Effect of Iron Chloride Addition on Softwood Lignin Nano-Fiber Stabilization and Carbonization
Abstract
1. Introduction
2. Materials and Methods
2.1. Lignin Fiber
2.2. Stabilization and Carbonization of Electrospun Lignin Fibers
2.3. Lignin Fiber Analysis
3. Results and Discussion
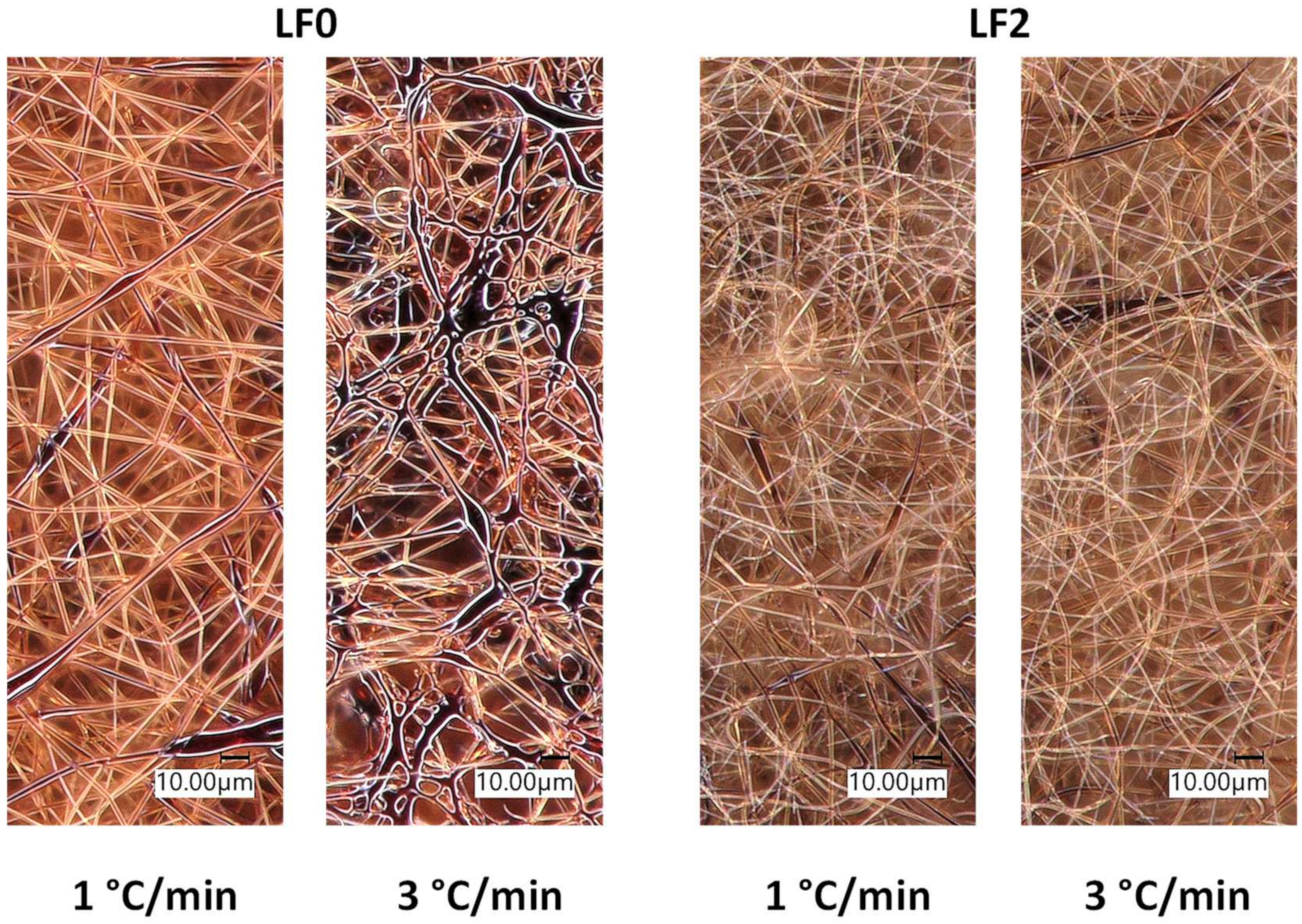
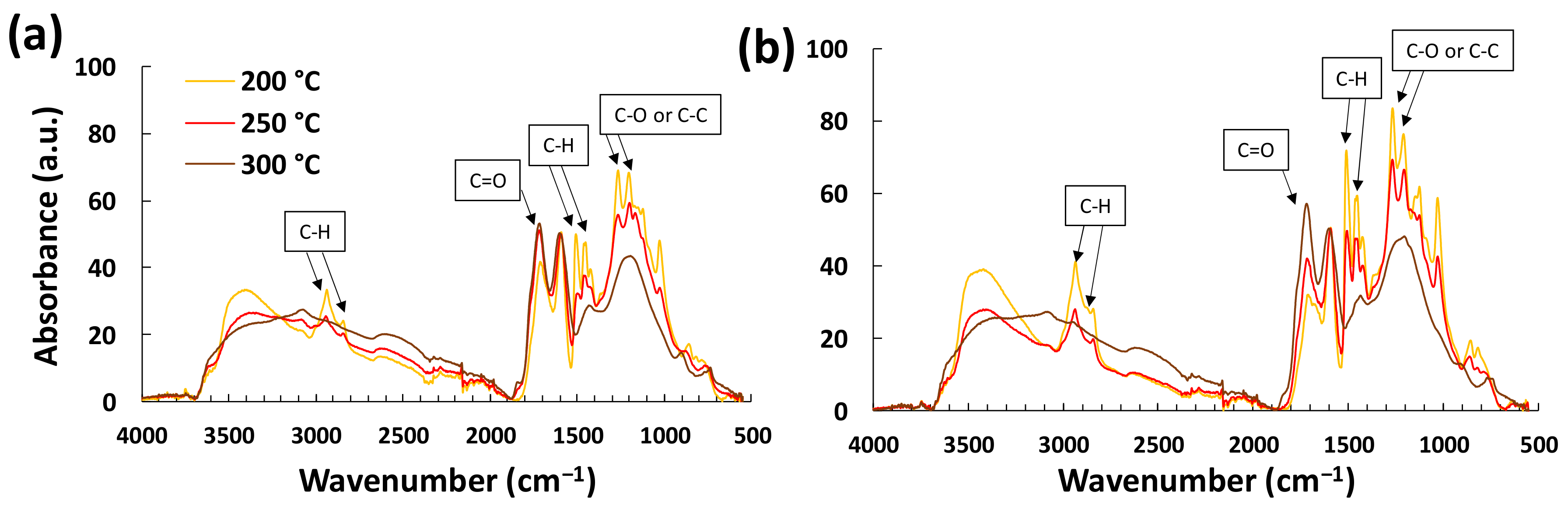
3.1. Effect of FeCl3 Addition on Lignin Fiber Stabilization
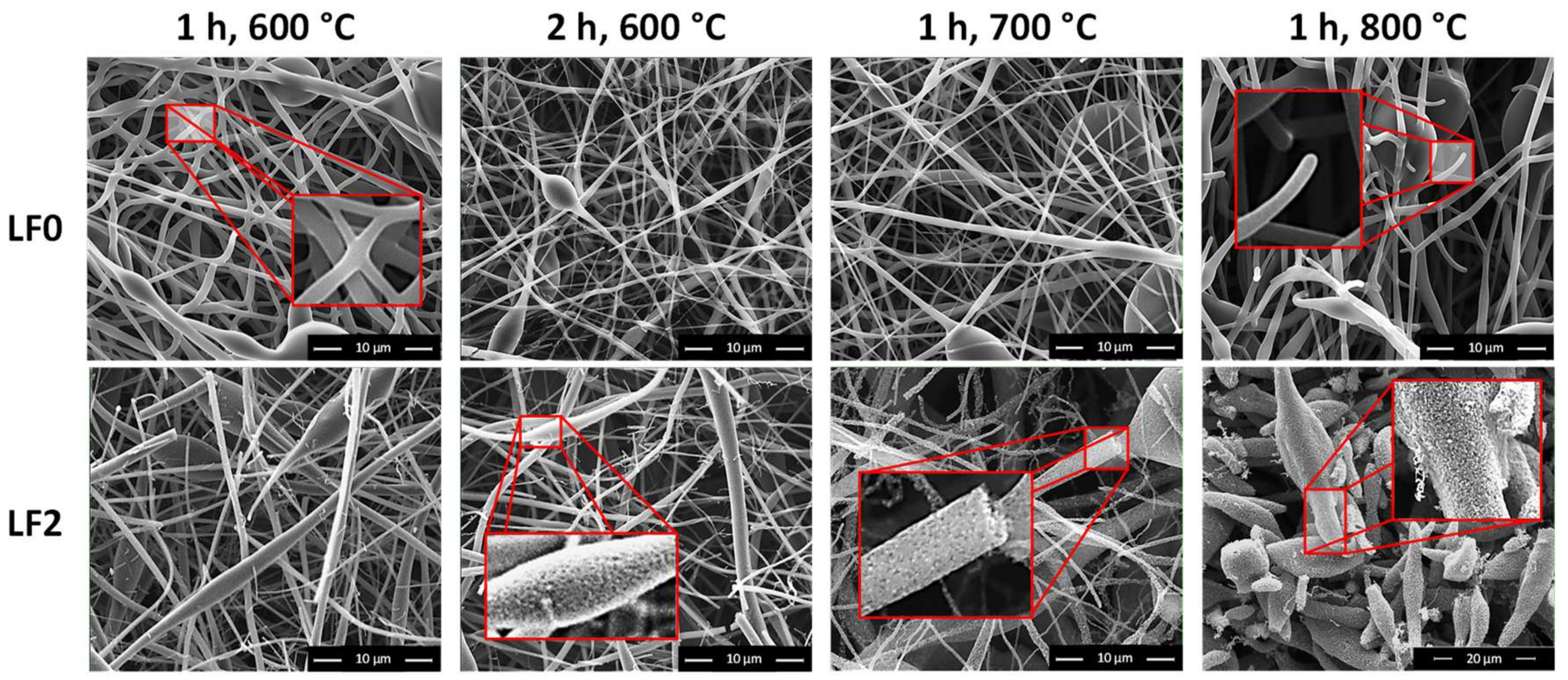
3.2. Effect of FeCl3 Addition on Lignin Fiber Carbonization
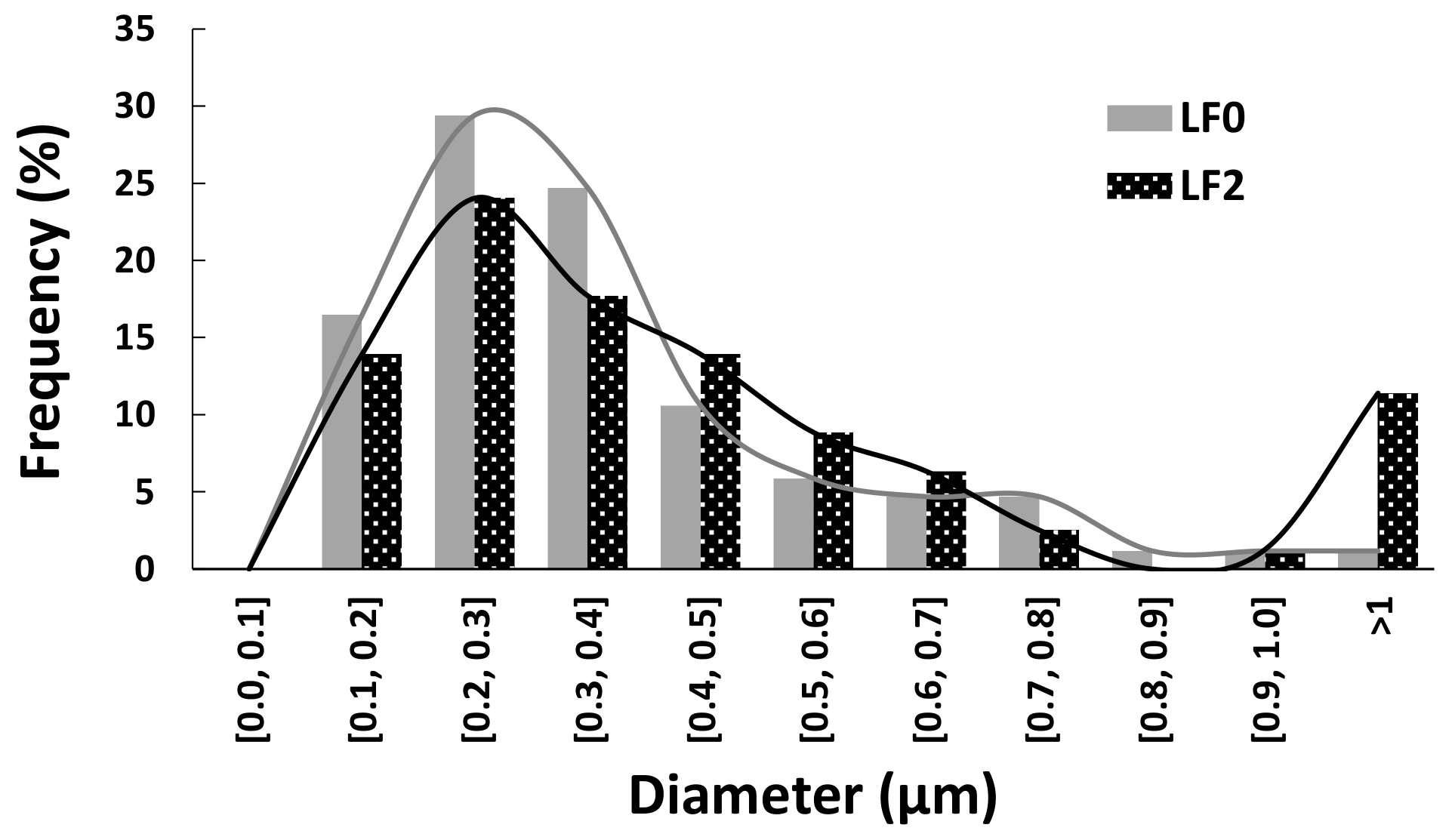
3.2.1. CF Diameter
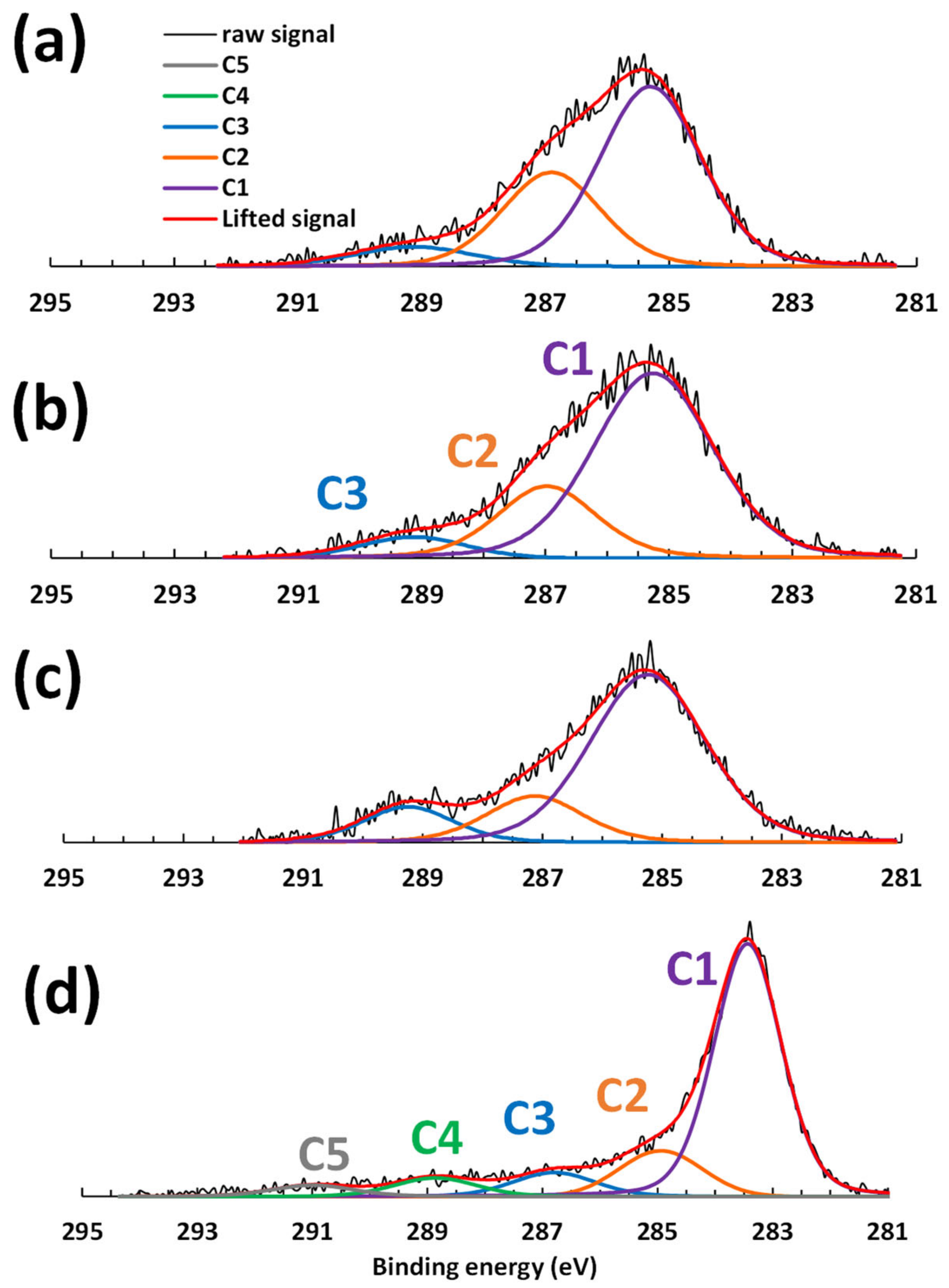
3.2.2. Surface Analysis of Carbon Fibers
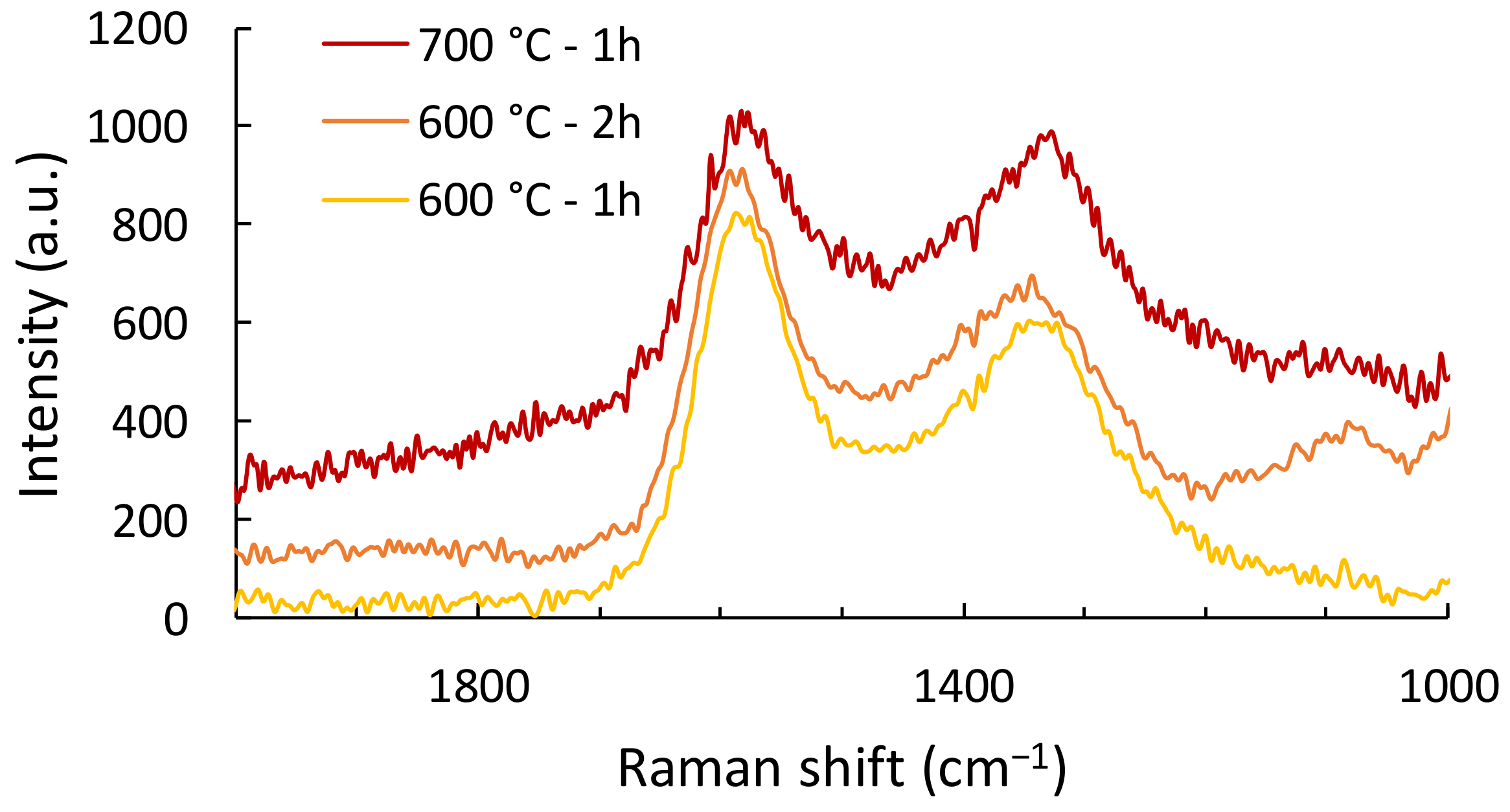
3.2.3. Structural Order Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamshaid, H.; Mishra, R. A green material from rock: Basalt fiber–a review. J. Text. Inst. 2016, 107, 923–937. [Google Scholar] [CrossRef]
- Lefeuvre, A.; Garnier, S.; Jacquemin, L.; Pillain, B.; Sonnemann, G. Anticipating in-use stocks of carbon fiber reinforced polymers and related waste flows generated by the commercial aeronautical sector until 2050. Resour. Conserv. Recycl. 2017, 125, 264–272. [Google Scholar] [CrossRef]
- Mainka, H.; Täger, O.; Körner, E.; Hilfert, L.; Busse, S.; Edelmann, F.T.; Herrmann, A.S. Lignin–an alternative precursor for sustainable and cost-effective automotive carbon fiber. J. Mater. Res. Technol. 2015, 4, 283–296. [Google Scholar] [CrossRef]
- Tang, S.; Hu, C. Design, Preparation and Properties of Carbon Fiber Reinforced Ultra-High Temperature Ceramic Composites for Aerospace Applications: A Review. J. Mater. Sci. Technol. 2017, 33, 117–130. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A.; Kaygusuz, K. Thermal conductivity improvement of stearic acid using expanded graphite and carbon fiber for energy storage applications. Renew. Energy 2007, 32, 2201–2210. [Google Scholar] [CrossRef]
- Snyder, J.F.; Wong, E.L.; Hubbard, C.W. Evaluation of Commercially Available Carbon Fibers, Fabrics, and Papers for Potential Use in Multifunctional Energy Storage Applications. J. Electrochem. Soc. 2009, 156, A215. [Google Scholar] [CrossRef]
- Lee, K.J.; Shiratori, N.; Lee, G.H.; Miyawaki, J.; Mochida, I.; Yoon, S.-H.; Jang, J. Activated carbon nanofiber produced from electrospun polyacrylonitrile nanofiber as a highly efficient formaldehyde adsorbent. Carbon 2010, 48, 4248–4255. [Google Scholar] [CrossRef]
- Xiong, W.; Tong, J.; Yang, Z.; Zeng, G.; Zhou, Y.; Wang, D.; Song, P.; Xu, R.; Zhang, C.; Cheng, M. Adsorption of phosphate from aqueous solution using iron-zirconium modified activated carbon nanofiber: Performance and mechanism. J. Colloid Interface Sci. 2017, 493, 17–23. [Google Scholar] [CrossRef]
- Khayyam, H.; Jazar, R.N.; Nunna, S.; Golkarnarenji, G.; Badii, K.; Fakhrhoseini, S.M.; Kumar, S.; Naebe, M. PAN precursor fabrication, applications and thermal stabilization process in carbon fiber production: Experimental and mathematical modelling. Prog. Mater. Sci. 2020, 107, 100575. [Google Scholar] [CrossRef]
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A review of heat treatment on polyacrylonitrile fiber. Polym. Degrad. Stab. 2007, 92, 1421–1432. [Google Scholar] [CrossRef]
- Frank, E.; Hermanutz, F.; Buchmeiser, M.R. Carbon Fibers: Precursors, Manufacturing, and Properties. Macromol. Mater. Eng. 2012, 297, 493–501. [Google Scholar] [CrossRef]
- Naito, K.; Tanaka, Y.; Yang, J.-M.; Kagawa, Y. Tensile properties of ultrahigh strength PAN-based, ultrahigh modulus pitch-based and high ductility pitch-based carbon fibers. Carbon 2008, 46, 189–195. [Google Scholar] [CrossRef]
- Baker, D.A.; Rials, T.G. Recent advances in low-cost carbon fiber manufacture from lignin. J. Appl. Polym. Sci. 2013, 130, 713–728. [Google Scholar] [CrossRef]
- Warren, C.D.; Eberle, C.C.; Naskar, A.K.; Paulauskas, F.L. Novel Precursor materials and approaches for producing lower cost carbon fiber for high volume industries. Mater. Sci. Eng. Environ. Sci. 2009, 18. [Google Scholar]
- Braun, J.L.; Holtman, K.M.; Kadla, J.F. Lignin-based carbon fibers: Oxidative thermostabilization of kraft lignin. Carbon 2005, 43, 385–394. [Google Scholar] [CrossRef]
- Kadla, J.F.; Kubo, S.; Venditti, R.A.; Gilbert, R.D.; Compere, A.L.; Griffith, W. Lignin-based carbon fibers for composite fiber applications. Carbon 2002, 40, 2913–2920. [Google Scholar] [CrossRef]
- Capanema, E.A.; Balakshin, M.Y.; Kadla, J.F. A Comprehensive Approach for Quantitative Lignin Characterization by NMR Spectroscopy. J. Agric. Food Chem. 2004, 52, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Rakotovelo, A.; Peruch, F.; Grelier, S. Lignine: Structure, Production et Valorisation Chimique, Technique de L’ingénieur. 2019. Available online: https://www.techniques-ingenieur.fr/base-documentaire/procedes-chimie-bio-agro-th2/chimie-du-vegetal-et-produits-biosources-42570210/lignine-structure-production-et-valorisation-chimique-in235/ (accessed on 14 April 2021).
- Shi, X.; Wang, X.; Tang, B.; Dai, Z.; Chen, K.; Zhou, J. Impact of lignin extraction methods on microstructure and mechanical properties of lignin-based carbon fibers. J. Appl. Polym. Sci. 2018, 135, 45580. [Google Scholar] [CrossRef]
- Norberg, I.; Nordström, Y.; Drougge, R.; Gellerstedt, G.; Sjöholm, E. A new method for stabilizing softwood kraft lignin fibers for carbon fiber production. J. Appl. Polym. Sci. 2013, 128, 3824–3830. [Google Scholar] [CrossRef]
- Cho, M.; Karaaslan, M.; Chowdhury, S.; Ko, F.; Renneckar, S. Skipping Oxidative Thermal Stabilization for Lignin-Based Carbon Nanofibers. ACS Sustain. Chem. Eng. 2018, 6, 6434–6444. [Google Scholar] [CrossRef]
- Ding, R.; Wu, H.; Thunga, M.; Bowler, N.; Kessler, M.R. Processing and characterization of low-cost electrospun carbon fibers from organosolv lignin/polyacrylonitrile blends. Carbon 2016, 100, 126–136. [Google Scholar] [CrossRef]
- Frank, E.; Steudle, L.M.; Ingildeev, D.; Spörl, J.M.; Buchmeiser, M.R. Carbon Fibers: Precursor Systems, Processing, Structure, and Properties. Angew. Chem. Int. Ed. 2014, 53, 5262–5298. [Google Scholar] [CrossRef] [PubMed]
- Huang, X. Fabrication and Properties of Carbon Fibers. Materials 2009, 2, 2369–2403. [Google Scholar] [CrossRef]
- Parot, M.; Rodrigue, D.; Stevanovic, T. Electrospinning of Softwood Organosolv Lignin without Polymer Addition. ACS Sustain. Chem. Eng. 2022, 11, 607–616. [Google Scholar] [CrossRef]
- Parot, M.; Rodrigue, D.; Stevanovic, T. High purity softwood lignin obtained by an eco-friendly organosolv process. Bioresour. Technol. Rep. 2022, 17, 100880. [Google Scholar] [CrossRef]
- Cho, M.; Ko, F.K.; Renneckar, S. Impact of Thermal Oxidative Stabilization on the Performance of Lignin-Based Carbon Nanofiber Mats. ACS Omega 2019, 4, 5345–5355. [Google Scholar] [CrossRef] [PubMed]
- Culebras, M.; Sanchis, M.J.; Beaucamp, A.; Carsí, M.; Kandola, B.K.; Horrocks, A.R.; Panzetti, G.; Birkinshaw, C.; Collins, M.N. Understanding the thermal and dielectric response of organosolv and modified kraft lignin as a carbon fibre precursor. Green Chem. 2018, 20, 4461–4472. [Google Scholar] [CrossRef]
- Brodin, I.; Ernstsson, M.; Gellerstedt, G.; Sjöholm, E. Oxidative stabilisation of kraft lignin for carbon fibre production. Holzforschung 2012, 66, 141–147. [Google Scholar] [CrossRef]
- MacDermid-Watts, K.; Adewakun, E.; Norouzi, O.; Abhi, T.D.; Pradhan, R.; Dutta, A. Effects of FeCl3 Catalytic Hydrothermal Carbonization on Chemical Activation of Corn Wet Distillers’ Fiber. ACS Omega 2021, 6, 14875–14886. [Google Scholar] [CrossRef]
- Svinterikos, E.; Zuburtikudis, I.; Al-Marzouqi, M. Electrospun Lignin-Derived Carbon Micro- and Nanofibers: A Review on Precursors, Properties, and Applications. ACS Sustain. Chem. Eng. 2020, 8, 13868–13893. [Google Scholar] [CrossRef]
- Wang, S.-X.; Yang, L.; Stubbs, L.P.; Li, X.; He, C. Lignin-Derived Fused Electrospun Carbon Fibrous Mats as High Performance Anode Materials for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2013, 5, 12275–12282. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-C.; Xu, Y.; Wen, J.-L.; Yuan, T.-Q.; Sun, R.-C. Recent advances in lignin-based carbon fibers (LCFs): Precursors, fabrications, properties, and applications. Green Chem. 2022, 24, 5709–5738. [Google Scholar] [CrossRef]
- Ruiz-Rosas, R.; Bedia, J.; Lallave, M.; Loscertales, I.G.; Barrero, A.; Rodríguez-Mirasol, J.; Cordero, T. The production of submicron diameter carbon fibers by the electrospinning of lignin. Carbon 2010, 48, 696–705. [Google Scholar] [CrossRef]
- Cao, Q.; Zhang, Y.; Chen, J.; Zhu, M.; Yang, C.; Guo, H.; Song, Y.; Li, Y.; Zhou, J. Electrospun biomass based carbon nanofibers as high-performance supercapacitors. Ind. Crops Prod. 2020, 148, 112181. [Google Scholar] [CrossRef]
- Schreiber, M.; Vivekanandhan, S.; Mohanty, A.K.; Misra, M. Iodine Treatment of Lignin–Cellulose Acetate Electrospun Fibers: Enhancement of Green Fiber Carbonization. ACS Sustain. Chem. Eng. 2015, 3, 33–41. [Google Scholar] [CrossRef]
- Svinterikos, E.; Zuburtikudis, I.; Al-Marzouqi, M. The nanoscale dimension determines the carbonization outcome of electrospun lignin/recycled-PET fibers. Chem. Eng. Sci. 2019, 202, 26–35. [Google Scholar] [CrossRef]
- García-Mateos, F.J.; Rosas, J.M.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T. Highly porous and conductive functional carbon fibers from electrospun phosphorus-containing lignin fibers. Carbon 2022, 200, 134–148. [Google Scholar] [CrossRef]
- Yang, C.; Wu, H.; Cai, M.; Zhou, Y.; Guo, C.; Han, Y.; Zhang, L. Valorization of Biomass-Derived Polymers to Functional Biochar Materials for Supercapacitor Applications via Pyrolysis: Advances and Perspectives. Polymers 2023, 15, 2741. [Google Scholar] [CrossRef]
- Kaciulis, S. Spectroscopy of carbon: From diamond to nitride films. Surf. Interface Anal. 2012, 44, 1155–1161. [Google Scholar] [CrossRef]
- Deng, L.; Young, R.J.; Kinloch, I.A.; Abdelkader, A.M.; Holmes, S.M.; De Haro-Del Rio, D.A.; Eichhorn, S.J. Supercapacitance from Cellulose and Carbon Nanotube Nanocomposite Fibers. ACS Appl. Mater. Interfaces 2013, 5, 9983–9990. [Google Scholar] [CrossRef]

| Code | Rate (°C/min) | Initial Temp. (°C) | Final Temp. (°C) | Holding Time (h) |
|---|---|---|---|---|
| Stabilization | ||||
| Ox1 | 1 | 30 | 200 | 2 |
| Ox2 | 1 | 30 | 250 | 2 |
| Ox3 | 1 | 30 | 300 | 2 |
| Ox4 | 1 | 30 | 400 | 2 |
| Ox5 | 2 | 30 | 200 | 2 |
| Ox6 | 2 | 30 | 250 | 2 |
| Ox7 | 2 | 30 | 300 | 2 |
| Ox8 | 3 | 30 | 200 | 2 |
| Ox9 | 3 | 30 | 250 | 2 |
| Ox10 | 3 | 30 | 300 | 2 |
| Carbonization | ||||
| Ca1 | 1 | R.T. | 500 | 1 |
| Ca2 | 5 | R.T. | 700 | 1 |
| Ca3 | 10 | R.T. | 600 | 1 |
| Ca4 | 10 | R.T. | 600 | 2 |
| Ca5 | 10 | R.T. | 700 | 1 |
| Ca6 | 10 | R.T. | 800 | 1 |
| Ca7 | 10 | R.T. | 900 | 1 |
| Sample | LF | LF after Oxidation | CF after Ca3 | CF after Ca4 | CF after Ca5 |
|---|---|---|---|---|---|
| Carbon (%) | 79.5 ± 0.5 | 74.2 ± 0.9 | 93.3 ± 0.6 | 92.5 ± 0.8 | 95.3 ± 0.9 |
| Oxygen (%) | 20.5 ± 0.5 | 25.8 ± 0.9 | 6.7 ± 0.6 | 7.5 ± 0.8 | 4.7 ± 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parot, M.; Rodrigue, D.; Stevanovic, T. Effect of Iron Chloride Addition on Softwood Lignin Nano-Fiber Stabilization and Carbonization. Polymers 2024, 16, 814. https://doi.org/10.3390/polym16060814
Parot M, Rodrigue D, Stevanovic T. Effect of Iron Chloride Addition on Softwood Lignin Nano-Fiber Stabilization and Carbonization. Polymers. 2024; 16(6):814. https://doi.org/10.3390/polym16060814
Chicago/Turabian StyleParot, Maxime, Denis Rodrigue, and Tatjana Stevanovic. 2024. "Effect of Iron Chloride Addition on Softwood Lignin Nano-Fiber Stabilization and Carbonization" Polymers 16, no. 6: 814. https://doi.org/10.3390/polym16060814
APA StyleParot, M., Rodrigue, D., & Stevanovic, T. (2024). Effect of Iron Chloride Addition on Softwood Lignin Nano-Fiber Stabilization and Carbonization. Polymers, 16(6), 814. https://doi.org/10.3390/polym16060814









