Eco-Friendly Biopolymer Composite Sheet Derived from Water Hyacinth Reinforced with Cassava Chip: Optimal Conditions for Mixing, Blending, and Forming
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Collection and Preparation of WH
2.3. Preparation of Cassava Chip (CC)
2.4. Characterizations of WH and CC
2.5. Extraction of Cellulose from WH Powder
2.5.1. Lignin Removal
2.5.2. Bleaching Step
2.6. Conversion of Cellulose into Carboxymethylcellulose (CMC)
2.7. Optimal Ratios of CMC:CC for Mixing, Blending and Forming
2.8. Characterizations of Composite Biopolymer Sheet
2.8.1. Scanning Electron Microscope (SEM) Analysis
2.8.2. X-Ray Diffraction (XRD)
2.8.3. Moisture Content
2.8.4. Water Solubility
2.8.5. Oxygen Transmission Rate (OTR)
2.8.6. Tensile Strength Testing
3. Results and Discussion
3.1. Raw Materials of Water Hyacinth (WH) and Cassava Chip (CC)
3.2. Characterizations of WH and CC
3.3. Characterizations of Cellulose Extract and Its Yield
3.4. Characterizations of Carboxymethylcellulose (CMC) Extract and Its Yield
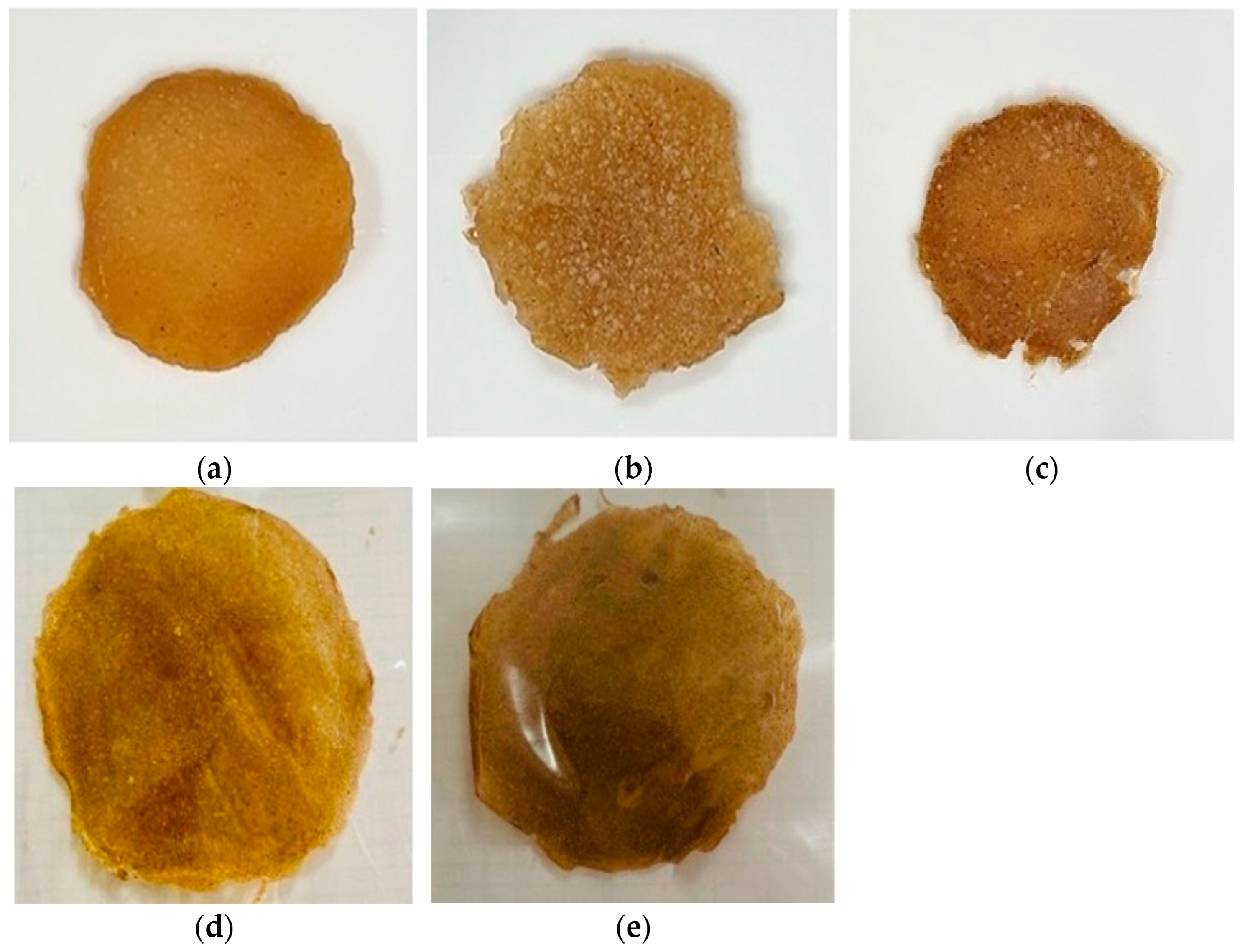
3.5. Composite Biopolymer Sheets
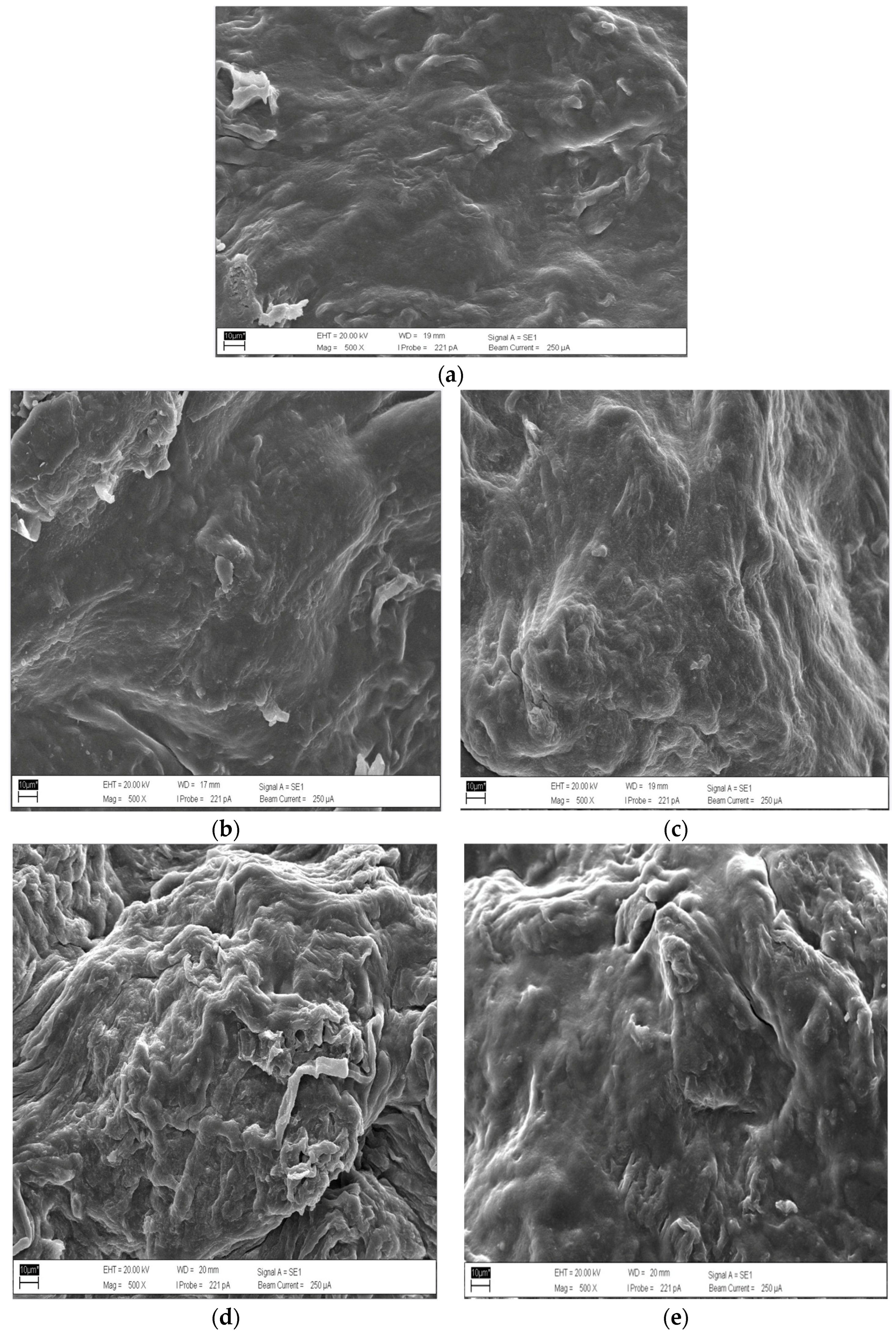
3.6. Scanning Electron Microscope (SEM) Technique
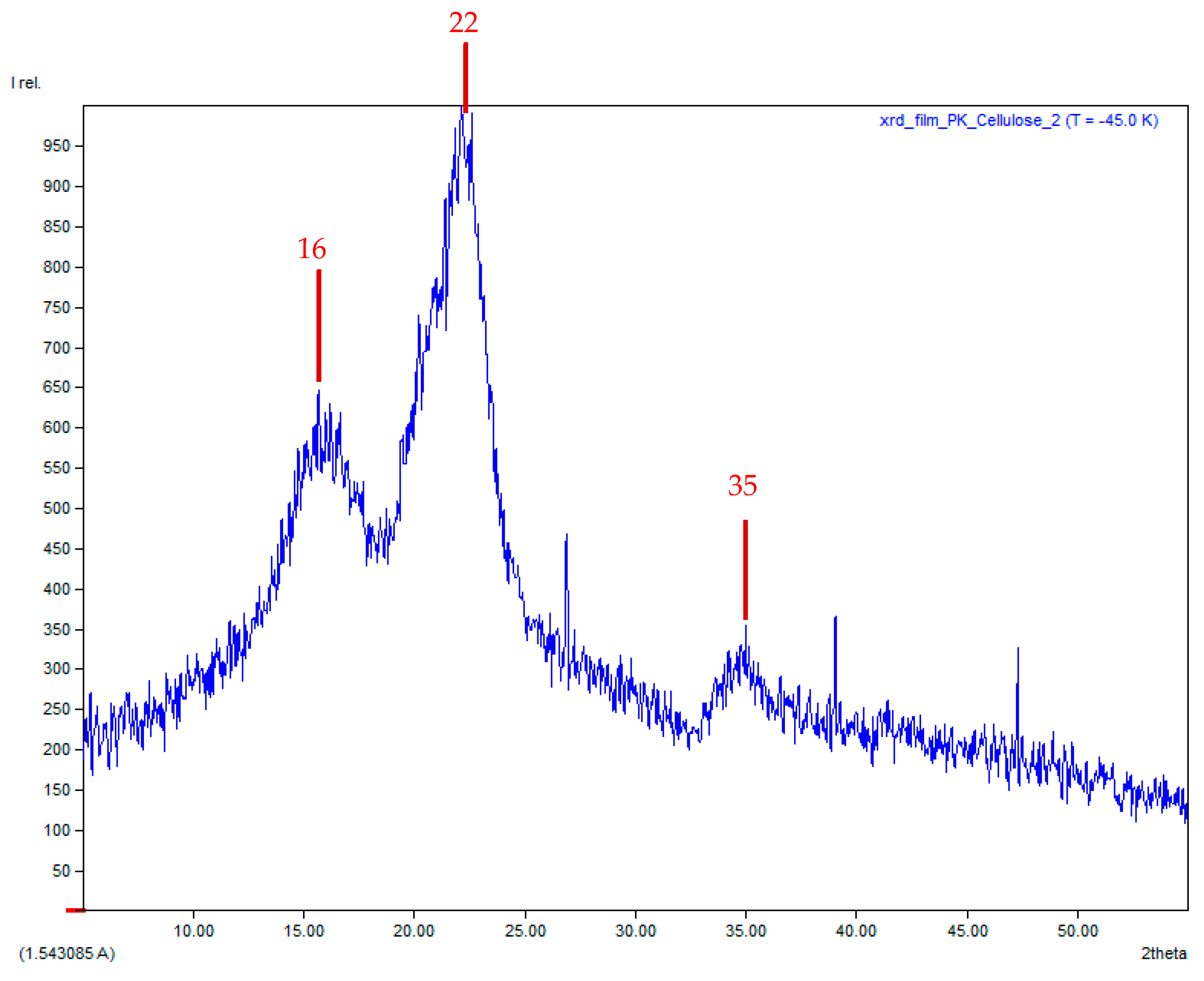
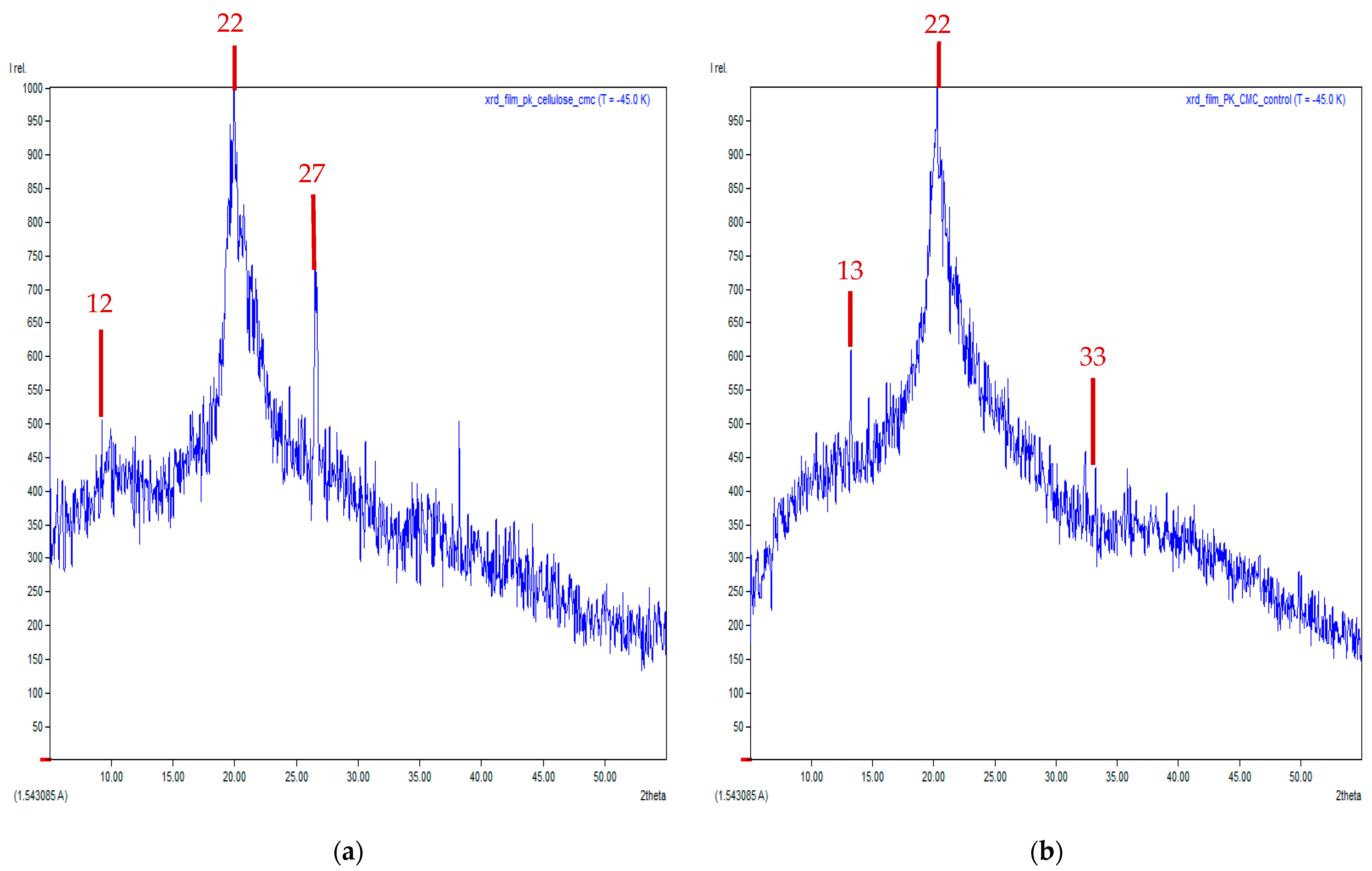
3.7. X-Ray Diffraction (XRD) Technique
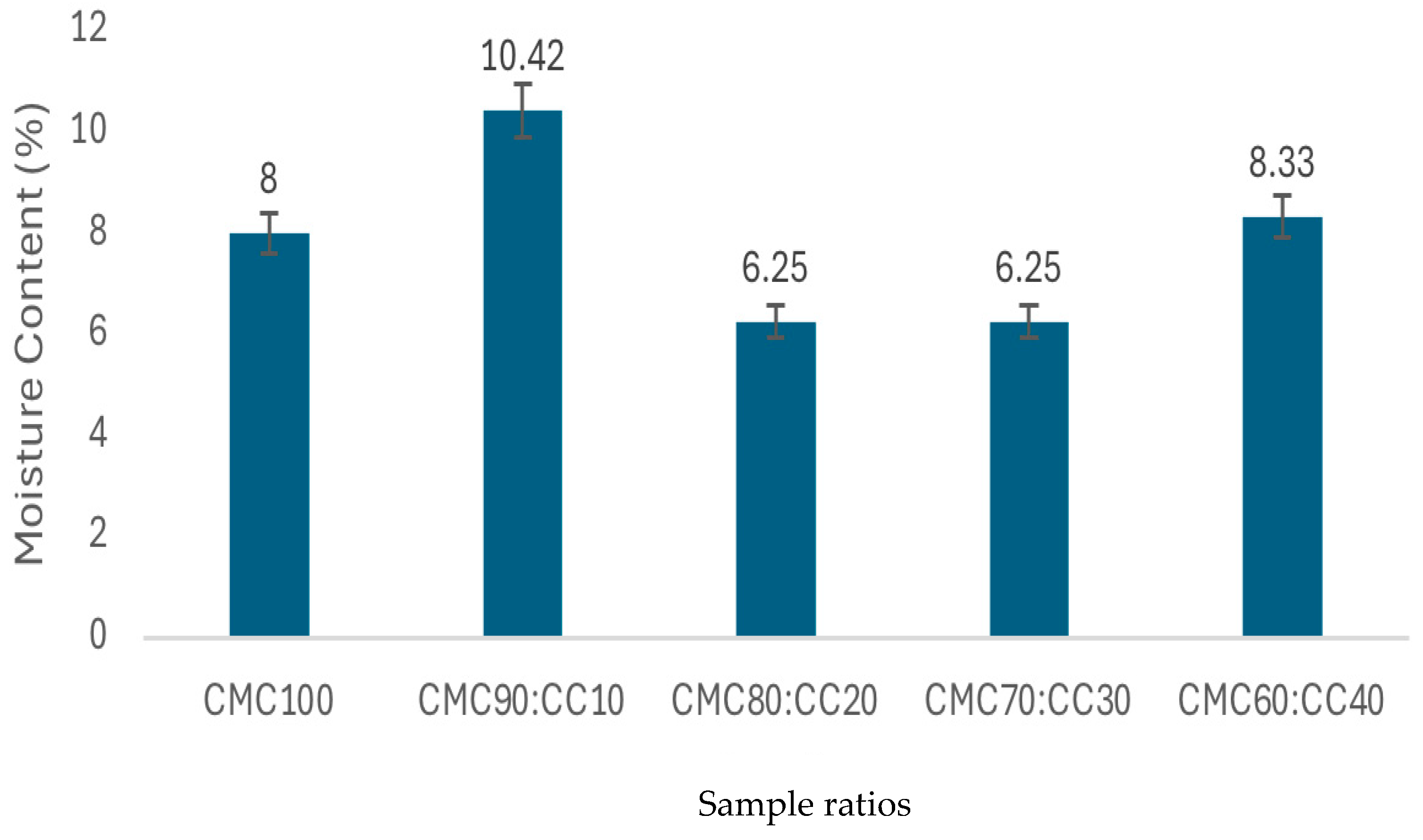
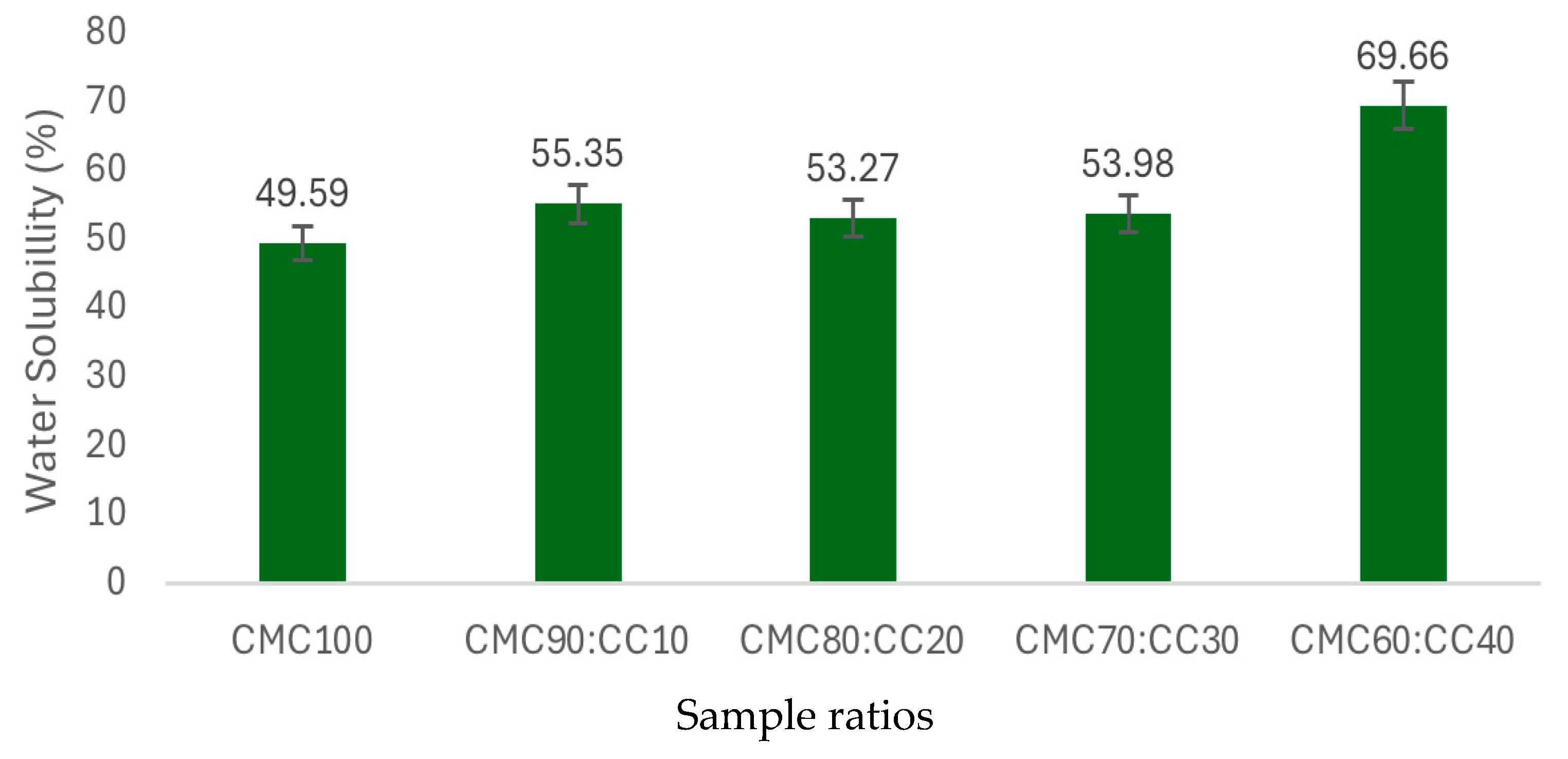
3.8. Moisture Content and Water Solubility
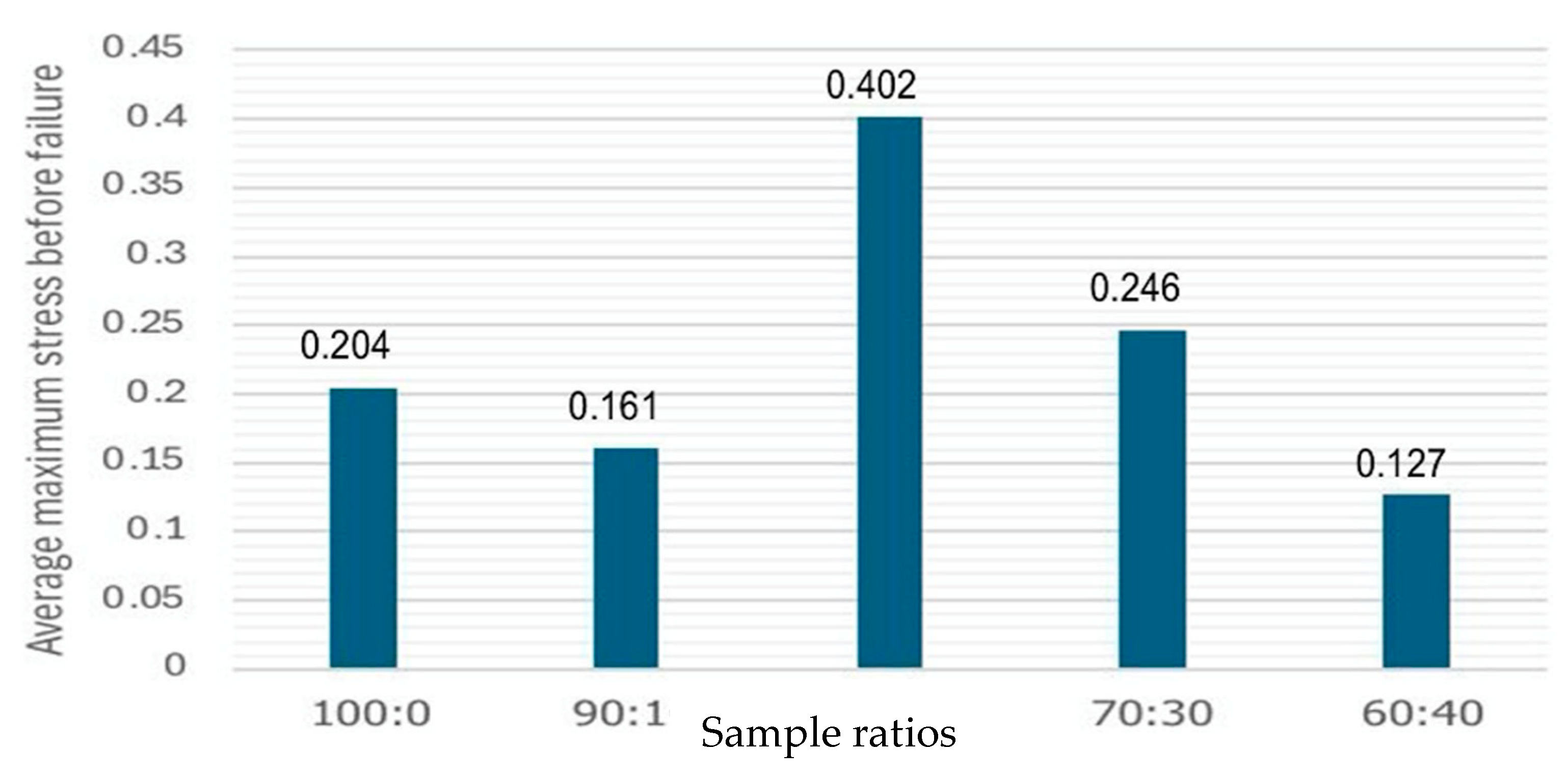
3.9. Tensile Strength
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdel-Sabour, M.F. Water hyacinth: Available and under-utilized biomass. J. Appl. Sci. Res. 2010, 6, 1245–1251. [Google Scholar]
- Bajpai, S.; Nemade, R.P. An integrated biorefinery approach for the valorization of water hyacinth towards circular bioeconomy: A review. Environ. Sci. Pollut. Res. 2023, 30, 39494–39536. [Google Scholar] [CrossRef]
- Patel, S.; Kalia, V.C.; Kumar, R.; Kataria, R.; Kumar, P. Water hyacinth: A potential source for value-added products: A review. Biores. Technol. Rep. 2021, 15, 100787. [Google Scholar]
- Villamagna, A.M.; Murphy, B.R. Ecological and socio-economic impacts of invasive water hyacinth (Eichhornia crassipes): A review. Freshw. Biol. 2010, 55, 282–298. [Google Scholar] [CrossRef]
- Gunnarsson, C.C.; Petersen, C.M. Water hyacinths as a resource in agriculture and energy production: A literature review. Waste Manag. 2007, 27, 117–129. [Google Scholar] [CrossRef]
- Rezania, S.; Ponraj, M.; Din, M.F.M.; Songip, A.R.; Sairan, F.M.; Chelliapan, S. The diverse applications of water hyacinth with main focus on sustainable energy and production for new era: An overview. Renew. Sustain. Energy Rev. 2015, 41, 943–954. [Google Scholar] [CrossRef]
- Mishima, D.; Kuniki, M.; Sei, K.; Soda, S.; Ike, M.; Fujita, M. Ethanol production from candidate energy crops: Water hyacinth (Eichhornia crassipes) and water lettuce (Pistia stratiotes L.). Bioresour. Technol. 2008, 99, 2495–2500. [Google Scholar] [CrossRef]
- Ofoefule, A.U.; Uzodinma, E.O.; Onukwuli, O.D. Comparative study of the effect of different pretreatment methods on the biogas yield from water hyacinth (Eichhornia crassipes). Int. J. Phys. Sci. 2009, 4, 535–539. [Google Scholar]
- Gajalakshmi, S.; Abbasi, S.A. High-rate composting–vermicomposting of water hyacinth (Eichhornia crassipes, Mart. Solms). Bioresour. Technol. 2002, 83, 235–239. [Google Scholar] [CrossRef]
- Hossain, M.T.; Rahman, M.M. Utilization of water hyacinth (Eichhornia crassipes) as compost: A review. Int. J. Environ. Agric. Res. 2015, 1, 33–39. [Google Scholar]
- Lata, N.; Dubey, V. Water hyacinth (Eichhornia crassipes) as a potential source of animal feed: A review. J. Exp. Zoo. India 2010, 13, 67–72. [Google Scholar]
- Valipour, A.; Raman, V.K.; Ghole, V.S. A new approach for wastewater treatment by flotation using Moringa oleifera and water hyacinth. Econ. Eng. 2015, 77, 76–84. [Google Scholar]
- Mishra, S.; Maiti, A. The efficacy of water hyacinth in the remediation of arsenic and chromium from contaminated water. Environ. Monit. Assess. 2017, 189, 559. [Google Scholar]
- Rezania, S.; Ponraj, M.; Talaiekhozani, A. Perspectives of phytoremediation using water hyacinth for removal of heavy metals, organic and inorganic pollutants in wastewater. J. Environ. Manag. 2015, 163, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Ayanda, I.O.; Tolulope, A.; Asuwaju, P.F. Eichhornia crassipes (Mart.) Solms: Uses, Challenges, Threats, and Prospects. Sci. World J. 2020, 2020, 3452172. [Google Scholar] [CrossRef]
- Santana, R.F.; Bonomo, R.C.F.; Gandolfi, O.R.R.; Capparelli Mattoso, L.H.; Bonomo, R. Cassava starch-based bio-composites reinforced with natural fibers: A review of recent developments. Composi. Part A Appl. Sci. Manufac. 2018, 115, 269–284. [Google Scholar] [CrossRef]
- Prachayawarakorn, J.; Chaiwatyothin, S.; Mueangta, S.; Hanchana, P. Effect of jute and kapok fibers on properties of thermoplastic cassava starch composites. Mater. Des. 2013, 47, 309–315. [Google Scholar] [CrossRef]
- Daramola, O.O.; Aransiola, S.A.; Adewumi, D.F.; Ogbonna, C.N. Bio-composite of cassava starch reinforced with plantain Pseudostem microfibrillated cellulose. Heliyon 2019, 5, e01564. [Google Scholar]
- Bertuzzi, M.A.; Castro Vidaurre, E.F.; Armada, M.; Gottifredi, J.C. Water vapor permeability of edible starch based-films. J. Food Eng. 2007, 80, 972–978. [Google Scholar] [CrossRef]
- Svamsuria, M.M.F.; Marzukia, H.; Setiawan, D.W.A.; Rusmania, T.A. Synthesis of water hyacinth/cassava starch composite as an environmentally friendly plastic solution. Equilib. J. Chem. Eng. 2023, 7, 137–146. [Google Scholar] [CrossRef]
- Palanisamy, C.P.; Cui, B.; Zhang, H.; Jayaraman, S.; Muthukaliannan, G.K. A comprehensive review on corn starch-based nanomaterials: Properties, simulations, and applications. Polymers 2020, 12, 2161. [Google Scholar] [CrossRef]
- Syafri, E.; Jamaluddin; Wahono, S.; Irwan, A.; Asrofi, M.; Sari, N.H.; Fudholi, A. Characterization and properties of cellulose microfibers from water hyacinth filled sago starch biocomposites. Int. J. Biol. Macromol. 2019, 137, 119–125. [Google Scholar] [CrossRef]
- Sanjay, M.R.; Madhu, P.; Jawaid, M.; Senthamaraikannan, P.; Senthil, S.; Pradeep, S. Characterization and properties of natural fiber polymer composites: A comprehensive review. J. Clean. Prod. 2018, 172, 566–581. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Grossmann, M.V.E.; Yamashita, F.; Pineda, E.A.G. Antimicrobial, mechanical, and barrier properties of cassava starch-chitosan films incorporated with oregano essential oil. J. Agri. Food Chem. 2019, 67, 5202–5213. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.A.; Nazir, M.S.; Wahjoedi, B.A. Development of value-added biomaterials from oil palm agro-wastes. Bio Res. 2013, 8, 2835–2851. [Google Scholar]
- Ramesh, M.; Palanikumar, K.; Reddy, K.H. Plant fiber base bio-composites: Sustainable and renewable green materials. Renew. Sustain. Energy Rev. 2017, 79, 558–584. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Gupta, R.K. Review: Raw natural fiber–based polymer composites. Int. J. Polym. Anal. Charact. 2014, 19, 256–271. [Google Scholar] [CrossRef]
- Ungprasoot, P.; Muanruksa, P.; Tanamool, V.; Winterburn, J.; Kaewkannetra, P. Valorization of aquatic weed and agricultural residues for innovative biopolymer production and their biodegradation. Polymers 2021, 13, 2838. [Google Scholar] [CrossRef]
- Sari, N.H.; Wardana, I.N.G.; Irawan, Y.S.; Siswanto, E. Characterization of the chemical, physical, and mechanical properties of NaOH-treated natural cellulosic fibers from corn husks. J. Nat. Fibers 2018, 15, 545–558. [Google Scholar] [CrossRef]
- Rozman, H.D.; Tan, K.W.; Kumar, R.N.; Abubakar, A.; Mohd Ishak, Z.A.; Ismail, H. The effect of lignin as a compatibilizer on the physical properties of coconut fiber–polypropylene composites. Eur. Polym. J. 2000, 36, 1483–1494. [Google Scholar] [CrossRef]
- Zhai, H.; Li, J.; Chen, X. Development of biodegradable films from cassava starch blended with natural fibers for packaging applications. Carbo. Polym. 2021, 251, 117026. [Google Scholar]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- French, D.A. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Nguyen, T.B.H. Treatment of water hyacinth fibers to improve mechanical and microstructural properties of green composite materials. Nano Hybrids Compos. 2022, 35, 111–122. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Processing and characterization of natural cellulose fibers/thermoset polymer composites. Carb. Polym. 2014, 109, 102–117. [Google Scholar] [CrossRef]
- Anggraini, L.; Setyawan, P.D.; Rahmaniah, W. Mechanical properties of cassava starch-based bio-composite reinforced with water hyacinth fiber. J. Nat. Fibers 2021, 18, 1166–1175. [Google Scholar]
- Bakrim, B.W.; Ezzariai, A.; Karouach, F.; Sobeh, M.; Kibret, M.; Hafidi, M.; Kouisni, L.; Yasri, A. Eichhornia crassipes (Mart.) Solms: A Comprehensive Review of Its Chemical Composition, Traditional Use, and Value-Added Products. Front. Pharmacol. 2022, 13, 842511. [Google Scholar] [CrossRef]
- Fajrin, J.; Zhuge, Y.; Bullen, F.; Wang, H. Significance of specimen configuration on dynamic mechanical analysis of pultruded fibre reinforced polymer. Compos. Part B Eng. 2016, 103, 31–39. [Google Scholar]



| Parameters | Analytical Technique |
|---|---|
| Cellulose | Acid Detergent Fiber (ADF) |
| Crude Fiber | Crude Fiber Analysis |
| Carbohydrate | Phenol-Sulfuric Acid Methods |
| Crude Protein | Lowry Method |
| Crude lipid | Acid Hydrolysis |
| Lignin | Acid Detergent Lignin (ADL) |
| Total ash | Muffle Furnace 550–600 °C |
| Composition | Water Hyacinth (%) | Cassava Chip (%) | Cassava Starch (%) | Cassava Pulp (%) |
|---|---|---|---|---|
| Cellulose | 35–40 | 4–5 | 0.1–0.5 | 10–28 |
| Crude fiber | 17.67 | 2–4 | 2–3 | 10–18 |
| Starch | 4.2 | 76–78 | 72–85 | 35–37 |
| Crude protein | 6.90 | 2–3 | 2–6 | 1.8–2.0 |
| Crude lipid | 1.18 | 0.5–0.8 | 0.05–0.45 | 0.4–0.5 |
| Lignin | 0.92 | 2–4 | * | 5–15 |
| Total ash | 33.92 | 2.6–3.0 | 1.5–3.0 | 3.5–3.7 |
| Characteristics | CMC (from Water Hyacinth) | CMC (Commercial Grade) |
|---|---|---|
| Color | Yellow-brown | Light cream |
| External appearance | Fluffy and light weight | Light weight |
| Gel information | Moderate | Good |
| Solubility | Moderate | Good |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dujjanutat, P.; Suwanrueng, W.; Kaewkannetra, P. Eco-Friendly Biopolymer Composite Sheet Derived from Water Hyacinth Reinforced with Cassava Chip: Optimal Conditions for Mixing, Blending, and Forming. Polymers 2025, 17, 2709. https://doi.org/10.3390/polym17192709
Dujjanutat P, Suwanrueng W, Kaewkannetra P. Eco-Friendly Biopolymer Composite Sheet Derived from Water Hyacinth Reinforced with Cassava Chip: Optimal Conditions for Mixing, Blending, and Forming. Polymers. 2025; 17(19):2709. https://doi.org/10.3390/polym17192709
Chicago/Turabian StyleDujjanutat, Praepilas, Woravut Suwanrueng, and Pakawadee Kaewkannetra. 2025. "Eco-Friendly Biopolymer Composite Sheet Derived from Water Hyacinth Reinforced with Cassava Chip: Optimal Conditions for Mixing, Blending, and Forming" Polymers 17, no. 19: 2709. https://doi.org/10.3390/polym17192709
APA StyleDujjanutat, P., Suwanrueng, W., & Kaewkannetra, P. (2025). Eco-Friendly Biopolymer Composite Sheet Derived from Water Hyacinth Reinforced with Cassava Chip: Optimal Conditions for Mixing, Blending, and Forming. Polymers, 17(19), 2709. https://doi.org/10.3390/polym17192709






