Isolation and Characterization of Cellulose Nanocrystals from Bacterial Cellulose Synthesized via Ancylobacter sp. STN1A Using Residual Glycerol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. BNC Synthesis and Purification
2.3. Preparation of BCNCs
2.4. Morphological, Physicochemical, Spectroscopic, and Thermal Characterization
2.4.1. Scanning Electron Microscopy (SEM) of BCN
2.4.2. MorFi Analysis
2.4.3. Optical Microscopy
2.4.4. Atomic Force Microscopy (AFM)
2.4.5. Dynamic Light Scattering (DLS) and Zeta Potential (ZP)
2.4.6. X-Ray Diffraction (XRD)
2.4.7. Fourier Transform Infrared (FTIR) Spectroscopy
2.4.8. Thermogravimetric Analysis (TGA)
2.5. Statistical Analysis
3. Results and Discussion
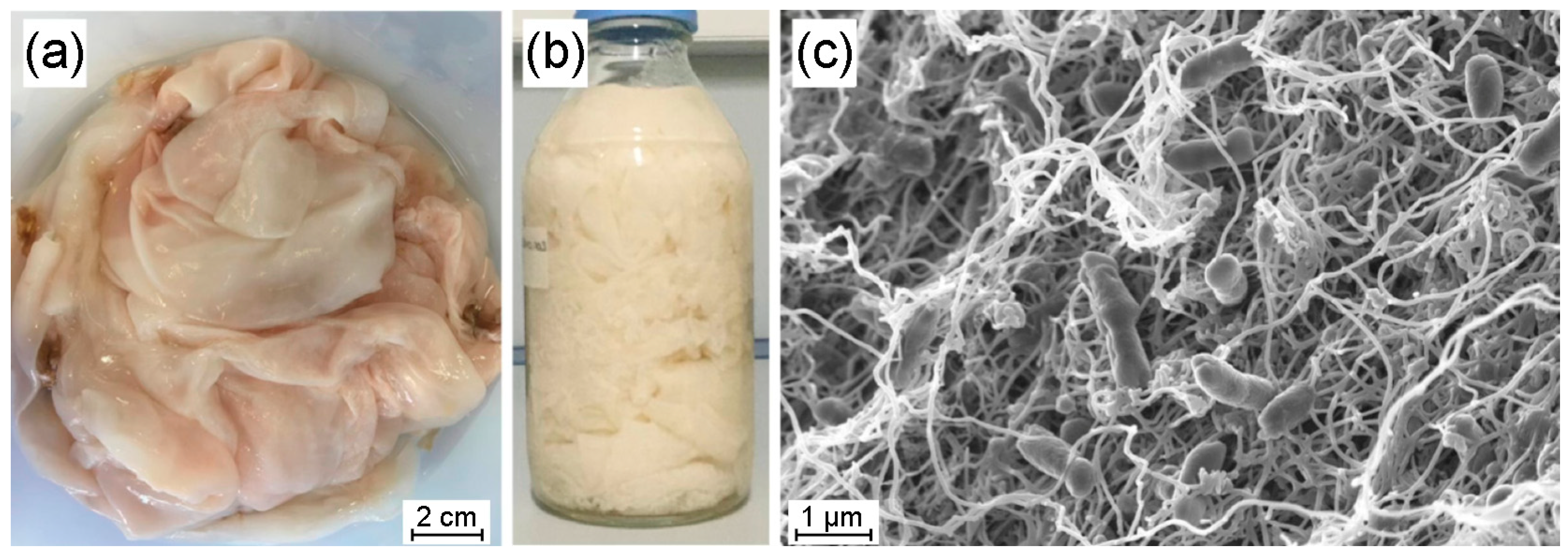
3.1. Bacterial Nanocellulose from Ancylobacter sp. STN1A
3.2. SEM Characterization of UCO-Gly
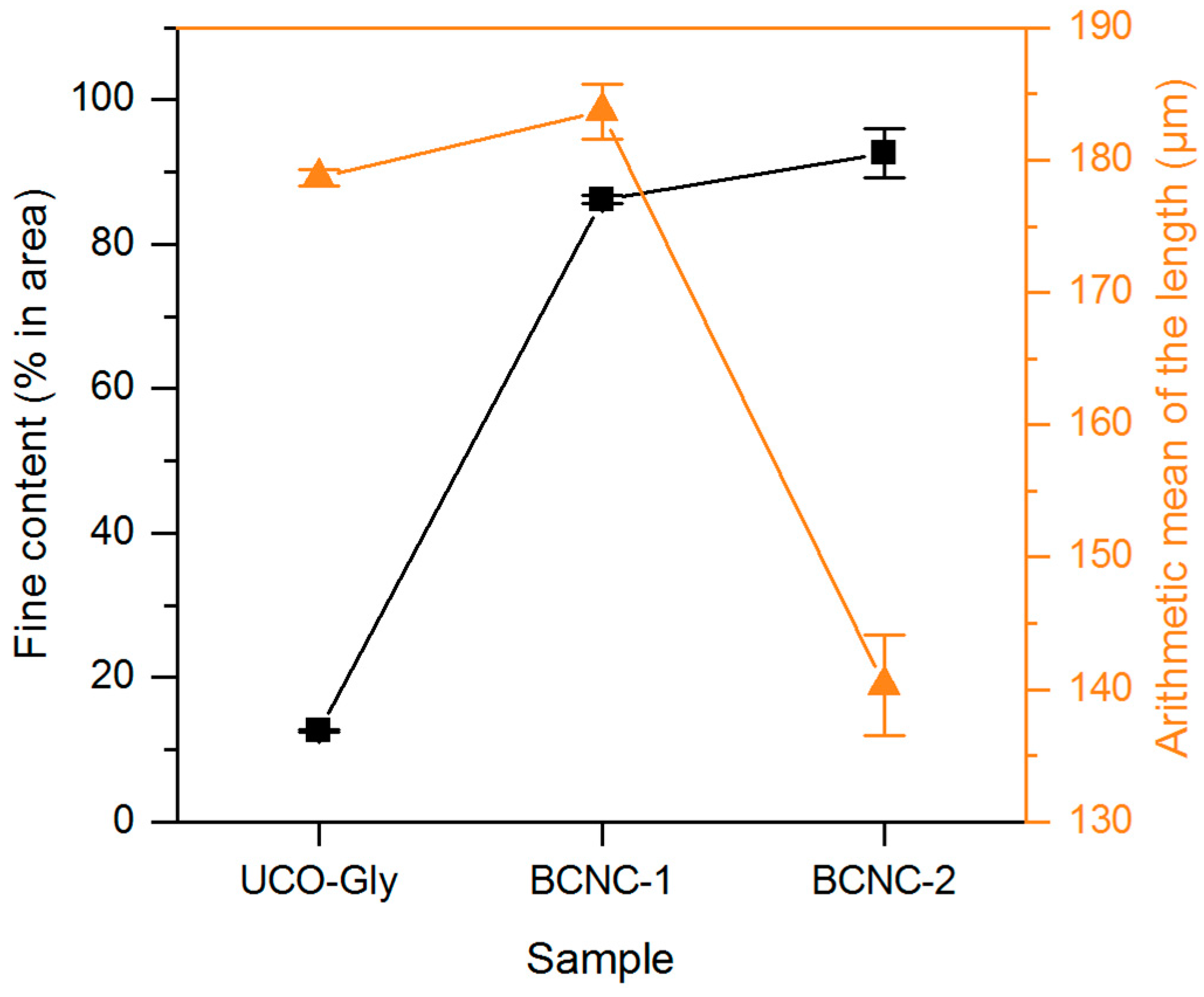
3.3. MorFi Evaluation
3.4. Optical Microscopy Evaluation
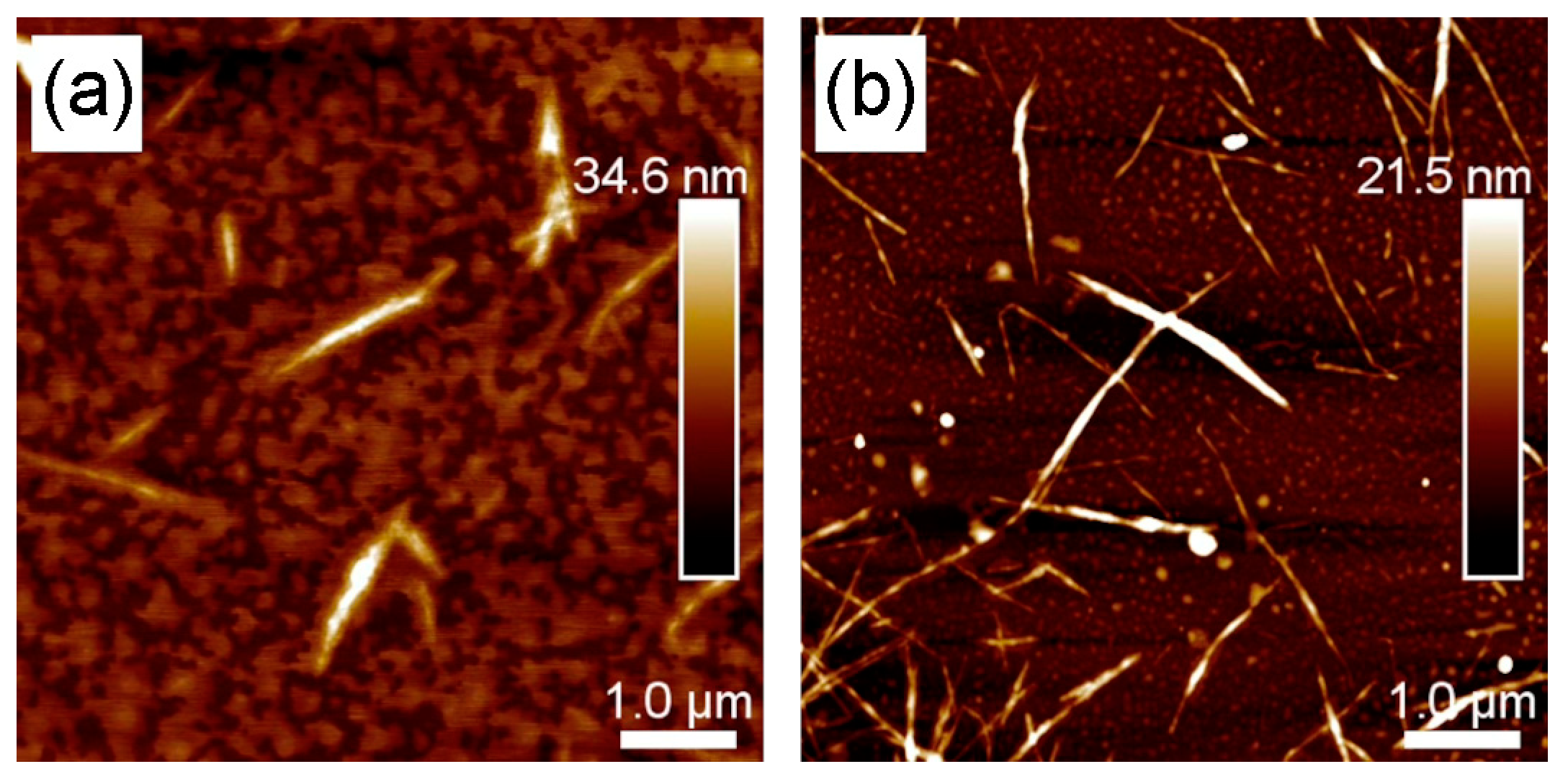
3.5. AFM Characterization of BCNC-1 and BCNC-2
3.6. DLS and Zeta Potential
3.7. XRD Analysis
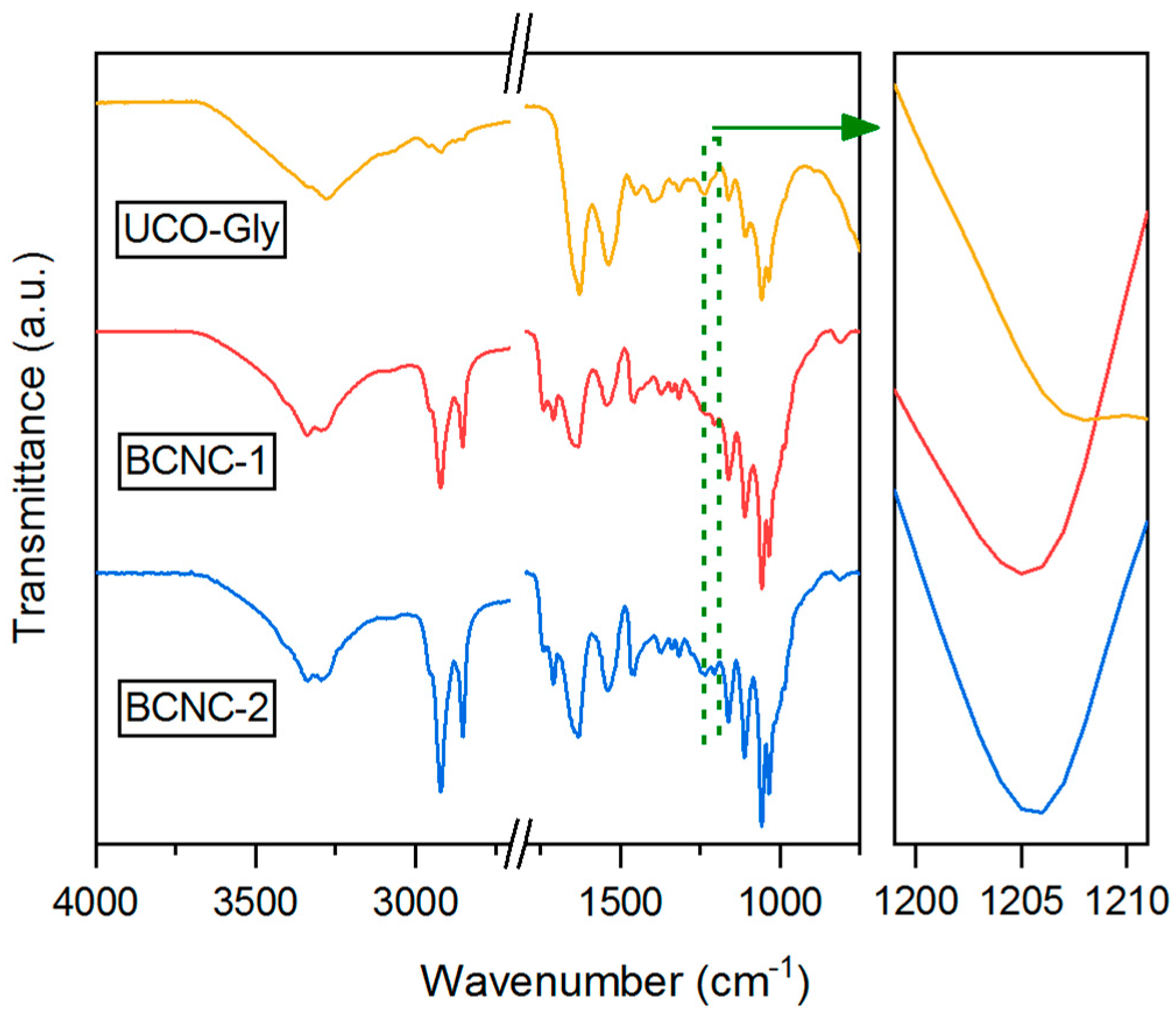
3.8. FTIR Characterization
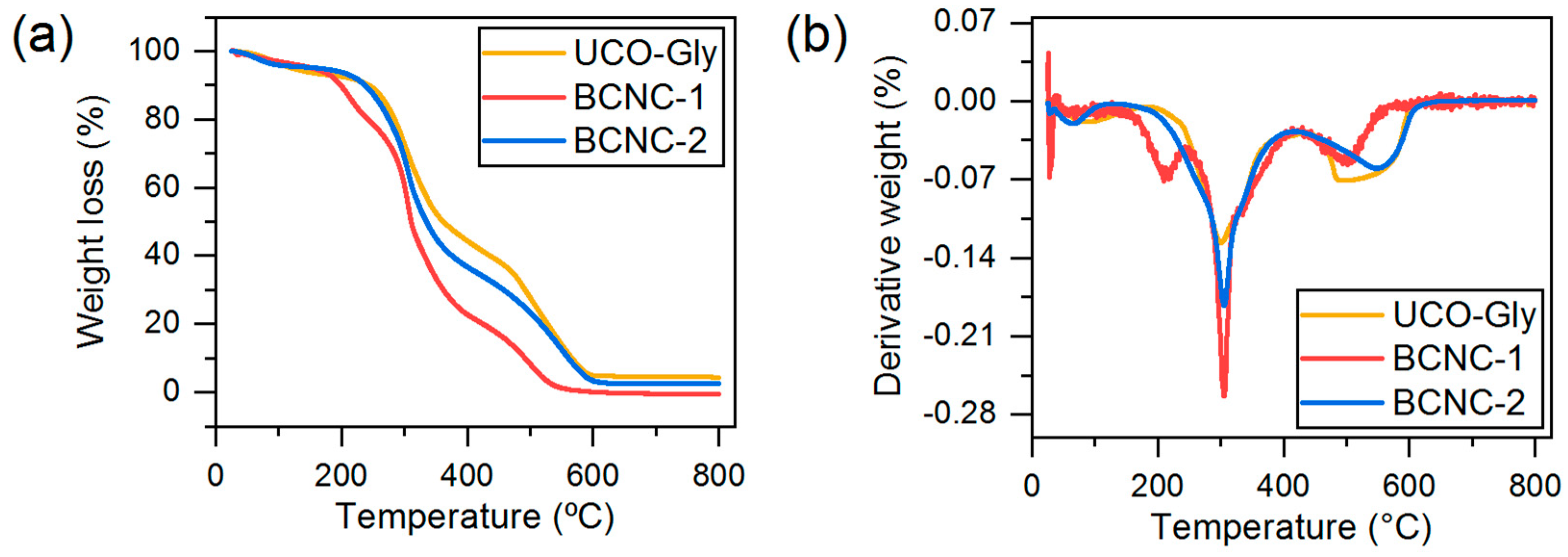
3.9. Thermal Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NFC | Nanofibrillated cellulose |
| CNCs | Cellulose nanocrystals |
| BNC | Bacterial nanocellulose |
| BCNCs | Bacterial cellulose nanocrystals |
| UCO-Glycerine | Used cooking oil-derived crude glycerine |
| WPM | Widdel production medium |
| WMM | Widdel mineral medium |
| SEM | Scanning electron microscopy |
| AFM | Atomic force microscopy |
| DLS | Dynamic light scattering |
| ZP | Zeta potential |
| XRD | X-ray diffraction |
| FTIR | Fourier transform infrared |
| ATR | Attenuated total reflectance |
| TGA | Thermogravimetric analysis |
| DTG | Derivative thermogravimetry |
References
- Yu, X.; Tong, S.; Ge, M.; Wu, L.; Zuo, J.; Cao, C.; Song, W. Adsorption of heavy metal ions from aqueous solution by carboxylated cellulose nanocrystals. J. Environ. Sci. 2013, 25, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.P.S.A.; Bhat, A.H.; Yusra, A.F.I. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Trache, D.; Hussin, M.H.; Haafiz, M.K.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Surface chemistry, morphological analysis and properties of cellulose nanocrystals with gradiented sulfation degrees. Nanoscale 2014, 6, 5384–5393. [Google Scholar] [CrossRef] [PubMed]
- Arantes, V.; Dias, I.K.R.; Berto, G.L.; Pereira, B.; Marotti, B.S.; Nogueira, C.F.O. The current status of the enzyme-mediated isolation and functionalization of nanocelluloses: Production, properties, techno-economics, and opportunities. Cellulose 2020, 27, 10571–10630. [Google Scholar] [CrossRef]
- Wang, X.; Guo, J.; Ren, H.; Jin, J.; He, H.; Jin, P.; Wu, Z.; Zheng, Y. Research progress of nanocellulose-based food packaging. Trends Food Sci. Technol. 2024, 143, 104289. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: From Nature to High Performance Tailored Materials, 2nd ed.; De Gruyter: Berlin, Germany, 2017. [Google Scholar]
- Mohomane, S.M.; Motloung, S.V.; Koao, L.F.; Motaung, T.E. Effects of acid hydrolysis on the extraction of cellulose nanocrystals (CNCs): A review. Cellul. Chem. Technol. 2022, 56, 691–703. [Google Scholar] [CrossRef]
- Tenaye, T.; Mohammed, S.A.; Jabasingh, S.A. Sustainable synthesis and characterization of Enset cellulose nanocrystals (E-CNp) from Enset ventricosum biomass and its application in the fabrication of Enset cellulose nanocomposite (E-CNc). Biomass Convers. Biorefin. 2024, 14, 5019–5034. [Google Scholar] [CrossRef]
- García-García, D.; Balart, R.; Lopez-Martinez, J.; Ek, M.; Moriana, R. Optimizing the yield and physico-chemical properties of pine cone cellulose nanocrystals by different hydrolysis time. Cellulose 2018, 25, 2925–2938. [Google Scholar] [CrossRef]
- Leong, S.L.; Tiong, S.I.X.; Siva, S.P.; Ahamed, F.; Chan, C.H.; Lee, C.L.; Chew, I.M.L.; Ho, Y.K. Morphological control of cellulose nanocrystals via sulfuric acid hydrolysis based on sustainability considerations: An overview of the governing factors and potential challenges. J. Environ. Chem. Eng. 2022, 10, 108145. [Google Scholar] [CrossRef]
- Ferreira, P.J.T.; Lourenço, A.F. Nanocelluloses: Production, Characterization and Market. In Nanotoxicology in Safety Assessment of Nanomaterials. Advances in Experimental Medicine and Biology; Louro, H., Silva, M.J., Eds.; Springer: Cham, Switzerland, 2022; Volume 1357, pp. 129–151. [Google Scholar] [CrossRef]
- Liu, A.; Wu, H.; Naeem, A.; Du, Q.; Ni, B.; Liu, H.; Li, Z.; Ming, L. Cellulose nanocrystalline from biomass wastes: An overview of extraction, functionalization and applications in drug delivery. Int. J. Biol. Macromol. 2023, 241, 124557. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, N.F.; Feitosa, J.P.A.; da Gama, F.M.P.; Morais, J.P.S.; Andrade, F.K.; de Souza Filho, M.D.S.M.; Rosa, M.D.F. Bacterial cellulose nanocrystals produced under different hydrolysis conditions: Properties and morphological features. Carbohydr. Polym. 2017, 155, 425–431. [Google Scholar] [CrossRef]
- Yan, H.; Chen, X.; Song, H.; Li, J.; Feng, Y.; Shi, Z.; Wang, X.; Lin, Q. Synthesis of bacterial cellulose and bacterial cellulose nanocrystals for their applications in the stabilization of olive oil pickering emulsion. Food Hydrocoll. 2017, 72, 127–135. [Google Scholar] [CrossRef]
- Martirani-Von Abercron, S.M.; Pacheco-Sánchez, D.; Castillo-Rodríguez, I.; Marín, P.; Aguilar, M.R.; Fernández-González, R.; Bertran-Llorens, S.; Marqués, S. Spontaneous loss of quorum sensing control selects a new high cellulose producing Ancylobacter strain. Int. J. Biol. Macromol. 2025, 304, 140620. [Google Scholar] [CrossRef]
- Patel, A.; Patel, P.; Shukla, A.; Wong, J.W.C.; Varjani, S.; Gosai, H. Sustainable Bioconversion of Industrial Wastes into Bacterial Cellulose for Diverse Applications: A Way Towards Pollution Control and Abatement. Curr. Pollut. Rep. 2023, 9, 226–242. [Google Scholar] [CrossRef]
- Volova, T.G.; Prudnikova, S.V.; Sukovatyi, A.G.; Shishatskaya, E.I. Production and properties of bacterial cellulose by the strain Komagataeibacter xylinus B-12068. Appl. Microbiol. Biotechnol. 2018, 102, 7417–7428. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, B.; Lawal, A. Recovery and utilization of crude glycerol, a biodiesel byproduct. RSC Adv. 2022, 12, 27997–28008. [Google Scholar] [CrossRef]
- Dikshit, P.K.; Kim, B.S. Bacterial cellulose production from biodiesel–derived crude glycerol, magnetic functionalization, and its application as carrier for lipase immobilization. Int. J. Biol. Macromol. 2020, 153, 902–911. [Google Scholar] [CrossRef]
- Gedarawatte, S.T.G.; Ravensdale, J.T.; Johns, M.L.; Li, M.; Al-Salami, H.; Dykes, G.A.; Coorey, R. Evaluation of the water-holding and anti-spoilage effect of a bacterial cellulose nanocrystal coating for the storage of vacuum-packaged beef. Food Packag. Shelf Life 2022, 31, 100818. [Google Scholar] [CrossRef]
- Sommer, A.; Staroszczyk, H. Bacterial cellulose vs. bacterial cellulose nanocrystals as stabilizer agents for O/W pickering emulsions. Food Hydrocoll. 2023, 145, 109080. [Google Scholar] [CrossRef]
- Hirai, A.; Inui, O.; Horii, F.; Tsuji, M. Phase Separation Behavior in Aqueous Suspensions of Bacterial Cellulose Nanocrystals Prepared by Sulfuric Acid Treatment. Langmuir 2009, 25, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Urbina, L.; Corcuera, M.Á.; Gabilondo, N.; Eceiza, A.; Retegi, A. A review of bacterial cellulose: Sustainable production from agricultural waste and applications in various fields. Cellulose 2021, 28, 8229–8253. [Google Scholar] [CrossRef]
- Urbina, L.; Corcuera, M.Á.; Eceiza, A.; Retegi, A. Stiff all-bacterial cellulose nanopaper with enhanced mechanical and barrier properties. Mater. Lett. 2019, 246, 67–70. [Google Scholar] [CrossRef]
- Anwar, B.; Bundjali, B.; Sunarya, Y.; Arcana, I.M. Properties of Bacterial Cellulose and Its Nanocrystalline Obtained from Pineapple Peel Waste Juice. Fibers Polym. 2021, 22, 1228–1236. [Google Scholar] [CrossRef]
- Efthymiou, M.N.; Tsouko, E.; Papagiannopoulos, A.; Athanasoulia, I.G.; Georgiadou, M.; Pispas, S.; Briassoulis, D.; Tsironi, T.; Koutinas, A. Development of biodegradable films using sunflower protein isolates and bacterial nanocellulose as innovative food packaging materials for fresh fruit preservation. Sci. Rep. 2022, 12, 6935. [Google Scholar] [CrossRef]
- Poddar, M.K.; Dikshit, P.K. Recent development in bacterial cellulose production and synthesis of cellulose based conductive polymer nanocomposites. Nano Sel. 2021, 2, 1605–1628. [Google Scholar] [CrossRef]
- Luo, X.L.; Zhu, J.Y.; Gleisner, R.; Zhan, H.Y. Effects of wet-pressing-induced fiber hornification on enzymatic saccharification of lignocelluloses. Cellulose 2011, 18, 1055–1062. [Google Scholar] [CrossRef]
- Salari, M.; Khiabani, M.S.; Mokarram, R.R.; Ghanbarzadeh, B.; Kafil, H.S. Preparation and characterization of cellulose nanocrystals from bacterial cellulose produced in sugar beet molasses and cheese whey media. Int. J. Biol. Macromol. 2019, 122, 280–288. [Google Scholar] [CrossRef]
- Marín, P.; Martirani-Von Abercron, S.M.; Urbina, L.; Pacheco-Sánchez, D.; Castañeda-Cataña, M.A.; Retegi, A.; Eceiza, A.; Marqués, S. Bacterial nanocellulose production from naphthalene. Microb. Biotechnol. 2019, 12, 662–676. [Google Scholar] [CrossRef]
- Du, R.; Zhao, F.; Peng, Q.; Zhou, Z.; Han, Y. Production and characterization of bacterial cellulose produced by Gluconacetobacter xylinus isolated from Chinese persimmon vinegar. Carbohydr. Polym. 2018, 194, 200–207. [Google Scholar] [CrossRef]
- Gond, R.K.; Gupta, M.K.; Jawaid, M. Extraction of nanocellulose from sugarcane bagasse and its characterization for potential applications. Polym. Compos. 2021, 42, 5400–5412. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr. Polym. 2012, 87, 564–573. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Pignon, F.; Belgacem, M.N. Morphological properties of nanofibrillated cellulose produced using wet grinding as an ultimate fibrillation process. J. Mater. Sci. 2014, 50, 531–541. [Google Scholar] [CrossRef]
- Vera-Loor, A.; Rigou, P.; Marlin, N.; Mortha, G.; Dufresne, A. Oxidation treatments to convert paper-grade Eucalyptus kraft pulp into microfibrillated cellulose. Carbohydr. Polym. 2022, 296, 119946. [Google Scholar] [CrossRef] [PubMed]
- Carter, N.; Grant, I.; Dewey, M.; Bourque, M.; Neivandt, D.J. Production and Characterization of Cellulose Nanofiber Slurries and Sheets for Biomedical Applications. Front. Nanotechnol. 2021, 3, 729743. [Google Scholar] [CrossRef]
- Banvillet, G.; Grange, C.; Curtil, D.; Putaux, J.L.; Depres, G.; Belgacem, N.; Bras, J. Cellulose nanofibril production by the combined use of four mechanical fibrillation processes with different destructuration effects. Cellulose 2023, 30, 2123–2146. [Google Scholar] [CrossRef]
- Desmaisons, J.; Boutonnet, E.; Rueff, M.; Dufresne, A.; Bras, J. A new quality index for benchmarking of different cellulose nanofibrils. Carbohydr. Polym. 2017, 174, 318–329. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Physical and/or Chemical Compatibilization of Extruded Cellulose Nanocrystal Reinforced Polystyrene Nanocomposites. Macromolecules 2013, 46, 5570–5583. [Google Scholar] [CrossRef]
- Ben Mabrouk, A.; Dufresne, A.; Boufi, S. Cellulose nanocrystal as ecofriendly stabilizer for emulsion polymerization and its application for waterborne adhesive. Carbohydr. Polym. 2020, 229, 115504. [Google Scholar] [CrossRef]
- Tan, L.; Dufresne, A.; Zhu, G.; Lin, N. Regioselective Modification at Reducing End Aldehydes of Cellulose Nanocrystals and Mercerization. ACS Sustain. Chem. Eng. 2023, 11, 4485–4497. [Google Scholar] [CrossRef]
- Khiari, R.; Rol, F.; Salon, M.C.B.; Bras, J.; Belgacem, M.N. Efficiency of Cellulose Carbonates to Produce Cellulose Nanofibers. ACS Sustain. Chem. Eng. 2019, 7, 8155–8167. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Kordoghli, B.; Khiari, R.; Mhenni, M.F.; Sakli, F.; Belgacem, M.N. Sulfonation of polyester fabrics by gaseous sulfur oxide activated by UV irradiation. Appl. Surf. Sci. 2012, 258, 9737–9741. [Google Scholar] [CrossRef]
- Boumediri, H.; Bezazi, A.; Del Pino, G.G.; Haddad, A.; Scarpa, F.; Dufresne, A. Extraction and characterization of vascular bundle and fiber strand from date palm rachis as potential bio-reinforcement in composite. Carbohydr. Polym. 2019, 222, 114997. [Google Scholar] [CrossRef] [PubMed]
- Serafica, G.; Mormino, R.; Bungay, H. Inclusion of solid particles in bacterial cellulose. Appl. Microbiol. Biotechnol. 2002, 58, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, G.; Srinikethan, G. Crude glycerol as a cost-effective carbon source for the production of cellulose by K. Saccharivorans. Biocatal. Agric. Biotechnol. 2018, 16, 326–330. [Google Scholar] [CrossRef]
- Lu, P.; Jin, L.; Liang, B.; Zhang, J.; Li, S.; Feng, Z.; Huang, X. Study of Biochemical Pathway and Enzyme Involved in Metsulfuron-Methyl Degradation by Ancylobacter Sp. XJ-412-1 Isolated from Soil. Curr. Microbiol. 2011, 62, 1718–1725. [Google Scholar] [CrossRef]
- Zikmanis, P.; Kolesovs, S.; Ruklisha, M.; Semjonovs, P. Production of bacterial cellulose from glycerol: The current state and perspectives. Bioresour. Bioprocess. 2021, 8, 116. [Google Scholar] [CrossRef]
- Lee, S.; Abraham, A.; Lim, A.C.S.; Choi, O.; Seo, J.G.; Sang, B.I. Characterisation of bacterial nanocellulose and nanostructured carbon produced from crude glycerol by Komagataeibacter sucrofermentans. Bioresour. Technol. 2021, 342, 125918. [Google Scholar] [CrossRef]
- de Souza Ferreira, A.; Rodrigues, H.C.; Montanher, P.F.; de Souza, S.S.; de Castilhos Ghisi, N. The current state of bacterial nanocellulose research: A scientometric analysis. Cellulose 2025, 32, 1469–1483. [Google Scholar] [CrossRef]
- Kosamia, N.M.; Samavi, M.; Uprety, B.K.; Rakshit, S.K. Valorization of Biodiesel Byproduct Crude Glycerol for the Production of Bioenergy and Biochemicals. Catalysts 2020, 10, 609. [Google Scholar] [CrossRef]
- Takayama, G.; Kondo, T. Quantitative evaluation of fiber network structure–property relationships in bacterial cellulose hydrogels. Carbohydr. Polym. 2023, 321, 121311. [Google Scholar] [CrossRef]
- de Amorim, J.D.P.; de Souza, K.C.; Duarte, C.R.; Duarte, I.D.S.; Ribeiro, F.D.A.S.; Silva, G.S.; de Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M.; et al. Plant and bacterial nanocellulose: Production, properties and applications in medicine, food, cosmetics, electronics and engineering. A review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Qian, H.; Liu, J.; Wang, X.; Pei, W.; Fu, C.; Ma, M.; Huang, C. The state-of-the-art application of functional bacterial cellulose-based materials in biomedical fields. Carbohydr. Polym. 2023, 300, 120252. [Google Scholar] [CrossRef] [PubMed]
- Gindl, W.; Keckes, J. Tensile properties of cellulose acetate butyrate composites reinforced with bacterial cellulose. Compos. Sci. Technol. 2004, 64, 2407–2413. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef]
- Pennells, J.; Heuberger, B.; Chaléat, C.; Martin, D.J. Assessing cellulose micro/nanofibre morphology using a high throughput fibre analysis device to predict nanopaper performance. Cellulose 2022, 29, 2599–2616. [Google Scholar] [CrossRef]
- Vanderfleet, O.M.; Osorio, D.A.; Cranston, E.D. Optimization of cellulose nanocrystal length and surface charge density through phosphoric acid hydrolysis. Phil. Trans. R. Soc. A 2018, 376, 20170041. [Google Scholar] [CrossRef]
- Lam, N.T.; Saewong, W.; Sukyai, P. Effect of varying hydrolysis time on extraction of spherical bacterial cellulose nanocrystals as a reinforcing agent for poly(vinyl alcohol) composites. J. Polym. Res. 2017, 24, 71. [Google Scholar] [CrossRef]
- Flauzino Neto, W.P.; Mariano, M.; da Silva, I.S.V.; Silvério, H.A.; Putaux, J.L.; Otaguro, H.; Pasquini, D.; Dufresne, A. Mechanical properties of natural rubber nanocomposites reinforced with high aspect ratio cellulose nanocrystals isolated from soy hulls. Carbohydr. Polym. 2016, 153, 143–152. [Google Scholar] [CrossRef]
- Wang, X.; Wang, P.; Liu, S.; Crouse, J.; Gardner, D.J.; Via, B.; Gallagher, T.; Elder, T.; Peng, Y. Material properties of spray-dried cellulose nanocrystal reinforced homopolymer polypropylene composites. Polym. Compos. 2023, 46, 1177–1191. [Google Scholar] [CrossRef]
- Chia, M.R.; Phang, S.W.; Ahmad, I. Influence of polyaniline and cellulose nanocrystals on starch biopolymer film for intelligent food packaging. Food Biosci. 2023, 56, 103212. [Google Scholar] [CrossRef]
- Diem, L.N.; Torgbo, S.; Banerjee, I.; Pal, K.; Sukatta, U.; Rugthaworn, P.; Sukyai, P. Sugarcane Bagasse-Derived Cellulose Nanocrystal/Polyvinyl Alcohol/Gum Tragacanth Composite Film Incorporated with Betel Leaf Extract as a Versatile Biomaterial for Wound Dressing. Int. J. Biomater. 2023, 2023, 9630168. [Google Scholar] [CrossRef]
- Kalhori, F.; Yazdyani, H.; Khademorezaeian, F.; Hamzkanloo, N.; Mokaberi, P.; Hosseini, S.; Chamani, J. Enzyme activity inhibition properties of new cellulose nanocrystals from Citrus medica L. pericarp: A perspective of cholesterol lowering. Luminescence 2022, 37, 1836–1845. [Google Scholar] [CrossRef]
- Rovera, C.; Fiori, F.; Trabattoni, S.; Romano, D.; Farris, S. Enzymatic Hydrolysis of Bacterial Cellulose for the Production of Nanocrystals for the Food Packaging Industry. Nanomaterials 2020, 10, 735. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, T.I.; Fouda, A. Green approach for one-pot synthesis of silver nanorod using cellulose nanocrystal and their cytotoxicity and antibacterial assessment. Int. J. Biol. Macromol. 2018, 106, 784–792. [Google Scholar] [CrossRef]
- Naduparambath, S.; Jinitha, T.V.; Shaniba, V.; Sreejith, M.P.; Balan, A.K.; Purushothaman, E. Isolation and characterisation of cellulose nanocrystals from sago seed shells. Carbohydr. Polym. 2018, 180, 13–20. [Google Scholar] [CrossRef]
- Pirich, C.L.; de Freitas, R.A.; Woehl, M.A.; Picheth, G.F.; Petri, D.F.S.; Sierakowski, M.R. Bacterial cellulose nanocrystals: Impact of the sulfate content on the interaction with xyloglucan. Cellulose 2015, 22, 1773–1787. [Google Scholar] [CrossRef]
- Pinheiro, J.A.; Marques, N.D.N.; Villetti, M.A.; Balaban, R.D.C. Polymer-Decorated Cellulose Nanocrystals as Environmentally Friendly Additives for Olefin-Based Drilling Fluids. Int. J. Mol. Sci. 2020, 22, 352. [Google Scholar] [CrossRef]
- Gabriel, T.; Belete, A.; Hause, G.; Neubert, R.H.H.; Gebre-Mariam, T. Isolation and Characterization of Cellulose Nanocrystals from Different Lignocellulosic Residues: A Comparative Study. J. Polym. Environ. 2021, 29, 2964–2977. [Google Scholar] [CrossRef]
- Kiziltas, E.E.; Kiziltas, A.; Blumentritt, M.; Gardner, D.J. Biosynthesis of bacterial cellulose in the presence of different nanoparticles to create novel hybrid materials. Carbohydr. Polym. 2015, 129, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, A.; Foresti, M.L.; Cerrutti, P.; Galvagno, M. Bacterial Cellulose from Simple and Low Cost Production Media by Gluconacetobacter xylinus. J. Polym. Environ. 2013, 21, 545–554. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Barile, C.; Casavola, C.; De Cillis, F. Mechanical comparison of new composite materials for aerospace applications. Compos. B Eng. 2019, 162, 122–128. [Google Scholar] [CrossRef]
- Anwar, B.; Bundjali, B.; Arcana, I.M. Isolation of Cellulose Nanocrystals from Bacterial Cellulose Produced from Pineapple Peel Waste Juice as Culture Medium. Procedia Chem. 2015, 16, 279–284. [Google Scholar] [CrossRef]
- Gea, S.; Reynolds, C.T.; Roohpour, N.; Wirjosentono, B.; Soykeabkaew, N.; Bilotti, E.; Peijs, T. Investigation into the structural, morphological, mechanical and thermal behaviour of bacterial cellulose after a two-step purification process. Bioresour. Technol. 2011, 102, 9105–9110. [Google Scholar] [CrossRef] [PubMed]
- Singhsa, P.; Narain, R.; Manuspiya, H. Bacterial Cellulose Nanocrystals (BCNC) Preparation and Characterization from Three Bacterial Cellulose Sources and Development of Functionalized BCNCs as Nucleic Acid Delivery Systems. ACS Appl. Nano Mater. 2018, 1, 209–221. [Google Scholar] [CrossRef]
- Wang, D.; Yang, T.; Li, J.; Zhang, J.; Yu, J.; Zhang, X.; Zhang, J. Thermostable and Redispersible Cellulose Nanocrystals with Thixotropic Gelation Behavior by a Facile Desulfation Process. ACS Sustain. Chem. Eng. 2020, 8, 11737–11746. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, Y.; Wang, Z.; Peng, Z. Effect of polymorphs of cellulose nanocrystal on the thermal properties of poly(lactic acid)/cellulose nanocrystal composites. Eur. Phys. J. E 2016, 39, 118. [Google Scholar] [CrossRef]
- Rasheed, M.; Jawaid, M.; Parveez, B.; Zuriyati, A.; Khan, A. Morphological, chemical and thermal analysis of cellulose nanocrystals extracted from bamboo fibre. Int. J. Biol. Macromol. 2020, 160, 183–191. [Google Scholar] [CrossRef]
- Arserim-Uçar, D.K.; Korel, F.; Liu, L.S.; Yam, K.L. Characterization of bacterial cellulose nanocrystals: Effect of acid treatments and neutralization. Food Chem. 2021, 336, 127597. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña-Ortiz, M.; García, A.; Martirani-Von Abercron, S.M.; Marín, P.; Marqués, S.; Khiari, R.; Dufresne, A.; Serrano, L. Isolation and Characterization of Cellulose Nanocrystals from Bacterial Cellulose Synthesized via Ancylobacter sp. STN1A Using Residual Glycerol. Polymers 2025, 17, 1240. https://doi.org/10.3390/polym17091240
Peña-Ortiz M, García A, Martirani-Von Abercron SM, Marín P, Marqués S, Khiari R, Dufresne A, Serrano L. Isolation and Characterization of Cellulose Nanocrystals from Bacterial Cellulose Synthesized via Ancylobacter sp. STN1A Using Residual Glycerol. Polymers. 2025; 17(9):1240. https://doi.org/10.3390/polym17091240
Chicago/Turabian StylePeña-Ortiz, Manuel, Araceli García, Sophie Marie Martirani-Von Abercron, Patricia Marín, Silvia Marqués, Ramzi Khiari, Alain Dufresne, and Luis Serrano. 2025. "Isolation and Characterization of Cellulose Nanocrystals from Bacterial Cellulose Synthesized via Ancylobacter sp. STN1A Using Residual Glycerol" Polymers 17, no. 9: 1240. https://doi.org/10.3390/polym17091240
APA StylePeña-Ortiz, M., García, A., Martirani-Von Abercron, S. M., Marín, P., Marqués, S., Khiari, R., Dufresne, A., & Serrano, L. (2025). Isolation and Characterization of Cellulose Nanocrystals from Bacterial Cellulose Synthesized via Ancylobacter sp. STN1A Using Residual Glycerol. Polymers, 17(9), 1240. https://doi.org/10.3390/polym17091240












