Abstract
A series of new fluorenylamido-ligated zirconium complexes bearing an electron-donating adamantyl group on the amido ligand were synthesized and characterized by elemental analysis, 1H NMR, and single crystal X-ray analysis. The coordination mode of the fluorenyl ligand to the zirconium metal was η3 manner, and all the complexes were Cs-symmetric in solution. The complexes showed moderate activity (1.0 × 105 g-polymer mol-Zr−1·h−1), even at a low Al/Zr ratio of 50. The increase of propylene pressure improved the activity by one order of magnitude (up to 1.0 × 106 g-polymer mol-Ti−1·h−1). All catalyst systems gave syndiotactic polypropylene, where the complex containing the 3,6-di-t-butyl fluorenyl ligand was more effective for the enhancement of the syndiospecificity. The increase of propylene pressure also improved the syndiospecificity with the syndiotactic pentad of 0.96 and the melting point of 159 °C.
1. Introduction
Developments of single-site catalysts based on group-4 metallocenes demonstrated a well-defined mechanism for the relationship between the symmetry of the complex and the stereospecificity, in which a little change of the ligand dramatically influenced the polymerization performances, such as the activity, stereospecificity, and molecular weight [1,2,3]. Ewen et al. first reported the preparation of syndiotactic polypropylene (syn-PP) by using Cs-symmetric metallocene in 1988 [4]. Since this report, much effort has been made towards achieving the synthesis of syn-PP with single-site catalysts, including doubly bridged metallocenes [5], constrained geometry catalysts (CGCs) [6], and nonmetallocene catalysts [7,8,9,10,11,12]. Among these catalysts, group-4 CGCs have attracted much attention for their capabilities of improving copolymerization ability [13,14,15,16,17,18,19,20,21,22], stereospecificity [23,24,25,26,27], and living polymerization characteristics [28,29]. Many attempts have been made to improve the catalytic performance of CGC catalysts by changing the electronic and steric properties of the ligand.
Razavi et al. reported that the introduction of t-butyl substituent on the fluorenyl ligand of [Me2Si(t-Bu-N)(di-t-Bu-Flu)ZrCl2] (Zr(a–c), Figure 1) improved both the activity and syn-specificity with syn-pentad (rrrr) of 0.87 [23,24]. Miller et al. demonstrated that sterically expanded zirconium complex (Zr(d), Figure 1) combined with methylaluminoxane (MAO) was strikingly active to give syn-PP with unsurpassed syn-specificity (rrrr > 0.99) and melting temperature (Tm up to 165 °C) [25]. The results indicated that alkyl substituents on the fluorenyl ligand of zirconium complexes play an important role in catalytic activity and syn-specificity. However, the electronic effect of the amido ligand was not investigated.
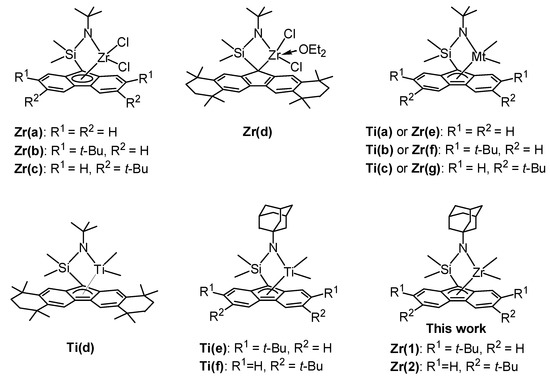
Figure 1.
Structures of ansa-(fluorenyl)(amido)-ligated complexes.
We previously synthesized the corresponding dimethyltitanium complexes (Ti(a–c), Figure 1) and found that the introduction of t-butyl group on the 3,6-position of the fluorenyl ligand improved both the activity and syn-specificity in the living polymerization of propylene [30]. However, sterically expanded titanium complex (Ti(d), Figure 1) showed low syn-specificity, which differed from that of the corresponding zirconium complex Zr(d) [31]. On the other hand, dimethylzirconium complexes [Me2Si(t-Bu-N)(di-t-Bu-Flu)ZrMe2] (Zr(e–g), Figure 1) showed very low activity for propylene polymerization [32]. Recently, we reported that the introduction of an electron-donating adamantyl substituent on the amido ligand of dimethyltitanium complexes (Ti(e–f), Figure 1) exhibited remarkably high activity with an Al/Ti ratio of 20 without changing syn-specificity or livingness [33,34]. In this paper, we synthesized dimethylzirconium complexes (Zr(1) and Zr(2), Figure 1) by using the same ligand to investigate the substitute effects of the adamantyl group on the amido ligand of fluorenylamido-ligated zirconium complexes in propylene polymerization.
2. Experimental Section
2.1. Materials
All operations were carried out under N2 by using standard Schlenk techniques, and all solvents were purified by a PS-MD-5 solvent purification system (Innovative Technology (China) Ltd., Hong Kong, China). A research grade propylene was purified by being passed through a dehydration column of ZHD-20 and a deoxidation column of ZHD-20A before use. Modified methylaluminoxane (MMAO) was donated by Tosoh-Finechem Co. (Shunan, Japan). The ligands and zirconium complexes were prepared according to the procedure reported in the literature [32,33].
2.2. Synthesis of Complexes
2.2.1. Synthesis of [(1-Adamantyl)NSiMe2(2,7-di-t-BuFlu)]ZrMe2 (Zr(1))
MeLi (1.6 M in ether 10.5 mL, 16.8 mmol) was added dropwise at −20 °C to a solution of ligand (2,7-di-t-BuFlu)SiMe2(1-Adamantyl) (1.94 g, 4.0 mmol) in 60 mL of diethylether. The resultant orange solution was stirred at room temperature for 4 h. To a solution of ZrCl4 (0.93 g, 4.0 mmol) in 30 mL pentane, the diethylether solution of the lithium salt was added, which gave a yellow suspension. After stirring for 12 h, the solvent was removed and the residue was extracted with hexane. Then the hexane solution was concentrated and cooled at −30 °C to yield Zr(1) as yellow crystals (0.72 g, 1.24 mmol, 31% yield).
1H NMR (CDCl3) (Figure S1): δ = 8.00 (d, 2H, Flu); 7.73 (s, 2H, Flu); 7.45 (dd, 2H, Flu); 2.07 (s, 3H, Ad); 1.80(d, 6H, Ad); 1.64 (d, 6H, Ad); 1.41 (s, 18H, t-Bu-Flu); 0.84 (s, 6H, SiCH3); −0.11 (s, 6H, ZrCH3). 13C NMR (CDCl3) (Figure S2): 150.8 (Flu); 136.0 (Flu); 122.9 (Flu); 122.4 (Flu); 122.1 (Flu); 120.5 (Flu); 56.1 (Flu); 48.0 (Zr-(CH3)2); 39.6 (Ad); 36.5 (Ad); 35.4 (Flu-(C(CH3)3)2); 31.8 (Ad); 31.5 (Flu-(C(CH3)3)2); 30.3 (Ad); 7.2 (Si-(CH3)2). Elemental analysis for C35H51NSiZr (calc/found, %): C, 69.47/69.26; H, 8.50/8.41; N, 2.31/2.26.
2.2.2. Synthesis of [(1-Adamantyl)NSiMe2(3,6-di-t-BuFlu)]ZrMe2 (Zr(2))
Complex Zr(2) was synthesized in a method similar to that for Zr(1), and yellow crystals were obtained in 33% yield.
1H NMR (CDCl3) (Figure S3): δ = 8.06 (s, 2H, Flu); 7.70 (d, 2H, Flu); 7.46 (dd, 2H, Flu); 2.06 (s, 3H, Ad); 1.82 (d, 6H, Ad); 1.64 (d, 6H, Ad); 1.46 (s, 18H, t-Bu-Flu); 0.82 (s, 6H, SiCH3); −1.05 (s, 6H, ZrCH3). 13C NMR (CDCl3) (Figure S4): 147.1 (Flu); 134.0 (Flu); 127.2 (Flu); 124.7 (Flu); 124.4 (Flu); 118.3 (Flu); 56.1 (Flu); 48.1 (Zr-(CH3)2); 39.6 (Ad); 36.5 (Ad); 35.1 (Flu-(C(CH3)3)2); 32.0 (Ad); 31.9 (Flu-(C(CH3)3)2); 30.3(Ad); 7.0 (Si-(CH3)2). Elemental analysis for C35H51NSiZr (calc/found, %): C, 69.47/69.38; H, 8.50/8.46; N, 2.31/2.29.
2.3. Polymerization Procedure
Atmospheric polymerization of propylene was performed in a 100-mL glass reactor equipped with a magnetic stirrer and carried out according to the semi-batch method. At first, the reactor was charged with prescribed amounts of MMAO/2,6-di-tert-butyl-4-methyl phenol (BHT) or dired MMAO (dMMAO) and solvent (heptane). After the solution of the cocatalyst was saturated with gaseous propylene under atmospheric pressure, polymerization was started by the addition of 1 mL solution of the zirconium complex in heptane, and the consumption rate of propylene was monitored by a mass flow meter.
High pressure polymerization of propylene was performed in a 200-mL Quick-Open Micro Autoclaves/Pressure Vessel purchased from Anhui Kemi Machinery Technology Co., Ltd. (Hefei, China) Before polymerization, the reactor was cleaned and evacuated at 110 °C for 1 h. Certain amounts of the MMAO/BHT, heptane were added into the reactor under a nitrogen atmosphere, and the mixture was stirred continuously. When the temperature was established, the catalyst solution of heptane was added into the reactor. The reactor was then pressurized with propylene. The polymerization was conducted for a certain time, and terminated with acidic alcohol. The polymers obtained were washed by alcohol to remove MMAO and ligand residue, and dried under vacuum at 80 °C for 6 h until a constant weight was reached.
2.4. Analytical Procedure
The single crystals were mounted under a nitrogen atmosphere at a low temperature, and data collection was made on a Bruker APEX2 diffractometer (Bruker, Karlsruhe, Germany) using graphite monochromated with Mo Ka radiation (=0.71073 Å). The SMART program package (University of Göttingen, Göttingen, Germany) was used to determine the unit cell parameters. The absorption correction was applied using the SADABS program (University of Göttingen, Göttingen, Germany) [35]. All structures were solved by direct methods and refined on F2 by full-matrix least-squares techniques with anisotropic thermal parameters for non-hydrogen atoms. Hydrogen atoms were placed at calculated positions and were included in the structure calculation. Calculations were carried out using the SHELXS-97, SHELXL-2014, or Olex2 program (Bruker AXS Inc., Madison, WI, USA) [36,37,38,39,40,41]. Crystallographic data are summarized in Table 1.

Table 1.
Crystallographic data and parameters for Zr(1) and Zr(2).
Molecular weights and molecular weight distributions of polymers were measured by a polymer laboratory PL GPC-220 chromatograph (Agilen, Santa Clara, CA, USA) equipped with one PL1110-1120 column and two PL MIXED-B 7.5 × 300 mm columns at 150 °C using 1,2,4-trichlorobenzene as a solvent. The parameters for universal calibration were K = 7.36 × 10−5, α = 0.75 for polystyrene standard and K = 1.03 × 10−4, α = 0.78 for PP samples. Differential scanning calorimeter (DSC) analyses were performed on a TA Q2000 instrument (Waters, New Castle, DE, USA) and the DSC curves of the samples were recorded under a nitrogen atmosphere at a heating rate of 10 °C/min from 40 to 200 °C. The 1H NMR spectra of complexes were recorded and the 13C NMR spectra of PPs were measured on a Bruker Asend™ 600 spectrometer (Bruker, Karlsruhe, Germany). The chemical shifts of the 1H NMR spectra were referenced to the residual proton resonance of chloroform-d (δ: 7.26), and the 13C NMR spectra of PPs were recorded at 110 °C and referenced to the resonance of 1,1,2,2-tetrachloroethane-d2 (δ: 74.47).
3. Results and Discussion
3.1. Molecular Structure of Complexes
The zirconium complexes were synthesized by a one-pot reaction of the corresponding ligand with 4 equivalent of methyl lithium and 1 equiv of ZrCl4. 1H NMR spectrum of the methyl groups bonded to Zr and Si atoms in both zirconium complexes indicated that the complexes exhibited a Cs-symmetric nature in solution, respectively. The molecular structures of Zr(1) and Zr(2) were characterized by single crystal X-ray analysis. The structures are shown in Figure 2, and the selected bond lengths and angles of complexes are shown in Table 2. The lengths between the zirconium metal and five-member carbons of fluorenyl ligand in Zr(1) and Zr(2) are very close to those previously reported for t-butyl amido complex (Zr(f)), in which the fluorenyl ligand was coordinated to the zirconium in an η3 manner. We applied Tolman cone angles of the amino ligand (θ value in Table 1) to evaluate the steric effect of the amido ligand [42]. The θ values of Zr(1) and Zr(2) were 71.06 and 68.80°, respectively, which were close to that of the previously reported Zr(f) (70.12°). These results indicated that t-butyl and 1-admantyl groups possess a similar steric environment around the cationic zirconium metal, thus maintaining the hapticity of the fluorenyl ligand. We therefore can investigate the true electronic effect of the adamantyl substituent on the amido ligand with these fluorenylamido-ligated zirconium complexes for propylene polymerization.
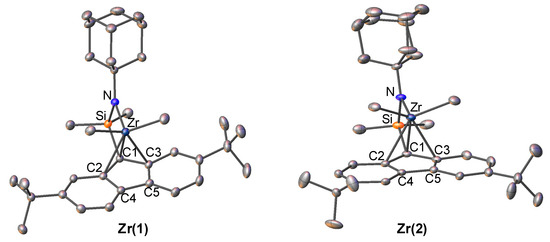
Figure 2.
Structure of fluorenylamidotitanium complexes Zr(1) and Zr(2). Hydrogen atoms are omitted for clarity. Atoms are drawn at the 40% probability level.

Table 2.
Selected bond lengths (Å) and bond angles (degrees) for related complexes.
3.2. Propylene Polymerization
Propylene polymerizations were performed by Zr(1) and Zr(2) activated with trialkylaluminum-free dried MMAO (dMMAO) under atmospheric pressure of propylene in heptane at 0 and 20 °C, and the results are summarized in Table 3. For comparison, the same polymerization conditions as those previously reported for propylene polymerization using Zr(f) and Zr(g) were employed [32]. The complexes Zr(f) and Zr(g) conducted propylene polymerization at 20 °C, although they did not show any activity at 0 °C. On the other hand, Zr(1) and Zr(2) carried out propylene polymerization with moderate activity of ~1.2 × 105 g-polymer mol-Zr−1·h−1 to produce low molecular weight PP, even at 0 °C. The activities of Zr(1) and Zr(2) were higher than those of Zr(f) and Zr(g) at 20 °C. The results testified that the electronic effect of the adamantyl group on the amido ligand plays an important role in this catalyst system rather than having a steric effect, since Zr(1), Zr(2), and Zr(g) possess a similar steric environment around the amido ligand. The high performance of Zr(1) and Zr(2) can be ascribed to the decreased electrophilicity of the zirconium cation caused by the electron-donating adamantyl group [43], which enhances the separation of the counter anion.

Table 3.
Results of propylene polymerization with zirconium complexes a.
The use of the modification of trialkylaluminum in MMAO with 2,6-di-tert-butyl-4-methyl phenol (BHT) as the cocatalyst [44,45] resulted in levels of activity approximately twice as high (up to 2.1 × 105 g-polymer mol-Zr−1·h−1). The same phenomenon was also observed in propylene polymerization with fluorenylamido-ligated dimethyl titanium catalysts (Ti(e–f)), where the presence of iBu2Al(OC6H2tBu2Me) derived from the reaction of iBu3Al and BHT promoted the efficient separation of the active ion pair to improve the activity [33].
The high cost of single-site catalysts, owing to the requirement of a very high Al/metal ratio to achieve high activity, is a serious limitation for industrial applications. The effect of the Al/Zr ratio in these catalysts was thus investigated (entries 9–12). Although the activity decreased according to the decrease of the Al/Zr ratio from 400 to 100, complex Zr(1) still showed moderate activity of 1.0 × 105 g-polymer mol-Zr−1·h−1 even with an Al/Zr ratio of 50 (entry 12), the value of which was comparable to those of Zr(1) and Zr(2) activated by dMMAO with Al/Ti = 400.
The increase of propylene pressure from 1.0 to 8.0 atm resulted in the increase of the activity by one order of magnitude (up to ~1.0 × 106 g-polymer mol-Ti−1 h−1, entries 13 and 14). We previously reported that the propagation rate of propylene polymerization with Ti(a–c)–dMMAO at 0 °C was increased linearly against the propylene pressure [46].
The melting temperatures of the PPs obtained are also shown in Table 2. All of the catalyst systems gave crystalline polymers with high Tm values. The PP obtained with Zr(2) showed a higher Tm value than that obtained with Zr(1) in the same polymerization conditions, and the Tm value slightly increased with the increase of the propylene pressure in each catalyst system. A higher Tm value should be ascribed to the higher syn-tacticity of PP.
The steric pentad distributions calculated by the 13C NMR spectra (Figures S5 and S6) of the methyl region of PPs are shown in Table 4. The results indicated that the PPs obtained were syndiotactic with high rrrr value, and the PP obtained by Zr(2) showed a higher rrrr value of 0.96. The syn-specific polymerization was conducted via an enantiomorphic site-controlled mechanism with a Cs-symmetric catalyst. In this system, two types of stereodefects are present: one is rmrr arising from the chain migration without monomer insertion, and the other is rmmr arising from the monomer mis-insertion [4]. Both rmrr and rmmr values were decreased in the following order: Zr(1) (0.026) > Zr(2) (0.003) and Zr(1) (0.021) > Zr(2) (0.007). These results indicated that the t-butyl groups at the 3,6-position of the fluorenyl ligand effectively improve the chain migration and enantioselectivity of the propylene monomer. This result is in agreement with that of propylene polymerization with Ti(b–c)–dMMAO, where the 3,6-position was more effective than the 2,7-position in improving syn-specificity [30].

Table 4.
Steric pentad distributions for samples in Table 3 (entries 13 and 14) a.
4. Conclusions
The substituent effects of an electron-donating adamantyl group on the amido ligand of fluorenylamido-ligated zirconium catalysts were investigated. Complexes Zr(1) and Zr(2) showed moderate activity of ~1.0 × 105 g-polymer mol-Zr−1·h−1 with a low Al/Ti ratio of 50. Complex Zr(2) containing 3,6-di-t-butyl fluorenyl ligand and the increase of propylene pressure were effective for the improvement of polymerization activity (up to 1.0 × 106 g-polymer mol-Zr−1·h−1) and syn-specificity to produce a highly syn-tactic PP with an rrrr value of 0.96 and a melting point of 159 °C. These results are in good agreement with the substituent effects of the adamantyl group on the amido ligand of the fluorenylamido-ligated titanium complex.
Supplementary Materials
The following are available online at www.mdpi.com/2073-4360/9/11/632/s1, Figure S1: 1H NMR spectrum of complex Zr(1), Figure S2: 13C NMR spectrum of complex Zr(1), Figure S3: 1H NMR spectrum of complex Zr(2), Figure S4: 13C NMR spectrum of complex Zr(2), Figure S5: 13C HMR spectrum of the methyl region of polypropylene obtained with Zr(1) (entry 13, Table 3), Figure S6: 13C HMR spectrum of the methyl region of polypropylene obtained with Zr(2) (entry 14, Table 3).
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant No. 21174026), the program for New Century Excellent Talents in University, the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, “Shu Guang” project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation, and the Fundamental Research Funds for the Central Universities. The authors thank Tosoh-Finechem Co. for generously donating the MMAO.
Author Contributions
All authors tried their best to contribute effectively to perform and analyze this experimental work. They all participated in the writing of the present manuscript. Yanjie Sun performed the experimental work. Shuhui Li participated in the analysis of structural data. The setup of the experimental protocol as well as the interpretation of the obtained results were performed under the supervision of Zhengguo Cai and Takeshi Shiono.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brintzinger, H.H.; Fischer, D.; Mülhaupt, R.; Rieger, B.; Waymouth, R.M. Stereospecific olefin polymerization with chiral metallocene catalysts. Angew. Chem. Int. Ed. 1995, 34, 1143–1170. [Google Scholar] [CrossRef]
- Resconi, L.; Cavallo, L.; Fait, A.; Piemontesi, F. Selectivity in propene polymerization with metallocene catalysts. Chem. Rev. 2000, 100, 1253–1346. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, W. Olefin polymerization catalyzed by metallocenes. Adv. Catal. 2001, 46, 89–159. [Google Scholar]
- Ewen, J.A.; Jones, R.L.; Razavi, A.; Ferrara, J.D. Syndiospecific propylene polymerizations with group 4 metallocenes. J. Am. Chem. Soc. 1988, 110, 6255–6256. [Google Scholar] [CrossRef] [PubMed]
- Veghini, D.; Henling, L.M.; Burkhardt, T.J.; Bercaw, J.E. Mechanisms of stereocontrol for doubly silylene-bridged Cs- and C1-symmetric zirconocene catalysts for propylene polymerization. Synthesis and molecular structure of Li2[(1,2-Me2Si)2{C5H2–4-(1R,2S,5R-menthyl)}{C5H-3,5-(CHMe2)2)}]·3THF and [(1,2-Me2Si)2{η5-C5H2–4-(1R,2S,5R-menthyl)}{η5-C5H-3,5-(CHMe2)2}]ZrCl2. J. Am. Chem. Soc. 1999, 121, 564–573. [Google Scholar]
- McKnight, A.L.; Waymouth, R.M. Group 4 ansa-cyclopentadienyl-amido catalysts for olefin polymerization. Chem. Rev. 1998, 98, 2587–2598. [Google Scholar] [CrossRef] [PubMed]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-metal catalysts for ethylene homo- and copolymerization. Chem. Rev. 2000, 100, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Gibson, V.C.; Spitzmesser, S.K. Advances in non-metallocene olefin polymerization catalysis. Chem. Rev. 2003, 103, 283–315. [Google Scholar] [CrossRef] [PubMed]
- Makio, H.; Terao, H.; Iwashita, A.; Fujita, T. FI catalysts for olefin polymerization—A comprehensive treatment. Chem. Rev. 2011, 111, 2363–2449. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Pan, L.; Song, D.; Li, Y. Neutral nickel catalysts for olefin homo- and copolymerization: Relationships between catalyst structures and catalytic properties. Chem. Rev. 2015, 115, 12091–12137. [Google Scholar] [CrossRef] [PubMed]
- Coates, G.W. Precise control of polyolefin stereochemistry using single-site metal catalysts. Chem. Rev. 2000, 100, 1223–1252. [Google Scholar] [CrossRef] [PubMed]
- Coates, G.W.; Hustad, P.D.; Reinartz, S. Catalysts for the living insertion polymerization of alkenes: Access to new polyolefin architectures using Ziegler–Natta chemistry. Angew. Chem. Int. Ed. 2002, 41, 2236–2257. [Google Scholar] [CrossRef]
- Xu, G. Copolymerization of ethylene with styrene catalyzed by the [η1:η5-tert-butyl(dimethylfluorenylsilyl)amido]methyltitanium “Cation”. Macromolecules 1998, 31, 2395–2402. [Google Scholar] [CrossRef]
- Irwin, L.J.; Reibenspies, J.H.; Miller, S.A. A sterically expanded “constrained geometry catalyst” for highly active olefin polymerization and copolymerization: An unyielding comonomer effect. J. Am. Chem. Soc. 2004, 126, 16716–16717. [Google Scholar] [CrossRef] [PubMed]
- Schwerdtfeger, E.D.; Miller, S.A. Intrinsic branching effects in syndiotactic copolymers of propylene and higher α-olefins. Macromolecules 2007, 40, 5662–5668. [Google Scholar] [CrossRef]
- Schwerdtfeger, E.D.; Price, C.J.; Chai, J.; Miller, S.A. Tandem catalyst system for linear low-density polyethylene with short and long branching. Macromolecules 2010, 43, 4838–4842. [Google Scholar] [CrossRef]
- Chai, J.; Abboud, K.A.; Miller, S.A. Sterically expanded CGC catalysts: Substituent effects on ethylene and α-olefin polymerization. Dalton Trans. 2013, 42, 9139–9147. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.Y.; Hong, S.D.; Jung, M.W.; Lee, H.; Park, Y.W. Norbornene copolymerization with α-olefins using methylene-bridged ansa-zirconocene. Polyhedron 2005, 24, 1269–1273. [Google Scholar] [CrossRef]
- Kirillov, E.; Razaci, A.; Carpentier, J.F. Syndiotactic-enriched propylene–styrene copolymers using fluorenyl-based half-titanocene catalysts. J. Mol. Catal. A 2006, 249, 230–235. [Google Scholar] [CrossRef]
- Na, S.J.; Wu, C.J.; Yoo, J.; Kim, B.E.; Lee, B.Y. Copolymerization of 5,6-dihydrodicyclopentadiene and ethylene. Macromolecules 2008, 41, 4055–4057. [Google Scholar] [CrossRef]
- Yu, S.T.; Na, S.J.; Lim, T.S.; Lee, B.Y. Preparation of a bulky cycloolefin/ethylene copolymer and its tensile properties. Macromolecules 2010, 43, 725–730. [Google Scholar] [CrossRef]
- Nakayama, Y.; Sogo, Y.; Cai, Z.; Shiono, T. Copolymerization of ethylene with 1,1-disubstituted olefins catalyzed by ansa-(fluorenyl)(cyclododecylamido)dimethyltitanium complexes. J. Polym. Sci. Part A 2013, 51, 1223–1229. [Google Scholar] [CrossRef]
- Razavi, A.; Thewalt, U. Preparation and crystal structures of the complexes (η5-C5H3TMS–CMe2–η5-C13H8)MCl2 and [3,6-ditButC13H6–SiMe2–NtBu]MCl2 (M = Hf, Zr or Ti): Mechanistic aspects of the catalytic formation of a isotactic–syndiotactic stereoblock-type polypropylene. J. Organomet. Chem. 2001, 621, 267–276. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R.; Cutillo, F.; Talarico, G.; Razavi, A. Syndiotactic poly(propylene) from [Me2Si(3,6-di-tert-butyl-9-fluorenyl)(N-tert-butyl)]TiCl2–based catalysts: Chain-end or enantiotopic-sites stereocontrol? Macromol. Chem. Phys. 2003, 204, 1269–1274. [Google Scholar] [CrossRef]
- Irwin, L.J.; Miller, S.A. Unprecedented syndioselectivity and syndiotactic polyolefin melting temperature: Polypropylene and poly(4-methyl-1-pentene) from a highly active, sterically expanded η1-Fluorenyl–η1-amido zirconium complex. J. Am. Chem. Soc. 2005, 127, 9972–9973. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Su, H.; Nakayama, Y.; Shiono, T.; Akita, M. Synthesis of C1 symmetrical ansa-cyclopentadienylamidotitanium complexes and their application for living polymerization of propylene. J. Organomet. Chem. 2014, 770, 136–141. [Google Scholar] [CrossRef]
- Tanaka, R.; Chie, Y.; Cai, Z.; Nakayama, Y.; Shinon, T. Structure-stereospecificity relationships of propylene polymerization using substituted ansa-silylene(fluorenyl)(amido) titanium complexes. J. Organomet. Chem. 2016, 804, 95–100. [Google Scholar] [CrossRef]
- Shiono, T. Living polymerization of olefins with ansa-dimethylsilylene(fluorenyl)(amido) dimethyltitanium-based catalysts. Polym. J. 2011, 43, 331–351. [Google Scholar] [CrossRef]
- Cai, Z.; Su, H.; Shiono, T. Precise synthesis of olefin block copolymers using a syndiospecific living polymerization system. Chin. J. Polym. Sci. 2013, 31, 541–549. [Google Scholar] [CrossRef]
- Cai, Z.; Ikeda, T.; Akita, M.; Shiono, T. Substituent effects of tert-butyl groups on fluorenyl ligand in syndiospecific living polymerization of propylene with ansa-fluorenylamidodimethyltitanium complex. Macromolecules 2005, 38, 8135–8139. [Google Scholar] [CrossRef]
- Shiono, T.; Harada, R.; Cai, Z.; Nakayama, Y. A highly active catalyst composed of ansa-fluorenylamidodimethyltitanium derivative for propene polymerization. Top. Catal. 2009, 52, 675–680. [Google Scholar] [CrossRef]
- Cai, Z.; Nakayama, Y.; Shiono, T. Substituent effects of tert-butyl groups on fluorenyl ligand of [t-BuNSiMe2Flu] ZrMe2. Chin. J. Polym. Sci. 2008, 31, 575–578. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, B.; Shiono, T.; Cai, Z. Highly active ansa-(Fluorenyl)(amido)titanium-based catalysts with low load of methylaluminoxane for syndiotactic-specific living polymerization of propylene. Organometallics 2017, 36, 3009–3012. [Google Scholar] [CrossRef]
- Song, X.; Ma, Q.; Yuan, H.; Cai, Z. Synthesis of hydroxy-functionalized ultrahigh molecular weight polyethylene using fluorenylamidotitanium complex. Chin. J. Polym. Sci. 2017. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS: An Empirical Absorption Correction Program for Area Detector Data; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, C71, 3–8. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- SAINT+, version 6.22a; Bruker AXS Inc.: Madison, WI, USA, 2002.
- SAINT+, version v7.68A; Bruker AXS Inc.: Madison, WI, USA, 2009.
- SHELXTL NT/2000, version 6.1; Bruker AXS Inc.: Madison, WI, USA, 2002.
- Tolman, C.A. Formation of three-coordinate nickel(0) complexes by phosphorus ligand dissociation from NiL4. J. Am. Chem. Soc. 1974, 96, 53–60. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R.; Cutillo, F.; Friederichs, N.; Ronca, S.; Wang, B. Improving the performance of methylalumoxane: A facile and efficient method to trap “free” trimethylaluminum. J. Am. Chem. Soc. 2003, 125, 12402–12403. [Google Scholar] [CrossRef] [PubMed]
- Cipullo, R.; Busico, V.; Fraldi, N.; Pellecchia, R.; Talarico, G. Improving the behavior of bis(phenoxyamine) Group 4 metal catalysts for controlled alkene polymerization. Macromolecules 2009, 42, 3869–3872. [Google Scholar] [CrossRef]
- Cai, Z.; Nakayama, Y.; Shiono, T. Facile synthesis of tailor-made stereoblock polypropylenes via successive variation of monomer pressure. Macromolecules 2008, 41, 6596–6598. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).