Physico-Chemical, Rheological, and Antiviral Properties of Poly(butylene succinate) Biocomposites with Terpene—Hydrophobized Montmorillonite
Abstract
1. Introduction
- (i)
- Prepare biocomposites with biodegradable polymer as the matrix and natural mineral filler are modified with natural, active compounds.
- (ii)
- Essential oils and terpenes are highly volatile, fragrant, and irritative and can cause corrosion of the processing machines and tools; therefore, the active compounds were introduced into the polymer matrix in the mineral carrier.
- (iii)
- The clay was modified via a solvent-free method.
- (iv)
- Due to terpenes exhibiting antiviral properties, also towards coronaviruses, antiviral properties of biocomposite films were investigated using bacteriophage Φ6 as a surrogate of SARS-CoV-2.
2. Materials and Methods
2.1. Materials
2.2. Modification of Montmorillonite
2.3. Preparation of the Biocomposite Films
2.4. Fourier Transformation Infrared Spectroscopy with Attenuated Total Reflection FTIR-ATR Analysis
2.5. X-Ray Diffraction (XRD) Analysis
2.6. Differential Scanning Calorimetry (DSC)
2.7. Dynamic Mechanical Thermal Analysis (DMTA)
2.8. Rheological Behavior
2.9. Thermogravimetric Analysis (TGA)
2.10. Scanning Electron Microscopy (SEM)
2.11. Testing of Mechanical Properties
2.12. Characterization of Barrier Properties
2.13. Contact Angle Measurement
2.14. Antioxidative Properties
2.15. Antiviral Tests
3. Results
3.1. Characterization of the Fillers
3.1.1. TGA of the Fillers
3.1.2. Characterization of the Fillers Using FTIR-ATR
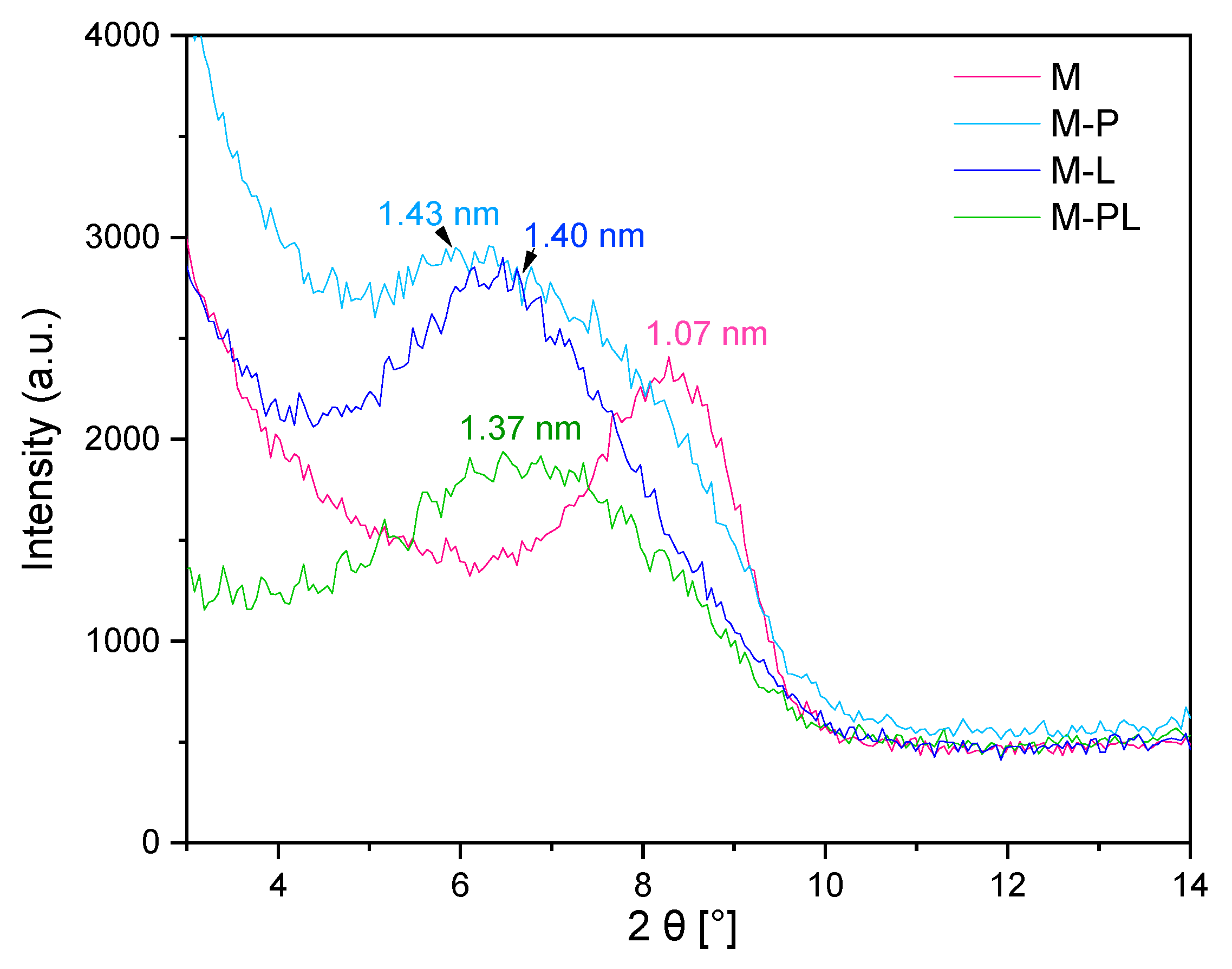
3.1.3. Analysis of the Fillers with XRD
3.2. Characterization of the PBS Films
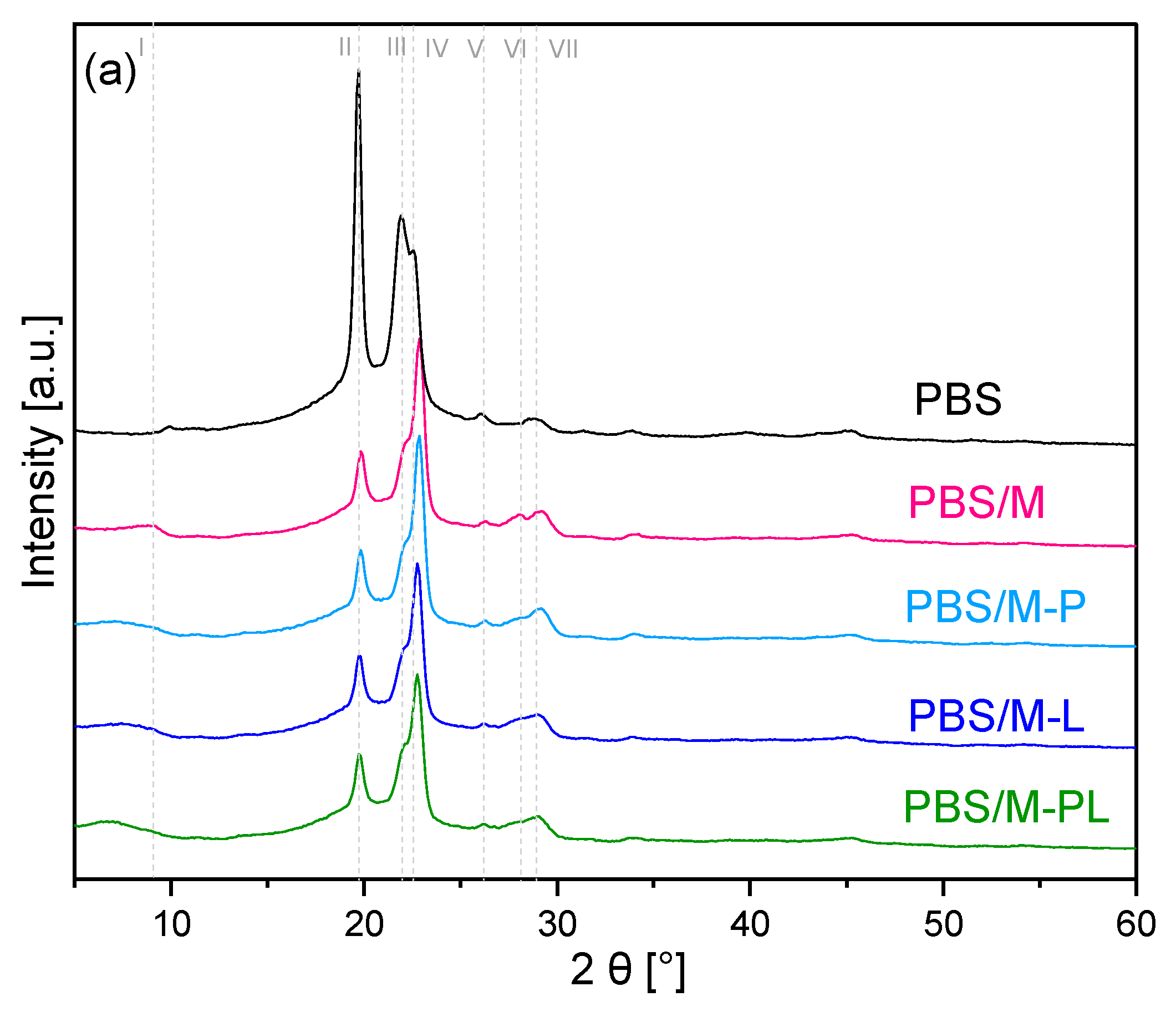
3.2.1. XRD Analysis of the Materials
3.2.2. DSC Characterization: Phase Transitions
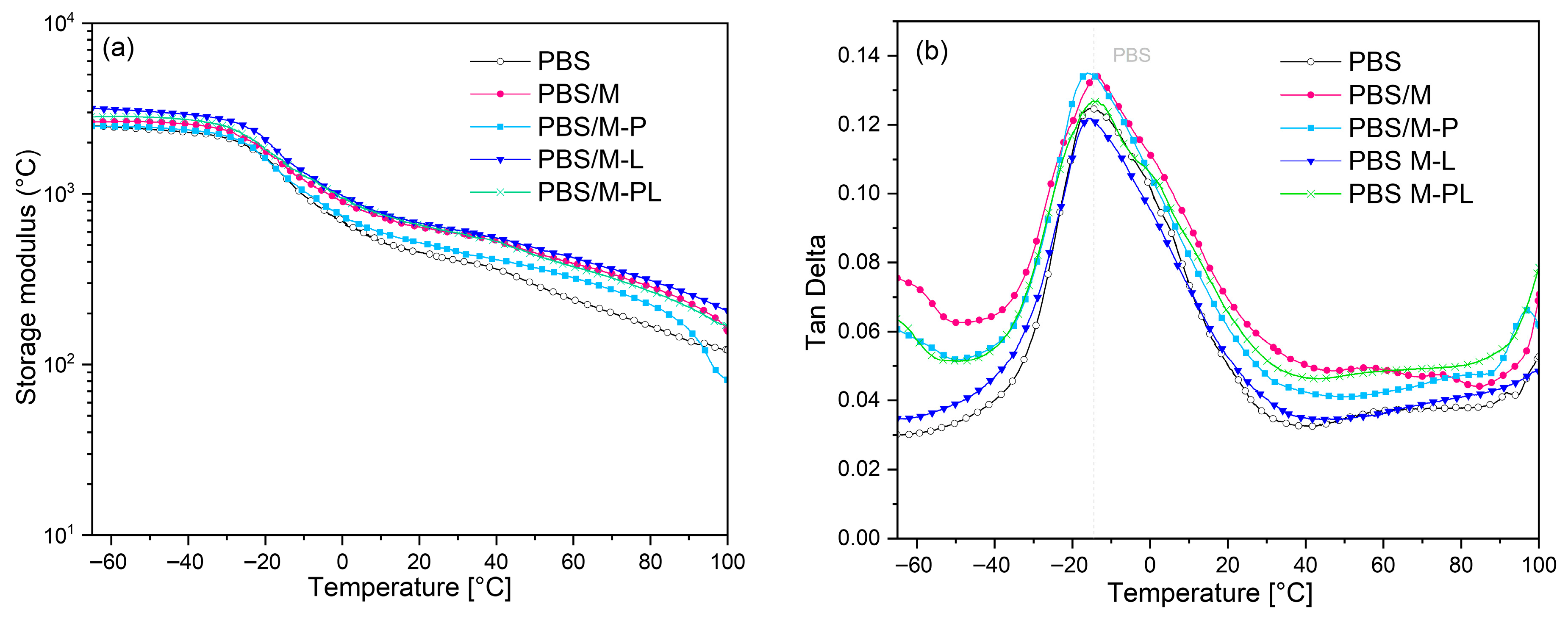
3.2.3. Thermomechanical Behavior
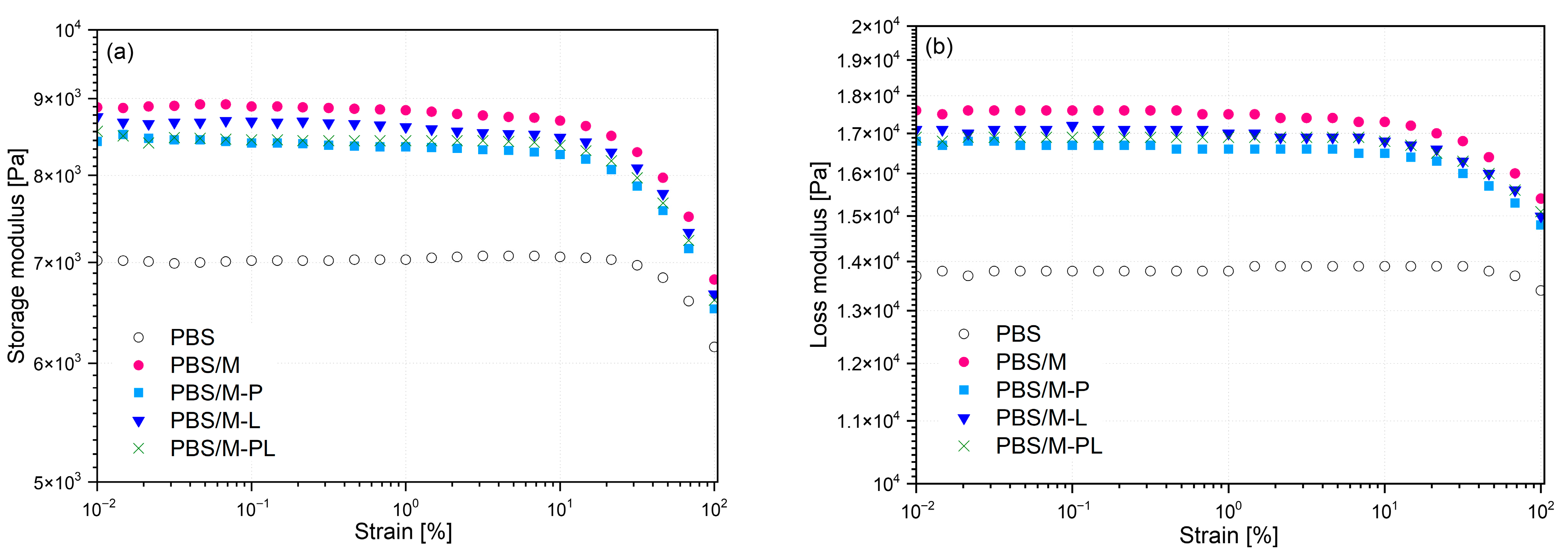
3.2.4. Results of the Rheological Studies
3.2.5. TGA Results
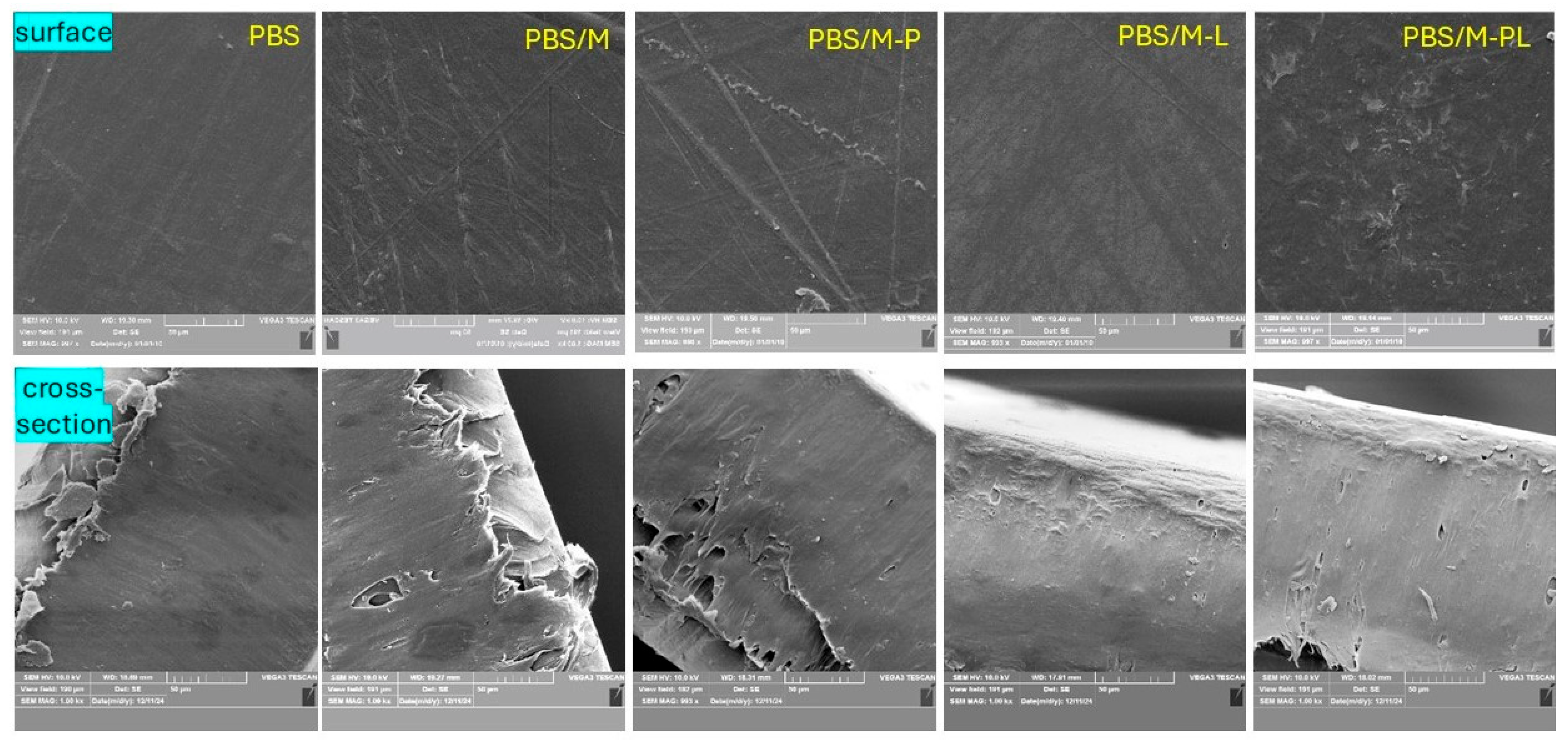
3.2.6. SEM Analysis
3.2.7. Mechanical Properties
3.2.8. Barrier Properties
3.2.9. Wettability—Surface Contact Angle
3.2.10. Antiviral Properties
4. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DSC | Diffraction scanning calorimetry |
| DMTA | Dynamic mechanical thermal analysis |
| FTIR | Fourier Transform Infrared Spectroscopy |
| L | Limonene |
| M | Montmorillonite |
| OTR | Oxygen transmission rate |
| WCA | Water contact angle |
| WVTR | Water vapor transmission rate |
| P | Pinene |
| PBS | Poly(butylene succinate) |
| SEM | Scanning Electron Microscopy |
| XRD | X-ray diffractometry |
References
- Available online: https://environment.ec.europa.eu/topics/circular-economy/green-claims_en (accessed on 27 December 2024).
- Punia Bangar, S.; Whiteside, W.S.; Chaudhary, V.; Parambil Akhila, P.; Sunooj, K.V. Recent functionality developments in Montmorillonite as a nanofiller in food packaging. Trends Food Sci. Technol. 2023, 140, 104148. [Google Scholar] [CrossRef]
- Barletta, M.; Aversa, C.; Ayyoob, M.; Gisario, A.; Hamad, K.; Mehrpouya, M.; Vahabi, H. Poly(butylene succinate) (PBS): Materials, processing, and industrial applications. Prog. Polym. Sci. 2023, 132, 101579. [Google Scholar] [CrossRef]
- Petchwattana, N.; Covavisaruch, S.; Wibooranawong, S.; Naknaen, P. Antimicrobial food packaging prepared from poly(butylene succinate) and zinc oxide. Measurement 2016, 93, 442–448. [Google Scholar] [CrossRef]
- Venkatesan, R.; Rajeswari, N. Preparation, Mechanical and Antimicrobial Properties of SiO2/ Poly(butylene adipate-co-terephthalate) Films for Active Food Packaging. Silicon 2019, 11, 2233–2239. [Google Scholar] [CrossRef]
- Rapacz-Kmita, A.; Szaraniec, B.; Mikołajczyk, M.; Stodolak-Zych, E.; Dzierzkowska, E.; Gajek, M.; Dudek, P. Multifunctional biodegradable polymer/clay nanocomposites with antibacterial properties in drug delivery systems. Acta Bioeng. Biomech. 2020, 22, 83–92. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, C.; Wang, Y.; Xie, L.; Li, W.; Li, B.; Guo, R.; Yan, H. Magnesium oxide/silver nanoparticles reinforced poly(butylene succinate-co-terephthalate) biofilms for food packaging applications. Food Packag. Shelf Life 2021, 30, 100748. [Google Scholar] [CrossRef]
- Belghazdis, M.; Hachem, E.-K. Clay and Clay Minerals: A Detailed Review. Int. J. Recent Technol. Appl. Sci. 2022, 4, 54–75. [Google Scholar] [CrossRef]
- Gao, F. Clay/polymer composites: The story. Mater. Today 2004, 7, 50–55. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Spychaj, T. Viscosity changes of aqueous carboxymethyl starch by partial crosslinking and montmorillonite addition. J. Appl. Polym. Sci. 2014, 131, app.40793. [Google Scholar] [CrossRef]
- Zhu, T.T.; Zhou, C.H.; Kabwe, F.B.; Wu, Q.Q.; Li, C.S.; Zhang, J.R. Exfoliation of montmorillonite and related properties of clay/polymer nanocomposites. Appl. Clay Sci. 2019, 169, 48–66. [Google Scholar] [CrossRef]
- Liu, P. Polymer modified clay minerals: A review. Appl. Clay Sci. 2007, 38, 64–76. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, F.; Zeng, G.; Jiang, M.; Yang, Z.; Yu, Z.; Zhu, M.; Shen, L. Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Sci. Total Environ. 2015, 518–519, 352–362. [Google Scholar] [CrossRef]
- Katti, D.R.; Ghosh, P.; Schmidt, S.; Katti, K.S. Mechanical Properties of the Sodium Montmorillonite Interlayer Intercalated with Amino Acids. Biomacromolecules 2005, 6, 3276–3282. [Google Scholar] [CrossRef]
- Demir, F.; Demir, B.; Yalcinkaya, E.E.; Cevik, S.; Odaci Demirkol, D.; Anik, U.; Timur, S. Amino acid intercalated montmorillonite: Electrochemical biosensing applications. RSC Adv. 2014, 4, 50107–50113. [Google Scholar] [CrossRef]
- Edraki, M.; Mousazadeh Moghaddampour, I.; Alinia-Ahandani, E.; Banimahd Keivani, M.; Sheydaei, M. Ginger intercalated sodium montmorillonite nano clay: Assembly, characterization, and investigation antimicrobial properties. Chem. Rev. Lett. 2021, 4, 120–129. [Google Scholar] [CrossRef]
- De Oliveira, L.H.; Trigueiro, P.; Souza, J.S.N.; De Carvalho, M.S.; Osajima, J.A.; Da Silva-Filho, E.C.; Fonseca, M.G. Montmorillonite with essential oils as antimicrobial agents, packaging, repellents, and insecticides: An overview. Colloids Surf. B Biointerfaces 2022, 209, 112186. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.R.; Farina, M.C.; Maia, A.; Torin, R.F.S. PLA Films Containing Montmorillonite Nanoclay–Citronella Essential Oil Hybrids for Potential Active Film Formulation. Macromol 2023, 3, 200–210. [Google Scholar] [CrossRef]
- Saad, H.; Ayed, A.; Srasra, M.; Attia, S.; Srasra, E.; Charrier-El Bouhtoury, F.; Tabbene, O. New Trends in Clay-Based Nanohybrid Applications: Essential Oil Encapsulation Strategies to Improve Their Biological Activity. In Nanoclay—Recent Advances, New Perspectives and Applications; Oueslati, W., Ed.; IntechOpen: London, UK, 2022. [Google Scholar]
- Kechagias, A.; Salmas, C.E.; Chalmpes, N.; Leontiou, A.A.; Karakassides, M.A.; Giannelis, E.P.; Giannakas, A. E Laponite vs. Montmorillonite as Eugenol Nanocarriers for Low Density Polyethylene Active Packaging Films. Nanomaterials 2024, 14, 1938. [Google Scholar] [CrossRef] [PubMed]
- El Miz, M.; Salhi, S.; El Bachiri, A.; Wathelet, J.P.; Tahani, A. Adsorption of essential oil components of Lavandula angustifolia on sodium modified bentonite from Nador (North-East Morocco). Afr. J. Biotechnol. 2014, 13, 3413–3425. [Google Scholar] [CrossRef][Green Version]
- Nguemtchouin, M.G.M.; Ngassoum, M.B.; Kamga, R.; Deabate, S.; Lagerge, S.; Gastaldi, E.; Chalier, P.; Cretin, M. Characterization of inorganic and organic clay modified materials: An approach for adsorption of an insecticidal terpenic compound. Appl. Clay Sci. 2015, 104, 110–118. [Google Scholar] [CrossRef]
- Giannakas, A.; Tsagkalias, I.; Achilias, D.S.; Ladavos, A. A novel method for the preparation of inorganic and organo-modified montmorillonite essential oil hybrids. Appl. Clay Sci. 2017, 14, 362–370. [Google Scholar] [CrossRef]
- De Souza, A.G.; Dos Santos, N.M.A.; Da Silva Torin, R.F.; Dos Santos Rosa, D. Synergic antimicrobial properties of Carvacrol essential oil and montmorillonite in biodegradable starch films. Int. J. Biol. Macromol. 2020, 164, 1737–1747. [Google Scholar] [CrossRef]
- Oliveira, L.H.; De Lima, I.S.; Da, S.; Neta, E.R.; Neta, E.R.; De Lima, S.G.; Trigueiro, P.; Osajima, J.A.; Da Silva-Filho, E.C.; Jaber, M.; et al. Essential oil in bentonite: Effect of organofunctionalization on antibacterial activities. Appl. Clay Sci. 2023, 245, 107158. [Google Scholar] [CrossRef]
- Tshibangu, D.S.T.; Matondo, A.; Lengbiye, E.M.; Inkoto, C.L.; Ngoyi, E.M.; Kabengele, C.N.; Bongo, G.N.; Gbolo, B.Z.; Kilembe, J.T.; Mwanangombo, D.T.; et al. Possible Effect of Aromatic Plants and Essential Oils Against COVID-19: Review of Their Antiviral Activity. J. Complement. Altern. Med. Res. 2020, 11, 10–22. [Google Scholar] [CrossRef]
- Reichling, J. Antiviral and Virucidal Properties of Essential Oils and Isolated Compounds—A Scientific Approach. Planta Medica 2022, 88, 587–603. [Google Scholar] [CrossRef]
- Da Silva, J.K.R.; Figueiredo, P.L.B.; Byler, K.G.; Setzer, W.N. Essential Oils as Antiviral Agents, Potential of Essential Oils to Treat SARS-CoV-2 Infection: An In-Silico Investigation. Int. J. Mol. Sci. 2020, 21, 3426. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb. Pathog. 2021, 152, 104620. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Seenipandi, A.; Javed, H.; Javed, H.; Sharma, C.; Hashiesh, H.M.; Goyal, S.N.; Jha, N.K.; Ojha, S. Can limonene be a possible candidate for evaluation as an agent or adjuvant against infection, immunity, and inflammation in COVID-19? Heliyon 2021, 7, e05703. [Google Scholar] [CrossRef] [PubMed]
- Astani, A.; Schnitzler, P. Antiviral activity of monoterpenes beta-pinene and limonene against herpes simplex virus in vitro. Iran. J. Microbiol. 2014, 6, 149–155. [Google Scholar] [PubMed]
- Zdanowicz, M.; Mizielińska, M.; Kowalczyk, A. Cast Extruded Films Based on Polyhydroxyalkanoate/Poly(lactic acid) Blend with Herbal Extracts Hybridized with Zinc Oxide. Polymers 2024, 16, 1954. [Google Scholar] [CrossRef]
- Zdanowicz, M. Influence of Epilobium parviflorum Herbal Extract on Physicochemical Properties of Thermoplastic Starch Films. Polymers 2023, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Bhetwal, A.; Maharjan, A.; Shakya, S.; Satyal, D.; Ghimire, S.; Khanal, P.R.; Parajuli, N.P. Isolation of Potential Phages against Multidrug-Resistant Bacterial Isolates: Promising Agents in the Rivers of Kathmandu, Nepal. BioMed Res. Int. 2017, 2017, 3723254. [Google Scholar] [CrossRef]
- Bonilla, N.; Rojas, M.I.; Netto Flores Cruz, G.; Hung, S.-H.; Rohwer, F.; Barr, J.J. Phage on tap–a quick and efficient protocol for the preparation of bacteriophage laboratory stocks. PeerJ 2016, 4, e2261. [Google Scholar] [CrossRef]
- ISO 22196-2011; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. International Organization for Standardization: Geneva, Switzerland, 2011.
- Elkhalifah, A.E.I.; Murugesan, T.; Bustam, M.A. Characterization of different cationic forms of montmorillonite by FTIR, XRD and TGA techniques. In Proceedings of the 2011 National Postgraduate Conference, Perak, Malaysia, 19–20 September 2011; IEEE: New York, NY, USA, 2011; pp. 1–6. [Google Scholar]
- Aghadavoud, A.; Rezaee Ebrahim Saraee, K.; Shakur, H.R.; Sayyari, R. Removal of uranium ions from synthetic wastewater using ZnO/Na-clinoptilolite nanocomposites. Radiochim. Acta 2016, 4, 809–819. [Google Scholar] [CrossRef]
- Li, K.-M.; Jiang, J.-G.; Tian, S.-C.; Chen, X.-J.; Yan, F. Influence of Silica Types on Synthesis and Performance of Amine–Silica Hybrid Materials Used for CO2 Capture. J. Phys. Chem. C 2014, 118, 2454–2462. [Google Scholar] [CrossRef]
- Danková, Z.; Mockovčiaková, A.; Dolinská, S. Influence of ultrasound irradiation on cadmium cations adsorption by montmorillonite. Desalination Water Treat. 2014, 52, 5462–5469. [Google Scholar] [CrossRef]
- Cataldo, F.; Keheyan, Y. Radiopolymerization of β(-)pinene: A case of chiral amplification. Radiat. Phys. Chem. 2006, 75, 572–582. [Google Scholar] [CrossRef]
- Essifi, K.; Hammani, A.; Berraaouan, D.; El Bachiri, A.; Fauconnier, M.-L.; Tahani, A. Montmorillonite nanoclay based formulation for controlled and selective release of volatile essential oil compounds. Mater. Chem. Phys. 2022, 277, 125569. [Google Scholar] [CrossRef]
- Masood, A.; Ahmed, N.; Razip Wee, M.F.M.; Patra, A.; Mahmoudi, E.; Siow, K.S. Atmospheric Pressure Plasma Polymerisation of D-Limonene and Its Antimicrobial Activity. Polymers 2023, 15, 307. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, M.; Heller-Kallai, L. Interlayer Cations as Reaction Directors in the Transformation of Limonene on Montmorillonite. Clays Clay Min. 1983, 31, 92–96. [Google Scholar] [CrossRef]
- Gomes, M.D.F.T.; Antunes, O.A.C. Autoxidation of limonene, α-pinene and β-pinene by dioxygen catalyzed by Co(OAc)2/bromide. J. Mol. Catal. A Chem. 1997, 121, 145–155. [Google Scholar] [CrossRef]
- Giannakas, A. Na-Montmorillonite Vs. Organically Modified Montmorillonite as Essential Oil Nanocarriers for Melt-Extruded Low-Density Poly-Ethylene Nanocomposite Active Packaging Films with a Controllable and Long-Life Antioxidant Activity. Nanomaterials 2020, 10, 1027. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G. Solid-state structure and thermal characteristics of a sustainable biobased copolymer: Poly(butylene succinate-co-furanoate). Thermochim. Acta 2017, 656, 112–122. [Google Scholar] [CrossRef]
- Charlon, S.; Marais, S.; Dargent, E.; Soulestin, J.; Sclavons, M.; Follain, N. Structure–barrier property relationship of biodegradable poly (butylene succinate) and poly [(butylene succinate)-co-(butylene adipate)] nanocomposites: Influence of the rigid amorphous fraction. Phys. Chem. Chem. Phys. 2015, 17, 29918–29934. [Google Scholar] [CrossRef]
- Pulikkalparambil, H.; Phothisarattana, D.; Promhuad, K.; Harnkarnsujarit, N. Effect of silicon dioxide nanoparticle on microstructure, mechanical and barrier properties of biodegradable PBAT/PBS food packaging. Food Biosci. 2023, 55, 103023. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Leontiou, A.; Moschovas, D.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Karakassides, A.; Avgeropoulos, A.; Proestos, C. Performance of Thyme Oil@Na-Montmorillonite and Thyme Oil@Organo-Modified Montmorillonite Nanostructures on the Development of Melt-Extruded Poly-L-lactic Acid Antioxidant Active Packaging Films. Molecules 2022, 27, 1231. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Bousmina, M. The relations between structure and mechanical properties of poly(butylene succinate-co-adipate)/montmorillonite nanocomposites. J. Polym. Eng. 2006, 26, 885–901. [Google Scholar] [CrossRef]
- Taleb, K.; Saidi-Besbes, S.; Pillin, I.; Grohens, Y. Biodegradable Poly(Butylene Succinate) Nanocomposites Based on Dimeric Surfactant Organomodified Clays with Enhanced Water Vapor Barrier and Mechanical Properties. ACS Omega 2022, 7, 43254–43264. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.F.; Wang, T.Y.; Jeng, R.J.; Wu, J.Y.; Teng, C.C. Biodegradable nanocomposites based on poly(butylene succinate)/organoclay. J. Polym. Environ. 2007, 15, 151–158. [Google Scholar] [CrossRef]
- Safakas, K.; Giotopoulou, I.; Giannakopoulou, A.; Katerinopoulou, K.; Lainioti, G.C.; Stamatis, H.; Barkoula, N.-M.; Ladavos, A. Designing Antioxidant and Antimicrobial Polyethylene Films with Bioactive Compounds/Clay Nanohybrids for Potential Packaging Applications. Molecules 2023, 28, 2945. [Google Scholar] [CrossRef]
- Someya, Y.; Nakazato, T.; Teramoto, N.; Shibata, M. Thermal and mechanical properties of poly(butylene succinate) nanocomposites with various organo-modified montmorillonites. J. Appl. Polym. Sci. 2004, 91, 1463–1475. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, K.; Okamoto, M. Structure−Property Relationship in Biodegradable Poly(butylene succinate)/Layered Silicate Nanocomposites. Macromolecules 2003, 36, 2355–2367. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, J.; Dong, Q.; Luan, D.; Tao, N.; Deng, S.; Wang, L.; Hao, Y.; Li, L. Development of organic-inorganic hybrid antimicrobial materials by mechanical force and application for active packaging. Food Packag. Shelf Life 2023, 37, 101060. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Zhang, S.; Ma, L.; Shi, G.; Yang, L. Crystallization and melting behavior of poly(butylene succinate)/silicon nitride composites: The influence of filler’s phase structure. Thermochim. Acta 2016, 627–629, 68–76. [Google Scholar] [CrossRef]
- Sasimowski, E.; Majewski, Ł.; Grochowicz, M. Efficiency of Twin-Screw Extrusion of Biodegradable Poly (Butylene Succinate)-Wheat Bran Blend. Materials 2021, 14, 424. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, J.; Li, L. Multiple melting behavior of poly(butylene succinate). Eur. Polym. J. 2007, 43, 3163–3170. [Google Scholar] [CrossRef]
- Proença, L.B.; Righetto, G.M.; Camargo, I.L.B.d.C.; Branciforti, M.C. Poly(acid lactic)-montmorillonite clay bionanocomposites loaded with tea tree oil for application in antibacterial wound healing. Hybrid. Adv. 2024, 6, 100201. [Google Scholar] [CrossRef]
- Bashir, M.A. Use of Dynamic Mechanical Analysis (DMA) for Characterizing Interfacial Interactions in Filled Polymers. Solids 2021, 2, 108–120. [Google Scholar] [CrossRef]
- Hato, M.J.; Ray, S.S.; Luyt, A.S. Melt-State Viscoelastic Properties of POSS-Containing Polyethylene Nanocomposites. Adv. Sci. Lett. 2011, 4, 3585–3589. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, J. Effects of filler–filler and polymer–filler interactions on rheological and mechanical properties of HDPE–wood composites. J. Appl. Polym. Sci. 2009, 111, 2806–2812. [Google Scholar] [CrossRef]
- Li, J.; Zhou, C.; Wang, G.; Zhao, D. Study on rheological behavior of polypropylene/clay nanocomposites. J. Appl. Polym. Sci. 2003, 89, 3609–3617. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Zhang, Y.; Yin, N. Rheological behavior of polypropylene/octavinyl polyhedral oligomeric silsesquioxane composites. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 526–533. [Google Scholar] [CrossRef]
- Coombs, S.J.; Kanso, M.A.; Giacomin, A.J. Cole–Cole relation for long-chain branching from general rigid bead–rod theory. Phys. Fluids 2020, 32, 093106. [Google Scholar] [CrossRef]
- Viñas-Ospino, A.; Anticona, M.; Esteve, M.J.; Blesa, J.; Mizielińska, M.; Zdanowicz, M. Poly(Butylene Succinate) Films Modified with Functional Deep Eutectic Solvent/Orange Peel Extract Systems. Food Packag. Shelf Life 2025, 52, 101614. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Sałasińska, K. Characterization of Thermoplastic Starch Plasticized with Ternary Urea-Polyols Deep Eutectic Solvent with Two Selected Fillers: Microcrystalline Cellulose and Montmorillonite. Polymers 2023, 15, 972. [Google Scholar] [CrossRef]
- Min, K.; Kim, M.; Choi, K.Y.; Lee, J.H.; Lee, S.-G. Effect of Layered Silicates on the Crystallinity and Mechanical Properties of HDPE/MMT Nanocomposite Blown Films. Polym. Bull. 2006, 57, 101–108. [Google Scholar] [CrossRef]
- Horst, M.F.; Quinzani, L.M.; Failla, M.D. Rheological and barrier properties of nanocomposites of HDPE and exfoliated montmorillonite. J. Thermoplast. Compos. Mater. 2014, 27, 106–125. [Google Scholar] [CrossRef]
- Deng, S.; Hou, M.; Ye, L. Temperature-dependent elastic moduli of epoxies measured by DMA and their correlations to mechanical testing data. Polym. Test. 2007, 26, 803–813. [Google Scholar] [CrossRef]
- Wong, K.J.; Yousif, B.F.; Low, K.O.; Ng, Y.; Tan, S.L. Effects of fillers on the fracture behaviour of particulate polyester composites. J. Strain Anal. Eng. Des. 2010, 45, 67–78. [Google Scholar] [CrossRef]
- Jajam, K.C.; Tippur, H.V. Quasi-static and dynamic fracture behavior of particulate polymer composites: A study of nano- vs. micro-size filler and loading-rate effects. Compos. Part B Eng. 2012, 43, 3467–3481. [Google Scholar] [CrossRef]
- Macieja, S.; Zdanowicz, M.; Mizielińska, M.; Jankowski, W.; Bartkowiak, A. Poly(butylene succinate) Film Coated with Hydroxypropyl Methylcellulose with Sea Buckthorn Extract and Its Ethosomes—Examination of Physicochemical and Antimicrobial Properties Before and After Accelerated UV Aging. Polymers 2025, 17, 1784. [Google Scholar] [CrossRef]
- Szymczyk, A.; Paszkiewicz, S.; Pawelec, I.; Lisiecki, S.; Jotko, M.; Špitalský, Z.; Mosnáček, J.; Rosłaniec, Z. Oxygen Barrier Properties and Melt Crystallization Behavior of Poly(Ethylene Terephthalate)/Graphene Oxide Nanocomposites. J. Nanomater. 2015, 2015, 382610. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Irska, I.; Walkowiak, K.; Włodarczyk, F.; Zdanowicz, M.; Piesowicz, E.; Barczewski, M. Sustainable and Eco-Friendly Single- and Multilayer Polyester Foils (Laminates) from Polylactide and Poly(Ethylene 2,5-Furandicarboxylate). Molecules 2025, 30, 178. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Szymczyk, A.; Pawlikowska, D.; Irska, I.; Piesowicz, E.; Jotko, M.; Lisiecki, S.; Bartkowiak, A.; Sieradzka, M.; Fryczkowski, R.; et al. Improvement of Barrier Properties of Glycol Modified Poly(Ethylene Terephthalate) Based Nanocomposites Containing Graphene Derivatives Forms. Polimery 2017, 62, 868–874. [Google Scholar] [CrossRef]
- Ilsouk, M.; Raihane, M.; Rhouta, B.; Meri, R.M.; Zicans, J.; Vecstaudža, J.; Lahcini, M. The relationship of structure, thermal and water vapor permeability barrier properties of poly(butylene succinate)/organomodified beidellite clay bionanocomposites prepared by in situ polycondensation. RSC Adv. 2020, 10, 7314–37326. [Google Scholar] [CrossRef]
- Delorme, A.E.; Radusin, T.; Myllytie, P.; Verney, V.; Askanian, H. Enhancement of Gas Barrier Properties and Durability of Poly(butylene succinate-co-butylene adipate)-Based Nanocomposites for Food Packaging Applications. Nanomaterials 2022, 12, 978. [Google Scholar] [CrossRef] [PubMed]
- Jaime, S.B.M.; Alves, R.M.V.; Bókoli, P.F.J. Moisture and oxygen barrier properties of glass, PET and HDPE bottles for pharmaceutical products. J. Drug Deliv. Sci. Technol. 2022, 71, 103330. [Google Scholar] [CrossRef]
- Ordon, M.; Zdanowicz, M.; Nawrotek, P.; Stachurska, X.; Mizielińska, M. Polyethylene Films Containing Plant Extracts in the Polymer Matrix as Antibacterial and Antiviral Materials. Int. J. Mol. Sci. 2021, 22, 13438. [Google Scholar] [CrossRef] [PubMed]
- Mizielińska, M.; Ordon, M.; Burdajewicz, W.; Nawrotek, P.; Sternal, J.; Okręglicki, M. The Antifungal and Antiviral Activity of Coatings Containing Zinc Oxide Nanoparticles and Verbascum L. or Formitopsis betulina Extracts and Their Influence on the Quality of Strawberries after Storage. Coatings 2024, 14, 260. [Google Scholar] [CrossRef]
- Mizielińska, M.; Bartkowiak, A. The Influence of Zinc Oxide and Zinc Stearate on the Antimicrobial Activity of Coatings Containing Raspberry and Chokeberry Extracts. Molecules 2024, 29, 3493. [Google Scholar] [CrossRef]
- Jankowski, W.; Mizielińska, D.; Mizielińska, M. Influence of Q-SUN Irradiation on Antimicrobial and Antiviral Activity of Tea Tree Oil-Based Coatings on Polypropylene Films. Appl. Sci. 2025, 15, 10017. [Google Scholar] [CrossRef]
- Ochirbat, E.; Zbonikowski, R.; Sulicka, A.; Bończak, B.; Bonarowska, M.; Łoś, M.; Malinowska, E.; Hołyst, R.; Paczesny, J. Heteroaggregation of virions and microplastics reduces the number of active bacteriophages in aqueous environments. J. Environ. Qual. 2023, 52, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Richter, Ł.; Księżarczyk, K.; Paszkowska, K.; Janczuk-Richter, M.; Niedziółka Jönsson, J.; Gapiński, J.; Łoś, M.; Hołyst, R.; Paczesny, J. Adsorption of bacteriophages on polypropylene labware affects the reproducibility of phage research. Sci. Rep. 2021, 11, 7387. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Carol, L.; Lednicky, J.A.; Eiguren-Fernandez, A.; Hering, S.; Fan, Z.H.; Wu, C.-Y. Determination of the distribution of infectious viruses in aerosol particles using water-based condensational growth technology and a bacteriophage MS2 model. Aerosol Sci. Technol. 2019, 53, 583–593. [Google Scholar] [CrossRef]
- Turgeon, N.; Toulouse, M.-J.; Martel, B.; Moineau, S.; Duchaine, C. Comparison of Five Bacteriophages as Models for Viral Aerosol Studies. Appl. Environ. Microbiol. 2014, 80, 4242–4250. [Google Scholar] [CrossRef]
- Prussin, A.J.; Schwake, D.O.; Lin, K.; Gallagher, D.L.; Buttling, L.; Marr, L.C. Survival of the Enveloped Virus Phi6 in Droplets as a Function of Relative Humidity, Absolute Humidity, and Temperature. Appl. Environ. Microbiol. 2018, 84, e00551-18. [Google Scholar] [CrossRef]






| Filler | Modifier Type | Modifier Content |
|---|---|---|
| M-P | P (pinene) | 13.8% |
| M-L | L (limonene) | 6.6% |
| M-PL | PL (pinene + limonene) | 8.8% |
| Sample | Peaks Assigned to MMT (d-Spacing) | Peaks Assigned to PBS Matrix | |||||
|---|---|---|---|---|---|---|---|
| Line (Figure 3a) | I (001) | VI (005) | II (020) | III (021) | IV (110) | V (121) | VII (111) |
| PBS | - | - | 19.62° | 21.92° | 22.55° | 26.1° | 29.17° |
| PBS/M | 7.05° (1.25 nm) | 28.01° | 19.82° | - | 22.97° | - | 29.07° |
| PBS/M-P | 6.79° (1.30 nm) | 28.01° | 19.82° | - | 22.76° | - | 29.07° |
| PBS/M-L | 6.72° (1.32 nm) | 28.01° | 19.82° | - | 22.76° | - | 29.07° |
| PBS/M-PL | 6.75° (1.31 nm) | 28.01° | 19.82° | - | 22.76° | - | 29.07° |
| Sample | Young’s Modulus [MPa] | Tensile Strength [MPa] | Elongation at Break [%] |
|---|---|---|---|
| PBS | 473 (±34) c | 28 (±1.5) a,b | 18 (±5.0) a |
| PBS/M | 511 (±72) c | 34 (±2.8) a | 11 (±1.2) b |
| PBS/M-P | 718 (±72) a,b | 30 (±3.4) a,b | 8 (±1.4) b |
| PBS M-L | 684 (±42) b | 27 (±3.6) b | 8 (±1.1) b |
| PBS/M-PL | 625 (±34) b | 32 (±3.3) a,b | 10 (±1.4) b |
| Sample | Barrier Properties | WCA [°] | ||
|---|---|---|---|---|
| OTR RH 0% [cm3/m2∙24 h] | WVTRRH 100% [g/m2∙24 h] | WVTRRH 90% * [g/m2/24 h] | ||
| PBS | 52 (±3.2) | 21 (±1.1) | 19 (±1.0) | 67 (±2.7) d |
| PBS/M | 39 (±3.4) | 11 (±0.2) | 10 (±0.1) | 73 (±2.8) b,c |
| PBS/M-P | 38 (±7.5) | 13 (±1.4) | 12 (±1.3) | 77 (±0.9) a,b |
| PBS M-L | 38 (±1.6) | 12 (±1.9) | 11 (±1.7) | 72 (±1.2) c |
| PBS/M-PL | 42 (±3.7) | 13 (±0.2) | 12 (±0.1) | 77 (±2.0) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdanowicz, M.; Barczewski, M.; Mizielińska, M.; Miądlicki, P. Physico-Chemical, Rheological, and Antiviral Properties of Poly(butylene succinate) Biocomposites with Terpene—Hydrophobized Montmorillonite. Polymers 2025, 17, 2984. https://doi.org/10.3390/polym17222984
Zdanowicz M, Barczewski M, Mizielińska M, Miądlicki P. Physico-Chemical, Rheological, and Antiviral Properties of Poly(butylene succinate) Biocomposites with Terpene—Hydrophobized Montmorillonite. Polymers. 2025; 17(22):2984. https://doi.org/10.3390/polym17222984
Chicago/Turabian StyleZdanowicz, Magdalena, Mateusz Barczewski, Małgorzata Mizielińska, and Piotr Miądlicki. 2025. "Physico-Chemical, Rheological, and Antiviral Properties of Poly(butylene succinate) Biocomposites with Terpene—Hydrophobized Montmorillonite" Polymers 17, no. 22: 2984. https://doi.org/10.3390/polym17222984
APA StyleZdanowicz, M., Barczewski, M., Mizielińska, M., & Miądlicki, P. (2025). Physico-Chemical, Rheological, and Antiviral Properties of Poly(butylene succinate) Biocomposites with Terpene—Hydrophobized Montmorillonite. Polymers, 17(22), 2984. https://doi.org/10.3390/polym17222984








