; Gierl, A. An herbivore elicitor activates the gene for indole emission in maize. Proc. Natl. Acad. Sci. USA 2000, 97, 14801–14806. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, J. Indole as an intercellular signal in microbial communities. FEMS Microbiol. Rev. 2010, 34, 426–444. [Google Scholar] [CrossRef]
- Fujii, Y. Strange Name of Plants-Does Skunkvine Really Stink? Kagaku Dojin: Kyoto, Japan, 2019. [Google Scholar]
- Calabrese, E.J.; Bachmann, K.A.; Bailer, A.J.; Bolger, P.M.; Borak, J.; Cai, L.; Cedergreen, N.; Cherian, M.G.; Chiueh, C.C.; Clarkson, T.W.; et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose–response framework. Toxicol. Appl. Pharmacol. 2007, 222, 122–128. [Google Scholar] [CrossRef]
- Chen, S.; Jeffrey, C. Gynostemma Blume. Available online: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=114356 (accessed on 15 September 2019).
- Hussain, M.I.; Reigosa, M.J. Allelochemical stress inhibits growth, leaf water relations, PSII photochemistry, non-photochemical fluorescence quenching, and heat energy dissipation in three C3 perennial species. J. Exp. Bot. 2011, 62, 4533–4545. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy; Academic Press: Orlando, FL, USA, 2012; ISBN 978-0-08-092539-4. [Google Scholar]
- Bhadoria, P. Allelopathy: A Natural Way towards Weed Management. Am. J. Exp. Agric. 2011, 1, 7–20. [Google Scholar] [CrossRef]
- Waddington, J. Growth of barley, bromegrass and alfalfa in the greenhouse in soil containing rapeseed and wheat residues. Can. J. Plant Sci. 1978, 58, 241–248. [Google Scholar] [CrossRef]
- Cheema, Z.A.; Farooq, M.; Wahid, A. Allelopathy: Current Trends and Future Applications; Springer Science & Business Media: Berlin, Germany, 2012; ISBN 978-3-642-30595-5. [Google Scholar]
- Pérez-González, S. Relationship between Parental Blossom Season and Speed of Seed Germination in Peach. HortScience 1990, 25, 958–960. [Google Scholar] [CrossRef]
- Novak, N.; Novak, M.; Barić, K.; Šćepanović, M.; Ivić, D. Allelopathic potential of segetal and ruderal invasive alien plants. J. Cent. Eur. Agric. 2018, 19, 408–422. [Google Scholar] [CrossRef]
- Wu, A.-P.; Li, Z.-L.; He, F.-F.; Wang, Y.-H.; Dong, M. Screening Allelochemical-Resistant Species of the Alien Invasive Mikania micrantha for Restoration in South China. PLoS ONE 2015, 10, e132967. [Google Scholar] [CrossRef] [PubMed]
- Putnam, A.R.; Tang, C.S. The Science of Allelopathy; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Leather, G.R.; Einhelling, F.A. Bioassays in the Study of Allelopathy. In The Science of Allelopathy; John Wiley and Sons: New York, NY, USA, 1986; pp. 133–145. [Google Scholar]
- Inderjit, O.M. Bioassays for Rice Allelopathy: Some Concerns. Allelopathy in Rice; Int. Rice Res. Inst. Pres: Manila, Philippines, 1998. [Google Scholar]
- Navarez, D.C.; Olofsdotter, M. Relay Seeding Technique for Screening Allelopathic Rice (Oryza sativa). In Proceedings of the 2nd International Weed Control Congress, Copenhagen, Denmark, 25–28 June 1996; pp. 1285–1290. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).