Abstract
Lipases were employed under solvent-free conditions to conjugate oligo-ricinoleic acid derivatives with 10-undecenoic acid, to incorporate a reactive terminal double bond into the resultant product. First, undecenoic acid was covalently attached to oligo-ricinoleic acid using immobilized Candida antarctica lipase (CAL) at a 30% yield. Thirty percent conversion also occurred for CAL-catalyzed esterification between undecenoic acid and biocatalytically-prepared polyglycerol polyricinoleate (PGPR), with attachment of undecenoic acid occurring primarily at free hydroxyls of the polyglycerol moiety. The synthesis of oligo-ricinoleyl-, undecenoyl- structured triacylglycerols comprised two steps. The first step, the 1,3-selective lipase-catalyzed interesterification of castor oil with undecenoic acid, occurred successfully. The second step, the CAL-catalyzed reaction between ricinoleyl-, undecenoyl structured TAG and ricinoleic acid, yielded approximately 10% of the desired structured triacylglycerols (TAG); however, a significant portion of the ricinoleic acid underwent self-polymerization as a side-reaction. The employment of gel permeation chromatography, normal phase HPLC, NMR, and acid value measurements was effective for characterizing the reaction pathways and products that formed.
1. Introduction
As concerns for the long-term economic and environmental sustainability of petroleum increase, the utilization of natural resources as sources of fuels, chemicals, and materials is becoming increasingly attractive. For these reasons, biobased polymers have received increased attention, with its capacity projected to increase from 0.4 million metric tons (MMT) in 2007 to 3.5 MMT in 2020 [1]. Vegetable oils are an important potential starting material for biobased polymers (reviewed in [2]). Particularly attractive are polymers based on long-chain hydroxy fatty acids, such as ricinoleic acid (R-18:19c–OH12) derived from the oil of castor (Ricinus communis L), which have numerous uses: lubricants, paints, inks, adhesives, and plasicizers to name a few (reviewed in [3,4]). In addition, polyglycerol polyricinoleate, PGPR, consisting of oligo-ricinoleic acid chains grafted onto polyglycerol via ester bond formation, is a well-known biobased and biocompatible emulsifier for foods and pharmaceuticals [5,6,7]. oligo-Ricinoleic acid and its derivatives (including PGPR [8]) are readily formed using lipases under nonaqueous media and moderate operating conditions; e.g., 50–80 °C and near-ambient pressure [9]. Reviewed elsewhere, enzymes, particularly lipases, are potentially valuable biocatalysts for catalyzing polymer synthesis, providing a “green” manufacturing route to polymer synthesis, and imparting unique selectivity toward polymerization, including the preparation of optically pure polymers (reviewed in [10,11,12,13,14,15]). Biocatalytic synthesis of polymers is very attractive from a sustainability perspective, due to its reduction of energy costs (through employment of moderate temperatures), safer operating conditions, and the reduction of waste materials enriched in acids, bases, and metal catalysts that can harm the environment.
A problem associated with ricinoleic acid homopolymers is the absence of functional groups available for covalent modification or attachment to other polymeric chains or surfaces. Although the carboxylic acid end-group of the homopolymer is a useful conjugation site, the other endgroup, a secondary hydroxyl, is much less reactive. To improve the reactivity of oligo-ricinoleic acid, the attachment of 10-undecenoic acid, a biobased fatty acid derived from the pyrolysis of ricinoleic acid [16], is proposed. Undecenoic acid’s terminal double bond is very reactive, readily participating in Friedel-Crafts acylation and metathesis reactions, and can undergo addition reactions to incorporate halogens or epoxide groups [2,16]. The objective being pursued in this paper is the covalent attachment of undecenoic acid onto oligo-ricinoleic acid and its derivatives using lipases under solvent-free conditions.
Our group’s interest in this reaction type stems from ongoing research in preparing unimolecular polymeric micelles (UPMs), amphiphilic dendrimer-inspired star polymers that typically form nanometer-sized spheres possessing a lipophilic core and a hydrophilic exterior, or “corona” , useful delivery vehicles for drugs, nutraceuticals, and other compounds [9]. The value of UPMs is that they remain stable upon dilution, as would be encountered when vehicles enter into biological fluids after their delivery. UPMs are typically prepared using chemical synthesis, and require several synthesis and separation steps. Chemo-enzymatic synthesis is expected to increase the biocompatibility of the vehicle, simplify and improve the environmental-friendliness of the synthetic procedure, lower the operational and materials costs, and perhaps lower the degree of polydispersity. The incorporation of undecenoic acid onto amphiphilic derivatives of oligo-ricinoleic acid (such as PGPR) would allow for the tethering together of the oligomeric chains via free radical polymerization, occurring within the lipophilic core of micelles formed by the amphiphilic derivatives [16].
In this paper, incorporation of undecenoic acid into three different oligo-ricinoleic acid derivatives using biocatalysis is described. The first synthesis involves the covalent attachment of undecenoic acid to the -OH end group of oligo-ricinoleic acid (Scheme I). A similar objective was achieved previously by us; moreover, lauric acid was successfully attached onto the -OH termini of ricinoleic acid oligomers using immobilized Candida antarctica lipase, CAL [17]. For the second synthesis, undecenoic was reacted with PGPR prepared via CAL-catalyzed esterification (Scheme II) [5,6,7].
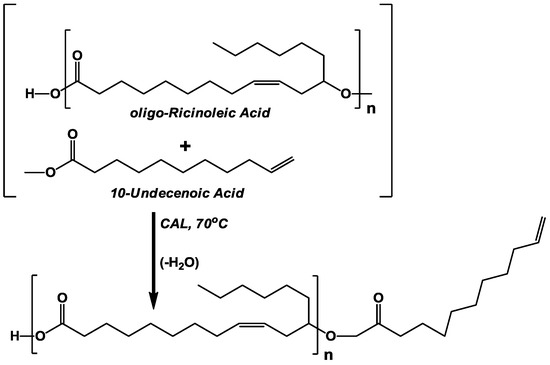
Scheme I.
Lipase-catalyzed solvent-free synthesis of oligo-ricinoleic acid containing a covalently attached 10-undecenoic acyl group at its -OH terminus. CAL refers to immobilized Candida antarctica lipase.
The third synthesis entails the formation of oligo-ricinoleyl-, undecenoyl structured triacylglycerols, or TAG (Scheme III). The product depicted in this scheme was produced at the 0.1 gram scale in a preliminary study [18]. In the preliminary study, the first stage of the synthesis, the preparation of ricinolyl-, undecenoyl- structured TAG, was achieved via two routes. For the first, triundecenoin, prepared via CAL-catalyzed transesterification between glycerol and vinyl undecenoin (>90% yield), was subjected to immobilized Rhizomucor miehei lipase, or RML,-catalyzed acidolysis with ricinoleic acid. Lipases possessing 1,3-positional selectivity such as RML are generally unable to utilize the secondary C12 hydroxyl group of ricinoleic acid as acyl acceptor [9]. The second approach entailed the RML-catalyzed transesterification between castor oil and vinyl undecenoin. For both approaches, the resultant structured TAG product was isolated via preparative thin layer chromatography at an approximately 50% yield. The structured TAG would then be reacted with further amounts of ricinoleic acid to prepare oligo-ricinoleic acyl chains within TAG. In this paper, a similar approach is employed; except, free undecenoic acid replaces vinyl ester as acyl donor due to the high cost associated with synthesizing the latter on a commercial scale; and, the scale is increased 300-fold.
A final objective is to determine the necessary characterization techniques required to analyze the time course of reaction and composition of products. The analysis contains many challenges, due to the possible occurrence of side reactions and the employment of multiple monomers (ricinoleic acid, undecenoic acid, glycerol, and polyglycerol). The employment of NMR, gel permeation chromatography, normal phase HPLC, and titration to measure the concentration of free COOH groups was determined to be effective.
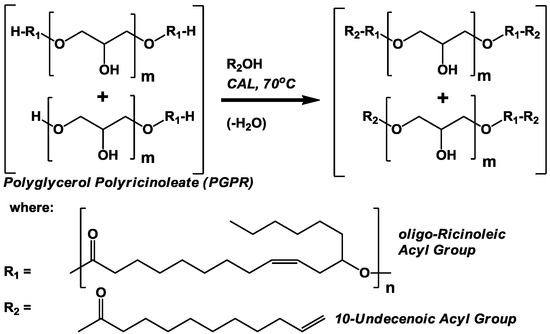
Scheme II.
Lipase-catalyzed solvent-free modification of polyglycerol polyricinoleate, PGPR, with 10-undecenoic acid. CAL is defined in Scheme I.
2. Experimental Section
2.1. Materials
Ricinoleic (R-12-hydroxy cis-9-octadecenoic) acid; technical grade, approximately 80% pure), castor oil, and both immobilized lipases (EC 3.1.1.3) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The biocatalyst CAL, Candida artarctica lipase expressed in Aspergillus niger and immobilized onto macroporous acrylic resin, is prepared by Novozymes, Inc. (Franklinton, NC, USA), with product name Novozym® 435). CAL possesses an average diameter in the range of 0.3–0.9 mm and a specific activity of >10,000 U·g−1, where 1 U is defined as refers to the amount of enzyme which produces 1 μmol of 1-propyl laurate per minute at 40 °C from a stoichiometric feed of acyl donor and acceptor. The biocatalyst RML, Rhizomucor miehei lipase immobilized onto macroporous anionic resin beads, is also a commercial product manufactured by Novozymes (Lipozyme RM IM®), possessing a particle size between 0.2 and 0.6 mm and 86.8 U·g−1 of activity, where 1 U refers to the amount of enzyme which releases 1 μmol stearic acid per minute from tristearin at pH 8.0 and 70 °C. Polyglycerol polyricinoleate, PGPR, was prepared via solvent-free biocatalysis by J.L. Gómez and co-workers at the University of Murcia, Spain, according to a published protocol [8]. It possesses an acid value (AV) of 5.31 mg KOH g−1, a hydroxyl value of 89.16 mg KOH g−1, a refractive index of 1.4655, an iodine value of 74.96 g I2 per 100 g, number-averaged molecular weight (Mn) of 2730, and a polydispersity index (PDI) of 1.1. 10-Undecenoic acid, 99% pure, was obtained from Acros (Geel, Belgium). Reagents employed in the measurement of AV: potassium hydroxide, potassium hydrogen phthalate, and phenolphthalein, were of high purity (>99%) and obtained from Fisher Scientific (Pittsburgh, PA USA). Solvents employed for chromatographic (chloroform, 2-propanol, and 1-hexane) and AV (ethyl ether and ethanol) analyses were HPLC grade and employed without further purification. Lesquerella fendleri oil, employed as a normal phase HPLC standard to identify mono- and di-hydroxyacyl TAG, was provided by International Floratechnologies (Gilbert, AZ, USA).
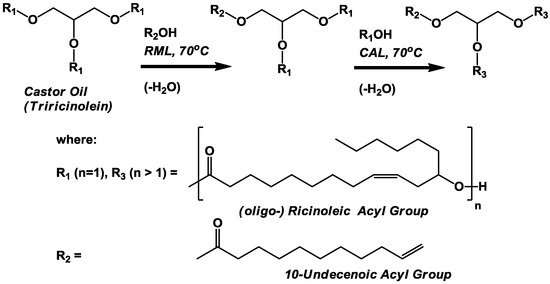
Scheme III.
Lipase-catalyzed solvent-free synthesis of oligo-ricinoleyl, undecenoyl structured TAG via a two-step procedure. RML and CAL refer to immobilized Rhizomucor miehei and Candida antarctica lipases, respectively.
2.2. Methods
2.2.1. Lipase-Catalyzed Conjugation of 10-Undecenoic Acid onto oligo-Ricinoleic Acid (Scheme I)
oligo-ricinoleic acid was prepared using the CAL-catalyzed solvent-free condensation of ricinoleic acid [9]. Technical grade monomer (20 g, approximately 61 mmol ricinoleic acid and 7 mmol of other fatty acids) was stirred with 7.1 wt % CAL in a 50 mL beaker at 300 rpm (radius = 2.0 cm) at 72 °C for 116 h, and stopped at the latter time due to the reaching of a plateau for molecular weight during the time course of reaction via removal of lipase. The degree of polymerization (DP) was determined to be 2.66 and 2.68 based on GPC and 1H-NMR analyses, respectively.
10-Undecenoic acid was added to oligo-ricinoleic acid at a ratio of 0.20 g per g (approximately 0.32 mol undecenoic acid per mol ricinoleic acyl group; 0.85 mol undecenoic acid per mol of -OH end groups of oligo-ricinoleic acid). The reaction conditions were identical to that described above, except for a slightly lower temperature that was employed (69 °C). Aliquots of reaction medium were collected periodically for GPC, HPLC, AV, and NMR analyses. Reaction product was isolated by removing the enzyme via centrifugation.
NMR spectra of the reaction mixture during the time course of reaction contained spectral peaks at the following ppm positions: 1H, (subscripts for C reflect carbon number in acyl group; boldface reflects H atom corresponding to the shift), 11-undecenoic acyl groups: 5.8 (m) C10H; 5.0 (s) and 4.93 (t) C11H2; 2.3 (t) C2H2; 2.0 (m) C9H2; 1.6 (m) C3H2; 1.3 (m) C4–8H2; ricinoleic acyl groups: 5.25–5.57 (m) HC9=C10H; 4.8 (m) C12HOR (ester linkage); 3.6 (m) C12H-OH (hydroxyl end group); 2.3 (t) C2H2; 2.2 (t) C8H2, 2.25 (t) and 2.0 (m) C11H2; 1.6 (m) C6H2; 1.4 (m) C13H2; 1.3 (m) C3–7,14–17H2; 0.9 (t) C18H3. 13C-NMR: 11-undecenoic acyl groups: 180 C1OOH; 174 C1OOR; 139 C10; 114 C11; 33 C2; 32.5 C9; 29 C4–8; 25 C3; ricinoleic acyl groups: 180 C1OOH; 174 C1OOR; 132 C9; 125 C10; 73.7 C12HOR (ester linkage); 71.5 C12HOH (hydroxyl end group); 37.9 C13; 36.1 C11; 34 C2; 25–30 C3–8,14–16; 23.0 C17; 14.1 C18.
2.2.2. Lipase-Catalyzed Esterification between Polyglycerol Polyricinoleate (PGPR) and 10-Undecenoic Acid (Scheme II)
PGPR (2.48 g) was mixed with 10-undecenoic acid (0.62 g, 3.4 mmol) and CAL (11 wt %) and placed in a 20 mL scintillation vial. The solvent-free mixture was magnetically stirred at 300 rpm (radius = 1.7 cm) and 68 °C. Aliquots of reaction medium were collected periodically for analysis. Reaction product was isolated by removing the enzyme via centrifugation.
NMR spectra contained spectral peaks at the following ppm positions during the time course of reaction are as follows. For 1H- and 13C-NMR, ppm positions for the ricinoleic and undecenoic acyl groups are consistent with those listed above in the previous section. For 1H-NMR, the following spectral bands occur, as associated with the polyglycerol moiety. A large band from 3.4 to 3.8 ppm represents (in addition to the C12HOH proton of ricinoleic acid end groups) H atoms attached to C atoms containing free OH groups (i.e., H-(OCH2-CHOH-CH2)n-OH). Upon esterification, H atoms associated with polyglycerol (e.g., ROCH2-CHOH-CH2O-) appear at 4.1 and 4.3 ppm. For the 13C-NMR spectra obtained, the resolution was insufficient to resolve spectral bands associated with polyglycerol (63–74 ppm).
2.2.3. Lipase-Catalyzed Synthesis of oligo-Ricinoleyl, 10-Undecenoyl Triacyglycerol (TAG) (Scheme III)
For the first step of the reaction, castor oil (consisting of approximately 80% triricinolein and 20% diricinoleyl TAG) underwent 1,3-selective acidolysis with 10-undecenoic acid. Castor oil (18.7 mg, 20.0 mmol) was mixed with 10-undecenoic acid (11.1 g, 60 mmol) and RML (9.5% w/w) in a 50 mL beaker. The reaction mixture was held at 67 °C and underwent stirring at 300 rpm. After 116 h, the reaction was stopped; and, RML was removed by centrifugation.
For the second step, the reaction mixture (after removal of RML) was mixed with CAL (5.8 wt %) to allow for ricinoleic acid released from the first step to serve as substrates. The reaction was conducted at 72 °C and 350 rpm magnetic stirring. Additional feeds of ricinoleic acid (9.5 g, 32 mmol) were added 10 h, 34 h, and 58 h after the addition of CAL to the reaction mixture. Aliquots of reaction medium were collected periodically for analysis. Reaction product was isolated by removing the enzyme via centrifugation.
NMR spectra of the reaction mixture during the time course of reaction contained spectral peaks at the following ppm positions: For 1H- and 13C-NMR, ppm positions for the ricinoleic and undecenoic acyl groups are consistent with those listed above. For 1H-NMR, the following spectral bands occur, as associated with the glycerol moiety: 5.24 (m) R1-OCH2-CHOR2-CH2OR3 (where Ri refer to acyl groups); 4.13 (m) and 4.27 (m) R1-OCH2-CHOR2-CH2OR3. 13C-NMR spectral bands associated with glycerol molecules that form triesters: 68.8 R1-OCH2-CHOR2-CH2OR3; 62.0 R1-OCH2-CHOR2-CH2OR3.
2.2.4. Gel Permeation Chromatographic (GPC) Analysis
GPC analysis was conducted using a dual-pump HPLC system obtained from Varian, equipped with model Mark III evaporative light scattering detector from Alltech Associates, a division of WR Grace (Deerfield, IL), and a 300 mm × 7.5 mm ID PL Gel mixed D column purchased from Agilent (Santa Clara, CA USA). The analysis was operated at room temperature (22 °C) using chloroform as solvent at a flow rate of 0.8 mL·min−1. Molecular weight was determined using the retention times of the partially resolved peaks for dimer, trimer, and tetramer of oligo-ricinoleic acid, yielding a straight line for molecular weight vs. retention time when plotted on semilog paper. The exception to this rule was for TAGs; their molecular weight was calculated based on the TAG compositional profile determined from normal phase HPLC. It was determined that the number-averaged molecular weight and the area percentage of ricinoleic acid-containing chemical species were independent of concentration over the concentration range employed for all reaction mixtures examined, suggesting the response factors for all chemical species was approximately the same. Response factors were also obtained for 10-undecenoic acid, which differed greatly from the others.
The chromatogram’s underlying area was divided into a series of N trapezoids; then, the number- and weight- averaged molecular weight (Mn and Mw, respectively) were calculated using the following formulae:
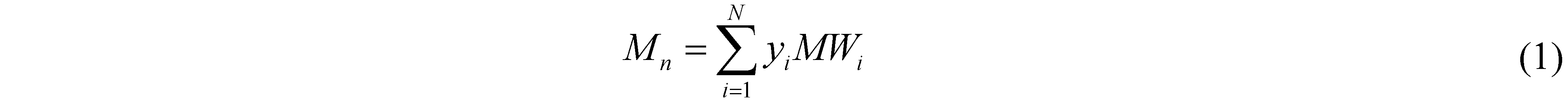
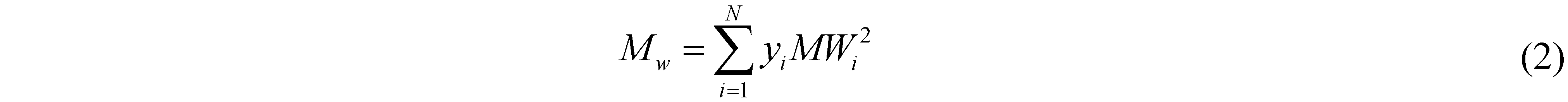
where the subscript i refers to the ith trapezoid and MWi to the average molecular weight of trapezoid i. The index of polydispersity, PDI, is then calculated from the ratio of the two:
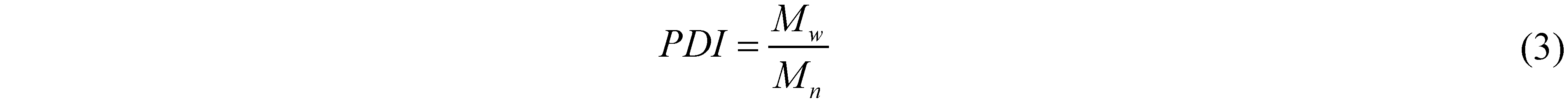
2.2.5. Normal Phase HPLC Analysis
Normal phase HPLC was conducted using the HPLC system described above, equipped with a Sunfire silica column (5µm, 4.6 × 250 mm) from Waters (Milford, MA, USA). The analysis was operated at room temperature (22 °C) using hexane/2-propanol/acetic acid (95/5/0.4, v/v/v) as solvent at a flow rate of 0.8 mL·min−1. Response factors were employed to convert area percentages into weight percentages.
2.2.6. NMR Analysis
1H- and 13C-NMR spectra were obtained using a 400 mHz spectrometer from Varian, Inc, using a pulse width of 90° and a delay time of 1.5 s. CDCl3 was employed as solvent for all samples.
2.2.7. Acid Value (AV) Measurement
AV was measured via titration of samples dissolved in ethanol/ethyl ether (1:1, v/v) using 0.040 N KOH, according to a standardized procedure [19]. Duplicate measurements were employed for most of the reported data, with standard deviations falling within 3.5% of the mean.
3. Results and Discussion
The objective of this investigation is to incorporate 10-undecenoic acyl groups into oligo-ricinoleic acid derivatives. Three approaches were employed. The first two, involving the esterification of undecenoic acid to the -OH end group of oligo-ricinoleic acyl chains, either as homopolymers or attached to polyglycerol (i.e., PGPR), Scheme Iand Scheme II, respectively, were performed successfully. The second approach, the formation of oligo-ricinoleyl, undecenoyl mixed TAG (Scheme III) was successful to a lesser extent. The analysis of the reactions was challenging due to the several different co-products that could form, and weak detector signal exhibited by undecenoic acid when analyzed by GPC and HPLC. Moreover, undecenoic acid’s detector signal was approximately two orders of magnitude lower than signals for ricinoleic acid-containing analytes under most of the situations encountered in this investigation. Therefore, additional chemical analyses (AV and NMR) were required. The analysis of each reaction will now be discussed separately.
3.1. CAL-Catalyzed Esterification of oligo-Ricinoleic Acid and 10-Undecenoic Acid (Scheme I)
Based on the modestly successful esterification of lauric acid to oligo-ricinoleic acid in our previous work [17], it was anticipated that the attachment of 10-undecenoic acid to -OH end groups would also occur readily. Both GPC and normal phase HPLC analysis demonstrated a slight increase of molecular weight, shown by the growth of peaks representing tetramers and multimers and a reduction in the peak area corresponding to monomers and dimers (Figure 1). Concurrently, the AV decreased from 121 to 107 mg KOH g−1 (Figure 1c), with the decrease attributed mainly to consumption of undecenoic acid, since the amount of free ricinoleic acid initially was <3 wt %. Formation of free ricinoleic acid via hydrolysis or chain scission probably did not occur. Moreover, the fraction of ricinoleic acyl groups that participated in intermolecular ester bonds did not change during the time course of reaction, as determined from 1H-NMR analysis using bands at 4.80 and 5.25–5.57 ppm to represent the fraction esterified and total number of ricinoleic acyl groups, respectively. The absence of chain scission for oligo-ricinoleic acid during its biocatalytic modification was previously reported by us [9]. (DP could not be calculated accurately by 1H-NMR due to the high degree of uncertainty in integrating the band at 3.6 ppm attributable to free OH end groups.) Therefore, under this assumption, the decrease in AV of 13.8 mg KOH g−1 represents a 28% conversion of undecenoic acid. In agreement, the increase of Mn for the oligomer, 61 g·mol−1 (Figure 1c), equates to 33% of undecenoic acid’s molecular weight, i.e., a 33% conversion of the latter. This level of conversion strongly agrees with the incorporation of lauric acid onto oligo-ricinoleic acid reported previously by us, 34% [17]. Both the reactions to incorporate undecenoic acid and lauric acid into oligo-ricinoleic acid contained 12–13 wt% of unreacted medium-chain free fatty acid (FFA) in the final product. PDI values remained low throughout the time course of reaction, residing between 1.1 and 1.2. Further research is needed to determine if the percent incorporation of undecenoic acid can be increased without increasing the final FFA concentration, perhaps by adding undecenoic acid into the reaction mixture in small batchwise increments throughout the time course of reaction, and/or by applying a more effective means of water removal than free evaporation, such as high vacuum pressure [7,8]. (It is known that the small amount of non-hydroxy fatty acid present in technical grade ricinoleic acid is readily incorporated as end groups for oligo-ricinoleic acid [9], suggesting the former approach may be successful.)
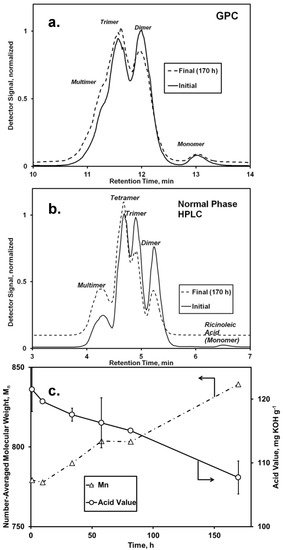
Figure 1.
Analysis of the Candida antarctica lipase (CAL)-catalyzed esterification of oligo-ricinoleic acid and 10-undecenoic acid (Scheme I). Chromatograms: (a) gel permeation chromatography (GPC) and (b) normal phase HPLC of the reaction mixture and initial and final conditions (170 h); (c) Number-averaged molecular weight (Mn), obtained from GPC and acid value (AV) vs. time. Reaction conditions: 10-undecenoic acid/oligo-ricinoleic acid 0.20 g·g−1 (0.32 mol·mol−1); 7.1% w/w CAL, 69 °C.
3.2. CAL-Catalyzed Esterification of Polyglyerol Polyricinoleate (PGPR) and 10-Undecenoic Acid (Scheme II)
Chromatographic analyses demonstrate the reaction between PGPR (prepared using lipases under solvent-free conditions [7,8]) and undecenoic acid greatly affected the chemical nature of the polymer (Figure 2). Normal phase HPLC provided the more useful data for interpretation, demonstrating the existence of two main bands for the starting material, a main and broad band between 3.0 and 4.5 min retention time and a second sharper band at 4.5–5.0 min. It is believed the former and latter represent di- and mono-esters of polyglycerol and polyricinoleic acid, respectively. The larger number of free hydroxyls of the monoester promote its increased polarity, hence its longer retention on the polar silica HPLC column. From a previous series of experiments performed by the authors examining the covalent attachment of oligo-ricinoleic acid to mono- di-, and tri-glycerol, it appears that the primary, or endgroup, hydroxyls of polyglycerol are the principal acyl acceptors [20]. Moreover, our research group has observed that secondary hydroxyls of polyols are poor acyl acceptors for oligo-hydroxy fatty acids. Therefore, it is probable that ester bonds between polyglycerol and oligo-ricinoleic acid form only at the former’s primary hydroxyl end groups.
The normal phase HPLC chromatogram reflects a decrease of the percentage of the monoester from 30% to 11% (Figure 2b, inset), and a concurrent increase of the diester percentage. Consistent with the conversion of monoester to diester is an increase of intensity for bands between 4.0 and 4.3 ppm, consistent with ester formation by the -OH groups of the polyglycerol moiety (Figure 3). The normal-phase HPLC diester peak shifted to slightly higher retention times as the reaction progressed, indicating increased polarity. This is attributed to the formation of oligo-ricinoleic acid-polyglycerol-undecenoic acid diester (Scheme II), anticipated to be more polar than PGPR diester.
During the time course of reaction, the AV decreased from 71 to 51 mg KOH g−1, reflecting the consumption of undecenoic acid (Figure 2b, inset). The measured AV for the initial reaction medium (71 mg KOH g−1) is reasonably close to a calculated value based on the initial amount of undecenoic acid (20 wt %; 60.9 mg KOH g−1), plus the AV reported for PGPR (5.3 mg KOH g−1), or, 65.2 mg KOH g−1. The decrease of 20 mg KOH g−1 is equivalent to a 31% consumption of undecenoic acid.
GPC analysis reveals a single peak for PGPR which shifts to higher retention times during the time course of reaction (Figure 2a). Also, the peak broadens slightly, shown by an increase of the PDI from 1.05 to 1.12. Typically, an increase of retention time would reflect a decrease of molecular weight. However, events that would lead to a decrease of molecular weight, such as chain scission or hydrolysis, appear to be absent. First, peaks representing ricinoleic acid and its dimer are absent in the chromatogram obtained by normal phase HPLC, Figure 2b (cf. Figure 1b). Second, the area underneath 1H-NMR bands at 4.8 ppm and 5.25–5.57 ppm, representing the fraction esterified and total number of ricinoleic acyl groups, respectively, do not change appreciably (Figure 3), similar to that discussed in the previous subsection. Third, the absence of major GPC chromatographic peaks associated with ricinoleic acid and its oligomers, 10.6–13.5 min (Figure 1a), further supports this hypothesis (Figure 2a). The shift in retention time may result from major differences in conformation of the biopolymer in solution resulting from the conjugation of undecenoic acyl groups to the polyglycerol moiety’s -OH groups.
The results are inconclusive whether the desired reaction, attachment of undecenoic acid to the hydroxyl endgroups of oligo-ricinoleic acyl chains occurred or not. However, the unintended result, the apparent attachment of FFA onto free hydroxyls of the polyglycerol moiety, may lead to improved homogeneity of the material’s hydrophilic-lipophilic balance, hence to improved performance, of PGPR prepared enzymatically for its employment as a food or pharmaceutical emulsifier.
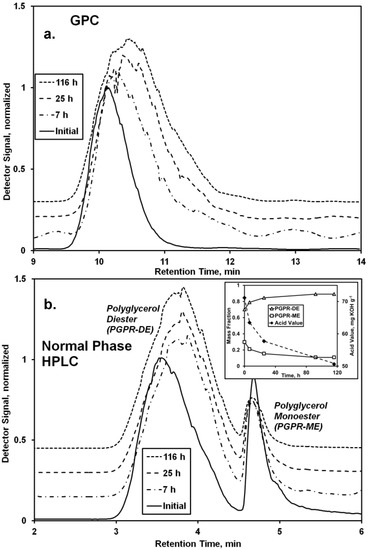
Figure 2.
Analysis of the CAL-catalyzed modification of polyglycerol polyricinoleate (PGPR) with 10-undecenoic acid. Chromatograms from (a) GPC and (b) normal phase HPLC analysis of the reaction mixture and initial and final conditions (170 h) are depicted. Figure b inset shows the change of oligo-fatty acid-polyglycerol mono- and di-esters and acid value (AV) vs. time. Reaction conditions: PGPR (2.48 g) was mixed with 10-undecenoic acid (0.62 g, 3.4 mmol) and CAL (11 wt %). The solvent-free mixture was magnetically stirred at 68 °C.
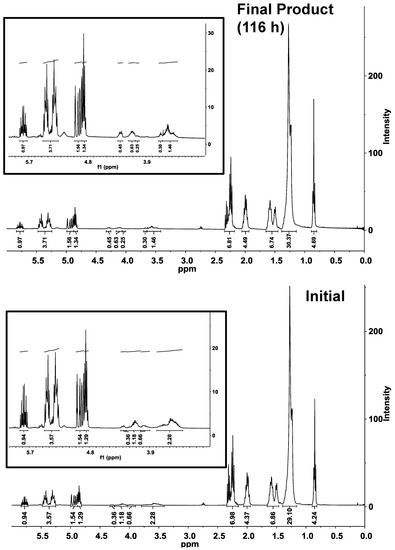
Figure 3.
1H-NMR analysis of the CAL-catalyzed esterification of polyglycerol polyricinoleate (PGPR) and 10-undecenoic acid under initial and final conditions. Inset shows expanded view of 3–6 ppm region. Reaction conditions are given in Figure 2.
3.3. Lipase-Catalyzed Synthesis of oligo-Ricinoleyl, Undecenoyl TAG (Scheme III)
The solvent-free synthesis of oligo-ricinoleyl, undecenoyl TAG entailed two steps, the 1,3-selective lipase (RML)-catalyzed interesterification of castor oil (consisting of >80% triricinolein and the remainder primarily as diricinoleyl TAG) with undecenoic acid, followed by the CAL-catalyzed formation and elongation of oligo-ricinoleyl acyl groups in TAG molecules. For the first step, a mole ratio of 1:1 for undecenoic acid to TAG acyl groups was employed initially; moreover a 50 mol% excess of undecenoic acid per acyl groups in the 1- and 3-TAG position was employed. These conditions would drive acidolysis in the forward position, to produce a mixture of monoundecenoyl and diundecenoyl TAGs. Normal-phase HPLC and GPC chromatograms for the first step are contained in Figure 4a,b, respectively. The former method provided very good resolution of non-, mono-, di- and tri-hydroxylacyl (i.e., -ricinoleyl) TAG, and ricinoleic acid. Peak positions for the former three were confirmed using Lesquerella fendleri oil as a standard, due to its prominence of mono- and di-hydoxyacyl TAG [21]. Undecenoic acid appears as a shoulder at 5.0 min, but disappears during the time course of reaction due in part to its consumption, and to the incommensurately large decrease of its detector response with a small decrease of concentration (detector signal α concentration5.5). The undecenoic acid concentration was calculated from the AV after subtracting the contribution attributable to ricinoleic acid, obtained chromatographically (Figure 4a,b). Of note, the absence of oligo-ricinoleic acyl groups in the TAG or in free acyl form was confirmed from chromatographic and NMR analyses, consistent with a previously study demonstrating the inability of RML to catalyze oligo-ricinoleic acid, and to utilize secondary hydroxyl acyl acceptors [9].
Quantitative analysis of the chromatographic and AV data for the first stage of the two-step synthesis (0–116 h) and the first portion of the second stage (up to 150 h) is given in Figure 5. As shown in Figure 5a, the relative amount of triricinoleyl TAG decreased, accompanied by a successive growth of diricinoleyl TAG, in agreement with the measured decrease of the undecenoic acid and increase of ricinoleic acid that would occur during of acidolysis. However, the relative amount of diricinoleyl TAG reached a plateau and then decreased as the reaction time increased past 100 h, due to its conversion into monoricinoleyl TAG. In addition, the amount of TAG containing no ricinoleyl groups slowly increased, probably reflecting the occurrence of acyl migration, where neutral lipids undergo isomerization, with acyl exchange occurring between the 2- and 1- + 3-positions on the glycerol backbone (thereby allowing for undecenoic acyl groups at the 1- and/or 3-glycerol positions to migrate to the 2-positon). The shift to higher retention times for the TAG peak of the GPC chromatogram (Figure 4b), representing a decrease of molecular weight, is consistent with the replacement of the higher molecular weight ricinoleic acyl groups with lower molecular weight undecenoic acyl groups attached to glycerol. There is good agreement between HPLC and GPC-derived estimates of the released ricinoleic acid, and between AV-derived concentration of undecenoic acid and the latter’s concentration calculated based on the moles of released ricinoleic acid, equated to moles of undecenoic acid incorporated into TAG. The approximate conversion of undecenoic acid for the first stage of reaction is therefore 38%. The latter calculation assumes that hydrolysis of TAG did not occur at appreciable levels, an assumption supported by chromatographic analysis which showed the absence of partial glycerides. In addition, 1H-NMR spectra did not contain bands in the 3.7–4.0 range, attributable to free -OH groups for glycerol. In sum, RML-catalyzed acidolysis of castor oil on a 30 g scale occurred successfully, consistent with the previously obtained successful interesterification of castor oil with vinyl undecenoin on a 0.1 gram scale [18].
The second stage of the reaction, the formation of oligo-ricinoleyl groups within the TAG formed from the first stage, was conducted in a manner that would minimize formation of free oligo-ricinoleic acid. Moreover, the ricinoleic acid concentration was kept intentionally low to allow the hydroxyls of ricinoleic acyl groups contained in the TAG to be as high as possible relative to free ricinoleic acid’s hydroxyl groups, thereby permitting the former to serve as the principal acyl acceptor. To begin the second stage of reaction, at 116 h, RML was replaced by CAL to enable oligomer formation. Additional ricinoleic acid was added in small batchwise increments (32 mmol) at 126, 150, and 174 h during the time course of reaction.
During the first 10 h of the second stage (116–126 h) the most major change was the depletion of tri- and diricinoleyl-TAG and increase of monoricinoleyl TAG (Figure 5b), reflecting the ability of CAL to catalyze acyl exchange at the 2-glycerol position. The amount of TAG containing no ricinoleic acyl groups also decreased, suggesting the acidolysis of the TAG by ricinoleic acid, resulting in additional monoricinoleyl TAG formation. In addition, the dimer of ricinoleic acid (diricinoleic acid) clearly forms by 126 h, as evidenced by the HPLC and GPC chromatograms (Figure 4c and d); however, monoricinoleyl TAG may contribute slightly to the GPC peak. Moreover, the retention times of the newly formed peak are identical with those of dimer present in oligo-ricinoleic acid (cf. Figure 1). As additional ricinoleic acid is added to the reaction mixture, additional dimer and trimer is formed, with little or no oligo-ricinoleyl TAG formed (Figure 4 and Figure 5). oligo-Ricinoleyl TAG did not form until the latter stages of the reaction, reaching approximately 10% when the reaction was stopped. The formation of this product by 285 h is evidenced by the occurrence of new peaks at lower retention times for both HPLC and GPC suggesting the new product is relatively hydrophobic and higher in molecular weight, respectively (Figure 4). The increase of Mn during the latter stage of the time course of reaction reflects the increased levels of oligo-ricinoleyl TAG. However, a small peak representing tetramer or multimer hidden within the oligo-ricinoleyl TAG band (GPC analysis) cannot be discounted (cf. Figure 1a), with its fraction being small, based on the DP value obtained for the final reaction mixture (2.3 according to 1H-NMR analysis). Concurrently, the amount of free ricinoleic acid decreased greatly; and, the concentration of dimer and trimer reach plateaus, with the amount of both being significantly higher than free ricinoleic acid. Therefore, it is believed the incorporation of oligo-ricinoleic acyl groups into TAG results from acidolysis of TAG by dimer and trimer.
The AV of the reaction mixture after the reaction was terminated via removal of CAL was 82.4 ± 1.0 mg KOH g−1. Using approximate concentrations for ricinoleic acid and its dimers and trimers derived via normal phase HPLC (Figure 5b), and subtracting their contributions to the AV, the remaining concentration of COOH groups, if attributed solely to free undecenoic acid, would be 13.0 wt %., i.e., a 65% conversion for undecenoic acid for the entire two-stage reaction.
In sum, the formation of oligo-ricinoleyl TAG appears to have been moderately successful. Perhaps the direct acidolysis of undecenoyl TAG by dimer, trimer, and multimer of ricinoleic acid may produce the desired higher yield. In addition, the extent of incorporation of undecenoic acid into oligo-ricinoleic TAG is not known. Due to the prominence of monoricinoleyl (diundecenoyl) TAG during the latter stages of reaction, it is believe that the majority of oligo-ricinoleyl TAG contain undecenoic acyl groups. The amount of undecenoic acid conjugated to dimers and trimers is also not clear. However, the HPLC chromatogram, Figure 4c, suggests column chromatography using a silica gel-based stationary phase can be used to isolate the oligo-ricinoleyl TAG, dimer, and trimer fractions, to allow for their more thorough analysis, and to obtain the former in purified form.
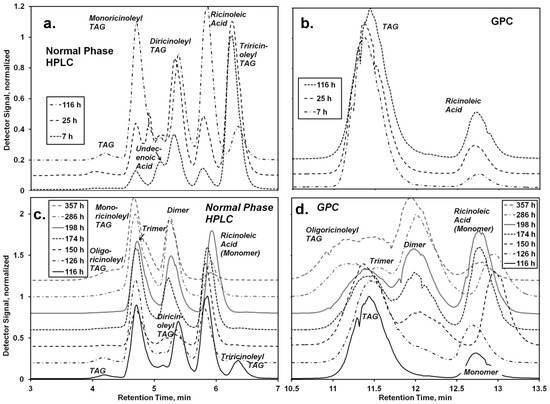
Figure 4.
Analysis of the lipase-catalyzed synthesis of TAG containing oligo-ricinoleic acid and 10-undecenoic acid. (a) and (b) refer to the first step, the Rhizomucor miehei lipase (RML) -catalyzed acidolysis of castor oil with 10-undecenoic acid; (c) and (d) to the second step of the reaction, the CAL-catalyzed reaction between ricinoleic acid and the product from the first step. Chromatograms are from (a,c) normal phase HPLC and (b,d) GPC analyses. Reaction conditions: For the first step, castor oil (18.7 mg, 20.0 mmol) was mixed with 10-undecenoic acid (11.1 g, 60 mmol) and RML (9.5% w/w). The reaction mixture was held at 67 °C. After 116 h, the reaction was stopped; RML was removed by centrifugation, and CAL (5.8 wt %) was added. Additional ricinoleic acid (9.5 g, 32 mmol) was added 10 h, 34 h, and 58 h after the addition of CAL to the reaction mixture. The reaction was conducted at 72 °C.
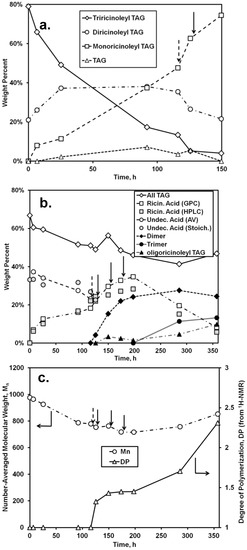
Figure 5.
Time course of reaction for the lipase-catalyzed synthesis of TAG containing oligo-ricinoleic acid and 10-undecenoic acid. (a) change in distribution of TAG species (determined from normal phase HPLC analysis; “TAG” refers to TAG not containing ricinoleic acyl groups); (b) change in concentration of TAG, fatty acid, and oligomer (determined from normal phase HPLC, unless noted otherwise; “AV” and “Stoich” refer to the wt % of 10-undecenoic acid determined from acid value measurements and from stoichiometry: equating the amount of released ricinoleic acid to the amount of undecenoic acid consumed, respectively); (c) change of number-averaged molecular weight and degree of polymerization for fatty acids (determined from, GPC and 1H-NMR, respectively). Dashed arrow refers to the replacement of RML with CAL; solid arrows refer to the addition of (9.5 g, 32 mmol) of ricinoleic acid. Reaction conditions are given in Figure 4.
4. Conclusions and Future Directions
The main objective of this paper was to incorporate 10-undecenoic acid into the molecular structure of oligo-ricinoleic acid and its derivatives to enable further modification for the biobased polymer via the reactive terminal double-bond of the former. Three specific reactions were pursued: Lipase-catalyzed esterification between undecenoic acid and either oligo-ricinoleic acid or polyglycerol polyricinoleate (PGPR), and formation of structured triacylglycerols containing oligo-ricinoleic and undecenoic acyl groups, obtained via a two-step process. For the first two approaches, approximately 30% incorporation of undecenoic acid was achieved. However, for the modification of PGPR, it appears the majority of undecenoic acyl incorporation occurred at free hydroxyls of the polyglycerol moiety (presumed to be the primary hydroxyl endgroups of the polyol) rather than at the desired secondary hydroxyl endgroups contained on the oligo-ricinoleic acyl chains. The ability to readily convert the monoester into diester via solvent-free enzyme-catalyzed acidolysis may improve the performance of PGPR prepared using lipases as emulsifier. The first step of the structured TAG synthesis, the 1,3-selective lipase-catalyzed acidolysis of castor oil by undecenoic acid, occurred readily, with the majority of TAG containing only one or two ricinoleic acyl groups. However, the second stage, the CAL-catalyzed formation and elongation of oligo-ricinoleic acyl chains in the structured TAG, was slightly successful, providing a yield of approximately 10%. Ricinoleic acid dimers and trimers formed from side-reactions occurred to a large extent, despite efforts to reduce their formation via the delivery of ricinoleic acid in small batchwise increments. Since analysis of the time course of reaction suggests the formation of oligo-ricinoleyl containing TAG occurred mainly during the latter stages, after the maximum formation of dimers and trimers occured, it appears that the former were formed through the CAL-catalyzed acidolysis of TAG by ricinoleic acid dimers and trimers.
Perhaps the most significant result was the development of an effective experimental methodology (i.e., the combination of GPC, normal phase HPLC, NMR, and measurement of AV) to sufficiently monitor the time course of reaction to understand the underlying reaction steps involved with the syntheses. The abovementioned “suite” of analyses will allow for further investigation of the reactions described in this paper, to obtain higher yields, and tailor the products’ structure and composition. It appears, based on normal phase HPLC analysis, that flash chromatography on silica stationary phase will enable the isolation and purification of PGPR and oligo-ricinoleyl TAG, to produce a more detailed analysis of the final product, and for the products’ employment in new syntheses.
Acknowledgments
Financial support was kindly supplied by the (US) National Institutes of Health grant 5R03EB005664-02. S. Ortega was beneficiary of a pre-doctoral scholarship from Fundación Séneca of Murcia.M.C. Montiel was beneficiary of a José Castillejo fellowship from Ministerio de Ciencia e Innovación of Spain. Financial support allowing for the visit of Haizhen Zhao to the Hayes laboratory through the “International Exchange Program for Agri-food Quality and Safety” (948 Program from Ministry of Agriculture, project No. 2011-S10) is gratefully acknowledged.
References
- Shen, L.; Worrell, E.; Patel, M. Present and future development in plastics from biomass. Biofuels Bioprod. Bioref. 2010, 4, 25–40. [Google Scholar]
- Ronda, J.C.; Lligadas, G.; Galia, M.; Cadiz, V. Vegetable oils as platform chemicals for polymer synthesis. Eur. J. Lipid Sci. Technol. 2011, 113, 46–58. [Google Scholar]
- Isbell, T.A. Chemistry and physical properties of estolides. Grasas Aceites 2001, 62, 8–20. [Google Scholar]
- Mutlu, H.; Meier, M.A.R. Castor oil as a renewable resource for the chemical industry. Eur. J. Lipid Sci. Technol. 2009, 112, 10–30. [Google Scholar]
- Jorgensen, L.; Vermehren, C.; Bjerregaard, S.; Frokjaer, S. In vitro release of insulin aspart incorporated into water-in-oil emulsions. J. Drug Deliver. Sci. Technol. 2004, 14, 455–459. [Google Scholar]
- Sapei, L.; Naqvi, M.A.; Rousseau, D. Stability and release properties of double emulsions for food applications. Food Hydrocolloid. 2012, 27, 316–323. [Google Scholar]
- Bodalo, A.; Bastida, J.; Maximo, M.F.; Montiel, M.C.; Gomez, M.; Ortega, S. Screening and selection of lipases for the enzymatic production of polyglycerol polyricinoleate. Biochem. Eng. J. 2009, 46, 217–222. [Google Scholar]
- Gomez, J.L.; Bastida, J.; Maximo, M.F.; Montiel, M.C.; Murcia, M.D.; Ortega, S. Solvent-free polyglycerol polyricinoleate synthesis mediated by lipase from Rhizopus arrhizus. Biochem. Eng. J. 2011, 54, 111–116. [Google Scholar] [CrossRef]
- Kelly, A.R.; Hayes, D.G. Lipase-catalyzed synthesis of polyhydric alcohol-poly(ricinoleic acid) ester star polymers. J. Appl. Polym. Sci. 2006, 101, 1646–1656. [Google Scholar]
- Guebitz, G.M. Hydrolases in polymer chemistry: Part III: Synthesis and limited surface hydrolysis of polyesters and other polymers. In Enzymatic Polymerisation (Advances in Polymer Science Series); Palmans, A.R.A., Heise, A., Eds.; Springer: Heidelberg, Germany, 2010; Volume 237, pp. 115–126. [Google Scholar]
- Palmans, A.; Veld, M. Chiral polymers by lipase catalysis. In Biocatalysis in Polymer Chemistry; Loos, K., Ed.; Wiley VCH: Weinheim, Germany, 2011; pp. 277–304. [Google Scholar]
- Puskas, J.E.; Sen, M.Y. Green polymer chemistry: Enzymatic functionalization of liquid polymers in bulk. In Green Polymer Chemistry: Biocatalysis and Biomaterials (ACS Symposium Series); Cheng, H.N., Gross, R.A., Eds.; American Chemical Society: Washington, DC, USA, 2010; Volume 1043, pp. 417–424. [Google Scholar]
- Scandola, M.; Focarete, M.L.; Gross, R.A. Polymers from biocatalysis: Materials with a broad spectrum of physical properties. In Green Polymer Chemistry: Biocatalysis and Biomaterials (ACS Symposium Series); American Chemical Society: Washington, DC, USA, 2010; Volume 1043, pp. 201–211. [Google Scholar]
- Yang, Y.; Yu, Y.; Zhang, Y.; Liu, C.; Shi, W.; Li, Q. Lipase/esterase-catalyzed ring-opening polymerization: A green polyester synthesis technique. Process Biochem. 2011, 46, 1900–1908. [Google Scholar]
- Yeniad, B.; Naik, H.; Heise, A. Lipases in polymer chemistry. In Biofunctionalization of Polymers and Their Applications (Advances in Biochemical Engineering Biotechnology Series); Nyanhongo, G.S., Steiner, W., Gübitz, G., Eds.; Springer: Heidelberg, Germany, 2011; Volume 125, pp. 69–95. [Google Scholar]
- Van der Steen, M.; Stevens Christian, V. Undecylenic acid: A valuable and physiologically active renewable building block from castor oil. ChemSusChem 2009, 2, 692–713. [Google Scholar]
- Hayes, D.G. Biocatalytic synthesis of ricinoleic acid star polymers: “Green” manufacturing of potentially valuable lubricant additives and drug delivery materials. In Degradable Polymers and Materials: Principles and Practice (ACS Symposium Series); Khemani, K., Scholz, C., Eds.; American Chemical Society: Washington, DC, USA, 2006; Volume 939, pp. 126–139. [Google Scholar]
- Mannam, V.K. Lipase-Catalyzed Synthesis of 2-Undecenoyl, 1,3-oligo(Ricinoleyl) Triacylglycerol and Chemical Conjugation of Poly(Ethylene) Glycol Monomethyl Ether to Hydroxyl End Groups of Poly(Hydroxyl) Fatty Acids to Achieve the “Green” Synthesis of Amphiphilic Star Polymers. Masters Thesis, University of Tennessee, Knoxville, TN, USA, 2009. [Google Scholar]
- ASTM D974–06:Standard Test Method for Acid and Base Number by Color-Indicator Titration; American Society for Testing and Materials International: West Conshohocken, PA, USA, 2006.
- Montiel, M.C.; Hayes, D.G. Biocatalytic synthesis of polyglycerol polyricinoleate, a value-added, biobased, food emulsifier. In Proceedings of the 16th Annual BioEnvironmental Polymer Society (BEPS) Meeting (Polymers and the Environment—Emerging Technologies and Science), Chicago, IL, USA, 16–19 June 2009.
- Hayes, D.G.; Kleiman, R. A detailed triglyceride analysis of Lesquerella fendleri oil: Column chromatographic fractionation followed by supercritical fluid chromatography. J. Am. Oil Chem. Soc. 1996, 73, 267–269. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).