Abstract
Gregarious desert locusts constitute very destructive agricultural pests. They aggregate and form collectively moving swarms that devastate vegetation and reduce crop production. To combat gregarious locusts, a bio-pesticide formulation that contains linseed oil as the main component was described recently. Since linseed oil is rich in fatty acids, some of which function as necromones that indicate injury or death in various insect species, we investigated the influence of linseed oil on the aggregation behaviour of sexually mature gregarious desert locusts. For this reason, we performed a series of aggregation experiments with six individuals of the same sex and brushed the wings of one individual (target individual) with linseed oil. The time the oil brushed target males spent close to any other individual was reduced in 76% of trials (average reduction of 18%), whereas the time target females spent in groups with members of the same sex did not alter. These results suggest that linseed oil may act as a bioactive agent that has the potential to disrupt swarm formation.
1. Introduction
Desert locusts (Schistocerca gregaria Forsskål, 1775) are considered to be among the most serious agricultural pests because of their polyphagous feeding behaviour, rapid reproduction rates and quick migration patterns [1,2]. Like other locust species, this species displays density-dependent phase polyphenism, which means that they can transform reversibly between two phases in response to population density: The solitary and gregarious phases. Individuals in either phase differ in terms of their morphology, physiology and behaviour [1,3]. Behavioural changes can occur quickly, appearing within just a few hours [4]. If the population density is low, locusts exist in a solitary phase and avoid each other, except when they are ready to mate. If the population density increases, even over a short time period, the behavioural transition to the gregarious phase is evoked [5,6]. This is the case, for example, after rainfalls that end long periods of drought, resulting in nymphs hatching from eggs laid in burrows in the ground [7]. As the population density increases, the locusts become social, aggregate and form marching bands on the ground (wingless hoppers); later, they form collectively moving swarms that can migrate over long distances [1]. Locust swarms can devastate entire fields and cause extensive crop damage over very short time periods. A small swarm of locusts contains thousands of individuals that spread out over several hundred square metres, but large swarms contain up to 80 million individuals per square kilometre. Since such swarms can cover a distance of 100 km per day [1], farmers regard gregarious locusts as one of the most destructive plagues on earth.
Locusts can sense swarm members using olfactory, visual and tactile cues [5,6,8]. Physical contact was found to be the most potent stimulus, causing solitary locusts to gregarise. Simpson et al. [8] discovered that touching the hind legs of others in a repetitive way induces phase transition. A patchy distribution of food plants also increases the probability of physical contact between locusts and boosts aggregation [9]. On the contrary, visual or olfactory stimulants have lesser or incompetent effects on phase transition in the desert locust [8,9], whereas the combination of them can lead to a behavioural gregarisation [10]. In addition, seeing other locusts for a longer period (24 h) can also mediate phase change, at least partially [10]. Agents that disrupt the formation of swarms have not yet been established on the market but may represent alternative measures that can be used against locust outbreaks.
The authors of this study recently developed a highly effective botanical pesticide formulation against two species of locusts that is mainly composed of linseed oil as a carrier oil and three essential oils [11]. A single spray treatment of locusts with this formulation killed all adults and nymphs of desert locusts (Schistocerca gregaria) and migratory locusts (Locusta migratoria) within 30 h [11,12]. In addition to this toxic effect, this botanical pesticide formulation may also exert a change in group formation behaviour after individuals have come in contact with linseed oil that contains 75–90% unsaturated fatty acids (50–55% linolenic acid, 15–20% oleic acid and 11–20% linoleic acid [13]). Yao et al. [14] described oleic and linoleic acid as necromones, substances that are associated with the injury and death of insects. These often evoke distinct behaviour patterns in other individuals of the same or different species. For example, eusocial and some semi-social species show necrophoric behaviour, including the removal of dead bodies from the nests (observed in bees, ants, spiders and aphids), burial (covering the dead with soil and/or other materials in ants and termites) and cannibalism (intraspecific necrophagy in ants and termites) [14,15,16]. In contrast, solitary and some sub-social species avoid dead or injured individuals (necrophobic behaviour) [14,16]. In this study, we performed aggregation experiments to test the following hypothesis: Linseed oil evokes a change in aggregation behaviour in gregarious desert locusts once one individual (“the target individual”) has come into contact with this oil. To test this hypothesis, we evaluated the time the target individual spent in close proximity to others in a group of six individuals before and after brushing its wings with linseed oil. Moreover, we tested the behavioural responses of desert locusts towards a stationary linseed oil target, dead and crushed locust bodies.
2. Materials and Methods
2.1. Insect Species and Oil
Desert locusts (Schistocerca gregaria) were purchased in the gregarious phase from a breeding stock provided by the Buchner Company in Austria. Locusts were maintained in a crowded colony at the Institute of Zoology in Graz. About 100 locusts were kept in a glass terrarium with the dimensions of 60 × 30 × 30 cm. The light:dark cycle was 12:12 h, and the average temperature in the terrarium was 28 °C at night and 35 °C during the day. The relative humidity was 45–60%. The individuals used in the behavioural experiments were of the same age (mature: about four or five weeks after their last moult).
Organic linseed oil (Linum usitatissimum, Natur-Pur brand) was purchased from Spar Österreich. It was stored in a dark bottle at 4 °C during testing.
2.2. Insects Food
The locusts’ diet consisted of organic wheat seedlings and organic wheat bran (DM-Drogeriemarkt, Karlsruhe, Germany). Pots of wheat seedlings (i.e., “cat grass”) were purchased from Zoo Muser, a local pet shop. These pots of grass were watered daily and exposed to a light:dark schedule of 12:12 h. The wheat seedlings were replaced every two days in the terrarium.
2.3. Experimental Setup
Aggregation experiments were performed inside an anechoic chamber, using an arena with the dimensions of 85 × 65 × 45 cm. The floor of the arena was covered by a piece of paper that was replaced after each trial. We placed a heater (model: PF320LCD, ewt) to maintain a constant temperature of 30 °C inside the anechoic chamber. To illuminate the arena, we use two lamps, an 8 W inspection lamp (Electronic Montage Lamp, SLV Elektronik GmbH: Löhne, Germany) mounted on the top of the arena and a standard lamp (type: 160312, lamp/Bulb: 65× LED, 8 W, SLV Elektronik GmbH: Löhne, Germany). A top-view video camera (CB-38075, GKB: Taichung, Taiwan, China) was used to record the movement of locust individuals inside the arena.
2.4. Insect Isolation
Before performing aggregation experiments, we isolated gregarious desert locusts for three days to simulate swarm disruption that has to be expected after treatment of a swarm with the linseed oil based botanical pesticide. Therefore, we caged individuals in plastic boxes with dimensions of 9 × 9 × 6 cm and placed them at a distance of 2 cm from one another. Boxes were located near to a glass terrarium that contains crowded-reared locusts. All individuals used in experiments were taken from isolation boxes, behaved normally and were able to jump. The walls of insect boxes provided air exchange and olfactory communication among the individuals (ninety-eight pores with a diameter of 1 mm). These boxes were placed on sheets of soft kitchen paper towel to absorb the vibrations generated by the locusts. Additionally, white paper tape was applied to the walls of the plastic boxes to prevent visual contact. To study the aggregation ability of locusts three days after isolation, we evaluated the time individuals came close to any other individual in our aggregation experiments (see Table 1). During the isolation period, the same amount of grass was offered to all individuals. On the third day of isolation, a small piece of reflecting tape was mounted on the pronotum of one individual to mark the target locust. This reflecting tape was fixed in place using a small drop of super glue (Loctite, Henkel Central Eastern Europe GmbH, Vienna, Austria), which is harmless for insects such as bees and locusts.
2.5. Behavioural Experiments
Three days after their isolation, the locusts were transferred to the arena to study group formation during the light phase of their day cycle. In all trials, we studied the group formation of six individuals of the same sex to prevent premating and mating behaviour. All individuals were inspected for dual sexual characteristics (gynandromorph) to prevent an influence arising from mixing different sexes. The inter-individual distance between the target individual and any other individual was observed over a time period of 30 min before linseed oil application. Then, the wings of the target locust were brushed with a thin film of linseed oil (provided at room temperature), and the locusts were monitored for another 30 min. The movements of locusts were recorded under bright conditions using the top-view video camera. This camera was connected to a frame grabber (Pixelsmart Inc.: Lewiston, NY, USA) that captured images in intervals of 5 s, resulting in 12 images per minute. In total, 26 trials were performed with 13 female groups and 13 male groups. Since tactile stimulation may affect the target locust behaviour, we also performed sham operation experiments to exclude an influence of being handled and brushed. In sham operation trials, locusts were monitored over a time period of 30 min before treatment during the light phase of their day cycle. Then, the wings of the target locust were brushed with a clean brush, and the locust group was monitored for another 30 min after treatment. In total, 24 trials were performed with 6 female groups and 8 male groups. The ambient temperature and relative humidity were measured with a hand-held device during the trial. On average, the ambient temperature was 28.7 ± 0.9 °C (mean ± SD), and the mean relative humidity was 36.3 ± 2.9% (mean ± SD).
2.6. Behavioural Responses Towards Linseed Oil and Dead Insect Bodies
Experiments were performed on mature desert locusts of both sexes to study the attraction/avoidance behaviour after exposing locusts to linseed-oil-soaked paper and dead locust bodies. Five males or females were taken from the crowded-rearing arena and were placed in a test arena with a dimension of 48 × 24 × 28 cm. Two clean pieces of plastic foil (dimension of 8 × 3.5 cm) were placed on opposite corners of the arena. The movement of locust individuals was monitored for 30 min using a USB-camera that captured images in intervals of 5 s. Then, a filter paper saturated with 0.5 mL of linseed oil (dimension of 7 × 2.5 cm) was placed on a randomly selected plastic foil of the arena and locust individuals were monitored for another 30 min. Then, the filter paper was replaced by a clean plastic foil and the locusts were given 5 min pause before the next experiment was performed with dead insect bodies. For this purpose, two freshly killed locust individuals (a male and a female) were placed on the clean plastic foil opposite the linseed oil corner and the locust individuals were monitored for another 30 min. Locusts were killed by putting them into a freezer for one hour. In total, 11 trials were performed with 5 female groups and 6 male groups.
2.7. Behavioural Responses to Crushed Male Bodies
While intact dead bodies can be an indicator of contagion in wood lice, the crushed bodies of conspecifics can be a sign of an injury caused by a predator [14]. Therefore, we exposed male locusts to the crushed bodies of conspecific males in a similar setup. Two males were killed by freezing and then their bodies were crushed. A clean piece of plastic foil was placed in a randomly selected corner of the arena and the movements of locusts were monitored for 30 min by means of a top view USB-camera. Then, the foil was replaced by another foil to expose locusts to crushed conspecific males and to monitor locust movements for another 30 min. Ten trials were performed with groups of 5 males.
2.8. Data Evaluation and Statistical Analysis
The obtained video frames were imported into ImageJ (version 1.51j8, National institutes of Health: Bethesda, MD, USA) to measure the distances between the target locust and the next individual. Frame-by-frame distance measurements allow us to quantify the tendency to form groups. We regarded two or more individuals as a group when the inter-individual distance from the target individual was shorter than or equal to its body length (see red scale bar in Figure 1, on average: 5.8 cm for male groups and 6.2 cm for female groups). Such a small distance allowed individuals to see and smell each other. Gillet defined the grouping of locusts by the distance of two body-lengths to each other [17,18]. To analyse the group formation over a period of 30 min, 360 images were imported into ImageJ, and the time the target locust spent in groups (aggregation time) was evaluated by hand. The distance between the body of the target individual (see blue circle in Figure 1) and the closest individual was measured manually after spatially calibrating the image. The first five minutes at the beginning of frame grabbing (equals to 60 image) were excluded from the evaluation to remove possible behavioural influences related to insect handling. The time spent in groups was calculated using Equation (1):

Figure 1.
Snapshot of the top view camera. Blue circle: The target individual with the reflecting tape on the thorax. The red scale bar indicates the body length of the target individual, which was used as the minimum distance to define group formation.
The term “sum of group formation” refers to the number of frames in which the inter-individual distance of the target individual from any group member was shorter than or equal to its body length. To calculate the number of minutes individuals spent in a state of aggregation, we multiplied the “sum of group formation” by 5, which refers to the inter-frame interval of 5 s, and divided the result by 60. Percentages of aggregation relative to an observation period of 25 min were calculated using Equation (2):
If the duration of group formation changed after the application of linseed oil or after sham operation by more than 10% (2.5 min), we considered this as a change in aggregation time. This threshold definition allowed us to discriminate between trials in which group formation either increased or decreased after linseed oil treatment.
To study the general tendency of the individuals to form groups after three days of isolation, the time any individual spent in groups of at least two individuals was quantified by measuring the distances between the closest parts of their bodies. We regarded two or more individuals as a group if their inter-individual distance was shorter than or equal to the average of the body lengths (on average: 5.6 cm for male groups and 6.2 cm for female groups) of all six locusts. The time any individual spent in groups was calculated according to Equation (1) (results are shown in Table 1).
To quantify the activity of the target individual before and after the application of linseed oil, the distance covered by the target individual was measured with the help of the MTrackJ plugin offered in ImageJ. The time spent in the target corner was measured by counting the frames in which any individual was within one body length to borders of the plastic foil. The time spent in the target corner was converted into minutes by using Equation (1).
The time spent by individuals in groups or in the target corner before and after the treatments was tested for statistically significant differences by performing a paired t-test. If data distribution deviated from a normal distribution, a Wilcoxon signed-rank test was performed. To test for statistically significant differences between the percentages of trials in which either an increased or decreased group formation time was observed after the application of linseed oil, we performed a z-test with Yates correction. All statistical tests were performed in Sigma Plot version 14 (Systat Software Inc.: Erkrath, Germany). The average amount of aggregation time and the proportion of aggregate formation relative to the observation period are given as the arithmetic mean ± standard deviation.
3. Results
3.1. Group Formation after Isolation
To study the general tendency of individuals to form groups after three days spent in isolation, we quantified the amount of time (see Equation (1) during which any individual maintained a distance to other individuals that was equal to or smaller than the average body length of all locusts in a trial (Table 1). Neither males nor females changed the time spent close to any other individual before and after the wings of target individuals were brushed with linseed oil (females: 21.2 ± 2.2 min before vs. 22 ± 2.7 min after treatment, p = 0.137, paired t-test, N = 13; males: 23.0 ± 2.1 min before vs. 21.8 ± 1.9 min after treatment; p = 0.112, paired t-test, N = 13).

Table 1.
Time any individual spent in groups of at least two individuals (including the target one) before and after the application of linseed oil. Males and females were tested in separate experiments. Total observation time = 25 min, Group size = 6 individuals, N = 26.
3.2. Linseed Oil Treatment of Male and Female Groups
The average amount of time target males spent in groups with members of the same sex was significantly reduced by 4.6 ± 5.3 min (18.2 ± 21.2% of the observation time, Figure 2A, p = 0.010, paired t-test, N = 13) after brushing their wings with linseed oil. In 76.7% of trials, males reduced the time spent in aggregation by more than 10%. In these trials, the average aggregation time among males significantly decreased by 6.8 ± 3.2 min (27 ± 12.6% of observation time) after oil treatment (red horizontal stripes in Figure 2B, p < 0.001, paired t-test, N = 10). In only two trials did the aggregation time among males increase by 4.9 ± 1.9 min (20 ± 7.8% of observation time, blue vertical stripes in Figure 2B), and no change was observed in one trial (black area in Figure 2B). Furthermore, the proportion of trials exhibiting a decreased aggregation time after treatment was significantly higher compared to the proportion of trials showing an increase (Figure 2B, p = 0.006, z-test, N = 13). Before the application of the linseed oil, the target males sometimes mounted other males or were mounted by other males. Interestingly, male–male mounting involving the target individual was never observed after the target locust’s wings were brushed with linseed oil. The average distance covered by the target males (activity) within 25 min did not change after linseed oil application (Table S1, p = 0.818, paired t-test, N = 13). Tracking brushed males revealed that, in 92.3% of trials, the target males were very active and moved towards other individuals in the arena, but other individuals were avoiding them.
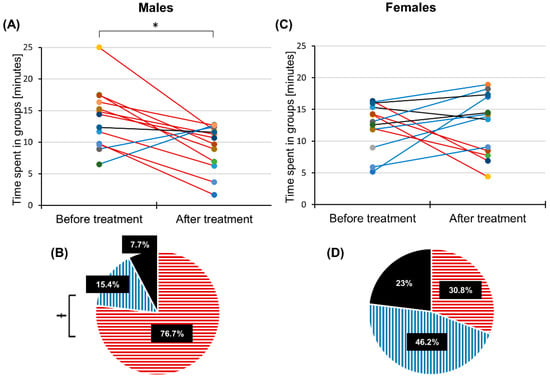
Figure 2.
Sex differences in group formation after linseed oil treatment. Time that the target males (A) and target females (C) spent in groups. Red lines: Time spent in groups decreased. Blue lines: Time spent in group increased. Black lines: Time spent in groups did not change by more than 10%. Total observation time = 25 min, N = 13 experiments. (B,D) Percentage of experiments in which aggregation time decreased (red horizontal stripes), increased (blue vertical stripes) or was left unchanged (black area) after linseed oil treatment. * indicates a p-value < 0.05 (paired t-test, N = 13). Ɨ indicates a significant difference between the percentage of experiments with a decreased and increased aggregation time related to the application of the linseed oil (p < 0.05, z-test, N = 13).
The average amount of time that the target females spent in groups with members of the same sex did not significantly differ before and after the application of the linseed oil (0.2 ± 6.2 min, Figure 2C, p = 0.962, paired t-test, N = 13). In six trials out of 13, the target females spent on average 5.0 ± 3.5 min longer in groups after oil treatment (blue vertical stripes in Figure 2D). However, the proportion of trials exhibiting an increased and decreased aggregation time did not differ significantly (Figure 2D, p = 0.687, z-test, N = 13). Egg pods laid by females were often found in the isolation boxes and one time inside the arena after the trial. The average distance covered by the target females did not change after linseed oil application (Table S1, p = 0.814, paired t-test, N = 13). Observations performed after linseed oil application revealed that, in 69.2% of trials performed with females, six females, including the target one, were active, behaved normally and moved towards each other. In the other 30.8%, the target females were less active and only walked along the borders of the arena.
3.3. Pooled Aggregation Time after Linseed Oil Treatment
In more than half of all trials performed with either males or females, the total amount of time that the target individual spent in groups of at least two individuals decreased by more than 10% after the application of linseed oil (Figure 3A,B). An increase in the amount of aggregation time, however, was found in only about one-third of the trials. Averaging over all trials and sexes, the time spent in an aggregation decreased by only 2.2 ± 6 min (8.7 ± 24.5% of the observation time), which indicates that the application of linseed oil did not significantly change the total aggregation time (p = 0.077, paired t-test, N = 26). However, in 53.8% of trials in which the target individuals spent less time in groups, the aggregation time was significantly reduced by 7 ± 2.9 min after the application of linseed oil (red striped segment in Figure 3B, p < 0.001, paired t-test, N = 14). In only 30.8% of trials, the target individuals significantly extended the average time spent in aggregation by 5 ± 3.1 min (blue-striped segment in Figure 3B, p = 0.008, Wilcoxon signed-rank test, N = 8) after treatment. In 15.4% of all trials, there was no change in the aggregation time of the target individuals of more than 10% (black segment in Figure 3B).
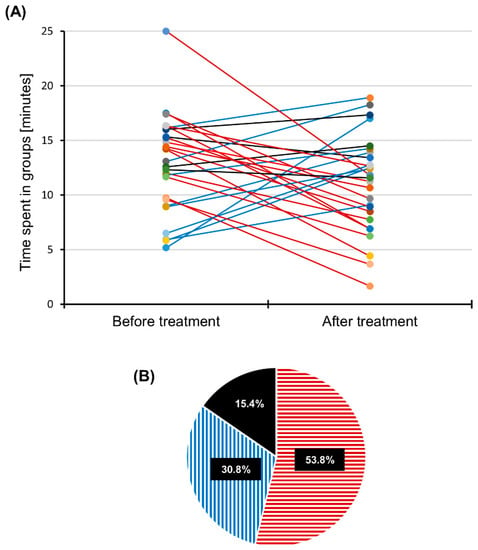
Figure 3.
Time that the target individual spent in male and female groups. (A) Aggregation time of the target individual before and after treatment with linseed oil. Red lines: Time spent in groups decreased. Blue lines: Time spent in groups increased. Black lines: Time spent in groups did not change by more than 10%. (B) Percentage of experiments in which aggregation time decreased (red horizontal stripes), increased (blue vertical stripes) or was left unchanged (black area) after linseed oil treatment. Total observation time = 25 min, N = 26 experiments.
3.4. Sham Operation
To exclude an influence caused by the handling of the target individual, we quantified the total amount of time the target locust spent in groups of at least two individuals before and after the wings were brushed with a clean brush (results are shown in Table S2). Averaging over all trials and sexes, the time spent in an aggregation decreased by only 0.8 ± 6.2 min (2.7 ± 20.8% of the observation time), which shows that the clean brush did not change the total aggregation time significantly (p = 0.538, paired t-test, N = 24). The average amount of time target males spent in groups with members of the same sex was reduced by only 1.2 ± 6.2 min (3.9 ± 20.7% of the observation time) after sham operation. The average amount of time the target females spent in groups with members of the same sex in this experiment was reduced by only 0.4 ± 6.5 min (1.4 ± 21.7% of the observation time).
3.5. Group Exposure to Linseed Oil and Dead Bodies
The average amount of time males or females spent in the target corner did not change significantly after the addition of the filter paper containing linseed oil (Table 2, males: p = 0.460, paired t-test, N = 6; females: p = 0.451, paired t-test, N = 5).

Table 2.
Time any individual spent in the target corner of the arena before and after the addition of the linseed oil. Males and females were tested in separate experiments. Total observation time = 25 min, Group size = 5 individuals, N = 11.
The average amount of time males or females spent in the target corner did not change significantly after the addition of the dead bodies (Table 3, males: p = 0.894, paired t-test, N = 6; females: p = 0.381, paired t-test, N = 5).

Table 3.
Time any individual spent in the target corner of the arena before and after the addition of the intact dead bodies. Males and females were tested in separate experiments. Total observation time = 25 min, Group size = 5 individuals, N = 11.
3.6. Responses of Males Towards Crushed Bodies
In an observation period of 25 min, the average amount of time males spent in the target corner was significantly decreased by 6.4 ± 4.9 min (Table 4, p = 0.003, paired t-test, N = 10) after the addition of the crushed male bodies.

Table 4.
Time any individual spent in the target corner of the arena before and after the addition of the crushed male bodies. Total observation time = 25 min, Group size = 5 individuals, N = 10.
4. Discussion
After isolating individuals for three days, we observed a general tendency for these individuals to form groups during the observation period (see Table 1), which is in contrast to solitary-reared locusts that are less active and freeze when they sense movement in the arena [5,10]. Furthermore, the amount of time any group member spent in groups was not influenced by brushing the wings of the target individuals with linseed oil (Table 1). Brushing the wings of the target locusts with linseed oil, however, affected the amount of time these individuals spent in proximity to other individuals in most trials (see Figure 3). In contrast, brushing the wings with a clean brush in the sham operation experiment did not change the average group formation time significantly (see Table S2). This suggests that the reduced group formation time observed in males is caused by linseed oil and less likely by physical stimulation. Brushing the wings of the target individuals with linseed oil did not change the average distance covered by the target locusts (see Table S1), which suggests that the reduced group formation time observed in males is not caused by an overall reduction in the activity of the target locust. Linseed oil is rich in unsaturated fatty acids, and some of these acids (especially oleic and linoleic acid) have been described as necromones that are released by injured and dead insects. These necromones are known to trigger distinct behaviour patterns in other individuals of the same or different species [14,15,16]. Therefore, we tested whether linseed oil could be used as an agent to control the aggregation behaviour of gregarious locusts by investigating the aggregation behaviour of members of both sexes in controlled experiments.
A reduced group formation time of males after the linseed oil application (see Figure 2A,B) may be the result of necrophobic avoidance behaviour, which may be absent in females most likely because of oviposition pheromone release. Males in this study showed a necrophobic avoidance and tended to move away from the target individual that released “the smell of death” in order to avoid possible infections that can be transmitted by swarm mates or indicates an injury from a predator attack [14]. This aversion to necromone fatty acids (especially oleic and linoleic acids) were reported in a large number of invertebrates [19]. However, locusts are also known to cannibalise other swarm mates in situations where food is scarce. We have not observed cannibalistic tendencies in our study because all individuals were fed with fresh grass during the isolation period. An interesting observation made in the course of this study was that the target individuals were no longer involved in mounting behaviours after being brushed with linseed oil. This male–male mounting behaviour is rather common in the absence of females, and our results indicate that the observed change in male behaviour was mediated by linseed oil. This result differs from that of Clancy et al. [20], who observed an increase in the frequency of male–male mounting (MMM) behaviour in desert locust males suffering from Metarhizium acridum, a fungal infection. This reduction in MMM is similar to the effect caused by “phenylacetonitrile” (PAN, also known as benzyl cyanide), which is a mature male volatile that prevents males from being mounted (homosexuality) by other males [5,21]. It is mainly released from the wings and the hind legs [22] and acts as a strong repellent for mature males to hide a female from other competing males (a courtship inhibition pheromone) [5,21]. Brushing the wings with linseed oil likely dilutes the concentration of PAN on the wings of the target male due to the high hydrophobicity of this pheromone. Therefore, we conclude that the repellent effect related to our treatment is mediated by fatty acids but less likely by PAN. However, Bashir et al. [23] proved that PAN has a solitarising effect (anti-gregarisation) on the hopper bands, and another recent study revealed that PAN serves as an antipredator defence in gregarious migratory locusts as it is converted into a hypertoxic cyanide (HCN) when they are under attack [24]. Since mature males exclusively produce aggregation pheromones that are attractive to members of both sexes [25,26,27], a reduction in male group formation caused by linseed oil may have important consequences for the formation and persistence of locust swarms.
We observed no significant difference in the average amount of time the target females spent in groups before and after the application of linseed oil (see Figure 2C,D). In almost half of all trials, there was a significant increase in the average amount of aggregation time by 5 ± 0.6 min. This result can be attributed to the attraction between female individuals mediated by the oviposition pheromone [25]. Since the target females were treated after the control recording ended, this might lead to an increase in the amount of oviposition pheromone released in the arena with time. This consequently increases the time the females spent in groups even in the presence of fatty acids necromones. This female–female attraction ensures the spatial aggregation of egg pods, which increases the survival rates and supports gregarious cohesion among members of the next generation. Furthermore, it has been also shown that ovipositing females of desert locusts aggregate responding to a pheromone produced by alive or dead individuals in all development stages [28]. In 30.8% of trials performed with females in this study, brushed target females stayed away, were less active and only walked on the borders of the arena. This may indicate that females carrying the smell of death seem to avoid being eaten by conspecifics because it is known that desert locusts show cannibalistic behaviour beginning in the fifth-instar stage, and especially adults may be regarded as an important source of proteins by conspecifics [29,30]. Bazazi et al. [31] studied collective motion and cannibalism in marching bands of desert locust nymphs and was able to show that individuals that were injured by conspecifics may suffer an increased risk of cannibalism. Since females have a higher demand for the proteins involved in egg development, they may respond to the linseed-oil-treated target individual in a different way than males.
To study possible attraction/avoidance behaviour of the desert locusts to necromones, we tested the time males and females spent in the presence of a stationary linseed oil target and dead bodies. While we found no difference in the average amount of time males and females spent in the target corner after the addition of the linseed oil or dead bodies (see Table 2 and Table 3), there was a significant reduction in this parameter in the experiment performed with crushed male bodies as males spent less time next to them (see Table 4). A response to freshly dead individuals is rather unlikely since the responses to fatty acid necromones increased over time [14,19,32]. According to our results, responses of some genera of isopods were very weak to intact dead bodies (disease) compared to crushed bodies or body extracts [14]. The latter indicates injury resulting from predation and swarm mates should avoid them.
Sex-specific responses to fatty acid necromones were also described in the cricket Acheta domesticus, where females respond to body extracts less than males, as females might be less risk averse because they seek out singing males and explore oviposition sites [19]. Another study investigated the repellent effect of various fatty acid necromones on cockroach males and females. In both sexes, the percentage of repellence was strictly dose dependent and the percentage of oleic acid repellence was more significant in cockroach males (70%) than females (43%) [33]. The results obtained from males in our study indicate that fatty acids from linseed oil may change the aggregation behaviour of desert locusts. A similar solitarising effect has been found in S. gregaria when nymphs were exposed to faeces of crowded locusts [34].
5. Conclusions
A novel botanical pesticide formulation uses linseed oil as the main component and was found to be highly effective against gregarious desert locusts as well as migratory locusts [11]. Since linseed oil contains unsaturated fatty acids that have been shown to act as necromone cues in different insect species, it was of interest to determine whether this oil affects group formation in gregarious desert locusts. Brushing the wings of single gregarious desert locust males with this oil significantly decreased the amount of aggregation time they spent in male groups in the majority of the trials (Figure 2). In contrast, most treated females either did not display alterations in aggregation time or the aggregation time increased (Figure 2). These sex differences in aggregation behaviour may be explained by the release of different pheromones produced by adult males and females in the gregarious phase. Since a reduction in the amount of aggregation time among males leads to a reduction in tactile stimuli that have been shown to be highly gregarizing [8,9], linseed oil seems to be a promising candidate agent for the control of aggregation behaviour in gregarious desert locusts, as it may even disrupt swarm formation once a certain percentage of individuals have come into contact with linseed oil in the course of botanical pesticide treatments. Therefore, fatty acids that act as necromones and are contained in linseed oil should be considered in pest management as previously suggested by Yao et al. [14]. Future studies need to reveal the origin of this behaviour by exposing males and females to different concentrations and types of necromones in controlled laboratory conditions.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4395/10/10/1458/s1, Table S1: Distance covered by the target individual before and after the application of linseed oil. Males and females were tested in separate experiments. Total observation time = 25 min, Group size = 6 individuals, N = 26. Table S2: Time the target individual spent in groups before and after sham operation. Males and females were tested in separate experiments. Total observation time = 25 min, Group size = 6 individuals, N = 14.
Author Contributions
Z.A.S.A. and M.H. conceived and designed the research. Z.A.S.A. conducted the experiments. M.H. supervised the experiments. Z.A.S.A. analysed the data and drafted the manuscript. Both authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Egyptian Ministry of Higher Education, Cultural Affairs and Mission Sector. Open Access Funding by the University of Graz.
Acknowledgments
Many thanks to Boris P. Chagnaud for his critical reading and valuable comments.
Conflicts of Interest
The authors declare no competing interests.
Data Availability
Data will be made accessible upon request.
Compliance with ethical standards
The experimental procedures were performed at the Institute of Biology (University of Graz). All experiments comply with the current Austrian and European Community laws for the ethical treatment of animals and are in line with the ASAB Guidelines for the Use of Animals in Research.
References
- Steedman, A. Locust Handbook. Overseas Development Natural Resources Institute, 2nd ed.; Overseas Development Natural Resources Institute: London, UK, 1988. [Google Scholar]
- Cressman, K. Desert Locust. In Biological and Environmental Hazards, Risks, and Disasters.; Shroder, J.F., Sivanpillai, R., Eds.; Elsevier: Amsterdam, The Netherland, 2016; pp. 87–105. [Google Scholar] [CrossRef]
- Uvarov, B. Grasshoppers and Locusts: A handbook of General Acridology; Cambridge University Press: Cambridge, UK, 1966; Volume 1. [Google Scholar]
- Gaten, E.; Huston, S.J.; Dowse, H.B.; Matheson, T. Solitary and gregarious locusts differ in circadian rhythmicity of a visual output neuron. J. Biol. Rhythm. 2012, 27, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Pener, M.P.; Simpson, S.J. Locust phase polyphenism: An update. Adv. Insect Physiol. 2009, 36, 1–286. [Google Scholar] [CrossRef]
- Ma, Z.; Guo, X.; Lei, H.; Li, T.; Hao, S.; Kang, L. Octopamine and tyramine respectively regulate attractive and repulsive behavior in locust phase changes. Sci. Rep. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Latchininsky, A.V. Locusts and remote sensing: A review. J. Appl. Remote Sens. 2013, 7, 075099. [Google Scholar] [CrossRef]
- Simpson, S.J.; Despland, E.; Hägele, B.F.; Dodgson, T. Gregarious behavior in desert locusts is evoked by touching their back legs. Proc. Natl. Acad. Sci. USA 2001, 98, 3895–3897. [Google Scholar] [CrossRef]
- Roessingh, P.; Bouaichi, A.; Simpson, S.J. Gregarious behavior in desert locusts is evoked by touching their back legs., Schistocerca gregaria. J. Insect Physiol. 1998, 44, 883–893. [Google Scholar] [CrossRef]
- Simpson, S.J.; McCaffery, A.R.; Hägele, B.F. A behavioural analysis of phase change in the desert locust. Biol. Rev. Camb. Philos. Soc. 1999, 74, 461–480. [Google Scholar] [CrossRef]
- Abdelatti, Z.A.S.; Hartbauer, M. Plant oil mixtures as a novel botanical pesticide to control gregarious locusts. J. Pest Sci. 2020, 93, 341–353. [Google Scholar] [CrossRef]
- Hartbauer, M.; Abdelatti, Z.A.S. Pesticidal compositions for pest control. P18417PCT/EP2019/056709 (WO 2019/179945 A1), 26 September 2019. [Google Scholar]
- Bayrak, A.; Kiralan, M.; Ipek, A.; Arslan, N.; Cosge, B.; Khawar, K.M. Fatty acid compositions of linseed (Linum usitatissimum L.) genotypes of different origin cultivated in Turkey. Biotechnol. Biotechnol. Equip. 2010, 24, 1836–1842. [Google Scholar] [CrossRef]
- Yao, M.; Rosenfeld, J.; Attridge, S.; Sidhu, S.; Aksenov, V.; Rollo, C.D. The ancient chemistry of avoiding risks of predation and disease. Evol. Biol. 2009, 36, 267–281. [Google Scholar] [CrossRef]
- Wilson, E.O.; Durlach, N.I.; Roth, L.M. Chemical releaser of necrophoric behavior in ants. Psyche 1958, 65, 108–114. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, X. Corpse management in social insects. Int. J. Biol. Sci. 2013, 9, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Gillett, S.D. Social determinants of aggregation behaviour in adults of the Desert locust. Anim. Behav. 1973, 21, 599–606. [Google Scholar] [CrossRef]
- Gillett, S.D. Changes in the social behaviour of the desert locust, Schistocerca gregaria, in response to the gregarizing pheromone. Anim. Behav. 1975, 23, 494–503. [Google Scholar] [CrossRef]
- Aksenov, V.; David Rollo, C. Necromone death cues and risk avoidance by the cricket Acheta domesticus: Effects of sex and duration of exposure. J. Insect Behav. 2017, 30, 259–272. [Google Scholar] [CrossRef]
- Clancy, L.M.; Cooper, A.L.; Griffith, G.W.; Santer, R.D. Increased male-male mounting behaviour in desert locusts during infection with an entomopathogenic fungus. Sci. Rep. 2017, 7, 5659. [Google Scholar] [CrossRef] [PubMed]
- Seidelmann, K.; Ferenz, H.J. Courtship inhibition pheromone in desert locusts, Schistocerca gregaria. J. Insect Physiol. 2002, 48, 991–996. [Google Scholar] [CrossRef]
- Seidelmann, K.; Weinert, H.; Ferenz, H.-J. Wings and legs are production sites for the desert locust courtship-inhibition pheromone, phenylacetonitrile. J. Insect Physiol. 2003, 49, 1125–1133. [Google Scholar] [CrossRef]
- Bashir, M.O.; Hassanali, A. Novel cross-stage solitarising effect of gregarious-phase adult desert locust (Schistocerca gregaria (Forskal)) pheromone on hoppers. J. Insect Physiol. 2010, 56, 640–645. [Google Scholar] [CrossRef]
- Wei, J.; Shao, W.; Cao, M.; Ge, J.; Yang, P.; Chen, L.; Wang, X.; Kang, L. Phenylacetonitrile in locusts facilitates an antipredator defense by acting as an olfactory aposematic signal and cyanide precursor. Sci. Adv. 2019, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ferenz, H.J.; Seidelmann, K. Pheromones in relation to aggregation and reproduction in desert locusts. The Royal Entomological Society. Physiol. Entomol. 2003, 28, 11–18. [Google Scholar] [CrossRef]
- Obeng-Ofori, D.; Njagi, P.G.N.; Torto, B.; Hassanali, A.; Amiani, H. Sex differentiation studies relating to releaser aggregation pheromones of the desert locust, Schistocerca gregaria. Entomol. Exp. Appl. 1994, 73, 85–91. [Google Scholar] [CrossRef]
- Obeng-Ofori, D.; Njagi, P.G.N.; Hassanali, A. Aggregation pheromone complex of the desert locust, Schistocerca gregaria (Forskal) (Orthoptera: Acrididae): Current Status. J. Ghana Sci. Ass. 1998, 1, 69–83. [Google Scholar] [CrossRef]
- Norris, M.J.; Richards, O.W. Aggregation response in ovipositing females of the desert locust, with special reference to the chemical factor. J. Insect Physiol. 1970, 16, 1493–1515. [Google Scholar] [CrossRef]
- Whitman, D.W.; Blum, S.M.; Slansky, F., Jr. Carnivory in phytophagous insects. In Functional Dynamics of Phytophagous Insects; Ananthakrisnan, T.N., Ed.; Oxford & IBH: New Delhi, India, 1994; pp. 161–205. [Google Scholar]
- Niassy, A. Interactions between Schistocerca gregaria (Forskal) and Locusta migratoria migratorioides (Reich & Farmaire) in Relation to Phase Polymorphism. Ph.D. Thesis, University of Ghana Legon, Accra, Ghana, 1996. [Google Scholar]
- Bazazi, S.; Buhl, J.; Hale, J.J.; Anstey, M.L.; Sword, G.A.; Simpson, S.J.; Couzin, I.D. Collective Motion and Cannibalism in Locust Migratory Bands. Curr. Biol. 2008, 18, 735–739. [Google Scholar] [CrossRef]
- Akino, T.; Yamaoka, R. Origin of oleic acid corpse recognition signal in the ant, Formica japonica Motschlsky (Hymenoptera: Formicidae). Jpn. J. Appl. Entomol. Zool. 1996, 40, 265–271. [Google Scholar] [CrossRef][Green Version]
- Rollo, C.D.; Czvzewska, E.; Borden, J.H. Fatty acid necromones for cockroaches. Naturwissenschaften 1994, 81, 409–410. [Google Scholar] [CrossRef]
- Gillett, S.D.; Phillips, M. Faeces as a source of a locust gregarisation stimulus. Effects on social gregarisation and on cuticular colour of nymphs of the desert locust, Schistocerca gregaria (Forsk.). Acrida 1977, 6, 279–286. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).