1. Introduction
Biodegradable materials are increasingly replacing biostable materials in biomedical applications, particularly in tissue engineering, due to their excellent biocompatibility and ability to degrade in physiological environments. These polymeric biomaterials can be classified into hydrolytically and enzymatically degradable materials based on their degradation mechanism, with most synthetic polymers undergoing hydrolytic degradation due to their biologically inert nature. Hydrolytic degradation primarily affects functional groups such as esters, amides, anhydrides, and carbonates, with polyester biomaterials being characterized by their tunable mechanical and thermal properties, as well as their reproducibility [
1].
Tissue engineering involves designing three-dimensional scaffolds that can temporarily replace the extracellular matrix. These scaffolds support cell adhesion, proliferation, and differentiation until new tissue forms. Polymeric materials such as PCL and PLA have been widely studied for this purpose because of their tunable degradation rates, biocompatibility, and ability to be processed into porous architectures. PCL in particular offers a favorable balance between mechanical strength and long-term degradability, making it suitable for bone, cartilage, and vascular regeneration applications. The microstructure of these scaffolds, particularly their porosity, pore size, and interconnectivity, plays a decisive role in regulating nutrient transport, vascularization, and mechanical integration with host tissue. Therefore, developing environmentally friendly processing routes that can be used to tailor these structural parameters remains a major focus in the fabrication of polymer-based scaffolds for tissue engineering.
Traditionally, polymer processing for scaffold manufacturing has relied on volatile organic solvents. However, these methods have several drawbacks, including the difficulty of completely removing solvent residues, environmental hazards due to solvent emissions, and the high temperatures required in many processes. In addition, the morphological properties of scaffolds produced using solvent-based methods are often inadequate, particularly in terms of achieving a highly interconnected porous structure with a uniform pore-size distribution [
2,
3].
To overcome these limitations, supercritical CO
2 foaming has become progressively more popular due to its success in producing functional scaffolds. This process relies on the unique physical properties of supercritical carbon dioxide that create a porous structure in the polymer for tissue engineering with tunable properties [
4]. In addition to the low toxicity of scCO
2, it is cost-effective, reusable, and avoids the use of organic solvents that can interfere with the polymers [
5]. In the first step, the polymer is saturated with CO
2 at a constant pressure and temperature. The dissolved CO
2 facilitates the mobility of the polymer chains, lowers the glass transition temperature, and promotes plasticization [
6]. The system is then brought to a supersaturated state, usually with a rapid reduction in pressure, although this can also be due to a sudden increase in temperature. A phase separation is produced, which induces cell nucleation by reducing the solubility of CO
2. Finally, cell growth takes place within the polymer matrix, gradually forming the foam structure [
7].
This foaming process requires the polymer to have a high affinity for CO
2. In this regard, polymers such as PLA, PGA, PLGA, and PCL are among the most widely used materials for tissue engineering scaffolds due to their excellent biocompatibility, biodegradability, suitable mechanical properties, and non-toxic degradation products, as well as their relatively high affinity for CO
2, making them particularly suitable for processing using supercritical foaming techniques [
8,
9]. PCL is a semi-crystalline aliphatic polyester with a glass transition temperature (Tg) close to −60 °C and a low melting point of 55–60 °C [
10]. Moreover, PCL has a slower degradation rate compared to other polymers, which makes it more suitable for controlled release systems and long-term degradable implants [
11]. Therefore, PCL has been widely used for medical purposes; for example, as nanofibers loaded with antibiotic drugs for controlled release [
11] and with therapeutic molecules such as proteins [
12,
13]. On the other hand, this polymer can be used as an additive in resins to improve their resistance or to coat stainless steel against corrosion [
14]. Furthermore, PCL has been successfully employed as a scaffold for tissue repair in cardiovascular, nerve, skin, cartilage, and bone engineering [
15].
An effective scaffold should provide the structural support and the porous morphology required for cell adhesion and consequent tissue regeneration. In this sense, the supercritical CO
2 foaming process allows for tuning of the porosity and pore size by adjusting the main foaming parameters (temperature, pressure, CO
2 contact time, and depressurization gradients) [
16,
17]. However, as the pore formation mechanisms are complex, it is difficult to obtain precise and predictable control of the pore sizes and distributions in the produced scaffolds, which causes certain limitations in the supercritical process [
18]. Therefore, the use of various types of pore-forming substances (e.g., bicarbonates, polyethylene oxide, sodium chloride, sucrose) has been studied in scCO
2 foaming processes to obtain a well-defined porosity and a good pore size distribution [
19,
20,
21,
22]. The type of porogen used, as well as its content and size, has a significant effect on the properties of the produced solid foams. Kosowska et al. analyzed hydroxyapatite, carboxymethylcellulose, nanocellulose, and graphene oxide as porogens, recommending the process of PCL foaming with 5% hydroxyapatite and 0.2% or graphene oxide using scCO
2 [
23]. Ammonium bicarbonate has also been used as a porogen in PCL scaffolds prepared using supercritical foaming [
18]. This substance, when incorporated in scaffold formulation, produces a dual porosity that is advantageous for regenerative medicine purposes. Nevertheless, an extra stage is needed to remove the porogen. This step is normally carried out using solvent leaching, usually water. Hence, in the case of drug-loaded scaffolds, porogen removal can reduce the drug load due to leaching of the bioactive substance incorporated into the scaffold formulation [
18,
24].
Therefore, new strategies are needed to promote the development of interconnected porous networks and the optimal values of the mean pore size without the incorporation of solid porogens. In this context, a hydrothermal treatment could favor these porous structures. This process involves using water at high pressure and temperature. The hydrothermal method is an efficient technique for the synthesis of crystals of hydroxyapatite with a uniform morphology and high crystallinity [
25]. The defect-free crystals obtained through hydrothermal processes present a crystallinity with a narrow particle size distribution [
26]. Hydrothermal treatment has also been investigated as an efficient technique for modifying polymer structures without the need for additives or cross-linking agents, making it an attractive strategy for tissue engineering applications [
27]. In this context, Wasupalli et al. demonstrated that increasing the temperature during hydrothermal treatment enhanced the porosity and interconnectivity of chitosan–polygalacturonic acid polyelectrolyte complex fibrous scaffolds, which are key characteristics for promoting cell adhesion and tissue regeneration [
28]. These findings suggest that hydrothermal treatment could be a valuable tool for optimizing the morphology of PCL scaffolds without the need for additional solid porogens, aligning with the objectives of the present study. Moreover, a higher swelling capacity and stiffness were obtained with this hydrothermal treatment in the scaffolds produced for bone tissue engineering. Meanwhile, cellulose nanocrystals hydrogels have also been prepared using hydrothermal treatment [
29]. In this study, it was concluded that these hydrogels can be dried to produce an effective scaffold system, supporting their use in different applications.
Although the use of high temperatures is often considered a drawback in conventional foaming or solvent-based processes due to the potential degradation of polymers and the need for additional purification steps, hydrothermal treatment represents a more environmentally friendly alternative. In this case, water at an elevated temperature and pressure acts as a clean and non-toxic medium, avoiding the use of organic solvents or porogens. Under these conditions, temperature plays a beneficial role by inducing chain mobility and partial recrystallization, which can promote the development of interconnected porous structures during subsequent foaming. There is evidence in the literature that higher hydrothermal temperatures improve porosity and interconnectivity in chitosan-based scaffolds and that hydrothermal gelation of cellulose nanocrystals produces stable porous networks [
28,
29]. Based on this evidence, the hydrothermal conditions used in this study (temperature, pressure, and time) were selected to exceed the glass transition temperature of PCL and approach its melting range [
10] without complete melting, thus promoting structural rearrangements favorable for scaffold formation.
Currently, there are no studies on the effects of hydrothermal treatment on the fibrous structure of PCL. In order to take advantage of the scCO2 foaming process, which promotes a structural support with a porous morphology necessary for cell adhesion and consequent tissue regeneration without the incorporation of solid porogens, the present work proposes the combination of both processes. Therefore, this study analyzed the effects of performing a hydrothermal treatment before or after the supercritical CO2 foaming process on pore size and distribution, and the interconnected porous networks in the PCL produced. In addition, the effect of the thermal treatment on the mechanical properties of the PCL scaffolds was investigated.
3. Results and Discussion
This study is based on a combined treatment system of scCO2 foaming and hydrothermal treatment of PCL polymer. The effect of performing a hydrothermal treatment before or after the supercritical CO2 foaming process was analyzed in terms of the pore morphology and mechanical properties of the produced scaffolds. Based on the results, the most complex characterization techniques were carried out on experimental samples 1B, 2B, 3B, and 4B (hydrothermal pretreatment), as these samples showed the most promising potential for improving the properties of the polymers.
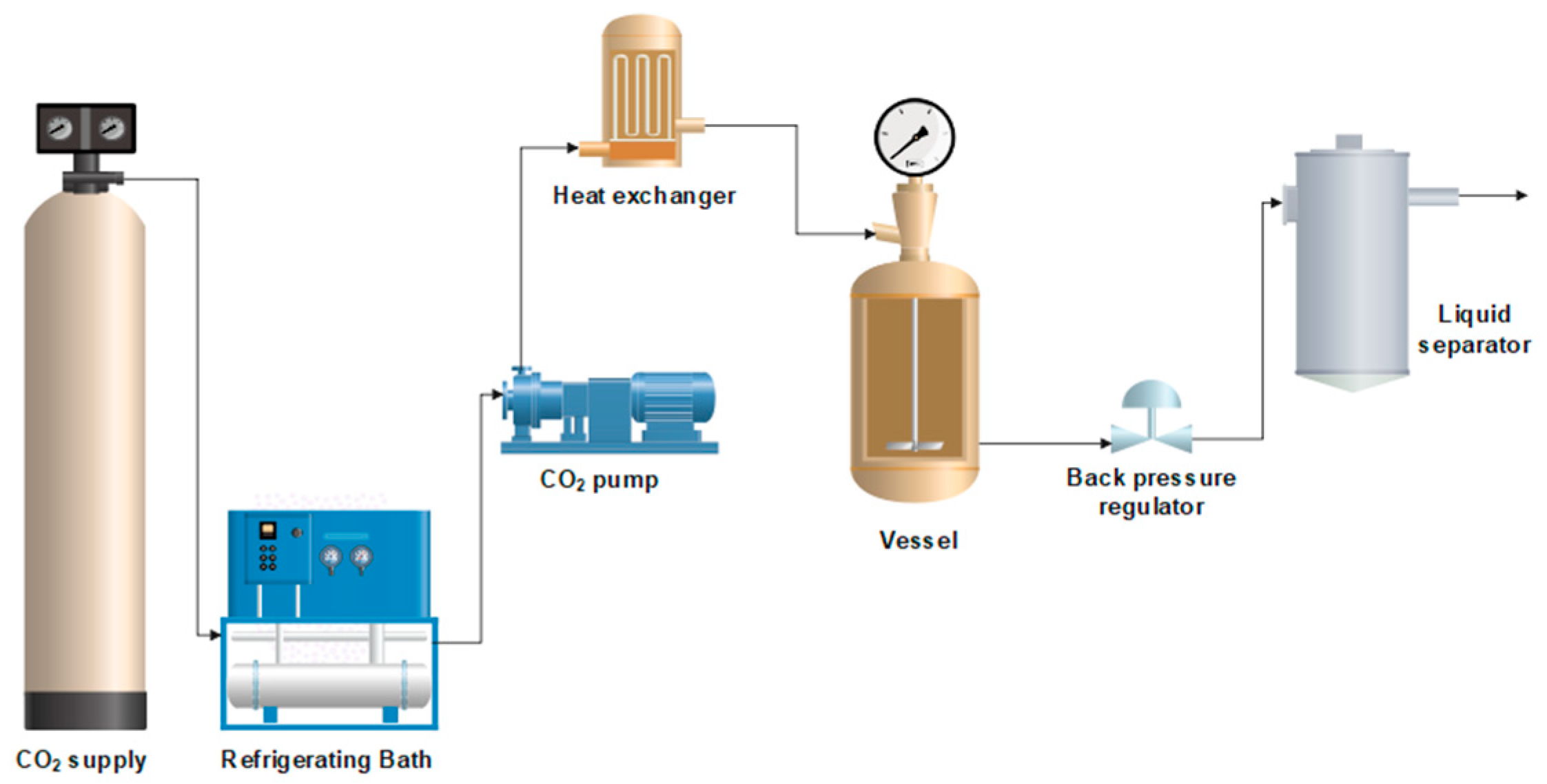
The preliminary experiments on scCO
2 foaming with PLC were carried out at 40 °C with a foaming time of 60 min, as these were determined as the better conditions in a previous study [
5]. Similarly, Satpayeva et al. [
7] achieved a higher porosity in the scaffolds produced at a lower temperature (40 °C) and with a one-step decompression. As previous studies [
6,
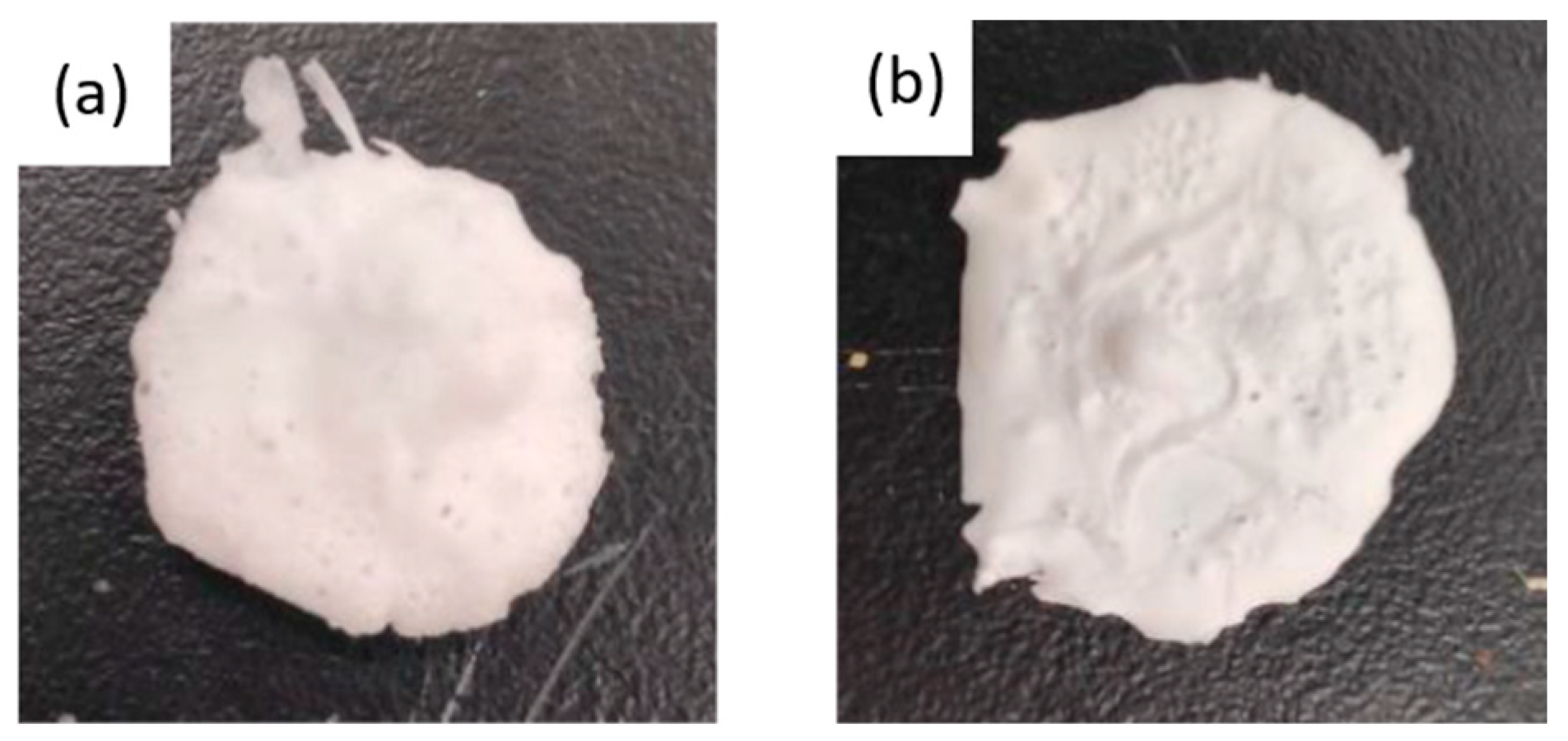
32] showed a significant effect of pressure on the level of porosity and morphology, two different pressures were analyzed (100 and 300 bar). In
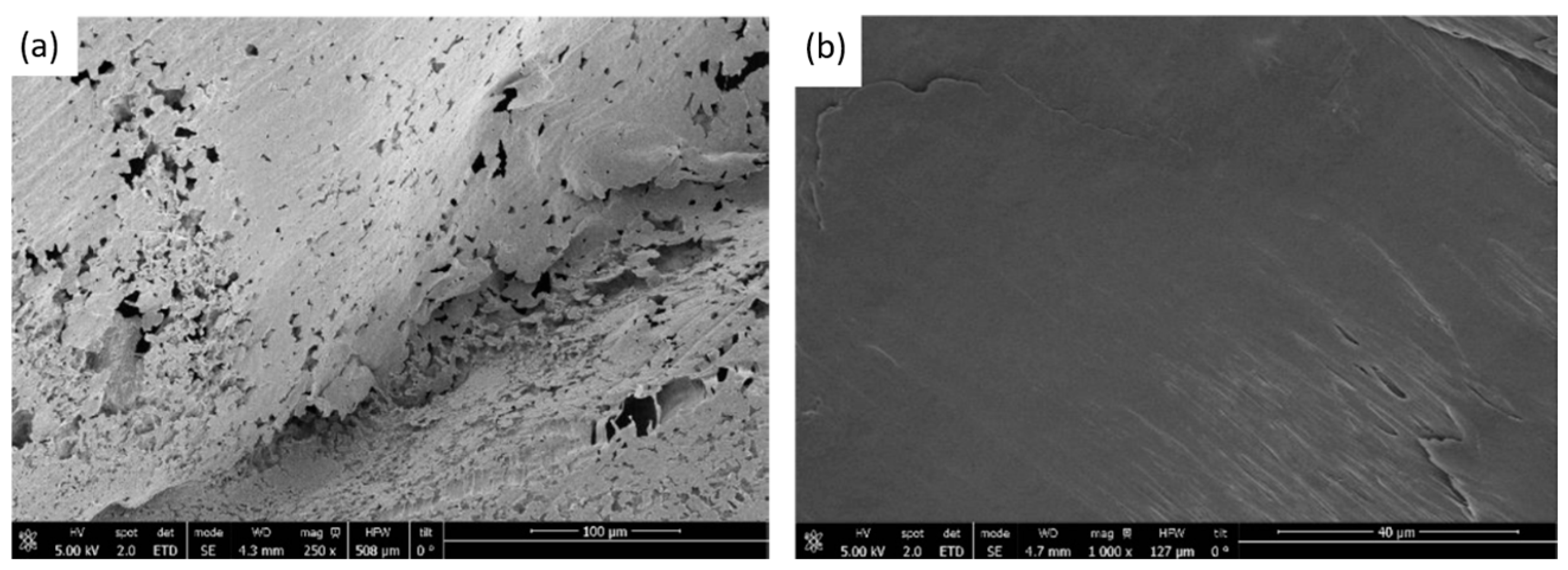
Figure 3, images of the scaffolds formed with these pressures are shown. In both cases, the final size of the polymer obtained was very similar. However, according to the SEM images shown in
Figure 4, it can be seen that the porosity obtained at a pressure of 300 bar is notably higher than that obtained under the 100 bar condition (porosities of approximately 30% were obtained at the higher pressure). This indicates that higher pressures promote more effective nucleation and pore growth under our processing conditions. Thus, the subsequent foaming experiments were carried out at 300 bar, 40 °C, and for 60 min. These findings are consistent with the trend reported by Chen et al. [
32], who also observed that increasing pressure favors porosity up to a certain threshold.
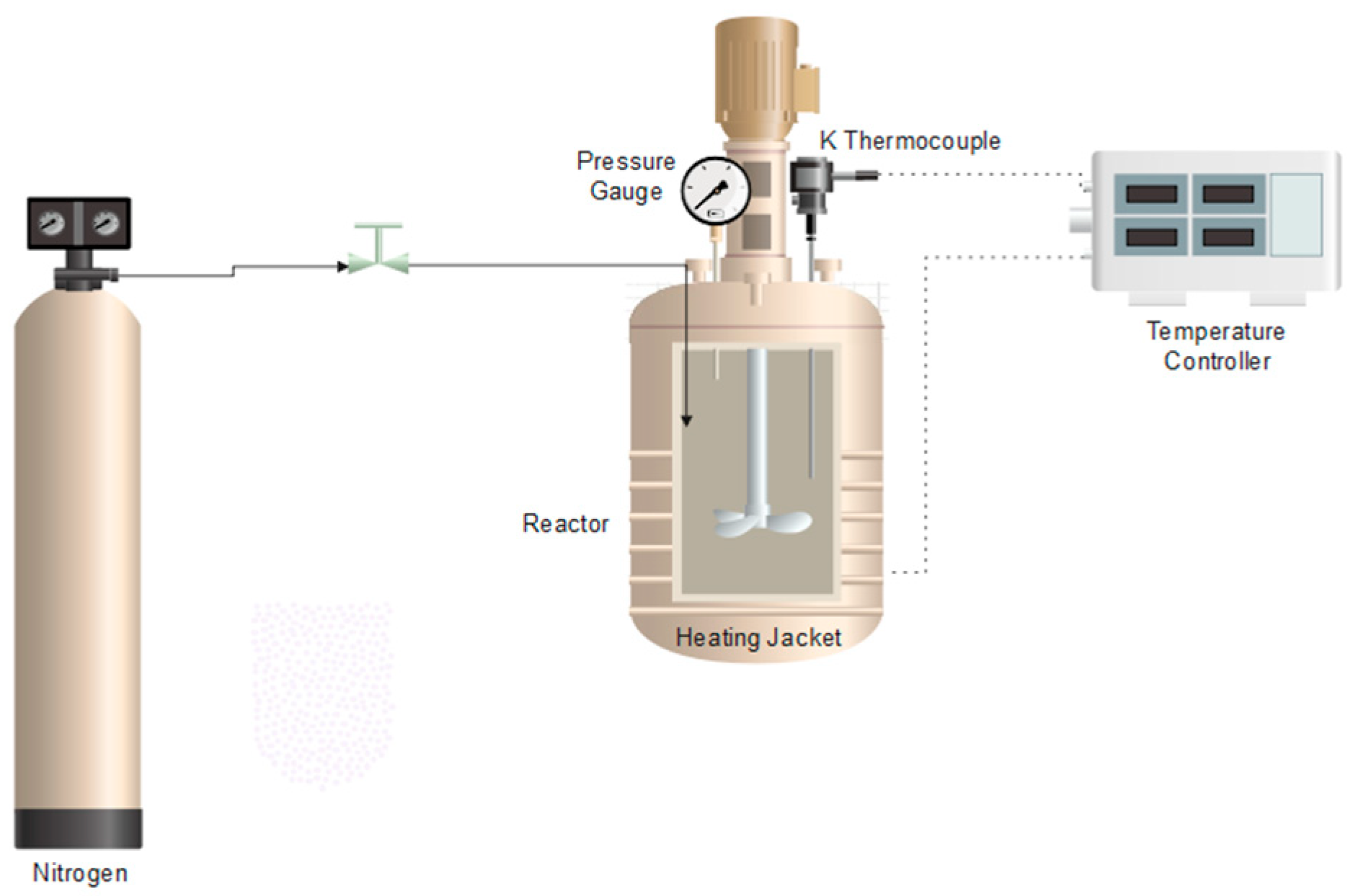
3.1. Effect of the Hydrothermal Treatment After Foaming Process
Once the polymers were treated with CO
2, the hydrothermal treatment was carried out under different conditions, as shown in
Table 2. The application of hydrothermal treatment following the supercritical CO
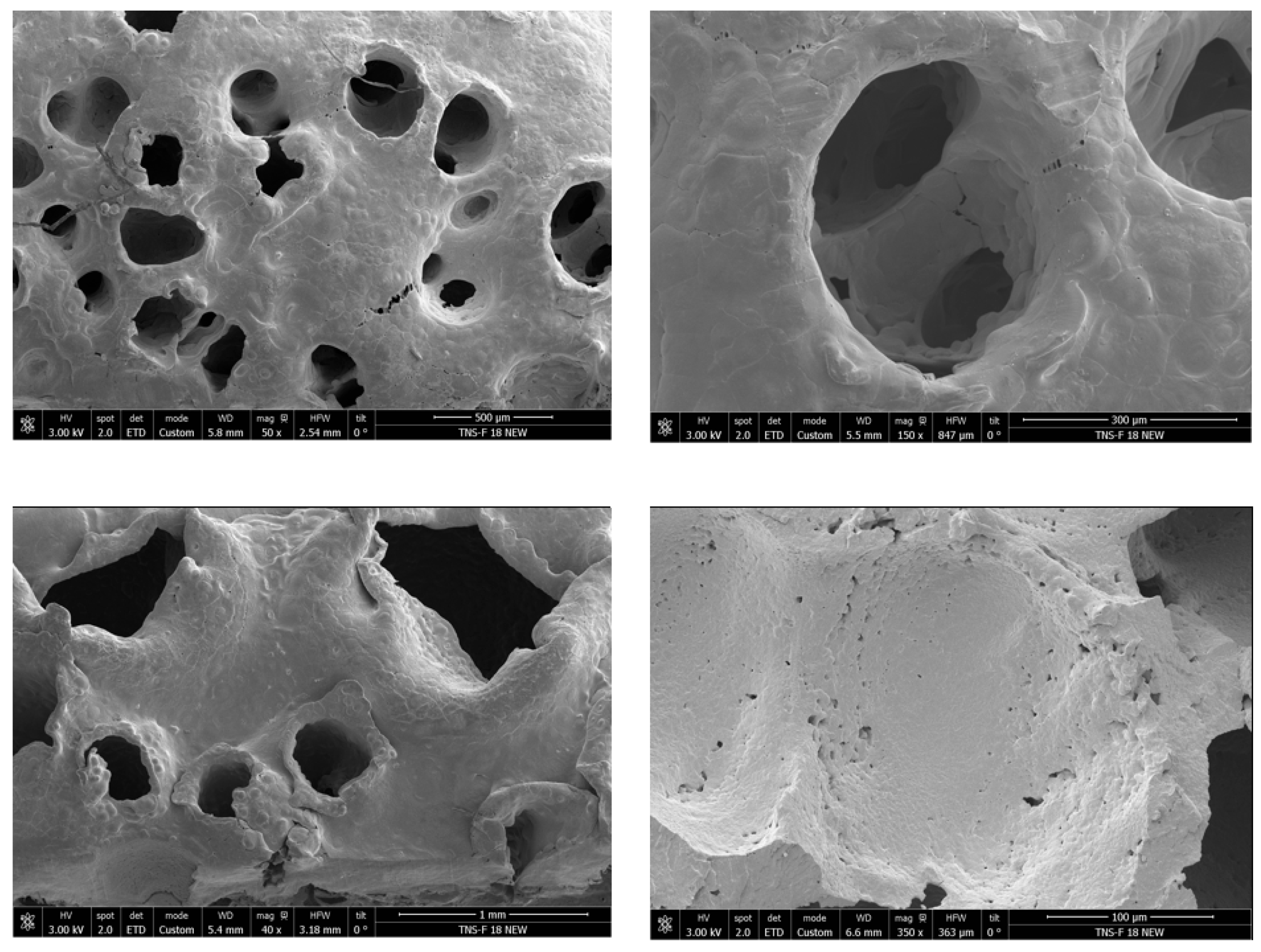
2 foaming process adversely affected the morphology and mechanical integrity of the polycaprolactone (PCL) scaffolds. Scanning electron microscopy images (
Figure 5) revealed a significant reduction in porosity and pore interconnectivity compared to untreated samples, with noticeable pore collapse and a denser, less porous structure. As a direct consequence, the scaffolds became structurally fragile and could not withstand minimal compressive loads, preventing mechanical testing. This observation highlights the critical importance of maintaining the pore architecture to preserve mechanical stability. Similar behavior has been reported in the literature for highly porous PCL scaffolds, where compression moduli as low as 0.24 MPa have been recorded [
33], supporting the relationship between excessive porosity and loss of strength. These findings suggest that post-foaming hydrothermal treatment may detrimentally impact the scaffold’s architecture and mechanical properties, limiting its potential application in tissue engineering, where both porosity and mechanical resilience are crucial.
Given the structural instability and poor morphological properties observed in the scaffolds subjected to post-foaming hydrothermal treatment, further analysis of these samples was deemed unfeasible. Therefore, the discussion will now center on the impact of pre-foaming hydrothermal treatment on scaffold morphology, porosity, interconnectivity, and mechanical performance, as these conditions have shown greater potential for generating structurally robust and well-defined porous architectures suitable for tissue engineering applications.
3.2. PCL Porosity, Pore, and Connectivity Analyses
As previously mentioned, the polymeric matrices formed by PCL were produced using a two-step process. The first step involved a hydrothermal treatment conducted at varying temperatures and pressures (
Table 3), followed by a foaming process using supercritical CO
2 under consistent operating conditions of 300 bar, 40 °C, and 60 min. The objective of this part of the study was to investigate the impact of the initial hydrothermal treatment on the morphology of the resulting scaffold. The key characteristics of macropores ranging from 100 to 1000 µm and good interconnectivity are crucial for achieving an optimal scaffold structure [
34]. A sample of PCL treated only with CO
2 was also analyzed as a control.
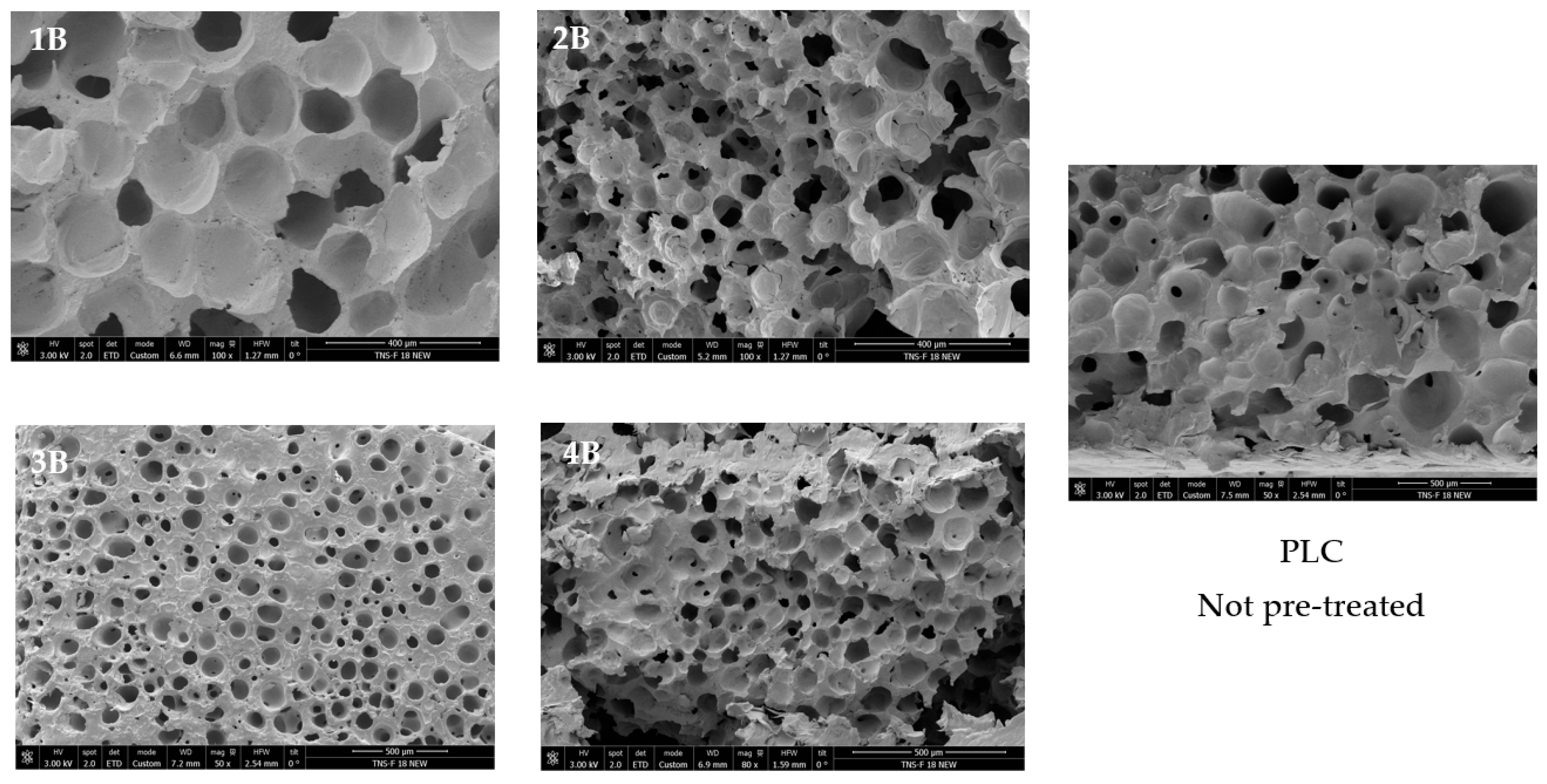
The SEM analysis (
Figure 6) revealed notable differences in the porous morphology between the pretreated samples (1B, 2B, 3B, and 4B) and the untreated PCL scaffold. The hydrothermally treated samples exhibit a higher number of pores that were distributed more homogeneously throughout the structure compared to the untreated PCL, which presented a less defined and more irregular pore distribution. Additionally, the pretreated scaffolds displayed a more ordered architecture, suggesting an enhanced control over the foaming process due to the hydrothermal step. This structural organization hints at a potentially higher degree of pore interconnectivity, which was further examined in the following part through X-ray microtomography analysis.
Based on the results shown in
Table 3, a significant influence on the porosity of the scaffolds was observed for the initial hydrothermal treatment, with the porosity increasing from 16.54% in the untreated PCL to a range of 41.67–57.90%.
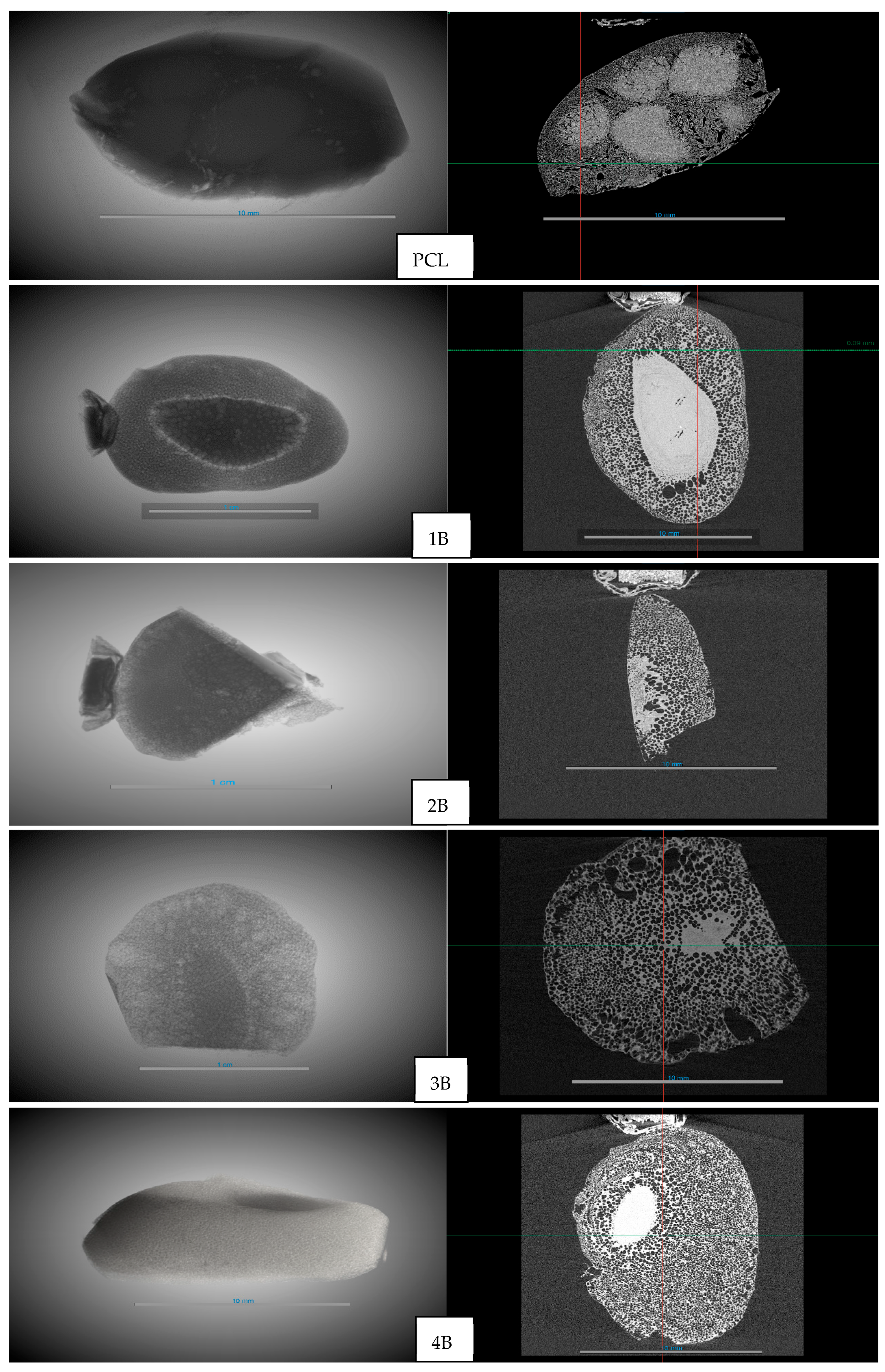
Figure 7 shows the 3D reconstruction of the formed frameworks and the YZ cross-section. In the PCL without hydrothermal treatment, it can be seen that the foaming process with supercritical CO
2 was less effective, with visible areas where the PCL had undergone foaming (white areas). In the other cases, with prior hydrothermal treatment, both the 3D images and the YZ cross-sections showed that the areas where the PCL had not undergone foaming were significantly smaller.
The apparent discrepancy between the overall porosity and pore density values can be explained by pore coalescence occurring at higher porosity levels. In these circumstances, neighboring pores tend to merge, creating larger interconnected voids that boost the total void fraction while reducing the number of individual pores per unit volume. This phenomenon is typical of supercritical CO2 foaming processes and indicates structural coalescence rather than sampling inhomogeneity.
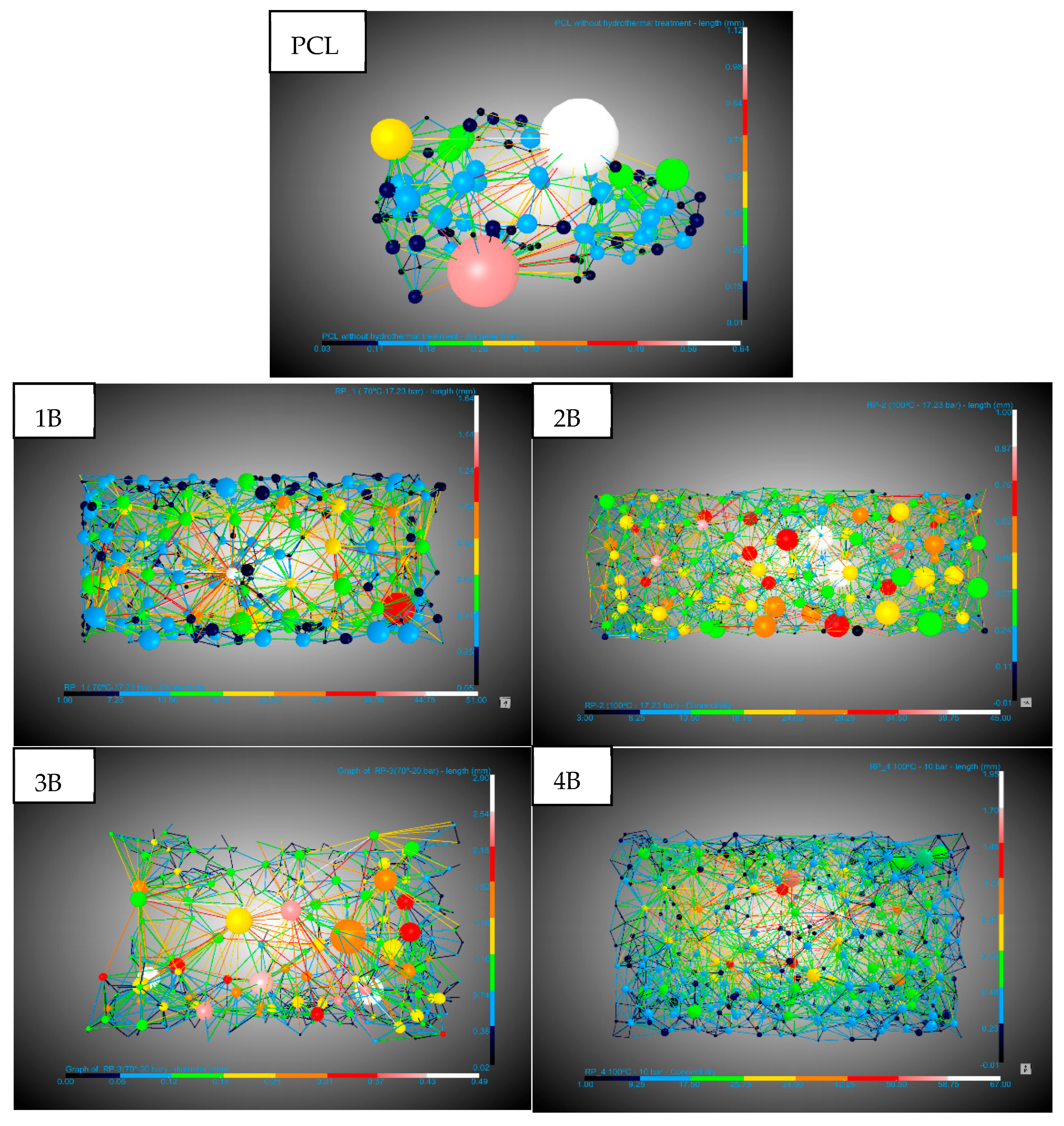
The areas of interest in the scaffolds where foaming and pore formation had occurred were analyzed. The main aspects of a scaffold, such as its pores and interconnectivity, were studied (
Figure 8 and
Table 3). There was a slight increase in pore diameter in the scaffolds generated with hydrothermal treatment, from an average of 0.12 mm for the untreated PCL to 0.17 mm for the scaffold whose pre-treatment was at the highest temperature (100 °C) and an average pressure of 17.23 bar. In terms of the pore density, a decrease was observed compared to untreated PCL. This reduction is likely associated with the combined effect of a lower nucleation site density and increased pore growth during the foaming process, resulting in fewer but larger pores within the scaffold structure. On the other hand, the connectivity decreased in samples 1B, 2B, and 4B, which also had the longest channel lengths (0.49–0.64 mm), while 2B and the untreated PCL showed a greater number of connections with average lengths of 0.29 mm and 0.34 mm, respectively. A good scaffold is defined by its porosity, pore density, and connectivity. The 2B experiment, with hydrothermal pre-treatment at the highest temperature (100 °C) and a pressure of 17.23 bar, shows a three-times higher porosity than that of the untreated PCL but also maintained a similar pore density (27.8 vs. 30.6 pores/mm
3) and high connectivity (156.8 vs. 126.7 throats/mm
3) compared to the small porous regions in the untreated PCL. This demonstrates the potential of hydrothermal pre-treatment in significantly enhancing the design of PCL scaffolds, offering a balance between increased porosity and preserved connectivity.
It should be noted that the micro-CT images presented correspond to individual slices of the reconstructed volume. Therefore, the compact white regions do not necessarily extend along the entire axis of the sample, but rather represent localized areas without foaming. The appearance of these regions has been previously described in PCL foaming with supercritical CO
2 [
17] and is associated with diffusion limitations and heterogeneous nucleation. However, the hydrothermal pretreatment significantly reduced the presence of these compact domains, resulting in scaffolds with more uniformly distributed pores.
3.3. Mechanical Properties
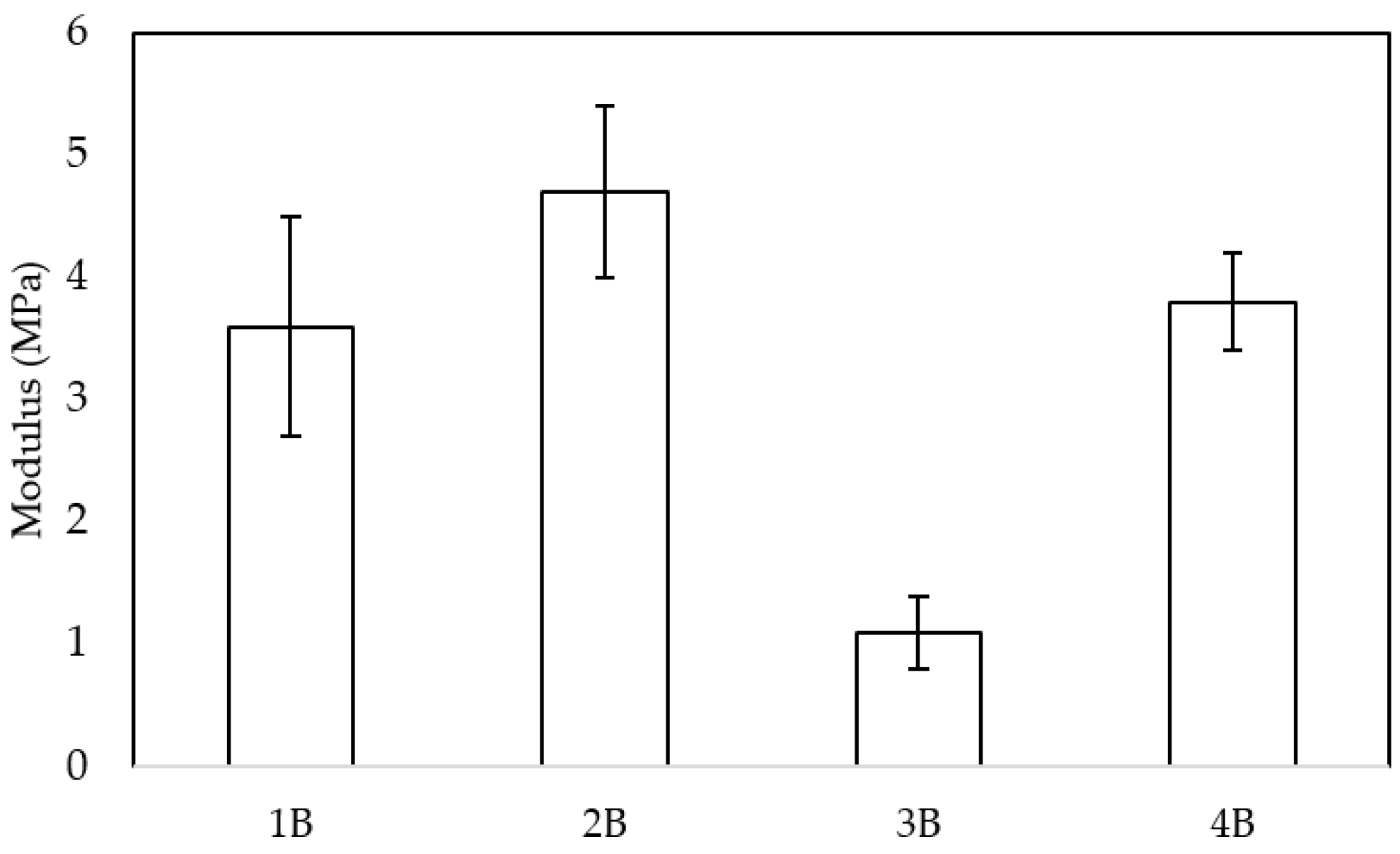
Figure 9 presents the compression modulus of the four scaffold samples. Notably, sample 2B exhibited the highest compression modulus, which aligns with its superior interconnectivity (156.8 throats/mm
3) as determined by the X-ray tomography analysis. This result is partially consistent with previous studies. For example, Murphy et al. [
35] demonstrated that increased pore interconnectivity can significantly alter mechanical performance, although the effect may depend on the processing route. In their work, solvent-molded scaffolds showed an increase in modulus with higher interconnectivity, while gas-foamed scaffolds showed the opposite trend. In our case, the higher interconnectivity of sample 2B appears to contribute to better stress distribution and a higher compressive modulus. In addition, Carr et al. [
36] indicated an inverse relationship between these two parameters, obtaining a decrease in the elastic modulus as porosity increased. Conversely, sample 3B, which had the highest porosity (57%) compared to the other samples, demonstrated the lowest compression modulus. This discrepancy may be attributed to the lower interconnectivity and pore density per unit volume in 3B, which implies the presence of larger pores. Larger pores can lead to reduced structural integrity, as the load-bearing capacity of the scaffold is weakened due to fewer supporting struts within the microstructure. Thus, while porosity plays a crucial role in scaffold architecture, the balance between pore size, density, and interconnectivity appears to be a key determinant of mechanical performance.
When assessing the mechanical properties of a material, both stiffness and strength are key factors, as their values can differ substantially based on the intended implantation site and its specific mechanical demands. In the field of bone tissue engineering, various studies have reported compression strength values in the range of 2–12 MPa for scaffolds fabricated using different techniques [
37,
38,
39]. These values serve as a reference for evaluating the mechanical feasibility of newly developed materials. Given this context, the mechanical performance of the pretreated samples in this study falls within an acceptable range, supporting their potential suitability for specific applications where similar mechanical properties are required. It is important to note that the mechanical properties of PCL scaffolds can vary significantly depending on the manufacturing technique used, the porosity, and the incorporation of other materials or treatments.
Although porosity is a key parameter that influences the mechanical behavior of scaffolds, the distribution of different pore sizes (polydispersity) and the presence of large defects can have a greater impact on strength than macroscopic porosity alone, particularly at low-to-moderate porosity levels. This was examined in the present study. Local heterogeneities and coalesced large pores act as stress concentrators, reducing the effective load-bearing cross-section. Consequently, samples with similar overall void fractions can exhibit markedly different compressive moduli when they differ in defect size and pore-size distribution [
40].
Conversely, pore interconnectivity and an evenly distributed network of throats can improve load transfer through the scaffold by offering several load paths and encouraging a more consistent stress distribution. This observation helps to explain why, despite its elevated porosity, sample 2B exhibited relatively high connectivity and the highest compression modulus. In other words, the mechanical responses of our samples appear to be governed by a balance between (i) the reduction in strength caused by large, inhomogeneous defects, which are dominant in the most porous or coalesced structures; and (ii) the mechanical reinforcement derived from a well-connected pore network, which distributes stresses more evenly [
41].
Therefore, porosity alone is not a sufficient predictor of compressive performance. To understand the mechanical differences between samples with comparable global porosity, the combined descriptors of pore size distribution, maximum defect size, and connectivity must be considered. A more detailed statistical analysis of defect size distributions and larger replicate numbers would clarify the relative importance of these factors using the current process [
42].
3.4. In Vitro Degradation Studies
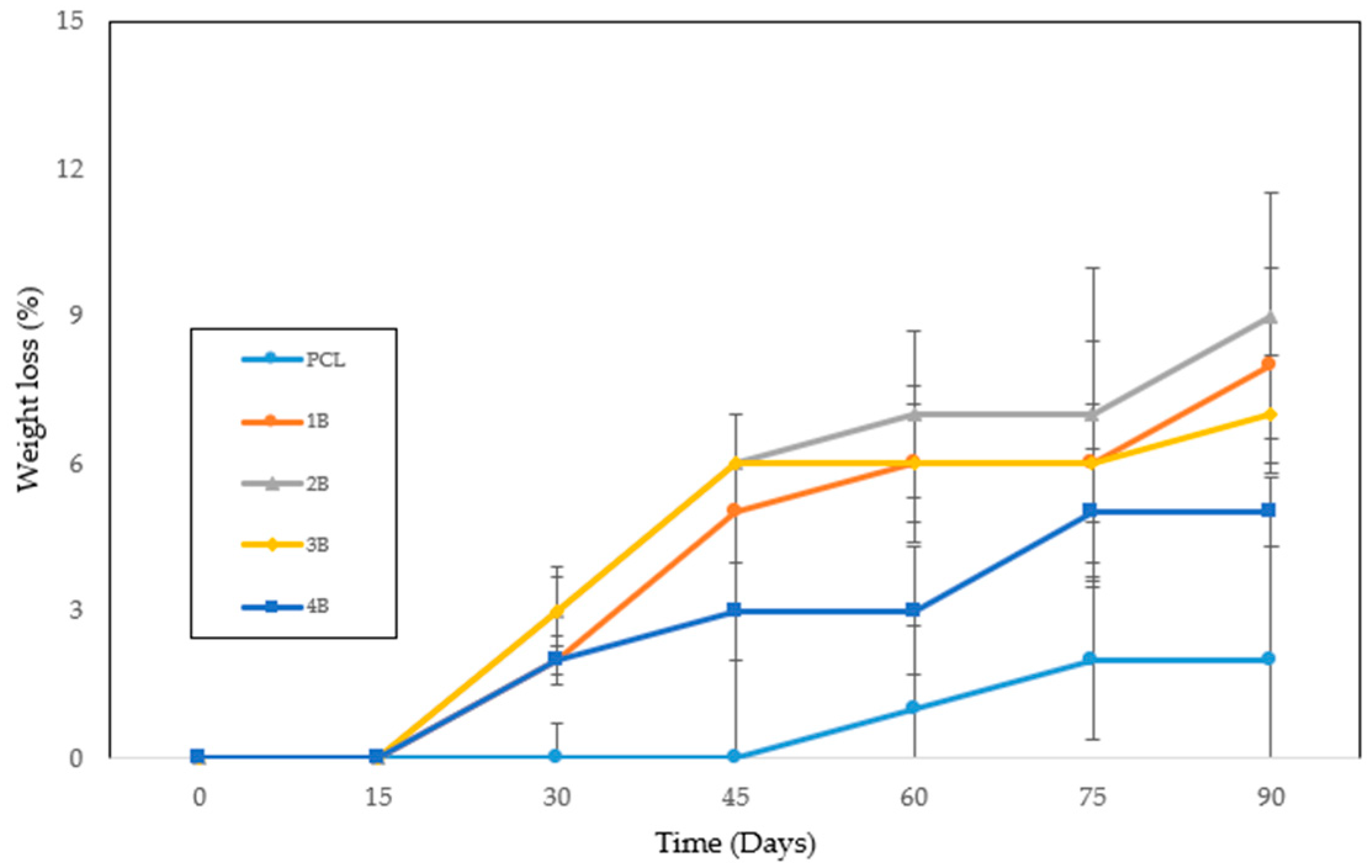
The in vitro degradation of polycaprolactone (PCL) scaffolds was assessed in phosphate-buffered saline (PBS) over a period of 90 days. Five different samples were studied: untreated PCL and four hydrothermally treated PCL samples (1B, 2B, 3B, and 4B) processed at temperatures ranging from 70 °C to 100 °C. The results (
Figure 10) indicate a slight increase in weight loss for the hydrothermally treated samples compared to the untreated PCL, suggesting that hydrothermal processing may influence the degradation behavior.
PCL is known for its slow degradation due to its high hydrophobicity and semicrystalline nature, with degradation mainly driven by hydrolytic cleavage of ester bonds. Studies consistently report that degradation rates are closely linked to polymer crystallinity, as crystalline domains hinder water penetration and enzymatic accessibility, while amorphous regions are more prone to hydrolysis [
1]. In our study, the moderate increase in weight loss observed for hydrothermally treated samples suggests that the treatment may induce subtle modifications to the crystalline organization of PCL [
43], thereby facilitating water uptake and accelerating hydrolytic attack. Although techniques such as FTIR or XRD could provide complementary confirmation at the molecular and structural levels, early-stage degradation often produces changes too subtle to be reliably detected within the 90-day window analyzed. Instead, the combined evidence from the morphological analysis, porosity characterization, and in vitro degradation behavior provides a robust indication that hydrothermal treatment alters scaffold microstructure in a way that impacts crystallinity and, consequently, degradation dynamics. In any case, there do not appear to be any significant differences between the treated samples, as can be seen in the overlap of the error bars at different times.
It should be noted that porosity and interconnectivity are not only crucial for internal tissue growth but also directly affect mass transport properties, such as nutrient diffusion and drug-loading capacity. Previous studies have shown that scaffold porosity regulates drug release kinetics and facilitates cellular nutrient transport within the 3D matrix [
17,
33]. In this work, we focused on the structural and physicochemical characterization of PCL scaffolds, which represent a necessary first step before evaluating their drug-loading and biological performance. Therefore, future research will extend this work towards in vitro drug release and cell compatibility studies in order to validate their potential in tissue engineering applications.
Alternative strategies, such as blending polymers with PLA or using ultrasonic irradiation, have been reported to improve the pore morphology and mechanical properties of PCL scaffolds. However, these approaches often require the incorporation of secondary components, whereas the hydrothermal process represents a solvent- and additive-free alternative that preserves the chemical composition of PCL. Importantly, our degradation results (
Figure 10) showed only a slight increase in weight loss of hydrothermally treated samples compared to untreated PCL, indicating that the treatment did not compromise the stability of the polymer. Therefore, hydrothermal pretreatment can be considered an environmentally friendly and scalable method for improving porosity and interconnectivity without the drawbacks associated with the removal of porogens or polymer blending.
4. Conclusions
In this study, we investigated the influence of hydrothermal treatment on the morphology and mechanical behavior of polycaprolactone scaffolds produced using supercritical CO2 foaming. The main outcomes highlight how pre- and post-foaming hydrothermal steps affect porosity, pore connectivity, and mechanical performance. Specifically, hydrothermal pre-treatment was shown to significantly enhance porosity and interconnectivity while maintaining mechanical integrity. This work demonstrates that hydrothermal treatment before supercritical CO2 foaming significantly enhances scaffold properties, while post-foaming treatment compromises structural integrity. Pre-foaming hydrothermal treatment at 373 K and 17.23 bar resulted in scaffolds with a porosity of 51.88%, a pore diameter of 0.17 mm, and a throat density of 156.8 throats/mm3, making them more suitable for tissue engineering applications. In contrast, post-foaming hydrothermal treatment led to pore collapse, mechanical weakness, and lower interconnectivity, rendering the scaffolds unsuitable for structural applications. Micro-computed tomography (µ-CT) confirmed an improved pore distribution, enhanced interconnectivity, and reduced non-foamed regions in the scaffolds subjected to pre-foaming treatment. Mechanical testing demonstrated that the pre-treated scaffolds maintained a compressive modulus within the range required for biomedical applications, ensuring their potential use in load-bearing environments. Furthermore, the in vitro degradation study revealed that the hydrothermally treated PCL scaffolds exhibited a slight increase in weight loss compared to the untreated PCL, suggesting that the hydrothermal treatment influences the degradation behavior and crystallinity of the polymer. Additionally, this study suggests that fine-tuning the hydrothermal parameters, such as temperature, pressure, and exposure time, could further optimize scaffold performance. These results highlight the importance of hydrothermal pre-treatment as a scalable and effective strategy for producing highly porous, mechanically stable scaffolds without additional porogens. Future research should investigate the in vitro and in vivo biological responses, scaffold degradation kinetics, and potential functionalization strategies to enhance bioactivity and promote tissue integration in clinical applications.